Abstract
Chronic traumatic encephalopathy (CTE) is a neurodegenerative disease confirmed at postmortem. Those at highest risk are professional athletes who participate in contact sports and military personnel who are exposed to repetitive blast events. All neuropathologically confirmed CTE cases, to date, have had a history of repetitive head impacts. This suggests that repetitive head impacts may be necessary for the initiation of the pathogenetic cascade that, in some cases, leads to CTE. Importantly, while all CTE appears to result from repetitive brain trauma, not all repetitive brain trauma results in CTE. Magnetic resonance imaging has great potential for understanding better the underlying mechanisms of repetitive brain trauma. In this review, we provide an overview of advanced imaging techniques currently used to investigate brain anomalies. We also provide an overview of neuroimaging findings in those exposed to repetitive head impacts in the acute/subacute and chronic phase of injury and in more neurodegenerative phases of injury, as well as in military personnel exposed to repetitive head impacts. Finally, we discuss future directions for research that will likely lead to a better understanding of the underlying mechanisms separating those who recover from repetitive brain trauma vs. those who go on to develop CTE.
Keywords: neuroimaging, repetitive head injury
Introduction
Concussion, or mild traumatic brain injury (mTBI), is a common injury that affects between 1.6 and 3.8 million people each year 37, 82. The symptoms following a concussion usually resolve within days to weeks, but for some postconcussive symptoms persist [see review in 141]. Most notably, there is evidence that those exposed to repetitive brain trauma may be at higher risk for persistent postconcussive symptoms, for structural alterations in the brain [see review in 119] as well as for developing a neurodegenerative disease such as chronic traumatic encephalopathy (CTE) [eg, 151; see also review by 156]. CTE is marked by a widespread accumulation of hyperphosphorylated tau (p‐tau) 107, 108. It is most frequently observed in professional athletes involved in contact sports (eg, boxing, American football) and in warfighters 55, 108. Particularly noteworthy, there is no evidence that a single mTBI or even a more severe single TBI leads to CTE 116, 150. In contrast, there are many cases of neuropathologically confirmed CTE without any reported or known symptomatic concussions but with significant exposure to repetitive subconcussive head impacts. In fact, to date, all cases of neuropathologically confirmed CTE have had a history of repetitive head impacts, including concussion or mTBI as well as subconcussive impacts, suggesting that repetitive head impacts may be necessary for the initiation of the pathogenetic cascade that eventually leads to neurodegeneration [eg, 13, 53, 107, 115, 116, 151].
Advanced neuroimaging techniques may lead to the early detection of brain alterations associated with repetitive head impacts, as well as to an improvement in our understanding of the underlying pathomechanisms that link exposure to repetitive head impacts to neurodegenerative disease.
The purpose of this review is to provide an overview of advanced neuroimaging findings in athletes and in military service members with repetitive head impacts (one civilian study was included that met repetitive head impact criteria, see below), and to elucidate possible trajectories such as chronic postconcussive syndrome and neurodegeneration.
In what follows, we first provide an introduction to different categories or classifications of brain trauma, including concussion (mTBI), subconcussive head impacts as well as the different trajectories observed, including chronic postconcussive syndrome and progressive neurodegeneration, such as CTE. Second, we provide a brief overview of advanced imaging techniques that are currently used to investigate repetitive brain trauma. Third, we review neuroimaging findings and we include comprehensive tables that list the major findings, along with specific information about the subjects evaluated and the methods used. Finally, we provide information regarding important future directions for research that will lead to a better understanding of the underlying mechanisms involved in those who recover vs. those who go on to experience postconcussive symptoms or a neurodegenerative disease such as CTE.
Definition of categories/classifications and stages of brain trauma
In what follows we introduce the concept of mTBI before describing repetitive concussive brain trauma and repetitive subconcussive head impacts/brain trauma. We also provide a figure that depicts how these categories and trajectories can be viewed in the context of repetitive brain trauma and its sequelae.
mTBI or concussion
mTBI (concussion) usually results from a bump or blow to the head that leads to temporary functional and structural alterations in the brain 26, 104, 110, 157. Common causes include sports‐related injuries, motor vehicle accidents and falls 134. In addition to a direct impact to the head, mTBI can also result from explosions 90. It is estimated that at least 6 per 1000 people experience mTBI each year [eg, 28]. Sports‐related concussion is particularly common with about a 20% risk per year for the individual contact sport athlete 35. However, the incidence of mTBI is likely to be much greater due to differences in the definition of mTBI and, more importantly, due to underreporting 82, 133.
Symptoms following mTBI include nausea, vomiting, headache, irritability, insomnia, anxiety, depression and sometimes personality change 19, 58, 74. These symptoms are typically most prominent immediately postinjury and typically resolve over days and weeks, although cognitive and behavioral sequelae may persist for months or even years 80, 81, 95, 127. These cognitive and behavioral deficits are generally mild and generally resolve on their own 18, 137. However, a significant number of those afflicted (15%–30%), the so‐called miserable minority 135, suffer from persistent postconcussive symptoms [see review in 141].
Although the precise pathomechanisms that link mTBI to neuropathological changes are not completely understood, they likely involve a series of multifocal axonal injuries set in motion by the initial trauma. During mTBI, the brain undergoes shear deformation that produces a stretch of axons resulting in alterations in axonal membrane permeability and ionic shifts, including a massive influx of calcium into the cell, which, in turn, leads to an accumulation in the mitochondria, which impairs oxidative metabolism, leading to energy failure and to the breakdown of microtubules 21, 54. Additive effects may include a decrease in total cerebral blood flow, activation of N‐methyl‐d‐aspartate receptors and a decrease in gamma‐aminobutyric acid and other inhibitory neurotransmitters 22, 54, 139. Trauma‐induced metabolic changes, however, may return to baseline within a relatively short period of time. Nonetheless, advanced neuroimaging techniques reveal structural and functional brain abnormalities following mTBI [see review in 120, 141].
Repetitive TBI
There is evidence that the brain is particularly vulnerable when recovering from mTBI. The most severe form of repetitive mTBI is referred to as second‐impact syndrome. It is a rare, often fatal, TBI that may occur when the first brain trauma is followed shortly by a second brain trauma before the symptoms of the first trauma have resolved 27. Although there have been several cases described in the literature 27, 105, 109, second‐impact syndrome remains a controversial diagnosis and the underlying mechanisms are still unclear 103.
The focus of this review is not on single mTBI or on second‐impact syndrome. It is instead on repetitive brain trauma, which may result from multiple head impacts over the course of months and years, as experienced most frequently by athletes who participate in contact sports as well as by soldiers who are exposed to multiple blast events. The incidence of repetitive subconcussive head impacts [ie, hits to the head with enough force to have an impact on neuronal integrity, but without associated symptoms] is much greater than that of impacts that may lead to concussion. For example, a recent study by Broglio et al 26 found that the average high school football player receives 652 hits to the head per season that exceed 15 g's of force. Similarly, repeat deployments, mission demands and the use of improvised explosive devices increase the exposure of military service members to repetitive mTBI. More specifically, one study reported 4623 combat blast exposures in military personnel deployed to Iraq between March 2004 and December 2007, where 273 (5.9%) were characterized by repetitive blast events, that is, up to four blast events per person 49. Those who experience repetitive brain trauma are particularly vulnerable to developing persistent postconcussive symptoms 117. Moreover, repetitive brain trauma may be a necessary condition for developing CTE. Further, and as noted previously, to date, all neuropathologically confirmed cases of CTE have had a history of repetitive brain trauma 151. Importantly, however, a history of repetitive brain trauma may also lead to other neurodegenerative disorders such as Parkinson's disease (PD), frontotemporal lobar degeneration (FTLD) as well as other neurodegenerative diseases characterized by tauopathies [see below; see also 65, 156].
Repetitive subconcussive brain trauma
Subconcussive head impacts are even more common than head impacts that lead to concussions. The term “subconcussive” was introduced to describe impact to the head that produces neuronal changes similar to those in concussion, but without the acute symptoms 9. Most concerning, however, is the fact that because there is often no evidence of clinical symptoms, subconcussive head impacts are generally considered to be harmless. Although little is known about the pathophysiology of subconcussive blows, recent neuroimaging literature has shown that this may not be the case. For example, in a study by our group we used advanced, sensitive neuroimaging techniques and demonstrated, for the first time, alterations in the brain's microstructure in soccer players who are at high risk for repetitive subconcussive head impacts when heading the ball 78 (Figure 1). The soccer players included in this study were selected specifically for having no history of concussive brain trauma 78. This finding is not only alarming, given the large number of soccer players worldwide, but, and most importantly, it challenges current concepts of the effects of repetitive head impacts by demonstrating that even repetitive subconcussive head impacts may lead to subtle alterations in the brain. Findings from this study are consistent with a very recent study using task‐based functional magnetic resonance imaging (MRI) that reports impaired brain function in college‐level contact sports athletes without a history of concussion 100. They are also consistent with a series of studies demonstrating cognitive 170, functional 1, 25, 154 and biochemical changes 128 in the brains of contact sport athletes, despite a lack of diagnosed concussion. It is unknown, however, whether or not these findings represent the direct cumulative effects of repetitive brain trauma (eg, axonopathy) or if these findings reflect a vulnerability to developing a neurodegenerative disease, including CTE 151.
Figure 1.
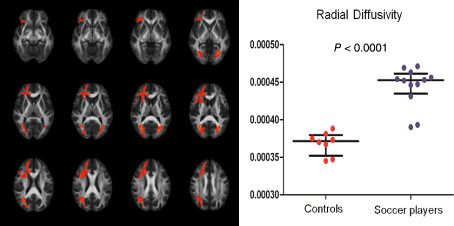
Soccer players experience repetitive subconcussive head impacts while heading the ball. A recent study investigated the white matter microstructure using diffusion tensor imaging in a group of professional soccer players compared with swimmers. Tract‐based spatial statistics revealed increased radial diffusivity in widespread white matter regions in soccer players. Journal of the American Medical Association [see 78].
Chronic traumatic encephalopathy
CTE, historically referred to as “punch drunk,” “dementia pugilistica” or “boxer's dementia” 39, 94, 112, is a neurodegenerative disease believed to be caused, in part, from exposure to repetitive head impacts. Bowman and Blau 24 were the first to use the term “chronic traumatic encephalopathy” in their description of a 28‐year‐old professional boxer who suffered from progressive changes in behavior, mood and cognition. Since that time, several others have used this term 41, 42, 111. For example, Critchley, in 1957, reviewed 69 cases of progressive neurodegenerative disease in professional boxers and suggested that the term “chronic progressive traumatic encephalopathy of boxers” be used to describe the neurodegenerative changes he observed 42. Some of the symptoms observed were similar to postconcussive symptoms and acute concussion such as headaches and dizziness, but other symptoms were more disabling including mood swings, euphoria, emotional lability, unsteady gait (see also below) as well as changes in behavior. Additionally, Miller 111 also used the term CTE to describe the kind of symptoms that he thought resembled neurodegenerative disorders such as Alzheimer's disease (AD) and FTLD 111. The diagnosis, however, as noted previously, is made postmortem. Symptoms may begin years or even decades following brain trauma exposure and include memory and other cognitive impairments, depression and suicidality, as well as problems with impulse control 150, 151. As the disease progresses, it may lead to dementia such as CTE or even to other neurological diseases [see 65, 156].
CTE has been reported most often in professional athletes involved in contact sports (eg, boxing, American football) who have been subjected to repetitive head impacts. CTE has also been reported in individuals with epilepsy, developmentally disabled individuals with head banging behavior and in victims of physical abuse 108, 151. Additionally, CTE has been reported in military personnel with a history of repetitive head impacts or blast events 55, 108. Thus, the number of those affected is potentially quite large. Besides exposure to repetitive brain trauma, it is not known to date what additional risk factors lead to CTE. There are, for example, cases of young athletes who show evidence of CTE, suggesting that there may be some who are more vulnerable to the cascade of neurodegenerative changes it involves. Genetic factors such as apolipoprotein 4 (AP0E4), which has been studied in AD, may also be a risk factor for CTE [eg, 44].
CTE is defined by a specific pattern of neuropathological changes, that is, the presence of p‐tau protein in the form of neurofibrillary tangles, glial tangles and neuropil threads, with widespread distribution throughout the brain, beginning primarily with focal perivascular deposits at the depths of the cortical sulci 107 (for review please see article by McKee in this special issue). While other neurodegenerative diseases also involve p‐tau (eg, AD, frontotemporal lobe dementia), the specific regional and cortical distribution of this protein in CTE is thought to be unique 108, 116, 151, although Tartaglia et al in an extensive review of CTE note that not all those who experience multiple head impacts and develop a neurodegenerative disease will have neuropathologically confirmed CTE 156. Thus, a better understanding of the differences and similarities of CTE with other neurodegenerative diseases, including possible coexisting neurodegenerative disorders, would help guide possible future interventions. Hazrati and colleagues, for example, examined, prior to death, six retired Canadian Football League players who had a history of concussions and symptoms 65. At postmortem, three showed a pattern consistent with CTE. Of the three, there was comorbid evidence of AD, PD or vasculopathy, and the other three showed evidence of PD, AD and amyotrophic lateral sclerosis, respectively.
Understanding concepts
Concussive head impacts may lead to acute symptoms that generally resolve within days or weeks (see Figure 2, as indicated with blue lines). Subconcussive head impacts may not lead to acute symptoms, as indicated by the horizontal dotted blue line in the acute/subacute stage of Figure 2. Following brain trauma, between 15% and 30% of patients develop chronic postconcussive symptoms that either resolve within months or years (blue line in Figure 2) or remain the same (yellow band in Figure 2). Those who are exposed to repetitive head impacts are particularly at high risk for developing chronic symptoms. Moreover, a small number of those exposed to repetitive head impacts will develop a progressive neurodegenerative disease such as CTE (red band in Figure 2).
Figure 2.
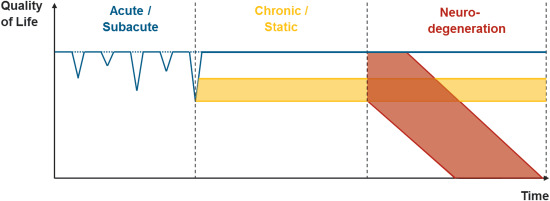
We present a multistage disease model of short‐ and long‐term consequence following repetitive brain trauma. Quality of life is indicated by symptom load, which is expressed as a function of time, thereby allowing for the differentiation between at least three main trajectories of the disease including an acute/subacute phase, a chronic/static phase and a phase of possible neurodegeneration.
Short primer on relevant imaging modalities
In acute hospital settings, both computed tomography (CT) and conventional MRI are used to rule out severe complications resulting from a TBI, including skull fracture, intracranial hemorrhage and/or brain edema. However, conventional CT and MRI have proven to be insensitive to the more subtle changes in the brain such as diffuse axonal injury following repetitive brain trauma. Moreover, conventional imaging modalities do not provide accurate information relevant to long‐term prognosis 70, 84, 114. Thus, highly sensitive as well as objective measures of early diagnosis and accurate prognosis are needed to detect structural and functional brain alterations following repetitive brain trauma.
In what follows, we provide a brief overview of advanced neuroimaging techniques that are currently being used in research studies to evaluate brain structure (high‐resolution structural MRI), micro‐hemorrhages (susceptibility‐weighted imaging, SWI), tissue microarchitecture (diffusion tensor imaging, DTI), brain metabolism (magnetic resonance spectroscopy, MRS; positron emission tomography, PET), brain function (functional and functional connectivity MRI) and, finally, regional blood flow (single‐photon emission computer tomography, SPECT).
High‐resolution structural imaging
Based on T1‐weighted MRI, the entire human brain volume, as well as specific white or gray matter structures, can be quantified and characterized. Methods of quantification include region of interest (ROI) (Figure 3), voxel‐based morphometry as well as almost fully automated software tools such as Statistical Parametric Mapping (http://www.fil.ion.ucl.ac.uk/spm/) and FreeSurfer 76 75 74 (Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, USA), all of which enable comparisons between groups. Quantitative analyses of brain volume, and characteristics of specific structures such as cortical thickness, provide useful information for the early diagnosis and prognosis of neurodegenerative diseases 31, 45, 69, 72, 123, 138, 146. For example, brain volume and cortical thickness have been shown to correlate with cognitive performance 31, 138, 146. Recent literature also suggests that the quantitative analysis of the brain's structure, such as cortical thickness, may be helpful in both diagnostic assessment and in determining the prognosis of mTBI 159 and repetitive brain trauma 145. Figure 4 depicts an image of the cortical surface of the brain where measures of cortical thickness may now be quantified.
Figure 3.
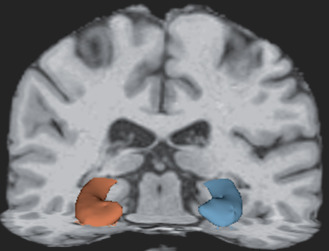
Three‐dimensional reconstruction of the hippocampus. The hippocampus has been associated with memory impairment in traumatic brain injury and repetitive brain trauma.
Figure 4.
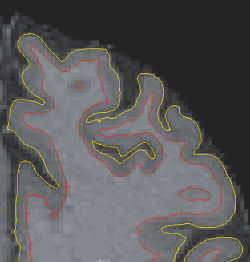
Surface lines of pial (yellow) and white matter (red) superimposed on a T1‐weighted image. Surface segmentation was performed using FreeSurfer 5.1 in a retired national football league player, the surfaces can then be used to calculate cortical thickness and perform group comparisons as well as associations with, for example, neuropsychological test evaluations.
Susceptibility Weighted Imaging
SWI is based on a gradient‐recalled echo MRI that takes advantage of the different responses of molecules to an applied magnetic field (susceptibility), which can be measured as phase shifts. These phase shifts are then superimposed on the conventional MRI, which primarily uses the signal magnitude. This overlaying accentuates local susceptibility changes in the final image. The resulting contrast is sensitive to venous blood, hemorrhage and iron in the brain 59. SWI has proven to be a sensitive technique to detect microhemorrhages and to predict severity of brain injury following TBI 6, 11, 17. The number and volume of microhemorrhages identified using SWI have been shown to be correlated with neuropsychological and clinical outcome 36. SWI is just beginning to be used to investigate repetitive brain trauma [see review of the literature below; see also 66]. Figure 5 shows evidence of microhemorrhages following repetitive head impacts detected using SWI.
Figure 5.
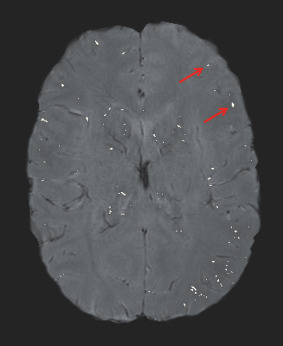
Susceptibility‐weighted image (SWI) with superimposed lesion mask. SWI is sensitive to detect microhemorrhages following brain trauma. Typically, the hemorrhages appear dark in SWI making them difficult to separate from blood vessels. A threshold mask is used such that lesions are hyperintense and can be used to calculate the hypointensity burden [see 66].
Diffusion Tensor Imaging
DTI is an advanced MRI technique that quantifies the diffusion properties of water molecules in tissue 12. Diffusion indices provide indirect information about the tissue's microstructure. Isotropic diffusion is characterized by water molecules that diffuse with the same probability and speed in all directions. This is found, for example, in the cerebrospinal fluid (CSF). In contrast, anisotropic diffusion results when the diffusion of water molecules is restricted as, for example, in restrictions resulting from axonal membranes, filaments and/or myelin sheaths, which lead to directionality, or anisotropy, in the motion of water molecules. Moreover, based on the diffusion of water molecules, the diffusion tensor can be quantified and characterized in each voxel. Common parameters are mean diffusivity (MD), which is the average of the diffusion, and fractional anisotropy (FA), which takes into account the directionality of the diffusion. In addition, radial (RD) and axial diffusivity (AD) can be calculated relative to the main vector of the diffusion 12. Accordingly, DTI has proven to be a particularly sensitive technique to evaluate the brain's white matter microstructure following mTBI. For reviews, see Niogi and Mukherjee 120 and Shenton et al 141. This makes sense given that the most common injury observed in mTBI is diffuse axonal injury, thus making DTI an important imaging technique for investigating brain alterations in mTBI. These findings will be reviewed below in the context of repetitive brain trauma.
Magnetic Resonance Spectroscopy
MRS differs from other imaging methods in TBI in that it measures the concentration of chemicals in the brain. These chemicals are often involved in metabolic processes in the brain and as such provide a window into the underlying pathological changes that can occur as a result of brain injury from acute to more chronic stages. This quantitative, noninvasive and objective technique has demonstrated its value in diagnosis and prognosis, particularly in severe brain injury. Specific chemical changes identified by MRS are also amenable to targeted treatment and treatment monitoring. This research technique is available across all MR scanners as a software option, and it shows great promise for translation into clinical practice [eg, 86]. The major metabolites measured by MRS include the following: (i) N‐acetyl aspartate (NAA), a putative marker of viable neurons and axons; (ii) glutamate (Glx), the primary excitatory neurotransmitter in the brain, tightly coupled to glutamine and found in astrocytes; (iii) glutathione (GSH), an anti‐oxidant that is reduced with oxidative stress; (iv) creatine (Cr), an energy marker that is often used as an internal reference for the measurement of other peaks; (v) choline (Cho), a membrane marker used to measure changes in brain tissue; and (vi) myoinositol (mI), an astrocyte marker and osmolyte (Figure 6). Specific patterns of change in these metabolites can provide insight into biological processes such as neuroinflammation, which has been shown not only to reduce GSH levels but also to increase Glx and mI levels. Of particular note, and as described below, MRS has been applied to mTBI and has shown significant changes in brain metabolism following repetitive brain trauma 119.
Figure 6.
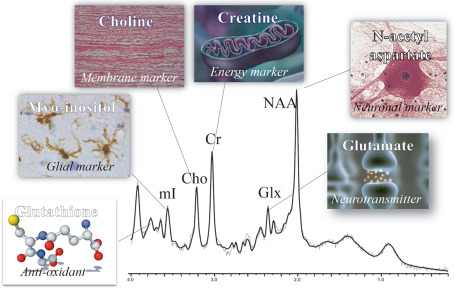
Spectrum obtained using magnetic resonance spectroscopy. Cho = choline; Cr = creatine; Glx = glutamate; mI = myoinositol; NAA = N‐acetyl aspartate. [see 86]
Functional MRI (fMRI) and resting state functional MRI
Functional MRI measures the cerebral blood flow via the blood oxygen level‐dependent contrast (BOLD contrast) due to the presence of deoxyhemoglobin in the blood. Functional MRI is based on the assumption that cerebral blood flow is directly linked to neuronal activity. For example, when a subject performs a visual activation or attention task, increased blood flows to the region of the brain responsible for the task. One method of fMRI is to subtract images acquired during the task from those acquired when not performing the task in order to determine which brain regions show signal changes that may indicate neuronal activity. Another method is to compare images acquired during tasks of increasing cognitive demand for a particular cognitive task, and yet another is to identify multiple tasks that rely on the same cognitive process and measure commonalities in activation across tasks. Tasks may include tests of memory, executive function or other brain functions. One unique method of fMRI examines activity during “resting state,” which presumably reveals connectivity between different brain regions. Although there have been a number of studies in mTBI [eg, 106], there have been very few studies in repetitive brain trauma that have used fMRI to understand brain function and dysfunction in repetitive brain injury [eg, 136, 154].
Positron Emission Tomography
PET uses positron‐emitting radionucleotides bound to ligands, which are molecules that have specific biological roles in the brain. When injected, these radiopharmaceuticals are taken up into the brain and imaged in three dimensions to provide a functional assessment of brain activity. A commonly used marker is 18‐fluorodeoxyglucose ([18F]‐FDG), an analog of glucose. Here, increased signal indicates an area of increased binding or uptake of the marker. The acquisition time of the data is relatively short due to high sensitivity, although this time may be limited by the half‐life of the radionucleotide that is used. Like fMRI, PET can be performed either in a resting state or with performance stimuli 52, 56, 68, 73.
Of quite recent origin is the development of PET ligands that specifically target tau 165. Shah and Catafau 140 reviewed six new tau PET radiotracers that have been tested in humans ([11C]‐PBB3, [18F]‐THK523, [18F]‐THK515, [18F]‐THK5119, [18F]‐T807 and [18F]‐T808). The first is not practical for clinical use given its short half‐life of 20 minutes. The family of arylquinolines that comprise the next three tau ligands is also not as amendable for clinical use because the first does not label tau lesions in non‐AD tauopathies and has high retention in white matter, whereas the latter two have more data in vitro and less data in vivo. Finally, [18F]‐T807 and [18F]‐T808 both show strong selectivity for tau vs. amyloid beta (Aβ) in vitro 165, 171, although in vivo studies in humans are more promising for [18F]‐T807 33 than [18F]‐T808 34 because human studies with [18F]‐T808 show bone uptake in skull due to defluorination, which may interfere with interpreting images. This is not seen in [18F]‐T807, which shows good clearance from normal gray and white matter, although it has only been tested in AD, with the exception of one case study of a retired NFL player by Mitsis et al 113 and one ex vivo study of a former NFL player with neuropathologically confirmed CTE, where the fluorescent probe T557 and the radiolabeled [18F]‐T807 were used and showed increased binding to paired helical filament (PHF‐tau) in brain sections in Alzheimer's and in one case of confirmed CTE 7 (note: abstract does not include the CTE case but it was presented at the meeting). Thus, a focus on PET tau ligands that can image tauopathy in living brains may be an important first step toward the diagnosis of CTE during lifetime, which may, in turn, lead to possible interventions that might decrease or eliminate hyperphosphorylated tau in the brain and prevent further neurodegenerative changes. (See also below under Neurodegenerative section, under American Football and under Future Directions for Research for a further discussion of tau and Aβ PET studies and their role in furthering our understanding of the specificity of tauopathy vs. amyloid neuritic plaques in CTE.)
Single Photon Emission Computed Tomography
SPECT is a functional imaging technique used to determine blood flow in the brain based on the distribution of radioactive pharmaceuticals in the brain. The most common radiopharmaceutical used for SPECT imaging in the brain is technetium‐99m‐hexamethylpropyleneamine oxime (99mTc‐HMPAO). 99mTc‐HMPAO is injected into the bloodstream of patients and as the radioisotope decays and accumulates in areas of blood flow, the photons emitted are detected and recorded by gamma‐cameras. Cameras rotate 360 degrees around the subject and produce three‐dimensional tomographic images of activity in the brain. Therefore, SPECT is utilized as a resting state functional neuroimaging technique similar to functional MRI methods. The radionuclides can be generated much more cheaply than PET isotopes, they have a longer half‐life and they are more readily available for clinical use. Despite these factors, SPECT has only seen very limited use in the study of repetitive brain trauma.
Imaging findings associated with different trajectories of repetitive brain trauma
Introduction
In this section, we present neuroimaging findings in the following categories: (i) acute/subacute injuries (Table 1); (ii) chronic injuries (Table 2); (iii) neurodegenerative changes (Table 3); and (iv) military (Table 4). Acute/subacute injuries include studies on athletes who are currently playing sports and who experienced repetitive subconcussive and/or concussive head impacts. Chronic injuries include imaging findings from studies of retired athletes who were examined months to years after their last concussion. In neurodegenerative changes, we include imaging findings from studies of athletes who are primarily retired and who experience symptoms of and are likely to be at risk for CTE (and one civilian study that met criteria). Finally, in military injuries, we include imaging findings from studies of military personnel who have experienced repetitive head impacts.
Table 1.
Acute/subacute injuries
| First author | Year | Title | Journal | Predominant injury mechanism | Sports/military | Subjects (n, gender, age) | Modality sequence | Analysis technique | Main findings |
|---|---|---|---|---|---|---|---|---|---|
| Levin HS | 1987 | Neurobehavioral functioning and magnetic resonance imaging findings in young boxers | J Neurosurg | Repetitive (sub)concussive head impacts | Boxing | 12 professional + 3 amateur boxers (male, mean age 20.5 ± 2.1 years), controls with frequent amateur sport participation (male, mean age 20.0 ± 2.2 years) |
0.35 T MRI T1w |
Reading by two neuroradiologists | No evidence of increased sulcal prominence or ventricular enlargement. Also no group difference in neurocognitive testing, suggestive of cerebral atrophy or cavum septum pellucidum. Qualitative study using clinical reads of scans. Advanced technology only beginning to be used but not in this area of research. |
| Jordan BD | 1990 | Computed tomography and magnetic resonance imaging comparisons in boxers | JAMA | Repetitive (sub)concussive head impacts | Boxing | 21 boxers (male, 1 retired, 16 active professionals, 4 active amateur, age range 21–66 years, mean age 28.3 years) | 0.5 T MRI T1w, T2w, CT | Clinical radiological read and neurologic examination | Goal: to compare effectiveness of MRI vs. CT imaging in evaluating brain trauma in boxers. 11 boxers had normal findings on CT and MRI, seven had abnormal findings on both. Concluded that MRI was superior to CT in delineating more clearly brain abnormalities, for example, small subdural hematoma that was visible on MRI was not visible on CT. Also MRI detected more abnormalities than did CT. There were no cases where CT was superior to MRI. Findings include, but were not limited to, periventricular white matter hyperintensities, CSP, hydrocephalus. |
| Kemp PM | 1995 | Cerebral perfusion and psychometric testing in military amateur boxers and controls | J Neurol Neurosurg Psychiatry | Repetitive (sub)concussive head impacts | Boxing | 34 amateur boxers (male, mean age 22.0 ± 2.9 years), controls with frequent amateur sport participation (male, mean age 22.2 ± 3.1 years) | Tc‐99m HMPAO SPECT | Comparison with “atlas of normality”, image was defined “abnormal” if it contained at least one deficit with 10 connected voxel above threshold of 2.9 SDs | Significantly more abnormalities in boxers (14 of 34 “abnormal”) on cerebral perfusion imaging compared with controls (5 of 34) “abnormal”), boxers performed worse in psychometric testing compared with controls, and boxers who fought more bouts/years performed worse than those with fewer bouts/years. |
| Zhang L | 2003 | Increased diffusion in the brain of professional boxers: a preclinical sign of traumatic brain injury? | AJNR Am J Neuroradiol | Repetitive (sub)concussive head impacts | Boxing | 24 professional boxers (male, age range 21–53 years, mean 32.2 ± 7.2 years), 14 controls (male, age‐matched to boxers) | 1.5 T MRI T1w, DTI | DTI: whole‐brain diffusion histogram, fitted to triple Gaussian curve to represent tissues and mixing; calculation of mean brain diffusion constant and the width of distribution in a histogram. T1: scoring of CSP, SWM, PWMD and volume loss (scoring confirmed by qualitative rating system) | Diffusion values were significantly higher in boxers. Increased diffusion coefficient was correlated with frequency of hospitalization for boxing injuries. The most common MR findings were volume loss, inappropriate to age, in cavum septi pellucidi, SWM and PWMD. Findings suggest that microstructural damage to the brain is associated with chronic, repetitive, brain injury even when clinical MRI is normal. |
| Chappell MH | 2006 | Distribution of microstructural damage in the brains of professional boxers: a diffusion MRI study | J Magn Reson Imaging | Repetitive (sub)concussive head impacts | Boxing | 81 professional boxers (male, 20–42 years, median age 28 years), 12 controls (male, 22–31 years, median age 25.5 years) | 1.5 T MRI DTI | Voxel‐wise based statistical analysis VBA and SPM | Increased ADC and decreased FA was observed in deep WM, whereas decreases in ADC were observed in cortical gray matter in boxers compared with controls. Clusters of positive correlation of ADC with age were found in both groups, although regions and strength of correlation differed. No subject had a history of moderate or severe head trauma. These findings suggest that cumulative brain injury results from nonsevere repetitive head trauma. |
| Zhang L | 2006 | Diffusion anisotropy changes in the brains of professional boxers | AJNR Am J Neuroradiol | Repetitive (sub)concussive head impacts | Boxing | 49 professional boxers (male, mean age 30 ± 4.5 years), 19 controls (male, mean age 32 ± 9.5 years) | 1.5 T MRI T1w, T2w, DTI | Voxel‐wise analysis (“pixel by pixel” … ) | 42 of 49 boxers had normal clinical MRI, and none had a neurological disorder. Nonetheless, group differences were evident in diffusion and anisotropy measurements in boxers compared with healthy controls, especially in the CC and in the posterior limb of internal capsule. These findings were interpreted as possible preclinical signs of subtle brain injury. |
| Tanriverdi F | 2008 | Brief communication: pituitary volume and function in competing and retired male boxers | Ann Intern Med | Repetitive (sub)concussive head impacts | Boxing | 21 young professional boxers (male, mean age 18 years, age range 17–19 years), 23 adult male professional boxers (mean age 22 years, age range 19–28 years), 17 retired male professional boxers (mean age 42 years, range 32–53 years), from Turkish National Boxing Team; no comparison group | 1.5 T MRI T1w | Volumetry of pituitary gland in 38 (of 61) randomly selected boxers. No description of exact method or software. Serum hormones measured using radioimmunoassay, immunoradiometric assay or chemiluminescent methods | 9 out of 61 (15%) boxers had growth hormone (GH) deficiency. All except 1 of the 9 was retired. Of the 17 retired boxers, eight (47%) had GH deficiency. Retired boxers without GH deficiency (n = 8) also had lower pituitary volume than retired boxers with normal GH (n = 9). Comparing 7 GH‐deficient boxers to 31 GH normals, pituitary volume was significantly lower in those with GH deficiency. Mean pituitary volume was significantly lower in adult and retired boxers than in young boxers. Limitations are lack of control group and small number of subjects with volume measures. |
| Chappell MH | 2008 | Multivariate analysis of diffusion tensor imaging data improves the detection of microstructural damage in young professional boxers | MRI | Repetitive (sub)concussive head impacts | Boxing | 59 professional boxer (male, age range 22–31 years), 12 controls (male, age range 22–31 years) | 1.5 T MRI DTI | 2 multivariate voxel‐wise analyses were employed, 1: Hotelling's T2 multivariate analysis using MD and FA, and 2: t‐test on linear discriminant analysis (LDA) that calculates a “separability metric” from MD, FA and mode | Previous univariate analyses were not able to differentiate between groups. Here, two multivariate methods were used where the LDA method demonstrated a better separation between groups than the Hotelling's method. This study uses interesting multivariate techniques, but the sample size for the controls is small (n = 12), which limits the power. |
| Hahnel S | 2008 | Prevalence of cerebral microhemorrhages in amateur boxers as detected by 3 T MR imaging | AJNR Am J Neuroradiol | Repetitive (sub)concussive head impacts | Boxing | 42 amateur boxers (male, age range 18–65 years; mean age 32.95 years), 37 healthy controls (male, group age‐matched to the boxers) | 3 T MRI T2*, TOF | Two radiologists reviewed images clinically to evaluate the presence or absence of microhemorrhage, blind to group membership | In the amateur boxers 3 out of 42 had microhemorrhages compared with 0 out of 37 healthy control, although this finding was not statistically significant. |
| Vagnozzi R | 2008 | Temporal window of metabolic brain vulnerability to concussion: a pilot 1H‐magnetic resonance spectroscopic study in concussed athletes—part III | Neurosurgery | Sports‐related concussion, in 3 cases 2 concussions | Mixed | 13 athletes of different sports (5 female, 8 male, age range 21–32 years), (10 athletes at three time points, three athletes with second concussion at four time points), five controls + case report of one professional boxer (35 years) studied at five time points (gender information not provided) | 3 T MRI MRS (SVS, TE = 135 ms) | MRS ROI on both sides in frontal WM, scan at 3, 15 30 days postinjury. Those with second concussion also scanned at 45 days postinitial injury. Professional boxer was scanned before and at 4, 7, 15 and 30 days after knockout | Longitudinal study of NAA/Cr in individuals with single and double concussions. Single concussions show that NAA/Cr is significantly lower than controls in the acute stages of injury; however, NAA/Cr begins to recover to normal levels 30 days postinjury. However in those subjects with two concussions, NAA/Cr rates were lower and took longer to recover to normal. This suggests that cumulative effects of head injury may play a significant role. |
| Orrison WW | 2009 | Traumatic brain injury: a review and high‐field MRI findings in 100 unarmed combatants using a literature‐based checklist approach | J Neurotrauma | Repetitive (sub)concussive head impacts (no information about concussion count) | Boxing, MMA | 85 male fighters (mean age 27.3 ± 5.0 years) | 3 T MRI T1w, T2w | Semi‐quantitative reading by one neuroradiologist | 76% of fighters showed at least one sign associated with TBI, 59% hippocampal atrophy, 43% CSP, 32% DPVS, 29% DAI, 24% cerebral atrophy, 19% increased lateral ventricles, 14% pituitary gland atrophy, 5% arachnoid cysts, 2% contusions; no sign of vascular damage in MRA. |
| Provenzano FA | 2010 | F‐18 FDG‐PET imaging of chronic traumatic brain injury in boxers: a statistical parametric analysis | Nuclear Med Comm | Repetitive (sub)concussive head impacts | Boxing | 19 active boxers who were all poor performers in boxing matches and all had been knocked out (male, mean age 30 ± 4.9 yrs), eight controls (five female; mean age 37 ± 8.5 years) | [18F] FDG‐PET | Voxel‐wise t‐tests using SPM and ROI analysis (manually drawn) | Goal was to determine whether there is reduced brain glucose metabolism in professional and amateur boxers using F‐18 FDG‐PET. Results showed an 8%–15% decreased uptake in bilaterally in posterior cingulate cortex, parieto‐occipital lobes, frontal lobes (Broca's area) and cerebellum compared with controls. Findings suggest that hypometabolism as shown in reduced brain glucose metabolism may reflect mechanisms relevant to repeated brain trauma in boxers. |
| Amen DG | 2011 | Impact of playing American professional football on long‐term brain function | J Neuropsychiatr | Repetitive (sub)concussive head impacts | Football | 100 active and retired NFL players (male, mean age 57.27 years), 20 right‐handed controls (male, mean age 50.0 years), 32 without loss of consciousness vs. 32 with multiple loss of consciousness | SPECT, qEEG | SPECT: general linear (Chang) methods qEEG compared with a nationally published normative database | SPECT: decreased perfusivity was found in the whole brain, especially in the prefrontal, temporal, parietal and occipital lobes, anterior and posterior cingulate gyrus and cerebellum. qEEG confirmed those results with significantly slower waves in the bilateral temporal regions and bilateral frontal lobes and reduced power at the higher frequencies. |
| Hasiloglu ZI | 2011 | Cerebral microhemorrhages detected by susceptibility‐weighted imaging in amateur boxers | AJNR Am J Neuroradiol | Repetitive (sub)concussive head impacts | Boxing | 21 amateur classic boxers (eight active, 13 retired, (male, mean age 42.5 years), 21 healthy nonboxing volunteers (male, age range 19–59 years) | 1.5 T MRI T1w, SWI | Visual inspection by two experienced neuroradiologists | No significant difference in microbleeds between boxers and controls, but significantly more CSPs in boxers. |
| Bazarian JJ | 2012 | Subject‐specific changes in brain white matter on diffusion tensor imaging after sports‐related concussion | Magn Reson Imaging | Repetitive (sub)concussive head impacts | Ice hockey, football | Four male high school‐level ice hockey players, five male high school‐level football players (mean age 17 years) and six controls (one female, mean age 23 years), one athlete sustained a concussion | 3 T MRI DTI | Voxel‐wise analysis using bootstrapping pre‐ vs. postseason. In case of concussion additional postinjury scan at 72 H | Percentage of voxels with significant pre‐post FA and MD changes was highest for concussed subject (n = 1), intermediate for remaining athletes with subconcussive head impacts and lowest for controls. Affected regions included the right corona radiata, and the right ILF. |
| Breedlove EL | 2012 | Biomechanical correlates of symptomatic and asymptomatic neurophysiological impairment in high school football | J Biomech | Repetitive (sub)concussive head impacts | Football | Season 1: 24 high school‐level football players (male, age range 15–18 years, mean age 17 years), season 2: 28 high school‐level football players (male, age range 14–18 years, mean 16.8 years), season 2 included 14 athletes from season 1 7 of 38 athletes sustained a concussion | 3 T MRI fMRI | AFNI after testing visual working memory in fMRI. Pre‐ vs. postseason comparison in 23 athletes, and correlation with head impact exposure | All athletes with concussion showed behavioral and functional abnormalities in follow‐up scans. A subgroup of those without a clinically diagnosed concussion showed changes in fMRI and ImPACT. There was an association between the number of head impacts and change in fMRI. |
| Koerte IK | 2012 | White matter integrity in the brains of professional soccer players without a symptomatic concussion | JAMA | Repetitive (sub)concussive head impacts. No previous history of reported concussion | Soccer | 12 professional soccer players (male, mean age 19.7 years) and 11 swimmers (male, mean age 21.4 years) | 3 T MRI DTI | TBSS, group comparison | Widespread white matter area with increased RD in soccer players without history of concussion consistent with findings observed in patients with mild TBI, and suggesting possible demyelination due to repetitive subconcussive head impacts. |
| Koerte IK | 2012 | A prospective study of physician‐observed concussion during a varsity university hockey season: white matter integrity in ice hockey players. Part 3 of 4 | Neurosurg Focus | Repetitive (sub)concussive head impacts | Ice hockey | 17 college‐level ice hockey players (male, mean age 22.2 years), three sustained a concussion during the study | 3 T MRI DTI | TBSS, pre‐ vs. postseason | Increase in trace, AD and RD over the course of one play season in the right precentral region, the right corona radiata and the anterior and posterior limb of the internal capsule. Results suggest microstructural alterations due to concussive and subconcussive head impacts. |
| Chamard, E | 2012 | A prospective study of physician‐observed concussion during a varsity university hockey season: metabolic changes in ice hockey players. Part 4 of 4 | Neurosurg Focus | Repetitive (sub)concussive head impacts. 11 sustained a concussion during the study | Ice hockey | 45 college‐level ice hockey players (25 male, mean age 22.2 years), (20 female, mean age 20.2 years) | 3 T MRI MRS (SVS TE = 35msROI:CC | LC model; ratios to Cr; pre‐ vs. postseason. In case of concussion additional postinjury scans at 72 h, 2 weeks and at 2 months | Female athletes without a diagnosed concussion showed a decrease in their NAA/Cr ratio over the course of the season, whereas all ratios stayed stable for male athletes over the season. No difference were found in changes over the season between athletes with concussive and those with subconcussive head impacts. These results show that the corpus callosum may not be sensitive to metabolic changes after TBI. |
| Lipton ML | 2013 | Soccer heading is associated with white matter microstructural and cognitive abnormalities | Radiology | Repetitive (sub)concussive head impacts | Soccer | 37 amateur soccer players (29 male, mean age 30.9 years, 8 female, mean age 30.8 years) | 3 T MRI DTI | Whole‐brain linear regression analysis ‐formed ROIs on which subsequent nonlinear analysis was performed | Nonlinear association between number of headings performed during the past 12 months and lower FA in temporo‐occipital white matter indicating a threshold dose–response relationship. Lower FA was also associated with poorer memory scores. Lifetime history of concussion did not correlate with neither FA nor memory function. |
| Marchi N | 2013 | Consequences of repeated blood–brain barrier disruption in football players | PLoS ONE | Repetitive subconcussive head impacts | Football | 67 college‐level football players (age range 18–23 years) a subgroup of 10 underwent MRI | 3 T MRI DTI | Bootstrapping permutation test, pre‐ vs. postseason comparison pre‐ and postgame blood test (S100B, S100B autoantibodies) | Postgame S100B correlated with number and severity of head impacts. Percentage of voxels with change in MD correlated with change in S100B autoantibodies. Results suggest that football players may experience repeated BBBD. |
| Abbas K | 2014 | Alteration of default mode network in high school football athletes due to repetitive subconcussive mild traumatic brain injury: a resting‐state functional magnetic resonance imaging study | Brain Connect | Repetitive (sub)concussive head impacts | Football |
22 high school‐level football players (male, age range 14–18 years, mean age 16.7 years) 10 male noncontact sport athletes (male, age range 14–18 years, mean age 16.7 years) |
3 T MRI rs fMRI | DMN, group comparison, pre‐, in‐, and 5 months postseason comparison | Hyperconnectivity of the DMN in asymptomatic footballers compared with athlete controls at pre‐, month 2, month 3 and postseason sessions, but lower or comparable number of DMN connections for month 1 and month 4 sessions. Changes in connectivity persisted 5 months postseason. |
| Helmer KG | 2014 | Hockey concussion education project, Part 1. Susceptibility‐weighted imaging study in male and female ice hockey players over a single season | J Neurosurg | Repetitive (sub)concussive head impacts | Ice hockey | 45 college‐level ice hockey players (25 males and 20 females; male mean age 23 years and female mean age 21 years) tested at beginning and end of season; 11 subjects (five men, six women) imaged 72 h, 2 weeks and 2 months postconcussion | 3 T MRI SWI | Matlab code for identifying hypointensity clusters with algorithm for ruling out vessels | Significant change at 2 weeks in postconcussed males suggesting potential cerebral microbleeds as evidenced by increased SWI hypointensity burden. No significant pre‐ vs. postseason changes in nonconcussed subjects of either sex. However, there was a significant difference in the burden index between males and females at both the beginning and end of season, with males having a higher hypointensity burden. The latter suggests gender differences in exposure to head impacts and their sequelae. |
| Davenport EM | 2014 | Abnormal white matter integrity related to head impact exposure in a season of high school varsity football | J Neurotrauma | Repetitive (sub)concussive head impacts | Football | 24 high school‐level football players (male, mean age 16.9 year [0.6]) | 3 T MRI DTI | Pre‐ vs. postseason comparison of diffusion measures. Voxel‐wise correlation with head impact exposure | RWE (risk‐weighted cumulative exposure including linear and rotational acceleration) as well as pre‐ vs. postseason difference in ImPACT verbal memory composite were correlated with number of voxels with change in diffusion measures (defined by ±2 SD differing from group mean). Diffusion measures did not correlate with number of impacts. Results suggest an association between strength of head impact and extent of microstructural alteration. |
| McAllister TW | 2014 | Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes | Neurology | Repetitive (sub)concussive head impacts | Football, ice hockey | 80 college‐level football and ice hockey player (64 male, 16 female, 18–22 years, mean age 19 ± 1.1 years), 79 noncontact sport athletes (56 male, 23 female, 18–22 years, mean age 19.5 ± 1.3 years) |
3 T MRI T1w, DTI |
Group comparison and pre‐ vs. postseason comparison of diffusion measures in ROIs based on FreeSurfer segmentation | Significant group difference for MD in the CC and FA in the amygdala at postseason scan. No difference between pre‐ and postseason in either group. Head impact exposure correlated with diffusion measures in CC, amygdala, cerebellum, hippocampus and thalamus. Poorer learning and memory task performance correlated with MD in CC at postseason scan in both groups. |
| Poole VN | 2014 | MR spectroscopic evidence of brain injury in the nondiagnosed collision sport athlete | Dev Neuropsychol | Repetitive (sub)concussive head impacts | Football | 34 high school‐level football players (male, age range 15–18 years), 10 noncontact sport athletes (male, age range 15–18 years) | 3 T MRI MRS (SVS TE = 30 ms); ROI: DLPFC, M1 | LC model concentrations with PV correction, and group comparison, pre‐ vs. postseason | Increased Glx in M1 postseason in all football players despite lack of concussion. Team‐specific decreases in Cr and mI. These results show that biochemical changes are occurring as a result of just subconcussive blows although it remains unclear how specific or sensitivity. |
| Singh R | 2014 | Relationship of collegiate football experience and concussion with hippocampal volume and cognitive outcomes | JAMA | Repetitive (sub)concussive head impacts | Football | 25 college‐level football players with concussion (age range 20.6–21.7 years, mean 21.2 ± 1.31 years, time since concussion: 1–1672 days, mean 270 days), 25 football players without concussion (male, age range 19.7–20.9 years, mean age 20.28 ± 1.43 years), 25 matched controls (age range 20–21.6 years, mean age 20.8 ± 1.87 years | 3 T MRI T1w | FreeSurfer segmentation of hippocampus and group comparison | Decreased bilateral hippocampal volumes in footballers compared with controls. Players with history of concussion had smaller volumes than those without a history of concussion. Number of years played was inversely correlated with left hippocampal volume. |
| Talavage TM | 2014 | Functionally detected cognitive impairment in high school football players without clinically diagnosed concussion | J Neurotrauma | Repetitive (sub)concussive head impacts | Football | 24 high school football players (male, age range 15–18 years, mean age 17 years), three experienced a concussion during the study | 3 T MRI fMRI | AFNI after testing visual working memory in fMRI. Pre‐ vs. postseason comparison in 11 athletes, and in case of a concussion or high HIT measure, additional scan was performed within 72 h after diagnosis | All three players who experienced a concussion showed abnormalities in postimpact scan. Four of 8 players without symptoms but with high HIT measures showed no change in postimpact scan. The remaining four showed changes in fMRI and ImPACT in the absence of a clinically diagnosed concussion. |
| Banks SJ | 2014 | Impulsiveness in professional fighters | J Neuropsychiatr Clin Neurosci | Repetitive (sub)concussive head impacts | Boxing, mixed martial arts |
60 boxers and 71 MMA fighters (6 female, age range 19–71 years, mean age 28.5 years) 393 controls (male, age range 17–45 years, mean age not provided) |
3 T MRI T1w | FreeSurfer segmentation of subcortical gray matter, correlation with BIS, group comparison to a previously published control group | Fighters demonstrated higher impulsiveness than previously published controls. MMA fighters reported more impulsiveness than boxers. Reductions in caudate and thalamic volume were associated with higher attention and self‐control scores. Smaller caudate volumes were related to higher impulsiveness. Smaller hippocampal volumes were associated with reduced self‐control. |
| Bazarian JJ | 2014 | Persistent, long‐term cerebral white matter changes after sports‐related repetitive head impacts | PLoS ONE | Repetitive (sub)concussive head impacts | Football | 10 college‐level football players (male, mean age 20.4 ± 1.08 years), five nonathlete controls (male, mean age 20.6 ± 1.14 years) | 3 T MRI DTI | Wild bootstrapping permutation test, comparison of pre‐, post‐ and 6 months postseason scans, with correlation with head impact exposure, and also serum ApoA1 and S100B autoantibodies, and cognitive function assessments | Athletes showed greater changes in FA and MD between pre‐ and postseason as well as between pre‐ and 6 months postseason. Within athletes, the percentage of voxels with decreased FA at postseason was positively correlated with head impact exposure. The persistence of WM changes between pre‐ and 6 months postseason was associated with changes in serum ApoA1 and S100B autoantibodies. WM changes were not associated with cognition or balance. |
| Shin W | 2014 | Diffusion measures indicate fight exposure‐related damage to cerebral white matter in boxers and mixed martial arts fighters | AJNR | Repetitive (sub)concussive head impacts | Boxers, mixed martial arts | 74 boxers (male, mean age 28 ± 6) and 80 MMA fighters (male, mean age 28 ± 5). No controls. | 3 T DTI | ROI analysis of WM, CC and GM regions of FA, LD, TD, MD. Multiple hierarchal linear regression model used to test if fight exposure predicted DTI values | In boxers, the number of knockouts predicted increased LD and TD in WM and GM leading to increased MD and decreased FA. In MMA fighters, decreased FA in the posterior CC and increased TD in the posterior cingulate were predicted by number of knockouts. The results suggest that the number of knockouts may predict microstructural brain damage. |
Predominant injury mechanism:
— repetitive (sub)concussive head impacts: more than one head impact, including subconcussive and concussive head impacts.
— repetitive subconcussive head impacts: more than one head impact, all of them subconcussive.
Abbreviations: AD = Alzheimer's disease; ADC = apparent diffusion coefficient; AFNI = analysis of functional neuroimages software; Apo A = apolipoprotein A‐I; BBBD = blood–brain barrier disruption; BIS = Barratt Impulsiveness Scale II; CC = corpus callosum; Cr = creatine; CSP = cavum septi pellucidi; CT = computed tomography; DAI = diffuse axonal injury; DLPFC = dorsolateral prefrontal cortex; DMN = default mode network; DPVS = dilated perivascular spaces; DTI = diffusion tensor imaging; FA = fractional anisotropy; FDG = fluorodeoxyglucose; fMRI = functional magnetic resonance imaging; GH = growth hormone; Glx = glutamate; HIT = head impact telemetry system; ILF = inferior longitudinal fasciculus; ImPACT = immediate postconcussion assessment and cognitive testing; LC model = linear combination model; LD = longitudinal diffusivity (also known as axial diffusivity); LDA = linear discriminant analysis; M1 = motor cortex; MD = mean diffusivity; mI = myoinositol; MMA = mixed martial arts; MRA = magnetic resonance angiography; MRI = magnetic resonance imaging; MRS = magnetic resonance spectroscopy; NAA = N‐acetylaspartate; NFL = national football league; PET = positron emission tomography; PV = partial volume; PWMD = periventricular white matter disease; qEEG = quantitative electroencephalography; RD = radial diffusivity; ROI = region of interest; rsfMRI = resting state fMRI; RWE = risk‐weighted cumulative exposure; S100B S100 = calcium binding protein B; SD = standard deviation; SPECT = single‐photon emission computed tomography; SPM = statistic parametric mapping; SVS = single‐voxel spectra; SWI = susceptibility‐weighted imaging; SWM = subcortical white matter disease; T1w = T1‐weighted image; T2* = T2 star; T2w = T2‐weighted image; TBI = traumatic brain injury; TBSS = tract‐based spatial statistics; Tc‐99m HMPAO Spect Technetium (99mTc) = exametazime hexamethylpropylene amine oxime spectroscopy; TD = transveral diffusivity (also known as radial diffusivity); TE = echo time; TOF = time of flight; VBA = voxel‐based analysis; WM = white matter.
Table 2.
Chronic injuries
| First author | Year | Title | Journal | Predominant injury mechanism | Sports/military | Subjects (n, gender, age) | Modality sequence | Analysis technique | Main findings |
|---|---|---|---|---|---|---|---|---|---|
| Haglund Y | 1990 | Does Swedish amateur boxing lead to chronic brain damage? 2. A retrospective study with CT and MRI | Acta Neurol Scand | Repetitive (sub)concussive head impacts | Boxers | 25 former amateur boxers with high exposure (mean age 32.6 years, range 26–41, 25 former amateur boxers with lower exposure (mean age 33.6 years, range 26–43), control group 1: 25 soccer athletes with heading skills (mean age 33.0 years, range 25–44) and control group 2: 25 track‐and‐field athletes who never had had head injury mean age 33.4 years, range 25–44; gender information NOT provided | 0.5 T MRI T1w, T2w CT | MRI reading by two neuroradiologists, quantitative measures: anterior horn index | No significant difference was found between boxers and controls and there were no group differences in the anterior horn index, width of sulci, nor were there signs of vermian atrophy. These negative results may be due to the study using older technology. |
| Amen DG | 2011 | Reversing brain damage in former NFL players: implications for traumatic brain injury and substance abuse rehabilitation | J Psychoactive Drugs | Repetitive (sub)concussive head impacts | Football | 30 retired NFL players (male, age not reported) who demonstrated brain damage and cognitive impairment, no control group. | SPECT | Neuropsychological testing, 99mTc‐HMPAO | Significant increases in brain perfusion were observed in the prefrontal cortex, anterior cingulate gyrus, parietal lobes, occipital lobes and in the cerebellum as well as improvements in cognitive tests after treatment (fish oil, vitamins, gingko, etc.). Results may be reflective of treatment success tempered by several study weaknesses |
| Terry DP | 2012 | Lack of long‐term fMRI differences after multiple sports‐related concussions | Brain injury | Repetitive concussions | Multiple sports | 20 male athletes with multiple sports‐related concussions (two or more mTBIs, at least 6‐month post‐TBI), and 20 male control athletes matched on age, IQ and handedness | 3 T MRI fMRI | Neuropsychological testing, Stroop test and operation span testing in fMRI | Although neuropsychological testing revealed some deficits in athletes with multiple concussions, the fMRI paradigm did not result in significant differences between the two groups. The authors concluded that young brains are more plastic but these fMRI tasks may not be sensitive to brain injury. Moreover, combining multiple sports injured athletes together in the concussed group adds to the variation in trying to detect differences between groups. |
| Ford JH | 2013 | Episodic memory in former professional football players with a history of concussion: an event‐related functional neuroimaging study | J Neurotrauma | Postconcussive, retired football player | Football | Two groups of former professional NFL players. 1. low concussion (n = 0–2 mean concussions = 1.07, male, mean age 64.1 ± 6.8 years) 2. high concussion (n > 3, mean concussions = 6.5, male, mean age 62.6 ± 5.0 years) , and 14 healthy controls (male, 62.2 ± 6.3 years) | 3 T MRI T1w, fMRI | Item and relational memory task‐related fMRI, SPM 8 | Former NFL players with 3 + concussion showed greater activation during memory tasks; no significant effect of concussion history on long‐term behavioral changes was observed. Data suggest that multiple concussions may be associated with less efficient memory performance. |
| Hampshire A | 2013 | Hypoconnectivity and hyperfrontality in retired American football players | Sci Rep | Repetitive (sub)concussive head impacts | Football | 13 retired NFL players (male, mean age 54 years) and 20 healthy controls (male, mean age 53 years) | 3 T MRI fMRI | Spatial planning task (tower of London) task, ROI analysis (DLPFC, PC, FPC), psychophysiological interaction model, SPM 8 | Retired players showed some executive deficits on neuropsychological measures. However, fMRI showed hyperactivation and hypoconnectivity in the frontal lobe cortices compared with controls that also correlated with number of concussions. The results suggest that there may be a cortical compensatory mechanism to counteract disrupted dorsal executive network connectivity. |
| Tremblay S | 2013 | Sports concussions and aging: a neuroimaging investigation | Cerebral Cortex | Repetitive (sub)concussive head impacts | Ice hockey, football | 15 male former university‐level athletes (mean age 60.87 years), with last concussion in early adulthood, and 15 male controls who were former university level athletes with no history of concussions (mean age 58.13 years, group matched on education and frequency of APOE4) | 3 T MRI T1w, T2w, MRS (PRESS TE = 30 ms; ROI: MTL, PFC) | MRI: FSL‐VBM, hippocampal volume, CIVET for cortical thickness, MRS: quantitative metabolite concentrations (LC model) | Former athletes with a history of multiple concussions examined 30 years after playing showed lateral ventricular enlargement, cortical thinning in the frontal, temporal and parietal lobes, increased mI and decreased Cho in the MTL. These findings also correlated strongly with episodic memory and verbal fluency tests. These results reflect an abnormal aging pattern when compared with former athletes with no history of concussions. |
| Albaugh MD | 2015 | Postconcussive symptoms are associated with cerebral Cortical thickness in healthy collegiate and preparatory school ice hockey players | J Pediatr | Repetitive (sub)concussive head impacts | Ice hockey | 29 ice hockey players (male, mean age 17.8 years); 16 with concussive and 13 with subconcussive head impacts | 3 T MRI T1w | FreeSurfer 5.3 for cortical thickness (ICV + age as covariate) | Cortical thickness in frontal, parietal and temporal regions was inversely associated with self‐reported postconcussive symptoms. Self‐reported attention problems were inversely associated with cortical thickness in the left anterior cingulate, left ventromedial prefrontal and left dorsomedial prefrontal areas as well as left temporal cortex. These results show that morphological changes occur at young age in athletes with multiple concussions. |
| Casson IR | 2014 | Is there chronic brain damage in retired NFL players? Neuroradiology, neuropsychology and neurology examinations of 45 retired players | Sports Health | Repetitive (sub)concussive head impacts concussions were self‐reported by participants | Football | 45 retired NFL player (male, mean age 45.6 ± 8.9 years), mean number of concussions 6.9 ± 6.2 ; n = 34 reported more than three concussions; no control group | 1.5 T MRI T1w, T2w, SWI, DTI | Semi‐quantitative reading for SWI lesions, quantification using in house software, global WM and FA mean analysis. Histogram approach, semi‐quantitative scores for anatomical reading | SWI lesions were found in 4/45 players, which correlated with number of concussions and family history of neurological disease. CSP found in 9/45 players but did not correlate with other findings. Using DTI, global WM FA was found to correlate with number of self‐reported concussions. The authors conclude there is no clear evidence of chronic brain injury; however, the lack of control group and other methodological issues weaken the conclusion. |
Predominant injury mechanism:
— repetitive (sub)concussive head impacts: more than one head impact, including subconcussive and concussive head impacts.
— repetitive subconcussive head impacts: more than one head impact, all of them subconcussive.
Abbreviation: APOE4 = apolipoprotein E‐4; CSP = cavum septi pellucidi; CT = computed tomography; DLPFC = dorsolateral prefrontal cortex; DTI = diffusion tensor imaging; FA = fractional anisotropy; fMRI = functional MRI; FPC = frontopolar cortex; FSL‐VBM = functional MRI of the brain (FMRIB) software library—voxel‐based morphometry; ICV = intracranial volume; IQ = intelligence quotient; mI = myoinositol; MRI = magnetic resonance imaging; MRS = magnetic resonance spectroscopy; mTBI = mild traumatic brain injury; MTL = medial temporal lobe; NFL = national football league; PC = parietal cortex; PFC = prefrontal cortex; PRESS = point‐resolved spectroscopy pulse sequence; ROI = region of interest; SPECT = single‐photon emission tomography; SPM 8 = statistic parametric mapping 8; SWI = susceptibility‐weighted imaging; T1w = T1‐weighted; T2w = T2‐weighted; Tc‐99m HMPAO Spect Technetium (99mTc) = exametazime hexamethylpropylene amine oxime spectroscopy; TBI = traumatic brain injury; WM = white matter.
Table 3.
Neurodegenerative injuries
| First author | Year | Title | Journal | Predominant injury mechanism | Sports/military | Subjects (n, gender, age) | Modality sequence | Analysis technique | Main findings |
|---|---|---|---|---|---|---|---|---|---|
| Hart J | 2013 | Neuroimaging of cognitive dysfunction and depression in aging retired national football league players: a cross‐sectional study | JAMA Neurol | Repetitive (sub)concussive head impacts | Football | 26 aging retired NFL players (male, mean age 62 years), 26 age‐, education and IQ‐matched controls | 3 T MRI T1w, FLAIR, hemosiderin scan, DTI, ASL | Flair: lesion volumetry and manual dividing into deep and periventricular WM lesions; DTI: TBSS, corrected for age; ASL: maps of cerebral blood flow, no exact method description | Comparison between NFL players that are cognitively impaired vs. those who did not show impairment revealed increase WM lesion volume, FA changes in frontal and parietal regions, corpus callosum and in the left temporal lobe, and increased blood flow in select cortical regions of the brain. Hemosiderin did not show differences. It is interesting to note that asymptomatic NFL players showed similar results to healthy controls and that cognitive impairment correlate strongly with DTI and ASL changes. |
| Small GW | 2013 | PET scanning of brain tau in retired national football league players: preliminary findings | Am J Geriatr Psychiatry | Repetitive (sub)concussive head impacts | Football | Five symptomatic retired NFL players (male, mean 59, range 45–73 years), five controls (male, mean age 60 years, age range 45–66 years) matched for BMI, age, gender, education | FDDNP‐PET | Logan graphical analysis—ROI was traced on coregistered MRI or CT scans | Players showed significantly higher FDDNP signals in caudate, putamen, thalamus, subthalamus, midbrain and cerebellar white matter regions. However, as FDDNP labels both amyloid and tau, it is unclear how specific this ligand is for CTE. |
| Strain J | 2013 | Depressive symptoms and white matter dysfunction in retired NFL players with concussion history | Neurology | Repetitive (sub)concussive head impacts | Football | 26 retired NFL players [male, mean age 57.8 years, age range 41–79 years; five symptomatic players sustained 3–11 concussions (mean 5.6), 21 asymptomatic athletes sustained 0–10 concussions (mean 3.43)] | 3 T MRI DTI | FSL, TBSS, ROI approach | As a follow‐up to the Hart study, this study examined a similar cohort but focusing on those with depression. Significant association between WM integrity as measured by FA and the presence as well as severity of depressive symptoms were found. These results show that DTI changes may be sensitive to depression. |
| Little DM | 2014 | Imaging chronic traumatic brain injury as a risk factor for neurodegeneration | Alzheimer's Dement | Repetitive (sub)concussive head impacts | Civilian | 29 subjects with single TBI (14 female, mean age 35 years, range 20–58 years), 14 with multiple TBIs (mean age 33 years, age range 21–51 years, 6 female), 37 controls (21 female, mean age 33 years, range 19–60 years) | MRI T1w magnet field strength not reported | Voxel‐based morphometry (VBM) | Participants with a single TBI had less changes compared with those with multiple events. Those with a history of multiple mTBIs showed decreased tissue density in the temporal lobes, parahippocampal gyri, ventrolateral prefrontal regions, external capsule and cerebellum. These findings suggest that a greater number of concussions results in greater neurodegeneration. |
| Mitsis EM | 2014 | Tauopathy PET and amyloid PET in the diagnosis of chronic traumatic encephalopathies: studies of retired NFL player and of a man with FTD and a severe head injury | Transl Psychiatry | Repetitive (sub)concussive head impacts | Football | Retired male NFL player (71 year) | PET, MRI, CT | Visual inspection of the CT/MRI and F‐18 Florbetapir/T807 imaging | Case report of retired NFL players with symptoms of PSP. Although results were negative for amyloid and positive for tau, it is unclear if tau deposition may be due to PSP. |
| Coughlin JM | 2014 | Neuroinflammation and brain atrophy in former NFL players: an in vivo multimodal imaging pilot study | Neurobiol Dis | Repetitive (sub)concussive head impacts | Football | 9 retired NFL player (male, mean age 65.7 ± 5.4 years), mean number of concussions 3.8 ± 3.0), 9 healthy controls (male, mean age 58.3 ± 3.8) | PET, [11C]DPA‐713; 3 T MRI T1w | PET: regional total distribution volume; MRI: FreeSurfer regional volumes and cortical thickness | Increased binding of [11C]DPA‐713 binds to translocator proteins, markers of brain injury and repair, was found in the supramarginal gyrus and right amygdala in the former NFL players when compared with controls. Significant atrophy in the right hippocampus of former NFL players after correction for multiple comparisons. Results appear to support signs of atrophy from MRI and potentially inflammation from PET results; however, it is unclear if the findings may be age‐related changes given the significantly younger control group. |
| Lin AP | 2015 | Changes in the neurochemistry of athletes with repetitive brain trauma: preliminary results using localized correlated spectroscopy | Alz Res and Therap | Repetitive (sub)concussive head impacts | Football | Five retired NFL players (male, mean age 44 ± 10 years), five controls (mean age 45 ± 13 years) matched for BMI, age and gender | 3 T MRI MRS (correlated spectroscopy; PCG) | FELIX‐NMR (crosspeak volumes relative to Cr), group comparison | NFL players showed increased Cho, Glx, phenylalanine and fucose reflective of diffuse axonal injury, excitotoxicity and neuroinflammation. NAA and mI were not significantly different that was unexpected as NAA is a neuronal marker and expected to decrease with neurodegeneration. |
Predominant injury mechanism:
— repetitive (sub)concussive head impacts: more than one head impact, including subconcussive and concussive head impacts.
— repetitive subconcussive head impacts: more than one head impact, all of them subconcussive.
ASL = arterial spin labeling; BMI = body mass index; Cho = choline; CT = computed tomography; CTE = chronic traumatic encephalopathy; DTI = diffusion tensor imaging; FA = fractional anisotropy; FDDNP‐PET = 2‐(1‐(6‐[(2‐[fluorine‐18] fluoroethyl) (methyl)amino]‐2‐naphthyl)–ethylidene)malononitrile positron emission tomography; FLAIR = fluid‐attenuated inversion recovery; FSL = functional MRI of the brain (FMRIB) software library; FTD = frontotemporal dementia; Glx = glutamate; IQ = intelligence quotient; MRI = magnetic resonance imaging; MRS = magnetic resonance spectroscopy; mTBI = mild traumatic brain injury; NAA = N‐acetylaspartate; NMR = nuclear magnetic resonance tomography; PCG = posterior cingulate gyrus; PET = positron emission tomography; PSP = progressive nuclear palsy; ROI = region of interest; T1w = T1‐weighted; TBI = traumatic brain injury; TBSS = tract‐based spatial statistics; VBM = voxel‐based morphometry.
Table 4.
Military injuries
| First author | Year | Title | Journal | Predominant injury mechanism | Subjects (n, gender, age) | Modality sequence | Analysis technique | Main findings |
|---|---|---|---|---|---|---|---|---|
| Peskind ER | 2011 | Cerebrocerebellar hypometabolism associated with repetitive blast exposure mild traumatic brain injury in 12 Iraq war veterans with persistent postconcussive symptoms | Neuroimage | Repetitive blast‐related mTBI (range 3–14) | 12 veterans (male, mean age 32 ± 4.6 years), 10 of 12 were comorbid for PTSD, 12 controls (seven males and five females, mean age 53 ± 4.6 years) | [18F] FDG‐PET | Stereotactically defined volume of interest (VOI) analyses | Regional hypometabolism was observed in infratentorial and medial temporal brain regions. Cognitive domain deficits and behavioral symptoms were also observed in the veterans compared with controls but no correlation with imaging was made. One of the drawbacks to this study was the use of and older control cohort. An important finding is there appeared to be no affect of comorbid PTSD on the imaging results indicating that these regional changes are likely tied to postconcussive symptoms of mTBI. |
| Scheibel RS | 2012 | Altered brain activation in military personnel with one or more traumatic brain injuries following blast | J Int Neuropsychol Soc | At least one blast‐related TBI (repetitive in 6 out of 15) | 15 veterans (male, mean age 29 ± 6 years) vs. 15 controls (1 female, mean age 31 ± 6 years) | 3 T MRI fMRI | SPM, voxel‐wise and cluster‐wise correlation with correct/incorrect response trial events, within‐group, between‐group comparisons | Between groups there was a greater activation during stimulus–response incompatibility within the anterior cingulate gyrus, medial frontal cortex and posterior cerebral areas, a negative relationship between symptoms of PTSD and activation of posterior regions, and increased task‐related activation following blast brain injury. These findings suggest impairment of executive function in those with blast injury. |
| Petrie EC | 2014 | Neuroimaging, behavioral and psychological sequelae of repetitive combined blast/impact mild traumatic brain injury in Iraq and Afghanistan war veterans | J Neurotrauma | (repetitive) blast‐related mTBI | 34 veterans (male, mean age 31.6 years) with a history of one or more blast mTBI, and 18 nonblast veterans (one female, mean age 32.8 years) |
3 T MRI DTI PET |
DTI voxel‐based analysis, PET whole‐brain voxel‐wise analysis | Chronic alterations in brain white matter structure and composition and increased cortical glucose metabolism were observed in the veterans with exposure to blasts. The study also showed there may be a retain threshold of number of concussions to elicit changes in DTI and PET. Furthermore, results also showed that comorbid PTSD did not affect results. |
Predominant injury mechanism:
— (repetitive) blast‐related mTBI: one or more than one mTBI.
— repetitive blast‐related mTBI: more than one mTBI.
Cho = choline; Cr = creatine; CSI = chemical shift imaging; DTI = diffusion tensor imaging; FDG‐PET = fluorodeoxyglucose—PET; fMRI = functional MRI; MRI = magnetic resonance imaging; MRS = magnetic resonance spectroscopy; mTBI = mild traumatic brain injury; NAA = N‐acetylaspartate; PET = positron emission tomography; PTSD = posttraumatic stress disorder; ROI = region of interest; SPM = statistic parametric mapping; T1 = T1‐weighted; TBI = traumatic brain injury; TE = echo time; VOI = volume of interest.
We used PubMed to locate these articles. The following keywords were used: (neuroimaging or imaging) AND (repetitive traumatic brain injury or repetitive brain trauma or chronic traumatic encephalopathy). The dates for the articles selected were inclusive to 1 December 2014. Studies were selected if: (i) repetitive brain trauma was explicitly stated and it was clear that subjects had sustained more than one concussion; or (ii) subjects of the study were contact sport athletes and/or military service members who experienced repetitive brain trauma via blasts or direct impact to the head; or (iii) repetitive brain trauma was explicitly stated and it was clear there was more than one subconcussive impact to the head. We did not include case studies (with the exception of a PET tau ligand case study), review articles, articles that focused on animal models (see article in this special issue by Margulies, which covers this topic) or articles that focused on repetitive head trauma resulting from head banging, epilepsy or physical abuse. One civilian study met these criteria and the remaining studies were either sports related or military (see descriptions below and see Tables 1, 2, 3, 4).
Acute/subacute (Table 1)
Boxing
As noted previously, CTE has been referred to historically as “punch drunk,” “dementia pugilistica” and “boxer's dementia” [see early descriptions by 39, 94, 112]. It is still not known, however, what additional risk factors lead to the long‐term complications of repetitive concussive and subconcussive brain trauma long recognized in boxing.
Martland introduced the term “punch drunk” in his early 1928 paper where the term was used to highlight a peculiar behavioral profile, including Parkinsonian motor features, as well as a tilt of the head and even a dragging of one side of the body, that was observed in some prizefighters. These physical signs resembled someone who was drunk, hence the term “punch drunk.” Based on his observations, Martland concluded that these symptoms were most likely the result of blows to the head and injuries to the brain.
It is only recently that neuroimaging techniques have been used to investigate brain abnormalities in boxers. Levin et al 85 did not detect any abnormal MRI findings in young boxers nor any relationship between behavior and brain structure on MRI. This early imaging study, however, used a 0.35 T magnet and MRI findings were based on the qualitative clinical read of the MR scan by two neuroradiologists. This study did not use quantitative measures that are better able to discern subtle changes in the brain.
Another study by Jordan and Zimmerman 71 was interested in determining the efficacy of MRI compared with CT in delineating brain abnormalities in a group of male professional boxers. Similar to the Levin et al 85 study, qualitative rather than quantitative measures were used, with clinical radiology reads and neurological exams performed. These investigators concluded that MRI was superior to CT in discerning visible abnormalities such as cavum septi pellucidi (CSP), periventricular white matter disease and small subdural hematomas, which were either not observed or were far less visible using CT. Orrison et al 121 investigated 85 boxers and used semi‐quantitative assessments by one neuroradiologist who reported that 76% of these boxers evinced some sign of TBI with 59% showing hippocampal atrophy, 43% showing CSP and 24% showing cerebral atrophy as well as other changes (see Table 1). These findings are consistent with those reported in neuropathologically confirmed CTE [see 107, 108].
In a study using more advanced techniques, Zhang et al 169 used DTI to investigate brain injuries in professional boxers and concluded that abnormalities in diffusion, which were correlated with frequency of hospitalization for boxing injuries, might be a preclinical sign of TBI in boxers who did not have any major symptoms.
In another DTI study by Chappell et al 32 boxers were selected who had nonsevere head trauma and these investigators also reported diffusion abnormalities, particularly in deep white matter and in cortical gray matter, compared with controls. Based on these findings, Chappell et al suggested that there was a pathological effect of cumulative repetitive head impacts on the brain, even when the impacts are nonsevere. Zhang et al 168 also reported diffusion abnormalities in professional boxers compared with controls in a group where 42 of 49 boxers had normal clinical MRI reads, and yet DTI revealed abnormalities in the corpus callosum and in the posterior limb of the internal capsule, again underscoring the presence of what might be preclinical signs of the brain alterations that precipitate further progression to CTE. In a separate study, Kemp et al 75 looked at cerebral perfusion in 34 male amateur boxers and 20 male controls and reported more cerebral perfusion abnormalities in the boxer group as well as more cognitive decline on psychometric tests. In addition, these changes were associated with more bouts and longer years boxing.
More recently, Shin et al 142 examined both boxers and mixed martial art (MMA) fighters to determine whether or not fight exposure would predict DTI abnormalities. Findings demonstrated that in boxers, the number of knockouts predicted increased axial diffusivity and RD in white matter and in subcortical gray matter regions, as well as increased MD and decreased FA. Interestingly, these investigators did not find that the number of fights predicted DTI changes, suggesting that the number of knockouts best predicts damage to the brain. In MMA fighters, the results were different. The number of knockouts did predict increased RD in the posterior cingulate. These results suggest that the mechanism of injury is different in MMA, which might be expected given the different striking and grappling techniques that are used. Finally, we note that we included this study in the acute/subacute category as it is part of an ongoing study and the DTI scans were reported as baseline scans; however, the investigators do specify that they “did not consider time after the onset of head injury” and therefore there may be some subjects in this cohort who may be better characterized as evincing chronic injury.
Other studies listed in Table 1 include a study by Tanriverdi et al 155 who examined the association between pituitary volume and growth hormone deficiency in retired boxers compared with younger and more active boxers. Growth hormone deficiency and pituitary volume changes were characteristic of some older retired boxers, although there was no control group and the comparisons for pituitary volume included only a subset of the subjects. Nonetheless findings here warrant further investigation. A study by Banks et al 10 using FreeSurfer to automatically segment brain regions also noted changes in pituitary volume in 60 boxers and 71 mixed martial artists, compared with 393 controls. Other regions were also observed to be abnormal, including the caudate, thalamus and hippocampus, all of which showed reductions associated with attention problems and reduced self‐control.
A study by Hahnel et al 61 investigated cerebral microhemorrhages in 42 amateur boxers and 37 controls. Two radiologists reviewed the images clinically for the presence or absence of microhemorrhages and reported 3 out of 42 boxers with microhemorrhages compared with 0 out of 37 controls. These findings suggest that microbleeds are present in a very small number of amateur boxers. Another study by Hasiloglu et al 64 also examined microbleeds in 21 amateur boxers and 21 controls using visual inspection of SWI by two neuroradiologists. Although there were no significant differences regarding microbleeds between groups, there were more CSPs observed in the boxers than in the controls.
Provenzano et al 129 used [18F] FDG‐PET imaging in active male boxers who were all performing poorly in the ring and all had received knockout blows to the head. These boxers, both professional and amateur, showed reduced brain glucose metabolism in the brain, where there was an 8%–15% decrease in uptake bilaterally in the posterior cingulate cortex, in the parieto‐occipital lobes, in frontal lobes (Broca's area) and in the cerebellum compared with controls. Such findings suggest hypometabolism and likely reflect mechanisms relevant to repetitive brain trauma in boxers. It would be of interest to know whether or not such findings are extant in boxers with no or fewer symptoms.
In summary, the number of studies with appropriate control groups and the use of more advanced imaging techniques are quite small in this population. This is somewhat surprising, particularly given that boxing has received a great deal of attention with respect to brain trauma. Further studies are needed to delineate the course of changes in the brain over time in boxers. Longitudinal studies over time would also be instructive to determine why some are more prone to neurodegenerative changes in the brain while others are not. Such knowledge might lead to possible interventions that would prevent further progressive changes over time.
American football
American football is a high‐speed collision contact sport. Players are at high risk for experiencing repetitive head impacts during training sessions and games. A study by Broglio et al 26 previously found that high school football players receive an average of 652 hits to the head per season that exceed 15 g's of force. Given that there are more than one million high school students playing football each year, the public health impact of repetitive head impacts may be quite significant. In a study of college‐level football players, Singh et al 145 studied brain structural characteristics in 25 football players with a history of concussion and compared them with both a group of 25 players without a history of concussion and a group of controls. Findings showed decreased bilateral hippocampal volumes in football players compared with controls. Within the football group, those players with a history of concussion had smaller hippocampal volumes than those without a history of concussion. Further, the number of years playing football was inversely correlated with left hippocampal volume, suggesting a dose–response relationship.
Recently, fMRI studies have investigated brain activation patterns in football players using task‐related approaches, as well as resting state fMRI over the course of a play season. For example, a study by Talavage et al 154 investigated high school‐level football players before and after a play season using task‐related fMRI. All three athletes who sustained a concussion during the play season showed behavioral and functional abnormalities in follow‐up scans. However, a subgroup of 4 of the 8 players without a clinically diagnosed concussion also showed changes in activation pattern as well as subtle impairment detected using immediate postconcussion assessment and cognitive testing (ImPACT).
Another study by Breedlove et al 25 that included the aforementioned cohort, plus an additional football team from the following season, confirmed that these results suggest that some of those who experience repetitive subconcussive head impact will show alterations in brain function even in the absence of a concussive event. Moreover, there was an association between the number of head impacts experienced during the play season and change in activation pattern between pre‐ and postseason evaluation. The authors concluded that the changes in brain activation might be related to exposure to repetitive head impacts. When investigating brain connectivity in this same cohort using resting state fMRI, Abbas et al 1 found a hyperconnectivity of the default mode network (DMN), a network of brain areas that are active when the brain is at wakeful rest. The subjects were 22 asymptomatic high school‐level football players compared with athlete controls at pre‐ and postseason evaluation. There were, however, an increased number of DMN connections in football players during the season at month 2 and month 3, and a lower or comparable number of DMN connections at month 1 and month 4 evaluations. Changes in connectivity persisted 5 months postseason suggesting neurophysiological alterations that persisted beyond the time of exposure to repetitive head impacts.
In the same cohort, Poole et al 128 used MRS to investigate pre‐ and postseason brain metabolism in 34 high school‐level football players compared with noncontact sport athletes. Results showed increased Glx in all football players at postseason evaluation, despite the fact that none experienced a concussion during the play season. There was also a significant decrease in Cr and mI in a subset of the subjects, based on team and season, but not found across all football players. These findings indicate that repetitive subconcussive head impacts may result in the activation of Glx pathways, although changes in other metabolites are less clear. Activation of Glx pathways may suggest potential cellular injury even in the absence of clinical symptoms.
Microstructural changes in the brains of football players have been investigated using DTI. Davenport et al 43, for example, evaluated 24 male high school‐level football players before and after a play season using DTI, head impact measures and ImPACT. Diffusion measures did not correlate with number of recorded subconcussive head impacts. However, the risk‐weighted cumulative exposure, which includes both linear and rotational accelerations, correlated with the number of voxel changes in the diffusion measures. These findings suggest an association between exposure to repetitive head impacts and white matter alteration. Further, pre‐ vs. postseason difference in ImPACT verbal memory composite score also correlated with diffusion measures. These results suggest an association between deficits in verbal memory and changes in white matter microstructure over the course of a play season.
Marchi et al 93 studied the blood serum of 67 college‐level football players to identify the pathomechanisms of brain structural changes in repetitive brain trauma and found increased postgame levels of S100B, indicative of blood–brain barrier disruption, in those players who experienced subconcussive head impacts. The increase in S100B levels positively correlated with number and severity of head impacts experienced during the game. A subset of 10 players also underwent DTI scans pre‐ and postseason. The percentage of voxels with change (positive or negative) in MD correlated with change in S100B autoantibodies. These results suggest that football players may experience repeated blood–brain barrier disruption associated with changes in white matter microstructure due to repetitive subconcussive brain trauma. This was further supported by a subsequent study by Bazarian et al 15 that included 10 college‐level football players and five nonathlete control subjects who were scanned before and after a play season as well as 6 months later. Athletes showed greater changes in FA and MD between pre‐ and postseason as well as between pre‐ and 6 months postseason evaluation compared with controls. Within the football group, the percentage of voxels with decreased FA at postseason was positively correlated with head impact exposure. The persistence of white matter changes between preseason and 6 months postseason was associated with changes in serum ApoA1 and S100B autoantibodies. White matter changes were not associated with cognition or balance.
In summary, there is evidence of alterations in brain structure, function and metabolism in football players who are exposed to repetitive concussive and subconcussive head impacts. Further, studies indicate an association between the frequency and strength of head impacts and extent of brain alterations as well as cognitive and behavioral sequelae.
Ice hockey
Ice hockey is a high‐speed contact sport 3 with a high rate of concussions 46, 47, 48, 101, 132. The number of concussions per 1000 athlete exposures is reported to be as high as 11.7 47. However, to date, there are only a small number of neuroimaging studies that have included ice hockey players with repetitive head impacts 14, 30, 79, 102.
A study by Bazarian et al 14 investigated four high school‐level ice hockey players and five high school‐level football players using pre‐ and postseason DTI. One of the contact sport athletes sustained a concussion during the time of the study. A group of six nonathletes served as controls. The percentage of voxels with significant changes in FA and MD between pre‐ and postseason evaluation was highest for the concussed subject (n = 1), intermediate for the remaining athletes who were exposed to repetitive subconcussive head impacts and lowest for controls. Another study by Koerte et al 79 investigated a cohort of 17 male college‐level ice hockey players before and after one play season using DTI. Three athletes sustained a concussion during the time of the study. An increase in trace (or MD), AD and RD in the right precentral region, the right corona radiata and the internal capsule was reported from pre‐ to postseason. Although two of the three concussed subjects showed the most pronounced increase in diffusion measures, most of the nonconcussed athletes also showed changes from pre‐ to postseason. Results from these two studies suggest a cumulative effect of repetitive head impacts on white matter microstructure.
Another pre‐ and postseason study by Helmer et al 66 used SWI to investigate a cohort of 45 college‐level hockey players (25 males/20 females) and reported significant increases in the hypointensity burden index suggesting potential cerebral microbleeds at 2 weeks postconcussion in male but not female concussed subjects. A smaller but nonsignificant increase in the hypointensity burden was observed for concussed females at the same time point. There were no significant changes pre‐ to postseason in nonconcussed players of either sex. However, there was a significant difference in the burden index between males and females at both the beginning and end of the play season, with males having a higher hypointensity burden, suggesting that gender influences must be considered in evaluating head impacts and their sequelae.
A recent study by McAllister et al 100 investigated a cohort of 80 college‐level football and ice hockey players and compared them with 79 noncontact sport athletes. Athletes underwent pre‐ and postseason DTI. Significant group differences were found for MD in the corpus callosum and FA in the amygdala at postseason scan. No differences, however, were reported between pre‐ and postseason evaluation in either group. However, head impact exposure during the season correlated with diffusion measures in corpus callosum, amygdala, cerebellum, hippocampus and thalamus at postseason evaluation. Poorer learning and memory task performance also correlated with MD in the corpus callosum at postseason scan in both groups. These results indicate a relationship between head impact exposure and white matter microstructure even in players who appear cognitively and clinically unaffected, suggesting that repetitive brain injury may be extant even in the absence of cognitive and clinical symptoms.
Charmard et al 30 investigated brain metabolic changes in the corpus callosum over the course of one play season in a sample of 45 college‐level ice hockey players. Female athletes without a diagnosed concussion showed a decrease in their NAA/Cr ratio over the course of the season, possibly indicative of neuronal loss, whereas all ratios remained stable for male athletes. This study provides the first indications that there may be gender differences in metabolic changes in the brain for those who are exposed to repetitive head impacts. No differences were observed in metabolic changes over the season between athletes with concussive and those with subconcussive head impacts. It is possible that the corpus callosum is not biochemically sensitive to these kinds of injury in the acute phase.
Soccer
For a long time soccer has not been the focus of research in repetitive brain trauma and much more attention has been given to other contact sports such as boxing, martial arts, football and ice hockey. Soccer players, however, are not only at a relatively high risk for sustaining a concussion while playing soccer, but they are also at high risk for repetitive subconcussive head impacts to their unprotected heads when heading the ball. On average, soccer players perform 6–12 headings per game, resulting in thousands of headings over a player's career 97, 160. A study initiated by the Fédération Internationale de Football (FIFA) showed head accelerations of up to 20 g when heading a soccer ball 118. These head accelerations are within the range of those that occur in a car crash at 30 km/h. Additionally, more severe head accelerations are expected to occur when a child with insufficient neck strength and posture control attempts to head a high‐velocity ball 130. This is very concerning given the fact that the young brain is still developing and may be even more vulnerable to brain injury. This concern is supported by a recent study that reports an increased risk for long‐term brain alterations depending on the age at start of exposure to repetitive brain trauma 149. Soccer players have also been shown to be at increased risk for impaired neurocognitive function later in life 96, 161. Earlier clinical studies from the 1970s have already suggested a link between frequent heading of a soccer ball and cognitive impairment in professional soccer players 98. Moreover, a recent study reports an association between heading the ball and reduced cognitive performance immediately after training 170. However, until recently there were no techniques available that were sufficiently sensitive to detect changes in the brain in vivo.
A study by Koerte et al 78 described earlier used DTI to investigate a small cohort of young professional soccer players who were selected specifically for having no history of a clinically symptomatic concussion compared with professional swimmers. An increase in RD was found in widespread white matter areas in soccer players consistent with findings observed in mTBI studies, suggesting possible demyelination due to repetitive subconcussive head impacts. This study is particularly important because it is the first study to show microstructural changes in the brains of athletes who experienced only repetitive subconcussive head impact in the absence of a symptomatic concussion. A study by Lipton et al 88 also used DTI to study white matter alterations in amateur players and found a nonlinear association between the number of headings performed during the previous 12 months and lower FA in temporo‐occipital white matter, suggesting a threshold dose–response relationship. In this study, lower FA was also associated with poorer performance in a memory task. Lifetime history of concussion did not correlate with either FA or memory function.
In summary, soccer players who are at high risk for repetitive subconcussive head impacts show brain microstructural alterations associated with subtle cognitive deficits. Given the great number of soccer players worldwide, future studies are needed to evaluate the association of cognitive impairments with strength and frequency of exposure to head impact as well as to characterize the time course of brain alterations and clinical sequelae.
Mixed sports
An important longitudinal spectroscopic study 162 utilized a mixed population of 14 athletes including kickboxers (n = 6), boxers (n = 4, of which one was a professional boxer), soccer players (n = 2), a rugby player (n = 1) and an alpine skier (n = 1) compared with five age‐matched healthy controls without a history of head injury. Single‐voxel spectroscopy was acquired in the frontal white matter bilaterally using a long echo time sequence. As a result, only NAA, Cho and Cr were available for analysis (metabolites such as Glx and mI are not visible at this longer echo time). Their results showed significantly lower NAA 3 days after injury, which was increased at 15 days postinjury, and recovered back to control levels at 30 days postinjury in those subjects who experienced a single concussion. Three subjects (boxers and kickboxer) received a second concussion 10–13 days after their initial concussion. These subjects manifested prolonging reduction in levels of NAA, but NAA did not recover back to normal levels even at 45 days postinjury. As NAA is a marker of neuronal health, this study demonstrates the cumulative effects of repetitive brain injury.
Chronic (Table 2)
The long‐term effects of repetitive brain trauma are among the greatest concerns in sports‐related head injury. In this next section, we highlight studies that examine individuals with a history of repetitive head injury 3 months to more than three decades after the last concussion. However, we note that some studies that would fall under the definition of “chronic” and that specifically focus on individuals with symptoms of CTE or neurodegeneration, or military subjects, are highlighted subsequently under Neurodegenerative and Military sections.
Boxers
The earliest study of chronic repetitive brain trauma was conducted in 1990 by a group in Sweden examining amateur boxers 60. A retrospective study examined CT and 0.5 T MRI scans for evidence of CSP, which is considered a potential marker for brain injury. In comparing 50 boxers (25 with high exposure with a large number of bouts, 25 with low exposure and few bouts) with matched soccer and track‐and‐field athletes, there were no significant differences in CSP observed. A more quantitative analysis that measured the anterior horn index and width of sulci also did not show significant differences. Although the authors concluded that there was a lack of evidence of chronic brain injury, advances in imaging technology that provide higher resolution images suggest that this conclusion requires reconsideration as increased rates of CSP have been reported among boxers and participants of other contact sports exposed to repetitive head impacts 121, 148. Additionally, recent studies suggest a possible causal relationship between repetitive head impacts and CSP. Aviv and colleagues, for example, studied 164 active boxers who underwent annual MRI for a boxing board license renewal 8. CSP was present in 49% of boxers and was found in the subsequent scans of eight boxers who did not exhibit CSP on their first scan. Moreover, three boxers showed an increasing extent of CSP in follow‐up scans. These findings demonstrate clear evidence of brain injury in boxers.
American football
It is interesting that similar controversies occur today in studies of American football players. In a study that was prematurely ended from the former NFL's MTBI Committee, 45 former NFL players were examined and the authors concluded a lack of evidence of chronic brain injury 29. Using 1.5 T MRI, SWI and DTI, Casson and colleagues found microhemorrhages in 9% of the players and 7% with CSP. Although they found correlations with global white matter FA and number of self‐reported concussions, the study on the whole was negative. Several limitations to this study such as the lack of a control group, relatively low resolution MRI (1.5 vs. 3.0 T) and early termination of the study raise questions about the strength of their conclusions, especially in light of the many previous studies to the contrary.
For example, two recent fMRI studies 50, 62 used fMRI to show patterns of hyperactivity that reflect deficits in episodic memory and executive function, respectively, in former NFL players. In both studies, the number of concussions correlated with the degree of hyperactivation. Ford et al compared NFL players with 0–2 concussions to those with greater than three concussions and found greater activation during memory tasks in the group with more concussions. Their data suggest that multiple concussions may be associated with less efficient memory performance. Similarly, Hampshire et al showed hyperactivation and hypoconnectivity of the dorsolateral frontal and frontopolar cortices both of which correlated with the number of documented concussions. Their results suggest that there may be a cortical compensatory mechanism to counteract disrupted dorsal executive network connectivity. In another fMRI paradigm that included the Stroop test and operation span testing, 28 male college athletes from several different sports who experienced two or more concussions were compared with 20 young college male controls. Results showed no differences between groups, which led to these investigators concluding younger brains have more plasticity in the face of multiple head impacts 158. This particular fMRI task, however, may be less sensitive to detecting differences between groups. Moreover, combining different athletic sports injuries may increase the variation in the sports injured group, making detection of differences between the concussed group and the controls more difficult to discern.
The question therefore is: are any of these changes reversible with treatment? An intriguing study conducted by Amen et al 5 used SPECT imaging to try to answer this question. One hundred retired NFL players had baseline SPECT and were then given a cocktail of fish oil (omega‐3 for memory and mood), ginkgo (enhance blood flow), vinpocetine (decrease cortisol), phosphatidylserine (enhance acetylcholine), n‐acetyl cysteine (anti‐oxidant) and various other homeopathic remedies for 2–12 months and were then re‐imaged. The authors report significant increases in brain perfusion in the prefrontal cortex, anterior cingulate gyrus, parietal lobes, occipital lobes and cerebellum. These results suggest that this treatment regimen was effective in improving brain perfusion. However, there are several limitations to the study including the lack of a control group, informal monitoring of the regimen and the method of statistical analysis performed was group as opposed to individual comparisons, making it unclear if individuals improved. Nonetheless, the study does provide inspiration for future clinical trials that can utilize neuroimaging as end points for treatment of repetitive brain injury.
Ice hockey
These changes in brain structure and function are not limited only to retired professional athletes as there appear to also be near‐term and long‐term effects in other contact sports such as ice hockey. Albaugh et al 4, for example, examined preparatory‐ and collegiate‐level ice hockey players approximately 3 months after their last concussion and found decreased cortical thickness in frontal, parietal and temporal regions that was inversely correlated with self‐reported postconcussive symptoms. Self‐reported attention problems were inversely correlated with cortical thickness in the left anterior cingulate, left ventromedial prefrontal and left dorsomedial prefrontal areas, as well as in the left temporal cortex. These results suggest that morphological changes occur at a young age in athletes with multiple concussions. Perhaps of greater concern is that these changes appear to persist. Tremblay et al 159 studied athletes who played in college but imaged them at least 30 years after their last concussion. Their aim was to understand better the effect of head injury upon aging. In addition to also finding cortical thinning in the frontal, temporal and parietal lobes, these former athletes also exhibited lateral ventricular enlargement. Using MRS, Tremblay found increased mI and decreased Cho in the mesial temporal lobe. Increased mI is associated with glial proliferation and decreased Cho with membrane turnover. Of particular interest, both are also changes found in mild cognitive impairment. These underlying biochemical changes and persistent changes in brain morphology are concerning.
Neurodegenerative (Table 3)
Repetitive head impacts are, as noted previously, a necessary risk factor for CTE. Studies listed in Table 3 are differentiated from Tables 1 and 2 in that these players have both a history of repetitive head impacts but also clinical symptoms that may be indicative of CTE. Given the postmortem diagnosis of CTE, these studies provide the current state‐of‐the‐art prospective studies of presumed CTE [and/or perhaps other neurodegenerative disease; see also 156], absent pathological confirmation.
American football
The study by Hart et al 63 recruited both symptomatic (cognitive impairment and/or depression) and asymptomatic retired NFL players, all with a history of at least one concussion during their professional career, and compared them with healthy age‐matched controls. Results showed that the symptomatic athletes showed increased total and deep white matter lesion volumes using fluid‐attenuated inversion recovery MRI as well as reduced FA in the left and right frontal and parietal regions, corpus callosum and left temporal lobe using DTI. Of particular interest, these same findings were found when comparing the symptomatic and asymptomatic athletes, indicating that differences between professional sports lifestyle and nonprofessional sports lifestyles did not impact these results. The study also incorporated the use of arterial spin labeling, a method of measuring cerebral blood flow, which showed significantly increased flow in the left inferior parietal lobe, posterior superior temporal gyrus, bilateral mid‐cingulate gyri and right middle frontal gyrus, and reduced flow in the left temporal pole and right occipital region in symptomatic retired NFL players when compared with controls. Comparison with asymptomatic players was not possible due to image coregistration issues. These findings also correlated with cognitive measures, although other neuroimaging measures did not. These results demonstrate that there are neuroimaging abnormalities that may be specific to the symptoms of CTE. In a second study by the same group 153, which focused primarily on depression, a cohort of noncognitively impaired retired NFL players with and without depression was compared with healthy controls. Voxel‐wise analysis found widely distributed FA changes in the frontal region that appeared to correlate negatively with depressive symptoms. Tract‐based analysis showed depressive symptoms that negatively correlated with forceps minor, right frontal aslant tract, right uncinate fasciculus and left superior longitudinal fasciculus tract ROIs. These results imply that the changes observed in these DTI changes are specific to depression and not necessarily to CTE.
One of the major questions is what is the underlying mechanism for the neurodegenerative decline? Neuroinflammation could be a potential suspect. Coughlin et al utilized [11C] DPA‐713, a PET ligand that binds to translocation proteins, which have been found to colocalize with regions of neuroinflammation to study retired NFL players with a history of multiple concussions 40. In addition, they acquired T1‐weighted MRI to measure brain volumes and cortical thickness. Their results showed increased binding in the hippocampus, amygdala, entorhinal cortex, parahippocampal cortex and supramarginal gyrus, all bilaterally. Of further note, MRI brain volumes showed atrophy in the right hippocampus as well as reduced total gray matter volume. These results appear to show widespread neuroinflammation as well as brain atrophy, reflective of neurodegeneration. However, one of the major weaknesses of this study is that the control subjects were not age matched and they were significantly younger than the NFL cohort. As neuroinflammation increases with age 16, the differences observed may be related to age rather than the injury itself.
The most recent study by Lin et al 87 also studied a cohort of symptomatic retired NFL players using an advanced MRS method called correlated spectroscopy (COSY), which can measure additional metabolites by utilizing a second chemical shift domain that provides greater spectral specificity. The authors identified increased Cho (indicative of diffuse axonal injury) and increased Glx/glutamine (indicative of excitotoxicity), which have been found in previous mTBI MRS studies. However, using the COSY method, they also identified additional cerebral metabolites that have not been previously shown, and which also link to neuroinflammation, providing further support of the mechanism described earlier. These metabolic changes may provide insight into the potential mechanisms of injury that underlie the structural changes observed in the previous study.
Because symptoms of presumed CTE overlap with many other disorders, the development of a method to detect and to measure tau in the brains of living individuals is critical for the early diagnosis of CTE, even prior to the onset of symptoms, as well as for quantifying the degree and progression of tau accumulation. Until there is an accurate in vivo biomarker for CTE, it will not be possible to examine incidence and prevalence of CTE, to characterize underlying structural and physiological changes, to determine genetic and other risk factors, or to conduct clinical trials for treatment and prevention. It has also been only quite recently that PET ligands that specifically target tau have been developed. One older ligand, [18F]‐FDDNP, has shown increased uptake in retired NFL players 147. However, this tracer is nonspecific as it binds to both Aβ and tau proteins. Agdeppa et al have also highlighted the binding characteristics of FDDNP and note that it binds to both Aβ and tau 2. There are now, however, as noted previously, several promising probes with good tau specificity that have been developed 33, 143, 171 and recently used, in vivo, in several studies of neurodegenerative diseases, including CTE. One of these probes, [18F]‐T807, was used in a recent case study report 113, where a retired NFL player showed not only subcortical binding of tau but also negative binding for [18F]‐florbetapir, demonstrating that CTE is likely separate from AD, which tends to show more Aβ and less tau accumulation 163, 164. It is important to note however that the subject also suffered from progressive nuclear palsy that also results in tauopathy; therefore, it is unclear if the patterns observed in this subject are wholly representative of CTE 51. Of further note, an ex vivo study by Attardo et al 7 has shown that the fluorescent probe T557 and the radiolabeled [18F]‐T807 bind to paired helical filament (PHF‐tau) in brain sections in AD and non‐AD samples, including, notably, samples with neuropathologically confirmed CTE, suggesting the selectivity of this tau ligand (note: published abstract did not include CTE case but this was included in the presentation). There is thus much promise in investigating, in vivo, tau protein accumulation in the brains of those suspected of having CTE.
Nonsports‐related concussion
Finally, rather than focusing on retired NFL players, Little et al 89 recruited civilians with a history of single and repetitive brain trauma for neuropsychological evaluation and volumetric imaging. Their results show that those subjects with a history of multiple mTBI have more neuroimaging changes compared with those with a single concussion including decreased tissue density in the temporal lobes, parahippocampal gyri, ventrolateral prefrontal regions, external capsule and cerebellum. They also showed significantly worse neuropsychological performance. These findings suggest that the greater the number of brain injuries, the greater the neurodegenerative changes that are seen.
Military‐related repetitive brain trauma (Table 4)
One of the difficulties with studying brain injury in military personnel are the comorbidities of posttraumatic stress, other mood and anxiety disorders, and substance misuse that may contribute to changes in brain morphometry and functionality 83. Also, given the difficulty in conducting imaging studies in acute or subacute time periods of brain injury in combat theater settings, most studies have focused on brain injury in the chronic phase or in veteran populations with repetitive exposures to blast and/or concussion. Further, because it is difficult to obtain an accurate estimate of the number of blast‐related exposures, many studies include individuals with a range of exposure in terms of both number of incidents and severity of incidents. Further, such injuries are less likely to utilize the label “repetitive” and so our search may have excluded these studies. There is also the issue of other complications for many military personnel including pre‐ and postdeployment concussion via other nonblast incidents that complicate the picture. Finally, the difficulties involved in retrospective and self‐report‐based determination of “TBI” or “concussion” contribute to the complexity in this area. Nonetheless, this is an important area of investigation, and there is an active line of research investigating whether or not repeated exposure to explosive events (both controlled, as with military breachers in training, and uncontrolled, as with unintentional exposure to improvised explosive devices) may lead to brain findings similar to those observed in former NFL players in both in vivo imaging studies and postmortem analyses (see 55.
Nevertheless, recent military conflicts have increased interest in the long‐term effects of exposure to repeated blast and nonblast concussive events sustained in combat. For example, an FDG‐PET study that examined Iraq war combat veterans exposed to up to 51 blast events during deployment, and 102 over their entire career, showed reduced uptake in the cerebellum vermis, pons and medial temporal lobes 125. Furthermore, the same patterns of hypometabolism were observed in veterans with and without a diagnosis of PTSD. These findings indicate that these changes are likely specific to repetitive brain trauma and may provide the biological basis of impairments in information processing, verbal fluency, attention and working memory. One of the weaknesses of the study, however, was the use of an older, nonmilitary control group.
In a follow‐up study by the same group 126, veterans without exposure to blast injury were included as controls and different results were reported from the previous study. In this study, reduced FDG uptake was noted in the bilateral parietal cortices, left somatosensory cortex and right visual cortex. This demonstrates the importance of using an appropriate comparison group. This study also examined DTI FA as well as a novel measure of macromolecular proton fraction (MPF), which quantifies white matter myelin compositional integrity using the magnetization transfer effect. DTI FA values were reduced ∼20% in the corpus callosum between groups. For MPF measures, significant differences between the blast and nonblast exposed veterans became more apparent after 20 blasts, suggesting a possible threshold for the number of exposures before changes can be detected. PTSD comorbidity did not change the results, suggesting that these changes are, as noted in the previous study, likely specific to repetitive brain trauma. However, the functional relevance of the PET, DTI and MPF measures is yet to be determined as there were no significant correlations observed with any of the neuropsychological measures.
Similar to sports‐related injury, there is also an element of subconcussive injury in military‐related repetitive brain injury. For example, there are several occupations in the military that result in being repeatedly exposed to blast events, such as bomb detonators, mortor men, field artillery personnel, infantrymen that fire hand‐held missiles, etc. Another such group are breachers, those who use explosives to breach buildings. Stone et al found significant differences in DTI and MRI measures in breachers with 7–15 years of experience compared with those with little to no experience and those with only 1–3 years of experience, demonstrating a potential cumulative effect of injury 152 (not included in Table 3 because it is an Abstract).
Another study of functional imaging in military subjects with repetitive brain trauma has shown greater activation in the anterior cingulate gyrus, medial frontal cortex and posterior cerebral areas involving visual and visual‐spatial functions 136. In this study, 15 subjects underwent a task‐related functional MRI examination that included a stimulus–response compatibility task that tests executive function including cognitive control. Increased activation was found with stimulus–response incompatibility after controlling for differences in reaction time and symptoms of PTSD and depression. It is difficult to compare the results of this study with other studies in single mTBI due to differences in the study design. However, these brain regions have been shown to be involved in fMRI studies of mTBI, and may be relevant to repetitive brain injury.
Finally, a recent study utilized MRS, albeit at 7 Tesla 67. Findings showed that hippocampal NAA/Cho and NAA/Cr ratios were decreased in veterans at least 1 year after 1–10+ blast exposures. Reduced hippocampus volume and deficits in neurocognitive testing were also found. Changes in NAA also appeared to be correlated with effort testing, although without a formal comparison. These findings point toward injury to the hippocampus in soldiers exposed to repetitive blast events. Interestingly, there were no significant differences between mTBI subjects with and without a diagnosis of PTSD, anxiety or alcohol abuse, demonstrating that these changes are, as noted above in other studies, likely specific to repetitive brain trauma.
Summary and future directions for research
Introduction
Today, advanced neuroimaging techniques can provide insights into brain injury following repetitive head impacts that go beyond self‐report and clinical measures. Diagnosing repetitive brain trauma or resulting neurodegeneration on the basis of objective radiological evidence sets the stage for a more complete understanding of the underlying pathophysiological mechanisms. This is thus an exciting new era of discovery with the hope that this greater understanding will lead to early diagnosis, efficacious treatment and monitoring of repetitive brain trauma. More studies are, nonetheless, needed to further elucidate the pathomechanisms underlying repetitive brain trauma. There are several directions for future research that we believe will improve our understanding of repetitive brain trauma and the different trajectories, as well as lead to possible interventions to prevent the cascade of progressive changes that characterize some of the outcomes observed to date.
Neuroimaging as a biomarker of neurodegeneration
A major issue with neuroimaging of any brain injury is the dynamic course of neuropathological changes that occur during the acute to subacute and chronic stages, including neurodegenerative changes that result in progressive volume loss 20. Little is known about how and under what circumstance repetitive head trauma results in neurodegeneration. Although most studies that have examined longitudinal neuroimaging findings in TBI are based on just a single traumatic event 167, two consistent findings from that literature have direct implications for the study of repetitive brain trauma 1: injury induced atrophic changes over time may be region specific (ie, hippocampus) and/or involve the whole brain, and 2 vulnerability of damage to white over gray matter. For example, focusing just within the mild end of the TBI spectrum (Glasgow Coma Scale ≥ 13), both MacKenzie et al 91 and Zhou et al 172 established an early postinjury baseline with various volumetric analyses, and then followed patients a year or more postinjury. Findings demonstrated that even at this mild end of brain injury, volumetric loss was particularly notable within white matter, which was also predictive of neuropsychological outcome. The implications of such findings are that even mild injury may set into motion degenerative changes 38, including neuroinflammation and neuroprotective factors, where advanced neuroimaging techniques may function as biomarkers of recovery and pathological outcomes. This may be particularly important if repetitive brain trauma has the potential to induce a subtle neuroinflammatory response that may set the stage for progressive neurodegenerative changes at some point during the life span [see previous discussions; see also 40, 166]. Of further note, a number of the imaging modalities discussed in this review provide quantitative metrics that would be sensitive to parenchymal loss associated with neurodegeneration. Additionally, as explained in this review, once a baseline is established in cases of repetitive head impacts either pre‐injury or as close to the time of injury as possible, such a neuroimaging reference point will provide the basis for determining if neurodegeneration occurs and whether brain changes are static or progressive. Finally, a further important area of future research is to understand the similarities and differences between CTE and other neurodegenerative diseases that may co‐occur so as to refine further our understanding of the underlying mechanisms that characterize CTE as well as other tauopathies [see also review 156].
Multimodal imaging, longitudinal study designs and standardized protocols
Each modality provides different information and using several different modalities, we enrich the information we have regarding an individual injury. Future research needs to include multimodal imaging to acquire different information from different modalities in the same subject in order to have a more complete picture of each subject's brain alterations. Findings from a multimodal imaging approach can be combined with information from clinical evaluations, neuropsychological evaluations as well as biomarkers from blood and CSF to identify sensitive biomarkers for diagnosis, therapeutic decisions and prognosis. To date, studies in this field have not used a multimodal approach.
Moreover, there is a need for longitudinal studies of repetitive brain trauma to understand the dynamic nature of repetitive brain trauma and the influence of possible modulating factors such as the biomechanics of the injury, lesion location, genetics and other factors that are currently not known. This may lead to the early identification of those individuals who are likely to develop neurodegeneration vs. those who will not. Studies of pre‐ and postseason injury are important but more instructive would be studying, for example, football players at the beginning of their careers and following them through several years of play.
In addition, given the heterogeneous nature of brain trauma, studies will likely need a large number of subjects to truly connect symptoms to brain pathology. Thus, multicenter studies will be needed and the development of standardized protocols that can produce similar measurements across MR platforms will be a key component of future research.
More specific measures of repetitive brain injury
Multi‐shell sampling
Advanced diffusion MRI (dMRI) protocols use a multi‐shell sampling scheme. Here, diffusion measurements are acquired at several different b‐values for a specific set of gradient directions. This enables the calculation of microstructural properties that are not available using standard dMRI protocols (single shell) 131. For example, one can compute additional diffusion parameters such as kurtosis, fast and slow diffusion fractions, mean‐squared displacement etc. 57, 122, 173.
Multi‐tensor tracing of fiber bundles
Tracing of multiple fiber components is now also possible using a two‐tensor tractography algorithm 92. This novel tractography algorithm improves the estimation of fiber directions in areas of fiber crossings and increased noise (Figure 7).
Figure 7.
Comparison between single‐tensor streamline tractography (A) and two‐tensor tractography (B) of the corpus callosum. The same region of interest was used to seed fibers (A) and to select fibers from a two‐tensor whole‐brain tractography (B). Note that two‐tensor fiber reconstruction includes numerous fibers in the periphery while the single‐tensor approach only detects more central fibers.
Free water (FW)
Another newly developed diffusion measure, FW, distinguishes the contribution of extracellular water, which is a potential measure of atrophy, edema and neuroinflammation 124, and neurodegenerative processes.
MRS
Although the biochemical changes observed by MRS can help elucidate underlying pathophysiology, they may not necessarily provide specific diagnostic information. The use of MRS with greater spectral resolution such as COSY might provide the needed additional biochemical specificity. Other advances in spatial resolution such as three‐dimensional chemical shift imaging may also provide specificity by allowing for the interrogation of brain biochemistry in all regions of the brain, which, importantly, may then be correlated with other imaging modalities.
PET tau ligands
By successfully imaging tau deposition, in vivo, along with ostensibly ruling out AD through negative Aβ imaging, we will be able to develop a potential biological marker of CTE in living individuals (to be neuropathologically confirmed). This method has the potential to quickly become a tool for aiding in differential diagnosis in cases of suspected CTE, much like [18F]‐DOPA is currently used in PD. Correlates of PET findings including cognitive/clinical and other imaging measures (MRI, DTI, MRS and resting state fMRI) will also likely provide noninvasive biomarkers for a putative measure of CTE, in vivo. Careful attention to other tauopathies and differentiating among them will, nonetheless, be an important challenge for future research studies.
Personalized medicine approach
The heterogeneity of repetitive brain trauma also calls for new postprocessing approaches to identify individual injury patterns. An example is the work by Bouix et al 23 who observed alterations in diffusion in gray matter in individuals with chronic postconcussive symptoms following a single mTBI [see also 77, 99, 144]. Bouix et al used individual profiles of injury based on developing a normative atlas, and then compared individual patients to the normative atlas to reveal individual profiles of injury. Using this approach provides a signature of injury for each patient that can be used to guide treatment, to correlate findings with cognitive deficits and to provide a more informed treatment plan. Such an approach could also be used to prospectively monitor changes over time in a single individual, which may be very important with respect to early detection of brain alterations, and to following those who are exposed to repetitive head impacts.
Summary
All of the above advances, such as in DTI, PET and MRS, have great promise for becoming highly sensitive markers of tissue structure that, in combination, will likely lead to greater specificity in diagnosing CTE (and differentiating it from other tauopathies), perhaps in the early stages, which may facilitate preventative strategies. These new advances and their application to longitudinal studies of repetitive head impacts will also provide invaluable information about the neurobiology of brain injuries and hopefully lead to an understanding of the underlying mechanisms that determine recovery vs. progression. Such information may, in the future, identify early indicators of those at risk for poor outcome so that early interventions can be introduced. Further, a personalized medicine approach to identify individual profiles of injury will provide the treating physician with helpful information about individual patients, as well as serve as a way to monitor treatment efficacy in that the profile of injury may be followed over time to determine change. All of these new advances are not beyond the horizon but are available today to begin a whole new era of investigating repetitive head trauma so as to change the trajectory of outcome in those most at risk for CTE.
Acknowledgments
This study was supported by the Else Kröner‐Fresenius‐Stiftung, Germany (IK, MM). This work was also partially funded by grants from the Department of Defense (W81XWH‐10‐1‐0835: APL; X81XWH‐07‐CC‐CSDoD; MES, RAS), the National Institutes of Health (R01‐NS078337: APL, MES, RAS; U01‐NS086659: RAS) and a VA Merit Award (MES).
References
- 1. Abbas K, Shenk TE, Poole VN, Breedlove EL, Leverenz LJ, Nauman EA et al (2014) Alteration of default mode network in high school football athletes due to repetitive subconcussive mild traumatic brain injury: a resting‐state functional magnetic resonance imaging study. Brain Connect. [DOI] [PubMed] [Google Scholar]
- 2. Agdeppa ED, Kepe V, Liu J, Flores‐Torres S, Satyamurthy N, Petric A et al (2001) Binding characteristics of radiofluorinated 6‐dialkylamino‐2‐naphthylethylidene derivatives as positron emission tomography imaging probes for beta‐amyloid plaques in Alzheimer's disease. J Neurosci 21:RC189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agel J, Harvey EJ (2010) A 7‐year review of men's and women's ice hockey injuries in the NCAA. Can J Surg 53:319–323. [PMC free article] [PubMed] [Google Scholar]
- 4. Albaugh MD, Orr C, Nickerson JP, Zweber C, Slauterbeck JR, Hipko S et al (2015) Postconcussive symptoms are associated with cerebral cortical thickness in healthy collegiate and preparatory school ice hockey players. J Pediatr 166:394–400. [DOI] [PubMed] [Google Scholar]
- 5. Amen DG, Wu JC, Taylor D, Willeumier K (2011) Reversing brain damage in former NFL players: implications for traumatic brain injury and substance abuse rehabilitation. J Psychoactive Drugs 43:1–5. [DOI] [PubMed] [Google Scholar]
- 6. Ashwal S, Babikian T, Gardner‐Nichols J, Freier MC, Tong KA, Holshouser BA (2006) Susceptibility‐weighted imaging and proton magnetic resonance spectroscopy in assessment of outcome after pediatric traumatic brain injury. Arch Phys Med Rehabil 87:S50–S58. [DOI] [PubMed] [Google Scholar]
- 7. Attardo G, Gomez F, Lin Y, Liang Q, Conway K, Kolb HC et al (2014) Detection of PHF‐tau pathology with [18F]T807 and T557 in brain sections from Alzheimer's and non‐Alzheimer's tauopathy patients. Eur J Nucl Med Mol Imaging 41:S427. [Google Scholar]
- 8. Aviv RI, Tomlinson G, Kendall B, Thakkar C, Valentine A (2010) Cavum septi pellucidi in boxers. Can Assoc Radiol J 61:29–32. [DOI] [PubMed] [Google Scholar]
- 9. Bailes JE, Petraglia AL, Omalu BI, Nauman E, Talavage T (2013) Role of subconcussion in repetitive mild traumatic brain injury. J Neurosurg 119:1235–1245. [DOI] [PubMed] [Google Scholar]
- 10. Banks SJ, Mayer B, Obuchowski N, Shin W, Lowe M, Phillips M et al (2014) Impulsiveness in professional fighters. J Neuropsychiatry Clin Neurosci 26:44–50. [DOI] [PubMed] [Google Scholar]
- 11. Barnes SR, Haacke EM (2009) Susceptibility‐weighted imaging: clinical angiographic applications. Magn Reson Imaging Clin N Am 17:47–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Basser PJ, Jones DK (2002) Diffusion‐tensor MRI: theory, experimental design and data analysis—a technical review. NMR Biomed 15:456–467. [DOI] [PubMed] [Google Scholar]
- 13. Baugh CM, Stamm JM, Riley DO, Gavett BE, Shenton ME, Lin A et al (2012) Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav 6:244–254. [DOI] [PubMed] [Google Scholar]
- 14. Bazarian JJ, Zhu T, Blyth B, Borrino A, Zhong J (2012) Subject‐specific changes in brain white matter on diffusion tensor imaging after sports‐related concussion. Magn Reson Imaging 30:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bazarian JJ, Zhu T, Zhong J, Janigro D, Rozen E, Roberts A et al (2014) Persistent, long‐term cerebral white matter changes after sports‐related repetitive head impacts. PLoS ONE 9:e94734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beal MF (1995) Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol 38:357–366. [DOI] [PubMed] [Google Scholar]
- 17. Beauchamp MH, Beare R, Ditchfield M, Coleman L, Babl FE, Kean M et al (2013) Susceptibility weighted imaging and its relationship to outcome after pediatric traumatic brain injury. Cortex 49:591–598. [DOI] [PubMed] [Google Scholar]
- 18. Belanger HG, Curtiss G, Demery JA, Lebowitz BK, Vanderploeg RD (2005) Factors moderating neuropsychological outcomes following mild traumatic brain injury: a meta‐analysis. J Int Neuropsychol Soc 11:215–227. [DOI] [PubMed] [Google Scholar]
- 19. Bigler ED (2004) Neuropsychological results and neuropathological findings at autopsy in a case of mild traumatic brain injury. J Int Neuropsychol Soc 10:794–806. [DOI] [PubMed] [Google Scholar]
- 20. Bigler ED (2013) Traumatic brain injury, neuroimaging, and neurodegeneration. Front Hum Neurosci 7:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bigler ED, Maxwell WL (2012) Neuropathology of mild traumatic brain injury: relationship to neuroimaging findings. Brain Imaging Behav 6:108–136. [DOI] [PubMed] [Google Scholar]
- 22. Binder LI, Guillozet‐Bongaarts AL, Garcia‐Sierra F, Berry RW (2005) Tau, tangles, and Alzheimer's disease. Biochim Biophys Acta 1739:216–223. [DOI] [PubMed] [Google Scholar]
- 23. Bouix S, Pasternak O, Rathi Y, Pelavin PE, Zafonte R, Shenton ME (2013) Increased gray matter diffusion anisotropy in patients with persistent post‐concussive symptoms following mild traumatic brain injury. PLoS ONE 8:e66205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bowman KM, Blau A (1940) Psychotic states following head and brain injury in adults and children. In: Injuries of the Skull, Brain and Spinal Cord: Neuropsychiatric, Surgical, and Medico‐Legal Aspects. Brock S (ed.), pp. 309–360. Williams & Wilkins Co: Baltimore, MD. [Google Scholar]
- 25. Breedlove EL, Robinson M, Talavage TM, Morigaki KE, Yoruk U, O'Keefe K et al (2012) Biomechanical correlates of symptomatic and asymptomatic neurophysiological impairment in high school football. J Biomech 45:1265–1272. [DOI] [PubMed] [Google Scholar]
- 26. Broglio SP, Eckner JT, Martini D, Sosnoff JJ, Kutcher JS, Randolph C (2011) Cumulative head impact burden in high school football. J Neurotrauma 28:2069–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cantu RC (1998) Second‐impact syndrome. Clin Sports Med 17:37–44. [DOI] [PubMed] [Google Scholar]
- 28. Cassidy JD, Carroll LJ, Peloso PM, Borg J, von Holst H, Holm L et al (2004) Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med 36(Suppl. 43):28–60. [DOI] [PubMed] [Google Scholar]
- 29. Casson IR, Viano DC, Haacke EM, Kou Z, LeStrange DG (2014) Is there chronic brain damage in retired NFL players? Neuroradiology, neuropsychology, and neurology examinations of 45 retired players. Sports Health 6:384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chamard E, Theoret H, Skopelja EN, Forwell LA, Johnson AM, Echlin PS (2012) A prospective study of physician‐observed concussion during a varsity university hockey season: metabolic changes in ice hockey players. Part 4 of 4. Neurosurg Focus 33:E4:1–E4:7. [DOI] [PubMed] [Google Scholar]
- 31. Chang YL, Jacobson MW, Fennema‐Notestine C, Hagler DJ Jr, Jennings RG, Dale AM et al (2010) Level of executive function influences verbal memory in amnestic mild cognitive impairment and predicts prefrontal and posterior cingulate thickness. Cerebral Cortex 20:1305–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chappell MH, Ulug AM, Zhang L, Heitger MH, Jordan BD, Zimmerman RD, Watts R (2006) Distribution of microstructural damage in the brains of professional boxers: a diffusion MRI study. J Magn Reson Imaging 24:537–542. [DOI] [PubMed] [Google Scholar]
- 33. Chien DT, Bahri S, Szardenings AK, Walsh JC, Mu F, Su MY et al (2013) Early clinical PET imaging results with the novel PHF‐tau radioligand [F‐18]‐T807. J Alzheimer's Dis 34:457–468. [DOI] [PubMed] [Google Scholar]
- 34. Chien DT, Szardenings AK, Bahri S, Walsh JC, Mu F, Xia C et al (2014) Early clinical PET imaging results with the novel PHF‐tau radioligand [F18]‐T808. J Alzheimer's Dis 38:171–184. [DOI] [PubMed] [Google Scholar]
- 35. Clay MB, Glover KL, Lowe DT (2013) Epidemiology of concussion in sport: a literature review. J Chiropr Med 12:230–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Colbert CA, Holshouser BA, Aaen GS, Sheridan C, Oyoyo U, Kido D, Ashwal S (2010) Value of cerebral microhemorrhages detected with susceptibility‐weighted MR imaging for prediction of long‐term outcome in children with nonaccidental trauma. Radiology 256:898–905. [DOI] [PubMed] [Google Scholar]
- 37. Coronado VG, McGuire LC, Sarmiento K, Bell J, Lionbarger MR, Jones CD et al (2012) Trends in traumatic brain injury in the U.S. and the public health response: 1995–2009. J Safety Res 43:299–307. [DOI] [PubMed] [Google Scholar]
- 38. Corps KN, Roth TL, McGavern DB (2015) Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Corsellis JA, Bruton CJ, Freeman‐Browne D (1973) The aftermath of boxing. Psychol Med 3:270–303. [DOI] [PubMed] [Google Scholar]
- 40. Coughlin JM, Wang Y, Munro CA, Ma S, Yue C, Chen S et al (2014) Neuroinflammation and brain atrophy in former NFL players: an in vivo multimodal imaging pilot study. Neurobiol Dis 74C:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Critchley M (1949) Punch‐drunk syndromes: the chronic traumatic encephalopathy of boxers. In: Hommage à Clovis Vincent. Vincent C (ed.), p. 131. Maloine: Paris. [Google Scholar]
- 42. Critchley M (1957) Medical aspects of boxing, particularly from a neurological standpoint. Br Med J 1:357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Davenport EM, Whitlow CT, Urban JE, Espeland MA, Jung Y, Rosenbaum DA et al (2014) Abnormal white matter integrity related to head impact exposure in a season of high school varsity football. J Neurotrauma 31:1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. De Meyer G, Shapiro F, Vanderstichele H, Vanmechelen E, Engelborghs S, De Deyn PP et al (2010) Diagnosis‐independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol 67:949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dickerson BC, Wolk DA, Alzheimer's Disease Neuroimaging I (2012) MRI cortical thickness biomarker predicts AD‐like CSF and cognitive decline in normal adults. Neurology 78:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Donaldson L, Asbridge M, Cusimano MD (2013) Body checking rules and concussion in elite hockey. PLoS ONE 8:e69122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Echlin PS, Skopelja EN, Worsley R, Dadachanji SB, Lloyd‐Smith DR, Taunton JA et al (2012) A prospective study of physician‐observed concussion during a varsity university ice hockey season: incidence and neuropsychological changes. Part 2 of 4. Neurosurg Focus 33:E2:1–E2:11. [DOI] [PubMed] [Google Scholar]
- 48. Echlin PS, Tator CH, Cusimano MD, Cantu RC, Taunton JE, Upshur RE et al (2010) A prospective study of physician‐observed concussions during junior ice hockey: implications for incidence rates. Neurosurg Focus 29:E4. [DOI] [PubMed] [Google Scholar]
- 49. Eskridge SL, Macera CA, Galarneau MR, Holbrook TL, Woodruff SI, MacGregor AJ et al (2012) Injuries from combat explosions in Iraq: injury type, location, and severity. Injury 43:1678–1682. [DOI] [PubMed] [Google Scholar]
- 50. Ford JH, Giovanello KS, Guskiewicz KM (2013) Episodic memory in former professional football players with a history of concussion: an event‐related functional neuroimaging study. J Neurotrauma 30:1683–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gandy S, DeKosky ST (2014) [18F]‐T807 tauopathy PET imaging in chronic traumatic encephalopathy. F1000Research.3:229. [DOI] [PMC free article] [PubMed]
- 52. Garcia‐Panach J, Lull N, Lull JJ, Ferri J, Martinez C, Sopena P et al (2011) A voxel‐based analysis of FDG‐PET in traumatic brain injury: regional metabolism and relationship between the thalamus and cortical areas. J Neurotrauma 28:1707–1717. [DOI] [PubMed] [Google Scholar]
- 53. Gavett BE, Cantu RC, Shenton M, Lin AP, Nowinski CJ, McKee AC, Stern RA (2011) Clinical appraisal of chronic traumatic encephalopathy: current perspectives and future directions. Curr Opin Neurol 24:525–531. [DOI] [PubMed] [Google Scholar]
- 54. Giza CC, Hovda DA (2001) The neurometabolic cascade of concussion. J Athl Train 36:228–235. [PMC free article] [PubMed] [Google Scholar]
- 55. Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA et al (2012) Chronic traumatic encephalopathy in blast‐exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med 4:134ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gross H, Kling A, Henry G, Herndon C, Lavretsky H (1996) Local cerebral glucose metabolism in patients with long‐term behavioral and cognitive deficits following mild traumatic brain injury. J Neuropsychiatry Clin Neurosci 8:324–334. [DOI] [PubMed] [Google Scholar]
- 57. Grossman EJ, Ge Y, Jensen JH, Babb JS, Miles L, Reaume J et al (2012) Thalamus and cognitive impairment in mild traumatic brain injury: a diffusional kurtosis imaging study. J Neurotrauma 29:2318–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Guskiewicz KM, Marshall SW, Bailes J, McCrea M, Harding HP Jr, Matthews A et al (2007) Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc 39:903–909. [DOI] [PubMed] [Google Scholar]
- 59. Haacke EM, Xu Y, Cheng YC, Reichenbach JR (2004) Susceptibility weighted imaging (SWI). Magn Reson Med 52:612–618. [DOI] [PubMed] [Google Scholar]
- 60. Haglund Y, Bergstrand G (1990) Does Swedish amateur boxing lead to chronic brain damage? 2. A retrospective study with CT and MRI. Acta Neurol Scand 82:297–302. [DOI] [PubMed] [Google Scholar]
- 61. Hahnel S, Stippich C, Weber I, Darm H, Schill T, Jost J et al (2008) Prevalence of cerebral microhemorrhages in amateur boxers as detected by 3T MR imaging. AJNR Am J Neuroradiol 29:388–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hampshire A, MacDonald A, Owen AM (2013) Hypoconnectivity and hyperfrontality in retired American football players. Sci Rep 3:2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hart J Jr, Kraut MA, Womack KB, Strain J, Didehbani N, Bartz E et al (2013) Neuroimaging of cognitive dysfunction and depression in aging retired National Football League players: a cross‐sectional study. JAMA Neurol 70:326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hasiloglu ZI, Albayram S, Selcuk H, Ceyhan E, Delil S, Arkan B, Baskoy L (2011) Cerebral microhemorrhages detected by susceptibility‐weighted imaging in amateur boxers. AJNR Am J Neuroradiol 32:99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hazrati LN, Tartaglia MC, Diamandis P, Davis KD, Green RE, Wennberg R et al (2013) Absence of chronic traumatic encephalopathy in retired football players with multiple concussions and neurological symptomatology. Front Hum Neurosci 7:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Helmer KG, Pasternak O, Fredman E, Preciado RI, Koerte IK, Sasaki T et al (2014) Hockey Concussion Education Project, Part 1. Susceptibility‐weighted imaging study in male and female ice hockey players over a single season. J Neurosurg 120:864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hetherington HP, Hamid H, Kulas J, Ling G, Bandak F, de Lanerolle NC, Pan JW (2014) MRSI of the medial temporal lobe at 7 T in explosive blast mild traumatic brain injury. Magn Reson Med 71:1358–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Humayun MS, Presty SK, Lafrance ND, Holcomb HH, Loats H, Long DM et al (1989) Local cerebral glucose abnormalities in mild closed head injured patients with cognitive impairments. Nucl Med Commun 10:335–344. [DOI] [PubMed] [Google Scholar]
- 69. Ibarretxe‐Bilbao N, Junque C, Segura B, Baggio HC, Marti MJ, Valldeoriola F et al (2012) Progression of cortical thinning in early Parkinson's disease. Mov Disord 27:1746–1753. [DOI] [PubMed] [Google Scholar]
- 70. Iverson GL (2005) Outcome from mild traumatic brain injury. Curr Opin Psychiatry 18:301–317. [DOI] [PubMed] [Google Scholar]
- 71. Jordan BD, Zimmerman RD (1990) Computed tomography and magnetic resonance imaging comparisons in boxers. JAMA 263:1670–1674. [PubMed] [Google Scholar]
- 72. Jubault T, Gagnon JF, Karama S, Ptito A, Lafontaine AL, Evans AC, Monchi O (2011) Patterns of cortical thickness and surface area in early Parkinson's disease. Neuroimage 55:462–467. [DOI] [PubMed] [Google Scholar]
- 73. Kato T, Nakayama N, Yasokawa Y, Okumura A, Shinoda J, Iwama T (2007) Statistical image analysis of cerebral glucose metabolism in patients with cognitive impairment following diffuse traumatic brain injury. J Neurotrauma 24:919–926. [DOI] [PubMed] [Google Scholar]
- 74. Kaufman MS, Concannon LG, Herring SA (2014) Evaluation and treatment of the concussed athlete—update. Phys Med Rehabil Clin N Am 25:707–722. [DOI] [PubMed] [Google Scholar]
- 75. Kemp PM, Houston AS, Macleod MA, Pethybridge RJ (1995) Cerebral perfusion and psychometric testing in military amateur boxers and controls. J Neurol Neurosurg Psychiatry 59:368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Khan AR, Wang L, Beg MF (2008) FreeSurfer‐initiated fully‐automated subcortical brain segmentation in MRI using Large Deformation Diffeomorphic Metric Mapping. Neuroimage 41:735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kim N, Branch CA, Kim M, Lipton ML (2013) Whole brain approaches for identification of microstructural abnormalities in individual patients: comparison of techniques applied to mild traumatic brain injury. PLoS ONE 8:e59382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Koerte IK, Ertl‐Wagner B, Reiser M, Zafonte R, Shenton ME (2012) White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA 308:1859–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Koerte IK, Kaufmann D, Hartl E, Bouix S, Pasternak O, Kubicki M et al (2012) A prospective study of physician‐observed concussion during a varsity university hockey season: white matter integrity in ice hockey players. Part 3 of 4. Neurosurg Focus 33:E3:1–E3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Konrad C, Geburek AJ, Rist F, Blumenroth H, Fischer B, Husstedt I et al (2011) Long‐term cognitive and emotional consequences of mild traumatic brain injury. Psychol Med 41:1197–1211. [DOI] [PubMed] [Google Scholar]
- 81. Kurca E, Sivak S, Kucera P (2006) Impaired cognitive functions in mild traumatic brain injury patients with normal and pathologic magnetic resonance imaging. Neuroradiology 48:661–669. [DOI] [PubMed] [Google Scholar]
- 82. Langlois JA, Rutland‐Brown W, Wald MM (2006) The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 21:375–378. [DOI] [PubMed] [Google Scholar]
- 83. Lanius RA, Bluhm R, Lanius U, Pain C (2006) A review of neuroimaging studies in PTSD: heterogeneity of response to symptom provocation. J Psychiatr Res 40:709–729. [DOI] [PubMed] [Google Scholar]
- 84. Le TH, Gean AD (2009) Neuroimaging of traumatic brain injury. Mt Sinai J Med 76:145–162. [DOI] [PubMed] [Google Scholar]
- 85. Levin HS, Lippold SC, Goldman A, Handel S, High WM Jr, Eisenberg HM, Zelitt D (1987) Neurobehavioral functioning and magnetic resonance imaging findings in young boxers. J Neurosurg 67:657–667. [DOI] [PubMed] [Google Scholar]
- 86. Lin A, Tran T, Bluml S, Merugumala S, Liao HJ, Ross BD (2012) Guidelines for acquiring and reporting clinical neurospectroscopy. Semin Neurol 32:432–453. [DOI] [PubMed] [Google Scholar]
- 87. Lin AP, Ramadan S, Stern RA, Box HC, Nowinski CJ, Ross BD, Mountford CE (2015) Changes in the neurochemistry of athletes with repetitive brain trauma: preliminary results using localized correlated spectroscopy. Alzheimer's Research & Therapy. [DOI] [PMC free article] [PubMed]
- 88. Lipton ML, Kim N, Zimmerman ME, Kim M, Stewart WF, Branch CA, Lipton RB (2013) Soccer heading is associated with white matter microstructural and cognitive abnormalities. Radiology 268:850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Little DM, Geary EK, Moynihan M, Alexander A, Pennington M, Glang P et al (2014) Imaging chronic traumatic brain injury as a risk factor for neurodegeneration. Alzheimer's Dement 10(Suppl. 3):S188–S195. [DOI] [PubMed] [Google Scholar]
- 90. Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS et al (2011) Detection of blast‐related traumatic brain injury in U.S. military personnel. NEJM 364:2091–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. MacKenzie JD, Siddiqi F, Babb JS, Bagley LJ, Mannon LJ, Sinson GP, Grossman RI (2002) Brain atrophy in mild or moderate traumatic brain injury: a longitudinal quantitative analysis. AJNR Am J Neuroradiol 23:1509–1515. [PMC free article] [PubMed] [Google Scholar]
- 92. Malcolm JG, Michailovich O, Bouix S, Westin CF, Shenton ME, Rathi Y (2010) A filtered approach to neural tractography using the Watson directional function. Med Image Anal 14:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Marchi N, Bazarian JJ, Puvenna V, Janigro M, Ghosh C, Zhong J et al (2013) Consequences of repeated blood‐brain barrier disruption in football players. PLoS ONE 8:e56805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Martland HS (1928) Punch drunk. J Am Med Assoc 91:1103–1107. [Google Scholar]
- 95. Maruta J, Lee SW, Jacobs EF, Ghajar J (2010) A unified science of concussion. Ann N Y Acad Sci 1208:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Matser EJ, Kessels AG, Lezak MD, Jordan BD, Troost J (1999) Neuropsychological impairment in amateur soccer players. JAMA 282:971–973. [DOI] [PubMed] [Google Scholar]
- 97. Matser JT, Kessels AG, Jordan BD, Lezak MD, Troost J (1998) Chronic traumatic brain injury in professional soccer players. Neurology 51:791–796. [DOI] [PubMed] [Google Scholar]
- 98. Matthews WB (1972) Footballer's migraine. Br Med J 2:326–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mayer AR, Bedrick EJ, Ling JM, Toulouse T, Dodd A (2014) Methods for identifying subject‐specific abnormalities in neuroimaging data. Hum Brain Mapp 35:5457–5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. McAllister T (2014) Task‐related cerebral activation pre and post season in a cohort of collegiate contact sport athletes brain injury. IBIA Abstract.
- 101. McAllister TW, Flashman LA, Maerlender A, Greenwald RM, Beckwith JG, Tosteson TD et al (2012) Cognitive effects of one season of head impacts in a cohort of collegiate contact sport athletes. Neurology 78:1777–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. McAllister TW, Ford JC, Flashman LA, Maerlender A, Greenwald RM, Beckwith JG et al (2014) Effect of head impacts on diffusivity measures in a cohort of collegiate contact sport athletes. Neurology 82:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. McCrory P (2001) Does second impact syndrome exist? Clin J Sport Med 11:144–149. [DOI] [PubMed] [Google Scholar]
- 104. McCrory P, Meeuwisse WH, Aubry M, Cantu B, Dvorak J, Echemendia RJ et al (2013) Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med 47:250–258. [DOI] [PubMed] [Google Scholar]
- 105. McCrory PR, Berkovic SF (1998) Second impact syndrome. Neurology 50:677–683. [DOI] [PubMed] [Google Scholar]
- 106. McDonald BC, Saykin AJ, McAllister TW (2012) Functional MRI of mild traumatic brain injury (mTBI): progress and perspectives from the first decade of studies. Brain Imaging Behav 6:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. McKee AC, Cantu RC, Nowinski CJ, Hedley‐Whyte ET, Gavett BE, Budson AE et al (2009) Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 68:709–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH et al (2013) The spectrum of disease in chronic traumatic encephalopathy. Brain 136(Pt 1):43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. McQuillen JB, McQuillen EN, Morrow P (1988) Trauma, sport, and malignant cerebral edema. Am J Forensic Med Pathol 9:12–15. [DOI] [PubMed] [Google Scholar]
- 110. Menon DK, Schwab K, Wright DW, Maas AI (2010) Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil 91:1637–1640. [DOI] [PubMed] [Google Scholar]
- 111. Miller H (1966) Mental after‐effects of head injury. Proc R Soc Med 59:257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Millspaugh J (1937) Dementia pugilistica. US Naval Med Bull 35:297–303. [Google Scholar]
- 113. Mitsis EM, Riggio S, Kostakoglu L, Dickstein DL, Machac J, Delman B et al (2014) Tauopathy PET and amyloid PET in the diagnosis of chronic traumatic encephalopathies: studies of a retired NFL player and of a man with FTD and a severe head injury. Transl Psychiatry 4:e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Mittl RL, Grossman RI, Hiehle JF, Hurst RW, Kauder DR, Gennarelli TA, Alburger GW (1994) Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. AJNR Am J Neuroradiol 15:1583–1589. [PMC free article] [PubMed] [Google Scholar]
- 115. Montenigro PH, Baugh CM, Daneshvar DH, Mez J, Budson AE, Au R et al (2014) Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimer's Res Ther 6:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Montenigro PH, Corp DT, Stein TD, Cantu RC, Stern RA (2015) Chronic traumatic encephalopathy: historical origins and current perspective. Annu Rev Clin Psychol. [DOI] [PubMed] [Google Scholar]
- 117. Mouzon B, Chaytow H, Crynen G, Bachmeier C, Stewart J, Mullan M et al (2012) Repetitive mild traumatic brain injury in a mouse model produces learning and memory deficits accompanied by histological changes. J Neurotrauma 29:2761–2773. [DOI] [PubMed] [Google Scholar]
- 118. Naunheim RS, Bayly PV, Standeven J, Neubauer JS, Lewis LM, Genin GM (2003) Linear and angular head accelerations during heading of a soccer ball. Med Sci Sports Exerc 35:1406–1412. [DOI] [PubMed] [Google Scholar]
- 119. Ng TS, Lin AP, Koerte IK, Pasternak O, Liao H, Merugumala S et al (2014) Neuroimaging in repetitive brain trauma. Alzheimer's Res Ther 6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Niogi SN, Mukherjee P (2010) Diffusion tensor imaging of mild traumatic brain injury. J Head Trauma Rehabil 25:241–255. [DOI] [PubMed] [Google Scholar]
- 121. Orrison WW, Hanson EH, Alamo T, Watson D, Sharma M, Perkins TG, Tandy RD (2009) Traumatic brain injury: a review and high‐field MRI findings in 100 unarmed combatants using a literature‐based checklist approach. J Neurotrauma 26:689–701. [DOI] [PubMed] [Google Scholar]
- 122. Ozarslan E, Koay CG, Shepherd TM, Komlosh ME, Irfanoglu MO, Pierpaoli C, Basser PJ (2013) Mean apparent propagator (MAP) MRI: a novel diffusion imaging method for mapping tissue microstructure. Neuroimage 78:16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Park H, Yang JJ, Seo J, Lee JM, ADNI (2013) Dimensionality reduced cortical features and their use in predicting longitudinal changes in Alzheimer's disease. Neurosci Lett 550:17–22. [DOI] [PubMed] [Google Scholar]
- 124. Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y (2009) Free water elimination and mapping from diffusion MRI. Magn Reson Med 62:717–730. [DOI] [PubMed] [Google Scholar]
- 125. Peskind ER, Petrie EC, Cross DJ, Pagulayan K, McCraw K, Hoff D et al (2011) Cerebrocerebellar hypometabolism associated with repetitive blast exposure mild traumatic brain injury in 12 Iraq war Veterans with persistent post‐concussive symptoms. Neuroimage 54(Suppl. 1):S76–S82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Petrie EC, Cross DJ, Yarnykh VL, Richards T, Martin NM, Pagulayan K et al (2014) Neuroimaging, behavioral, and psychological sequelae of repetitive combined blast/impact mild traumatic brain injury in Iraq and Afghanistan war veterans. J Neurotrauma 31:425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Ponsford J, Cameron P, Fitzgerald M, Grant M, Mikocka‐Walus A (2011) Long‐term outcomes after uncomplicated mild traumatic brain injury: a comparison with trauma controls. J Neurotrauma 28:937–946. [DOI] [PubMed] [Google Scholar]
- 128. Poole VN, Abbas K, Shenk TE, Breedlove EL, Breedlove KM, Robinson ME et al (2014) MR spectroscopic evidence of brain injury in the non‐diagnosed collision sport athlete. Dev Neuropsychol 39:459–473. [DOI] [PubMed] [Google Scholar]
- 129. Provenzano FA, Jordan B, Tikofsky RS, Saxena C, Van Heertum RL, Ichise M (2010) F‐18 FDG PET imaging of chronic traumatic brain injury in boxers: a statistical parametric analysis. Nucl Med Commun 31:952–957. [DOI] [PubMed] [Google Scholar]
- 130. Queen RM, Weinhold PS, Kirkendall DT, Yu B (2003) Theoretical study of the effect of ball properties on impact force in soccer heading. Med Sci Sports Exerc 35:2069–2076. [DOI] [PubMed] [Google Scholar]
- 131. Rathi Y, Gagoski B, Setsompop K, Michailovich O, Grant PE, Westin CF (2013) Diffusion propagator estimation from sparse measurements in a tractography framework. Med Image Comput Comput Assist Interv 16(Pt 3):510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Rishiraj N, Lloyd‐Smith R, Lorenz T, Niven B, Michel M (2009) University men's ice hockey: rates and risk of injuries over 6‐years. J Sports Med Phys Fitness 49:159–166. [PubMed] [Google Scholar]
- 133. Robbins CA, Daneshvar DH, Picano JD, Gavett BE, Baugh CM, Riley DO et al (2014) Self‐reported concussion history: impact of providing a definition of concussion. Open Access J Sports Med 5:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Ropper AH, Gorson KC (2007) Clinical practice. Concussion. N Engl J Med 356:166–172. [DOI] [PubMed] [Google Scholar]
- 135. Ruff RM, Camenzuli L, Mueller J (1996) Miserable minority: emotional risk factors that influence the outcome of a mild traumatic brain injury. Brain Injury 10:551–565. [DOI] [PubMed] [Google Scholar]
- 136. Scheibel RS, Newsome MR, Troyanskaya M, Lin X, Steinberg JL, Radaideh M, Levin HS (2012) Altered brain activation in military personnel with one or more traumatic brain injuries following blast. J Int Neuropsychol Soc 18:89–100. [DOI] [PubMed] [Google Scholar]
- 137. Schretlen DJ, Shapiro AM (2003) A quantitative review of the effects of traumatic brain injury on cognitive functioning. Int Rev Psychiatry 15:341–349. [DOI] [PubMed] [Google Scholar]
- 138. Seo SW, Ahn J, Yoon U, Im K, Lee JM, Tae Kim S et al (2010) Cortical thinning in vascular mild cognitive impairment and vascular dementia of subcortical type. J Neuroimaging 20:37–45. [DOI] [PubMed] [Google Scholar]
- 139. Serbest G, Burkhardt MF, Siman R, Raghupathi R, Saatman KE (2007) Temporal profiles of cytoskeletal protein loss following traumatic axonal injury in mice. Neurochem Res 32:2006–2014. [DOI] [PubMed] [Google Scholar]
- 140. Shah M, Catafau AM (2014) Molecular imaging insights into neurodegeneration: focus on tau PET radiotracers. J Nucl Med 55:871–874. [DOI] [PubMed] [Google Scholar]
- 141. Shenton ME, Hamoda HM, Schneiderman JS, Bouix S, Pasternak O, Rathi Y et al (2012) A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav 6:137–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Shin W, Mahmoud SY, Sakaie K, Banks SJ, Lowe MJ, Phillips M et al (2014). Diffusion measures indicate fight exposure‐related damage to cerebral white matter in boxers and mixed martial arts fighters. AJNR Am J Neuroradiol 35:285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Shoup TM, Yokell DL, Rice PA, Jackson RN, Livni E, Johnson KA et al (2013) A concise radiosynthesis of the tau radiopharmaceutical, [(18) F]T807. J Labelled Comp Radiopharm 56:736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Singh M, Jeong J, Hwang D, Sungkarat W, Gruen P (2010) Novel diffusion tensor imaging methodology to detect and quantify injured regions and affected brain pathways in traumatic brain injury. Magn Reson Imaging 28:22–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Singh R, Meier TB, Kuplicki R, Savitz J, Mukai I, Cavanagh L et al (2014) Relationship of collegiate football experience and concussion with hippocampal volume and cognitive outcomes. JAMA 311:1883–1888. [DOI] [PubMed] [Google Scholar]
- 146. Singh V, Chertkow H, Lerch JP, Evans AC, Dorr AE, Kabani NJ (2006) Spatial patterns of cortical thinning in mild cognitive impairment and Alzheimer's disease. Brain 129(Pt 11):2885–2893. [DOI] [PubMed] [Google Scholar]
- 147. Small GW, Kepe V, Siddarth P, Ercoli LM, Merrill DA, Donoghue N et al (2013) PET scanning of brain tau in retired national football league players: preliminary findings. Am J Geriatr Psychiatry 21:138–144. [DOI] [PubMed] [Google Scholar]
- 148. Smith DH, Johnson VE, Stewart W (2013) Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol 9:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Stamm JM, Bourlas AP, Baugh CM, Fritts NG, Daneshvar DH, Martin BM et al (2015) Age of first exposure to football and later‐life cognitive impairment in former NFL players. Neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Stern RA, Daneshvar DH, Baugh CM, Seichepine DR, Montenigro PH, Riley DO et al (2013) Clinical presentation of chronic traumatic encephalopathy. Neurology 81:1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Stern RA, Riley DO, Daneshvar DH, Nowinski CJ, Cantu RC, McKee AC (2011) Long‐term consequences of repetitive brain trauma: chronic traumatic encephalopathy. PM R 3(10 Suppl. 2):S460–S467. [DOI] [PubMed] [Google Scholar]
- 152. Stone JR, Tustison NJ, Wassermann EM, Polejaeva L, Tierney M, McCarron RM et al (2014) Neuroimaging correlates of repetitive blast exposure in experienced military breachers. J Neurotrauma 30:A120–A121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Strain J, Didehbani N, Cullum CM, Mansinghani S, Conover H, Kraut MA et al (2013) Depressive symptoms and white matter dysfunction in retired NFL players with concussion history. Neurology 81:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Talavage TM, Nauman EA, Breedlove EL, Yoruk U, Dye AE, Morigaki KE et al (2014) Functionally‐detected cognitive impairment in high school football players without clinically‐diagnosed concussion. J Neurotrauma 31:327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Tanriverdi F, Unluhizarci K, Kocyigit I, Tuna IS, Karaca Z, Durak AC et al (2008) Brief communication: pituitary volume and function in competing and retired male boxers. Ann Intern Med 148:827–831. [DOI] [PubMed] [Google Scholar]
- 156. Tartaglia MC, Hazrati LN, Davis KD, Green RE, Wennberg R, Mikulis D et al (2014) Chronic traumatic encephalopathy and other neurodegenerative proteinopathies. Front Hum Neurosci 8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Tator CH (2013) Concussions and their consequences: current diagnosis, management and prevention. CMAJ 185:975–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Terry DP, Faraco CC, Smith D, Diddams MJ, Puente AN, Miller LS (2012) Lack of long‐term fMRI differences after multiple sports‐related concussions. Brain Injury 26:1684–1696. [DOI] [PubMed] [Google Scholar]
- 159. Tremblay S, De Beaumont L, Henry LC, Boulanger Y, Evans AC, Bourgouin P et al (2013) Sports concussions and aging: a neuroimaging investigation. Cerebral Cortex 23:1159–1166. [DOI] [PubMed] [Google Scholar]
- 160. Tysvaer A, Storli O (1981) Association football injuries to the brain. A preliminary report. Br J Sports Med 15:163–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Tysvaer AT, Lochen EA (1991) Soccer injuries to the brain. A neuropsychologic study of former soccer players. Am J Sports Med 19:56–60. [DOI] [PubMed] [Google Scholar]
- 162. Vagnozzi R, Signoretti S, Tavazzi B, Floris R, Ludovici A, Marziali S et al (2008) Temporal window of metabolic brain vulnerability to concussion: a pilot 1H‐magnetic resonance spectroscopic study in concussed athletes—part III. Neurosurgery 62:1286–1295. [DOI] [PubMed] [Google Scholar]
- 163. Villemagne VL, Furumoto S, Fodero‐Tavolette M, Harada R, Mulligan RS, Kudo Y et al (2012) The challenges of tau imaging. Future Neurology 7:409–421. [Google Scholar]
- 164. Villemagne VL, Okamura N (2014) In vivo tau imaging: obstacles and progress. Alzheimer's Dement 10(Suppl. 3):S254–S264. [DOI] [PubMed] [Google Scholar]
- 165. Xia CF, Arteaga J, Chen G, Gangadharmath U, Gomez LF, Kasi D et al (2013) [(18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer's disease. Alzheimer's Dement 9:666–676. [DOI] [PubMed] [Google Scholar]
- 166. Xu L, Nguyen JV, Lehar M, Menon A, Rha E, Arena J et al (2014) Repetitive mild traumatic brain injury with impact acceleration in the mouse: multifocal axonopathy, neuroinflammation, and neurodegeneration in the visual system. Exp Neurol. [DOI] [PubMed] [Google Scholar]
- 167. Yuh EL, Hawryluk GW, Manley GT (2014) Imaging concussion: a review. Neurosurgery 75(Suppl. 4):S50–S63. [DOI] [PubMed] [Google Scholar]
- 168. Zhang L, Heier LA, Zimmerman RD, Jordan B, Ulug AM (2006) Diffusion anisotropy changes in the brains of professional boxers. AJNR Am J Neuroradiol 27:2000–2004. [PMC free article] [PubMed] [Google Scholar]
- 169. Zhang L, Ravdin LD, Relkin N, Zimmerman RD, Jordan B, Lathan WE, Ulug AM (2003) Increased diffusion in the brain of professional boxers: a preclinical sign of traumatic brain injury? AJNR Am J Neuroradiol 24:52–57. [PMC free article] [PubMed] [Google Scholar]
- 170. Zhang MR, Red SD, Lin AH, Patel SS, Sereno AB (2013) Evidence of cognitive dysfunction after soccer playing with ball heading using a novel tablet‐based approach. PLoS ONE 8:e57364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Zhang W, Arteaga J, Cashion DK, Chen G, Gangadharmath U, Gomez LF et al (2012) A highly selective and specific PET tracer for imaging of tau pathologies. J Alzheimer's Dis 31:601–612. [DOI] [PubMed] [Google Scholar]
- 172. Zhou Y, Kierans A, Kenul D, Ge Y, Rath J, Reaume J et al (2013) Mild traumatic brain injury: longitudinal regional brain volume changes. Radiology 267:880–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Zhuo J, Xu S, Proctor JL, Mullins RJ, Simon JZ, Fiskum G, Gullapalli RP (2012) Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. Neuroimage 59:467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]


