Summary
Background
The standard for selecting unrelated umbilical cord blood units for transplantation for nonmalignant diseases rely on antigen-level (lower resolution) human leukocyte antigen (HLA) typing for HLA-A and –B and allele-level for HLA-DRB1. Our aim was to study the effects of allele-level matching at HLA-A, -B, -C and –DRB1, the standard for adult unrelated volunteer donor transplantation for nonmalignant diseases for umbilical cord blood transplantation.
Methods
We retrospectively studied 1199 pediatric donor-recipient pairs with allele-level HLA matching who received a single unit umbilical cord blood transplant for nonmalignant diseases reported to the Center for International Blood and Marrow Transplant Research or Eurocord/European Group for Blood and Marrow Transplant. Transplantations occurred between January 1, 2000 and December 31, 2012. The primary outcome was overall survival. The effect of HLA matching on survival was studied using a Cox regression model.
Findings
Compared to HLA-matched transplants, mortality was higher with transplants mismatched at two (hazard ratio [HR] 1·55, 95% CI 1·08 – 2·21, p=0·018), three (HR 2·04, 95% CI 1·44 – 2·89, p=0·0001) and ≥four alleles (HR 3·15, 95% CI 2·16 – 4·58, p<0·0001). There were no significant differences in mortality between transplants that were matched and mismatched at one allele (HR 1·18, 95% CI 0·80 – 1·72, p=0·388). Other factors associated with higher mortality included recipient cytomegalovirus seropositivity (HR 1·40, 95% CI 1·13 – 1·74, p=0·002), reduced intensity compared to myeloablative conditioning regimens (HR 1·36, 95% CI 1·10 – 1·68, p=0·004), transplantation of units with total nucleated cell dose >21 × 107/kg compared to ≤>21 × 107/kg (HR 1·47, 95% CI 1·11 – 1·95, p=0·008) and transplants performed in 2000 – 2005 compared to 2006 – 2012 (HR 1·64, 95% CI 1·31 – 2·04, p<0·0001). The 5-year overall survival adjusted for recipient cytomegalovirus serostatus, conditioning regimen intensity, total nucleated cell dose and transplant period was 79% (95% CI 74 – 85) after HLA matched, 76% (95% CI 71 – 81) after 1 allele mismatched, 70% (95% CI 65 – 75) after 2 allele mismatched, 62% (95% CI 57 – 68) after 3 allele mismatched and 49% (95% CI 41 – 57) after ≥4 allele mismatched transplants. Graft failure was the predominant cause of mortality.
Conclusion
These data support a change from current practice in that selection of unrelated umbilical cord blood units for transplantation for nonmalignant diseases must consider allele-level HLA matching at HLA-A, -B, -C and –DRB1.
Funding
National Cancer Institute, National Heart, Lung, and Blood Institute, National Institute for Allergy and Infectious Diseases, US Department of Health and Human Services - Health Resources and Services Administration and US Department of Navy
Introduction
In the absence of a suitable human leukocyte antigen (HLA) matched sibling, transplantation from a suitably HLA matched adult donor or banked umbilical cord blood is potentially lifesaving treatment for children with non-malignant diseases such as severe combined immunodeficiency (SCID), non-SCID primary immunodeficiency, inborn errors of metabolism, severe aplastic anemia, Fanconi anemia, other inherited bone marrow failure diseases and hemoglobinopathy.1–6 Current standards for HLA matching for transplantation of bone marrow and peripheral blood from unrelated adult volunteer donors for non-malignant diseases support matching donors to recipients at HLA-A, -B, -C and –DRB1 at the allele-level.7 Survival was highest after HLA matched transplants at 65%, and lower after transplants mismatched at one allele (57%; p=0·07) and two alleles (46%; p=0·001).7 Similarly, graft failure was also higher at 28% after one allele (p<0·0001) and 24% after two alleles (p=0·008) mismatched transplants compared to 11% after matched transplants.7
An important difference when selecting unrelated adult donors and umbilical cord blood units for transplantation is the criteria for HLA matching. Conventionally umbilical cord blood unit selection relies on low resolution HLA typing (antigen-level) for HLA-A and –B and allele-level for DRB1 and matching at the HLA–C locus is not considered.8 Recent reports on HLA matching for hematologic malignancy in umbilical cord blood transplantation support selecting units on high resolution HLA typing (allele-level) for HLA-A, -B, -C and –DRB1.9,10 Those studies reported higher non-relapse mortality after transplantations mismatched at one or more alleles and higher graft failure after transplantations mismatched at three or more alleles.9,10
It cannot be assumed that HLA matching criteria for umbilical cord blood transplants derived from reports on transplants for hematologic malignancy are appropriate for non-malignant diseases. Therefore, the current analysis sought to establish the importance of allele-level HLA matching at HLA-A, -B, -C and –DRB1 when selecting umbilical cord blood units for transplantation for non-malignant diseases.
Methods
Participants
The study population includes children (≤16 years at transplantation) with SCID, non-SCID primary immunodeficiency, inborn errors of metabolism, aplastic anemia/inherited bone marrow failure, Fanconi anemia and hemoglobinopathy who received a first single unrelated unmanipulated umbilical cord blood transplant between January 1, 2000 through December 31, 2012. Transplants were reported to Eurocord / European Society for Blood and Marrow Transplant (EBMT) or the Center for International Blood and Marrow Transplant Research (CIBMTR). One thousand two hundred and sixty-one eligible patients were screened and 62 were excluded (HLA mismatch at more than 2 HLA loci considering lower resolution match at HLA-A, -B and allele-level at DRB1 (n=29), HLA not reported (n=4), graft versus host disease prophylaxis not reported (n=24) and follow up less than 3 months (n=5)). The Institutional Review Boards of the National Marrow Donor Program and Eurocord approved this study.
Procedure
Donor and recipient HLA typing was completed using molecular techniques with a minimum of antigen split-level resolution for HLA-A, -B and –C and allele-level resolution for DRB1. For transplantations in North and South America (n=831), recipient HLA typings were provided by the transplant center and umbilical cord blood typings were from a centralized confirmatory typing laboratory or through retrospective typing of stored research samples.11 For transplantations reported to Eurocord (n=368) donor-recipient HLA typings were obtained from cord blood banks or transplant centers. A subset of the available typing included less than allele-level typing at HLA-A (323 of 1199; 27%), -B (275 of 23%) and/or –C (371 of 1199; 31%) loci. A validated HLA high-resolution imputation algorithm, Haplogic III developed by the National Marrow Donor Program was used to impute allele-level match status at HLA-A, -B, and -C for these donor-recipient pairs.10,11
The primary endpoint was overall survival. Death from any cause was considered an event and surviving patients were censored at last follow up. Transplant-toxicity mortality considered death from any cause other than from graft failure as an event (graft failure was the competing risk). Primary and secondary graft failure were considered together as a single outcome. Primary graft failure was defined as failure to achieve an absolute neutrophil count of ≥ 0·5 × 109/L for 3 consecutive days or donor chimerism less than 5% (unsorted blood or marrow or peripheral blood T cell).7 Secondary graft failure was defined as initial donor engraftment followed by graft loss, evidenced by a persistent decline in the absolute neutrophil count (<0·5 × 109/L) or loss of donor chimerism to less than 5%.7 Lineage-specific chimerism was not available for all patients. Grades II–IV acute and chronic graft versus host disease (GVHD) were scored based on reports from each transplant center using standard criteria.12,13
Statistical Analysis
Patient and transplantation characteristics by donor-recipient HLA matching were compared using the Chi-square test for categorical variables and Wilcoxon test for continuous variables (Table 1). Median values and ranges are reported for continuous variables and percentages for categorical variables. The incidences of transplant-toxicity mortality, acute and chronic GVHD were calculated using the cumulative incidence estimator to accommodate competing risks.14 95% confidence intervals (CI) were calculated with log transformation.
Table 1.
Patient, disease and transplant characteristics
| HLA match | ||||||
|---|---|---|---|---|---|---|
| Matched | 1 allele mismatch (7/8) |
2 allele mismatch (6/8) |
3 allele mismatch (5/8) |
≥4 allele mismatch (3/8 or 4/8) |
p-value | |
| Number | 234 | 266 | 303 | 261 | 135 | |
| Age, years | <0·001 | |||||
| <1 | 96 (41%) | 108 (41%) | 111 (37%) | 76 (29%) | 40 (30%) | |
| 1 – 5 | 100 (43%) | 117 (44%) | 131 (43%) | 112 (43%) | 57 (42%) | |
| 6 – 10 | 36 (15%) | 30 (11%) | 49 (16%) | 57 (22%) | 25 (19%) | |
| 11 – 16 | 2 (<1%) | 11 (4%) | 12 (4%) | 16 (6%) | 13 (10%) | |
| Sex | 0·548 | |||||
| Male | 143 (61%) | 154 (58%) | 188 (62%) | 167 (64%) | 77 (57%) | |
| Female | 91 (39%) | 112 (42%) | 115 (38%) | 94 (36%) | 58 (43%) | |
| CMV serostatus | 0·040 | |||||
| Negative | 124 (53%) | 150 (56%) | 162 (53%) | 111 (43%) | 71 (53%) | |
| Positive | 92 (39%) | 97 (36%) | 126 (42%) | 132 (51%) | 59 (44%) | |
| Not reported | 18 (8%) | 19 (7%) | 15 (5%) | 18 (7%) | 5 (4%) | |
| Disease | <0·001 | |||||
| SCID | 39 (17%) | 30 (11%) | 27 (9%) | 22 (8%) | 13 (10%) | |
| Non-SCID PIDD | 57 (24%) | 71 (27%) | 73 (24%) | 66 (25%) | 22 (16%) | |
| IEM | 92 (39%) | 110 (41%) | 136 (45%) | 94 (36%) | 52 (39%) | |
| Marrow failure | 17 (7%) | 20 (8%) | 29 (10%) | 30 (11%) | 18 (13%) | |
| Fanconi anemia | 8 (3%) | 16 (6%) | 26 (9%) | 28 (11%) | 22 (16%) | |
| Hemoglobinopathy | 21 (9%) | 19 (7%) | 12 (4%) | 21 (8%) | 8 (6%) | |
| Conditioning regimen | 0·889 | |||||
| Myeloablative | 153 (65%) | 169 (64%) | 202 (67%) | 164 (63%) | 88 (65%) | |
| Bu + Cy ± other | 131 | 140 | 163 | 140 | 74 | |
| Bu + flud ± other | 26 | 26 | 36 | 18 | 7 | |
| Mel + flud ± other | 3 | 8 | 9 | 3 | 5 | |
| TBI + Cy ± other | 3 | 2 | 3 | 5 | 3 | |
| Reduced intensity | 81 (35%) | 97 (36%) | 101 (33%) | 97 (37%) | 47 (35%) | |
| Bu + Cy ± other | 1 | 4 | 7 | 3 | 3 | |
| Bu + flud ± other | 6 | 5 | 2 | 4 | 1 | |
| Mel + flud ± other | 18 | 27 | 30 | 21 | 4 | |
| Cy + flud | 7 | 11 | 12 | 27 | 4 | |
| Cy ± other | 5 | 8 | 11 | 4 | – | |
| Flud + other | 10 | 10 | 11 | 11 | 7 | |
| TBI + Cy + flud | 5 | 13 | 13 | 18 | 18 | |
| TBI + other | 7 | 5 | 3 | 6 | 5 | |
| None | 11 | 6 | 3 | 2 | 3 | |
| In vivo T cell depletion | 0·013 | |||||
| None | 37 (16%) | 38 (14%) | 36 (12%) | 33 (13%) | 7 (5%) | |
| Yes | 181 (77%) | 215 (81%) | 255 (84%) | 218 (84%) | 127 (94%) | |
| Not reported | 16 (7%) | 13 (5%) | 12 (4%) | 10 (4%) | 1 (1%) | |
| GVHD prophylaxis | 0·623 | |||||
| Cyclosporine ± steroid | 106 (45%) | 120 (45%) | 140 (46%) | 128 (49%) | 72 (53%) | |
| Cyclosporine + MTX | 19 (8%) | 17 (6%) | 25 (8%) | 29 (11%) | 8 (6%) | |
| Cyclosporine + MMF | 83 (35%) | 90 (34%) | 100 (33%) | 70 (27%) | 33 (24%) | |
| Tacrolimus | 5 (2%) | 7 (3%) | 7 (2%) | 9 (3%) | 9 (7%) | |
| Tacrolimus + MTX | 10 (4%) | 15 (6%) | 10 (3%) | 11 (4%) | 5 (4%) | |
| Tacrolimus + MMF | 11 (5%) | 17 (6%) | 21 (7%) | 14 (5%) | 8 (6%) | |
| Pre cryopreserved TNC | 0·213 | |||||
| Median (range) | 10 (2 – 58) |
10 (2 – 61) |
11 (2 – 77) |
12 (2 – 74) |
11 (1 – 66) |
|
| < 5 × 107/kg | 23 (10%) | 21 (8%) | 19 (6%) | 22 (8%) | 15 (11%) | |
| 5 – 10 × 107/kg | 76 (32%) | 68 (26%) | 110 (36%) | 100 (38%) | 53 (39%) | |
| 11 – 15 × 107/kg | 36 (15%) | 48 (18%) | 44 (15%) | 40 (15%) | 21 (16%) | |
| 16 – 20 × 107/kg | 25 (11%) | 25 (9%) | 20 (7%) | 22 (8%) | 14 (10%) | |
| ≥ 21 × 107/kg | 27 (12%) | 41 (15%) | 36 (12%) | 33 (13%) | 16 (12%) | |
| Not reported | 47 (20%) | 63 (24%) | 74 (24%) | 44 (17%) | 16 (12%) | |
| Transplant period | 0·005 | |||||
| 2000 – 2005 | 62 (26%) | 65 (24%) | 87 (29%) | 100 (38%) | 45 (33%) | |
| 2006 – 2012 | 172 (74%) | 201 (76%) | 216 (71%) | 161 (62%) | 90 (67%) | |
| Follow up, median (inter quartile range), months | 60 (33–81) |
61 (36–84) |
59 (36–78) |
70 (48–105) |
65 (48–95) |
|
Number in parenthesis = column percent
Abbreviations:
Diseases: SCID = severe combined immunodeficiency; non-SCID PIDD = non- severe combined immunodeficiency primary immunodeficiency diseases; IEM: inborn errors of metabolism Conditioning regimen: Bu = busulfan; Cy = cyclophosphamide; flud = fludarabine; mel = melphalan; TBI = total body irradiation
GVHD = graft versus host disease; MTX = methotrexate; MMF = mycophenolate
To analyze the association between clinical outcomes and donor-recipient HLA-matching, Fine and Gray models were built for graft failure, acute and chronic graft-versus-host disease to accommodate competing risks and a Cox regression model was built for overall mortality.15,16 Results are reported as hazard ratio (HR). Donor-recipient HLA match was examined as matched vs. mismatched at one allele vs. two alleles vs. three alleles vs. ≥four alleles. Other variables tested included age (<1 vs. 1 – 5 vs. 6 – 10 vs. 10–16 years), recipient sex (female vs. male), recipient cytomegalovirus (CMV) serostatus (positive vs. negative), disease type (SCID/ non-SCID primary immunodeficiency vs. inborn errors of metabolism vs. marrow failure/hemoglobinopathy), transplant conditioning regimen intensity (myeloablative vs. reduced intensity), GVHD prophylaxis (cyclosporine-containing vs. tacrolimus-containing), in vivo T-cell depletion (yes vs. no), pre-cryopreserved total nucleated cell dose (>21 vs. ≤21 × 107/kg) and transplant period (2000 – 2005 vs. 2006 – 2012). The optimal cut-point for total nucleated cell dose (21 × 107/kg) was determined based on the maximum likelihood from the Cox regression model for overall survival. Models were built using a forward stepwise selection procedure and confirmed with the use of a backward selection procedure. The proportional hazards assumption was tested for each variable individually; stratification was used to adjust for variables that violated this assumption. Interactions between each variable and donor-recipient HLA match were tested to ensure the effect of HLA matching was independent of other variables held in the final multivariate model. Adjusted incidence of graft failure and probability of overall survival were generated from the final Fine and Gray and Cox models, respectively.17,18 All p-values are two-sided and p-values ≤ 0·05 were considered statistically significant. Analyses were performed using SAS 9·4 (SAS Institute, Cary, NC).
Role of the funding source
The sponsors of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
Table 1 shows the characteristics of the study population by donor-recipient HLA match (n=1199). Only 20% (234 of 1199) of donor-recipient pairs were HLA-matched at the allele-level for A, B, C and DRB1. Most donor-recipient pairs were mismatched at two or three alleles accounting for 47% (564 of 1199) of transplantations; 22% (266 of 1199) of donor-recipient pairs were mismatched at one allele and 11% (135 of 1199) at four or five alleles. The median age at transplantation was 2 years for transplants mismatched at two, three and ≥four alleles and 1 year for transplants mismatched at one allele or HLA-matched. Only 54 of 1199 patients (4·5%) were aged 11 – 16 years. Thirty-five percent (420 of 1199) of transplants were for SCID / non-SCID primary immunodeficiency, 40% (484 of 1199) for inborn errors of metabolism, 18% (214 of 1199) for aplastic anemia / inherited marrow failure including Fanconi anemia and 7% (81 of 1199) for hemoglobinopathy. Myeloablative transplant conditioning was more common than reduced intensity conditioning regimens. Busulfan with cyclophosphamide was the predominant myeloablative regimen and cyclophosphamide or melphalan with fludarabine were the predominant reduced intensity regimens. In vivo T cell depletion was common and the predominant GVHD prophylaxis regimen contained cyclosporine. Increasing numbers of transplants were performed between 2006 and 2012 compared to the earlier period and these were more likely to be HLA-matched or mismatched at one or two alleles. Table 2 shows the distribution of allele-level HLA-matching compared with lower resolution HLA-matching. Although 63% (234 of 372) of transplants matched at low resolution HLA-A, -B and allele-level at DRB1 (the usual umbilical cord blood matching criteria) were also matched at the allele-level for HLA-A, -B, -C and –DRB1, only 29% (170 of 584) of transplants mismatched at one HLA-locus by low resolution were mismatched at only one of eight alleles with the majority mismatched at two and three alleles.
Table 2.
Allele-level HLA match (HLA-A, -B, -C, -DRB1) compared with low resolution HLA match (HLA-A, -B at the antigen-level and -DRB1 at allele-level)
| Low resolution HLA match | Allele-level HLA match | ||||
|---|---|---|---|---|---|
| Matched (N=234) |
1 allele mismatch (N=266) |
2 allele mismatch (N=303) |
3 allele mismatch (N=261) |
≥4 allele mismatch (N=135) |
|
| 2 HLA loci mismatch (N=243) |
– | – | 29 (12%) |
128 (53%) |
72 (30%) |
| 1 HLA locus mismatch (N=584) |
– | 170 (29%) |
244 (42%) |
123 (21%) |
47 (8%) |
| HLA-matched (N=372) |
234 (63%) |
96 (26%) |
30 (8%) |
10 (3%) |
2 (<1%) |
Number in parenthesis = row percent
N = number
HLA mismatching was negatively associated with overall survival. In multivariate analysis, the risk of overall mortality was independently associated with the number of HLA mismatches (Table 3). Compared to HLA-matched transplants, mortality risks were significantly higher with transplants mismatched at two, three and ≥four alleles. Mortality risks were not different after HLA-matched and one allele mismatched transplants. Compared to two allele mismatched transplants, mortality risks were higher after three (HR 1·32, 95% CI 0·99 – 1·76, p=0·054) and ≥four allele (HR 2·02, 95% CI 1·47 – 2·77, p<0·0001) mismatched transplants. Risks were also higher after ≥four allele compared to three allele mismatched transplants (HR 1·52, 95% CI 1·12 – 2·07, p=0·008). Other factors associated with higher mortality included recipient CMV seropositivity (HR 1·40, 95% CI 1·13 – 1·74, p=0·002), reduced intensity conditioning compared to myeloablative conditioning regimens (HR 1·36, 95% CI 1·10 – 1·68, p=0·004), transplantation of units with total nucleated cell dose >21 × 107/kg compared to ≤21 × 107/kg (HR 1·47, 95% CI 1·11 – 1·95, p=0·008) and transplants performed in 2000–2005 compared to 2006–2012 (HR 1·64, 95% CI 1·31 – 2·04, p<0·0001). The 5-year adjusted overall survival is shown in Figure 1, p<0.0001.
Table 3.
Adjusted clinical outcomes by degree of HLA-A, -B, -C and –DRB1 mismatch
| Number Events/Evaluable |
Hazard ratio (95% confidence interval) |
P-value | |
|---|---|---|---|
| Mortality* | |||
| HLA matched | 44/234 | 1·00 | |
| 1 allele mismatch | 63/266 | 1·18 (0·80 – 1·72) | 0·388 |
| 2 allele mismatch | 88/303 | 1·55 (1·08 – 2·21) | 0·018 |
| 3 allele mismatch | 105/261 | 2·04 (1·44 – 2·89) | 0·0001 |
| ≥4 allele mismatch | 69/135 | 3·15 (2·16 – 4·58) | <0·0001 |
| Graft failure† | |||
| HLA matched | 39/233 | 1·00 | |
| 1 allele mismatch | 59/264 | 1·34 (0·90 – 1·98) | 0·149 |
| 2 allele mismatch | 72/302 | 1·50 (1·02 – 2·20) | 0·037 |
| 3 allele mismatch | 78/260 | 1·86 (1·27 – 2·73) | 0·001 |
| ≥4 allele mismatch | 35/135 | 1·62 (1·02 – 2·57) | 0·041 |
| Grade II-IV acute GVHD¶ | |||
| HLA matched | 52/228 | 1·00 | |
| 1 allele mismatch | 68/249 | 1·24 (0·87 – 1·78) | 0·235 |
| 2 allele mismatch | 85/291 | 1·36 (0·97 – 1·92) | 0·077 |
| 3 allele mismatch | 81/249 | 1·55 (1·09 – 2·20) | 0·014 |
| ≥4 allele mismatch | 47/130 | 1·72 (1·17 – 2·53) | 0·006 |
| Chronic GVHD§ | |||
| HLA matched | 51/228 | 1·00 | |
| 1 allele mismatch | 52/252 | 0·92 (0·63 – 1·34) | 0·659 |
| 2 allele mismatch | 68/296 | 1·06 (0·74 – 1·52) | 0·757 |
| 3 allele mismatch | 57/253 | 1·00 (0·69 – 1·47) | 0·982 |
| ≥4 allele mismatch | 33/133 | 1·15 (0·74 – 1·77) | 0·539 |
Abbreviations: GVHD = graft versus host disease
Mortality risks for degree of HLA mismatch were adjusted for recipient cytomegalovirus serostatus, transplant conditioning regimen intensity, total nucleated cell dose of the cord blood unit and transplant period
Graft failure risks for degree of HLA mismatch were adjusted for transplant conditioning regimen intensity
Model adjusted for transplant period and stratified by transplant conditioning regimen intensity and GVHD prophylaxis
Chronic GVHD risks were adjusted for GVHD prophylaxis
Figure 1.
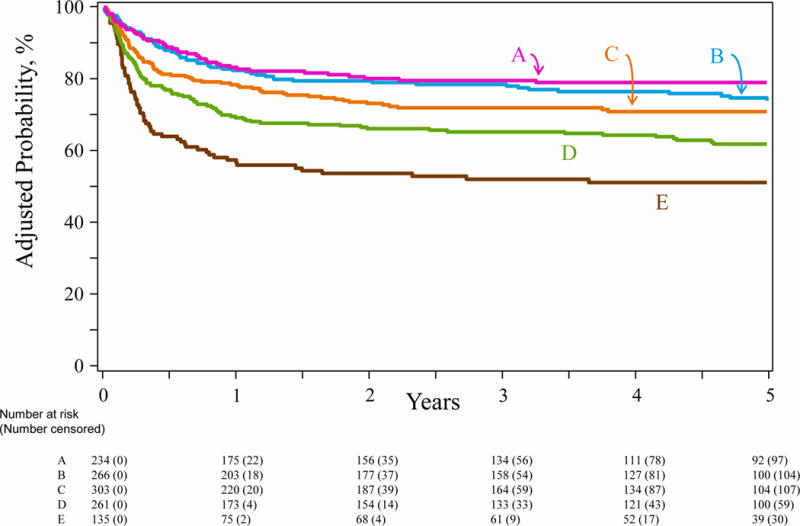
Overall survival by degree of HLA-A, -B, -C, -DRB1 mismatch: The 5-year overall survival adjusted for recipient cytomegalovirus serostatus, conditioning regimen intensity, total nucleated cell dose and transplant period was 79% (95% CI 74 – 85) after HLA matched (A), 76% (95% CI 71 – 81) after 1 allele mismatched (B), 70% (95% CI 65 – 75) after 2 allele mismatched, (C) 62% (95% CI 57 – 68) after 3 allele mismatched (D) and 49% (95% CI 41 – 57) after ≥4 allele mismatched (E) transplants
Age at transplantation and disease type were not associated with survival. Compared to patients aged <1 year, mortality risks for those aged 1 – 5 years were (HR 1·07, 95% CI 0·82 – 1·41, p=0·606), 6 – 10 years (HR 0·85, 95% CI 0·59 – 1·24, p=0·411) and 11 – 16 years (HR 1·55, 95% CI 0·82 – 1·41, p=0·606). Similarly, compared to patients with SCID/non-SCID immunodeficiency, mortality risks for those with inborn errors of metabolism were (HR 1·27, 95% CI 0·97 – 1·65, p=0·079) and marrow failure/hemoglobinopathy (HR 1·22, 95% CI 0·88 – 1·70, p=0·230). Nevertheless, the effect of HLA mismatch on mortality was examined with age and disease type forced into the final model (Supplemental Table 1, page 1) and the effect of HLA mismatch on survival was consistent with that reported without adjustment for these factors.
Graft failure was independently associated with the number of HLA mismatches (Table 3). Compared to HLA-matched transplants, graft failure was significantly higher with transplants mismatched at two, three and ≥four alleles. Graft failure risks after HLA-matched and one allele mismatched transplants were not different. There were no differences in graft failure risks between transplants mismatched at three and ≥four alleles compared to two-allele mismatched transplants (Supplemental Table 2, page 2). Among patients with graft failure, primary graft failure was more common with transplants mismatched at two (65 of 73; 78%), three (60 of 79; 76%), ≥four (34 of 61; 83%) alleles compared to one allele mismatched (23 of 40; 56%) and matched transplants (p=0·006). The only other factor associated with higher graft failure was reduced intensity conditioning compared to myeloablative conditioning regimens (HR 1·96, 95% CI 1·55 – 2·48, p<0·0001). The adjusted 2-year incidence of graft failure is shown in Figure 2, p=0.025.
Figure 2.
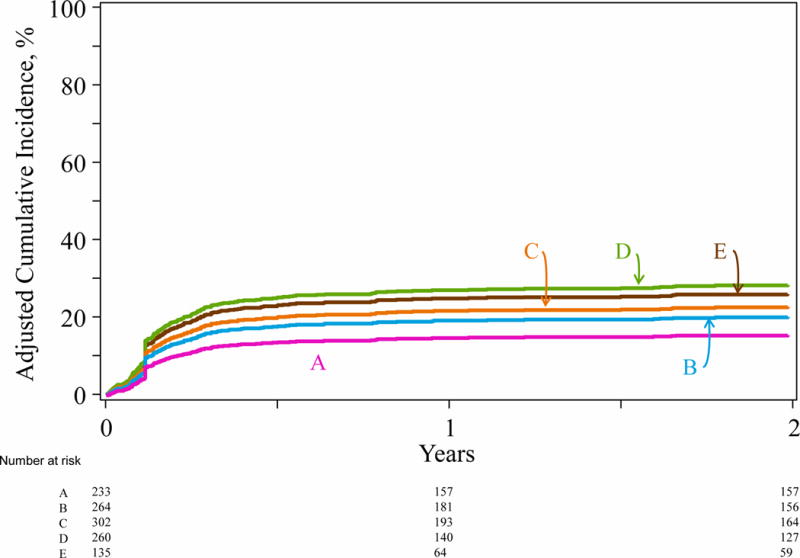
Graft failure by degree of HLA-A, -B, -C, -DRB1 mismatch: The 2-year incidence of graft failure adjusted for transplant conditioning regimen intensity was 16% (95% CI 12 – 21) after HLA matched transplants (A), 21% (95% CI 17 – 26) after 1 allele mismatched (B), 23% (95% CI 19 – 28) after 2 allele mismatched (C), 28% (95% CI 23 – 33) after 3 allele mismatched (D) and 25% (95% CI 18 – 32) after ≥4 allele mismatched (E) transplants
Grade II-IV acute GVHD was independently associated with HLA mismatch. Compared to HLA-matched transplants, grade II–IV acute GVHD was higher with transplants mismatched at three and ≥four alleles (Table 3). The day-100 incidence of grade II–IV acute GVHD was 22% (95% CI 17 – 28) after HLA matched transplants and 27% (95% CI 22 – 33), 29% (95% CI 24 – 35), 33% (95% CI 27 – 39) and 35% (95% CI 27 – 44) after one, two, three and ≥four allele mismatched transplants, respectively. The corresponding incidence of grade III–IV acute GVHD were 7% (95% CI 4 – 11), 11% (95% CI 7 – 15), 11% (95% CI 7 – 14), 14% (95% CI 10 – 19) and 18% (12 – 25). Compared to HLA-matched transplants, grade III–IV acute GVHD was higher with transplants mismatched at three (p=0·010) and ≥four alleles (p=0·005). Risks for grade II – IV acute GVHD were higher for transplants between 2000 and 2005 compared to the later period (HR 1·28, 95% CI 1·02 – 1·62, p=0·031). HLA mismatching was not associated with chronic GVHD (Table 3). The 5-year incidence of chronic GVHD was 24% (95% CI 19 – 30) after HLA matched transplants, 23% (95% CI 18 – 29), 26% (95% CI 21 – 32), 25% (95% CI 20 – 31) and 27% (95% CI 20 – 36), after one, two, three and ≥four allele mismatched transplants, respectively. The only factor associated with higher risk for chronic GVHD was tacrolimus-containing GVHD prophylaxis regimen (HR 1·54, 95% CI 1·12 – 2·11, p=0·007). The effect of HLA mismatch on acute and chronic GVHD was examined with all variables forced into the final model and findings consistent with that reported without these variables (Supplemental Tables 3, 4, pages 3,4)
The causes of death are shown in Table 4. Graft failure was the predominant cause of death in all HLA groups and there were no differences across the groups (p=0·824). Graft failure was also the predominant cause of death by disease type. The 2-year incidence of transplant-toxicity mortality after HLA-matched, one and two allele mismatched transplants were 12% (95% CI 8 – 17), 11% (95% CI 8 – 15) and 13% (95% CI 10 – 18), respectively. Corresponding incidences after three and ≥four allele mismatched transplants were higher at 21% (95% CI 17 – 27; p<0.001) and 30% (95% CI 22 – 38, p<0.001), respectively.
Table 4.
Causes of death by degree of HLA-A, -B, -C and –DRB1 mismatch
| HLA match | |||||
|---|---|---|---|---|---|
| Matched | 1 allele mismatch | 2 allele mismatch | 3 allele mismatch | ≥4 allele mismatch | |
| Number | 46 | 63 | 88 | 105 | 69 |
| Graft failure | 19 (41%) | 29 (46%) | 42 (48%) | 43 (41%) | 27 (39%) |
| Graft versus host disease | 1 (2%) | 4 (6%) | 3 (3%) | 11 (10%) | 7 (10%) |
| Interstitial pneumonitis | 5 (11%) | 3 (5%) | 6 (7%) | 8 (8%) | 4 (6%) |
| Infection | 8 (17%) | 4 (6%) | 7 (8%) | 12 (11%) | 10 (14%) |
| Organ failure | 7 (15%) | 10 (16%) | 14 (16%) | 14 (13%) | 11 (16%) |
| Haemorrhage | 1 (2%) | 3 (5%) | 5 (6%) | 6 (6%) | 2 (3%) |
| Progressive disease* | 2 (4%) | 2 (3%) | 5 (6%) | 3 (3%) | 1 (1%) |
| EBV-PTLD | – | – | 2 (2%) | 1 (1%) | 1 (1%) |
| Other | 2 (4%) | 4 (6%) | 1 (1%) | 2 (2%) | 2 (3%) |
| Not reported | 1 (2%) | 4 (6%) | 3 (3%) | 5 (5%) | 4 (6%) |
Number in parenthesis = column percent
Death from progression of primary disease despite donor chimerism ≥95%: Tay-Sachs, Sanfilipo, globoid cell leukodystrophy, metachromatic leukodystrophy and adrenoleukodystrophy
EBV-PTLD = Ebstein-Barr post transplant lymphoproliferative disease
Discussion
Our study showed allele-level matching at HLA-A, -B, -C and –DRB1 between the umbilical cord blood unit and its recipient is associated with best survival and lowest rate of graft failure in children with non-malignant diseases. Survival rates are substantially lower and graft failure higher for transplants mismatched at two or more alleles, though still impressive when considering the lack of alternative therapies available for many of these patients. A unit that is mismatched at one allele provides similar likelihood of survival and graft failure. Grade II-IV acute GVHD risk were higher with transplants mismatched at three and ≥four alleles. Although the incidence of transplant-toxicity mortality was higher with transplants mismatched at three and ≥four alleles, graft failure and not GVHD or another transplant-toxicity was the predominant cause of mortality in all HLA groups. Our findings are consistent with that recorded after adult unrelated volunteer donor transplantation for non-malignant diseases.7,19 The absence of a significant difference in survival after HLA-matched and one allele mismatched transplants should be interpreted cautiously as our power calculations indicated that with a larger sample size (n=2248), a significant association might be detected. Higher survival after allele-level matched transplants is also in keeping with a report on 106 patients with inherited metabolic diseases.20
With the exception of patients with severe T-cell immune deficiency diseases, the immune systems of patients with non-malignant diseases are more likely to reject a graft, regardless of donor source, than the immune systems of patients with hematologic malignancy who typically receive multiple courses of immunosuppressive chemotherapy prior to transplantation. Therefore, with 80% of transplants mismatched and only 10% of patients transplanted for severe T-cell immune deficiency diseases, the high incidence of graft failure in our study is not surprising and underscores the importance of HLA matching in donor selection algorithms. Use of reduced intensity conditioning regimens resulted in higher graft failure and mortality independent of HLA mismatch. Although 83% of the study population was exposed anti-thymocyte globulin, this was not associated with outcomes. Yet, others have recorded better immune reconstitution and survival with reduced exposure to anti-thymocyte globulin in a mixed population21 or substitution with fludarabine for hematologic malignancy.22 It is plausible some of the excess mortality may be mitigated by eliminating anti-thymocyte globulin or its dosing based on pharmacokinetics. We are limited by lack of these data and beyond the scope of the current study. Pharmacokinetic dosing of busulfan may also mitigate mortality23 but we recorded higher mortality with melphalan- and cyclophosphamide-containing regimens rather than with busulfan-containing regimens.
Prior reports on umbilical cord blood transplantation demonstrate the importance of total nucleated cells of the unit as a predictor for graft failure and survival.8,10 For marrow failure/ hemoglobinopathy the recommended pre-cryopreserved total nucleated cell dose is 4 – 5 × 107/kilogram recipient body weight.24–26 Further, in reports of HLA matched sibling cord blood transplants for non-malignant diseases the median total nucleated cell dose was approximately 4 × 107/kg and graft failure rates are low and survival is generally higher than 85%.27,28 We were unable to study the effect of low cell dose on graft failure and survival as fewer than 10% of transplants utilized units with total nucleated cell dose less than 5 × 107/kg and 2%, less than 3 × 107/kg. Notably, mortality risks were higher for transplants with units containing greater than 21 × 107/kg total nucleated cells. The median age at transplantation for this group was 6 months and almost all were transplanted for SCID, non-SCID primary immunodeficiency or inborn errors of metabolism. As these transplants accounted for only 13% (n=151) of the study population it is difficult to conclude whether the observed adverse effect on survival can be attributed to cell dose per se or another unknown or unmeasured factor associated with this group. GVHD rates did not differ by cell dose and graft failure was the predominant cause of death in this group. Acute GVHD risks were higher after transplants mismatched at three or more alleles but not chronic GVHD consistent with that recorded by others.10,19,29 Although GVHD was not the predominant cause of mortality after mismatched transplants its treatment results in delayed immune reconstitution and may decrease the chance of survival.
Our report has several limitations. First, our modest sample size prevented us from studying the effect of HLA mismatching at individual HLA loci as was undertaken for malignant diseases.10,29 Second, some patients may have acquired HLA antibodies that could be donor-directed.30 An effect of donor-directed antibodies on graft failure for non-malignant disease transplants is yet to be defined and could not be studied in the current analysis. Third, the effect of host-versus-graft mismatching on graft failure was not tested because of our modest sample size although an effect was not recorded after adult unrelated volunteer transplants for nonmalignant diseases.7 Finally, ours is a pediatric population. Most patients (80%) studied were aged 5 years or younger at transplantation with immune deficiency diseases or inborn errors of metabolism and the recorded results may be not be generalizable to adolescents and adults for whom additional mortality risks are mitigated by older age and other co-morbidities.
Data reported to the CIBMTR confirm that allele-level HLA typing was available for 90% of umbilical cord blood transplants for non-malignant diseases in 2015 and 2016 implying access to allele-level HLA typing is not a limitation. Yet, only 13% of transplants were HLA matched and 20% mismatched at one allele suggesting either difficulties in identifying suitably matched units or prioritization based on the highest total nucleated cell dose rather than HLA match. Our findings support a change in practice to prioritization of units on allele-level HLA matching at HLA-A, -B, -C and –DRB1. As most children with immune deficiency diseases and inborn errors of metabolism are transplanted aged ≤5 years, we recommend selecting the best available allele-level HLA-matched unit as cell dose is unlikely to be a barrier and to avoid units containing greater than 21 × 107/kg total nucleated cells. For marrow failure and hemoglobinopathy we recommend selecting units with total nucleated cell dose of 4 – 5 × 107/kg26–28 and, thereafter, prioritize based on the best available allele-level HLA matched unit.
Research in context
Evidence before the study
We searched work published after 2010 for articles on umbilical cord blood transplantation with Medline. Our search terms were “HLA match”, “unrelated donor transplantation”, “umbilical cord blood” and “nonmalignant disease”. We identified two reports describing HLA-matching at HLA-A, -B, -C, -DRB1 at the allele-level on outcomes of adult unrelated donor transplantation for nonmalignant diseases and one report on allele-level matching after cord blood transplantation for inherited metabolic disorders. There were two other reports on HLA matching and umbilical cord blood transplantation, one that reported outcomes when matching at the HLA-C locus was considered and the other, allele-level matching at HLA-A, -B, -C, -DRB1 for hematologic malignancy. Therefore, our aim was to test HLA matching at HLA-A, -B, -C, -DRB1 at the allele-level between umbilical cord blood units and patients with the hypothesis that better matching would lead to better survival and lower graft failure.
Added value of this study
In a population of 1199 donor-recipient pairs, the largest to-date, we recorded higher graft failure and lower survival after transplantation of umbilical cord blood units that were mismatched to the patient at two or more alleles compared to HLA-matched transplants.
Implications of all the available evidence
The data suggest that we reassess the present strategy for umbilical cord blood unit selection for nonmalignant diseases and support the need for even greater investment in public cord blood banks to expand the current inventory because of the importance of HLA matching on graft failure and survival.
Supplementary Material
Acknowledgments
The Center for International Blood and Marrow Transplant Research is supported by U24-CA76518 from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute for Allergy and Infectious Diseases, HHSH234200637015C from Health Resources and Services Administration/Department of Health and Human Services and the Office of Naval Research, US Department of Navy (N00014-15-1-0848 and N00014-16-1-2020).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
We declare we have no conflicts of interest.
Author contribution
ME, TW, PAV and ASM designed the study with input from all other authors. ASM assembled data from both registries with input from all other authors. ASM and TW analyzed the data which was interpreted by all other authors. ME wrote the first draft of the manuscript which was reviewed, modified and approved by all other authors. ME had unrestricted access to the data and final responsibility for modifying the manuscript and to submit for publication.
Contributor Information
Prof Mary Eapen, Medical College of Wisconsin, Milwaukee, WI, USA.
Prof Tao Wang, Medical College of Wisconsin, Milwaukee, WI, USA.
Prof Paul A. Veys, Hospital for Sick Children, Great Ormond Street, London, UK.
Prof Jaap J. Boelens, University Medical Center Utrecht, Utrecht, Netherlands.
Prof Andrew St Martin, Medical College of Wisconsin, Milwaukee, WI, USA.
Stephen Spellman, National Marrow Donor Program, Minneapolis, MN, USA.
Carmem Sales Bonfim, Hospital de Clinicas, Curitiba, Brazil.
Colleen Brady, National Marrow Donor Program, Minneapolis, MN, USA.
Prof Andrew Cant, Newcastle General Hospital, Newcastle-upon-Tyne, UK.
Prof Jean-Hugues Dalle, Hopital Robert Debre, Paris, France.
Prof Stella M. Davies, Cincinnati Children’s Hospital, Cincinnati, OH, USA.
John Freeman, National Marrow Donor Program, Minneapolis, MN, USA.
Prof Katherine C. Hsu, Memorial Sloane Kettering Cancer Center, New York, NY, USA.
Prof Katharina Fleischhauer, Essen University Hospital, Essen, Germany.
Chantal Kenzey, Hopital Saint Louis, Paris, France.
Prof Joanne Kurtzberg, Duke University, Durham, NC, USA.
Prof Gerard Michel, University Hospital of Marseille, Marseille, France.
Prof Paul J. Orchard, University of Minnesota, Minneapolis, MN, USA.
Annalisa Paviglianiti, Hopital Saint Louis, Paris, France.
Prof Vanderson Rocha, Churchill Hospital, Oxford, UK.
Prof Michael R. Veneris, University of Colorado, Denver, CO, USA.
Fernanda Volt, Hopital Saint Louis, Paris, France.
Prof Robert Wynn, Central Manchester University Hospitals, Manchester, UK.
Prof Stephanie J. Lee, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Prof Mary M. Horowitz, Medical College of Wisconsin, Milwaukee, WI, USA.
Prof Eliane Gluckman, Hopital Saint Louis, Paris, France.
Annalisa Ruggeri, Hopital Saint Antoine, Paris, France.
References
- 1.Pai SY, Logan BR, Griffith LM, et al. Transplantation of outcomes for severe combined immunodeficiency, 2000 – 20009. N Engl J Med. 2014;371:434–46. doi: 10.1056/NEJMoa1401177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orchard PJ, Fasth AL, Le Rademacher J, et al. Hematopoietic stem cell transplantation for infantile osteopetrosis. Blood. 2015;126:270–6. doi: 10.1182/blood-2015-01-625541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boelens JJ, Aldenhoven M, Purtill D, et al. Outcomes of transplantation using various hematopoietic cell sources in children with Hurler syndrome after myeloablative conditioning. Blood. 2013;121:3981–7. doi: 10.1182/blood-2012-09-455238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eapen M, Le Rademacher J, Antin JH, et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood. 2011;118:2618–21. doi: 10.1182/blood-2011-05-354001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacMillan ML, DeFor TE, Young JA, et al. Alternative donor hematopoietic cell transplantation for Fanconi anemia. Blood. 2015;125:3798–804. doi: 10.1182/blood-2015-02-626002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shenoy S, Eapen M, Panepinto JA, et al. A trial of unrelated donor marrow transplantation for children with severe sickle cell disease. Blood. 2016;128:2561–7. doi: 10.1182/blood-2016-05-715870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horan J, Wang T, Haagenson M, et al. Evaluation of HLA matching in unrelated hematopoietic stem cell transplantation for nonmalignant diseases. Blood. 2012;120:2918–24. doi: 10.1182/blood-2012-03-417758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocha V, Gluckman E. Improving outcomes of cord blood transplantation: HLA matching, cell dose and other graft- and transplantation-related factors. B J Haematol. 2009;147:262–74. doi: 10.1111/j.1365-2141.2009.07883.x. [DOI] [PubMed] [Google Scholar]
- 9.Eapen M, Klein JP, Sanz GF, et al. Effect of donor-recipient HLA matching at HLA A, B, C and DRB1 on outcomes after umbilical cord blood transplantation for leukemia and myelodysplastic syndrome. Lancet Oncol. 2011;12:1214–21. doi: 10.1016/S1470-2045(11)70260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eapen M, Klein JP, Ruggeri A, et al. Impact of allele-level HLA matching after myeloablative single unit umbilical cord blood transplantation for hematologic malignancy. Blood. 2014;123:133–40. doi: 10.1182/blood-2013-05-506253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spellman S, Setterholm M, Maiers M, et al. Advances in the selection of HLA-compatible donors: refinements in HLA typing and matching over the first 20 years of the National Marrow Donor Program Registry. Biol Blood Marrow Transplant. 2008;14(Suppl):37–44. doi: 10.1016/j.bbmt.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 13.Flowers ME, Kansu E, Sullivan KM. Pathophysiology and treatment of graft-versus-host disease. Hematol Oncol Clin North Am 1999. 13:1091–112. doi: 10.1016/s0889-8588(05)70111-8. [DOI] [PubMed] [Google Scholar]
- 14.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–10. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 15.Fine JR, Gray RJ. A proportional hazards model for the sub-distribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 16.Cox DR. Regression Models and Life-Tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 17.Zhang X, Zhang M-J. SAS macros for estimation of direct adjusted cumulative incidence curves under proportional subdistribution hazards models. Comput Methods Programs Biomed. 2011;101:87–93. doi: 10.1016/j.cmpb.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Loberiza FR, Klein JP, Zhang M-J. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88:95–101. doi: 10.1016/j.cmpb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Yagasaki H, Kojima S, Yabe H, et al. Acceptable HLA-mismatching in unrelated donor bone marrow transplantation for patients with acquired severe aplastic anemia. Blood. 2011;118:3186–90. doi: 10.1182/blood-2011-04-349316. [DOI] [PubMed] [Google Scholar]
- 20.Malhi KK, Smith AR, DeFor TE, Lund TC, Orchard PJ, Miller WP. Allele-level HLA matching impacts key outcomes following umbilical cord blood transplantation for inherited metabolic disorders. Biol Blood Marrow Transplant. 2017;23:119–25. doi: 10.1016/j.bbmt.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Admiraal R, Lindemans C, van Kersten C, et al. Excellent T cell reconstitution and survival depend on low ATG exposure after pediatric cord blood transplantation. Blood. 2016;128:2734–41. doi: 10.1182/blood-2016-06-721936. [DOI] [PubMed] [Google Scholar]
- 22.Wagner JE, Eapen M, Carter S, et al. One-unit versus two-unit cord-blood transplantation for hematologic cancers. N Engl J Med. 2014;371:1685–94. doi: 10.1056/NEJMoa1405584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartelink IH, Lalmohamed A, van Reij EM, et al. Association of buslfan exposure with survival and toxicity after haematopoietic cell transplantation in children and young adults: a multicenter, retrospective cohort analysis. Lancet Haematol. 2016;3:e526–e536. doi: 10.1016/S2352-3026(16)30114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gluckman E, Rocha V, Ionescu I, et al. Results of unrelated cord blood transplant in Fanconi anemia patients: risk factor analysis for engraftment and survival. Biol Blood Marrow Transplant. 2007;13:1073–82. doi: 10.1016/j.bbmt.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Peffault de Latour R, Purtill D, Ruggeri A, et al. Influence of nucleated cell dose on overall survival of unrelated cord blood transplantation for patients with severe acquired aplastic anemia: A study by Eurocord and the Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2011;17:78–85. doi: 10.1016/j.bbmt.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Fernandes JF, Rocha V, Labopin M, et al. Transplantation in patients with SCID: mismatched related stem cells or unrelated cord blood? Blood. 2012;119:2949–55. doi: 10.1182/blood-2011-06-363572. [DOI] [PubMed] [Google Scholar]
- 27.Boelens JJ, Aldenhoven M, Purtill D, et al. Outcomes of transplantation using various hematopoietic cell sources in children with Hurler syndrome after myeloablative conditioning. Blood. 2013;121:3981–7. doi: 10.1182/blood-2012-09-455238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Locatelli F, Kabbara N, Ruggeri A, et al. Outcome of patients with hemoglobinopathies given either cord blood or bone marrow transplantation from an HLA-identical sibling. Blood. 2013;122:1072–8. doi: 10.1182/blood-2013-03-489112. [DOI] [PubMed] [Google Scholar]
- 29.Pidala J, Lee SJ, Ahn KW, et al. Nonpermissive HLA-DPB1 mismatch increases mortality after myeloablative unrelated allogeneic hematopoietic cell transplantation. Blood. 2014;124:2596–606. doi: 10.1182/blood-2014-05-576041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takanashi M, Atsuta Y, Fujiwara K, et al. The impact of anti-HLA antibodies on unrelated cord blood transplantations. Blood. 2010;116:2839–46. doi: 10.1182/blood-2009-10-249219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


