Abstract
Catalytic enantioselective conjunctive cross-coupling between 9-BBN borate complexes and aryl electrophiles can be accomplished with Ni salts in the presence of a chiral diamine ligand. The reactions furnish chiral 9-BBN derivatives in an enantioselective fashion and these are converted to chiral alcohols and amines, or engaged in other stereospecific C-C bond forming reactions.
Keywords: Cross Coupling, Boron, Asymmetric Catalysis, Nickel
Table of Contents
Conjunctive Coupling: Ni-Catalyzed conjunctive couplings furnish chiral 9-BBN derivatives in an enantioselective fashion and these are converted to chiral alcohols and amines, or they can be engaged in other stereospecific C-C bond forming reactions.
Organoboron compounds are broadly useful reagents in organic chemistry. From the standpoint of enantioselective synthesis, considerable recent research has focused on the development of processes for the construction of chiral non-racemic organoboronic esters, in particular pinacol boronates. These reagents have the attractive features that they are chemically and configurationally stable – indeed, pinacol boronic esters are often isolable, storable materials – and they engage in a broad range of chemical transformations.[1] However, less common trialkylborane reagents hold two distinct advantages relative to their alkyl boronic ester counterparts. First, trialkylboranes are readily available by hydroboration of alkenes. Second, the enhanced electrophilicity of trialkylboranes relative to boronic esters allows the former to engage in reactions that are unavailable to the latter. Especially powerful reactions that apply to 9-BBN derivatives include an inexpensive CO-based homologation[2], homologations with α-haloacetate derivatives[3], and O'Donnell catalytic asymmetric amino acid syntheses.[4] Despite this synthesis utility, there is a lack of methods available for the asymmetric synthesis of 9-BBN derivatives. Indeed, as far as we are aware, the powerful Aggarwal homologation is the only method for the direct preparation of nonracemic 9-BBN reagents.[5] In this report, we address this short-coming through the development of a Ni-catalyzed conjunctive-cross-coupling with 9-BBN-derived boron ate complexes.
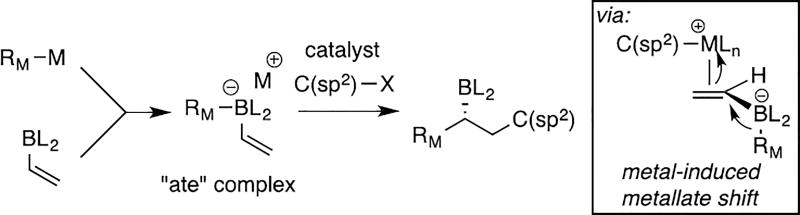
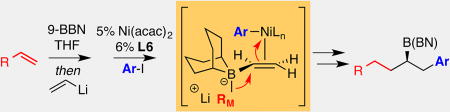
Recently, our laboratory developed a Pd-catalyzed conjunctive cross-coupling reaction (Scheme 1) that merges pinacol boronic esters, organolithium or Grignard reagents, and organic electrophiles to furnish chiral organoboron products.[6,7] Preliminary mechanistic studies suggest that this process occurs by π-activation of a reacting alkenyl boron "ate" complex by a Pd(II) intermediate (see box, Scheme 1), followed by 1,2-metalate shift.[8] This elementary step results in simultaneous addition of the migrating group and the Pd complex to opposite faces of the reacting alkene and is followed by reductive elimination. In an effort to expand the scope of the reaction towards 9-BBN derivatives, we studied the reaction of borate complexes formed by hydroboration of 1-octene, followed by addition of vinyllithium (Table 1). When this complex was treated with the Pd/Mandyphos complex that is effective for pinacolato boronate substrates (entry 1), the reaction proceeded efficiently; however, the coupling product 1 was racemic. After a survey of other chiral ligands provided only modest improvement in selectivity we considered the efficacy of Ni catalysts. While Ni complexes are less prolific than their Pd counterparts in terms of alkene π-activation, important precedents in the arena of Heck reactions[9], olefin polymerization[10] and oligomerization[11], suggested that Ni(II) complexes might be effective for conjunctive coupling.
Scheme 1.
Catalytic Conjunctive Cross-Coupling Reaction.
Table 1.
Catalyst Studies in 9-BBN Borate Conjunctive Coupling
| |||||
|---|---|---|---|---|---|
| Entry | MX | Ligand | PhX | Yielda | e.r. |
| 1b | Pd(OAc)2 | MandyPhos (L1) | PhOTf | 50 | 50:50 |
| 2 | Ni(cod)2 | none | PhI | <5 | – |
| 3 | Ni(cod)2 | bipyridine | PhI | 70 | – |
| 4 | Ni(cod)2 | DMEDA | PhI | 24 | – |
| 5 | Ni(cod)2 | TMEDA | PhI | <5 | – |
| 6 | Ni(cod)2 | i-PrPybox (L2) | PhI | <5 | – |
| 7 | Ni(cod)2 | t-BuBox (L3) | PhI | <5 | – |
| 8 | Ni(cod)2 | L4 | PhI | 87 | 79:21 |
| 9 | Ni(cod)2 | L5 | PhI | 60 | 93:7 |
| 10 | Ni(cod)2 | L6 | PhI | 58 | 95:5 |
Yield is after isolation and purification by silica gel chromatography.
Experiment employed 1-hexene and PhOTf as substrates.
Initial studies with Ni catalysts employed aryl halide electrophiles and simple achiral Ni complexes. Of note, while the reaction in the absence of ligand provides <5% yield (entry 2), when the bipyridine-derived catalyst was employed with iodobenzene electrophile (entry 3), the coupling product was isolated in 70% yield. In addition to bipyridine, N,N'-dimethyl-1,2-ethylene diamine was also mildly effective (entry 4). With these promising observations, several chiral ligands bearing sp2-hybridized N donors were examined for stereoselective conjunctive cross-coupling reactions. While elevated levels of selectivity were observed with pyridyl oxazoline ligands L4, much higher levels of selectivity were observed when chiral aliphatic diamines were employed (see Supplementary Material for complete ligand survey), with N,N'-dimethyl-1,2-stilbene diamine (L6) proving most selective.[12] A survey of Ni precatalysts revealed that Ni(acac)2 was the most effective and the most convenient catalyst precursor.
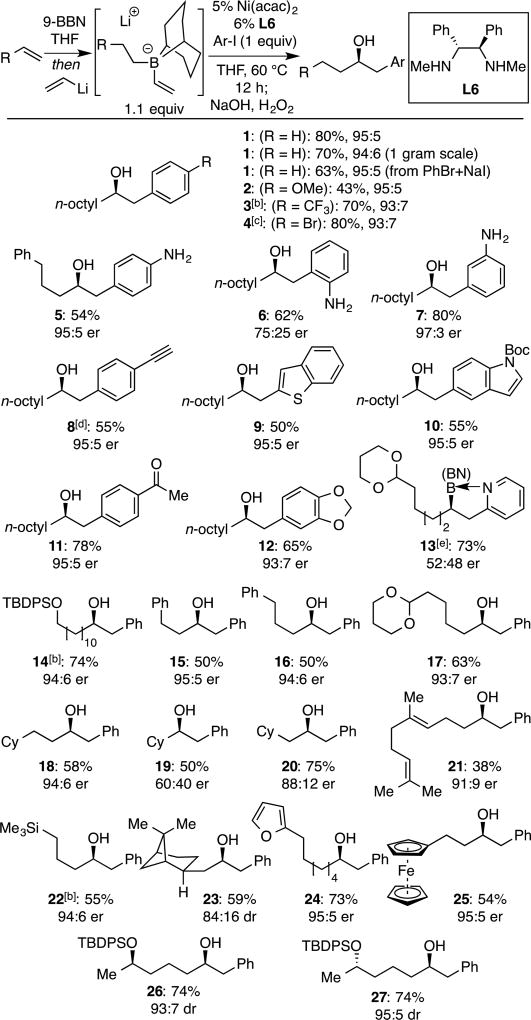
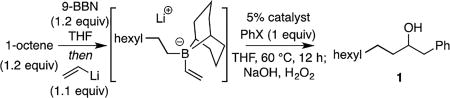
To survey the substrate scope, a range of 9-BBN-derived borate complexes was prepared from appropriate alkenes (hydroboration with 9-BBN in THF, followed by addition of vinyllithium[13]) and employed in a one-pot fashion. Treatment of the ate complex with pre-mixed Ni(acac)2 and ligand L6 in the presence of aryl halide electrophiles, followed by heating for 12 h at 60 °C and subsequent oxidative work-up afforded the derived alcohols. As noted in Table 2, good yield and selectivity can be obtained for many substrates. For the coupling with phenyl iodide, 80% yield is obtained on small scale (0.2 mmol), with a slight erosion of efficiency on gram scale. Bromobenzene could also be employed as long as an equivalent of sodium iodide is included. Both electron-poor (3, 4, 11) and electron-rich arenes (2, 5) can serve as competent electrophiles, although the reaction is more efficient with electron-withdrawing groups attached to the electrophilic substrate (cf. 3 and 2). Notably, electrophiles with sensitive functionality, such as ketones (11), halides (4) and even free amines (5–7) are tolerated in the reaction. Although Lewis basic functional groups are tolerated, electrophiles with an ability to coordinate to a linked transition metal center (2-pyridyl and ortho-anilido; 6 and 13) react with diminished selectivity. Migrating groups with different substitution patterns were also tolerated in the reaction. Interestingly, products derived from migration of the bicyclooctyl group of the 9-BBN ligand were not observed as a byproduct, even when secondary borane ligands (i.e. cyclohexyl) were employed as a migrating group (substrate 19).[14] In addition, substrates bearing appended arenes (5, 15, 16), silyl ethers (14), alkenes (21), acetals (13, 17), silanes (22), and ferrocenyl groups (25) are processed effectively in these reactions. Lastly, catalyst control operates with chiral substrates furnishing compounds 23, 26, and 27 with useful levels of diastereoselection.
Table 2.
Scope of Ni-Catalyzed Enantioselective Conjunctive Coupling[a]
See text and Supporting Information details. Vinyllithium prepared from n-BuLi and tetravinyl stannane. Yields are of purified material and represent the average value for two experiments.
Vinyllithium prepared from n-BuLi and vinyl iodide.
Reaction conducted at room temperature.
Substrate was (4-iodophenyl)triethylsilyl acetylene, which undergoes desilylation during oxidation.
Product difficult to oxidize; isolated as the borane after silica gel chromatography.
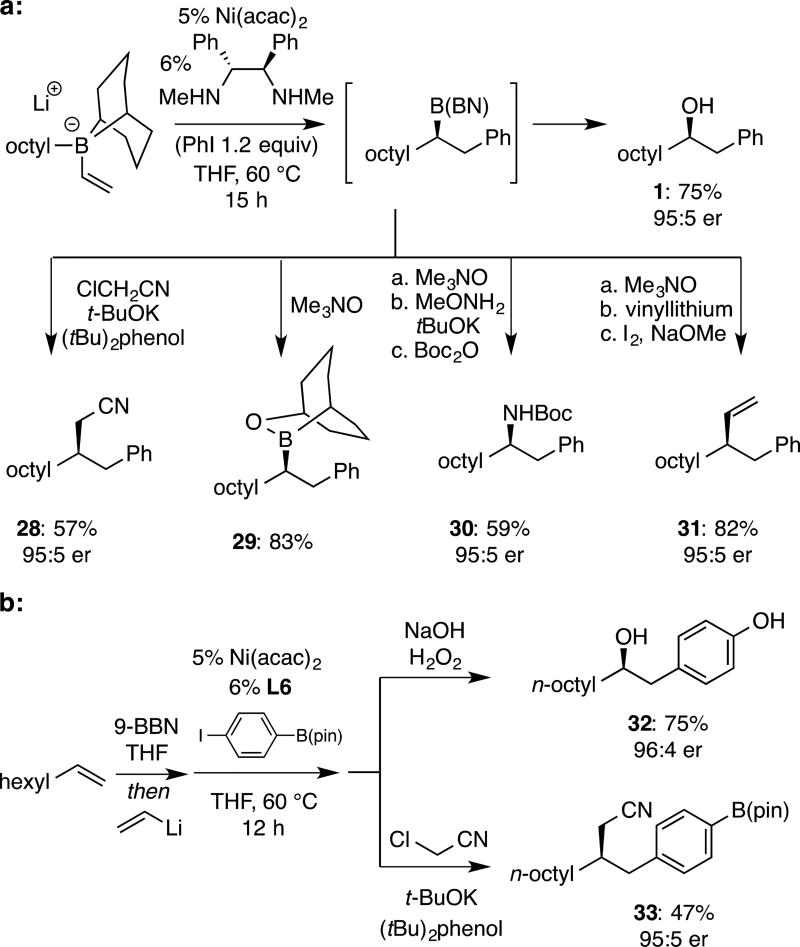
As mentioned above, 9-BBN derivatives are not commonly generated as products of asymmetric processes. Thus, it was of interest to examine transformations that might apply to Ni-catalyzed conjunctive coupling products. As depicted in Figure 2, while well-established oxidation[15] delivers alcohol 1 effectively, C-C bond-forming cyanomethylation[3c] was also found to apply in a one pot protocol, directly with the 9-BBN derivative, delivering 28 with excellent stereospecificity. In connection to other reactions, important studies by Aggarwal[14a] revealed a strong migratory aptitude of the 9-BBN bicyclooctane ring that often outcompetes migration of other alkyl substituents. To avoid this, we considered that the derived borinic ester, readily obtained by monooxidation with trimethylamine N-oxide (TMANO) as described by Soderquist[16], might provide a solution. The Soderquist oxidation applied directly on product mixtures and the resulting secondary borinic ester 29 was found to be stable in the open atmosphere and may be purified by column chromatography (83% isolated yield). Of note, the inexpensive hydrated form of TMANO proved equally effective (82% yield 29). Also of note, the borinic ester is a useful intermediate and can be employed directly, without isolation, allowing the alkene starting material to be converted directly, in a one-pot fashion, to the derived stereospecific amination[17] (30) or vinylation[18] (31) products in good yield and selectivity. The enhanced electrophilicity of borane derivatives can also be used to advantage in the selective functionalization of polyboryl species as demonstrated in Figure 1b.
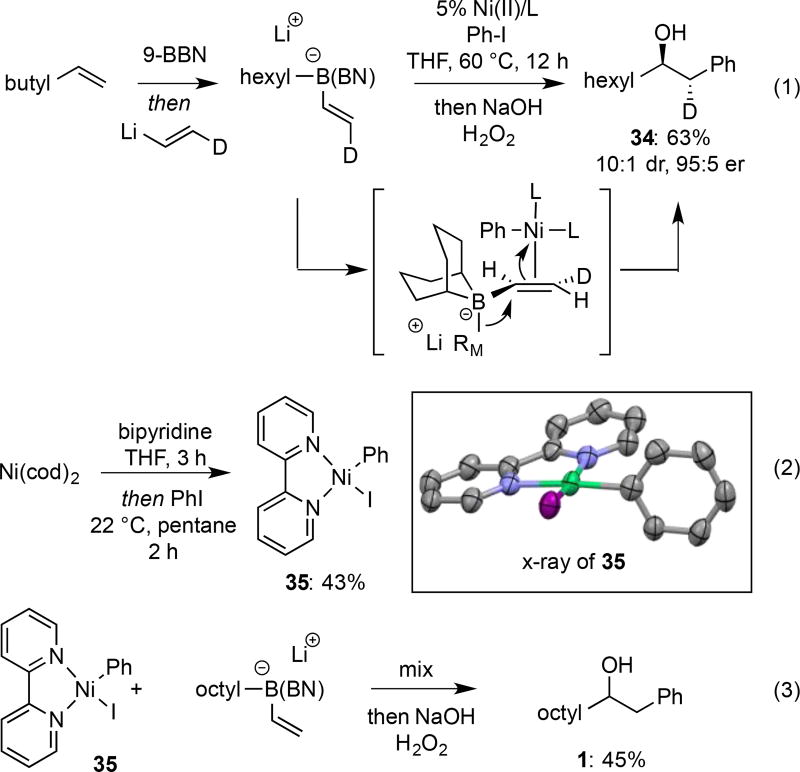
Figure 2.
Mechanistic experiments on Ni-catalyzed conjunctive coupling.
Figure 1.
a: Direct transformation of conjunctive coupling intermediate to chiral alcohol, amine, nitrile, and alkene products. b: Conjunctive coupling followed by selective functionalization of the borane in preference to the B(pin) group.
Ni-catalyzed cross coupling reactions can occur by a number of different mechanisms, in particular those that involve Ni(I)/Ni(III) and Ni(0)/Ni(II) redox cycles.[19] In addition to probing these possibilities, it was also of interest to learn about the overall topology of the Ni-catalyzed process. As depicted in equation 1 (Figure 2), by employing isotopically labeled vinyllithium, it was found that the Ni-catalyzed coupling occurs stereospecifically by overall anti addition across the alkene and delivers 34; this outcome is consistent with a catalyst-promoted metallate shift as opposed to radical-based addition reactions that appear to operate in processes recently developed by Studer[7h] and Aggarwal.[7i] Stoichiometric experiments with plausible catalytic intermediates were also undertaken. For the reaction with Ni/bipyridine, it was considered that oxidative addition adduct 35 (eq. 2) might be a catalytic intermediate. To investigate this, compound 35 was prepared by reacting iodobenzene with bipyridine•Ni(cod)2 complex in pentane and then triturating the resulting precipitate to remove unreacted electrophile.[20] To confirm the structure, Ni(II)(aryl)iodide adduct 35 was recrystallized and its structure determined by X-ray crystallography (inset, Figure 2).[21] Importantly, the solid obtained from the oxidative addition reaction, when treated with one equivalent of the octene-derived borate complex, furnished the conjunctive coupling product in 45% yield (eq. 3). This observation suggests that oxidative addition takes place before the 1,2-metallate shift, a feature consistent with the observed dependence of enantioselectivity on the structure of the aryl iodide. It also suggests that a Ni(0)-Ni(II) redox cycle may operate although it remains to be determined whether this species dissociates iodide prior to "ate" complex binding or whether five-coordinate complexes may be reactive species.
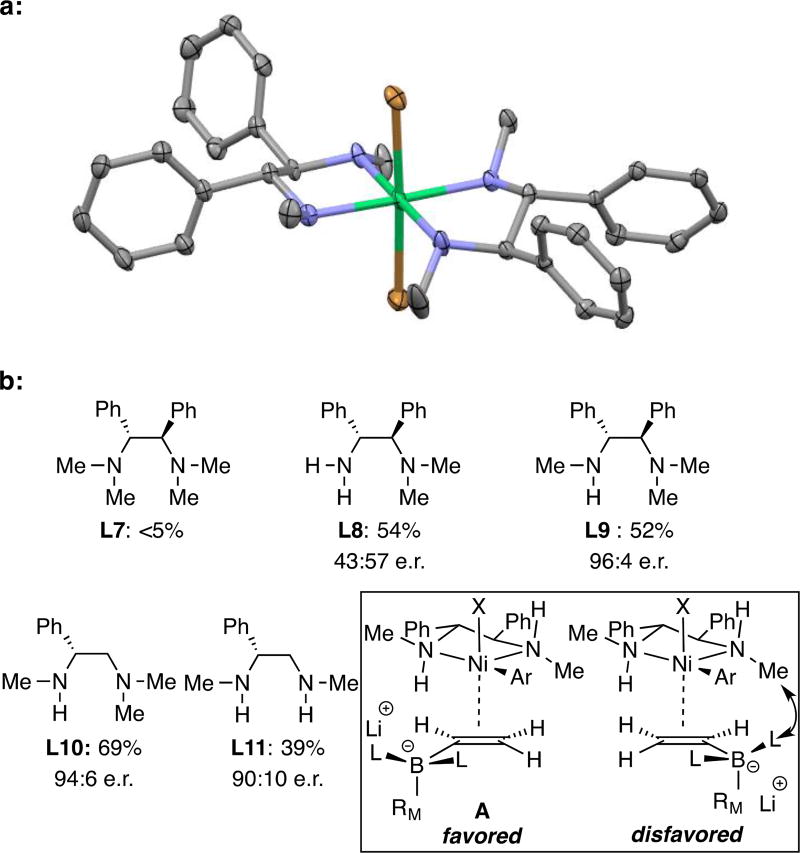
To aid in the understanding of the stereochemistry of conjunctive coupling, we prepared a coordination complex from NiBr2 and ligand L6. The crystal structure[21] is depicted in Figure 3 where it can be seen that the Ni chelate adopts a non-planar conformation with the phenyl groups and N-methyl substituents in equatorial positions. Along with this structure, we also analysed the stereoselectivity obtained with ligand variants L7–L11 (Figure 3). On the basis of this data, and noting the correlation between ligand and product configuration, we propose a model (A, Figure 3) where alkene binding occurs with the large borate group directed outside the cyclic framework of the catalyst and with the more substituted alkene carbon positioned away from the pseudoequatorial N-methyl group of the ligand. With permethylated ligand L7, binding of the alkene to the Ni complex is blocked, whereas with primary amino ligand (L8), approach of the olefin to Ni can occur from above or below the square plane leading to non-selective reaction. The stereoselectivity observed with ligands L9 and 10 is consistent with this model as well. Notably, the high level of selectivity observed with L11, a compound identical to L6 but with only one backbone substituent, reveals a likely function of the phenyl group: rather than gear the orientation of the adjacent N-methyl group, it more likely establishes the ring conformation such that both N-methyl groups in L11 occupy pseudoequatorial positions and a selective reaction results. This latter observation holds the practical implication that a broad array of readily available α-amino acid-derived ligands may prove effective in this reaction and thereby serve as a ligand platform to improve selectivity with problem substrates.
Figure 3.
a. X-ray structure of (L6)2NiBr2. b. Effect of ligand structure on conjunctive coupling reactions to furnish compound 1 (conditions of Table 2). Also depicted is a stereochemical model for the reaction (inset).
In conclusion, we have demonstrated the ability of Ni(II) catalysts to promote conjunctive cross-coupling of alkenyl borates and aryl halide electrophiles. Preliminary studies suggest that this process operates by a net Ni(0)/Ni(II) redox cycle similar to Pd-based catalysts. Further studies expanding the scope of this reaction are under way.
Supplementary Material
Acknowledgments
This work was supported by the NIH (GM-118641). We thank Dr. Bo Li (Boston College) for assistance with X-ray structures.
References
- 1.Review: Sandford C, Aggarwal VK. Chem. Commun. 2017;53:5481. doi: 10.1039/c7cc01254c.
- 2.Brown HC, Hubbard JL, Smith K. Synthesis. 1969:701. [Google Scholar]
- 3.(a) Brown HC, Rogic MM, Rathke MW, Kabalka GW. J. Am. Chem. Soc. 1968;90:818. [Google Scholar]; (b) Brown HC, Rogic MM. J. Am. Chem. Soc. 1969;91:2146. [Google Scholar]; (c) Brown HC, Nambu H, Rogic MM. J. Am. Chem. Soc. 1969;91:6854. [Google Scholar]
- 4.O'Donnell MJ, Cooper JT, Mader MM. J. Am. Chem. Soc. 2003;125:2370. doi: 10.1021/ja0298794. [DOI] [PubMed] [Google Scholar]
- 5.(a) Stymiest JL, Dutheuil G, Mahmood A, Aggarwal VK. Angew. Chem. Int. Ed. 2007;46:7491. doi: 10.1002/anie.200702146. [DOI] [PubMed] [Google Scholar]; (b) Stymiest JL, Bagutski V, French RM, Aggarwal VK. Nature. 2008;456:778. doi: 10.1038/nature07592. [DOI] [PubMed] [Google Scholar]; (c) Binanzer M, Fang GY, Aggarwal VK. Angew. Chem., Int. Ed. 2010;49:4264. doi: 10.1002/anie.201001223. [DOI] [PubMed] [Google Scholar]
- 6.(a) Zhang L, Lovinger GJ, Edelstein EK, Szymaniak AA, Chierchia MP, Morken JP. Science. 2016;351:70. doi: 10.1126/science.aad6080. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lovinger GJ, Aparece MD, Morken JP. J. Am. Chem. Soc. 2017;139:3153. doi: 10.1021/jacs.6b12663. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Edelstein EK, Namirembe S, Morken JP. J. Am. Chem. Soc. 2017;139:5027. doi: 10.1021/jacs.7b01774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For related processes: Fillion E, Carson RJ, Trépanier VE, Goll JM, Remorova AA. J. Am. Chem. Soc. 2004;126:15354. doi: 10.1021/ja045783t.Fillion E, Trépanier VE, Heikkinen JJ, Remorova AA, Carson RJ, Goll JM, Seed A. Organometallics. 2009;28:3518.Ishida N, Shimamoto Y, Murakami M. Org. Lett. 2009;11:5434. doi: 10.1021/ol902278q.Ishida N, Shinmoto T, Sawano S, Miura T, Murakami M. Bull. Chem. Soc. Jpn. 2010;83:1380.Ishida N, Narumi M, Murakami M. Org. Lett. 2008;10:1279. doi: 10.1021/ol8001305.Ishida N, Shimamoto Y, Murakami M. Org. Lett. 2010;12:3179. doi: 10.1021/ol1011136.Ishida N, Ikemoto W, Narumi M, Murakami M. Org. Lett. 2011;13:3008. doi: 10.1021/ol2008439.Kischkewitz M, Okamoto K, Mück-Lichtenfeld C, Studer A. Science. 2017;355:936. doi: 10.1126/science.aal3803.Silvi M, Sandford C, Aggarwal VK. J. Am. Chem. Soc. 2017;139:5736. doi: 10.1021/jacs.7b02569.Panda S, Ready JM. J. Am. Chem. Soc. 2017;139:6038. doi: 10.1021/jacs.7b01410.
- 8.For recent examples involving electrophile promoted metallate shifts of alkenyl boronate complexes, see: ref. 7j, Armstrong RJ, Sandford C, García-Ruiz C, Aggarwal VK. Chem. Commun. 2017;53:4922. doi: 10.1039/c7cc01671a.Armstrong RJ, García-Ruiz C, Myers EL, Aggarwal VK. Angew. Chem., Int. Ed. 2017;56:786. doi: 10.1002/anie.201610387.
- 9.(a) Boldrini GP, Savoia D, Tagliavini E, Trombini C, Ronchi AU. J. Organomet. Chem. 1986;301:C62. [Google Scholar]; (b) Lebedev SA, Lopatina VS, Petrov ES, Beletskaya IP. J. Organomet. Chem. 1988;344:253. [Google Scholar]; (c) Kelkar AA, Hanaoka T, Kubota Y, Sugi Y. Catal. Lett. 1994;29:69. [Google Scholar]; (d) Iyer S, Ramesh C, Ramani A. Tetrahedron Lett. 1997;38:8533. [Google Scholar]; (e) Bhanage BM, Zhao F, Shirai M, Arai M. Catal. Lett. 1998;54:195. [Google Scholar]; (f) InamotoJ K, Kuroda I, Hiroya K, Noda Y, Watanabe M, Sakamoto T. Organometallics. 2006;25:3095. [Google Scholar]; (g) Ma S, Wang H, Gao K, Zhao F. J. Mol. Catal. A. 2006;248:17. [Google Scholar]; (h) Wang ZX, Chai ZY. Eur. J. Inorg. Chem. 2007:4492. [Google Scholar]; (i) Lin PS, Jeganmohan M, Cheng CH. Chem. Asian J. 2007;2:1409. doi: 10.1002/asia.200700128. [DOI] [PubMed] [Google Scholar]; (j) Matsubara R, Gutierrez AC, Jamison TF. J. Am. Chem. Soc. 2011;133:19020. doi: 10.1021/ja209235d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Gøgsig TM, Kleimark J, Nilsson Lill SO, Korsager S, Lindhardt AT, Norrby PO, Skrydstrup T. J. Am. Chem. Soc. 2011;134:443. doi: 10.1021/ja2084509. [DOI] [PubMed] [Google Scholar]; (l) Ehle AR, Zhou Q, Watson MP. Org. Lett. 2012;14:1202. doi: 10.1021/ol203322v. [DOI] [PubMed] [Google Scholar]; (m) Standley EA, Jamison TF. J. Am. Chem. Soc. 2013;135:1585. doi: 10.1021/ja3116718. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Tasker SZ, Gutierrez AC, Jamison TF. Angew. Chem. Int. Ed. 2014;53:1858. doi: 10.1002/anie.201308391. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Desrosiers J-N, Hie L, Biswas S, Zatolochnaya OV, Rodriguez S, Lee H, Grinberg N, Haddad N, Yee NK, Garg NK, Senanayake CH. Angew. Chem. Int. Ed. 2016;55:11921. doi: 10.1002/anie.201606955. [DOI] [PubMed] [Google Scholar]
- 10.Reviews: Ittel SD, Johnson LK, Brookhart M. Chem. Rev. 2000;100:1169. doi: 10.1021/cr9804644.Gibson VC, Spitzmesser SK. Chem. Rev. 2003;103:283. doi: 10.1021/cr980461r.Mu HL, Pan P, Song DP, Li YS. Chem. Rev. 2015;115:12091. doi: 10.1021/cr500370f.; Seminal Report: Johnson LK, Killian CM, Brookhart M. J. Am. Chem. Soc. 1995;117:6414.
- 11.(a) Killian CM, Johnson LK, Brookhart M. Organometallics. 1997;16:2005. [Google Scholar]; (b) Svejda SA, Brookhart M. Organometallics. 1999;18:65. [Google Scholar]; Review: Speiser F, Braunstein P, Saussine L. Acct. Chem. Res. 2005;38:784. doi: 10.1021/ar050040d.
- 12.For selected enantioselective Ni-catalyzed cross-coupling with diamine ligands, see: Schmidt J, Choi J, Liu AT, Slusarczyk M, Fu GC. Science. 2016;354:1265. doi: 10.1126/science.aai8611.Wilsily A, Tramutola F, Owston NA, Fu GC. J. Am. Chem. Soc. 2012;134:5794. doi: 10.1021/ja301612y.Lu Z, Wilsily A, Fu GC. J. Am. Chem. Soc. 2011;133:8154. doi: 10.1021/ja203560q.Saito B, Fu GC. J. Am. Chem. Soc. 2007;129:9602. doi: 10.1021/ja074008l.
- 13.On small scale (<200 mg), pure vinyllithium in THF and halide-containing vinyllithium prepared from lithium-iodine exchange proved equally efficacious. On larger scale, use of halide free vinyl lithium (prepared by Li/Sn exchange from tetravinyl tin) provided superior results.
- 14.Selectivity in migration with mixed boranes is often governed by a subtle balance of steric and electronic effects. For a discussion, see: Aggarwal VK, Fang GY, Ginesta X, Howells DM, Zaja M. Pure Applied Chem. 2006;78:215.Bottoni A, Lombardo M, Neri A, Trombini C. J. Org. Chem. 2003;68:3397. doi: 10.1021/jo026733e.
- 15.Zweifel G, Brown HC. Org. React. 1963;13:1. [Google Scholar]
- 16.Soderquist JA, Najafi MR. J. Org. Chem. 1986;51:1330. [Google Scholar]
- 17.This is a modification of the following reference and its details will be reported separately: Mlynarski SN, Karns AS, Morken JP. J. Am. Chem. Soc. 2012;134:16449. doi: 10.1021/ja305448w.
- 18.(a) Zweifel G, Arzoumanian H, Whitney CC. J. Amer. Chem. Soc. 1967;89:3652. [Google Scholar]; (b) Evans DA, Thomas RC, Walker JA. Tetrahedron Lett. 1976;18:1427. [Google Scholar]
- 19.For an overview of cross-coupling with Ni catalysts, see: Hu X. Chem. Soc. Rev. 2011;2:1867.Tasker SZ, Standley EA, Jamison TF. Nature. 2014;509:299. doi: 10.1038/nature13274.
- 20.Compound 34 has been reported with limited characterization data (mp, CH analysis): Uchino M, Asagi K, Yamamoto A, Ikeda S. J. Organomet. Chem. 1975;84:93.
- 21.X-ray analysis has been deposited. CCDC-#will be assigned upon acceptance.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.