Abstract
Mesenchymal stem cells (MSCs) are multipotent cells and are the most widely studied cell type for stem cell therapies. In-vivo cell tracking of MSCs labeled with an FDA-approved superparamagnetic iron-oxide (SPIO) particle by magnetic resonance imaging (MRI) provides essential information, e.g., MSC engraftment, survival, and fate, thus improving cell therapy accuracy. However, current methodology for labeling MSCs with Ferumoxytol (Feraheme®), the only FDA-approved SPIO particle, needs transfection agents. This unit describes a new “bio-mimicry” protocol to prepare more native MSCs by using more “in-vivo environment” of MSCs, so that the phagocytic activity of cultured MSCs is restored and expanded MSCs can be labeled with Ferumoxytol, without the need for transfection agents and/or electroporation. Moreover, MSCs re-size to a more native size, reducing from 32.0 to 19.5 μm. The MSCs prepared from this protocol retain more native properties and would be useful for biomedical applications and MSC-tracking studies by MRI.
Keywords: mesenchymal stem cells, stem cell culture and expansion, Ferumoxytol (Feraheme®), stem cell tracking, magnetic resonance imaging
INTRODUCTION
In this unit, we describe a “bio-mimicry” method making use of more “in-vivo environment” of mesenchymal stem cells (MSCs) to prepare MSCs and to label them with Ferumoxytol (Feraheme® injection; AMAG Pharmaceuticals), the only currently FDA-approved superparamagnetic iron-oxide (SPIO) particle, with no need for the use of transfection agents. The reason that we name this new method as “bio-mimicry method” is because we use non-MSC components in the bone marrow extractions, namely, non-adherent cells and supernatant liquid, during cell culturing and labeling steps to mimic the “in-vivo cellular environment” of MSCs. MSCs have the ability to self-renew and differentiate into several different cell types, such as adipocytes, chondrocytes, osteoblasts, neurons, and myocytes (Bianco 2014, Ma et al., 2014). Currently, there are at least 493 MSC-based clinical trials (in the database of the US National Institutes of Health) for the investigation of their therapeutic potential for over 12 types of disease conditions (Squillaro et al., 2016), e.g., myocardial infarction, graft-versus-host disease (GVHD), diabetes, multiple sclerosis, etc.
Our new protocol aims to help overcome two challenges facing current MSC therapy: (i) MSC properties change during ex-vivo culture and expansion, i.e., their phagocytic activity; and (ii) one wants to know whether engraftment occurs at the appropriate site, and the subsequent fate of the transplanted MSCs. In fact, cellular magnetic resonance imaging (MRI) combined with superparamagnetic iron-oxide (SPIO)-based contrast agents provides a safe, effective, and non-invasive way to investigate the in-vivo engraftment and the survival of transplanted stem cells (Noad et al., 2013, Nguyen et al., 2014, Nejadnik et al., 2016). However, currently, Ferumoxytol (Feraheme® injection) is the only intravenous FDA-approved SPIO nanoparticle (Bashir et al., 2015) for clinical applications, and previous studies show that Ferumoxytol does not effectively label MSCs ex vivo (in cell culture). The Ferumoxytol-heparin-protamine (HPF) nanocomplex methodology is the current method for labeling MSCs ex vivo (Thu et al., 2012). A recent study shows that MSCs are phagocytic in nature and can be labeled by Ferumoxytol through an in-vivo cell-labeling method (i.v. injection) (Khurana et al., 2013). Thus, we have developed the following protocol to recover the phagocytic activity of the ex-vivo cultured MSCs by using “in-vivo cellular environment” of MSCs, so that MSCs can be labeled with Ferumoxytol in cell culture, without the need for using transfection agents. The protocols below have been briefly described in our recent publication (Liu et al., 2016).
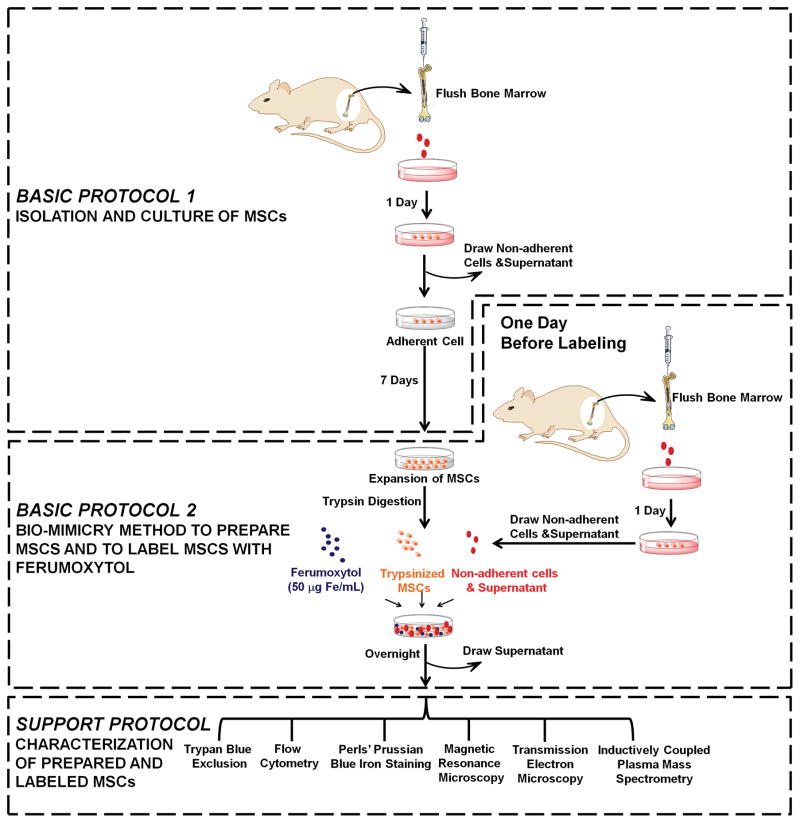
This unit begins with the isolation and culture of MSCs (Basic Protocol 1). We then describe our “bio-mimicry” method for preparing MSCs and labeling them with Ferumoxytol (Basic Protocol 2). We compare different conditions to mimic the “in-vivo environment” by using: (i) supernatant from adhering cultured MSCs plus non-adherent cells; (ii) supernatant alone; and (iii) non-adherent cells alone. In the Support Protocol, we describe the procedures for characterization of prepared and labeled MSCs by immunofluorescent staining followed by flow cytometry analysis, Perls’ Prussian blue iron staining, magnetic resonance microscopic (MRM) imaging, and transmission electron microscopy (TEM). The experimental flowchart of our protocol and the organization of this unit are shown in Figure 1.
Figure 1.
Experimental flow chart describing our “bio-mimicry” method for preparing mesenchymal stem cells and labeling with Ferumoxytol. [Modified from Figure 1 of (Liu et al., 2016)].
NOTE: All glassware, plastic ware, pipette tips, surgical instruments, sponges, and towels are sterilized by autoclaving at a steam pressure of ~15 psi at ~127 °C (260 °F) for 30 min. All sterilized tubes, culture media, buffers, and reagents are purchased at cell-culture grade. Solutions are prepared in sterilized Nanopure water. All the procedures are performed aseptically in a class II biological safety cabinet.
BASIC PROTOCOL 1. ISOLATION AND CULTURE OF MSCs
This protocol describes the isolation of MSCs from bone marrow and culture of MSCs using the untreated whole bone marrow extraction. A “direct adherence” method as described in previous publications from our laboratory (Chen et al., 2011a) and others (Soleimani et al., 2009, Khurana et al., 2013, Li et al., 2013, Noad et al., 2013, Zhang et al., 2014), is used to prepare MSCs with some modifications.
NOTE: All protocols involving live animal subjects are reviewed and approved by an Institutional Animal Care and Use Committee (IACUC). Animal care must be provided in accordance with government regulations for the care and use of laboratory animals.
Materials
Brown Norway (BN) rat
Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific, cat. no. 12491-015)
Heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific, cat. no. 16000044)
Penicillin and streptomycin (GIBCO, cat. no. 15140)
Glutamine (GIBCO, cat. no. 25030)
Phosphate-buffered saline (PBS; Mediatech, cat. no. 21-040-CV)
70% isopropyl alcohol (First Priority Inc., cat. no. MS073)
0.25% trypsin/1 mM ethylenediaminetetraacetic acid (EDTA) (Thermo Fisher Scientific, cat. no. 25200056)
Trypan Blue solution (Sigma-Aldrich, cat. no. T8154)
Isoflurane (Bulter Schein, cat no. 11695-6776-1)
Steel surgical scissors, sterilized
Steel surgical forceps (straight and curved), sterilized
Gloves, sterilized
Sponges, sterilized
27-gauge needles (Becton, Dickinson and Company, cat. no. 301629)
10-ml syringes (Sigma-Aldrich, cat. no. Z248029)
15-ml and 50-ml sterile conical centrifuge tubes (Sigma-Aldrich, cat. no. CLS430791 and CLS430829, respectively)
2-ml and 5-ml pipettes (Sigma-Aldrich, cat. no. CLS4021 and P7615, respectively)
6-well culture dishes (10-cm; Sigma-Aldrich, cat. no. CLS3516)
1.5-ml eppendorf tubes (Sigma-Aldrich, cat. no. T9661)
10 μl, 100 μl, and 1 ml pipettes and tips
Inverted light microscope
Hemacytometer with cover slips (Sigma-Aldrich, cat. no. Z359629)
Centrifuge (no temperature control is needed)
Incubator with both temperature and gas composition controls
Preparation
-
1
Prepare complete DMEM medium (see recipe). Put 20 ml of cold complete medium in a 50-ml conical centrifuge tube and keep the tube on ice.
-
2
Equip the safety cabinet with the following items: 15-ml and 50-ml conical centrifuge tubes, 2-ml and 5-ml pipettes, 6-well culture dishes, 10-ml syringe with 27-gauge needle, forceps, sponges, and cold complete DMEM medium. Spray alcohol for decontamination. Turn on laminar flow and UV light.
Isolate femurs and tibias
-
3
Euthanize the BN rat by means of an overdose (5%) of isoflurane inhalation. Use a pair of surgical scissors to make an incision around the perimeter of the limbs. Using a pair of forceps, remove the skin by pulling toward the end, and cutting it at the anklebone. Then, dissect the limbs from the trunk of the body by cutting along the spinal cord. Remove the muscle and connective tissue from both the tibia and the femur by pulling the tissue toward the ends of the bone using sponges. After cleaning, store the bones in a 50-ml tube with 20 ml of complete DMEM medium on ice.
Bone marrow aspiration and bone marrow cell culture
-
4
Turn off the UV light of the hood. Under the hood, break the ends of the tibia and femur just below the end of the marrow cavity using a pair of bone forceps. Remove the growth plate to expose the spongy bone. Insert a 27-gauge needle attached to a 10-ml syringe containing complete DMEM medium into the spongy bone and flush the marrow plug out of the cut end of the bone with ~5 ml of complete DMEM medium. Collect the bone marrow extraction in a 50-ml tube on ice.
If a lot of muscle and cell clumps or bone spicules are visible in the bone marrow extraction, a 70-mm filter mesh can be used to remove them. -
5
Check the viability of the harvested cells by using Trypan Blue staining and count cell number by using a hemacytometer.
Typically, ~300 × 106 mononuclear cells can be collected from one rat. -
6
Culture bone marrow cells in 6-well culture dishes with 1.5 ml of complete DMEM per well at a density of ~20 × 106 cell ml−1. Cells are incubated at 37°C with 5% CO2 in a humidified (85% humidity) cell culture incubator.
-
7
After 24 hr of incubation, remove the non-adherent cells and supernatant by using a 5-ml pipette. Wash the adherent cells carefully with 1.5 ml of PBS twice and replace the medium with 1.5 ml of fresh complete DMEM medium.
-
8
Thereafter, remove the previous medium, wash the adherent cells with PBS, and add fresh medium every 72 hr.
BASIC PROTOCOL 2. BIO-MIMICRY METHOD TO PREPARE MSCS AND TO LABEL MSCS WITH FERUMOXYTOL
In this protocol, we describe a new methodology to label MSCs with Ferumoxytol directly, with no need of using transfection agents and/or electroporation. Previous studies showed that cultured MSCs cannot take up Ferumoxytol effectively (Thu et al., 2012). Recently, MSCs were found to be able to take up Ferumoxytol in-vivo after i.v. injection of Ferumoxytol (Khurana et al., 2013). Our hypothesis is that the “in-vivo cellular environment” is important for MSC functions and can reverse the “changes”, e.g., decreasing of phagocytic activity of the ex-vivo expanded MSCs.
In our new labeling protocol, we use the supernatant and “non-adherent” cells, or non-MSCs, to mimic the “in-vivo cellular environment” of MSCs. Using our new method, 7-day cultured MSCs regain the capability to take up Ferumoxytol and exhibit an intracellular iron concentration of 2.50 ± 0.50 pg/MSC. Furthermore, we have found that MSCs re-size to a more native size, i.e., reducing from 32.0 ± 7.2 to 19.5 ± 5.2 μm, by our “bio-mimicry” method.
Materials
All the materials as described in Basic Protocol 1
MSC cells prepared as above, incubated 7 days
A second BN rat
Ferumoxytol (Feraheme® injection; AMAG Pharmaceuticals)
Sterilized Nanopure water (prepared by using Barnstead Nanopure Diamond system)
Mouse anti-rat ED1: Alexa Fluor 647 antibody (AbD SeroTec, cat. no. MCA341A647)
Leucoperm™ (AbD SeroTec, cat. no. BUF09)
Accustain® iron stain kit (Sigma-Aldrich, cat. no. HT20)
Low melting point agarose (Fisher Scientific, cat. no. BP16525)
Paraformaldehyde (Sigma-Aldrich, cat. no. 158127)
Nitric acid (70%; Sigma-Aldrich, cat. no. 438073)
Osmium tetroxide (OsO4; Sigma-Aldrich, cat. no. 419494)
5-mm NMR tubes (Sigma-Aldrich, cat. no. NORS55007)
One day before labeling
-
1
After MSC expansion (we have tested MSCs on day 7 of culture and the cell confluence is over 70%) and one day before labeling, prepare fresh non-adherent cells and supernatant by repeating steps 1 to 6 in Basic Protocol 1, using the bone marrow from the second BN rat.
-
2
Allow these bone marrow cells to incubate and attach in the cell culture incubator for 24 hr.
Day of labeling
-
3
Wash the expanded MSCs cultured for 7 days with PBS twice. Detach the MSCs by incubation with warm trypsin-EDTA (0.5 ml) for 2 min at room temperature. Transfer the cells to Eppendorf tubes and spin down at 350 g for 5 min. Wash the MSCs with PBS once.
The time and temperature of trypsin-EDTA digestion is very important. Do not exceed the time of 2 min and do not use a higher temperature than 25 °C. The purpose of detaching is to increase the interactions of MSCs with Ferumoxytol and their “in-vivo environment”, i.e., non-adherent cells and supernatant as described in the next step. -
4
In order to mimic the “in-vivo environment” of MSCs, the non-adherent cells and supernatant from step 2 of Basic Protocol 2 are removed from the overnight culture and mixed with the expanded and detached MSCs. One well of the non-adherent cells and supernatant is mixed with one well of MSCs.
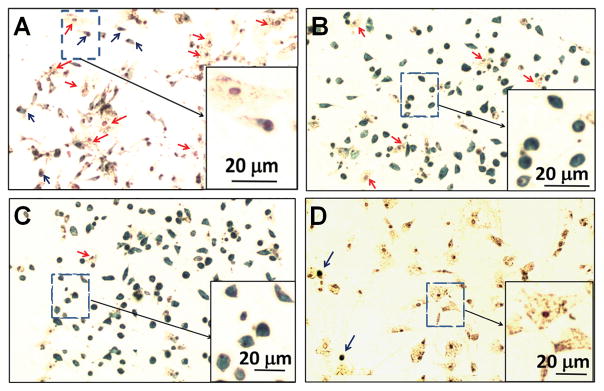
At this step, we have compared three different conditions to mimic the in-vivo environment by using: (i) non-adherent cells and supernatant (Figure 2B); (ii) non-adherent cells (Figure 2C); and (iii) supernatant liquid (Figure 2D). We have found that the non-adherent cells are the key component to recover the phagocytic activity of MSCs and to change the cell size (comparing Figure 2C and Figure 2D). We suggest using condition (i), supernatant and non-adherent cells, which gives the best results and saves one step of separation.At this step, attempting to avoid another bone marrow aspiration, we have tested non-adherent cells and supernatant, from step 7 of Basic Protocol 1 stored at −80 °C. We have found that the fresh ones work more efficiently than the frozen ones. While the intracellular iron concentration is 2.50 ± 0.50 pg/MSC when fresh ones are used, the intracellular iron concentration is 0.91 ± 0.22 pg/MSC when the thawed ones are used. -
5
Add Ferumoxytol (final concentration 50 μg Fe/ml; see recipe for preparation of Ferumoxytol stock solution) to the MSCs, non-adherent cells, and supernatant mixture. Mix well.
-
6
Allow the cell mixture to incubate overnight.
Figure 2.
Perls’ Prussian blue iron staining of MSCs labeled with Ferumoxytol under different conditions: (A) using the traditional method (Protocol 1); (B) co-incubation with non-adherent cells and supernatant liquid (Protocol 2); (C) co-incubation with non-adherent cells; and (D) co-incubation with supernatant liquid. Blue arrows point to Prussian blue positive cells. Red arrows show Prussian blue negative cells. [Modified from Figures 3, 4, and 6 of (Liu et al., 2016)].
Day of harvesting
-
7
Draw off the supernatant. Wash the adherent cells (MSCs) with 1.5 ml of PBS three times to clean up the residual Ferumoxytol particles.
-
8
Ferumoxytol-labeled MSCs are attached to the bottom and ready to be collected by trypsin-EDTA digestion (as described in step 3) followed by washing with PBS.
-
9
Examine the cell size using an inverted microscope equipped with a suitable camera and cell-size measuring software.
-
10
Examine the MSC viability by the Trypan Blue exclusion assay. Examine the presence of monocytes/macrophages by immunofluorescence staining followed by flow cytometry analysis. Examine the labeling efficiency by Perls’ Prussian blue iron staining assay, MR microscopic (MRM) imaging, and transmission electron microscopic (TEM) imaging. Measure the intracellular iron concentration by inductively coupled plasma-mass spectrometry (ICP-MS, NexION 300X, PerkinElmer Inc.). The protocols of these assays and measurements are described below in the Support Protocol.
As shown in Figure 2, we have observed that Prussian blue positive cells (blue arrows) usually are small and round. Most of the Prussian blue negative cells (red arrows) are bigger and flat.
NOTE: Our new method is different from the traditional or conventional method. In the traditional labeling method, Ferumoxytol (final concentration 50 μg Fe/ml) is added to the cell culture medium after MSC expansion. Using the traditional method, the intracellular iron concentration is 0.56 ± 0.29 pg Fe/MSC (also indicated by Prussian blue iron staining as shown in Figure 2A). Using our new method, the iron concentration increases to 2.50 ± 0.50 pg Fe/MSC (also indicated by Prussian blue iron staining as shown in Figure 2B), which is comparable to that obtained with the use of HPF nanocomplexes (2.12 ± 0.11 pg Fe/human MSC) (Thu et al., 2012).
SUPPORT PROTOCOL. CHARACTERIZATION OF PREPARED AND LABELED MSCs
Trypan Blue exclusion assay
-
1
Add 0.9 ml of Trypan Blue solution (0.4% w/v; Sigma-Aldrich, cat. no. T8154) to a test tube. Add 0.1 ml of cell suspension and mix thoroughly. Allow to stand for 5 min.
-
2
With the cover slip in place, load 10 μl of Trypan Blue-cell suspension into a hemacytometer (Sigma-Aldrich, cat. no. Z359629). Do not overfill or underfill the chamber.
-
3
Examine immediately under a microscope at low magnification.
-
4Count the number of blue staining cells and the total number of cells. Dead (non-viable) cells take up the dye and show a blue color.
-
5
Calculate the total number of cells.
Flow cytometry analysis of MSCs: the presence of monocytes/macrophages
The purity and phenotype of MSCs prepared through the “direct adherence” method have been investigated in our previous publication (Chen et al., 2011a). Briefly, MSCs are stained with CD166, CD105, CD44, CD29, MHC-I, and CD34. Flow cytometry results show that the purity of the MSCs is 92–95%. In this new labeling protocol, we are extremely cautious about the presence of phagocytic cells, i.e., monocytes/macrophages, in our labeled MSCs. Thus, we have tested for the presence or contamination of monocytes/macrophages by staining with ED1, which is the rat homologue of human CD68.
NOTE: The expression of the ED1 antigen is predominantly cytoplasmic. Leucoperm™ (AbD SeroTec, cat. no. BUF09) is used as a permeabilization reagent for ED1 detection.
Collect the Ferumoxytol labeled MSCs from step 8 of Basic Protocol 2 by trypsin-EDTA digestion. Spin down the cells at 350 g for 5 min. Wash the MSCs with PBS twice.
Incubate 5 × 105 collected cells with 100 μl of Reagent A (Fixation Medium in the Leucoperm™ kit) for 15 min.
Wash with 3 ml of PBS and centrifuge for 5 min at 350 g. Remove the supernatant.
Resuspend the cells in 100 μl of Reagent B (Permeabilization Medium).
Immediately add 10 μl of mouse anti-rat ED1:Alexa Fluor 647 antibody (Mouse anti-rat ED1: Alexa Fluor 647 antibody (AbD SeroTec, cat. no. MCA341A647). Mouse IgG1: Alexa Fluor 647 (BioLegend) is used as our isotype control. Vortex and incubate for 30 min at room temperature.
Flow cytometry is performed on a FACSVantage (Becton Dickinson). The data are processed with the use of FlowJo software (TreeStar).
Perls’ Prussian blue iron staining
The labeling efficiency of MSCs by Ferumoxytol through our new “bio-mimicry” method is examined by Perls’ Prussian Blue Iron Staining assay by using the iron staining kit purchased from Sigma-Aldrich (cat. no. HT20).
Prepare 20 ml of Working Iron Stain Solution by mixing 10 ml of Potassium Ferrocyanide Solution (potassium ferrocyanide, 4% w/v) and 10 ml of Hydrochloric Acid Solution (hydrochloric acid, 1.2 mmol/l) in a 50-ml tube.
-
Prepare 25.5 ml of Working Pararosaniline Solution by adding 0.5 ml of Pararosaniline Solution (pararosaniline hydrochloride, 1% w/v) to 25 ml of Nanopure water in a 50-ml tube.
This solution needs to be prepared fresh daily. Use once and discard. Add 1 ml of Working Iron Stain Solution to each well of cells (from step 7 of Basic Protocol 2) and co-incubate for 5 min at room temperature.
Draw off the supernatant and wash the cells with 1.5 ml of Nanopure water three times.
Counterstain in Working Pararosaniline Solution (1 ml per well) for 3 min at room temperature.
-
Draw off the supernatant and wash the cells with 1.5 ml of Nanopure water three times. The cells are ready to be studied by using an inverted light microscope.
The background is much cleaner if the cells are kept in 1.5 ml of Nanopure water at room temperature for 2 hr.
MR microscopy of Ferumoxytol labeled MSCs
-
Prepare 1% agarose gel (w/v; see recipe) and degas for at least 30 min.
Air bubbles in the gel will give dark spots in the T2* weighted MRI, thus producing artificial results. Degasing is very important for the preparation of cell-gelatin phantoms. When the temperature of the agarose gel decreases to 35 °C to 40 °C (the flask feels warm to the touch), mix 2 × 105 MSCs with 1 ml of gel evenly by pipetting up and down a few times.
-
Transfer the cell-gel mixture into a 5-mm NMR tube before it solidifies.
Avoid producing air bubbles during mixing and transfer. NMR tubes need to be cut to ~2 inches long for easier transfer of the cell-gel mixture. High-resolution 3D MR images are acquired with a Bruker 11.7-T scanner, equipped with a Micro 2.5 gradient set (Biospec, Avance-DBX, Bruker) with the following parameters: repetition time (TR) = 2500 ms; echo time (TE) = 7.5 ms; number of averages (NA) = 4; and isotropic resolution = 55 μm.
TEM
Fix MSCs in a fixation solution (2% paraformaldehyde buffered with PBS, see recipe) at 4°C overnight, followed by washing with PBS twice.
Fix the cells in 1% OsO4 buffered with PBS for 1 hr and then wash the cells three times with distilled water.
Dehydrate the samples using a gradient series of ethanol and embed the samples in an Epon-Araldite resin. 100-nm sections are cut using a DDK diamond knife on a Reichert-Jung Ultracut-E ultramicrotome (Leica, Wetzlar, Germany). The sections are not stained with lead citrate or uranyl acetate.
Mount the sections onto copper grids and image the cells on a Hitachi 7100 transmission electron microscope (Pleasanton, CA) operated at 75 kv.
Measurement of iron concentrations by ICP-MS
Add 70% nitric acid (250 μL) to 2 × 106 cells. Record the weight of the 70% nitric acid added and the total sample weight.
Incubate at 60 °C overnight.
Centrifuge the samples at 400 g for 15 min and collect the supernatant in a separate test tube.
-
Dilute samples with 2% HNO3 (prepared with Nanopure water) to reach the final concentration in the range of 0.02 to 1 part per million (ppm) with respect to iron. Record the mass of 2% HNO3 added.
If the iron concentration in the cells is expected to be low (e.g., unlabeled MSCs, or MSCs labeled with the traditional method), adjust the final volume to ~2 ml. Concentrated acid can damage the ICP; therefore samples with a higher iron concentration must be diluted further for a safe measurement. Analyze samples for iron concentration by ICP-MS (NexION 300X, PerkinElmer Inc.). Calculate the iron concentration per cell.
REAGENTS AND SOLUTIONS
Complete Dulbecco’s modified Eagle’s medium (DMEM)
Supplement Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific, cat. no. 12491-015) with 10% heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific, cat. no. 16000044), 100 U/ml penicillin and 100 μg/ml streptomycin (Penicillin and streptomycin mixture; GIBCO, cat. no. 15140), and 2 mM glutamine (GIBCO, cat. no. 25030). Complete DMEM can be stored for 1 week at 4 °C.
Heat inactivation of FBS
Prepare a 56 °C water bath. Allow FBS to warm up to 56 °C and heat for 30 min.
Preparation of Ferumoxytol stock solution
Prepare Ferumoxytol stock solution (3 mg Fe/ml) by adding 100 μl of Ferumoxytol (original concentration 30 mg Fe/ml) to 900 μl of sterilized Nanopure water. Mix well.
Agarose gel 1% (w/v)
Dissolve 1 g of low melting point agarose (Fisher Scientific, cat. no. BP16525) in 100 ml of Nanopure water in a 250-ml glass flask. Melt the agarose by heating on a hot plate with stirring. The gel can be stored at room temperature indefinitely. Degas for at least 30 min each time before use.
Fixation solution (2% paraformaldehyde)
Formaldehyde is toxic. All the following procedures are conducted inside a fume hood. Gloves and safety glasses are worn. For 1000 ml of 2% formaldehyde, add 800 ml of PBS to a glass beaker on a stir plate in a ventilated hood. Heat while stirring to approximately 60 °C. Make sure that the solution does not boil. Add 20 g of paraformaldehyde powder to the heated PBS solution. Slowly raise the pH by adding 1 N NaOH drop wise from a pipette until the solution clears. Once paraformaldehyde is dissolved, the solution should be cooled and filtered. Adjust the volume of the solution to 1000 ml with PBS. Adjust the pH to approximately 6.9. The solution can be aliquoted and frozen or stored at 2–8 °C for up to one month.
COMMENTARY
Background Information
MSCs are among the major stem cells used for cell therapy and regenerative medicine (Chen et al., 2011b, Wang et al., 2015, Squillaro et al., 2016). Scientists have studied the isolation, culture, expansion, characterization, and therapeutic applications of MSCs for many years (Friedenstein et al., 1968, Nadri et al., 2007, Bianco 2014, Zhang et al., 2014, Karantalis et al., 2015). About 500 MSC trials have been carried out (Squillaro et al., 2016). One of the challenges facing current stem cell therapies is that individual variation is significant during clinical trials (Bang et al., 2005, Alper 2009, Bolli et al., 2011, Hare et al., 2012, Perin et al., 2012, Heldman et al., 2014, Nguyen et al., 2014). It is possible that the transplanted cells did not engraft or survive in those trials that did not respond to therapy. In-vivo cell tracking by MRI provides an effective way to answer these crucial questions and to improve current clinical trial protocols. In order to track transplanted MCSs in the body, MSCs need to be labeled with an FDA-approved agent. The only FDA-approved SPIO particle, Ferumoxytol (Feraheme®), has been applied as the contrast agent in several clinical trials for cellular therapy (https://clinicaltrials.gov). Our laboratory is very interested in developing novel methods for labeling and tracking cells with superparamagnetic iron-oxide particles as MRI contrast agents. We have also developed a new MRI contrast agent that can label MSCs efficiently (Chen et al., 2011a). The present protocol, aiming to develop a new method to label MSCs with Ferumoxytol with no need of using transfection agents, is derived from our recent discoveries (Liu et al., 2016).
The procedure for ex-vivo expansion of MSCs greatly affects MSC properties, thus is a critical issue for MSC therapy (Hoch et al., 2015, Squillaro et al., 2016). Phenotype and functional of differences between minimally-cultured MSCs (2 hr) and conventionally-cultured MSCs (7 days or longer) have been reported (Kumamoto et al., 2009, Ng et al., 2014). Notably, cell size has been found to be an important characteristic of MSCs (Colter et al., 2000, Colter et al., 2001, Christodoulou et al., 2013, Ng et al., 2014). Smaller MSCs exhibit better self-renewal and differentiation capacity and bigger MSCs show signs of senescence (Christodoulou et al., 2013, Estrada et al., 2013). Recently, it has been found that the gene expressions of STRO-1, TWIST-1 and DERMO-1 are correlated with the cell size and potency of MSCs (Samsonraj et al., 2015). In our study (Liu et al., 2016), we have found that MSCs gradually enlarge the cell size (17.2 ± 1.9 μm on day 4 of culture vs. 32.0 ± 7.2 μm on day 7) and lose the capability to take up Ferumoxytol (3.2 ± 0.6 pg Fe/MSC on day 4 vs. 0.56 ± 0.29 pg Fe/MSC on day 7) during the ex-vivo cell culture and expansion.
Our new protocol makes use of the “in-vivo environment” of MSCs to recover their phagocytic activity and to re-size them. The “in-vivo environment” indicates the non-MSC components in the bone marrow extractions, namely “non-adherent cells and supernatant” (Figure 1, Basic Protocol 2). We use these non-MSC components to mimic the cellular environment of MSCs, thus we also call this “transfection-free method” as “bio-mimicry method”. Historically, there are two published methodologies to label MSCs with Ferumoxytol: (i) the ex-vivo labeling method using protamine and hepartin to form an HPF nanocomplex (Thu et al., 2012). MSCs show an iron content of 2.12 ± 0.11 pg Fe/human MSC. The advantage of this method is that it is a general one and can be used to label different types of cells with Ferumoxytol, e.g., MSCs, neural stem cells, hematopoietic stem cells, T-cells, and monocytes. However, the addition of transfection agents could cause undesired effects; (ii) the in-vivo labeling method by administering rats intravenously a dose of 28 mg of iron per kilogram of Ferumoxytol 48 hr before extraction, resulting in an iron content of 4.28 ± 0.19 pg Fe/MSC (Khurana et al., 2013). The advantage of this method is that it reduces the risk of contamination and biologic alterations of the MSCs between harvest and transplantation. However, this approach is not applicable to autologous MSC transplants for cell-tracking studies, because the macrophages in the MSC donor will also be labeled with Ferumoxytol and cannot be distinguished from the transplanted MSCs. This method is also not applicable to procedures requiring cell expansion, because cell divisions will dilute the Ferumoxytol label to below cellular MRI detection levels. More importantly, this in-vivo labeling method indicates that MSCs are phagocytic in nature and can take up Ferumoxytol. However, during ex-vivo cell culture and expansion, MSCs become “less phagocytic” and lose the ability to take up Ferumoxytol.
The “in-vivo environment” in our new protocol can help prepare more “native” MSCs: (i) to restore the phagocytic activity of cultured MSCs, so that MSCs can be labeled by Ferumoxytol (2.50 ± 0.50 pg Fe/MSC) after cell culture and expansion, with no need of transfection agents and/or electroporation; (ii) to re-size the cultured MSCs (32.0 ± 7.2 μm vs 19.5 ± 5.2 μm). Our protocol can be very useful for preparing MSCs and labeling with Ferumoxytol, improving MSC-tracking by MRI in both pre-clinical and clinical studies.
Critical Parameters and Troubleshooting
During the preparation of MSCs or “in-vivo environment” (non-adherent cells and supernatant), untreated whole bone marrow adherent culture should be used. The conditions for MSC isolation and culture have been studied for many years and controversies are reported (Horn et al., 2008, Soleimani & Nadri 2009, Peterbauer-Scherb et al., 2010, Al Battah et al., 2011). A recent study systematically compared several conditions to isolate and culture MSCs: untreated whole bone marrow adherent culture, 3 volumes of red blood cells (RBC) lysed with ammonium chloride, 6 volumes of RBC lysed with ammonium chloride, and Ficoll density gradient centrifugation (Zhang et al., 2014). It was found that the untreated whole bone marrow adherent cultures, which contain the MSC “in-vivo environment” as described in this protocol, are best for MSC isolation and culture and the resulting cells have the strongest proliferation capacity. The Ficoll purified cultures, which eliminate most of the “in-vivo environment”, give the weakest proliferation capacity. In this preparation, if a lot of cell debris, clumps, or bone spicules are observed in the bone marrow extraction, a 70-mm filter mesh can be used to remove them. Our method also shows advantage compared to the recent published in-vivo labeling method (Khurana et al., 2013). The labeled MSCs from the in-vivo labeling method (Khurana et al., 2013) cannot be expanded to reach the large cell number required for clinical use, because cell expansion and division will dilute the SPIO labeling. However, our new method allows MSCs expansion in cell culture prior to Ferumoxytol labeling, thus solving the above problem.
Anticipated Results
The labeling efficiency of MSCs by Ferumoxytol through this protocol is 2.50 ± 0.50 pg Fe/MSC. The cell size is 19.5 ± 5.2 μm. The majority of Ferumoxytol nanoparticles are localized in the cytoplasmic vacuoles of MSCs. The Ferumoxytol-labeled MSCs from our new method show over 95% viability. We have not observed cell aggregation after Ferumoxytol labeling. The impurity from monocytes/macrophages is ~2%.
Time Considerations
It takes ~4 hr to isolate femurs and tibias and to extract bone marrow cells. In this study, we culture the MSCs for 7 days. If more cells are needed, MSCs can be cultured and expanded for a longer time. On the day of labeling, the procedures will take ~3 hr. On the harvest day, the procedures will take ~2 hr.
Significance Statement.
Mesenchymal stem cells (MSCs) have generated great interest for regenerative medicine. In-vivo tracking of engrafted MSCs by magnetic resonance imaging (MRI) can provide needed information for cell therapy, ensuring that the MSCs engraft and survive after transplantation. Several clinical trials for cell therapy have incorporated MRI cell tracking into their trial protocols, using the only FDA-approved superparamagnetic iron-oxide particle, Ferumoxytol (Feraheme®), as the contrast agent. However, Ferumoxytol does not effectively label MSCs ex vivo; thus transfection agents have to be used. Another challenge facing MSC research is that MSC phenotype and function can change during ex-vivo expansion. In this unit, we describe a new “bio-mimicry” method to prepare more “native” MSCs to help resolve the two above-mentioned problems.
Acknowledgments
This work is supported by grants from the National Institutes of Health (P41EB-001977). We thank Dr. Chih-Lung Chen from MegaPro Biomedical Co. Ltd for excellent suggestions in the MSC culture experiment. We thank Ms. Lanya Tseng (Carnegie Mellon University), Dr. Yijen L. Wu (University of Pittsburgh), Dr. Qing Ye (University of Pittsburgh), Dr. Daniel J. Bain (University of Pittsburgh), and Mr. Joseph P. Suhan (Carnegie Mellon University) for their assistance and suggestions in MRM, ICP-MS, and TEM experiments. We also thank Dr. E. Ann Pratt for helpful discussions to improve this unit.
Literature Cited
- Al Battah F, De Kock J, Ramboer E, Heymans A, Vanhaecke T, Rogiers V, Snykers S. Evaluation of the multipotent character of human adipose tissue-derived stem cells isolated by Ficoll gradient centrifugation and red blood cell lysis treatment. Toxicol In Vitro. 2011;25:1224–1230. doi: 10.1016/j.tiv.2011.05.024. [DOI] [PubMed] [Google Scholar]
- Alper J. Geron gets green light for human trial of ES cell-derived product. Nat Biotechnol. 2009;27:213–214. doi: 10.1038/nbt0309-213a. [DOI] [PubMed] [Google Scholar]
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- Bashir MR, Bhatti L, Marin D, Nelson RC. Emerging Applications for Ferumoxytol as a Contrast Agent in MRI. Journal of Magnetic Resonance Imaging. 2015;41:884–898. doi: 10.1002/jmri.24691. [DOI] [PubMed] [Google Scholar]
- Bianco P. "Mesenchymal" stem cells. Annu Rev Cell Dev Biol. 2014;30:677–704. doi: 10.1146/annurev-cellbio-100913-013132. [DOI] [PubMed] [Google Scholar]
- Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chen CL, Zhang H, Ye Q, Hsieh WY, Hitchens TK, Shen HH, Liu L, Wu YJ, Foley LM, Wang SJ, Ho C. A new nano-sized iron oxide particle with high sensitivity for cellular magnetic resonance imaging. Mol Imaging Biol. 2011a;13:825–839. doi: 10.1007/s11307-010-0430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PM, Yen ML, Liu KJ, Sytwu HK, Yen BL. Immunomodulatory properties of human adult and fetal multipotent mesenchymal stem cells. J Biomed Sci. 2011b;18:49. doi: 10.1186/1423-0127-18-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou I, Kolisis FN, Papaevangeliou D, Zoumpourlis V. Comparative Evaluation of Human Mesenchymal Stem Cells of Fetal (Wharton’s Jelly) and Adult (Adipose Tissue) Origin during Prolonged In Vitro Expansion: Considerations for Cytotherapy. Stem Cells Int. 2013;2013:246134. doi: 10.1155/2013/246134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A. 2000;97:3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci U S A. 2001;98:7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada JC, Torres Y, Benguria A, Dopazo A, Roche E, Carrera-Quintanar L, Perez RA, Enriquez JA, Torres R, Ramirez JC, Samper E, Bernad A. Human mesenchymal stem cell-replicative senescence and oxidative stress are closely linked to aneuploidy. Cell Death Dis. 2013;4:e691. doi: 10.1038/cddis.2013.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. [PubMed] [Google Scholar]
- Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, Foo CW, da Silva J, Anderson E, Schwarz R, Mendizabal A, Hare JM. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch AI, Leach JK. Concise review: optimizing expansion of bone marrow mesenchymal stem/stromal cells for clinical applications. Stem Cells Transl Med. 2015;4:412. doi: 10.5966/sctm.2013-0196erratum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn P, Bork S, Diehlmann A, Walenda T, Eckstein V, Ho AD, Wagner W. Isolation of human mesenchymal stromal cells is more efficient by red blood cell lysis. Cytotherapy. 2008;10:676–685. doi: 10.1080/14653240802398845. [DOI] [PubMed] [Google Scholar]
- Karantalis V, Hare JM. Use of Mesenchymal Stem Cells for Therapy of Cardiac Disease. Circulation Res. 2015;116:1413–1430. doi: 10.1161/Circresaha.116.303614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana A, Chapelin F, Beck G, Lenkov OD, Donig J, Nejadnik H, Messing S, Derugin N, Chan RC, Gaur A, Sennino B, McDonald DM, Kempen PJ, Tikhomirov GA, Rao J, Daldrup-Link HE. Iron administration before stem cell harvest enables MR imaging tracking after transplantation. Radiology. 2013;269:186–197. doi: 10.1148/radiol.13130858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto M, Nishiwaki T, Matsuo N, Kimura H, Matsushima K. Minimally cultured bone marrow mesenchymal stem cells ameliorate fibrotic lung injury. Eur Respir J. 2009;34:740–748. doi: 10.1183/09031936.00128508. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang Y, Qi G. Evaluation of isolation methods and culture conditions for rat bone marrow mesenchymal stem cells. Cytotechnology. 2013;65:323–334. doi: 10.1007/s10616-012-9497-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Tseng L, Ye Q, Wu YL, Bain DJ, Ho C. A New Method for Preparing Mesenchymal Stem Cells and Labeling with Ferumoxytol for Cell Tracking by MRI. Sci Rep. 2016;6:26271. doi: 10.1038/srep26271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21:216–225. doi: 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadri S, Soleimani M, Hosseni RH, Massumi M, Atashi A, Izadpanah R. An efficient method for isolation of murine bone marrow mesenchymal stem cells. Int J Dev Biol. 2007;51:723–729. doi: 10.1387/ijdb.072352ns. [DOI] [PubMed] [Google Scholar]
- Nejadnik H, Lenkov O, Gassert F, Fretwell D, Lam I, Daldrup-Link HE. Macrophage phagocytosis alters the MRI signal of ferumoxytol-labeled mesenchymal stromal cells in cartilage defects. Sci Rep. 2016;6:25897. doi: 10.1038/srep25897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CP, Sharif AR, Heath DE, Chow JW, Zhang CB, Chan-Park MB, Hammond PT, Chan JK, Griffith LG. Enhanced ex vivo expansion of adult mesenchymal stem cells by fetal mesenchymal stem cell ECM. Biomaterials. 2014;35:4046–4057. doi: 10.1016/j.biomaterials.2014.01.081. [DOI] [PubMed] [Google Scholar]
- Nguyen PK, Riegler J, Wu JC. Stem cell imaging: from bench to bedside. Cell Stem Cell. 2014;14:431–444. doi: 10.1016/j.stem.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noad J, Gonzalez-Lara LE, Broughton HC, McFadden C, Chen Y, Hess DA, Foster PJ. MRI tracking of transplanted iron-labeled mesenchymal stromal cells in an immune-compromised mouse model of critical limb ischemia. NMR Biomed. 2013;26:458–467. doi: 10.1002/nbm.2884. [DOI] [PubMed] [Google Scholar]
- Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, Silva GV, Lai D, Thomas JD, Kronenberg MW, Martin AD, Anderson RD, Traverse JH, Penn MS, Anwaruddin S, Hatzopoulos AK, Gee AP, Taylor DA, Cogle CR, Smith D, Westbrook L, Chen J, Handberg E, Olson RE, Geither C, Bowman S, Francescon J, Baraniuk S, Piller LB, Simpson LM, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD Cardiovascular Cell Therapy Research N. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA. 2012;307:1717–1726. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterbauer-Scherb A, van Griensven M, Meinl A, Gabriel C, Redl H, Wolbank S. Isolation of pig bone marrow mesenchymal stem cells suitable for one-step procedures in chondrogenic regeneration. J Tissue Eng Regen Med. 2010;4:485–490. doi: 10.1002/term.262. [DOI] [PubMed] [Google Scholar]
- Samsonraj RM, Rai B, Sathiyanathan P, Puan KJ, Rotzschke O, Hui JH, Raghunath M, Stanton LW, Nurcombe V, Cool SM. Establishing criteria for human mesenchymal stem cell potency. Stem Cells. 2015;33:1878–1891. doi: 10.1002/stem.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009;4:102–106. doi: 10.1038/nprot.2008.221. [DOI] [PubMed] [Google Scholar]
- Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25:829–848. doi: 10.3727/096368915X689622. [DOI] [PubMed] [Google Scholar]
- Thu MS, Bryant LH, Coppola T, Jordan EK, Budde MD, Lewis BK, Chaudhry A, Ren J, Varma NR, Arbab AS, Frank JA. Self-assembling nanocomplexes by combining ferumoxytol, heparin and protamine for cell tracking by magnetic resonance imaging. Nat Med. 2012;18:463–467. doi: 10.1038/nm.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WB, Yen ML, Liu KJ, Hsu PJ, Lin MH, Chen PM, Sudhir PR, Chen CH, Chen CH, Sytwu HK, Yen BL. Interleukin-25 Mediates Transcriptional Control of PD-L1 via STAT3 in Multipotent Human Mesenchymal Stromal Cells (hMSCs) to Suppress Th17 Responses. Stem Cell Rep. 2015;5:392–404. doi: 10.1016/j.stemcr.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhang F, Shi H, Tan R, Han S, Ye G, Pan S, Sun F, Liu X. Comparisons of rabbit bone marrow mesenchymal stem cell isolation and culture methods in vitro. PLoS One. 2014;9:e88794. doi: 10.1371/journal.pone.0088794. [DOI] [PMC free article] [PubMed] [Google Scholar]