Abstract
Autologous, patient-specific chimeric antigen receptor T cell (CART) therapy has emerged as a powerful and potentially curative therapy for cancer, especially for CD19-positive hematological malignancies. Indeed, CD19-directed CART (CART-19) cell therapy (tisagenlecleucel-t) was Food and Drug Administration (FDA) approved for acute lymphoblastic leukemia on August 30, 2017 and approval of CART-19 in B-cell lymphomas is expected in late 2017. The development of this technology and its wider application is partly limited by the patient-specificity nature of such a platform and by the time required for CART manufacturing. The efficacious generation of universal allogeneic CART cells would overcome these limitations and represent a major advance in the field. However, several obstacles in the generation of universal CART cells need to be overcome, namely the risk of rejection of CART by the recipient and the risk of graft versus host disease mediated by the allogeneic CART. In this review, we discuss the different strategies being employed to generate universal CART and discuss our perspective on the successful development of a truly off-the-shelf CART product.
1. Background
It took more than 25 years from the initial conceptualization in the late ‘80s of a “chimeric antigen receptor” (CAR) as a system to redirect T cell specificity, to FDA approval of the first genetically engineered cellular product. [1] Chimeric antigen receptors are synthetic proteins generated by the fusion of a single chain variable fragment (scFv) derived from a monoclonal antibody with the signaling and co-stimulatory machinery of the T cell receptor (TCR). In their most commonly used form in the clinic, CART are redirected to recognize CD19, a protein expressed in B-cell leukemias and lymphomas. CART19 are composed of an anti-CD19 scFv linked through a hinge/transmembrane sequence to a costimulatory domain (most commonly CD28 or 4-1BB) and then to the CD3ζ signaling domain. [2] This construct is able to recognize a defined tumor surface antigen like a monoclonal antibody and trigger full T cell activation.
To generate clinical grade CART cells, T cells are collected from the patient by leukapheresis (or peripheral blood), activated, transduced with the CAR constructs using viral vectors (or with transposons/sleeping beauty systems), expanded, and then reinfused to the patient after lymphodepleting chemotherapy. This procedure is carried out in specialized good manufacturing processes (GMP) compliant facilities. During this process, the formerly non-tumor specific T cells acquire the ability to recognize CD19-positive tumors and form potent activating synapses. This T cell-tumor interaction includes both signal 1 (TCR triggering) and signal 2 (costimulation, e.g. 4-1BB). Subsequently CAR T cells become activated, exert their effector functions, proliferate, traffic around the body and can establish immunological memory. This paradigm was proven to be particularly successful when CART19 were used to treat patients with relapsing/refractory B-cell acute lymphoblastic leukemia (r/r B-ALL), as demonstrated by multiple groups. [3] The initial results of a global multicentric registration trial of the University of Pennsylvania/Novartis CART19 product (CTL019, tisagenlecleucel-t) showed 83% complete response (CR) rate in 29 pediatric and young adult patients with r/r B-ALL [4], illustrating the power of this therapy. Similar results have been observed by other groups with other CART19 products in both adult and pediatric patients with r/r B-ALL [5–7] but also, to a lesser extent, in other B-cell neoplasms as non-Hodgkin lymphoma [8, 9] and chronic lymphocytic leukemia. [10]
However, despite the fact that CTL019 (tisagenlecleucel-t) is approved, significant challenges remain regarding the scalability and feasibility of such a platform. First, adoptive cell transfer is still a fairly complicated process that requires high-level cell production expertise and clinical management together with substantial economic and structural resources. Secondly, many patients are unable to receive CART treatment because of rapid disease progression during T cell manufacturing and lastly prior therapies can limit the ability to manufacture CAR T cells. Since these issues represent a major hurdle to the wider application of this approach, investigators from both Industry and Academia are working together to find the best strategy for delivering this treatments to patients. [11] A very appealing possibility is the generation of “allogeneic” CART products that could be used “off-the-shelf” for most of the patients with a relatively short waiting time. However, to accomplish this goal a fundamental paradigm of immunology need to be changed: the fact that main goal of our immune system is to preserve the “self” by attacking and destroying “non-self” cells. Therefore T cells are designed to recognize and kill allogeneic cells through their TCR and vice versa T cells can be recognized through their MHC and destroyed by an allogeneic immune system. For these reasons, CART cell production has been thus far patient-specific and CART production takes between three and four weeks. This is a drug-manufacturing model that is very different from the one of small molecules or monoclonal antibodies where a single drug can be produced in large amounts and used to treat several patients with a defined disease. Therefore developing a “universal” or “off-the-shelf” T cell product would represent a vertical advance in the field and would significantly widen the number of patients eligible to this treatment. To this aim, in the last few years several groups have pursued the generation of universal T cell products that could be produced on large scale and used for several patients in a timely and cost-effective manner. [12]
The successful track record of using allogeneic virus specific T cells provides a compelling rationale for the development of allogeneic off the shelf CART cells. The application of third party, off the shelf virus specific T cells has been proven to be an effective strategy in the prophylaxis and treatment of viral infections, specially post allogeneic transplantation. [13, 14] Methods for the production of multivirus-specific T cells have become simplified over time and virus specific T cells are successfully isolated today from seropositive donors, seronegative donors or cord blood [15]. In a recent study, banks of T-cell lines specific for 12 viral antigens from five viruses (EBV, CMV, AdV, HHV-6, and BKV) were generated and successfully used to treat infections post allogeneic transplantation.[16] Tzannou and colleagues reported this treatment in 38 patients with 45 infections post allogeneic transplantation. Thirty one patients treated for one infection and seven treated for multiple coincident infections experienced a clinical benefit, including a complete resolution of 13 of the 14 patients treated for BKV-associated hemorrhagic cystitis. Importantly, most infusions of third party virus specific T cells are safe without significant GVHD. In this study, five patients developed recurrent or de novo grade 1 to 2 skin GVHD, which resolved with the administration of topical treatments or the re-initiation of corticosteroid treatment after a taper (n = 1). [16] This highlights the feasibility of the adoptive transfer of allogeneic T cells as well as provide a backbone for the development of third party off the shelf CART cells.
2. Strategies to generate universal CART
In order to generate universal, third-party, off-the-shelf T cells two main issues should be addressed: i. graft-versus-host disease (GVHD): the attack of recipient tissues by the infused allogeneic CART. This is mediated by the presence of the alloreactive TCR on donor CAR T cells; ii. graft-rejection: the rejection of infused allogeneic CART by the recipient immune system. This is mediated by the presence of the class I major histocompatibility complex (MHC, or human leukocyte antigens (HLA)) on donor T cells and HLA class II that is overexpressed upon activation. It is long known that rejection is a major problem after hematopoietic cell transplantation. Furthermore, 3rd party allogeneic CAR-T cells have also been shown to cause GVHD in animal models. [17] Therefore, a number of strategies are being developed to overcome these problems. These can be summarized as follows:
2.1 Donor-derived allogeneic CAR-T cells
When a patient receives an allogeneic transplantation and subsequently relapses, CART cells can be generated from the original bone marrow transplant donor and infused into the patient. In a recent report, 20 patients with B cell malignancies received donor derived CD-19 directed CAR-T cells derived from the original donor. All patients had prior allogeneic transplantation and relapsed after transplantation. CAR-T cells were generated from the original donor and no lymphodepleting chemotherapy was given, due to concerns of increased GVHD. Remarkably, 6 of the 20 patient achieved a CR and 2 had a partial response (PR). The response rate was higher in patients with B-ALL where minimal residual disease (MRD)-negative CR was achieved in 80% of patients. Most importantly, no cases of GVHD were recorded. [18] This report demonstrates the clinical feasibility, safety and initial efficacy of donor derived allogeneic CART19 and suggests that genetically targeted T cells could be an integral part of allogeneic transplant in an attempt to separate graft versus leukemia (GVL) from GVHD. Recent data suggest that allogeneic CART19 that use CD28 co-stimulation exert potent GVL with diminished GVHD. In contrast, 1st generation and 4-1BB co-stimulated 2nd generation CART have increased the occurrence of GVHD at least in preclinical setting. [19] The safety of allogeneic CART therapy in this setting could be further enhanced by the incorporation of a suicide system to control the potential uncontrolled GVHD. Several suicide systems like thymidine kinase (TK) from herpes simplex virus 1, induced caspase 9 (iCasp9), thetetracycline-inducible systems [20–24] and antibody-based T cell depletion strategies [25, 26]have been developed and shown to effectively deplete CART cells. In the clinic, the iCasp9 suicide system was able to stop the GVHD caused by the infused haploidentical T cells. [27] Therefore allogeneic CART19 represent an attractive option for relapses after allogeneic transplantation but are still patient-specific, limited to a restricted subset of patients (transplanted) and cannot be used if the patient has a baseline GVHD.
2.2 Selection of non-alloreactive T cells
Another strategy to reduce the likelihood of GVHD after infusion of off-the-shelf CART is the selection of non-alloreactive T cells. Virus specific T cells have been long used after allogeneic transplantation for the treatment of viral infections. [28–30] The main advantage for using virus specific T cells instead of polyclonal T cells is the known specificity of the TCR and therefore the risk for GVHD is minimal. In fact, their application to date has been safe without any reports of serious GVHD. [28, 31] Therefore, it is compelling to harness these properties for the generation of allogeneic CART combining the antigen specificity of the CAR with the TCR specificity towards viral antigens. This approach has been used to generate virus-specific CART19 for the treatment of B-cell malignancies relapsed after allogeneic transplantation. In a study reported by Cruz et al., [32] 8 patients were treated with allogeneic, donor derived virus specific CART19 for relapsed B-cell malignancies after allogeneic transplantation. Of the 6 patients with relapsed disease, 2 had an objective response that was transient. Notably, no patients developed GVHD. [32] CART cell expansion was noted after viral reactivation suggesting that TCR activity is enabled in CART. However, the manufacturing of these T cells required 5–6 weeks that is a significantly longer time as compared to standard CART19 (about 2 weeks), possibly reducing the applicability of this approach for patients with rapidly progressing disease. Lastly, co-activation of the TCR and the CAR may actually be detrimental for T cell function and persistence as demonstrated by Ghosh et al. [19]
Another strategy to select for non-alloreactive T cells is to generate CART from memory T cells. As compared to naïve T cells, memory T cells are associated with less GVHD because of their limited TCR specificity. [33, 34] Memory-derived CART cells have indeed shown to induce less GVHD in preclinical models. [35] Moreover, these T cells have demonstrated a potent anti-leukemic activity when used in autologous setting against non-Hodgkin lymphoma. [36] However, the therapeutic efficacy of adoptive T cell transfer appears to be correlated with the presence of less-differentiated T cell subset, such as naïve and stem-cell memory T cells. [37] Therefore the selection of memory T cells for CART therapy might lead to diminished in vivo proliferation and anti-tumor activity. Lastly, a recent report shows that patients transplanted with naïve T cell-depleted stem cell grafts do not actually have reduced GVHD, suggesting that naïve-derived CART. [38] Lastly, CAR T cell that have low GHV reactivity could be potentially generated from induced pluripotent stem cells (iPSC), although more studies need to be conducted to assess the potency and safety of this approach. [39]
2.3 Use of alternative effector cells
Other components of the immune system could be potentially employed to generate universal cell products for adoptive immunotherapy. NK cells represent an alternative backbone to the use of T cells in the generation of CARs for adoptive immunotherapy. They do not require HLA matching and can be used as allogeneic effector cells [40]. Clinical studies of post allogeneic transplantation NK cell infusion demonstrated the safety of using such an approach in an off-the-shelf fashion. [41] Additionally, CAR expression in NK cells increased their specificity and enhanced their anti-tumor activity. In preclinical studies, potent antitumor activity has been demonstrated using NK CAR cells generated from NK cell lines as well as NK cells derived from patients, [42] and early phase clinical trials are ongoing (please refer to Table 1). Additional immune cells that have been demonstrated not to cause GHVD are NKT cells [43] and γδ T cells. [44] More recently, our group demonstrated that human macrophages can be also redirected to kill cancer cells using a CAR; interestingly, as part of the innate immune system, macrophages would not cause GVHD therefore representing a fascinating cell type for off-the-shelf adoptive immunotherapy [45]. However, for a successful use of these effector cells the issue of rejection should be addressed.
Table 1.
Completed, ongoing or planned clinical trials involving allogenic or universal CAR T cells for cancer (as of August 24, 2017).
| Product | Target | Site | Disease | Notes | Clinical Trials | |
|---|---|---|---|---|---|---|
| Allogeneic CART cells | CAR-T | CD19 | NCI | B cell malignancies relapsed after allogeneic transplantation | CART cells are generated from the original donors | NCT01087294 |
| CAR-T | CD33 | The Affiliated Hospital of the Chinese Academy of Military Medical Sciences and the Chinese PLA General Hospital, China | AML | CART cells are generated from the original donors | NCT02799680 | |
| CAR-T | CD19 | The Affiliated Hospital of the Chinese Academy of Military Medical Sciences and the Chinese PLA General Hospital, China | CD19+ ALL | CART cells are generated from the original donors | NCT02799550 | |
| CAR-T | CD19 | Baylor College of Medicine | CD19+ malignancies relapsed after allogeneic transplantation | CART cells are generated from the original donors | NCT02050347 | |
| 4G7-CARD T-cells | CD19 | UCL, London, UK | CD19+ B-cell malignancies relapsed after Allo-SCT | CART cells are generated from the original donors | NCT02893189 | |
| CART123 | CD123 | City of Hope Medical Center and NCI | AML | Autologous or allogeneic | NCT02159495 | |
| CART123 | CD123 | Affiliated Hospital to Academy of Military Medical Sciences | AML | Allogeneic, after transplant | NCT03114670 | |
| CAR-T | CD19 | Seattle Children’s Hospital | CD19+ leukemia | CART cells are generated from the original donors, has EGFR as a suicide molecule | NCT02028455 | |
| Selection for non allo-reactive T cells | CAR-T | GD2 | Children’s Mercy Hospital Kansas City | Neuroblastoma | CART cells were generated with multi- virus specific cytotoxic T-cells after allogeneic transplantation | NCT01460901 |
| CART19 | CD19 | FHCRC/NCI, US | CD19+ B-cell malignancies after allogeneic transplantation | Donor-derived CD8+ central memory- derived CMV/CD19 or EBV/CD19 bi- specific T cells | NCT01475058 | |
| γδCART19 | CD19 | Beijing DOING Biomedical | CD19+ ALL, CLL, NHL, relapsed after allogeneic transplantation | CART19 are generated using allogeneic γδ T cells | NCT02656147 | |
| CAR19 EBV-CTL | CD19 | MSKCC, US | CD19+ B-ALL with MRD+ after ASCT or r/r B-NHL | Epstein-Barr specific T cells | NCT01430390 | |
| NK-CART cells | CAR-pNK | CD7 | The First People’s Hospital of Hefei | CD7+ leukemia and lymphoma | NK-92 cell line are engineered to contain anti-CD7 CAR | NCT02742727 |
| CAR-pNK | CD33 | The First People’s Hospital of Hefei | CD33+ AML | NK-92 cell line are engineered to contain anti-CD33 CAR | NCT02944162 | |
| PCAR-119 | CD19 | The First People’s Hospital of Hefei | CD19+ leukemia | NK-92 cell line are engineered to contain anti-CD19 CAR | NCT02159495 | |
| Edited CARTs | UCAR-T | CD19 | KCL, London, UK | CLL and ALL | TCR and CD52 knock-out | NCT02735083; NCT02746952 |
| UCAR-T | CD123 | Weill College Medical Cornell | AML | TCR and CD52 knock-out | NCT03190278 | |
| UCAR-T | CD123 | MD Anderson Cancer Center | BPDCN | TCR and CD52 knock-out | NCT03203369 |
CART: chimeric antigen receptor T cells, NCI: National Cancer Institute, AML: acute myeloid leukemia, ALL: acute lymphoblastic leukemia, ASCT: allogeneic stem cell transplantation, FHCRC: Fred Hutchinson Cancer Research Center, CLL: chronic lymphocytic leukemia, NHL: non Hodgkin lymphoma, CMV: cytomegalovirus, EBV: Epstein Barr Virus, MRD: minimal residual disease, ASCT: autologous stem cell transplantation, NK: natural killer cell, KCL: King College of London, TCR: T cell receptor BPDCN: Blastic Plasmacytoid Dendritic Cell Neoplasm
2.4 Gene-editing to generate universal CART
In the last few years several novel genome engineering tools have been developed and optimized to allow the specific and efficient modification of the human genome. [46] Zinc finger nucleases (ZFN), [47] transcription activator-like effector nucleases (TALEN) and megaTAL nucleases [48–50] and clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 [51] systems have all been applied to modify T cells. [52] In particular, these techniques are poised to be ideal tools to generate universal off-the-shelf CART. [50] Most efforts to date are aimed at targeting the native TCR to reduce GVHD and only few studies are focused on modifying the native HLA to reduce graft rejection. The MD Anderson Cancer Center (MDACC) group generated ZFN that can knock out the endogenous TCR in order to avoid GVHD. [53] Investigators from the company Cellectis developed and reported an off-the-shelf TCR-negative CART19 product (UCART19) where the TALEN technology was used to disrupt TCRα and CD52 genes. [54] This therapy was used in 2 pediatric patients with relapsed leukemia as a bridge to allogeneic transplantation. Both patients achieved MRD negative CR without significant GVHD. Importantly, UCART19 cells persisted until the start of the pre-transplant conditioning chemotherapy. [55] The CRISPR/Cas9 system allows for efficient and specific genomic disruption of multiple gene loci. Therefore this approach was used to generate off the shelf allogeneic donor cells, as well as potent effector T cells resistant to inhibitory pathways such as PD-1 and CTLA4. To increase the efficiency of targeting multiple loci, our group used a single protocol that incorporated multiple guide RNAs in a CAR lentiviral vector. [56, 57] Importantly, the CRISPR/Cas9 technology together with an adeno-associated virus (AAV) vector repair matrix was recently employed to directly insert the CAR encoding DNA into the TCR alpha chain locus, simultaneously generating a TCR-negative CAR-positive T cell. These cells were shown to be more potent than conventional lentivirally transduced CART cells because of a more physiological – TCR-like regulation of CAR expression. [58, 59]
However, TCR-negative off-the-shelf T cells may still be subjected to killing by the patient’s own T cells that recognize non-self HLA if there is mismatch, causing rejection. On this regard, lymphodepletion with chemotherapy or irradiation before universal CART infusion could help delay the rejection until the recipient immune system recovers. However it is likely that the persistence of universal HLA-positive CART would be short and since CART19 persistence has been associated with increased responses in several trials [60, 61], early CART rejection could lead to short lasting responses. Therefore, it has been proposed to eliminate the HLA molecules from CART using gene-editing technologies like zinc finger nucleases. [62] Meganucleases can also be used to knock out beta-2-microglobuline (together with the TCR) to obtain HLA class I negative T cells and therefore avoid T cell mediated rejection ([63], abstract #200). High efficiency of double knockout of endogenous TCR and HLA class I as well as PD1 were achieved to generate allogeneic universal CAR T cells. Fas-resistant universal CAR T cells were also generated using this triple gene disruption approach. The gene-edited T cells were as potent as the non-modified CAR T cells, had reduced alloreactivity and did not cause GVHD. [56, 64] Naïve TCR- and PD1-negative anti-NYESO T cell (NYCE cells) will be tested in phase I clinical trials for patients with myeloma, sarcoma and melanoma at the University of Pennsylvania, University of Maryland and at the MDACC.
On the other side, the complete absence of HLA class I on the off-the-shelf T cells, although avoid T cell-mediated rejection, would not prevent their recognition by recipient NK cells as “missing self”, potentially leading again to early rejection. To prevent activation of natural killer cells through “missing self” recognition would be circumvented by enforced expression of non-classical HLA molecules such as HLA-E and HLA-G that can protect universal CART from NK-cell–mediated lysis. [62, 65] Another recent approach to reduce NK-cell toxicity to HLA-negative universal T cells is the overexpression of Siglec-7 and -9 ligands. [66] Another strategy to avoid rejection of HLA mismatched CART is the use of HLA homozygous donors to generate a bank of universal CART products. It was calculated that with limited numbers of donors homozygous for at HLA-A/B/DRB1 it is possible to generate compatible products to cover the majority of the population. [67, 68]
Although gene-editing technologies are certainly the most promising approach to generate universal off-the-shelf CART, additional studies are needed to integrate the gene-editing in the clinical-grade CART expansion protocol. Moreover, the efficacy and most importantly the safety of these highly-engineered CART need to be carefully assessed in early phase clinical trials.
3. Conclusions and future perspectives
CART cell therapy is one of the most promising novel therapies for the treatment of cancer, and specifically hematological malignancies. Autologous CART cells have demonstrated unprecedented clinical results in B-cell malignancies and CART19 was the first genetically modified cellular product to gain FDA approval in August 2017. However, the possibility to generate universal off-the-shelf CART products would immensely increase the feasibility and diffusion of this approach. In particular, the successful generation of off-the-shelf universal CAR-T cells would lead to the following advantages:
Easier and cost-effective CART manufacturing: CART cell manufacturing could be readily undertaken in a centralized facility and off-the-shelf T cells can be generated and cryopreserved for future needs; there would be no need for patient-specific leukapheresis and CART production, drastically reducing the costs.
Reduced time to CART cell infusion: in highly proliferative diseases (such as acute leukemia), a 2–4 week wait is detrimental and in some cases not feasible. Therefore a readily available CART product could be increase the number of candidates for this therapy.
Increased probability of healthy CART cell generation: an off-the-shelf approach would overcome challenges in CART manufacturing from patients with diseases that are heavily pretreated with chemotherapy and in whom the quantity and quality (exhaustion, senescence, autoimmunity) of T cells is suboptimal. This standardization of the CART product could potentially lead also to higher predictability of clinical response.
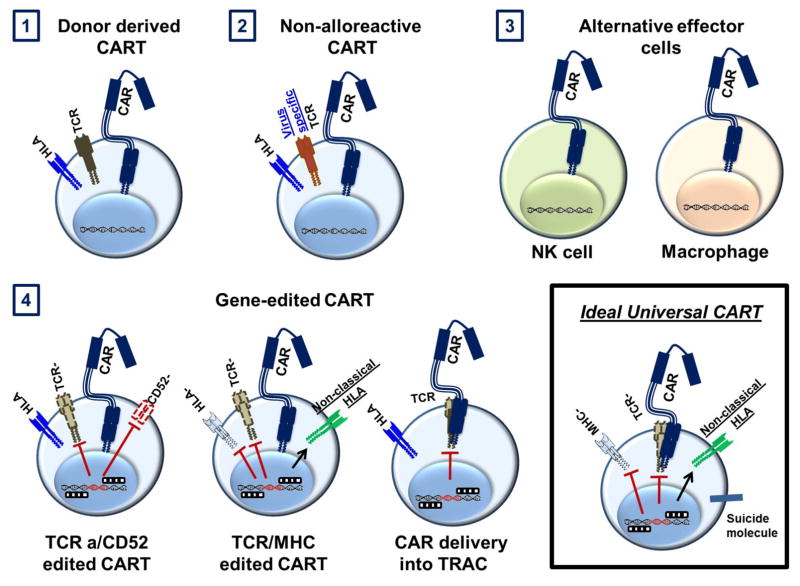
The ideal universal CART product should: i. lack naïve TCRs to avoid GVHD; ii. have matched or absent HLA to avoid rejection; iii. include NK inhibitory strategies (non-classical HLA or siglec-7/-9 ligands) and iv. include a significant amount of naïve and stem cell memory T cells to ensure adequate T cell expansion and persistence (see Figure 1).
Figure 1. Schema of the currently used strategies to generate universal off the shelf or allogeneic CART cells.
1) CART cells derived from original hematopoietic donors for patients relapsing after allogeneic transplantation; 2) Selection for non- alloreactive T cells to generate CART cells (such as virus specific CART cells) 3) Use of alternative effector cells, e.g. macrophages and NK cells; 4) Gene-edited CART. E.g.: TALEN technology used to generate TCR negative, CD52-negative CART cells; Zinc-finger nucleases and CRISPR-Cas9 to knock out the TCR and HLA; The CAR construct is directly delivered into the TCR locus with CRISPR-Cas9 and an AAV template, generating TCR-negative CART cells. The ideal universal CART should be HLA and TCR negative and include non-classical HLA to avoid NK cell lysis. It should also include a suicide system to control for potential toxicity.
Several strategies are currently being developed (see Figure 1) and many of these are in early phase clinical studies (see Table 1). Genome editing of T cells provides a wider application for the engineered T cells and the potential to generate truly off-the-shelf products, in large due to the capability of multiplex knockout and targeted transduction. While ZFN, TALEN and CRISPR technologies are used, we believe the CRISPR/Cas9 system is one most promising way to develop off-the-shelf CART products and to advance T cell immunotherapy because of the high specificity of this technology, the relative ease and the limited cost. Despite the complexity of the application of such tools, the possible benefits justify the development of a path to a broader clinical application.
Key points.
CART cells represent an exciting novel treatment modality for cancer but they require patient-specific manufacturing
Patient-specific manufacturing is costly and time-consuming, therefore universal CART products would be highly valuable
Gene-engineering and cell selection techniques allow the generation of off-the-shelf CART
Acknowledgments
This work was supported by grants from the SITC (EMD-Serono Cancer Immunotherapy Clinical Fellowship, PI: M.R.), the AACR (Bristol-Myers Squibb Oncology Fellowship in Clinical Cancer Research, PI: M.R.), the Gabrielle’s Angel Foundation (PI: M.R.), the SIES-AIL (PI: M.R.), the ASH Scholar Award (PI: M.R.), the NCI (K99 CA212302-01A1, PI: M.R. and K12CA166039, PI: S.S.K.), the Predolin Foundation (PI: S.S.K.), the NCCN Young Investigator Award (PI: S.S.K.), and the Mayo Clinic Center for Individualized Medicine (PI: S.S.K.)
Footnotes
Competing interests:
M.R. works under a research collaboration involving the University of Pennsylvania and the Novartis Institute of Biomedical Research, Inc. M.R. and S.S.K. are inventors of intellectual property licensed by the University of Pennsylvania to Novartis.
Authorship contribution. M.R. and S.S.K. wrote, reviewed and accepted the contents of the article.
References
- 1.Ruella M, Kalos M. Adoptive immunotherapy for cancer. Immunological reviews. 2014 Jan;257(1):14–38. doi: 10.1111/imr.12136. [DOI] [PubMed] [Google Scholar]
- 2.Kenderian SS, Ruella M, Gill S, Kalos M. Chimeric Antigen Receptor T-cell Therapy to Target Hematologic Malignancies. Cancer research. 2014 Nov 4; doi: 10.1158/0008-5472.CAN-14-1530. [DOI] [PubMed] [Google Scholar]
- 3.Ruella M, June CH. Chimeric Antigen Receptor T cells for B Cell Neoplasms: Choose the Right CAR for You. Curr Hematol Malig Rep. 2016 Jul 30; doi: 10.1007/s11899-016-0336-z. [DOI] [PubMed] [Google Scholar]
- 4.Grupp SA, Laetsch TW, Buechner J, Bittencourt H, Maude SL, Verneris MR, et al. Analysis of a Global Registration Trial of the Efficacy and Safety of CTL019 in Pediatric and Young Adults with Relapsed/Refractory Acute Lymphoblastic Leukemia (ALL) Blood. 2016;128(22):221. [Google Scholar]
- 5.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014 Feb 19;6(224):224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. The Journal of clinical investigation. 2016 Apr 25; doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015 Feb 7;385(9967):517–28. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-Refractory Diffuse Large B-Cell Lymphoma and Indolent B-Cell Malignancies Can Be Effectively Treated With Autologous T Cells Expressing an Anti-CD19 Chimeric Antigen Receptor. J Clin Oncol. 2015 Feb 20;33(6):540–9. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuster SJ, Svoboda J, Dwivedy Nasta S, Porter DL, Chong EA, Landsburg DJ, et al. Sustained Remissions Following Chimeric Antigen Receptor Modified T Cells Directed Against CD19 (CTL019) in Patients with Relapsed or Refractory CD19+ Lymphomas. Blood 2015. 2015 Dec 03;126(23):183. 00:00:00. [Google Scholar]
- 10.Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Science translational medicine. 2015 Sep 2;7(303):303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine BL, June CH. Perspective: assembly line immunotherapy. Nature. 2013 Jun 27;498(7455):S17. doi: 10.1038/498S17a. [DOI] [PubMed] [Google Scholar]
- 12.Torikai H, Cooper LJ. Translational Implications for Off-the-shelf Immune Cells Expressing Chimeric Antigen Receptors. Molecular therapy: the journal of the American Society of Gene Therapy. 2016 Aug;24(7):1178–86. doi: 10.1038/mt.2016.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rooney CM, Aguilar LK, Huls MH, Brenner MK, Heslop HE. Adoptive immunotherapy of EBV-associated malignancies with EBV-specific cytotoxic T-cell lines. Curr Top Microbiol Immunol. 2001;258:221–9. doi: 10.1007/978-3-642-56515-1_14. [DOI] [PubMed] [Google Scholar]
- 14.Peggs KS, Verfuerth S, Pizzey A, Khan N, Guiver M, Moss PA, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003 Oct 25;362(9393):1375–7. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- 15.Hanley PJ, Bollard CM, Brunstein CG. Adoptive immunotherapy with the use of regulatory T cells and virus-specific T cells derived from cord blood. Cytotherapy. 2015 Jun;17(6):749–55. doi: 10.1016/j.jcyt.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tzannou I, Papadopoulou A, Naik S, Leung K, Martinez CA, Ramos CA, et al. Off-the-Shelf Virus-Specific T Cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2017 Aug 07; doi: 10.1200/JCO.2017.73.0655. JCO2017730655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alcantar-Orozco EM, Gornall H, Baldan V, Hawkins RE, Gilham DE. Potential limitations of the NSG humanized mouse as a model system to optimize engineered human T cell therapy for cancer. Hum Gene Ther Methods. 2013 Oct;24(5):310–20. doi: 10.1089/hgtb.2013.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brudno JN, Somerville RP, Shi V, Rose JJ, Halverson DC, Fowler DH, et al. Allogeneic T Cells That Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-Cell Malignancies That Progress After Allogeneic Hematopoietic Stem-Cell Transplantation Without Causing Graft-Versus-Host Disease. J Clin Oncol. 2016 Apr 1;34(10):1112–21. doi: 10.1200/JCO.2015.64.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh A, Smith M, James SE, Davila ML, Velardi E, Argyropoulos KV, et al. Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nature medicine. 2017 Feb;23(2):242–9. doi: 10.1038/nm.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakemura R, Terakura S, Watanabe K, Miyao K, Koyama D, Goto T, et al. A Novel Strategy of Switching on/Off CD19CAR Expression Under Tetracycline-Based System. Blood 2015. 2015 Dec 03;126(23):4424. 00:00:00. [Google Scholar]
- 21.Tasian SK, Kenderian SS, Shen F, Ruella M, Shestova O, Kozlowski M, et al. Optimized Depletion of Chimeric Antigen Receptor T-Cells in Murine Xenograft Models of Human Acute Myeloid Leukemia. Blood. 2017 Feb 28; doi: 10.1182/blood-2016-08-736041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997:276. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 23.Straathof KC, Pule MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, et al. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005 Jun 1;105(11):4247–54. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gargett T, Brown MP. The inducible caspase-9 suicide gene system as a “safety switch” to limit on-target, off-tumor toxicities of chimeric antigen receptor T cells. Front Pharmacol. 2014:5. doi: 10.3389/fphar.2014.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Chang WC, Wong CW, Colcher D, Sherman M, Ostberg JR, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011 Aug 4;118(5):1255–63. doi: 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Philip B, Kokalaki E, Mekkaoui L, Thomas S, Straathof K, Flutter B, et al. A highly compact epitope-based marker/suicide gene for easier and safer T-cell therapy. Blood. 2014 Aug 21;124(8):1277–87. doi: 10.1182/blood-2014-01-545020. [DOI] [PubMed] [Google Scholar]
- 27.Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011 Nov 3;365(18):1673–83. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013 Jun 27;121(26):5113–23. doi: 10.1182/blood-2013-02-486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanley PJ, Cruz CR, Savoldo B, Leen AM, Stanojevic M, Khalil M, et al. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009 Aug 27;114(9):1958–67. doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossig C, Bollard CM, Nuchtern JG, Rooney CM, Brenner MK. Epstein-Barr virus-specific human T lymphocytes expressing antitumor chimeric T-cell receptors: potential for improved immunotherapy. Blood. 2002 Mar 15;99(6):2009–16. doi: 10.1182/blood.v99.6.2009. [DOI] [PubMed] [Google Scholar]
- 31.Melenhorst JJ, Leen AM, Bollard CM, Quigley MF, Price DA, Rooney CM, et al. Allogeneic virus-specific T cells with HLA alloreactivity do not produce GVHD in human subjects. Blood. 2010 Nov 25;116(22):4700–2. doi: 10.1182/blood-2010-06-289991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cruz CR, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013 Oct 24;122(17):2965–73. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foster AE, Marangolo M, Sartor MM, Alexander SI, Hu M, Bradstock KF, et al. Human CD62L- memory T cells are less responsive to alloantigen stimulation than CD62L+ naive T cells: potential for adoptive immunotherapy and allodepletion. Blood. 2004 Oct 15;104(8):2403–9. doi: 10.1182/blood-2003-12-4431. [DOI] [PubMed] [Google Scholar]
- 34.Robins HS, Campregher PV, Srivastava SK, Wacher A, Turtle CJ, Kahsai O, et al. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood. 2009 Nov 5;114(19):4099–107. doi: 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan WK, Suwannasaen D, Throm RE, Li Y, Eldridge PW, Houston J, et al. Chimeric antigen receptor-redirected CD45RA-negative T cells have potent antileukemia and pathogen memory response without graft-versus-host activity. Leukemia. 2015 Feb;29(2):387–95. doi: 10.1038/leu.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turtle CJ, Hanafi LA, Berger C, Hudecek M, Pender B, Robinson E, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Science translational medicine. 2016 Sep 07;8(355):355ra116. doi: 10.1126/scitranslmed.aaf8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer 2012. 2012:12. doi: 10.1038/nrc3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bleakley M, Heimfeld S, Loeb KR, Jones LA, Chaney C, Seropian S, et al. Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts. J Clin Invest. 2015 Jul 01;125(7):2677–89. doi: 10.1172/JCI81229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Themeli M, Kloss CC, Ciriello G, Fedorov VD, Perna F, Gonen M, et al. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nature biotechnology. 2013 Oct;31(10):928–33. doi: 10.1038/nbt.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nature immunology. 2008 May;9(5):503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 41.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005 Apr 15;105(8):3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 42.Romanski A, Uherek C, Bug G, Seifried E, Klingemann H, Wels WS, et al. CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies. J Cell Mol Med. 2016 Jul;20(7):1287–94. doi: 10.1111/jcmm.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heczey A, Liu D, Tian G, Courtney AN, Wei J, Marinova E, et al. Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood. 2014 Oct 30;124(18):2824–33. doi: 10.1182/blood-2013-11-541235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deniger DC, Switzer K, Mi T, Maiti S, Hurton L, Singh H, et al. Bispecific T-cells Expressing Polyclonal Repertoire of Endogenous γδ T-cell Receptors and Introduced CD19-specific Chimeric Antigen Receptor. Molecular Therapy. 2013 Mar;21(3):638–47. doi: 10.1038/mt.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klichinsky M, Ruella M, Shestova O, Kenderian SS, Kim MY, O’Connor R, et al. Abstract 4575: Chimeric antigen receptor macrophages (CARMA) for adoptive cellular immunotherapy of solid tumors. Cancer research. 2017;77(13 Supplement):4575. [Google Scholar]
- 46.Yin H, Kauffman KJ, Anderson DG. Delivery technologies for genome editing. Nat Rev Drug Discov. 2017 Jun;16(6):387–99. doi: 10.1038/nrd.2016.280. [DOI] [PubMed] [Google Scholar]
- 47.Perez EE, Wang J, Miller JC, Jouvenot Y, Kim KA, Liu O, et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol. 2008 Jul;26(7):808–16. doi: 10.1038/nbt1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller JC, Tan S, Qiao G, Barlow KA, Wang J, Xia DF, et al. A TALE nuclease architecture for efficient genome editing. Nat Biotechnol. 2011 Feb;29(2):143–8. doi: 10.1038/nbt.1755. [DOI] [PubMed] [Google Scholar]
- 49.Sather BD, Romano Ibarra GS, Sommer K, Curinga G, Hale M, Khan IF, et al. Efficient modification of CCR5 in primary human hematopoietic cells using a megaTAL nuclease and AAV donor template. Science translational medicine. 2015 Sep 30;7(307):307ra156. doi: 10.1126/scitranslmed.aac5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osborn MJ, Webber BR, Knipping F, Lonetree CL, Tennis N, DeFeo AP, et al. Evaluation of TCR Gene Editing Achieved by TALENs, CRISPR/Cas9, and megaTAL Nucleases. Molecular therapy: the journal of the American Society of Gene Therapy. 2016 Mar;24(3):570–81. doi: 10.1038/mt.2015.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013 Feb 15;339(6121):819–23. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ren J, Zhao Y. Advancing chimeric antigen receptor T cell therapy with CRISPR/Cas9. Protein Cell. 2017 Apr 22; doi: 10.1007/s13238-017-0410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Torikai H, Reik A, Liu PQ, Zhou Y, Zhang L, Maiti S, et al. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood. 2012 Jun 14;119(24):5697–705. doi: 10.1182/blood-2012-01-405365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poirot L, Philip B, Schiffer-Mannioui C, Le Clerre D, Chion-Sotinel I, Derniame S, et al. Multiplex Genome-Edited T-cell Manufacturing Platform for “Off-the-Shelf” Adoptive T-cell Immunotherapies. Cancer Res. 2015 Sep 15;75(18):3853–64. doi: 10.1158/0008-5472.CAN-14-3321. [DOI] [PubMed] [Google Scholar]
- 55.Qasim W, Zhan H, Samarasinghe S, Adams S, Amrolia P, Stafford S, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. 2017 Jan 25;9(374) doi: 10.1126/scitranslmed.aaj2013. [DOI] [PubMed] [Google Scholar]
- 56.Ren J, Zhang X, Liu X, Fang C, Jiang S, June CH, et al. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget. 2017 Feb 09; doi: 10.18632/oncotarget.15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ren J, Liu X, Fang C, Jiang S, June CH, Zhao Y. Multiplex Genome Editing to Generate Universal CAR T Cells Resistant to PD1 Inhibition. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016 Nov 04; doi: 10.1158/1078-0432.CCR-16-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eyquem J, Mansilla-Soto J, Giavridis T, van der Stegen SJ, Hamieh M, Cunanan KM, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017 Mar 02;543(7643):113–7. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hale M, Lee B, Honaker Y, Leung WH, Grier AE, Jacobs HM, et al. Homology-Directed Recombination for Enhanced Engineering of Chimeric Antigen Receptor T Cells. Mol Ther Methods Clin Dev. 2017 Mar 17;4:192–203. doi: 10.1016/j.omtm.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Science translational medicine. 2011 Aug 10;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine. 2014 Oct 16;371(16):1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Torikai H, Reik A, Soldner F, Warren EH, Yuen C, Zhou Y, et al. Toward eliminating HLA class I expression to generate universal cells from allogeneic donors. Blood. 2013 Aug 22;122(8):1341–9. doi: 10.1182/blood-2013-03-478255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.ASGCT 19th Annual Meeting: Abstracts. Molecular therapy: the journal of the American Society of Gene Therapy. 2016 Apr;24( Suppl 1):S1–S304. doi: 10.1038/mt.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ren J, Liu X, Fang C, Jiang S, June CH, Zhao Y. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016 Nov 04; doi: 10.1158/1078-0432.CCR-16-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gornalusse GG, Hirata RK, Funk SE, Riolobos L, Lopes VS, Manske G, et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nature biotechnology. 2017 Aug;35(8):765–72. doi: 10.1038/nbt.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jandus C, Boligan KF, Chijioke O, Liu H, Dahlhaus M, Demoulins T, et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J Clin Invest. 2014 Apr;124(4):1810–20. doi: 10.1172/JCI65899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011 May;8(5):409–12. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- 68.Taylor CJ, Peacock S, Chaudhry AN, Bradley JA, Bolton EM. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell. 2012 Aug 03;11(2):147–52. doi: 10.1016/j.stem.2012.07.014. [DOI] [PubMed] [Google Scholar]