ABSTRACT
Apolipoprotein B mRNA Editing Catalytic Polypeptide-like 1 or APOBEC1 was discovered in 1993 as the zinc-dependent cytidine deaminase responsible for the production of an in frame stop codon in apoB mRNA through modification of cytidine at nucleotide position 6666 to uridine. At the time of this discovery there was much speculation concerning the mechanism of base modification RNA editing which has been rekindled by the discovery of multiple C to U RNA editing events in the 3′ UTRs of mRNAs and the finding that other members of the APOBEC family while able to bind RNA, have the biological function of being DNA mutating enzymes. Current research is addressing the mechanism for these nucleotide modification events that appear not to adhere to the mooring sequence-dependent model for APOBEC1 involving the assembly of a multi protein containing editosome. This review will summarize our current understanding of the structure and function of APOBEC proteins and examine how RNA binding to them may be a regulatory mechanism.
KEYWORDS: ADAR, adenosine deaminase active on RNA, antiviral, APOBEC, apolipoprotein B editing catalytic subunit, ADAT, adenosine deaminase active on tRNA, cancer, cytidine deaminase, DNA mutation, epigenetics, HIV, human immunodeficiency virus, host defense, lncRNA, long noncoding RNA, mRNA, miRNA, microRNA, retrovirus, RNA editing, RNA modification
Historical preface: What if any distinction is there between RNA editing and RNA modification
Throughout the 3 domains of life there are over one hundred ribonucleotide modifications and several deoxyribonucleotide modifications identified in various classes of RNA as well as in genomic and organellar DNAs1-4 (Fig 1). The identification of RNA modifications and associated phenotypic studies predates the first use of the term RNA editing.4,5 Rob Benne introduced the term ‘RNA editing’ in the 1980s in reference to Trypanosome guide-RNA dependent, insertion and deletion of uridines within mitochondrial mRNAs (reviewed in6). Editing through numerous U insertions and deletions into mitochondrial transcripts precisely timed during the life cycle of the organism created the initiation codons, sense codons and open reading frames for many mitochondrial proteins and enabled mitochondrial function (reviewed in6,7). Subsequently, adenosine to inosine (A to I) and cytidine to uridine (C to U) base transitions through deamination in mRNAs of plants, insects and mammals became known as mRNA editing as these too created translation start codons, sense changes, nonsense codons as well as premRNA splice junctions (reviewed in8,9).
Figure 1.
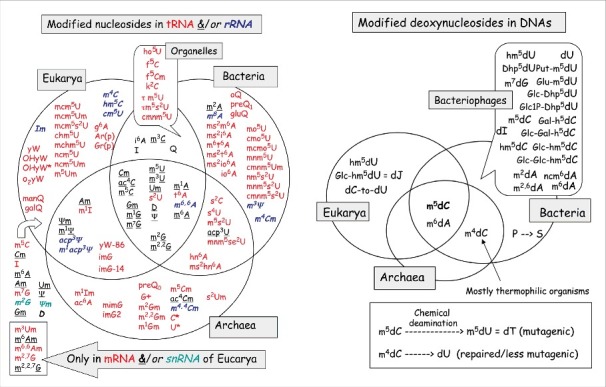
RNA Modifications Unique and Common to the Three Orders of Life. The Venn diagram shows the known modifications of ribonucleotides (indicated by standard abbreviations) found in naturally occurring RNAs in Archea, Bacteria and Eukarya. Nucleotide modifications common to 2 or all orders of life are indicated in the overlapping areas. Abbreviations for the modification are provide in.4 Figure modified from Figs. 4 and 6 in reference 4 (Landes BioSciences).
Adenosine deaminase active on RNA (ADAR)10,11 and adenosine deaminase active on tRNA (ADAT)12 are responsible for A to I editing and have deaminase domains that are structurally related to, but distinct from, those of apolipoprotein B mRNA editing catalytic subunit (APOBEC) family of cytidine deaminases13,14 responsible for C to U editing.8 ADAR1 and ADAR2 were first shown to edit mRNAs encoding glutamate and kainate receptor gated ion channels in excitatory tissue.6,7,15,16 In different excitatory tissues and through the brain mRNAs encoding ion channel subunits may not all be edited, nor are they edited at the same sites. The resulting protein heterogeneity in subunits comprising the multi subunit ion channels for Ca2+ and K+ enables regional control of plasticity in ion conduction rates and ion gradient recovery rates. APOBEC1 (A1) editing of the mRNA encoding apolipoprotein B created protein variants that are required for serum transport and tissue uptake of fats and cholesterol. ApoB protein variants have markedly different half lives in the blood and therefore have different implications in the production of low density lipoproteins (LDL) and atherogenic diseases.8,17
Both ADAR and APOBEC catalytic domains known as the zinc-dependent deaminase domain (ZDD) require a protein fold that coordinates zinc through histidine and cysteine residues and places a glutamic acid for proton shuttling proximal to the adenosine/cytosine targeted for deamination.13,18-23 The structure of APOBEC3F (A3F) C-terminal deaminase domain in the absence of zinc suggested that metal chelation is not essential for the general fold of the ZDD.23 ADAR1, ADAR2 and ADAT1 contain one or more double stranded RNA binding domains with selective affinities for imperfect RNA duplexes that contain the target adenosine to be edited; and hence edit different RNA substrates.10,11,24 ADAT2 and ADAT3 have no recognizable double-stranded RNA binding domains. In contrast, A1 has weak affinity for RNA25 and is moored to the RNA editing site through its interaction with the RNA binding protein A1CF that recognizes a cis-acting, 11 nucleotide RNA recognition element (the mooring sequence) 3′ of the edited cytidine26,27 (reviewed in14,28). As will be discussed below, APOBEC deamination of dC in ssDNA may not require auxiliary proteins as they bind ssDNA with nanomolar affinity using residues surrounding catalytic cleft and residues along exposed surfaces adjacent to the catalytic domain.18,20,29-32
By 1997 ADARs and A1 became the mammalian paradigms of editing enzymes as a growing list of examples of A to I and C to U mRNA editing sites emerged from studies in plants, protozoa, insects and viruses.9 Since that time, numerous non-genomically encoded A to I and C to U transitions have been identified through advances in computation biology and RNA and DNA sequencing technology.33-39 Although the ADAR and APOBEC editing enzymes are likely to be responsible for transcriptome and genome wide A to I and C to U transitions, the functional significance of editing these nucleotides is usually not apparent. Sequence analyses show that under in vitro conditions ADAR and APOBEC family members can edit numerous sites within RNAs (and DNA in the case of AID/APOBEC) as long as they satisfy the nearest neighbor nucleotide and/or secondary structure requirements; so called ‘hot spots’. And yet the vast majority of sites that qualify as ADAR or APOBEC editing sites are not edited in vivo. So while under defined in vitro conditions the cis-acting requirements for ADAR and APOBEC editing site selection can be readily described, we do not understand why nucleotides predicted to be editing sites are or are not utilized in vivo.
It has become increasingly difficult to think of all of the sites affected by ADAR or APOBEC in the historical strict definition of a nucleic acid editing site; namely as a form of nucleotide modification that enables phenotypic diversity. One might speculate that most of these modifications are determined by random access of the enzymes to RNA or ssDNA regions of the genome made single stranded during RNA transcription and DNA replication. But this cannot entirely be true as the chemical nature and frequency of particular DNA and RNA sequences harboring these nucleotide modifications are known to vary with changes in physiologic or disease states,16,33,34,40-44 reviewed in.14 Alternatively, if there is a clear phenotype associated with a modification, then there probably was an underlying regulatory process for modifying that site; hence it may be considered to be an editing event even though the function of other nucleotide modifications that occur simultaneously might not be clearly purposeful.
As case in point, the APOBEC field discovered early on that overexpression of editing enzymes for experimental purposes can lead to high levels of editing at physiologically relevant sites but also hyperediting of multiple RNAs that otherwise would not have been significantly edited45 and promiscuous editing of multiple cytidines proximal to the native editing site.46 Activation induced deaminase (AID) in the APOBEC family (described in greater detail below) is responsible for the diversity of antibodies that can be produced as part of the acquired immune system.47 Upon B lymphocyte activation, AID induces numerous dC to dU transitions within genomic DNA encoding the variable region of the immunoglobulin locus and within the immunoglobulin class switch region.47-49 In each activated B cell the deoxycytidines deaminated to deoxyuridine are repaired to any one of the 4 bases. This leads to a large diversity of new functionalities in immunoglobulins expressed within the activated B cell population in germinal centers throughout the body.47,50-52 While there are potentially numerous nucleotide modifications within some of the activated B cells that do not enable expression of functional immunoglobulins, AID modification of DNA produces diversity in the proteome and therefore arguably is DNA editing. Moreover, despite exquisite regulation of this process, there are numerous mutations within genomic DNA with AID/APOBEC signature nearest neighbor preferences.33,53 In some instances these ‘hyperediting’ sites lead to alterations in protein expression and are associated with particular cancers and cancer progression (reviewed in14,54,55).
Broadening the inclusivity of the RNA editing nomenclature, the functionality of several tRNAs is enabled by A to I transitions catalyzed by ADAT affecting codon structure, codon usage or tRNA availability.1,56 In addition, A to I transitions catalyzed by ADARs disrupts base pairing in RNA secondary structure such as those required for recognition and processing of microRNAs (miRNA) by the RISC complex.24,40,57 Therefore RNA editing also might be an appropriate nomenclature for the reduction of miRNA abundance by ADARs leading to altered gene expression through chromatin modification and condensation40,58-60 as well as reduction in the capacity of cells to produced miRNA that silence mRNA.24,40,61 ADAR colocalization with long noncoding (lnc) RNAs and unspliced HIV RNA in nuclear para speckles catalyzes A to I transitions in lncRNA secondary structure. This may be RNA editing as well based on the hypothesis that it is essential for controlling nuclear export of unspliced HIV RNA to the cytoplasm for translation of viral proteins and viral packaging.62,63 Future research no doubt will show that there are additional instances where nucleotide modifications in RNA (or DNA) lead to a gain or loss of function. Should these nucleic acid modifications be thought of as RNA editing once their functional consequences have been identified? For the moment, the field has taken a ‘middle of the road’ position in adopting the moniker ‘editing and modification’ when referring to all of these nucleotide transitions in RNA and DNA.
Development of the APOBEC field through the discovery of new genes and new editing mechanisms
In the decade spanning the discovery of apoB mRNA C to U editing and APOBEC1 (A1) as the enzyme responsible for this base modification editing, a question frequently posed by the scientific community was why would an apparently dedicated enzyme like A1 be conserved in mammalian evolution given that it catalyzes a single editing event in only one mRNA? If the truncated protein product expressed from edited mRNA is important, why over time has the C to U change responsible for the nonsense mutation not have been selected in the apoB gene? The answer put forth then and still in use today is that editing affords tissue-specific flexibility in protein expression and the ability to regulate the proportion of the ApoB proteins translated from edited and unedited mRNA; and consequently modulate serum lipid transport through metabolic and developmental regulation (reviewed in8,17).
A broader role for A1 in regulating protein diversity, mRNA expression and stability was suggested in the discovery of A1-dependent editing of the mRNAs encoding the tumor suppressor NF164 and the translation repressor factor eIF4G45 along with numerous C to U edits within the 3′ UTRs of RNAs36 (see other predicted mRNA substrates in65,66). The biological role of APOBEC proteins has come under the spotlight again following the discovery that A1 is one of 11 proteins in a family of cytidine deaminase active on nucleic acids.14,67 Many of proteins in the APOBEC family have essential deaminase activity-dependent and deaminase activity-independent functions in determining innate and acquire immunity, host cell antiviral defense65,68 and if unregulated could become oncogenic33,34,48,54,55,69,70 or potential reduce cancers by enabling immune surveillance.71
The ability to bind to nucleic acids and to catalyze dC to dU base modification on single stranded DNA is a family characteristic that many but not all of the APOBEC family members have in common,53,67,72,73 reviewed in.74 The reader is referred to recent reviews for the structures and functions of the APOBEC family13,14,34 as this review will provide only a brief overview of the APOBEC family for context in establishing the hypothesis that RNA binding to APOBEC proteins regulates their deaminase activity.
There are 11 known members in the human APOBEC family
A1 and AID are both encoded on human chromosome 12. A1 expression is most abundant in mammalian small intestine and liver.75 Expression of mammalian AID is highly regulated in B lymphocytes within germinal centers in response to foreign antigens. Its tissue-specific expression and ssDNA mutagenic activity of the immunoglobulin gene locus are essential in determining adaptive immunity through class switch recombination (CSR), somatic hypermutation (SMH) and gene conversion.47-49 Deletion of A1 has a weak phenotype in lipid metabolism in mice76 but loss of AID function leads to an autosomal recessive, immune disorder known as hyper-IgM syndrome (HIGM2) where IgM accumulates in the blood because class switch recombination cannot be performed.77 APOBEC2 (A2) and all 7 APOBEC3 proteins (A3A, A3B, A3C, A3D, A3F, A3G and A3H) are encoded on human chromosomes 6 and 22, respectively.67 A3 proteins have diverse deaminase activities on RNA and ssDNA; mostly related to the control of retroviruses and endogenous retroviral elements (13,39 and reviewed in14). APOBEC4 is encoded on chromosome 1.78 A2 and APOBEC4 (A4) have no known catalytic functions.78-80
AID and A2 may have been the ancestral genes from which all other APOBEC were derived. This may have occurred through gene duplication and divergence over the course of ∼500 million years of vertebrate evolution. AID ssDNA deaminase activity emerged with jawed fish and its DNA mutagenic activities would have been selected for in overcoming infections, immune surveillance in controlling cancer cell proliferation and maintaining homeostasis.53,70,81-84 For the A3 proteins, the leading hypothesis is that the driving force for A3 gene expansion and diversification was selective pressure on the innate immune system through the genotoxic effects resulting from infection with rapidly mutating retroviral genomes and/or transpositions of endogenous retroviral-like elements within the genome,38,84-92 reviewed in.14 This is an intriguing hypothesis considering present day diversification within the human APOBEC family, notably polymorphisms in A3C,93,94 the loss of A3B expression in various human populations55,93 and the expansion of alternatively spliced variants of AID in the progression of cancer95,96 and of A3H that have different capacities to suppress HIV infections88,97,98 or contribute to cancer.99 The importance of A3 in host cell defense is evident in that HIV-1 and -2 encode the accessory protein known as viral infectivity factor (Vif) whose primary function is to suppress the antiviral activity of A3C, 3D, 3F, 3G and 3H by mediating their ubiquitination and proteosomal degradation.68,88,94,97,100-102
APOBEC have a conserved catalytic domain
All APOBEC proteins can be identified through database searches by their signature zinc-coordinating deaminase domain (ZDD) motif (H x E—X25–30—PC X2–4 C) (Fig 2).8 The underlined residues are an absolute requirement for APOBEC binding to and use of zinc as a Lewis acid for a nucleophylic attack of the C4 position of cytidine or deoxycytidine during hydrolytic deamination.23 The ZDD is found within the tertiary fold comprising the cytidine deaminase catalytic domain that is composed of a 5-stranded mixed β-sheet that is stabilized by α helices packing against both faces of the β sheet due to their sequential arrangement as α1-β1-β2-α2-β3-α3-β4-α4-β5-α5-α6 in the primary sequence (Fig 2). This organization is conserved in the crystal structure of the yeast homolog of AID/A1 known as Cdd1 that has both RNA and ssDNA editing activity103,104 and with very little variation, is a key structural landmark observed in all known crystal or NMR structures of mammalian APOBEC.13,19,20,23,30,32,105-108 AID, A1, A2, A3A, A3C, A3H and A4 are single ZDD whereas A3B, D, F and G are dual deaminase domain proteins. With the possible exception of A3B and D, there is only one catalytically active deaminase domain in proteins with 2 ZDD. There is no explanation for the structural or catalytic advantage that single versus dual ZDD-containing proteins have although ssDNA substrates bind to residues in both domains in APOBEC with dual ZDD23,31,108,109 and both N- and C-terminal ZDD are required for robust ssDNA editing.109,110 The structure of APOBECs have the greatest divergence within the primary sequence and secondary structure of loop domains outside of the ZDD as well as their quaternary interactions such as homo multimerization, interaction with other cellular or viral proteins and nucleic acid binding that affects APOBEC oligomerization and subcellular distribution.14,74
Figure 2.
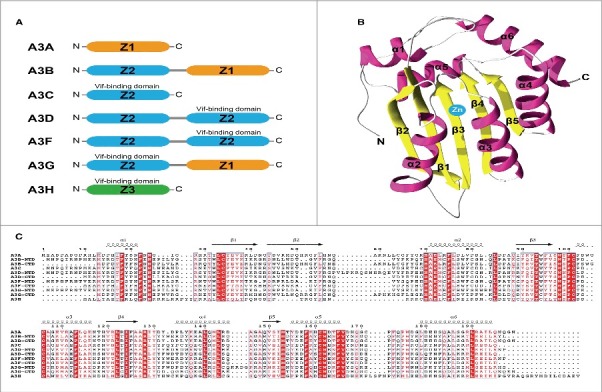
Structural Organization of Zinc Dependent Deaminase Domains and the Catalytic Fold for the APOBEC3 family. (A) Cartoon of the occurrence and position of evolutionarily related ZDD (color coded in orange, blue and green) in APOBEC3 a through H. Regions where the HIV Vif protein binds to A3 proteins is indicated. (B) Three dimensional fold of the A3 Z1 showing the distribution of α helices and β sheets relative to the catalytic zinc atom. (C) Primary amino acid sequence alignment of each individual ZDD domain in the A3 family showing the locations of conserved residues (in red vertical stripes) and homologous residues (in red text). Amino acids sequences forming α helices and β sheets are indicated about the text. Reproduced with permission from reference 13.
Most APOBEC have deaminase activity on ssDNA substrates
Nucleic acid binding is common to all APOBECs and with the exception of A2 and A4, all APOBEC will support in vitro deamination of deoxycytidine (dC) to deoxyuridine (dU) in ssDNA.67,72 Within single stranded DNA stretches of 25 nucleotides or longer dC's are targeted and deaminated do dU with apparently very lax nearest neighbor sequence preferences.111-114 The resultant dU's are repaired, first by rendering them to abasic sites and then through low fidelity of DNA repair converting them to dA, dC, dG or T.47,48,52 AID mutagenic activity targeting to the variable region and class switch region of transcriptionally active immunoglobulin genes may be facilitated through a mechanism that involves cofactor binding to DNA, AID post-translation modification or DNA structure restrictions.51,52,115-122
A3D, F, G and H provide host defense against retroviral infection by hypermutating proviral ssDNA during reverse transcription,72,101,123,124 reviewed in.125 Paleo-DNA databases reveal numerous dG to dA mutations throughout primate genomes with nearest neighbor target preferences characteristic of modern day A3 family deaminases.86,90,92 Evolutionary models predict that A3 proteins, especially A3G and A3F, have served a significant role as antiviral vanguards, in many species, for millions of years by mutating retroviral genomes and impairing their productive replication and retrotransposition.86,92,100,126-128
Crystal and NMR structures are only available for single ZDD APOBEC and for individual ZDD from double domain APOBEC. These have been modeled for ssDNA binding to A3B, 3F and 3G along a shallow groove on the protein surface punctuated with patches of positively charged (basic) and aromatic (hydrophobic) residues that respectively accommodate the negative charge on the backbone of nucleic acids backbone and enabling their stacking with the nucleic acid bases.13,20,29,30,109,129-131 In this regard the first crystal structures of A3B C-terminal half with bound dCMP130 and A3G N-terminus half with mono and oligo deoxynucleotides bound108 have begun to suggest the residues required to restrain nucleic acid in proximity to the catalytic site. A3B, 3F and 3G have greater processivity in deaminating multiple dC along ssDNA compared with A3A and this may be the result of a greater number of contacts made with ssDNA by their ZDD.109,132 Modeling suggests that single deaminase domain A3 such as A3A with fewer contacts with nucleic acid and may bend ssDNA substrates to position the targeted dC into the catalytic pocket.32 In this regard it is interesting that a rare S188I mutation in A3C expressed in a subset of Africans was shown to increase RNA bridged multimerization of the single domain A3C and activate of the mutant protein's deaminase activity on HIV-1.94
Mass spectroscopy of tryptic peptides from full length A3G cross linked to ssDNA 25 nt to 99 nt in length revealed that ssDNA binds to peptides within the C-terminus where the catalytic domain resides as well as to peptides within the N-terminus, containing the pseudo catalytic domain.31,133 Cross-linking coupled with mass spectroscopy peptide analysis suggested A3 residues that were bound to nucleic acid and corroborated biochemical studies suggesting that residues in the loop domains of both ZDD in dual deaminase domain A3 such as A3F were required for ssDNA binding and robust deaminase activity.109
APOBEC proteins commonly form homo multimers
The relative contribution of ssDNA in bridging APOBEC monomeric proteins to one another as higher-order oligomers vs. protein-protein interactions remains to be determined. Protein-protein interactions may alone be sufficient for homodimerization of A167,134,135 and A3F and A3G109,136-138 as these APOBEC bind to ssDNA as such multimers.109,114,139 Consistent with these data, in-cell fluorescence fluctuation spectroscopy demonstrated that A2, A3A, and A3C were monomeric in cells whereas A3B, A3D, A3F, A3G, as A3H were multimeric.50 The prerequisite of a homo oligomeric state for AID/APOBEC to recognize target dC and bind to ssDNA remains an area of active research.94,106,107,140-147 We do not known whether both subunits of a homodimer are directly bound to ssDNA or in fact whether the catalytic activity of holoenzyme complexes require more than one active site; in other words, how many ZDD are engaged to edit a single dC? What is more certain is that full length A3G monomers has approximately >100-fold higher affinity of ssDNA than does the C-terminal ZDD half molecule alone.29,109,141,148 The date suggest that APOBEC binding to ssDNA, the recognition of dC to be deaminated and movement of APOBEC along the ssDNA to adjacent editing sites requires more than the catalytic ZDD and the interaction with more than one APOBEC protein monomer.31,112-114,145
RNAs bound to APOBEC have important functions even when the RNAs are not substrates for deamination
After several years of controversy homomultimeric and heteromeric complexes of AID/APOBEC proteins have been accepted as critical for regulating APOBEC subcellular localization and their activity on nucleic acid substrates (reviewed in14,74). Almost every member of the AID/APOBEC family interacts with a variety of RNAs that bridge APOBEC monomers together to form megaDalton sized ribonucleoprotein particles (RNP) that also contain a variety of other RNA binding proteins involved in RNP structure and processing RNAs.31,94,149-158
Dual deaminase domain APOCECs were once thought to bind to RNA through their non-catalytic N-terminal CDA18,128,141,150,155,159-161 based on an early report suggesting that RNA and ssDNA bound to different termini.110 MS analysis of full length and native A3G cross linked to nucleic acid cross revealed that RNA bound to both N- and C-terminal peptides31 (Fig 3). RNA bound to the sites within the C-terminus of A3G that ssDNA also bound to. The N-terminal sites in A3G cross-linked exclusively to RNA were part of a continuous groove extending to the C-terminus and proximal to those that bound to both RNA and DNA in the C-terminus (Fig 3). The MS data and model in Fig. 3 were supported by data demonstrating that RNA displaced ssDNA from A3G and inhibited its deaminase activity in an RNA concentration-dependent manner.31,151 Given that RNA can bind to A3G at sites were ssDNA does not bind, the model suggested that in addition to being a competitive inhibitor, RNA ligands may noncompetitively inhibit ssDNA deaminase activity or have alternative structural or regulatory functions. It remains to be determined whether RNA and ssDNA can bind simultaneously to an APOBEC monomer or multimer.
Figure 3.
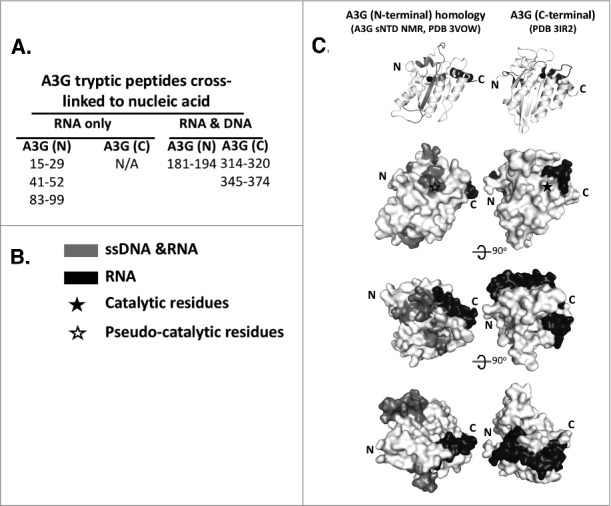
RNA and ssDNA Binding Surfaces on A3G. (A) Tryptic peptides of A3G bound to RNA or ssDNA were identified by mass spectroscopy following cross linking of native and full length A3G to short nucleic acids. (B) Peptides that only bound RNA (black) or bound to both RNA and ssDNA (gray) were mapped relative to the C-terminal ZDD catalytic domain and the N-terminal ZDD catalytically inactive pseudo-catalytic domain. (C) Grey scale coded RNA binding peptides and RNA and ssDNA binding peptides were mapped onto the NMR structure for the N-terminal ZCC and the crystal structure of the C-terminal ZDD of A3G shown as a ribbon diagram (top) and progressively rotated (top to bottom) space filling models. Black star and open star mark the location of the the catalytic and pseudo-catalytic ZDD, respectively. Reproduced with permission from reference 31.
A1 must interact with RNA binding proteins AC1F27,162-164 or RBM47165 for site-specific editing of apoB mRNA but A1 itself, has a low affinity for binding to RNA and is nonselective.25 A1 predominantly is a cytoplasmic protein125,166 sequestered with A1CF and apoB mRNA as editing-inactive 60S complexes along the exterior of the rough endoplasmic reticulum.27 Hormone regulated, reversible phosphorylation of A1CF enables nuclear import of A1164 and de novo assembly of 27S active editing complexes containing A1CF27 bound to unedited but spliced apoB mRNA.66,167 AID also must be imported to the nucleus for CSR and SHM125,168-170 and then is rapidly exported and degraded171,172 to control its mutagenic and potentially oncogenic activity.53,54,173,174 Regulation of AID occurs at many levels including alternative splicing173,174 and retention of AID in the cytoplasm through its interactions with HSP90,168,175 and cytoplasmic RNAs176 and binding to replication protein A.120
A3 proteins also form high molecular mass homo- and hetero- oligomers94,154,155,157,177-179 within the cytoplasm of cells. A3 complexes bridged by RNA form soon after A3 protein translation.179 This has led many in the field to conclude that A3 binding to RNAs only serves to inhibit A3 antiviral properties including their deaminase activity and assembly with viral particles.157,177-181 With additional studies the functional significance of A3 RNP formation became more nuanced. A3 binding to cellular coding and noncoding RNAs is now thought to be critical to maintaining their antiviral activity against HIV.150,153-156,182,183 Other studies have confirmed these interactions but concluded that A3 binding to HIV genomic RNA alone94,153,158,184 or with HIV p24 Gag protein185-188 is the most critical RNP for the assembly of A3D, F, G, and H with nascent viral particles. It remains unclear which residues within the N- and C-terminus bind to RNA but regional selectivity for RNA binding to A3G is supported by data showing that bulk cellular RNA and noncoding hY1 and hY3 RNAs required A3G residues W94 and W127 and to a lesser degree, S28 and Y124 but binding to Alu and 7SL RNAs did not require these residues.128 It is also apparent that A3 interaction with cellular RNAs is dynamic and reversible177,178 and upon viral infection can become more selective for HIV RNA and noncoding RNAs leading to their redistribution to cellular sites of viral particle assembly.153
Prospective on the APOBEC frontier
As the field pushes forward for structural characterization of APOBECs and their interactions with substrates and other macromolecules, an overarching focus has become the search for regulatory processes that limit or enhance APOBEC expression and their functions (reviewed in43,52,53,68,101). Given that the evolutionary forces leading to present day APOBEC proteins may be difficult to prove, a priority will be to understand the function of the present day APOBEC family. If we do come to appreciate the strengths and potential limitations of this family of proteins, there may be opportunities to use these enzymes in cell engineering and for the development of therapeutics for disease intervention.
From this perspective, there is a need to keep in mind what the native and biological context of APOBEC proteins and their enzymology is when inferring their functions from experimental systems. It can be very informative to overexpress cDNAs encoding APOBEC or mutants thereof in cells or animals to assess their maximum potential to deaminate viral or host cell genomic DNAs or bind to RNAs in cells. It is not appropriate to infer a proof of their biological role or activity in situ on nucleic acid substrates when the experimental expression of protein far exceeds that observed naturally. Promiscuous and hyperediting of substrates and the induction of neoplasia by overexpressing A1 has been known since 1996. The natural expression of A1 in small intestine is below western blot detection limits but edits 100% of the apoB mRNA produced in enterocytes. Following this analogy, many of the proposed antiviral roles for overexpressed A3 proteins and variants thereof that are naturally expressed at an intracellular abundance much lower than A3F and A3G may have to be reconsidered.
Many laboratories are pursuing computational methods and sequencing of genomic DNA and transcriptomes from cell types where AID/APOBEC are not only expressed but where changes in their expression or alternative apobec mRNA splicing correlate with phenotypic changes. These are important studies but beyond cataloging mutations, greater efforts need to be made in quantitative biology. Detecting APOBEC C to U editing in a particular RNA is undoubtedly of interest. What becomes biological significant is going on to quantify the proportion of edited RNAs in the transcriptome, determining whether the function of edited RNA and its translation product is altered and demonstrating how the frequency of editing site utilization varies in response to or drives cell phenotype.
How do APOBEC recognize editing sites in RNA and ssDNA? Other than the mooring sequence for A1 editing of RNA substrates, most of what we know suggests that AID/APOBEC prefer editing cytidine or deoxycytidine within single stranded regions of RNA or DNA with only lax requirements for the sequence immediately flanking the editing site. An area for future research will be to determine what are the intrinsic structural features of AID/APOBEC monomers of multimers that determine the selection of editing sites. We also will need to identify cellular auxiliary proteins, RNAs or post-translational modifications that determine selection of RNA and DNA substrates and editing site preferences. There is ample evidence that what every these control mechanisms are, they can be disrupted, and for AID, A1 and A3 proteins lead to disease-associated promiscuous editing within known targets and hyperediting of novel RNAs and chromosomal sites.
APOBEC enzymology, with few exceptions, is an underdeveloped area of research. The structural features of AID/APOBEC are being cataloged but high resolution structural determination of enzyme-substrate complexes and of full length APOBEC with 2 ZDD has not been achieved. There are conflicting reports as to whether AID/APOBEC monomers or multimers form before or after binding to nucleic acid substrates. An error in the past has been to conclude functionality of monomers or multimers of AID/APOBEC without reassessing the complexes that formed once the proteins had been added to nucleic acid substrates in a test tube. Holoenzyme complexes and the structural constraints they impose on the enzyme-substrate interactions are an important area for future enzymology research. While recent data for most APOBEC, particularly A3, are consistent with homomultimers being required for enhanced deaminase activity on ssDNA, addition studies will be required to determine the role of RNA binding and protein-protein interactions in multimerization.
New opportunities for discoveries exist in the area of the functional significance of RNAs that bind to AID/APOBEC as ligands that are not used as editing substrates. AID/APOBEC bound to a variety of coding and noncoding RNAs may have deaminase-independent functions in regulating endogenous retrovirus-like elements, redistributing A3 to the viral particle assembly process and sequestering RNAs in P-bodies and stress granules during a cellular response to changing environmental signals. Current data suggest that APOBEC interactions with non substrate RNAs inactive catalytic capability. Given that A1 employs an RNA binding protein to ‘find’ apoB mRNA within the vast abundance of cellular RNAs, RNA inhibition of AID/APOBEC may be driven by mass action of cellular RNAs that bind to these proteins but do not have appropriate editing sites.
Recent research has suggested that A3 binding to RNAs may not be nonspecific and that there may be competitive and noncompetitive interactions with RNAs that determine A3 oligomerization, RNP formation and the subcellular redistribution of A3 to sites of retroviral particle assembly. That later interaction with RNA must have been a critical feature of A3 antiviral activity in host cell defense for millennia. High resolution studies coupled to functional studies will be necessary to define the amino acid residues and protein folds of AID/APOBEC that bind to RNA and the RNA sequences that are bound to them. It is apparent from the literature that these interactions are not static and change during cell differential and development and in response to hormone stimulation, cell stress and viral infection. Here again, our understanding of RNA ligand binding to APOBEC will only be complete when the functional consequences of these interactions and their regulation has been revealed.
Conflict of Interest Statement
Dr. HC Smith is a full-time and tenured professor at the University of Rochester School of Medicine and Dentistry. He is the founder and CEO of the University of Rochester spinout company OyaGen, Inc. The company has a financial interest in the development of antiviral and anti-cancer drugs based on APOBEC technology.
Acknowledgments
The author wishes to acknowledge the contributions to the advancement of science in the APOBEC field made by investigators across the globe whose work may not have been adequately referenced here due to the limitations on the number of citations. Preparation of this review was supported in part by a Public Health Services Grant GM110568 and GM110038 awarded to HCS.
References
- 1.Jackman JE, Alfonzo JD. Transfer RNA modifications: nature's combinatorial chemistry playground. Wiley Interdiscip Rev RNA 2013; 4(1):35-48; PMID:23139145; http://dx.doi.org/ 10.1002/wrna.1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machnicka MA, Milanowska K, Osman Oglou O, Purta E, Kurkowska M, Olchowik A, Januszewski W, Kalinowski S, Dunin-Horkawicz S, Rother KM, et al.. MODOMICS: a database of RNA modification pathways–2013 update. Nucleic Acids Res 2013; 41:D262-7; PMID:23118484; http://dx.doi.org/ 10.1093/nar/gks1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grosjean H. RNA modification: the Golden Period 1995-2015. RNA 2015; 21:625-6; PMID:25780166; http://dx.doi.org/ 10.1261/rna.049866.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grosjean H. Nuclei Acids are Not Boring Long Polymers of Only Four Types of Nucleotides: A Guided Tour DNA and RNA modification enzymes: structure, mechanism, function and evolution, ed. Grosjean H. 2009, Austin, TX: Landes BioScience. [Google Scholar]
- 5.Grosjean H., Moddification and Editing of RNA: Historical Overview and Important Facts to Remember. Topics in Current Genetics, ed. Grosjean H. 2005; 12, New York: Springer:442. [Google Scholar]
- 6.Read LK, Lukes J, Hashimi H. Trypanosome RNA editing: the complexity of getting U in and taking U out. Wiley Interdiscip Rev RNA 2016; 7(1):33-51; PMID:26522170; http://dx.doi.org/ 10.1002/wrna.1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stuart KD, Schnaufer A, Ernst NL, Panigrahi AK. Complex management: RNA editing in trypanosomes. Trends Biochem Sci 2005; 30(2):97-105; PMID:15691655; http://dx.doi.org/ 10.1016/j.tibs.2004.12.006 [DOI] [PubMed] [Google Scholar]
- 8.Wedekind JE, Dance GS, Sowden MP, Smith HC. Messenger RNA editing in mammals: new members of the APOBEC family seeking roles in the family business. Trends Genet 2003; 19(4):207-16; PMID:12683974; http://dx.doi.org/ 10.1016/S0168-9525(03)00054-4 [DOI] [PubMed] [Google Scholar]
- 9.Smith HC, Gott JM, Hanson MR. A guide to RNA editing. RNA 1997; 3(10):1105-23; PMID:9326486 [PMC free article] [PubMed] [Google Scholar]
- 10.Mannion N, Arieti F, Gallo A, Keegan LP, O'Connell MA. New Insights into the Biological Role of Mammalian ADARs; the RNA Editing Proteins. Biomolecules 2015; 5(4):2338-62; PMID:26437436; http://dx.doi.org/ 10.3390/biom5042338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savva YA, Rieder LE, Reenan RA. The ADAR protein family. Genome Biol 2012; 13(12):252; PMID:23273215; http://dx.doi.org/ 10.1186/gb-2012-13-12-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rafels-Ybern A, Attolini CS, Ribas de Pouplana L. Distribution of ADAT-Dependent Codons in the Human Transcriptome. Int J Mol Sci 2015; 16(8):17303-14; PMID:26230688; http://dx.doi.org/ 10.3390/ijms160817303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aydin H, Taylor MW, Lee JE. Structure-guided analysis of the human APOBEC3-HIV restrictome. Structure 2014; 22(5):668-84; PMID:24657093; http://dx.doi.org/ 10.1016/j.str.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 14.Salter JD, Bennett RP, Smith HC. The APOBEC Protein Family: United by Structure, Divergent in Function. Trends Biochem Sci 2016; 41(7):578-94; PMID:27283515; http://dx.doi.org/ 10.1016/j.tibs.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li JB, Church GM. Deciphering the functions and regulation of brain-enriched A-to-I RNA editing. Nat Neurosci 2013; 16(11):1518-22; PMID:24165678; http://dx.doi.org/ 10.1038/nn.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmgren M, Rosenthal JJ. Regulation of ion channel and transporter function through RNA editing. Curr Issues Mol Biol 2015; 17:23-36; PMID:25347917 [PMC free article] [PubMed] [Google Scholar]
- 17.Driscoll DM, Innerarity TL. RNA Editing by Cytidine Deamination in Mammals, in RNA Editing, Bass BL, Editor. 2001, Oxford University Press: Oxford: p. 61-76. [Google Scholar]
- 18.Chelico L, Prochnow C, Erie DA, Chen XS, Goodman MF. A structural model for deoxycytidine deamination mechanisms of the HIV-1 inactivation enzyme APOBEC3G. J Biol Chem 2010; 285(21):16195-205; PMID:20212048; http://dx.doi.org/ 10.1074/jbc.M110.107987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitamura S, Ode H, Nakashima M, Imahashi M, Naganawa Y, Kurosawa T, Yokomaku Y, Yamane T, Watanabe N, Suzuki A, et al., The APOBEC3C crystal structure and the interface for HIV-1 Vif binding. Nat Struct Mol Biol 2012; 19(10):1005-10; PMID:23001005; http://dx.doi.org/ 10.1038/nsmb.2378 [DOI] [PubMed] [Google Scholar]
- 20.Shandilya SM, Nalam MN, Nalivaika EA, Gross PJ, Valesano JC, Shindo K, Li M, Munson M, Royer WE, Harjes E, et al.. Crystal structure of the APOBEC3G catalytic domain reveals potential oligomerization interfaces. Structure 2010; 18(1):28-38; PMID:20152150; http://dx.doi.org/ 10.1016/j.str.2009.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuratani M, Ishii R, Bessho Y, Fukunaga R, Sengoku T, Shirouzu M, Sekine S, Yokoyama S. Crystal structure of tRNA adenosine deaminase (TadA) from Aquifex aeolicus. J Biol Chem 2005; 280(16):16002-8; PMID:15677468; http://dx.doi.org/ 10.1074/jbc.M414541200 [DOI] [PubMed] [Google Scholar]
- 22.Goodman RA, Macbeth MR, Beal PA. ADAR proteins: structure and catalytic mechanism. Curr Top Microbiol Immunol 2012; 353:1-33; PMID:21769729 [DOI] [PubMed] [Google Scholar]
- 23.Shaban NM, et al.. 1.92 Angstrom Zinc-Free APOBEC3F Catalytic Domain Crystal Structure. J Mol Biol 2016; 428(11):2307-16; PMID:27139641; http://dx.doi.org/ 10.1016/j.jmb.2016.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deffit SN, Hundley HA. To edit or not to edit: regulation of ADAR editing specificity and efficiency. Wiley Interdiscip Rev RNA 2016; 7(1):113-27; PMID:26612708; http://dx.doi.org/ 10.1002/wrna.1319 [DOI] [PubMed] [Google Scholar]
- 25.Anant S, MacGinnitie AJ, Davidson NO. apobec-1, the catalytic subunit of the mammalian apolipoprotein B mRNA editing enzyme, is a novel RNA-binding protein. J Biol Chem 1995; 270(24):14762-7; PMID:7782342; http://dx.doi.org/ 10.1074/jbc.270.24.14762 [DOI] [PubMed] [Google Scholar]
- 26.Backus JW, Smith HC. Three distinct RNA sequence elements are required for efficient apolipoprotein B (apoB) RNA editing in vitro. Nucleic Acids Res 1992; 20(22):6007-14; PMID:1461733; http://dx.doi.org/ 10.1093/nar/20.22.6007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sowden MP, Ballatori N, de Mesy Jensen KL, Hamilton Reed L, Smith HC. The editosome for cytidine to uridine mRNA editing has a native complexity of 27S: identification of intracellular domains containing active and inactive editing factors. J Cell Science 2002; 115(1027-1039):1027-1039. [DOI] [PubMed] [Google Scholar]
- 28.Blanc V, Henderson JO, Newberry EP, Kennedy S, Luo J, Davidson NO. Targeted deletion of the murine apobec-1 complementation factor (acf) gene results in embryonic lethality. Mol Cell Biol 2005; 25(16):7260-9; PMID:16055734; http://dx.doi.org/ 10.1128/MCB.25.16.7260-7269.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen KM, Harjes E, Gross PJ, Fahmy A, Lu Y, Shindo K, Harris RS, Matsuo H. Structure of the DNA deaminase domain of the HIV-1 restriction factor APOBEC3G. Nature 2008; 452(7183):116-9; PMID:18288108; http://dx.doi.org/ 10.1038/nature06638 [DOI] [PubMed] [Google Scholar]
- 30.Harjes E, Gross PJ, Chen KM, Lu Y, Shindo K, Nowarski R, Gross JD, Kotler M, Harris RS, Matsuo H. An extended structure of the APOBEC3G catalytic domain suggests a unique holoenzyme model. J Mol Biol 2009; 389(5):819-32; PMID:19389408; http://dx.doi.org/ 10.1016/j.jmb.2009.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polevoda B, McDougall WM, Tun BN, Cheung M, Salter JD, Friedman AE, Smith HC. RNA binding to APOBEC3G induces the disassembly of functional deaminase complexes by displacing single-stranded DNA substrates. Nucleic Acids Res 2015; 43(19):9434-45; PMID:26424853; http://dx.doi.org/ 10.1093/nar/gkv970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Byeon IJ, Ahn J, Mitra M, Byeon CH, Hercík K, Hritz J, Charlton LM, Levin JG, Gronenborn AM. NMR structure of human restriction factor APOBEC3A reveals substrate binding and enzyme specificity. Nat Commun 2013; 4:1890; PMID:23695684; http://dx.doi.org/ 10.1038/ncomms2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasar S, Kim J, Improgo R, Tiao G, Polak P, Haradhvala N, Lawrence MS, Kiezun A, Fernandes SM, Bahl S, et al.. Whole-genome sequencing reveals activation-induced cytidine deaminase signatures during indolent chronic lymphocytic leukaemia evolution. Nat Commun 2015; 6:8866; PMID:26638776; http://dx.doi.org/ 10.1038/ncomms9866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knisbacher BA, Gerber D, Levanon EY. DNA Editing by APOBECs: A genomic preserver and transformer. Trends Genet 2016; 32(1):16-28; PMID:26608778; http://dx.doi.org/ 10.1016/j.tig.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 35.Bazak L, Haviv A, Barak M, Jacob-Hirsch J, Deng P, Zhang R, Isaacs FJ, Rechavi G, Li JB, Eisenberg E, et al.. A-to-I RNA editing occurs at over a hundred million genomic sites, located in a majority of human genes. Genome Res 2014; 24(3):365-76; PMID:24347612; http://dx.doi.org/ 10.1101/gr.164749.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenberg BR, Hamilton CE, Mwangi MM, Dewell S, Papavasiliou FN. Transcriptome-wide sequencing reveals numerous APOBEC1 mRNA-editing targets in transcript 3′ UTRs. Nat Struct Mol Biol 2011; 18(2):230-6; PMID:21258325; http://dx.doi.org/ 10.1038/nsmb.1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Licht K, Jantsch MF. Rapid and dynamic transcriptome regulation by RNA editing and RNA modifications. J Cell Biol 2016; 213(1):15-22; PMID:27044895; http://dx.doi.org/ 10.1083/jcb.201511041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knisbacher BA, Levanon EY. DNA Editing of LTR Retrotransposons reveals the impact of APOBECs on Vertebrate Genomes. Mol Biol Evol 2016; 33(2):554-67; PMID:26541172; http://dx.doi.org/ 10.1093/molbev/msv239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma S, Patnaik SK, Taggart RT, Kannisto ED, Enriquez SM, Gollnick P, Baysal BE. APOBEC3A cytidine deaminase induces RNA editing in monocytes and macrophages. Nat Commun 2015; 6:6881; PMID:25898173; http://dx.doi.org/ 10.1038/ncomms7881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomaselli S, Bonamassa B, Alisi A, Nobili V, Locatelli F, Gallo A. ADAR enzyme and miRNA story: a nucleotide that can make the difference. Int J Mol Sci 2013; 14(11):22796-816; PMID:24256817; http://dx.doi.org/ 10.3390/ijms141122796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Witkin KL, Hanlon SE, Strasburger JA, Coffin JM, Jaffrey SR, Howcroft TK, Dedon PC, Steitz JA, Daschner PJ, Read-Connole E. RNA editing, epitranscriptomics, and processing in cancer progression. Cancer Biol Ther 2015; 16(1):21-7; PMID:25455629; http://dx.doi.org/ 10.4161/15384047.2014.987555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harris RS, Dudley JP. APOBECs and virus restriction. Virology 2015; 479-480:131-45; PMID:25818029; http://dx.doi.org/ 10.1016/j.virol.2015.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zipeto MA, Jiang Q, Melese E, Jamieson CH. RNA rewriting, recoding, and rewiring in human disease. Trends Mol Med 2015; 21(9):549-59; PMID:26259769; http://dx.doi.org/ 10.1016/j.molmed.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 44.Avesson L, Barry G. The emerging role of RNA and DNA editing in cancer. Biochim Biophys Acta 2014; 1845(2):308-16; PMID:24607277 [DOI] [PubMed] [Google Scholar]
- 45.Yamanaka S, Poksay KS, Arnold KS, Innerarity TL. A novel translational repressor mRNA is edited extensively in livers containing tumors caused by the transgene expression of the apoB mRNA- editing enzyme. Genes Dev 1997; 11(3):321-33; PMID:9030685; http://dx.doi.org/ 10.1101/gad.11.3.321 [DOI] [PubMed] [Google Scholar]
- 46.Sowden M, Hamm JK, Spinelli S, Smith HC. Determinants involved in regulating the proportion of edited apolipoprotein B RNAs. RNA 1996; 2(3):274-88; PMID:8608451 [PMC free article] [PubMed] [Google Scholar]
- 47.Honjo T, Muramatsu M, Fagarasan S. AID: how does it aid antibody diversity? Immunity 2004; 20(6):659-68; PMID:15189732; http://dx.doi.org/ 10.1016/j.immuni.2004.05.011 [DOI] [PubMed] [Google Scholar]
- 48.Longerich S, et al.. AID in somatic hypermutation and class switch recombination. Curr Opin Immunol 2006; 18(2):164-74; PMID:16464563; http://dx.doi.org/ 10.1016/j.coi.2006.01.008 [DOI] [PubMed] [Google Scholar]
- 49.Arakawa H, Saribasak H, Buerstedde JM. Activation-induced cytidine deaminase initiates immunoglobulin gene conversion and hypermutation by a common intermediate. PLoS Biol 2004; 2(7):E179; PMID:15252444; http://dx.doi.org/ 10.1371/journal.pbio.0020179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li J, Potash MJ, Volsky DJ. Functional domains of APOBEC3G required for antiviral activity. J Cell Biochem 2004; 92(3):560-72; PMID:15156567; http://dx.doi.org/ 10.1002/jcb.20082 [DOI] [PubMed] [Google Scholar]
- 51.Dickerson SK, Market E, Besmer E, Papavasiliou FN. AID mediates hypermutation by deaminating single stranded DNA. J Exp Med 2003; 197(10):1291-6; PMID:12756266; http://dx.doi.org/ 10.1084/jem.20030481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Papavasiliou FN, Schatz DG. Somatic hypermutation of immunoglobulin genes: merging mechanisms for genetic diversity. Cell 2002; 109:S35-44; PMID:11983151; http://dx.doi.org/ 10.1016/S0092-8674(02)00706-7 [DOI] [PubMed] [Google Scholar]
- 53.Rebhandl S, Huemer M, Greil R, Geisberger R. AID/APOBEC deaminases and cancer. Oncoscience 2015; 2(4):320-33; PMID:26097867; http://dx.doi.org/ 10.18632/oncoscience.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robbiani DF, Bothmer A, Callen E, Reina-San-Martin B, Dorsett Y, Difilippantonio S, Bolland DJ, Chen HT, Corcoran AE, Nussenzweig A, et al.. AID is required for the chromosomal breaks in c-myc that lead to c-myc/IgH translocations. Cell 2008; 135(6):1028-38; PMID:19070574; http://dx.doi.org/ 10.1016/j.cell.2008.09.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henderson S, Fenton T. APOBEC3 genes: retroviral restriction factors to cancer drivers. Trends Mol Med 2015; 21(5):274-84; PMID:25820175; http://dx.doi.org/ 10.1016/j.molmed.2015.02.007 [DOI] [PubMed] [Google Scholar]
- 56.Alfonzo JD, Blanc V, Estévez AM, Rubio MA, Simpson L. C to U editing of the anticodon of imported mitochondrial tRNA(Trp) allows decoding of the UGA stop codon in Leishmania tarentolae. Embo J 1999; 18(24):7056-62; PMID:10601027; http://dx.doi.org/ 10.1093/emboj/18.24.7056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reid JG, Nagaraja AK, Lynn FC, Drabek RB, Muzny DM, Shaw CA, Weiss MK, Naghavi AO, Khan M, Zhu H, et al.. Mouse let-7 miRNA populations exhibit RNA editing that is constrained in the 5′-seed/ cleavage/anchor regions and stabilize predicted mmu-let-7a:mRNA duplexes. Genome Res 2008; 18(10):1571-81; PMID:18614752; http://dx.doi.org/ 10.1101/gr.078246.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fernandez HR, Kavi HH, Xie W, Birchler JA. Heterochromatin: on the ADAR radar? Curr Biol 2005; 15(4):R132-4; PMID:15723784; http://dx.doi.org/ 10.1016/j.cub.2005.02.012 [DOI] [PubMed] [Google Scholar]
- 59.Benetti R, Gonzalo S, Jaco I, Muñoz P, Gonzalez S, Schoeftner S, Murchison E, Andl T, Chen T, Klatt P, et al.. A mammalian microRNA cluster controls DNA methylation and telomere recombination via Rbl2-dependent regulation of DNA methyltransferases. Nat Struct Mol Biol 2008; 15(9):998; PMID:18769471; http://dx.doi.org/ 10.1038/nsmb0908-998b [DOI] [PubMed] [Google Scholar]
- 60.Alon S, Mor E, Vigneault F, Church GM, Locatelli F, Galeano F, Gallo A, Shomron N, Eisenberg E. Systematic identification of edited microRNAs in the human brain. Genome Res 2012; 22(8):1533-40; PMID:22499667; http://dx.doi.org/ 10.1101/gr.131573.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cui Y, Huang T, Zhang X. RNA editing of microRNA prevents RNA-induced silencing complex recognition of target mRNA. Open Biol 2015; 5(12):150126; PMID:26674414; http://dx.doi.org/ 10.1098/rsob.150126 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Doria M, Neri F, Gallo A, Farace MG, Michienzi A. Editing of HIV-1 RNA by the double-stranded RNA deaminase ADAR1 stimulates viral infection. Nucleic Acids Res 2009; 37(17):5848-58; PMID:19651874; http://dx.doi.org/ 10.1093/nar/gkp604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lazar DC, Morris KV, Saayman SM. The emerging role of long non-coding RNAs in HIV infection. Virus Res 2016; 212:114-26; PMID:26221763; http://dx.doi.org/ 10.1016/j.virusres.2015.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skuse GR, Cappione AJ, Sowden M, Metheny LJ, Smith HC. The neurofibromatosis type I messenger RNA undergoes base-modification RNA editing. Nucleic Acids Res 1996; 24(3):478-85; PMID:8602361; http://dx.doi.org/ 10.1093/nar/24.3.478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith HC. Deaminase-dependent and Deaminase-independent Functions of APOBEC1 and APOBEC1 Complementation Factor in the Context of the APOBEC Family RNA Editing, ed. Maas S. 2013, Norfolk, UK: Caister Academic Press. [Google Scholar]
- 66.Smith HC, Wedekind JE, Kefang X, Sowden MP. Mammalian C to U Editing. Topics Curr Genetics 2005; 12:365-400; http://dx.doi.org/ 10.1007/b105432 [DOI] [Google Scholar]
- 67.Jarmuz A, Chester A, Bayliss J, Gisbourne J, Dunham I, Scott J, Navaratnam N. An Anthropoid-Specific Locus of Orphan C to U RNA-Editing Enzymes on Chromosome 22. Genomics 2002; 79(3):285-96; PMID:11863358; http://dx.doi.org/ 10.1006/geno.2002.6718 [DOI] [PubMed] [Google Scholar]
- 68.Smith HC. APOBEC3G: a double agent in defense. Trends Biochem Sci 2011; 36(5):239-44; PMID:21239176; http://dx.doi.org/ 10.1016/j.tibs.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klein IA, Resch W, Jankovic M, Oliveira T, Yamane A, Nakahashi H, Di Virgilio M, Bothmer A, Nussenzweig A, Robbiani DF, et al.. Translocation-capture sequencing reveals the extent and nature of chromosomal rearrangements in B lymphocytes. Cell 2011; 147(1):95-106; PMID:21962510; http://dx.doi.org/ 10.1016/j.cell.2011.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ichikawa HT, Sowden MP, Torelli AT, Bachl J, Huang P, Dance GS, Marr SH, Robert J, Wedekind JE, Smith H, et al., Structural phylogenetic analysis of activation-induced deaminase function. J Immunol 2006; 177(1):355-61; PMID:16785531; http://dx.doi.org/ 10.4049/jimmunol.177.1.355 [DOI] [PubMed] [Google Scholar]
- 71.Leonard B, Starrett GJ, Maurer MJ, Oberg AL, Van Bockstal M, Van Dorpe J, De Wever O, Helleman J, Sieuwerts AM, Berns EM, et al.. APOBEC3G Expression Correlates with T-Cell infiltration and improved clinical outcomes in High-grade Serous Ovarian Carcinoma. Clin Cancer Res 2016; 22(18):4746-55; PMID:27016308; http://dx.doi.org/ 10.1158/1078-0432.CCR-15-2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harris RS, Petersen-Mahrt SK, Neuberger MS. RNA editing enzyme APOBEC1 and some of its homologs can act as DNA mutators. Mol Cell 2002; 10(5):1247-53; PMID:12453430; http://dx.doi.org/ 10.1016/S1097-2765(02)00742-6 [DOI] [PubMed] [Google Scholar]
- 73.Beale RC, Petersen-Mahrt SK, Watt IN, Harris RS, Rada C, Neuberger MS. Comparison of the differential context-dependence of DNA deamination by APOBEC enzymes: correlation with mutation spectra in vivo. J Mol Biol 2004; 26(337):585-96; http://dx.doi.org/ 10.1016/j.jmb.2004.01.046 [DOI] [PubMed] [Google Scholar]
- 74.Prohaska KM, Bennett RP, Salter JD, Smith HC. The multifaceted roles of RNA binding in APOBEC cytidine deaminase functions. Wiley Interdiscip Rev RNA 2014; 5(4):493-508; PMID:24664896; http://dx.doi.org/ 10.1002/wrna.1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anant S, Davidson NO. Molecular mechanisms of apolipoprotein B mRNA editing. Curr Opin Lipidol 2001; 12(2):159-65; PMID:11264987; http://dx.doi.org/ 10.1097/00041433-200104000-00009 [DOI] [PubMed] [Google Scholar]
- 76.Nakamuta M, Chang B.H.J, Zsigmond E, Kobayashi K, Lei H, Ishida BY, Oka K, Li E, Chan L. Complete phenotypic characterization of the apobec-1 knockout mice with a wild-type genetic background and a human apolipoprotein B transgenic background, and restoration of apolipoprotein B mRNA editing by somatic gene transfer of Apobec-1. J Biol Chem 1996; 271:25981-25988; PMID:8824235; http://dx.doi.org/ 10.1074/jbc.271.42.25981 [DOI] [PubMed] [Google Scholar]
- 77.Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, Catalan N, Forveille M, Dufourcq-Lagelouse R, Gennery A, et al.. Activation-Induced Cytidine Deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM Syndrome (HIGM2). Cell 2000; 102(5):565-575 ; PMID:11007475; http://dx.doi.org/ 10.1016/S0092-8674(00)00079-9 [DOI] [PubMed] [Google Scholar]
- 78.Rogozin IB, Basu MK, Jordan IK, Pavlov Y, Koonin EV. APOBEC4, a new member of the AID/APOBEC family of polynucleotide (deoxy)cytidine deaminases predicted by computational analysis. Cell Cycle 2005; 4(9):1281-5; PMID:16082223; http://dx.doi.org/ 10.4161/cc.4.9.1994 [DOI] [PubMed] [Google Scholar]
- 79.Mikl MC, Watt IN, Lu M, Reik W, Davies SL, Neuberger MS, Rada C. Mice deficient in APOBEC2 and APOBEC3. Mol Cell Biol 2005; 25(16):7270-7; PMID:16055735; http://dx.doi.org/ 10.1128/MCB.25.16.7270-7277.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sato Y, Probst HC, Tatsumi R, Ikeuchi Y, Neuberger MS, Rada C. Deficiency in APOBEC2 leads to a shift in muscle fiber type, diminished body mass, and myopathy. J Biol chem 2010; 285(10):7111-8; PMID:20022958; http://dx.doi.org/ 10.1074/jbc.M109.052977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kato L, Stanlie A, Begum NA, Kobayashi M, Aida M, Honjo T. An evolutionary view of the mechanism for immune and genome diversity. J Immunol 2012; 188(8):3559-66; PMID:22492685; http://dx.doi.org/ 10.4049/jimmunol.1102397 [DOI] [PubMed] [Google Scholar]
- 82.Zhao Y, Pan-Hammarström Q, Zhao Z, Hammarström L. Identification of the activation-induced cytidine deaminase gene from zebrafish: an evolutionary analysis. Dev Comp Immunol 2005; 29(1):61-71; PMID:15325524; http://dx.doi.org/ 10.1016/j.dci.2004.05.005 [DOI] [PubMed] [Google Scholar]
- 83.Wakae K, Magor BG, Saunders H, Nagaoka H, Kawamura A, Kinoshita K, Honjo T, Muramatsu M. Evolution of class switch recombination function in fish activation-induced cytidine deaminase, AID. Int Immunol 2006; 18(1):41-7; PMID:16291656; http://dx.doi.org/ 10.1093/intimm/dxh347 [DOI] [PubMed] [Google Scholar]
- 84.Franchini DM, Petersen-Mahrt SK. AID and APOBEC deaminases: balancing DNA damage in epigenetics and immunity. Epigenomics 2014; 6(4):427-43; PMID:25333851; http://dx.doi.org/ 10.2217/epi.14.35 [DOI] [PubMed] [Google Scholar]
- 85.Horn AV, Klawitter S, Held U, Berger A, Vasudevan AA, Bock A, Hofmann H, Hanschmann KM, Trösemeier JH, Flory E, et al.. Human LINE-1 restriction by APOBEC3C is deaminase independent and mediated by an ORF1p interaction that affects LINE reverse transcriptase activity. Nucleic Acids Res 2013; 42(1):396-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sawyer SL, Emerman M, Malik HS. Ancient adaptive evolution of the primate antiviral DNA-editing enzyme APOBEC3G. PLoS Biol 2004; 2(9):E275; PMID:15269786; http://dx.doi.org/ 10.1371/journal.pbio.0020275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kinomoto M, Kanno T, Shimura M, Ishizaka Y, Kojima A, Kurata T, Sata T, Tokunaga K. All APOBEC3 family proteins differentially inhibit LINE-1 retrotransposition. Nucleic Acids Res 2007; 35(9):2955-64; PMID:17439959; http://dx.doi.org/ 10.1093/nar/gkm181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hultquist JF, Lengyel JA, Refsland EW, LaRue RS, Lackey L, Brown WL, Harris RS. Human and Rhesus APOBEC3D, APOBEC3F, APOBEC3G, and APOBEC3H demonstrate a conserved capacity to Restrict Vif-deficient HIV-1. J Virol 2011; 85(21):11220-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tan L, Sarkis PT, Wang T, Tian C, Yu XF. Sole copy of Z2-type human cytidine deaminase APOBEC3H has inhibitory activity against retrotransposons and HIV-1. FASEB J 2009; 23(1):279-87; http://dx.doi.org/ 10.1096/fj.07-088781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Conticello SG, Thomas CJ, Petersen-Mahrt SK, Neuberger MS. Evolution of the AID/APOBEC Family of Polynucleotide (Deoxy)cytidine Deaminases. Mol Biol Evol 2005; 22(2):367-77; PMID:15496550; http://dx.doi.org/ 10.1093/molbev/msi026 [DOI] [PubMed] [Google Scholar]
- 91.Pace C, Keller J, Nolan D, James I, Gaudieri S, Moore C, Mallal S. Population level analysis of human immunodeficiency virus type 1 hypermutation and its relationship with APOBEC3G and vif genetic variation. J Virol 2006; 80(18):9259-69; PMID:16940537; http://dx.doi.org/ 10.1128/JVI.00888-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Munk C, Willemsen A, Bravo IG, An ancient history of gene duplications, fusions and losses in the evolution of APOBEC3 mutators in mammals. BMC Evol Biol 2012; 12:71; PMID:22640020; http://dx.doi.org/ 10.1186/1471-2148-12-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kidd JM, Newman TL, Tuzun E, Kaul R, Eichler EE. Population stratification of a common APOBEC gene deletion polymorphism. PLoS Genet 2007; 3(4):e63; PMID:17447845; http://dx.doi.org/ 10.1371/journal.pgen.0030063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wittkopp CJ, Adolph MB, Wu LI, Chelico L, Emerman M. A single nucleotide polymorphism in Human APOBEC3C enhances restriction of lentiviruses. PLoS Pathog 2016; 12(10):e1005865; PMID:27732658; http://dx.doi.org/ 10.1371/journal.ppat.1005865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McCarthy H, Wierda WG, Barron LL, Cromwell CC, Wang J, Coombes KR, Rangel R, Elenitoba-Johnson KS, Keating MJ, Abruzzo LV. High expression of activation-induced cytidine deaminase (AID) and splice variants is a distinctive feature of poor-prognosis chronic lymphocytic leukemia. Blood 2003; 101(12):4903-8; PMID:12586616; http://dx.doi.org/ 10.1182/blood-2002-09-2906 [DOI] [PubMed] [Google Scholar]
- 96.Wu X, Darce JR, Chang SK, Nowakowski GS, Jelinek DF. Alternative splicing regulates activation-induced cytidine deaminase (AID): implications for suppression of AID mutagenic activity in normal and malignant B cells. Blood 2008; 112(12):4675-4682; PMID:18684869; http://dx.doi.org/ 10.1182/blood-2008-03-145995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.OhAinle M, Kerns JA, Malik HS, Emerman M, Adaptive evolution and antiviral activity of the conserved mammalian cytidine deaminase APOBEC3H. J Virol 2006; 80(8):3853-62; PMID:16571802; http://dx.doi.org/ 10.1128/JVI.80.8.3853-3862.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feng Y, Love RP, Ara A, Baig TT, Adolph MB, Chelico L. Natural polymorphisms and oligomerization of human APOBEC3H contribute to single-stranded DNA scanning ability. J Biol Chem 2015; 290(45):27188-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Starrett GJ, Luengas EM, McCann JL, Ebrahimi D, Temiz NA, Love RP, Feng Y, Adolph MB, Chelico L, Law EK, et al., The DNA cytosine deaminase APOBEC3H haplotype I likely contributes to breast and lung cancer mutagenesis. Nat Commun 2016; 7:12918; PMID:27650891; http://dx.doi.org/ 10.1038/ncomms12918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang J., Webb DM. Rapid evolution of primate antiviral enzyme APOBEC3G. Hum Mol Genet 2004; 13(16):1785-91; PMID:15198990; http://dx.doi.org/ 10.1093/hmg/ddh183 [DOI] [PubMed] [Google Scholar]
- 101.Refsland EW, Stenglein MD, Shindo K, Albin JS, Brown WL, Harris RS. Quantitative profiling of the full APOBEC3 mRNA repertoire in lymphocytes and tissues: implications for HIV-1 restriction. Nucleic Acids Res 2010; 38(13):4274-84; PMID:20308164; http://dx.doi.org/ 10.1093/nar/gkq174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smith JL, Pathak VK. Identification of specific determinants of human APOBEC3F, APOBEC3C, and APOBEC3DE and African green monkey APOBEC3F that interact with HIV-1 Vif. J Virol 2010; 84(24):12599-608; PMID:20943965; http://dx.doi.org/ 10.1128/JVI.01437-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dance GS, Beemiller P, Yang Y, Mater DV, Mian IS, Smith HC. Identification of the yeast cytidine deaminase CDD1 as an orphan C–>U RNA editase. Nucleic Acids Res 2001; 29(8):1772-80; PMID:11292850; http://dx.doi.org/ 10.1093/nar/29.8.1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xie K, Sowden MP, Dance GS, Torelli AT, Smith HC, Wedekind JE. The structure of a yeast RNA-editing deaminase provides insight into the fold and function of activation-induced deaminase and APOBEC-1. Proc Natl Acad Sci U S A 2004; 101(21):8114-9; PMID:15148397; http://dx.doi.org/ 10.1073/pnas.0400493101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bohn MF, Shandilya SM, Albin JS, Kouno T, Anderson BD, McDougle RM, Carpenter MA, Rathore A, Evans L, Davis AN, et al., Crystal structure of the DNA cytosine deaminase APOBEC3F: the catalytically active and HIV-1 Vif-binding domain. Structure 2013; 21(6):1042-50; PMID:23685212; http://dx.doi.org/ 10.1016/j.str.2013.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Prochnow C, Bransteitter R, Klein MG, Goodman MF, Chen XS, The APOBEC-2 crystal structure and functional implications for the deaminase AID. Nature 2007; 445(7126):447-51; PMID:17187054; http://dx.doi.org/ 10.1038/nature05492 [DOI] [PubMed] [Google Scholar]
- 107.Krzysiak TC, Jung J, Thompson J, Baker D, Gronenborn AM. APOBEC2 is a monomer in solution: implications for APOBEC3G models. Biochemistry 2012; 51(9):2008-17; PMID:22339232; http://dx.doi.org/ 10.1021/bi300021s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xiao X, Li SX, Yang H, Chen XS. Crystal structures of APOBEC3G N-domain alone and its complex with DNA. Nat Commun 2016; 7:12193; PMID:27480941; http://dx.doi.org/ 10.1038/ncomms12193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen Q, Xiao X, Wolfe A, Chen XS. The in vitro Biochemical Characterization of an HIV-1 Restriction Factor APOBEC3F: Importance of Loop 7 on Both CD1 and CD2 for DNA Binding and Deamination. J Mol Biol 2016; 428(13):2661-70; PMID:27063502; http://dx.doi.org/ 10.1016/j.jmb.2016.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Navarro F, et al., Complementary function of the two catalytic domains of APOBEC3G. Virology 2005; 333(2):374-86; PMID:15721369; http://dx.doi.org/ 10.1016/j.virol.2005.01.011 [DOI] [PubMed] [Google Scholar]
- 111.Pham P, et al.. Processive AID-catalysed cytosine deamination on single-stranded DNA simulates somatic hypermutation. Nature 2003; 424(6944):103-7; PMID:12819663; http://dx.doi.org/ 10.1038/nature01760 [DOI] [PubMed] [Google Scholar]
- 112.Chelico L, Pham P, Goodman MF. Mechanisms of APOBEC3G-catalyzed processive deamination of deoxycytidine on single-stranded DNA. Nat Struct Mol Biol 2009; 16(5):454-5; author reply 455-6; PMID:19421154; http://dx.doi.org/ 10.1038/nsmb0509-454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Senavirathne G, et al.. Single-stranded DNA scanning and deamination by APOBEC3G cytidine deaminase at single molecule resolution. J Biol Chem 2012; 287(19):15826-35; PMID:22362763; http://dx.doi.org/ 10.1074/jbc.M112.342790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McDougall WM, Okany C, Smith HC. Deaminase Activity on Single-stranded DNA (ssDNA) Occurs in Vitro when APOBEC3G Cytidine Deaminase Forms Homotetramers and Higher-order Complexes. J Biol Chem 2011; 286 (35):30655-61; PMID:21737457; http://dx.doi.org/ 10.1074/jbc.M111.269506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ta VT, Nagaoka H, Catalan N, Durandy A, Fischer A, Imai K, Nonoyama S, Tashiro J, Ikegawa M, Ito S, et al.. AID mutant analyses indicate requirement for class-switch-specific cofactors. Nat Immunol 2003; 4(9):843-8; PMID:12910268; http://dx.doi.org/ 10.1038/ni964 [DOI] [PubMed] [Google Scholar]
- 116.Ramiro AR, et al.. Transcription enhances AID-mediated cytidine deamination by exposing single-stranded DNA on the nontemplate strand. Nat Immunol 2003; 4(5):452-6; PMID:12692548; http://dx.doi.org/ 10.1038/ni920 [DOI] [PubMed] [Google Scholar]
- 117.Bross L, et al.. DNA Double-Strand Breaks: Prior to but not Sufficient in Targeting Hypermutation. J Exp Med 2002; 195(9):1187-1192; PMID:11994423; http://dx.doi.org/ 10.1084/jem.20011749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Basu U, et al.. The AID antibody diversification enzyme is regulated by protein kinase A phosphorylation. Nature 2005; 438(7067):508-11; PMID:16251902; http://dx.doi.org/ 10.1038/nature04255 [DOI] [PubMed] [Google Scholar]
- 119.Chatterji M, Unniraman S, McBride KM, Schatz DG. Role of activation-induced deaminase protein kinase A phosphorylation sites in Ig gene conversion and somatic hypermutation. J Immunol 2007; 179(8):5274-80; PMID:17911613; http://dx.doi.org/ 10.4049/jimmunol.179.8.5274 [DOI] [PubMed] [Google Scholar]
- 120.Chaudhuri J, Khuong C, Alt FW. Replication protein A interacts with AID to promote deamination of somatic hypermutation targets. Nature 2004; 430(7003):992-8; PMID:15273694; http://dx.doi.org/ 10.1038/nature02821 [DOI] [PubMed] [Google Scholar]
- 121.Chaudhuri J., Tian M, Khuong C, Chua K, Pinaud E, Alt FW. Transcription-targeted DNA deamination by the AID antibody diversification enzyme. Nature 2003; 422(6933):726-30; PMID:12692563; http://dx.doi.org/ 10.1038/nature01574 [DOI] [PubMed] [Google Scholar]
- 122.Chelico L, Pham P, Goodman MF. Stochastic properties of processive cytidine DNA deaminases AID and APOBEC3G. Philos Trans R Soc Lond B Biol Sci 2009; 364(1517):583-93; PMID:19022738; http://dx.doi.org/ 10.1098/rstb.2008.0195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Koning FA, Newman EN, Kim EY, Kunstman KJ, Wolinsky SM, Malim MH. Defining APOBEC3 expression patterns in human tissues and hematopoietic cell subsets. J Virol 2009; 83(18):9474-85; PMID:19587057; http://dx.doi.org/ 10.1128/JVI.01089-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 2002; 418:646-650; PMID:12167863; http://dx.doi.org/ 10.1038/nature00939 [DOI] [PubMed] [Google Scholar]
- 125.Smith HC, Bennett RP, Kizilyer A, McDougall WM, Prohaska KM. Functions and regulation of the APOBEC family of proteins. Semin Cell Dev Biol 2012; 23(3):258-68; PMID:22001110; http://dx.doi.org/ 10.1016/j.semcdb.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Krupp A, McCarthy KR, Ooms M, Letko M, Morgan JS, Simon V, Johnson WE. APOBEC3G polymorphism as a selective barrier to cross-species transmission and emergence of pathogenic SIV and AIDS in a primate host. PLoS Pathog 2013; 9(10):e1003641; PMID:24098115; http://dx.doi.org/ 10.1371/journal.ppat.1003641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Perez-Caballero D, Soll SJ, Bieniasz PD. Evidence for restriction of ancient primate gammaretroviruses by APOBEC3 but not TRIM5alpha proteins. PLoS Pathog 2008; 4(10):e1000181; PMID:18927623; http://dx.doi.org/ 10.1371/journal.ppat.1000181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bulliard Y, Turelli P, Röhrig UF, Zoete V, Mangeat B, Michielin O, Trono D. Functional analysis and structural modeling of human APOBEC3G reveal the role of evolutionarily conserved elements in the inhibition of human immunodeficiency virus type 1 infection and Alu transposition. J Virol 2009; 83(23):12611-21; PMID:19776130; http://dx.doi.org/ 10.1128/JVI.01491-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Siu KK, Sultana A, Azimi FC, Lee JE. Structural determinants of HIV-1 Vif susceptibility and DNA binding in APOBEC3F. Nat Commun 2013; 4:2593; PMID:24185281; http://dx.doi.org/ 10.1038/ncomms3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shi K., Carpenter MA, Kurahashi K, Harris RS, Aihara H. Crystal Structure of the DNA Deaminase APOBEC3B Catalytic Domain. J Biol Chem 2015; 290(47):28120-30; PMID:26416889; http://dx.doi.org/ 10.1074/jbc.M115.679951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stauch B, Hofmann H, Perkovic M, Weisel M, Kopietz F, Cichutek K, Münk C, Schneider G. Model structure of APOBEC3C reveals a binding pocket modulating ribonucleic acid interaction required for encapsidation. Proc Natl Acad Sci U S A 2009; 106(29):12079-84; PMID:19581596; http://dx.doi.org/ 10.1073/pnas.0900979106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Logue EC, Bloch N, Dhuey E, Zhang R, Cao P, Herate C, Chauveau L, Hubbard SR, Landau NR. A DNA sequence recognition loop on APOBEC3A controls substrate specificity. PLoS One 2014; 9(5):e97062; PMID:24827831; http://dx.doi.org/ 10.1371/journal.pone.0097062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Polevoda B, McDougall WM, Bennett RP, Salter JD, Smith HC. Structural and functional assessment of APOBEC3G macromolecular complexes. Methods 2016; 107:10-22; PMID:26988126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lau PP, Zhu HJ, Baldini A, Charnsangavej C, Chan L. Dimeric structure of a human apolipoprotein B mRNA editing protein and cloning and chromosomal localization of its gene. Proc Natl Acad Sci U S A 1994; 91(18):8522-6; PMID:8078915; http://dx.doi.org/ 10.1073/pnas.91.18.8522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Navaratnam N, Fujino T, Bayliss J, Jarmuz A, How A, Richardson N, Somasekaram A, Bhattacharya S, Carter C, Scott J. Escherichia coli cytidine deaminase provides a molecular model for ApoB RNA editing and a mechanism for RNA substrate recognition. J Mol Biol 1998; 275(4):695-714; PMID:9466941; http://dx.doi.org/ 10.1006/jmbi.1997.1506 [DOI] [PubMed] [Google Scholar]
- 136.Bennett RP, Salter JD, Liu X, Wedekind JE, Smith HC. APOBEC3G subunits self-associate via the C-terminal deaminase domain. J Biol Chem 2008; 283(48):33329-36; PMID:18842592; http://dx.doi.org/ 10.1074/jbc.M803726200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Salter JD, Krucinska J, Raina J, Smith HC, Wedekind JE. A hydrodynamic analysis of APOBEC3G reveals a monomer-dimer-tetramer self-association that has implications for anti-HIV function. Biochemistry 2009; 48(45):10685-7; PMID:19839647; http://dx.doi.org/ 10.1021/bi901642c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wedekind JE, Gillilan R, Janda A, Krucinska J, Salter JD, Bennett RP, Raina J, Smith HC. Nanostructures of APOBEC3G support a hierarchical assembly model of high molecular mass ribonucleoprotein particles from dimeric subunits. J Biol Chem 2006; 281:38122-38126; PMID:17079235; http://dx.doi.org/ 10.1074/jbc.C600253200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shlyakhtenko LS, Lushnikov AY, Miyagi A, Li M, Harris RS, Lyubchenko YL. Atomic force microscopy studies of APOBEC3G oligomerization and dynamics. J Struct Biol 2013; 184(2):217-25; PMID:24055458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bohn MF, Shandilya SM, Silvas TV, Nalivaika EA, Kouno T, Kelch BA, Ryder SP, Kurt-Yilmaz N, Somasundaran M, Schiffer CA. The ssDNA Mutator APOBEC3A Is Regulated by Cooperative Dimerization. Structure 2015; 23(5):903-11; PMID:25914058; http://dx.doi.org/ 10.1016/j.str.2015.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Iwatani Y, Takeuchi H, Strebel K, Levin JG. Biochemical activities of highly purified, catalytically active human APOBEC3G: correlation with antiviral effect. J Virol 2006; 80(12):5992-6002; PMID:16731938; http://dx.doi.org/ 10.1128/JVI.02680-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang J, Shinkura R, Muramatsu M, Nagaoka H, Kinoshita K, Honjo T. Identification of a specific domain required for dimerization of activation-induced cytidine deaminase. J Biol Chem 2006; 281(28):19115-23; PMID:16687409; http://dx.doi.org/; http://dx.doi.org/ 10.1074/jbc.M601645200 [DOI] [PubMed] [Google Scholar]
- 143.Shlyakhtenko LS, Lushnikov AJ, Li M, Harris RS, Lyubchenko YL. Interaction of APOBEC3A with DNA assessed by atomic force microscopy. PLoS One 2014; 9(6):e99354; PMID:24905100; http://dx.doi.org/ 10.1371/journal.pone.0099354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brar SS, Sacho EJ, Tessmer I, Croteau DL, Erie DA, Diaz M. Activation-induced deaminase, AID, is catalytically active as a monomer on single-stranded DNA. DNA Repair (Amst) 2008; 7(1):77-87; PMID:17889624; http://dx.doi.org/ 10.1016/j.dnarep.2007.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chelico L., Sacho EJ, Erie DA, Goodman MF. A model for oligomeric regulation of APOBEC3G cytosine deaminase-dependent restriction of HIV. J Biol Chem 2008; 283(20):13780-91; PMID:18362149; http://dx.doi.org/ 10.1074/jbc.M801004200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Opi S, Takeuchi H, Kao S, Khan MA, Miyagi E, Goila-Gaur R, Iwatani Y, Levin JG, Strebel K. Monomeric APOBEC3G is catalytically active and has antiviral activity. J Virol 2006; 80(10):4673-82; PMID:16641260; http://dx.doi.org/ 10.1128/JVI.80.10.4673-4682.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Siriwardena SU, Guruge TA, Bhagwat AS. Characterization of the Catalytic Domain of Human APOBEC3B and the Critical Structural Role for a Conserved Methionine. J Mol Biol 2015; 427(19):3042-55; PMID:26281709; http://dx.doi.org/ 10.1016/j.jmb.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Furukawa A, Nagata T, Matsugami A, Habu Y, Sugiyama R, Hayashi F, Kobayashi N, Yokoyama S, Takaku H, Katahira M. Structure, interaction and real-time monitoring of the enzymatic reaction of wild-type APOBEC3G. EMBO J 2009; 28(4):440-51; PMID:19153609; http://dx.doi.org/ 10.1038/emboj.2008.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gallois-Montbrun S, Holmes RK, Swanson CM, Fernández-Ocaña M, Byers HL, Ward MA, Malim MH. Comparison of cellular ribonucleoprotein complexes associated with the APOBEC3F and APOBEC3G antiviral proteins. J Virol 2008; 82(11):5636-42; PMID:18367521; http://dx.doi.org/ 10.1128/JVI.00287-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Huthoff H, Autore F, Gallois-Montbrun S, Fraternali F, Malim MH, Hope TJ. RNA-dependent oligomerization of APOBEC3G is required for restriction of HIV-1. PLoS Pathog 2009; 5(3):e1000330; PMID:19266078; http://dx.doi.org/ 10.1371/journal.ppat.1000330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.McDougall WM, Smith HC. Direct evidence that RNA inhibits APOBEC3G ssDNA cytidine deaminase activity. Biochem Biophys Res Commun 2011; 412(4):612-7; PMID:21856286; http://dx.doi.org/ 10.1016/j.bbrc.2011.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wichroski MJ, Robb GB, Rana TM. Human retroviral host restriction factors APOBEC3G and APOBEC3F localize to mRNA processing bodies. PLoS Pathog 2006; 2(5):e41; PMID:16699599; http://dx.doi.org/ 10.1371/journal.ppat.0020041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.York A, Kutluay SB, Errando M, Bieniasz PD. The RNA Binding Specificity of Human APOBEC3 Proteins Resembles That of HIV-1 Nucleocapsid. PLoS Pathog 2016; 12(8):e1005833; PMID:27541140; http://dx.doi.org/ 10.1371/journal.ppat.1005833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Apolonia L, Schulz R, Curk T, Rocha P, Swanson CM, Schaller T, Ule J, Malim MH. Promiscuous RNA binding ensures effective encapsidation of APOBEC3 proteins by HIV-1. PLoS Pathog 2015; 11(1):e1004609; PMID:25590131; http://dx.doi.org/ 10.1371/journal.ppat.1004609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Friew YN, Boyko V, Hu W-S, Pathak VK. Intracellular interactions between APOBEC3G, RNA, and HIV-1 Gag: APOBEC3G multimerization is dependent on its association with RNA. Retrovirology 2009; 6:56; PMID:19497112; http://dx.doi.org/ 10.1186/1742-4690-6-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Khan MA, Goila-Gaur R, Opi S, Miyagi E, Takeuchi H, Kao S, Strebel K. Analysis of the contribution of cellular and viral RNA to the packaging of APOBEC3G into HIV-1 virions. Retrovirology 2007; 4:48; PMID:17631688; http://dx.doi.org/ 10.1186/1742-4690-4-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kozak SL, Marin M, Rose KM, Bystrom C, Kabat D. The anti-HIV-1 editing enzyme APOBEC3G binds HIV-1 RNA and messenger RNAs that shuttle between polysomes and stress granules. J Biol Chem 2006; 281(39):29105-19; PMID:16887808; http://dx.doi.org/ 10.1074/jbc.M601901200 [DOI] [PubMed] [Google Scholar]
- 158.Svarovskaia ES, Xu H, Mbisa JL, Barr R, Gorelick RJ, Ono A, Freed EO, Hu WS, Pathak VK. Human APOBEC3G is incorporated into HIV-1 virions through interactions with viral and nonviral RNAs. J Biol Chem 2004; 279(34):35822-8; PMID:15210704 [DOI] [PubMed] [Google Scholar]
- 159.Huthoff H., Malim MH. Identification of amino acid residues in APOBEC3G required for regulation by human immunodeficiency virus type 1 Vif and Virion encapsidation. J Virol 2007; 81(8):3807-15; PMID:17267497; http://dx.doi.org/ 10.1128/JVI.02795-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Belanger K, Langlois MA. RNA-binding residues in the N-terminus of APOBEC3G influence its DNA sequence specificity and retrovirus restriction efficiency. Virology 2015; 483:141-148; PMID:25974865; http://dx.doi.org/ 10.1016/j.virol.2015.04.019 [DOI] [PubMed] [Google Scholar]
- 161.Lavens D, Peelman F, Van der Heyden J, Uyttendaele I, Catteeuw D, Verhee A, Van Schoubroeck B, Kurth J, Hallenberger S, Clayton R, Tavernier J. Definition of the interacting interfaces of Apobec3G and HIV-1 Vif using MAPPIT mutagenesis analysis. Nucleic Acids Res 2010; 38(6):1902-12; PMID:20015971; http://dx.doi.org/ 10.1093/nar/gkp1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dance GS, Sowden MP, Cartegni L, Cooper E, Krainer AR, Smith HC. Two proteins essential for apolipoprotein B mRNA editing are expressed from a single gene through alternative splicing. J Biol Chem 2002; 277(15):12703-9; PMID:11815617; http://dx.doi.org/ 10.1074/jbc.M111337200 [DOI] [PubMed] [Google Scholar]
- 163.Mehta A, Driscoll DM. Identification of Domains in APOBEC-1 Complementation Factor Required for RNA Binding and Apolipoprotein B mRNA editing. RNA 2002; 8:69-82; PMID:11871661; http://dx.doi.org/ 10.1017/S1355838202015649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lehmann D.M., Galloway CA, Sowden MP, Smith HC. Metabolic regulation of apoB mRNA editing is associated with phosphorylation of APOBEC-1 complementation factor. Nucleic Acids Res 2006; 34(11):3299-308; PMID:16820530; http://dx.doi.org/ 10.1093/nar/gkl417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fossat N., Tourle K, Radziewic T, Barratt K, Liebhold D, Studdert JB, Power M, Jones V, Loebel DA, Tam PP. C to U RNA editing mediated by APOBEC1 requires RNA-binding protein RBM47. EMBO Rep 2014; 15(8):903-10; PMID:24916387; http://dx.doi.org/ 10.15252/embr.201438450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yang Y, Smith HC. Multiple protein domains determine the cell type-specific nuclear distribution of the catalytic subunit required for apolipoprotein B mRNA editing. Proc Natl Acad Sci U S A 1997; 94(24):13075-80; PMID:9371802; http://dx.doi.org/ 10.1073/pnas.94.24.13075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sowden MP, Smith HC. Commitment of apolipoprotein B RNA to the splicing pathway regulates cytidine-to-uridine editing-site utilization. Biochem J 2001; 359(Pt 3):697-705; PMID:11672445; http://dx.doi.org/ 10.1042/bj3590697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Patenaude AM, Di Noia JM. The mechanisms regulating the subcellular localization of AID. Nucleus 2010; 1(4):325-331; PMID:21327080; http://dx.doi.org/ 10.4161/nucl.1.4.12107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brar SS, Watson M, Diaz M. Activation-induced cytosine deaminase (AID) is actively exported out of the nucleus but retained by the induction of DNA breaks. J Biol Chem 2004; 279(25):26395-401; PMID:15087440; http://dx.doi.org/ 10.1074/jbc.M403503200 [DOI] [PubMed] [Google Scholar]
- 170.Bennett RP, Diner E, Sowden MP, Lees JA, Wedekind JE, Smith HC. APOBEC-1 and AID are nucleo-cytoplasmic trafficking proteins but APOBEC3G cannot traffic. Biochem Biophys Res Commun 2006; 350(1):214-9; PMID:16999936; http://dx.doi.org/ 10.1016/j.bbrc.2006.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ellyard JI, Benk AS, Taylor B, Rada C, Neuberger MS. The dependence of Ig class-switching on the nuclear export sequence of AID likely reflects interaction with factors additional to Crm1 exportin. Eur J Immunol 2011; 41(2):485-90; PMID:21268017; http://dx.doi.org/ 10.1002/eji.201041011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Aoufouchi S, Faili A, Zober C, D'Orlando O, Weller S, Weill JC, Reynaud CA. Proteasomal degradation restricts the nuclear lifespan of AID. J Exp Med 2008; 205(6):1357-68; PMID:18474627; http://dx.doi.org/ 10.1084/jem.20070950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.McCarthy H, Wierda WG, Barron LL, Cromwell CC, Wang J, Coombes KR, Rangel R, Elenitoba-Johnson KS, Keating MJ, Abruzzo LV. High expression of activation-induced cytidine deaminase (AID) and splice variants is a distinctive feature of poor-prognosis chronic lymphocytic leukemia. Blood 2003; 101(12):4903-8; PMID:12586616; http://dx.doi.org/ 10.1182/blood-2002-09-2906 [DOI] [PubMed] [Google Scholar]
- 174.Wu X, Darce JR, Chang SK, Nowakowski GS, Jelinek DF. Alternative splicing regulates activation-induced cytidine deaminase (AID): implications for suppression of AID mutagenic activity in normal and malignant B cells. Blood 2008; 112(12):4675-82; PMID:18684869; http://dx.doi.org/ 10.1182/blood-2008-03-145995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Methot SP, Litzler LC, Trajtenberg F, Zahn A, Robert F, Pelletier J, Buschiazzo A, Magor BG, Di Noia JM. Consecutive interactions with HSP90 and eEF1A underlie a functional maturation and storage pathway of AID in the cytoplasm. J Exp Med 2015; 212(4):581-96; PMID:25824822; http://dx.doi.org/ 10.1084/jem.20141157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nonaka T, Doi T, Toyoshima T, Muramatsu M, Honjo T, Kinoshita K. Carboxy-terminal domain of AID required for its mRNA complex formation in vivo. Proc Natl Acad Sci U S A 2009; 106(8):2747-51; PMID:19196959; http://dx.doi.org/ 10.1073/pnas.0812957106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kreisberg JF, Yonemoto W, Greene WC. Endogenous factors enhance HIV infection of tissue naive CD4 T cells by stimulating high molecular mass APOBEC3G complex formation. J Exp Med 2006; 203(4):865-70; PMID:16606671; http://dx.doi.org/ 10.1084/jem.20051856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Stopak KS, Chiu YL, Kropp J, Grant RM, Greene WC. Distinct patterns of cytokine regulation of APOBEC3G expression and activity in primary lymphocytes, macrophages, and dendritic cells. J Biol Chem 2007; 282(6):3539-46; PMID:17110377; http://dx.doi.org/ 10.1074/jbc.M610138200 [DOI] [PubMed] [Google Scholar]
- 179.Soros VB, Yonemoto W, Greene WC. Newly synthesized APOBEC3G is incorporated into HIV virions, inhibited by HIV RNA, and subsequently activated by RNase H. PLoS Pathog 2007; 3(2):e15; PMID:17291161; http://dx.doi.org/ 10.1371/journal.ppat.0030015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gallois-Montbrun S, Kramer B, Swanson CM, Byers H, Lynham S, Ward M, Malim MH. Antiviral protein APOBEC3G localizes to ribonucleoprotein complexes found in P bodies and stress granules. J Virol 2007; 81(5):2165-78; PMID:17166910; http://dx.doi.org/ 10.1128/JVI.02287-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chaurasiya KR, McCauley MJ, Wang W, Qualley DF, Wu T, Kitamura S, Geertsema H, Chan DS, Hertz A, Iwatani Y, et al.. Oligomerization transforms human APOBEC3G from an efficient enzyme to a slowly dissociating nucleic acid-binding protein. Nat Chem 2014; 6(1):28-33; PMID:24345943; http://dx.doi.org/ 10.1038/nchem.1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang T, Tian C, Zhang W, Sarkis PT, Yu XF. Interaction with 7SL RNA but not with HIV-1 genomic RNA or P bodies is required for APOBEC3F virion packaging. J Mol Biol 2008; 375(4):1098-112; PMID:18067920; http://dx.doi.org/ 10.1016/j.jmb.2007.11.017 [DOI] [PubMed] [Google Scholar]
- 183.Zhen A, Du J, Zhou X, Xiong Y, Yu XF. Reduced APOBEC3H variant anti-viral activities are associated with altered RNA binding activities. PLoS One 2012; 7(7):e38771; PMID:22859935; http://dx.doi.org/ 10.1371/journal.pone.0038771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bogerd HP, Cullen BR. Single-stranded RNA facilitates nucleocapsid: APOBEC3G complex formation. RNA 2008; 14(6):1228-36; PMID:18456846; http://dx.doi.org/ 10.1261/rna.964708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Alce TM, Popik W. APOBEC3G is incorporated into virus-like particles by a direct interaction with HIV-1 Gag nucleocapsid protein. J Biol Chem 2004; 279(33):34083-6; PMID:15215254; http://dx.doi.org/ 10.1074/jbc.C400235200 [DOI] [PubMed] [Google Scholar]
- 186.Cen S, Guo F, Niu M, Saadatmand J, Deflassieux J, Kleiman L. The interaction between HIV-1 Gag and APOBEC3G. J Biol Chem 2004; 279(32):33177-84; PMID:15159405; http://dx.doi.org/ 10.1074/jbc.M402062200 [DOI] [PubMed] [Google Scholar]
- 187.Luo K, Liu B, Xiao Z, Yu Y, Yu X, Gorelick R, Yu XF. Amino-terminal region of the human immunodeficiency virus type 1 nucleocapsid is required for human APOBEC3G packaging. J Virol 2004; 78(21):11841-52; PMID:15479826; http://dx.doi.org/ 10.1128/JVI.78.21.11841-11852.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Schafer A, Bogerd HP, Cullen BR. Specific packaging of APOBEC3G into HIV-1 virions is mediated by the nucleocapsid domain of the gag polyprotein precursor. Virol 2004; 328(2):163-8; PMID:15464836; http://dx.doi.org/ 10.1016/j.virol.2004.08.006 [DOI] [PubMed] [Google Scholar]


