Abstract
As an innovative non-antibiotic approach, antimicrobial blue light in the spectrum of 400–470 nm has demonstrated its intrinsic antimicrobial properties resulting from the presence of endogenous photosensitizing chromophores in pathogenic microbes and, subsequently, its promise as a counteracter of antibiotic resistance. Since we published our last review of antimicrobial blue light in 2012, there have been a substantial number of new studies reported in this area. Here we provide an updated overview of the findings from the new studies over the past 5 years, including the efficacy of antimicrobial blue light inactivation of different microbes, its mechanism of action, synergism of antimicrobial blue light with other angents, its effect on host cells and tissues, the potential development of resistance to antimicrobial blue light by microbes, and a novel interstitial delivery approach of antimicrobial blue light. The potential new applications of antimicrobial blue light are also discussed.
Keywords: antimicrobial blue light, non-antibiotic approach, endogenous photosensitizer, antibiotic resistance, microbe, bacterium, fungus, infection
1. Introduction
Antibiotic resistance of pathogenic microbes is a quickly growing and extremely dangerous health threat. It is now indisputable that antibiotic resistance is life-threatening in the same sense as cancer, both in the number of cases and the likely outcome (Bush et al., 2011). The extensive use of antibiotics is the single most important factor leading to antibiotic resistance (Cowen et al., 2014; Hampton, 2013; Rice, 2003). There is consequently a critical need for the development of new approaches to tackle antibiotic resistance. Recently, Dr. Karen Bush and 29 other scientists who are experts in antibiotic resistance pointed out that: “The investigation of novel non-antibiotic approaches for the prevention of and protection against infectious diseases should be encouraged, and such approaches must be high-priority research and development projects.” (Bush et al., 2011).
As an innovative non-antibiotic approach, antimicrobial blue light (aBL) in the spectrum of 400–470 nm has demonstrated its intrinsic antimicrobial properties resulting from the presence of endogenous photosensitizing chromophores in pathogenic microbes. It is envisioned that microbes are less able to develop resistance to aBL than to traditional antibiotics, because of the multi-target characteristics of aBL (Dai et al., 2012). In addition, it is well accepted that aBL is much less detrimental to host cells than UVC irradiation (Kleinpenning et al., 2010; Liebmann et al., 2010). Since we published our last review of aBL in 2012 (Dai et al., 2012), there have been a substantial number of new studies reported in this area. This review aims to update the findings from the new studies, including the efficacy of aBL inactivation of different microbes, its mechanism of action, synergism of aBL with other antimicrobials, its effect on host cells and tissues, the potential development of resistance to aBL by microbes, and a novel interstitial delivery approach of aBL. The potential new applications of aBL are also discussed.
2. Efficacy of antimicrobial blue light inactivation of pathogenic microbes
A wide range of microbial species was studied for aBL inactivation in the past five years, including Gram-positive bacteria, Gram-negative bacteria, mycobacteria, molds, yeasts and dermatophytes. The studies were carried out in vitro using planktonic cells or biofilms, ex vivo, and in vivo using animal models (pre-clinical) and in patients (clinical trials).
2.1. In vitro studies
2.1.1. Antimicrobial blue light inactivation of planktonic microbial cells
In vitro studies of planktonic microbial cells are usually performed in buffer suspensions or on agar plates.
a) Nosocomial bacterial pathogens
Healthcare-acquired infections (HAIs) are infections that patients acquire during the course of receiving treatment for other conditions in healthcare facilities (NIH, 2015). These infections are a leading cause of morbidity and mortality in the United States (Magill et al., 2014; McFee, 2009). According to recent studies, on any given day, about 1 in 25 patients has at least one HAI. There are an estimated 722,000 HAIs annually and about 75,000 patients with HAI die during their hospitalization (Magill et al., 2014). The total annual costs for HAI are as high as $10 billion in the United States (Zimlichman et al., 2013). The situation is exacerbated by the continued emergence of multidrug-resistant pathogenic microbes (Hidron et al., 2008; Sievert et al., 2013).
Halstead et al. (Halstead et al., 2016) recently assessed the effect of aBL at 400 nm from a light-emitting diode (LED) array on 34 bacterial strains commonly causing HAI, including Acinetobacter baumannii, Enterobacter cloacae, Stenotrophomonas maltophilia, Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Enterococcus faecium, Klebsiella pneumoniae, and Elizabethkingia meningoseptica. All the bacteria in phosphate-buffered saline (PBS) suspensions were found to be susceptible to aBL inactivation, with the majority (71%) demonstrating a >5-log10 decrease in colony-forming units (CFU) after exposures of 54 to 108 J/cm2 aBL.
The result of the above study is quantitatively in line with the observations in our group using aBL at 415 nm from LED arrays for inactivation of P. aeruginosa, A. baumannii, methicillin-resistant Staphylococcus aureus (MRSA), and Candida albicans in PBS suspensions. For P. aeruginosa, when 110 J/cm2 aBL was delivered, an approximately 7.64-log10 CFU inactivation was achieved (Dai et al., 2013b). For A. baumannii and C. albicans, over 4-log10 CFU were inactivated after an exposure of 70 J/cm2 aBL (Zhang et al., 2016; Zhang et al., 2014). The inactivation curves of the above pathogens approximately followed first-order kinetics (Xiong et al., 1999), a linear relation between the log-transformed cell survival fraction log10 and the aBL exposure. aBL inactivation of MRSA resulted in a survival kinetic with a shoulder or lag phase (Dai et al., 2013a). Only modest inactivation (0.17-log10) was observed in the lag phase up until 56 J/cm2 aBL was delivered. In the linear phase, approximately 4.75-log10 inactivation was achieved after 112 J/cm2 more aBL was delivered.
Similar observations were also reported by Barneck et al. (Barneck et al., 2016). It was evidenced by the investigators that 405-nm aBL successfully inactivated S. aureus, Streptococcus pneumoniae, E. coli, and P. aeruginosa seeded on agar plates. At an exposure of 133 J/cm2, reductions in log10 CFU were 6.27 for E. coli, 6.10 for S. aureus, 5.20 for P. aeruginosa, and 6.01 for S. pneumoniae. In another study from the same group of authors, the investigators found that β-lactam-resistant E. coli, which is resistant to penicillins, cephamycin, and carbapenems, is sensitive to aBL inactivation (Rhodes et al., 2016). Over 6-log10 CFU reduction of the β-lactam-resistant E. coli strain on agar plates was achieved after an exposure of 68 J/cm2 aBL.
In another study, a panel of microbial isolates from cases of infected joint arthroplasty were tested (Gupta et al., 2015), including 39 isolates of Gram-positive bacteria (S. aureus, S. epidermidis, E. faecalis, S. pneumoniae, Corynebacterium striatum, Coagulase negative Staphylococcus, etc), 11 isolates of Gram-negative bacteria (E. coli, K. pneumoniae, P. aeruginosa, and Serratia marcescens) and one isolate of C. albicans. aBL was emitted from a high-intensity narrow-spectrum light at 405 nm. Complete inactivation (> 4-log10 CFU) in suspensions was achieved in all of the isolates tested. The aBL exposures required for the complete inactivation of these pathogens ranged between 118 and 2214 J/cm2, with E. coli showing the highest tolerance and S. epidermidis the lowest. In this study, the authors reported that Gram-positive bacteria were generally found to be more susceptible to aBL than Gram-negative bacteria.
Makdoumi et al. compared the antimicrobial efficacy of 412 and 450 nm against MRSA planktonic cells (Makdoumi et al., 2017). Fifteen (15) or 40 μL MRSA suspensions were placed on microscope slides, creating fluid layers with 0.40 mm thick. At an exposure of 5.4 J/cm2, the reduction of MRSA CFU was minimal for both wavelengths. At an exposure of 28.5 J/cm2, the CFU reductions were 72% with 412 nm and 81% with 450 nm. The investigators also showed that the addition of riboflavin increased the antimicrobial efficacy of both aBL wavelengths.
de Sousa analyzed the influence of aBL emitted from a blue laser on the bacterial growth of S. aureus, P. aeruginosa, and E. coli (de Sousa et al., 2015b). Bacterial suspensions were exposed to a single exposure of a 450-nm blue laser at varying radiant exposures from 0 to 24 J/cm2. It was shown that the blue laser exhibited the bacterial inhibitory effect against S. aureus and P. aeruginosa at the exposures > 6 J/cm2, and against E. coli >3 J/cm2.
Fang et al employed 470-nm aBL emitted from a LED-array to inactivate P. aeruginosa (Fang et al., 2015). Suspensions of P. aeruginosa were exposed to aBL at the irradiance of 100 mW/cm2. After 80-min aBL exposure, 92.4% inactivation of P. aeruginosa was produced. The less effective inactivation of P. aeruginosa using 470-nm aBL is possibly due to the lack of sufficient amount of flavins in the bacterial strain used.
b) Periodontal bacterial pathogens
Periodontal diseases include infections that affect the periodontium. Bacterial plaque, a biofilm or mass of bacteria that develops over the surface of teeth, is the most common cause of periodontal disease. Left untreated, periodontal diseases can eventually result in tooth loss, and may have effects on general health; for example, periodontal disease has been associated with an increased risk of stroke, heart attack, and other health problems (Bouchard et al., 2017; Mira et al., 2017).
In a recent study, two anaerobic periodontal pathogens, Fusobacterium nucleatum and Porphyromonas gingivalis, were found to be susceptible to aBL with the spectrum of 400–520 nm (Song et al., 2013). Results exhibited that complete inactivation (6-log10 CFU) of bacteria on agar plates was achieved at 30 J/cm2 for F. nucleatum and 7.5 J/cm2 for P. gingivalis. In another study, Yoshida et al. showed that P. gingivalis in suspensions was effectively inactivated with 460-nm LED, and an exposure of 100 J/cm2 produced approximately 4-log10 CFU inactivation of P. gingivalis (Yoshida et al., 2017).
Cieplik et al. treated another periodontal pathogen, Aggregatibacter actinomycetemcomitans, with aBL at 460 nm derived from a LED (Cieplik et al., 2014). The investigators observed a ≥ 5-log10 reduction in CFU of A. actinomycetemcomitans in suspensions after an exposure of 150 J/cm2 aBL, whereas no effect of aBL was found against E. coli at the same aBL exposure. Spectrally resolved measurements of singlet oxygen luminescence showed clearly that a singlet oxygen signal was generated from lysed A. actinomycetemcomitans upon excitation at 460 nm.
Chui et al. investigated whether exposure to aBL kills P. gingivalis or only inhibits its growth (Chui et al., 2012) under anaerobic conditions. P. gingivalis suspensions were exposed to aBL (425–500 nm) at 135 J/cm2 anaerobically. The investigators observed delayed growth of P. gingivalis after the aBL exposure. The RNA integrity number value indicated no RNA degradation in the aBL-treated cultures. The expression levels of stress-related genes remained either constant or increased 15 min after the aBL exposure compared to that before the aBL exposure, thus suggesting that aBL may not kill P. gingivalis cells anaerobically. The investigators further proved that the inhibition of P. gingivalis after aBL exposure was induced by suppressing the expression of genes associated with chromosomal DNA replication and cell division (Chui et al., 2012).
In a similar study carried out by Hope et al. (Hope et al., 2013), a series of experiments were undertaken to inactivate P. gingivalis in suspensions under strict anaerobic conditions using two different 405-nm light sources: a hand-held light source (11.4 mW/cm2) or a laser pointer (328.5 mW/cm2). The lowest radiant exposure of the hand-held light source (0.34 J/cm2; 30 s irradiation) yielded an inactivation of 63.4% which increased to 98.1% (1.72-log10) at a higher radiant exposure (3.42 J/cm2; 300 s irradiation). Similarly, the laser pointer achieved an inactivation of 90.2% (1.01-log10) at a lower radiant exposure tested (9.86 J/cm2; 30 s) and 99.5% (2.36-log10) at the highest radiant exposure (98.55 J/cm2; 300 s).
c) Foodborne bacterial pathogens
Bacterial foodborne illnesses, or food poisoning, present a widespread and growing public health problem of recent times. The US FDA estimates that there are about 48 million cases of foodborne illness annually in the US, a number that translates into 1 in 6 Americans was sickened by foodborne illnesses each year. Each year in the US foodborne illnesses result in an estimated 128,000 hospitalizations and 3,000 deaths (CDC, 2016).
Salmonella enterica Heidelberg and Salmonella enterica Typhimurium are common serovars associated with foodborne infections. Bumah et al. reported the efficacy of aBL inactivation of the two species (Bumah et al., 2015b). Bacterial cultures on agar plates were exposed to aBL at 470 nm. For S. Typhimurium, 31% inactivation was achieved at the exposure of 55 J/cm2 and 93% inactivation at 110 J/cm2; while S. Heidelberg was inactivated by 11% and 84%, respectively, by the two exposures. Complete inactivation (≥2-log10 CFU) of each Salmonella strain was achieved using 165 or 220 J/cm2.
Kim and Yuk (Kim and Yuk, 2017) determined the effectiveness of aBL against Salmonella at 4°C, which is the ideal condition for food storage. First, 18 strains of Salmonella plated on the surface of agar were exposed to 405-nm LED at a set temperature of 4°C. S. Enteritidis ATCC 13076 and S. Saintpaul ATCC 9712 were found to be the most and the least susceptible strain to aBL, respectively. When the aBL exposure reached 576 J/cm2, 5.6- and 1.7-log10 CFU reductions were observed in S. Enteritidis and S. Saintpaul, respectively.
Listeria monocytogenes is another bacterial foodborne pathogen that can cause life-threatening infections in humans. O’Donoghue and his colleagues examined the effects of aBL (460–470 nm) on the growth and survival of this pathogen (O’Donoghue et al., 2016). Bacterial cultures were either spotted on agar plates or suspended in Brain-heart infusion (BHI) medium. The investigators reported that the growth of L. monocytogenes was inhibited at comparatively low aBL irradiance of 1.5–2 mW/cm2 (24 h irradiation), and that at higher irradiance of 8 mW/cm2, L. monocytogenes cells were killed. The investigators also presented evidence suggesting that aBL inhibited L. monocytogenes by the production of reactive oxygen species (ROS), such as hydrogen peroxide. Finally, the investigators showed that activation of the general stress response by aBL had a negative effect on growth, probably because cellular resources were diverted into protective mechanisms rather than growth.
Murdoch and co-workers exploited the effect of 405 nm aBL on taxonomically diverse bacterial pathogens, including three species of enteric facultatively anaerobic Gram-negative bacilli (S. enterica, Shigella sonneil, and E. coli), facultatively anaerobic Gram-positive coccobacillus L. monocytogenes, and aerobic, acid fast Gram-positive bacillus Mycobacterium terrae (Murdoch et al., 2012). In suspensions, S. enteritidis and E. coli were inactivated by 3.5-log10 CFU and 5-log10 CFU, respectively, at an aBL exposure of 288 J/cm2, and S. sonnei was inactivated by 5-log10 CFU at a lower exposure of 180 J/cm2. M. terrae was inactivated by 4–5 log10 CFU between an exposure of 144 and 288 J/cm2, and L. monocytogenes was inactivated by 5-log10 CFU at 108 J/cm2. For bacteria seeded on agar plates, almost complete (100%) inactivation was achieved in all the four bacterial species studied. L. monocytogenes again showed the highest sensitivity to aBL, with 100% reduction at an average exposure of 128 J/cm2. S. enterica, S. sonnei, and E. coli were inactivated by 2.28- (100%), 2.10- (99.3%) and 2.18-log10 CFU (99.8%), respectively, at an average exposure of 192 J/cm2.
Kim et al illustrated the antimicrobial effect of 405-nm LED on three Gram-positive foodborne pathogens B. cereus, L. monocytogenes, and S. aureus under refrigerated condition (4 °C) (Kim et al., 2015). The refrigerated condition was chosen to mimic an ideal food storage condition. After an exposure of 486 J/cm2 (7.5 h irradiation at 18 mW/cm2), approximately 1.9-, 2.1-, and 1.0-log10 CFU reductions were observed in the suspensions of B. cereus, L. monocytogenes, and S. aureus, respectively.
Roh et al. successfully demonstrated the antimicrobial activity of 405 and 465-nm LEDs against major bacterial pathogens that affect fish and shellfish in aquaculture (Roh et al., 2016). Seven bacterial pathogens, including Vibrio anguillarum, Edwardsiella tarda, Aeromonas salmonicida, Vibrio harveyi, Photobacterium damselae, Streptococcus iniae, and Streptococcus parauberis, were studied. All seven pathogens were observed to be susceptible to 405 nm aBL, with P. damselae, V. anguillarum, and E. tarda showing the highest susceptibility. Approximately 3.2- to 3.8-log10 CFU inactivation in bacterial suspensions was achieved after 137–262 J/cm2 aBL at 405 nm was delivered. In this study, Gram-negative bacteria (P. damselae, V. anguillarum, and E. tarda) were found to be more susceptible to 405 nm aBL than were Gram-positive bacteria (S. parauberis and S. iniae). The antimicrobial efficacy of 465-nm aBL is generally lower than that of 405-nm. In addition, the authors observed that higher initial bacterial concentrations in the suspensions were associated with lower antimicrobial effect of aBL.
d) Waterborne pathogens
Waterborne disease is a global burden which causes 2.2 million mortality and innumerable cases of morbidity every year (Ramirez-Castillo et al., 2015). As such, Schmid et al. investigated the photoinactivation of Legionella using 405-nm aBL (Schmid et al., 2017). After an aBL exposure of 125 J/cm2 was delivered, over 5-log10 CFU of Legionella rubrilucens on agar plates was inactivated. Due to safety and regulatory issues, the investigators used a non-pathogenic species L. rubrilucens. However, the results are probably transferable to pathogenic Legionella species based on the observation that the UVC susceptibility of different Legionella species is only slightly different (Schmid et al., 2017).
e) Plant pathogens
Plant pathogens cause many serious diseases of plants throughout the world. It was documented that aBL at 470 nm was able to reduce the viability of plant pathogens including Leuconostoc mesenteroides, Bacillus atrophaeus, P. aeruginosa, and Fusarium graminearum (De Lucca et al., 2012). Complete inactivation of these pathogens on agar plates (≥2.87-log10 CFU) was observed after 80 J/cm2 exposure for B. atrophaeus; 96% inactivation after 8 J/cm2 for P. aeruginosa; 80% inactivation after 180 J/cm2 for L. mesenteroides, and 47% inactivation after 100 J/cm2 for F. graminearum. In contrast, aBL had no effect on the viability of P. digitatum.
f) Veterinary pathogens
Schnedeker et al. measured the bactericidal activity of 465 nm aBL from LEDs on methicillin-susceptible Staphylococcus pseudintermedius (MSSP) and methicillin-resistant Staphylococcus pseudintermedius (MRSP), which are the most common pathogens of bacterial skin infections in dogs, and compared it with that on MRSA (Schnedeker et al., 2017). After an exposure of 112.5 J/cm2, complete eradication (>2-log10 CFU) of MRSA on agar plates was observed. In contrast, the reduction of MSSP and MRSP on agar plates was minimal with maximum reduction of 11.7% for MSSP and 21.2% for MRSP at the highest aBL exposure of 225 J/cm2. The low susceptibility of MSSP and MRSP might be attributed to the lack of endogenous flavins with the light absorption peak at around 460 nm in these two species.
g) Intracellular bacteria
Chlamydia trachomatis is an intracellular bacterium that resides in the conjunctival and reproductive tract mucosae and is responsible for an array of acute and chronic diseases. Using 405-nm aBL at varying exposures of 0–20 J/cm2, Wasson et al treated C. trachomatis infected endocervical epithelial cells, HeLa (Wasson et al., 2012). The results demonstrated a significant dose-dependent inhibitory effect on the growth of C. trachomatis following aBL exposure (41% at 5 J/cm2 and 75% at 20 J/cm2). Diminished bacterial load corresponded to lower IL-6 concentrations in the HeLa cells.
f) Bacterial endospores
Endospores are hardy, defensive structures that enable some bacteria to survive harmful environmental conditions, such as chemical disinfectants. The extraordinary drug-resistance properties of endospores make them of particular importance. Maclean et al determined the susceptibility of endospores of Bacillus cereus, Bacillus subtilis, Bacillus megaterium, and Clostridium difficile to aBL at 405 nm (Maclean et al., 2013). Up to a 4-log10 CFU reduction of spore population in suspensions was achieved after an exposure of 1.73 kJ/cm2. The exposures required for inactivation of B. cereus and C. difficile endospores were significantly higher than those required for inactivation of the counterparts of vegetative cells, where 4-log10 CFU reductions were achieved after exposures of 108 and 48 J/cm2, respectively. The significant increase in the aBL exposure required for the inactivation of endospores compared with vegetative cells is consistent with the notorious resilience of these microbial structures to many antibacterial agents.
g) Mycobacteria
Guffey et al. reported that aBL at 405 nm was effective in inactivating Mycobacterium smegmatis, which is a non-pathogenic model system for the pathogenic Mycobacterium tuberculosis (Guffey et al., 2013a). All aBL exposures from 60 to 250 J/cm2 produced a significant inactivation rate with the highest exposure (250 J/cm2) demonstrating 100% inactivation.
h) Fungal pathogens (dermatophytes, molds, and yeast)
It is also well documented that aBL was effective against several species of fungal pathogens, such as dermatophytes, molds, and yeasts. Moorhead et al. used 405-nm aBL for inhibiting the growth of Trichophyton rubrum, Trichophyton mentagrophytes, and Aspergillus niger (Moorhead et al., 2016b). On agar plates, the growth of the microconidia of T. rubrum and T. mentagrophytes was completely inhibited after an exposure of 504 J/cm2 aBL. A. niger conidia showed greater resistance, and colonial growth developed after aBL exposure. In suspensions, an exposure of 360 J/cm2 resulted in complete inactivation of T. rubrum microconidia, whereas A. niger showed greater resistance. At an exposure of 1440 J/cm2, although A. niger hyphae were completely inactivated, only a 3-log10 reduction of a 5-log10 conidial suspension was achieved. The high resistance of A. niger to aBL is most likely due to the multilayered pigmented spore coat containing aspergillin (Ray and Eakin, 1975).
Guffey et al. irradiated T. mentagrophytes on agar plates with multiple applications of a relatively low exposure of aBL (20 J/cm2) at 405 nm (Guffey et al., 2017). The authors observed that 3–5 applications of aBL over 28 h resulted in significant inactivation of T. mentagrophytes; 4 and 5 applications produced the greatest inactivation of 84.7% and 93.6%, respectively.
In another study, 405-nm light was successfully applied for the inactivation of Saccharomyces cerevisiae, C. albicans, and dormant and germinating conidia of A. niger (Murdoch et al., 2013). To achieve a 5-log10 CFU inactivation in fungal suspensions, the required aBL exposures were 288 J/cm2 for S. cerevisiae, 576 J/cm2 for C. albicans, and a much higher value of 2.3 kJ/cm2 for dormant conidia of A. niger. Upon germination, the susceptibility of A. niger conidia to aBL significantly increased. The investigators speculated this increase in susceptibility of A. niger conidia to aBL was related to morphological changes, e.g., increased light penetration associated with stretching or fracture of the dense pigmented spore coat, or to an increased metabolic vulnerability to light-induced ROS or to a combination of these effects. The study also revealed that aBL inactivation of fungi involved an oxygen-dependent mechanism, as previously described for bacteria.
Trzaska et al tested the efficacy of aBL against six common fungal pathogens, including Rhizopus microsporus, Mucor circinelloides, Scedosporium apiospermum, Scedosporium prolificans, Fusarium oxysporum, and Fusarium solani (Trzaska et al., 2017). The authors showed that aBL at 405 nm from LEDs effectively inactivated S. apiospermum, S. prolificans, F. oxysporum, and F. solani. When an exposure of 216 J/cm2 aBL had been delivered, fungal conidia in suspensions were almost completely inactivated, equivalent to ≥ 3-log10 CFU inactivation. However, aBL showed no effect on R. microspores and M. circinelloides. Interestingly, the authors observed a morphological change of fungal cells during germination into yeast-like budding, which was suppressed by aBL. In addition, the authors found that aBL could effectively stop the germination (growth from conidia to hyphae) of all fungal pathogens, including R. microspores and M. circinelloides.
i) Virus
In contrast to a substantial number of studies of aBL inactivation of bacteria and fungi, very few studies have been reported on aBL inactivation of viruses. Recently, Tomb et al. determined the viricidal efficacy of 405-nm aBL using feline calicivirus suspended in a minimal medium (DPBS) or several organically-rich media (Tomb et al., 2016). In DPBS, a 4-log10 plaque forming units (PFU) reduction was observed after an aBL exposure of 2.8 kJ/cm2 (irradiance 155.8 mW/cm2). In the organically-rich media, such as artificial faeces, artificial saliva, blood plasma, etc., feline calicivirus exhibited an equivalent level of inactivation under 50–85% less aBL exposures. The authors hypothesized that the increased aBL susceptibility of feline calicivirus in the organically-rich media was due to the photosensitizing components presenting in the organically-rich media.
In another study, the same investigators used the bacteriophage ϕC31, as a surrogate for non-enveloped double-stranded DNA viruses, to investigate whether aBL (405 nm) was viricidal (Bumah et al., 2015c). ϕC31 suspended in minimal media, nutrient-rich media, and porphyrin solution demonstrated differing sensitivity of the phage to aBL. Significant reductions in phage titer occurred when exposed to aBL in nutrient-rich media, with approximate 3-, 5-, and 7-log10 reductions achieved after aBL exposures of 0.3, 0.5, and 1.4 kJ/cm2, respectively. When suspended in minimal media, only 0.3-log10 reduction occurred after an aBL exposure of 306 J/cm2, which was much lower than the 2.7- and > 2.5-log10 reductions achieved under the same aBL exposure in nutrient-rich or porphyrin-supplemented media, suggesting that the accelerated inactivation was due to the aBL-excitation of photosensitizing components in the media. The reduced susceptibility of viruses in minimal media, compared with that of bacteria and fungi, provides further evidence that the antimicrobial action of aBL was predominantly attributed to the aBL-excitation of endogenous photosensitizing molecules such as porphyrins within aBL-susceptible microbes.
2.1.2. Parameters influencing the efficacy of aBL
a) Initial inoculum of microbial suspension
The higher initial microbial inoculum results in higher optical density in the microbial suspension, and subsequently induces higher light attenuation in the microbial suspension according to the Beer-Lambert law. In addition, at higher microbial inoculum oxygen might be consumed faster than it is re-supplied by diffusion from the surface of the initial suspension (Hessling et al., 2017; Vollmerhausen et al., 2017). Bumah et al. investigated the effect of bacterial inoculum on the efficacy of aBL at 405 and 470 nm (Bumah et al., 2015a). At the inoculum of 3×106 CFU/mL, nearly 40% and 50% growth of MRSA were suppressed with as low as 3 J/cm2 of 405 nm and 470 nm wavelengths. Moreover, 100% of the colonies were suppressed with a single exposure to 55 or 60 J/cm2 aBL at 470 nm or double exposures with 50, 55 or 60 J/cm2 aBL at 405 nm. At the inoculum of 5×106 CFU/mL, irradiating twice with 50, 55 or 60 J/cm2 aBL of either wavelength suppressed bacterial growth completely. The culture at the higher inoculum 7×106 CFU/mL required higher exposures to achieve 100% suppression, either one treatment with 220 J/cm2 at 470 nm or two treatments of the same exposure using 405 nm.
In a similar study, Vollmerhausen et al determined the efficacy of 420-nm aBL for inactivation of uropathogenic E coli in suspensions of urine mucin media at varying initial bacterial inocula from 103 to 107 CFU/mL (Vollmerhausen et al., 2017). At the inoculum of 103 CFU/mL, aBL reduced E. coli to below the detection level after an aBL exposure of 86.4 J/cm2 (6 h irradiation). At 105 CFU/mL, although there was an initial decrease of E coli CFU observed after an exposure of 115.2 J/cm2 (8 h irradiation), the CFU recovered by 24 h and reached the same population as the dark control. This was associated with a decline in dissolved oxygen levels in E coli suspensions after 8 h. At 107 CFU/mL, no reduction in bacterial CFU was detected after an aBL exposure of 28.8 J/cm2 (2 h irradiation). After 2 h, the dissolved oxygen in E coli suspensions decreased from an initial concentration of 18.0% to 7.3%.
b) Growth phase of microbes
Keshishyan et al. compared the response of bacteria to aBL (450 nm) inactivation in lag and log phase (Keshishyan et al., 2015). E. coli, S. aureus, and P. aeruginosa in suspensions containing 150 CFU/mL were studied. In latent phase (5 °C), complete inactivation was achieved for all three bacterial species after an exposure of 117 J/cm2. The effectiveness of aBL inactivation was lower for log-phase (37 °C) bacteria than latent-phase bacteria, and 14–50% inactivation of the bacteria was observed under the same aBL exposure. The investigators suggested that intracellular flavins acted as photosensitizers.
Abana et al. delineated the effectiveness of aBL at 455 nm throughout different growth phases of E coli, i.e., log, transition, and early stationary phases (Abana et al., 2017). Five E coli strains belonging to different phylogenetic groups and in suspensions were studied. Of all the strains tested, only DH5α displayed 1.5 to 2.5-log10 CFU inactivation following an aBL exposure of 120 J/cm2, and this reduction of CFU was conserved during all phases of bacterial growth. During stationary and transition phase, MG1655 exhibited statistically insignificant changes in CFU as a result of aBL exposure; though, nearly 1-log10 CFU inactivation of this strain was observed during exponential phase. The commensal E343 and E402 and multidrug-resistant UPEC strain EC958 showed modest and statistically insignificant susceptibility to aBL during all growth phases. The enterotoxigenic (ETEC) E9034A was more susceptible to aBL in exponential phase compared to transition and stationary phases where CFU were minimally reduced. UPEC strains UTI89 and EC958, and EHEC strain Sakai were most susceptible in stationary phase.
c) pH of suspensions
Ghate et al. revealed that pH played a role in the efficacy of aBL (Ghate et al., 2015). In their study, E. coli, S. Typhimurium, and L. monocytogenes in suspensions were exposed to 461-nm aBL at pH values of 4.5, 6.0, 7.3, 8.0, and 9.5, and temperature of 15 °C. After an exposure of 597 J/cm2 (irradiance 22.1 mW/cm2), the populations of E. coli decreased by averagely 2.1-, 1.2-, and 4.1-log10 at pH 4.5, 7.3, and 9.5 respectively. For S. Typhimurium, treatment at pH 8.0 and 9.5 reduced the bacterial population by 1.8- and 4.7-log10, respectively, after the same exposure of 597 J/cm2, and this was significantly higher than 0.4- and 2.0-log10 CFU reductions obtained at pH values of 6.0 and 4.5, respectively under the same aBL exposure. For L. monocytogenes, approximately 5.8-log10 CFU reduction was observed after 239 J/cm2 at pH 4.5, while the bacterial population was reduced by only 1.8-log10 at pH 9.5 after 597 J/cm2. The results highlight the enhanced effectiveness of aBL under acidic and alkaline pH conditions, suggesting its potential synergism with acidic and alkaline antimicrobials.
In a similar study, McKenzie et al. assessed the efficacy of 405 nm light for inactivation of E. coli and L. monocytogenes under sub-lethally stressed environmental conditions (McKenzie et al., 2014). Bacteria in suspensions were exposed to aBL under various temperature (4°C, 22°C, and 45°C), salt (0%, 0.8%, 10%, and 15% NaCl) and acid (pH 3, 3.5, and 7) conditions. Enhanced aBL inactivation of both E. coli and L. monocytogenes was observed under each of the sub-lethal stresses. The greatest enhancement of aBL inactivation for both the bacterial species was achieved under the sub-lethal acid stress conditions of pH 3. This effect was demonstrated by a 5-log10 CFU inactivation of E. coli following an aBL exposure of 84 J/cm2 compared to 378 J/cm2 for non-stressed populations; and by a 5-log10 CFU reduction of L. monocytogenes achieved with an aBL exposure of 42 J/cm2 compared to 84 J/cm2 required for the equivalent reduction of non-stressed populations.
d) Culture temperature
Ghate et al. also studied the effect of culture temperature on the efficacy of aBL inactivation of foodborne pathogens including E. coli, S. Typhimurium, L. monocytogenes and S. aureus (Ghate et al., 2013). For all of these bacterial species, 4.6 to 5.2-log10 CFU reductions were achieved at 10 and 15 °C after 596.7 J/cm2 aBL at 461 nm. The efficacy of aBL inactivation was greater at the temperatures of 10 and 15 °C than at 20 °C. Regardless of the culture temperature, sub-lethal injury was observed in all bacterial species during the aBL exposure and the percentage of injured cells increased with the treatment time.
Contradictory findings were, however, reported by Kumar et al. (Kumar et al., 2016, 2015). In their first study, the investigators found that aBL (405 nm) inactivation of S. aureus in suspensions was significantly higher at 25 °C (4.0-log10 CFU after 306 J/cm2 exposure) compared to 10 and 4 °C (2.1- and 1.9-log10 CFU, respectively, after 306 J/cm2 exposure). A similar effect was observed for aBL inactivation of S. Typhimurium. In contrast, aBL inactivation of B. cereus and L. monocytogenes was found to be independent of change in temperature. In the second study of aBL inactivation of Lactobacillus plantarum, S. aureus, and Vibrio parahaemolyticus, variation in the temperature from 4 to 10 and 25°C did not result in any noticeable effect.
e) Pulsed aBL exposure vs. continuous aBL exposure
Gillespie et al. compared the antimicrobial and operational efficacy of pulsed and continuously operated 405-nm LEDs (Gillespie et al., 2016). S. aureus suspensions containing 103 CFU/mL were exposed to continuous-wave 405 nm light for different lengths of time or pulsed 405-nm light of different frequencies, duty cycles, and intensities and for different lengths of time. Pulsed exposures with the same average irradiance of 16 mW/cm2 and varying duty cycle (25%, 50%, 75%) showed very similar efficacy compared with continuous exposures, with 95–98% reductions of S. aureus achieved for all duty cycles. The pulsing frequency was varied in intervals from 100 Hz to 0 kHz and appeared to have little effect on the antimicrobial efficacy. When comparing pulsed with continuous exposure, however, an improvement in inactivation per unit optical energy was achieved, with results showing an increase of approximately 83% in optical efficiency.
f) Single application vs. double/multiple applications of aBL under equivalent radiant exposures
Biener et al. demonstrated in their work that two applications of aBL were more effective in inactivating MRSA than a single application with the equivalent exposure (Biener et al., 2017). Two groups of MRSA cultures on agar plates were exposed to a single exposure of 405-nm aBL at 121 J/cm2 and to two exposures of aBL from the same light source at 60.5 J/cm2 for each exposure with 30 min between, and approximately 1.05- and 1.71-log10 inactivation of MRSA CFU were observed, respectively. According to fluorescence analysis, the authors indicated that MRSA produced endogenous photosensitizers only during certain phases of the cell cycle and therefore were susceptible to aBL inactivation only while expressing endogenous photosensitizers. Thirty (30) min time interval was long enough for more bacteria to enter the appropriate cell cycle phase and produce endogenous photosensitizers.
g) Non-coherent LED vs. coherent laser
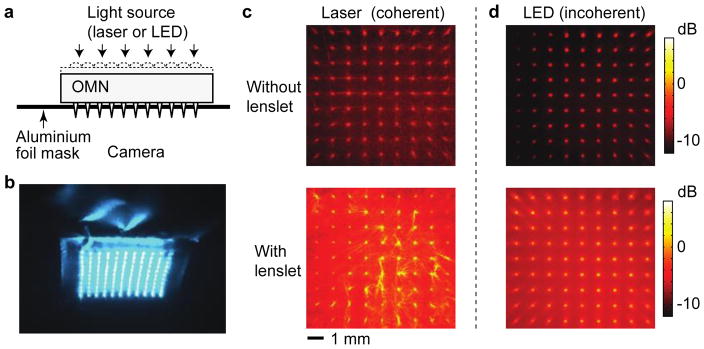
Masson-Meyers et al. compared the relative antimicrobial effect of non-coherent 405 nm LED and coherent 405 nm laser on MRSA (Masson-Meyers et al., 2015). Both the LED and laser irradiation at the exposures of 40–121 J/cm2 resulted in statistically significant bacterial growth suppression. The antimicrobial effect of the two light sources, LED and laser, was not statistically different at each radiant exposure in 35 of the 36 experimental trials. The investigators, thus, concluded that light coherence does not seem to play a significant role in the cellular response of bacteria to aBL.
h) High irradiance with short exposure time of aBL vs. low irradiance with long exposure time of aBL
Investigators also determined the potential difference in antimicrobial effect between high irradiance with short exposure time and low irradiance with long exposure time under equivalent radiant exposures (Barneck et al., 2016; Murdoch et al., 2012). Barneck et al. reported that with the equivalent radiant exposures of 130 J/cm2, 250 min irradiation at 9 mW/cm2 and 1000 min at 2.25 mW/cm2 resulted in similar log10-CFU reduction in all the bacterial species tested (S. aureus, S. pneumoniae, E. coli, and P. aeruginosa) (Barneck et al., 2016). However, in another study, Mudoch et al. observed a significant difference in the antimicrobial effect of aBL on L. monocytogenes at equivalent radiant exposures while adjusting irradiance and exposure time with longer exposure exhibiting a greater effect of aBL inactivation (Murdoch et al., 2012).
i) Blue light vs. other visible wavelengths
A study aiming to compare the antimicrobial efficacy of 405, 460, and 520-nm (green) LEDs was carried out by Kumar et al. (Kumar et al., 2016). Three foodborne pathogens L. plantarum, S. aureus, and V. parahaemolyticus were tested. It was shown that exposure to 405 and 460 nm LEDs produced significant inactivation of V. parahaemolyticus in suspensions (>4 log10 CFU) after 600 and 1800 J/cm2 aBL were delivered. Exposure to 405 nm also produced significant inactivation of L. plantarum in suspensions (2–3 log10 CFU), while S. aureus showed relatively less susceptibility to aBL. The 520 nm LED, in contrast, produced negligible inactivation in all the three bacteria at the exposures of up to 1800 J/cm2.
In another study, the same investigators compared the antimicrobial efficacy between 405- and 520-nm LED by using six other strains of foodborne pathogens (Kumar et al., 2015), including three Gram-positive strains (B. cereus, L. monocytogenes, and S. aureus) and three Gram-negative strains (P. aeruginosa, S. Typhimurium, and E. coli). Exposure of 306 J/cm2 aBL from the 405-nm LED brought 4.0-log10 CFU inactivation of S. aureus in suspensions. However, with the 405-nm LED, less than 0.6-log10 CFU and 0.5-log10 CFU inactivation was observed in S. Typhimurium and E. coli, respectively. With the 520-nm LED at ten-fold higher radiant exposure 3,060 J/cm2, the inactivation of S. aureus was 1.2-log10 CFU. The 520-nm LED was ineffective against E. coli and S. Typhimurium. Treatment of B. cereus and L. monocytogenes with the 405-nm LED reduced the bacterial population by 2.3- and 1.9-log10, respectively, while the 520-nm LED reduced the L. monocytogenes population by 0.7-log10. Both the 405- and 520-nm LEDs failed to bring about any significant inactivation in the population of P. aeruginosa (strain ATCC 10145).
Kim et al. compared the antimicrobial effect of 425 nm (aBL) with 525 (green) and 625 nm (red) light (Kim et al., 2013). P. gingivalis, S. aureus, and E. coli were tested. The investigators found that P. gingivalis and E. coli were killed by 425 nm, and S. aureus growth was inhibited by 525 nm. However, 625 nm had no antimicrobial effect on any of the three bacterial species.
In general, the blue spectrum showed higher antimicrobial efficacy than other visible spectra.
Table 1 summarizes the studies of aBL inactivation of planktonic microbial cells.
Table 1.
Summary of antimicrobial blue light inactivation of planktonic microbes
| Microbes | Wavelength (nm) | Radiant exposure (J/cm2) | Inactivation efficacy | Reference |
|---|---|---|---|---|
| A. baumannii, E. cloacae, S. maltophilia, P. aeruginosa, E. coli, S. aureus, E. faecium, K. pneumoniae, E. meningoseptica | 400 | 50–108 | >5-log10 CFU | (Halstead et al., 2016) |
| P. aeruginosa | 415 | 110 | 7.64-log10 CFU | (Dai et al., 2013b) |
| P. aeruginosa | 470 | 480 | 92.4% | (Fang et al., 2015) |
| A. baumannii | 415 | 70 | > 4-log10 CFU | (Zhang et al., 2014) |
| S. aureus, S. pneumoniae, E. coli, P. aeruginosa | 405 | 133 | 5.20–6.27 log10 CFU | (Barneck et al., 2016) |
| E. coli | 405 | 67.5 | > 6-log10 CFU | (Rhodes et al., 2016) |
| S. aureus, S. epidermidis, E. faecalis, S. pneumoniae, C. striatum, E. coli, K. pneumoniae, P. aeruginosa, S. marcescens, etc. | 405 | 118–2214 | > 4-log10 CFU | (Gupta et al., 2015) |
| MRSA | 415 | 168 | 4.82-log10 CFU | (Dai et al., 2013a) |
| MRSA | 412 | 28.5 | 72% | (Makdoumi et al., 2017) |
| MRSA | 450 | 28.5 | 81% | (Makdoumi et al., 2017) |
| MRSA | 405 | 121 | 91.2% | (Biener et al., 2017) |
| MRSA | 465 | 112.5 | >2-log10 CFU | (Schnedeker et al., 2017) |
| F. nucleatum, P. gingivalis | 400–520 | 7.5–30 | 6-log10 CFU | (Song et al., 2013) |
| P. gingivalis | 460 | 100 | 4-log10 CFU | (Yoshida et al., 2017) |
| A. actinomycetemcomitans | 460 | 150 | >5-log10 CFU | (Cieplik et al., 2014) |
| P. gingivalis | 405 | 98.6 | 2.3-log10 CFU | (Hope et al., 2013) |
| S. Heidelberg, S. Typhimurium | 470 | 165–220 | >2-log10 CFU | (Bumah et al., 2015b) |
| L. monocytogenes | 460–470 | 75.6–101 | > 6-log10 CFU | (O’Donoghue et al., 2016) |
| S. enterica, S. sonneiI, E. coli, L. monocytogenes, M. terrae | 405 | 108–288 | 3.5–5 log10 CFU | (Murdoch et al., 2012) |
| V. anguillarum, E. tarda, A. salmonicida, V. harveyi, P. damselae, S. iniae, S. parauberis | 405 | 137–260 | 3.2–4.3 log10 CFU | (Roh et al., 2016) |
| V. anguillarum, E. tarda, A. salmonicida, P. damselae, S. iniae, S. parauberis | 465 | 247–2178 | 2.9–4 log10 CFU | (Roh et al., 2016) |
| L. mesenteroides, B. atrophaeus, P. aeruginosa, F. graminearum | 470 | 80–180 | 47% to 2.87-log10 CFU | (De Lucca et al., 2012) |
| C. trachomatis | 405 | 5–20 | 40%–75% | (Wasson et al., 2012) |
| B. cereus, B. subtilis, B. megaterium, C. difficile (vegetative cells) | 405 | 48–108 | 4-log10 CFU | (Maclean et al., 2013) |
| B. cereus, B. subtilis, B. megaterium, C. difficile (endospores) | 405 | 1730 | 4-log10 CFU | (Maclean et al., 2013) |
| M. smegmatis | 405 | 250 | 100% | (Guffey et al., 2013a) |
| T. rubrum, T. mentagrophytes | 405 | 504 | 100% | (Moorhead et al., 2016b) |
| T. mentagrophytes | 405 | 60–100 | 84.7%–93.6% | (Guffey et al., 2017) |
| A. niger | 405 | 1440 | 3-log10 CFU | (Moorhead et al., 2016b) |
| S. cerevisiae, C. albicans | 405 | 288–576 | 5-log10 CFU | (Murdoch et al., 2013) |
| A. niger | 405 | 2300 | 5-log10 CFU | (Murdoch et al., 2013) |
| C. albicans | 415 | 70 | 5.42-log10 CFU | (Zhang et al., 2016) |
| C. albicans | 405 | 332 | 4.52-log10 CFU | (Gupta et al., 2015) |
| S. apiospermum, S. prolificans, F. oxysporum, F. solani | 405 | 216 | ≥ 3-log10 CFU | (Trzaska et al., 2017) |
2.1.3. Antimicrobial blue light inactivation of biofilms
It has been estimated that many microbial infections in humans are associated with biofilms (Romling and Balsalobre, 2012). Biofilms are characterized as highly resistant to antibiotic treatment and immune responses. Therefore, it is important to study the effectiveness of aBL on biofilms.
Rosa et al determined the effectiveness of 455-nm aBL to reduce the load of S. aureus and C. albicans biofilms applied to compact bone tissue specimens (Rosa et al., 2016). For the groups of S. aureus biofilms, all samples treated with aBL at the exposures of 4.5–45.2 J/cm2 showed reductions in bacterial load compared with the untreated samples. The largest reduction, 3.2-log10 CFU, was obtained in the group receiving 45.2 J/cm2 aBL. For the groups of C. albicans biofilms, those samples receiving 22.6, 31.6 and 45.2 J/cm2 aBL presented a significant difference in the reduction of bacterial load compared with the untreated samples, with the largest CFU reduction of 2.3-log10 when receiving 45.2 J/cm2 aBL.
In another study, Halstead et al. investigated a panel of 34 bacterial isolates comprising A. baumannii, E. cloacae, S. maltophilia, P. aeruginosa, E. coli, S. aureus, E. faecium, K. pneumoniae, and E. meningoseptica (Halstead et al., 2016). Biofilms were formed by seeding bacterial suspensions in 96-well microtiter plates and incubating at 33 °C for 72 h. After an exposure of 216 J/cm2 aBL at 400 nm, 34.6–96.4% reduction in the viability of bacteria in biofilms was observed depending on the bacterial strains. Biofilms formed by Gram-negative species were more susceptible to aBL inactivation than those formed by Gram-positive species.
McKenzie et al. investigated the efficacy of aBL at 405 nm for the inactivation of bacteria attached as biofilms to glass and acrylic (McKenzie et al., 2013). The reductions in bacterial population achieved after an exposure of 168 J/cm2 were similar between the two Gram-negative species (P. aeruginosa and E. coli, 3.6-log10 CFU), with which complete inactivation was achieved after an aBL exposure of 504 J/cm2, and between the two Gram-positive bacteria (L. monocytogenes and S. aureus, 2.6-log10 CFU). Successful inactivation was also observed in the poly-microbial biofilms containing both E. coli and S. aureus, with a 2.19-log10 CFU reduction after an exposure of 252 J/cm2.
Our group performed studies of aBL inactivation of P. aeruginosa and A. baumannii biofilms using bioluminescent strains (Wang et al., 2016). Biofilms were formed by incubating bacterial suspensions in 96-well microtiter plates for 24 to 72 h. Viability of bacterial cells in biofilms were quantified based on the bacterial luminescence. Exposure of 24-h-old and 72-h-old A. baumannii biofilms to 432 J/cm2 aBL resulted in inactivation of 3.59 log10 and 3.18-log10 CFU, respectively. For P. aeruginosa biofilms, similar levels of inactivation, 3.02-log10 and 3.12-log10 CFU, respectively, were achieved under the same aBL exposure.
Vollmerhausen et al. employed 420-nm aBL to treat uropathogenic E. coli attached to the silicone matrix of a urinary catheter (Vollmerhausen et al., 2017). First, the investigators showed that aBL was able to prevent E. coli biofilm formation by measuring bacterial adherence to the silicone surfaces. When the biofilms formed after 24 h incubation of E. coli on the silicone surfaces, 1.3-log10 CFU inactivation of biofilm in PBS was observed after an aBL exposure of 216 J/cm2 was delivered (24 h irradiation).
In areas related to oral health care, bacterial biofilms are found in dental unit water lines, on tooth surfaces and dental prosthetic appliances, and on oral mucous membranes. Fontana et al. evaluated the effect of aBL at 455 nm on periodontal biofilm growth in vitro (Fontana et al., 2015). Biofilms were formed by inoculating 150 μL bacterial suspensions into blood agar wells of 96-well microtiter plates, and were then exposed to aBL at 50 mW/cm2 for 4 min (or 12 J/cm2) immediately following bacterial inoculation, and 1, 2 and 3 days post-inoculation (i.e., total aBL exposure of 48 J/cm2). Following aBL exposure, the mean survival fraction of bacteria was reduced by 48.2% in biofilms. Except for P. gingivalis, the relative abundances of F. nucleatum ss. nucleatum, F. nucleatum ss. polymorphum, F. nucleatum ss. vincentii, Fusobacterium periodonticum, Prevotella intermedia, P. melaninogenica, and P. nigrescens were reduced after aBL exposure. However, only the relative abundances of F. nucleatum ss. vincentii were reduced significantly. When all the eight aBL-sensitive species were grouped together, their 3 % reduction of relative abundance was statistically significant. The effect of aBL on biofilms also included a statistically significant increase in the relative abundances of Streptococcus gordonii and Streptococcus mitis and a statistically significant reduction of Parvimonas micra. In a similar study, Song et al. observed a decreasing tendency of P. gingivalis CFU in biofilms with increasing aBL exposure, but not A. actinomycetemcomitans and F. nucleatum (Song et al., 2013).
It was reported that the surface roughness of dental implant has a significant impact on the amount of bacterial biofilm. Therefore, using a 405-nm LED, Giannelli et al. treated S. aureus biofilm adherent to titanium discs with moderately rough titanium oxide coating similar to the osseoconductive surface of dental implants (Giannelli et al., 2017). An aBL exposure of 315 J/cm2 resulted in 1.55-log10 CFU reduction in S. aureus biofilms. Moreover, aBL also inhibited lipopolysaccharide bioactivity up to 42%, thereby blunting host inflammatory response.
De Sousa et al. determined how daily treatment with aBL affects the development and composition of a matrix-rich Streptococcus mutans biofilm, which is known as the major etiological agent in dental caries (de Sousa et al., 2015a). The biofilms were exposed twice-daily to aBL at 420 nm. For each treatment, 72 J/cm2 aBL was delivered. It was observed that bacterial viability in biofilms and dry mass of biofilms were reduced after aBL exposure. Morphology change was also visible in the biofilms after aBL treatment. In addition, insoluble extracellular polysaccharides were reduced significantly.
Another study of aBL inactivation of S. mutans biofilm was carried out by Gomez et al. (Gomez et al., 2016). In the study, biofilms cultured in 96-well microtiter plates were exposed to aBL (380–440 nm, peak emission 405 nm) at an exposure of 9.26 J/cm2. The results indicated a significant reduction in the growth rate of the aBL-treated groups in comparison to the untreated group. Biofilm viability assays confirmed a statistically significant difference between aBL-treated and non-treated groups (50–70% decrease).
Chebath-Taub et al. assessed the viability and structure of new biofilm formed by S. mutans that was previously exposed to aBL (400–500 nm) while immobilized in biofilm (Chebath-Taub et al., 2012). Under all light exposure conditions, re-growth of new biofilm after irradiation was not affected during the first 2 and 4 h. However, after 6 h incubation, the viability of the new bacterial biofilms varied markedly as a result of the different pre-exposures to aBL. A statistically significant decrease in CFU was observed in the 6-h biofilms formed by bacteria that had been previously exposed to aBL for 476 and 680 J/cm2. As examined with the confocal scanning laser microscopy, the new biofilm formed by previously exposed bacteria to aBL for more than 204 J/cm2 demonstrated a significant death of bacteria in the outer layer of the biofilm.
In a later study, the same investigators determined the sustained effects of aBL on the pathogenicity of the newly formed biofilm (Cohen-Berneron et al., 2016). The investigators found that bacterial growth in the regrown biofilms was increased when samples were previously exposed to aBL; however, a higher proportion of dead bacteria and a reduction in polysaccharide production was observed. The acidogenicity from the regrown biofilm was lowered as the exposures of aBL increased. The aciduricity of the regrown biofilm was decreased, indicating less growth of bacteria into biofilm in low pH with increasing exposures.
Table 2 shows the summary of the in vitro studies on aBL inactivation of microbes in biofilms.
Table 2.
Summary of antimicrobial blue light inactivation of microbes in biofilm
| Microbes | Wavelength (nm) | Radiant exposure (J/cm2) | Inactivation efficacy | Reference |
|---|---|---|---|---|
| S. aureus, C. albicans | 455 | 45 |
S. aureus: 3.2-log10 CFU C. albicans: 2.3-log 10 CFU |
(Rosa et al., 2016) |
| A. baumannii, E. cloacae, S. maltophilia, P. aeruginosa, E. coli, S. aureus, E. faecium, K. pneumoniae, E. meningoseptica | 400 | 216 | 34.6%–96.4% | (Halstead et al., 2016) |
| Uropathogenic E. coli | 420 | 216 | 1.3-log10 CFU (95.86%) | (Vollmerhausen et al., 2017) |
| P. aeruginosa, E. coli, L. monocytogenes, S. aureus | 405 | 168 |
P. aeruginosa, E. coli: 3.6-log10 CFU L. monocytogenes, S. aureus: 2.6-log10 CFU |
(McKenzie et al., 2013) |
| P. aeruginosa, A. baumannii | 415 | 432 |
P. aeruginosa: 3.02–3.12 log10 CFU A. baumannii: 3.18–3.59 log 10 CFU |
(Wang et al., 2016) |
| P. gingivalis, F. nucleatum ss. nucleatum, F. nucleatum ss. polymorphum, F. nucleatum ss. vincentii, Fusobacterium periodonticum, Prevotella intermedia, P. melaninogenica, P. nigresce | 455 | 48 | average 48.2% | (Fontana et al., 2015) |
| S. aureus | 405 | 315 | 1.55-log10 CFU | (Giannelli et al., 2017) |
| S. mutans | 380–440 | 9 | 50%–70% | (Gomez et al., 2016) |
2.2. Ex vivo studies
Gunther et al. investigated the use of 405-nm aBL to reduce Campylobacter jejuni and Campylobacter coli in poultry products (Gunther et al., 2016). Campylobacter in chicken exudate were placed onto chicken skin or food-grade stainless steel before aBL exposure. A range of aBL exposures were applied to cocktails of six C. jejuni or six C. coli strains in exudate. At the maximal radiant exposure of 185 J/cm2, the CFU reductions of C. jejuni and C. coli on poultry skin were 1.7- and 2.1-log10 CFU, respectively. Significant higher inactivation of Campylobacter was observed when the samples were placed on stainless steel, producing 4.9-log10 CFU reduction for C. jejuni and 5.1-log10 CFU for C. coli. The investigators noted, however, that significant aBL-mediated reductions in Campylobacter CFU required exposure times that might be impractical under processing conditions.
In a later study, the same investigators evaluated the ability of 405-nm aBL to inactivate multi-isolate cocktails of either Salmonella spp., pathogenic E. coli, Staphylococcus spp., or L. monocytogenes suspended in chicken purge or skin (Sommers et al., 2017). When exposed to 180 J/cm2 aBL at two separate irradiances (300 mW/cm2 or 150 mW/cm2) the maximum pathogen reduction on chicken skin was ca. 0.4-log10. When the pathogens were suspended in chicken purge the maximum reductions ranged from 0.23- to 0.68-log10 (180 J/cm2; 150 mW/cm2) versus 0.69- to 1.01-log10 (180 J/cm2; 300 mW/cm2). Log10 reductions in CFU of each pathogen, when they were subjected to heat shock prior to aBL exposure, were reduced, indicating that thermal effects accounted for much of the bacterial inactivation.
Susan et al. evaluated the effect of aBL at 405 and 464 nm on the growth of L. monocytogenes in a Ready-to Eat meat product (Motts et al., 2016). Ten (10) μL bacterial suspensions were instilled on the surface of the meat product, and then exposed to aBL at 10, 30, 60, 90, and 120 J/cm2, respectively. At each exposure of the two wavelengths, significant inhibition of L. monocytogenes was achieved with inactivation rate ranging from 69.55–85.25%. It is interesting that 405 nm at 60 J/cm2 exhibited the highest inactivation efficacy.
In a recent study of our group, we developed an ex vivo model of keratitis using rabbit eye balls and a bioluminescent strain of P. aeruginosa, and investigated the effectiveness of aBL at 415 nm for treating this disease (Zhu et al., 2017). When aBL was delivered 6 h post-inoculation, the bacterial load decreased with increasing aBL exposure and approximately 3-log10 CFU inactivation was achieved, compared to the untreated control, by exposure to 84 J/cm2 aBL. In contrast, when aBL was delivered 24 h post-inoculation, exposure to 84 J/cm2 aBL elicited less than 1-log10 inactivation. To achieve approximately 3-log10 CFU inactivation, exposure to 304 J/cm2 aBL was required.
2.3. In vivo studies
In vitro and ex vivo studies have been proved useful for the assessment of the effect of aBL. However, ultimate clinical outcome depends on many parameters besides in vitro and ex vivo susceptibility. There are many uncontrolled factors that may influence relevance of the in vitro conditions to the in vivo conditions. For example, local or host defense mechanisms in the in vivo conditions may act in synergism or antagonism with aBL. The micro-environment could also be quite different between the in vivo and in vitro conditions and, as a consequence, may affect the biosynthesis of the endogenous photosensitizing chromophores and subsequently affect the efficacy of aBL. In addition, the optical properties of human tissue are quite different from those of in vitro media and, thus, affect the light distribution in vivo. Microbial cells in vivo usually expose to lower aBL exposures since aBL attenuates significantly more in tissue. For biofilms, there are considerable differences in filamentation patterns, extracellular lipase activity and expression levels of genes encoding factors involved in adhesion and virulence, which may affect the efficacy of aBL (Coenye and Nelis, 2010). As a result, the clinical relevance will ultimately need to be confirmed by in-vivo studies (Fantin et al., 1991). Since 2012, one of the significant advances in the area of aBL has been the in vivo studies using murine models of infections and clinical trials.
2.3.1. Animal studies
Our group carried out several in vivo studies of aBL (415 nm) therapy for burns and wounds in mice infected with bioluminescent microbial strains, including P. aeruginosa (Amin et al., 2016; Dai et al., 2013b), A. baumannii (Zhang et al., 2014), MRSA (Dai et al., 2013a), and C. albicans (Zhang et al., 2016). The bacteria load was quantified using a bioluminescence imaging system. aBL therapy was initiated at 30 min to 24h after bacterial inoculation. We found that aBL significantly reduced bacterial load in mouse burns or wounds (>2-log10 CFU) and saved the lives of mice in the event of potentially lethal infections, after 55.8 to 432 J/cm2 aBL had been delivered.
Yang et al. reported the effectiveness of aBL at 460 nm on the treatment of excision wounds in mice infected with MRSA (Yang et al., 2017). aBL was started 3 h after bacterial inoculation, and the aBL-treated group received a daily exposure of 120 J/cm2 for 2 weeks. The results showed that the differences in bacterial load of the wounds between the aBL-treated and the untreated control groups were statistically significant at all the time points measured (day 1, 3, 7, and 14 post-inoculation). In addition, the survival rate of mice in the aBL-treated group was significantly higher than that in the control group (100% vs. 50%). Moreover, the treatment with aBL led to a faster wound healing on day 14 compared with the control group (healing population 90% vs. 18%).
In a very recent study, our group investigated aBL therapy at 415 nm for P. aeruginosa keratitis in mice (Zhu et al., 2017). When aBL was delivered 6 h post-inoculation, approximately 2.0-log10 inactivation of P. aeruginosa was achieved after an exposure of 36 J/cm2 aBL. It was also observed that the corneas of aBL-treated mice had significantly lower mean corneal pathology scores (indicating lower bacterial load) as compared to the corneas of the untreated mice. In addition, the analysis of the interaction between “aBL treatment” and “time post-infection” showed that the effect of “aBL treatment” increased with the “time post-infection”. When aBL was delivered 24 h after P. aeruginosa inoculation, the bacterial luminescence was eliminated after an exposure of 144 J/cm2 aBL. The aBL inactivation curve of P. aeruginosa revealed that a >2.5-log10 inactivation was achieved after an exposure of 144 J/cm2 aBL, compared to the untreated controls.
2.3.2. Clinical studies
In addition to the above in vivo studies using murine models, two pilot clinical studies of dental application were reported recently. In the first study, the effect of aBL at 455 nm on the bacterial composition of human dental plaque was investigated (Soukos et al., 2015). Eleven subjects (patients) who refrained from brushing for 3 days before and during aBL therapy participated in the study. aBL was applied to the buccal surfaces of premolar and molar teeth on one side of the mouth twice daily for 2 min (70 mW/cm2) over a period of 4 days (or 8.4 J/cm2 each treatment). The proportions of black-pigmented species P. gingivalis and P. intermedia were significantly reduced on the exposed side from their original proportions by 25 and 56 %, respectively, while no change was observed on the unexposed side. The proportional reduction of the aBL-exposed side was also observed in five other species (Streptococcus intermedius, F. nucleatum ss. vincentii, F. nucleatum ss. polymorphum, F. periodonticum, and Capnocytophaga sputigena) relative to the unexposed side, but statistical significance was not achieved. No aBL-induced adverse effect was observed.
The second study aimed to evaluate the efficacy of a low intensive aBL (405–420 nm) emitting toothbrushes (Genina et al., 2015). Selected subjects were randomly divided into two groups of 30 persons each. One group was treated with the aBL toothbrushes, and the other group (control) used a standard Braun oral-B manual toothbrush. After 4 weeks, a statistically significant improvement of all dental indices in comparison with the baseline (by 59%, 66%, and 82% for plaque, gingival bleeding, and inflammation, respectively) was observed. The aBL-treated group demonstrated up to 50% improvement relative to the control group. Microbiological analysis showed that, in addition to partial mechanical removal of bacteria, aBL inactivated up to 97.5% of the bacterial population.
3. Mechanism of action of antimicrobial blue light
The mechanism of action of aBL is still not fully understood. A common hypothesis is that aBL excites naturally occurring endogenous photosensitizing chromophores (iron-free porphyrins or/and flavins) in microbial cells, and subsequently leads to the production of cytotoxic ROS (Dai et al., 2012; Hamblin et al., 2005). However, to our knowledge, this hypothesis has yet to be rigorously tested until recently, especially for the pathogenic microbes commonly identified in infections.
3.1. Presence of endogenous photosensitizers
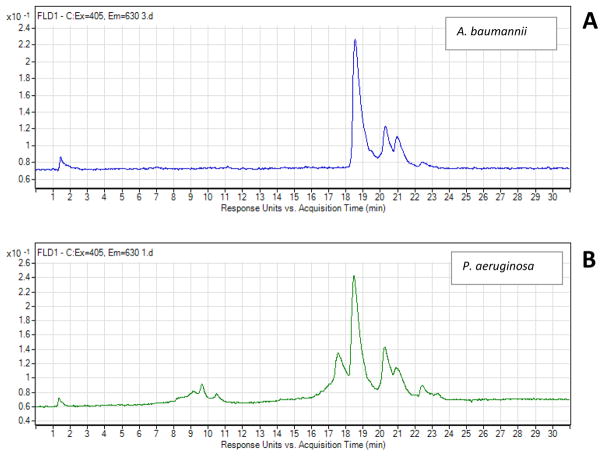
Recently, our group carried out several studies to determine the presence of endogenous photosensitizing chromophores in the aBL-sensitive microbial strains. P. aeruginosa (Dai et al., 2013b; Wang et al., 2016), A. baumannii (Wang et al., 2016; Zhang et al., 2014) and C. albicans (Zhang et al., 2016) were investigated by using fluorescence spectroscopy or HPLC analysis. All results implied the presence of endogenous porphyrins and/or flavins in the microbial cells. Figure 1 shows the HPLC chromatograms of P. aeruginosa and A. baumannii at the excitation of 405 nm and the emission of 630 nm. The emission peaks in the chromatograms indicate the presence of endogenous porphyrins. Similar studies from other investigators suggested the presence of both porphyrins and flavins in A. actinomycetemcomitans (Cieplik et al., 2014; Fyrestam et al., 2015), porphyrins, flavin adenine dinucleotide (FAD), and nicotinamide adenine dinucleotide (NADH) in MRSA (Biener et al., 2017), porphyrins in P. gingivalis (Fyrestam et al., 2015; Yoshida et al., 2017), porphyrins in S. cerevisiae (Fyrestam et al., 2015), and porphyrins (a mixture of coproporphyrin I, coproporphyrin III, and PpIX) in Helicobacter pylori (Battisti et al., 2017a; Battisti et al., 2017b) according to the spectroscopic or chromatographic analyses. Some other studies provided the evidence that the cytotoxicity of aBL to microbes is mediated by the aBL-induced production of ROS (Cieplik et al., 2014; Galbis-Martinez et al., 2012; O’Donoghue et al., 2016; Ramakrishnan et al., 2016; Yoshida et al., 2017).
Figure 1.
HPLC chromatograms of porphyrin extracts from A. baumannii (A) and P. aeruginosa (B) (Wang et al., 2016).
Nitzan et al. reported that, in response to 405-nm aBL, endogenous coproporphyrin, a precursor of protoporphyrin IX (PPIX), produces the majority of free radicals, and a higher amount of coproporphyrin in bacterial cells leads to higher inactivation of the bacteria, irrespective of the total porphyrin (different species of endogenous porphyrins) amount (Nitzan et al., 2004). Therefore, Kumar et al. determined the amount of endogenous coproporphyrins presenting in bacterial cells by using HPLC to understand the extent of inactivation of the bacteria (Kumar et al., 2015). Gram-positive bacteria had significantly higher coproporphyrin content per CFU (B. cereus: 5.25 ag/CFU, S. aureus: 3.38 ag/CFU, and L. monocytogenes: 2.03 ag/CFU) compared to the Gram-negative bacteria (S. Typhimurium: 0.21 ag/CFU, E. coli O157:H7: 0.14 ag/CFU, and P. aeruginosa: 0.67 ag/CFU).
Kim and Yuk quantified the amounts of endogenous coproporphyrin in S. Enteritidis ATCC 13076 and S. Saintpaul ATCC 9712, which were observed to be the most and the least susceptible strain to aBL among 18 strains of Salmonella tested, respectively, by using HPLC (Kim and Yuk, 2017). The investigators observed no significant difference in the amount of endogenous coproporphyrin between the two strains (P < 0.05). Therefore, the investigators concluded that the total coproporphyrin amount in bacteria was not a contributing factor to the differences in susceptibility to aBL.
The in vitro profiles of endogenous porphyrins (the amount and the species of porphyrins) were found to be affected by the culturing conditions of microbes, such as time of culturing, passaging, and growth medium (Fyrestam et al., 2016). For example, at day 3 of incubation, coproporphyrin III, PPIX, and coproporphyrin I were detected in A. actinomycetemcomitans with 80%, 16% and 4%, respectively, of molar total porphyrin content. However, at day 9, PPIX increased to be the most abundant porphyrin with 85% of the total porphyrin content, while coproporphyrin III decreased to 15% and coproporphyrin I was below limit of quantification. When cultivated colonies of A. actinomycetemcomitans were passaged onto a new, fresh growth medium, uroporphyrin and 7-carboxylporphyrin were detected in the passaged cultures at day 7 in addition to coproporphyrin III, PPIX and coproporphyrin I, but not in the non-passaged cultures. The total porphyrin amount increased by a factor of 28 from the non-passaged cultures to the passaged cultures.
3.2. DNA manipulation for elucidating the mechanism of aBL
Grinholc et al. (Grinholc et al., 2015) compared the aBL efficacy towards a wild-type S. aureus reference strain, which is capable of producing endogenous porphyrins, and its isogenic knockout mutant, which lacks the ability to produce endogenous porphyrins due to the knockout of porphyrin biosynthetic pathway. Exposure of the wildtype S. aureus strain to 10 J/cm2 aBL at 405 nm resulted in >2.5-log10 CFU reduction. In contrast, the mutant strain displayed no decrease in viability following the same aBL exposure, supporting the hypothesis that the antimicrobial effect of aBL is due to the photo-excitation of endogenously produced porphyrins.
In a similar study, Galbis-Martínez et al. compared the susceptibility to aBL between wide type Myxococcus xanthus, a mutant strain lacking of endogenous protoporphyrin IX (PPIX) due to deletion of hemB (ΔhemB mutant), and a mutant strain accumulating PPIX due to deletion of hemH (ΔhemH mutant) (Galbis-Martinez et al., 2012). HPLC analysis confirmed the presence of PPIX in the wild-type and ΔhemH strain and its absence in the ΔhemB mutant. Fluorescence and mass spectrometry revealed that the PPIX content (dry weight) in the ΔhemH mutant was about 30-fold higher than that in the wild type. It was further demonstrated that PPIX accumulation in ΔhemH mutant remarkably enhanced its susceptbility to aBL in comparison to the wide type strain, while the ΔhemB mutant showed no cellular response to aBL. The authors also proved that the interaction between endogenous PPIX and aBL led to the generation of singlet oxygen 1O2.
3.3. Cellular response of microbes to aBL
McKenzie et al. investigated the specific mechanism of cellular damage in response to aBL (405 nm) exposure (McKenzie et al., 2016). E. coli and S. aureus were selected as the representative Gram-negative and Gram-positive species in the study. Results of microscopic imaging showed membrane damage to both species after aBL exposure, with loss of salt and bile tolerance by S. aureus and E. coli, respectively, consistent with reduced membrane integrity. Increased nucleic acid release was also observed from the measurement of spectrophotometer, with up to 50 % increase in DNA concentration into the extracellular media in the case of both microbes. The above results are endorsed by the work of Biener et al., in which the authors suggested that the inactivation of bacteria by using aBL is due to the aBL-induced marked changes in the transmembrane potential, such as alterations in membrane integrity or disabling ion pumps that made the membrane more permeable to ions (Biener et al., 2017).
Using Fourier transform infrared (FTIR) spectroscopy coupled with principal component analysis followed by linear discriminant analysis (PCA-LDA), Bumah et al. aimed to elucidate the interaction between aBL (470 nm) and MRSA (Bumah et al., 2016). Loadings plot from PCA-LDA analysis revealed important functional groups in proteins, lipids, and nucleic acids region of the spectrum that are responsible for the classification of aBL irradiated spectra and unirradiated control spectra. Distinctive changes in DNA conformation in response to aBL exposure was detected. These findings indicated that exposure of MRSA to aBL induced A-DNA cleavage, leading to cell death. In addition, the investigators found that aBL inactivation of MRSA is complementary to and distinct from the known antimicrobial effect of vancomycin.
In agreement with the findings of Bumah et al. (Bumah et al., 2016), Yoshida et al suggested that the mechanism of aBL inactivation of P. gingivalis is due to the oxidative DNA damage according to their result of oxidative stredd assay (Yoshida et al., 2017). In addition, the authors demonstrated that the excitation of endogenous porphyrins (PPIX) led to the production of singlet oxygen 1O2 based on the measurement of electron spine resonance.
Fila and his colleagues (Fila et al., 2016) recently reported the first time that lethal aBL exposures at 405-nm (exposures that induced >3-log10 CFU inactivation) inactivated multiple virulence factors of P. aeruginosa including both cell-associated determinates and numerous secreted factors, indicating that aBL targets the pathogenic mechanism of P. aeruginosa. It was suggested that aBL inactivation of virulence factors is due to the activity of aBL-induced cytotoxic ROS. Cytotoxic ROS interacts with various biomolecules, leading to their destruction. The ability of aBL inactivation of virulence factors of pathogens renders aBL an advantageous approach over conventional antibiotics.
aBL (445 nm) was also shown to be effective in reducing the ochratoxin A production in Aspergillus carbonarius and Aspergillus westerdijkiae (Cheong et al., 2016). Ochratoxin A is classified as a Class 2B possible human carcinogen; it also has nephrotoxic, immunotoxic, teratogenic, genotoxic, and possibly neurotoxic properties in mammals. It occurs as a food contaminant in a wide range of products including cereals, grapes, cocoa, coffee, and spices.
3.4. aBL-induced genetic changes in microbes
Yang et al. (Yang et al., 2017) discovered that a total of 162 genes in MRSA genome were different between the aBL-treated (460 nm) cultures and the untreated control cultures. Of those 162 genes, 115 were up-regulated and 47 were down-regulated by aBL. Interestingly, 36 of the 115 up-regulated genes were phage-related, suggesting phage-related gene expressions are associated with aBL exposure. Therefore, 16 of the 36 phage-related genes were randomly selected to further confirm using real-time PCR. The results showed that the 16 phage-related genes in MRSA genome were up-regulated by aBL exposure.
Similarly, to better understand the mechanism of aBL inactivation of S. aureus, Adair and Drum performed a whole transcriptome analysis of S. aureus using RNA-Seq and analyzed the differential gene expression in response to aBL exposure (Adair and Drum, 2016). Bacterial suspensions were exposed to 250 J/cm2 aBL at 465 nm and then RNA was extracted from the S. aureus cultures. Transcriptomic comparisons using a cutoff of 5-fold identified a total of 28 genes were down-regulated and 6 genes up-regulated in the aBL-treated samples. Twenty-eight (28) out of the 34 differentially regulated genes fall into 8 functional categories: amino acid biosynthesis, cell envelope components, cellular processes, central intermediary metabolism, energy metabolism, protein synthesis, regulatory function, and transport and binding proteins. Five (5) genes encoded conserved proteins of unknown function.
Kim and Yuk observed that aBL induced genomic DNA oxidation and the loss of membrane functions in Salmonella cells, preferentially in efflux pump and glucose uptake activities (Kim and Yuk, 2017). TEM images clearly showed that genomes were one of the major targets of aBL. However, there is only slight damage to membrane potentials and integrity. Additionally, at the refrigeration temperature only oxyR was upregulated in non-irradiated and irradiated S. Enteritidis cells, whereas transcription levels of all the genes tested (oxyR, recA, rpoS, sodA, and soxR) were upregulated in non-irradiated and irradiated S. Saintpaul cells. As a result, the investigators suggested that gene expression levels might be altered by exposure to refrigeration temperature, by starving the cells, or by both conditions, rather than by aBL irradiation.
4. Synergism of antimicrobial blue light with other agents
Synergism is the effect of two drugs combined is stronger than that of the individual drug in the equivalent dose (Cokol et al., 2011), thereby increasing the therapeutic efficacy. Several studies revealed the synergistic effect between aBL and other agents, including nanoparticles, medicinal plants and their extracts, antibiotics, disinfectants, zinc salts, low intensity ultrasound, magnetic fields, and near infrared irradiation.
4.1. Synergism of antimicrobial blue light with nanoparticles
In a study of the combination therapy using aBL and silver nanoparticles, Akram et al. found that the antimicrobial activity of the combination therapy was significantly higher than each agent alone (Akram et al., 2016). MRSA suspensions with AgNPs at ½ to 1/128 minimum inhibitory concentration (MIC) were exposed to aBL at 460 nm and 250 mW/cm2 for 1 h. Complete inactivation of MRSA was achieved at 8 h after exposure to the combination therapy at all AgNPs concentrations tested.
In a similar study from the same group, Nour El Din et al. investigated the therapeutic application of aBL in combination with AgNPs against P. aeruginosa (Nour El Din et al., 2016). The antimicrobial activity of AgNPs and aBL at 460 nm (250 mW/cm2 for 1 h) was significantly enhanced when both agents were combined compared to each agent alone when AgNPs were tested at MIC, 1/2, or 1/4 MIC. Transmission electron microscopy showed significant damage to the bacterial cells that were treated with the combined therapy compared to the cells that received either the aBL or AgNPs alone. In addition, the combination treatment significantly inhibited biofilm formation by P. aeruginosa on gelatin discs compared to each agent individually. Finally, the combined therapy effectively treated a horse suffering from a chronic wound caused by polymicrobial infection.
In another study, Lipovsky et al. examined the antimicrobial efficacy of metal-oxide nanoparticles combined with aBL against S. aureus and S. epidermidis, known for their high prevalence in infected wounds (Lipovsky et al., 2011). Electron-spin resonance measurements revealed that metal-oxide (0.05 mg/ml ZnO or 0.1 mg/ml TiO2) nanoparticles were able to produce ROS in water suspension. A remarkable enhancement of ROS production by the nanoparticles was observed following exposure to aBL (415 nm, 100 mW/cm2, 5 min). In addition, aBL significantly enhanced the antimicrobial activity of the nanoparticles with 80–90% reduction in bacterial CFU. Nanoparticles alone and aBL alone showed no antimicrobial effect.
4.2. Synergism of antimicrobial blue light with the extracts from medicinal plants
4.2.1. Polyphenols
Recently, Nakamura et al. discovered the synergistic effect between aBL and polyphenols, which are naturally occurring polyphenolic compounds in fruits, nuts, and flowers (Nakamura et al., 2015; Nakamura et al., 2012). The polyphenols tested include caffeic acid, gallic acid, chlorogenic acid, epigallocatechin, epigallocatechin gallate, and proanthocyanidin. The bacteria studied were Gram-positive E. faecalis, S. aureus, and S. mutans, and Gram-negative A. actinomycetemcomitans, E. coli, and P. aeruginosa. Bacteria suspended in 1 mg/mL polyphenol aqueous solution were exposed to 78 or 156 J/cm2 aBL at 400 nm. Results showed that caffeic acid and chlorogenic acid had the highest antimicrobial activity against both Gram-positive and -negative bacteria followed by gallic acid and proanthocyanidin. In contrast, neither aBL alone nor polyphenols alone exhibited substantial bactericidal effect (Nakamura et al., 2012). Electron-spin resonance analysis demonstrated the generation of hydroxyl radicals by aBL-irradiation of polyphenols. Thus, the investigators suggested that aBL enhanced the antimicrobial activity of polyphenols via hydroxyl radical generation. The investigators also demonstrated that the disinfection treatment induced oxidative damage of bacterial DNA, which suggests that polyphenols are incorporated into bacterial cells.
As hydroxyl radicals cannot diffuse very far because they are so active, the affinity of polyphenols to bacterial is speculated to play an important role in the antimicrobial activity of aBL-irradiated polyphenols. It has been verified that polyphenols are more alible to penetrate into the cytoplasm and/or bound to the cytoplasmic membrane of Gram-negative bacteria than Gram-positive bacteria, resulting the fact that Gram-negative bacteria are more susceptible to aBL-irradiation of polyphenols than Gram-positive bacteria. The detailed mechanism about how polyphenols penetrate into bacterial cells needs further study.
Tsukada et al. verified the synergistic effects of 400-nm LED irradiation in combination with polyphenolic compounds extracted from wine lees against S. aureus, P. aeruginosa, and C. albicans (Tsukada et al., 2016a; Tsukada et al., 2016b). The study showed that the combination of aBL with polyphenolic compounds resulted in 3–4 log10 and >5-log10 CFU reductions in bacterial suspensions within 10 and 20 min, respectively. aBL alone also killed the bacteria, but the efficacy was 2–4 log10 CFU lower than that of the combination therapy. In contrast, almost no bacterial viability change occurred in the suspensions without aBL irradiation. The authors also analyzed the chemical composition and prooxidative profile of polyphenolic compounds.
4.2.2. Curcumin
Neelakantan et al. determined the anti-biofilm efficacy of the combination therapy using blue light (380–515 nm) with curcumin, a medicinal plant extract exhibiting antimicrobial effect, and compared it with the combination therapy of using ultrasonic files with curcumin (Neelakantan et al., 2015). E. faecalis biofilms grown within root canals irradiated with blue light (1200 mW/cm2 for 4 min) or activated with ultrasonic files (30 s cycles for 4 min). While both treatment groups showed a significantly higher percentage of dead bacteria in E. faecalis biofilms in comparison to the untreated controls, aBL activation exhibited significantly higher antimicrobial efficacy (97.3%) than ultrasonic agitation (39.0%).
A clinical study using the combination of aBL (450 ± 10nm) with curcumin for oral decontamination in orthodontic patients was carried out by Panhoca et al. (Panhoca et al., 2016). The result showed that aBL irradiated curcumin significantly reduced the S. mutans survival rate (from 6.33 ± 0.92 to 5.78 ± 0.96 log10 CFU, P < 0.05). When surfactant was further added to the combination, bacterial survival rate was further reduced (from 5.44 ± 0.94 to 3.83 ± 0.71, P < 0.01). The authors concluded that curcumin combined with aBL can be used as an adjutant and a convenient agent to promote the oral decontamination in clinical practice.
Recently, the antiviral activity of aBL in combination with curcumin for improving food safety was reported (Randazzo et al., 2016). At 37 °C, Randazzo et al found that aBL (464–476 nm LED, 3 J/cm2) irradiated curcumin (50 μg/mL) reduced feline calicivirus (FCV) titers by almost 5-log10. Lower antiviral activity (0.73-log10 TCID50/mL reduction) was observed for murine norovirus (MNV). At room temperature, curcumin at 5 μg/mL reduced FCV titers by 1.75-log10 TCID50/mL.
4.2.3. Essential oils
Essential oils are a type of mixtures extracted from aromatic plants. These extracts are currently used to inactivate the bacteria causing foodborne disease (Diao et al., 2013). In a study exploring the combination therapy of aBL with some essential oils, Marques-Calvo et al reported that aBL at 460–470 nm enhanced the antimicrobial activities of the clove and thyme oils against S. epidermidis, P. aeruginosa, and C. albicans (Marques-Calvo et al., 2016). In the study, essential oils were at 0.5% or 5% concentration. Microbial suspensions were incubated with essential oils for 15 min, then exposed to aBL for 15 min corresponding to 21.6 J/cm2.
4.3. Synergism of antimicrobial blue light with antibiotics
aBL was also found to be synergistic with some antibiotics. For example, the exposure of P. aeruginosa to sub-lethal aBL at 405 nm increased the susceptibility of P. aeruginosa to different antibiotics (gentamicin, ceftazidime, and meropenem), a phenomenon called hypersusceptibility (Fila et al., 2016). After being pretreated with a sub-lethal aBL exposure of 12.5 J/cm2, the MIC values of P. aeruginosa decreased by 2-, 8-, and even 64-fold lower.
Similar results were obtained in the study of Yasukawa et al. (Yasukawa et al., 2012). After being pre-treated with aBL (470 nm), E. coli cells expressing BsPAC became more susceptible to fosfomycin, nalidixic acid, and streptomycin than the cells incubated in the dark. In addition, the E. coli cells incubated with aBL formed more biofilms than the E. coli cells without being treated with aBL. The authors concluded from their observation that it is possible to determine the importance of cAMP in antibiotic susceptibility and biofilm formation of E. coli by photo-manipulating the cellular cAMP level using BsPAC. Most recently, Pereira et al. also reported the synergism between 470-nm aBL with ciprofloxacin or norfloxacin against E. coli and S. aureus (Pereira et al., 2017a; Pereira et al., 2017b).
However, in another study, Ramírez et al. reported that aBL reduced the susceptibility to minocycline and tigecycline of A. baumanni, E. coli, and S. aureus (Ramirez et al., 2015). The investigators assumed that the phenomenon was triggered by the generation of singlet oxygen 1O2, which ultimately led to the activation of resistance genes such as those coding for the efflux pump AdeABC.
4.4. Synergism of antimicrobial blue light with zinc salts
Zinc salts, such as zinc acetate, are commonly used as astringents and irritants. They also possess mild antibacterial effects. Sterer et al. reported that zinc acetate enhanced the effectiveness of aBL against malodour-producing bacteria in an experimental oral biofilm (Sterer et al., 2014). Biofilms were exposed to aBL (400–500 nm) at the exposures of 41, 82, and 164 J/cm2, respectively with or without the addition of zinc acetate. After aBL exposure, biofilm samples were examined for malodour production and Volatile Sulfur Compounds (VSC) production, and VSC-producing bacteria were quantified. Results showed that exposing experimental oral biofilm to the combination of aBL with zinc reduced malodour production, which coincided with a reduction in VSC-producing bacteria in the biofilm.
4.5. Synergism of antimicrobial blue light with disinfectants
Studies have also been carried out to explore the synergistic effect of aBL with hospital disinfectants, H2O2, Fenton regent, and HBO. Moorhead et al. established the efficacy of the combination therapy of aBL with low concentration chlorinated disinfectants for enhanced inactivation of C. difficile spores (Moorhead et al., 2016a). C. difficile spores suspended in the hospital disinfectants sodium hypochlorite, Actichlor or Tristel at non-lethal concentrations were exposed to aBL at 405 nm. Enhanced sporicidal activity was achieved when spores were exposed to aBL in the presence of the disinfectants, with a 99.9% reduction achieved following exposure to 33% less light exposure than required when exposed to aBL alone.
Feuerstein et al. evaluated the potential of enhancing the antimicrobial effect of aBL (450–490 nm) on S. mutans by making use of a potentially synergic antibacterial effect between aBL and H2O2 (Feuerstein et al., 2006). The investigators observed that the combination of aBL exposure for 20 s (23 J/cm2) with a concentration of 0.3 mM H2O2 yielded 96% growth inhibition, whereas, when applied separately, aBL exposure only decreased bacterial growth by 3% and H2O2 by 30% compared with the control. No direct effect of aBL on H2O2 degradation was observed, a partial protective effect of ROS scavengers on S. mutans and a non-lethal increase in the medium temperature after aBL exposure.
In another study, Lagori et al tested the disinfection efficacy of Fenton reagent (1.5% hydrogen peroxide and 0.15% iron gluconate) in combination with 400-nm aBL by using an endodontic pathogen E. faecalis (Lagori et al., 2017). When Fenton reagent was applied simultaneously with 4.0 J/cm2 aBL (i.e., photo-Fenton reaction), the bacterial population on discs was reduced from approximately 1.15×104 CFU to 0.43 CFU (i.e., >4.0-log10 CFU inactivation). In contrast, a pre-test determined that treatment with Fenton reagent or aBL alone at the equivalent doses had no effect on the bacterial growth.
Bumah et al. determined if combined treatment of 470-nm aBL (55 J/cm2) with hyperbaric oxygen (HBO) would eradicate MRSA infection (Bumah et al., 2015c). The investigators found that at no bacterial concentration did combined aBL and HBO treatment yield a statistically better inactivation efficacy than aBL alone. As a result, the investigators concluded that HBO did not act synergistically with aBL to heighten the inactivation efficacy.
4.6. Synergism of antimicrobial blue light with low intensity ultrasound
Schafer and McNeely proposed an approach to treat bacterial biofilms by simultaneous exposure to ultrasound and aBL (Schafer and McNeely, 2015). The investigators hypothesized that ultrasound would “activate” the bacteria within the biofilm such that they would become susceptible to aBL. aBL was delivered using 405-nm LEDs at an irradiance of 30 mW/cm2, and a coincident ultrasound source (450kHz) created a pressure field at less than 100 mW/cm2. S. epidermidis, S. aureus, and P. acnes were studied. Over 1-log10 CFU and 3-log10 CFU reduction were observed in bacterial biofilms after 5 and 30 min treatment, respectively. An additional 2-log10 CFU reduction was observed 24 h after treatment. In contrast, 24 h exposure to erythromycin only reduced biofilm CFU by less than 1-log10.
4.7. Synergism of antimicrobial blue light with magnetic fields
Magnetic fields was reported to cause stress in bacterial cells and activates genes ALA dehydratase, which is a key enzyme from a synthesis porphyrin, and subsequently increase the production of endogenous photosensitizing porphyrin (Fonseca et al., 2012). As a result, there might be a synergistic effect between aBL and magnetic fields. Astuti et al investigated the combination of aBL with magnetic fields for the inactivation of E. coli (Astuti et al., 2017). Wavelengths 469 (blue), 541(green), and 626 nm (red) and magnetic fields 1.8 mT were tested. It was reported that the combination therapy increased the antimicrobial efficacy by ca.14.2% (from 70% to 80% reduction in bacterial CFU after a radiant exposure of 18.81 J/cm2).
4.8. Synergism of antimicrobial blue light with near infrared irradiation
Guffey et al. reported that the combination of aBL at 464 nm with near infrared irradiation at 850 nm was super to either wavelength delivered alone in terms of inactivation of K. pneumoniae (Guffey et al., 2014b). aBL exposures of 3, 10, 30, 45, and 60 J/cm2 were employed. An inactivation of 96.2% CFU was achieved in the bacterial suspensions at the combination of 45 J/cm2 aBL with 60 J/cm2 near infrared irradiation. While aBL alone at 45 J/cm2 had almost no effect on the inactivation of bacteria, and near infrared irradiation alone at 60 J/cm2 only resulted in 60.1% inactivation of bacteria. The investigators suggested that the use of near infrared irradiation with aBL changed redox properties of the respiratory components of the bacteria cells and increased superoxide anion production with subsequent increase in concentration of H2O2.
4.9. Synergism of antimicrobial blue light with photoacids
In a very recent study, Simkovitch et al found that, in the presence of a photoacid (8-hydroxy-1,3,6-pyrenetrisulfonate) in agar hydorgel, continuous irradiation of 405-nm aBL for 40 min at the irradiance of 25 mW/cm2 (60 J/cm2) induced collapse of hyphea in Colletotrichum gloeosporioides, a plant pathogen (Simkovitch et al., 2017). Without the photoacid, a similar effect on the morphology of C. gloeosporioides was only observed after 4.5 h irradiation of 405-nm aBL at 25 mW/cm2 (405 J/cm2). The authors noted that, upon aBL irradiation, the photoacid coverts the photon of aBL to acid protons, which lower the pH at the interface of fungal infection and the digested polysaccharide, and subsequently curb fungal infections.
5. Effect of antimicrobial blue light on host cells and tissues
Toxicity testing is essential for drug development process and is also an important part of the mechanistic studies to understand how drugs or potentially toxic chemicals affect target organs. Many types of mammalian cells have been studied for the cytotoxicity and genotoxicity of blue light to cells as well as the effect of blue light on host immune response, such as keratinocytes, fibroblasts, osteoblasts, retinal cells, epithelial cells, dendritic cells, lymphocytes, monocytes, macrophages, etc. A number of parameters were used as end-points to measure toxicity, such as cell growth and cloning efficiency, cytosolic enzyme release, etc.
5.1. Cytotoxicity
5.1.1. In vitro studies
It is speculated that aBL can cause direct generation of ROS through excitation of flavins and/or flavin-containing oxidases within the peroxisomes and mitochondria of mammalian cells (Del Olmo-Aguado et al., 2016; Losi, 2007; Vandersee et al., 2015; Yoshida et al., 2015). To evaluate the feasibility of aBL for decontamination in orthopeadic surgery, Ramakrishnan et al. compared the susceptibilities to aBL at 405 nm between osteoblasts and bacterial cells including S. aureus, E. coli, P. aeruginosa, K. pneumoniae, S. epidermidis, and A. baumannii (Ramakrishnan et al., 2014). It was observed that aBL exposures of up to 36 J/cm2 had no significant effect on the viability, function, and proliferation rate of osteoblasts. In contrast, exposure of a variety of clinically related bacteria to 36 J/cm2 aBL resulted in up to 100% inactivation of bacteria.
Recently, our group compared “side-by-side” the susceptibilities to aBL at 415 nm between human keratinocytes and P. aeruginosa, A. baumannii, or C. albicans (Dai et al., 2013b; Zhang et al., 2016; Zhang et al., 2014). Our results showed that the inactivation rates of P. aeruginosa, A. baumannii, and C. albicans cells by aBL were approximately 35-, 21-, and 42-fold higher, respectively, than that of human keratinocytes, suggesting the existence of a therapeutic window in which pathogenic microbes are selectively inactivated by aBL while host cells are preserved.
Mamalis et al. reported the effects of blue light at 415 nm on normal human skin fibroblast proliferation, viability, migration speed, and ROS generation (Mamalis et al., 2015). After exposures of 5 to 80 J/cm2 blue light, relative proliferation and migration speed decreased to 8.4%–55.1% and 95%–32.3%, respectively compared to the untreated controls; and the production of ROS increased to 110.4%–130%. Cell viability was not altered under all the exposures of aBL.
Blue light also exhibited effects on human gingival fibroblasts in vitro (Yoshida et al., 2013). After 5 min blue light (460 nm) irradiation at an irradiance of 372.3 mW/cm2 (or 111.7 J/cm2), cell-proliferation activity decreased by approximately 45%, intracellular ROS increased by approximately 60%. In addition, the morphological cytotoxic effect was observed in the cell organelles, especially the mitochondria.
Lee et al. investigated if blue light affects human corneal epithelial cells (Lee et al., 2014). Cells were seeded in 35-mm tissue culture dishes and were exposed to 410 or 480 nm blue light. The viability of corneal epithelial cells was diminished after an exposure of 10 J/cm2 at 410 nm or 50 J/cm2 at 480 nm. ROS production was induced by blue light of both wavelengths at exposures of 5 J/cm2 and higher.
In another study using primary retina cells cultured in 96-well microtiter plates, Kuse et al. reported that, after a blue light (464 nm) exposure of 32.8 J/cm2 (24 h irradiation at the irradiance of 0.38 mW/cm2), there was an approximately 60% increase of ROS level in cells, a 40% decrease in cell viability, a 7% increase in the cleaved caspase-3 positive cells, and a 40% increase in the S-opsin and cleaved caspase-3 double positive cells (Kuse et al., 2014).
The effect of blue light on the viability of human primary retinal epithelial cells was explored by Godley et al. (Godley et al., 2005). In the study, confluence cultures of cells were exposed to blue light at 390–550 nm and 2.8 mW/cm2. During the first 3 h of light exposure (or 30.2 J/cm2), no significant change in cell viability was detected. At 6 h (or 60.5 J/cm2) and 9 h (90.7 J/cm2), approximately 6% and 10% decrease in cell viability was observed, respectively.
Fischer et al. studied the effect of blue light on human dendritic cells (Fischer et al., 2013). Cells cultured in RPMI media were exposure to blue light (400–500 nm) at the exposures up to 15 J/cm2. The investigators observed that blue light did not affect cell viability but only suppressed cell activation and subsequently reduced their allogeneic stimulatory potential. In another study, Monfrecola et al. reported that blue light (400–500 nm, up to 15 J/cm2) did not interfere with the differentiation and maturation of dendritic cells, whereas it was effective in reducing the production of IL-6 and TNF-a, pro-inflammatory cytokines (Monfrecola et al., 2014).
Several assays are available for assessing the viability of mammalian cells, but there is a dearth of studies comparing the results obtained with each test. To this end, Masson-Meyers et al. compared the capability of four viability assays MTT, neutral red, trypan blue, and live/dead fluorescence, to detect potential toxicity in fibroblasts exposed to 470 nm blue light (Masson-Meyers et al., 2016b). Cells were irradiated at 3, 55, 110, and 220 J/cm2, then incubated for 24 h and viability assessed using each assay. Overall, irradiation with 3 J/cm2 or 55 J/cm2 did not adversely affect cell viability, as evidenced by all the four assays. MTT assay showed significant decreases in viability when cells were irradiated with 110 and 220 J/cm2 exposure (89% and 57% viable cells post-aBL, respectively); likewise, the trypan blue assay showed 42% and 46% viable cells. Neutral red assay revealed significant decrease in viability when cells were irradiated with 220 J/cm2 (84% viable cells post-aBL). The live/dead fluorescence assay was less sensitive, showing 91% and 95% viable cells after aBL exposures of 110 and 220 J/cm2, respectively. Thus, the investigators concluded that aBL exposures below 110 J/cm2 appear safe.
Subsequently, the same authors investigated the effect of aBL (470 nm) on wound healing by using an in vitro scratch wound model (Masson-Meyers et al., 2016a). The results showed that wound closure was similar for the aBL irradiated (<55 J/cm2) wounds and the control wounds without being irradiated, indicating that aBL does not impair wound healing. Irradiated skin cells remained viable. Hydroxyproline levels, bFGF, and Il-10 concentrations did not differ between aBL-treated wounds and untreated control wounds. The IL-8 concentration decreased progressively with the increase of aBL exposure, suggesting that aBL is anti-inflammatory.
5.1.2. In vivo studies
Yoshida and colleagues investigated the effect of direct blue light irradiation on the rat palatal gingiva in vivo (Yoshida et al., 2015). For 4 days, rat palatal gingiva was exposed to blue light (460 nm, 400 mW/cm2) for 15 min (or 360 J/cm2) each day or left un-irradiated. The generation of singlet oxygen 1O2 was detected after blue light exposure. In addition, blue light significantly accelerated oxidative stress and increased the oxidized glutathione levels in gingival tissue.
In order to assess the influence that blue light can potentially exert on the antioxidant status of the skin, a pilot clinical trial was conducted by Vandersee et al. (Vandersee et al., 2015). Nine healthy volunteers were exposed to varying exposures of blue light (389–495 nm). The exposure of the human skin to blue light resulted in a dose-dependent degradation of the epidermal antioxidants as was shown by in vivo measurements of the carotenoid concentration using resonance Raman spectroscopy. The mean magnitude of the carotenoid destruction was determined to be 13.5% after an exposure of 50 J/cm2 blue light and 21.2% after an exposure of 100 J/cm2 blue light. Depending on the radiant exposure of blue light, the restoration time was measured to be 1 h for the exposure of 50 J/cm2 and 24 h for the exposure of 100 J/cm2. The investigators suggested that free radicals and most probably ROS are generated in the human skin after blue light exposure.
In another clinical trial, aBL at 450 nm was found to be able to improve skin hydration (Menezes et al., 2015). Twenty patients were randomized in two treatment groups. The first group was irradiated at forearm skin with 3 J/cm2 aBL (277 s at 10.8 mW/cm2) and the second with 6 J/cm2 (555 s at 10.8 mW/cm2). The treatment was performed twice a week for 30 days. The measurements were collected at 7, 14, 21, and 30 days during the treatment. Both aBL exposures improved the skin hydration; however, 6 J/cm2 kept this hydration for 30 days.
Adamskaya et al. recently showed that aBL significantly influenced biological systems, improving perfusion by release of nitric oxide from nitrosyl complexes with haemoglobin in a skin flap model in rats (Adamskaya et al., 2011). Circular excision wounds were surgically created on the dorsum of rats and were exposed to aBL (470 nm) post-surgery and on five consecutive days for 10 min at the irradiance of 50 mW/cm2 (or 30 J/cm2 each day). It was reported that aBL significantly decreased wound size on day 7, which correlated with enhanced epithelialisation. aBL also decreased keratin-1 mRNA on day 7 post-surgery, while keratin-10 mRNA level was elevated in aBL-treated group compared to the untreated controls.
aBL was also found to be effective in stimulating bone regeneration in critical sized defects in an study using a rat model (Dereci et al., 2016). aBL at 400–490 nm with an exposure of 13 J/cm2 was applied to 8-mm defects on the calvaria of rats each day for 6 days. At day 21, there was a statistically significant increase in the total horizontal length (20.50 mm vs. 7.70 mm) and the total vertical length of the newly produced bone tissue (20.50 mm vs. 6.70 mm) in comparison to the untreated controls. The investigators suggested that aBL activated osteogenic factors and proteins.
Blue light was also shown to be effective in reducing the organ injury from ischemia and reperfusion (I/R) in an animal study (Yuan et al., 2016). Exposure to blue light at 442 nm for 24 h (illuminance 1400 lux) before I/R reduced hepatocellular injury and necrosis, and reduced acute kidney injury and necrosis. In both cases, blue light reduced neutrophil influx, as evidenced by reduced myeloperoxidase within each organ, and reduced the release of high-mobility group box 1, a neutrophil chemotactant and key mediator in the pathogenesis of I/R injury. This protective mechanism of blue light appeared to involve an optic pathway and was mediated, in part, by a sympathetic (β3 adrenergic) pathway that functioned independent of significant alterations in melatonin or corticosterone concentrations to regulate neutrophil recruitment.
5.2. Genotoxicity
To identify the conditions that minimize the genotoxicity to live mammalian cells in standard fluorescent imaging, Ge et al. compared the potential genotoxic impact of UV irradiation (340–380 nm) and blue light (460–500 nm) (Ge et al., 2013). UV irradiation is normally used to excite fluorophores that emits blue fluorescence, and blue light is commonly used to excite fluorescent molecules that gives green fluorescence. The investigators observed that exposure to the UV irradiation (0.011 mW/cm2) for 15 min (or 9.9×10−3 J/cm2) caused significant DNA damage, while the blue light (0.652 mW/cm2) produced low to minimal damage during the equivalent period of time (0.562 J/cm2) (Figure 5). Although blue light possessed significantly higher irradiance than the UV irradiation, it appeared to be much less genotoxic.
Figure 5.
Arrayed micro-well comets with varying exposure times to UV irradiation (340–380 nm) and blue light (460–500 nm), respectively (Ge et al., 2013). Monochrome images were collected from microscope camera and colored with Red Hot lookup Table using Image J. Bars=100 μm.
However, mitochondrial DNA (mtDNA) damage was observed in the confluent cultures of human primary epithelial cells after blue light exposure (390–550 nm at 2.8 mW/cm2), as assessed using the quantitative polymerase chain reaction (Godley et al., 2005). Maximum damage occured at 3 h (or 30.24 J/cm2), which was 0.7 lesion per 10 kb mtDNA. The number of DNA lesion decreased to 0.29 lesion per 10 kb at 6 h (or 60.48 J/cm2), indicating that DNA repair was initiated. No nuclear DNA lesions were detected in cells exposed to blue light at any of the time points studied.
Using in vivo normal mouse skin, our group evaluated the genotoxic effect of 415 nm aBL on skin cells using TUNEL assay (Wang et al., 2016). Immunofluorescence pictures of representative mouse skin before, 0 h, and 24 h after a single aBL exposure (540 J/cm2), shown in Figure 6, revealed no presence of apoptotic cells in aBL-irradiated mouse skin up to 24 h after aBL exposure. Fluorescence shown in the positive control (mouse skin section treated with DNase I) indicated the presence of apoptotic cells.
Figure 6.
TUNEL assay of apoptotic cells in mouse skin before, 0 h, and 24 h after aBL exposure (540 J/cm2) (Wang et al., 2016). The positive control was treated with DNase I. Nuclei were stained blue with DAPI.
5.3. Effect on host immune response
The toxicity of aBL on inflammatory cells is important when investigating the application of aBL to infections. However, to our knowledge, there have been no published studies of the cytotoxicity and genotoxicity of blue light at the antimicrobial exposures to inflammatory cells.
A few studies investigating the light effects on immune response indicated that low level blue light did not induce toxicity to lymphocytes (Chen et al., 2016; Li et al., 2015). Phan et al. reported that T lymphocytes possess intrinsic capacity to sense and respond to light, and this photosensitivity may enhance the motility of T cells in skin (Phan et al., 2016). Blue light irradiation at low radiant exposures (4–30 mW/cm2, <300 mJ/cm2) triggered the synthesis of hydrogen peroxide (H2O2) in T cells in vitro. H2O2 enhanced T-cell motility by activating certain signal pathway and Ca2+ mobilization. Activated T cells were much more photosensitive than naive T cells.
In another recently published paper, Trotter et al studied the modulating effects of violet/blue light on anti-inflammatory and antioxidative signaling pathways in THP-1 monocytes cells (Trotter et al., 2017). The results showed that blue light (400–500 nm) activated the phase 2 response in cultured THP-1 monocytes cells and was protective against LPS (lipopolysaccharide) - induced cytotoxicity. The investigators noted that cellular responses to blue light energies were worth further study and might provide future therapeutic interventions for inflammation.
Also recently, Kushibiki et al. measured the intracellular ROS generation in various types of cells induced by several wavelengths of low-level laser therapy including a 405-nm blue laser (Kushibiki et al., 2013). It was found that the intracellular ROS levels in the macrophages differentiated from lymphocytes increased after the blue laser irradiation (405 nm, 100 mW/cm2 for 60 or 120 s), while this phenomenon was not observed after the irradiation with a red laser or a near-infrared laser.”
6. Development of resistance to antimicrobial blue light by microbes
An important question regarding the use of aBL as a therapeutic is: can pathogenic microbes develop resistance to aBL? This answer has not yet been broadly investigated. To this end, our laboratory explored this question by carrying out repeated sub-lethal aBL (415 nm, 9 J/cm2) inactivation of microbes including P. aeruginosa (Amin et al., 2016), A. baumanii (Zhang et al., 2014), and C. albicans (Zhang et al., 2016). No evidence of aBL-resistance development was observed after 10 cycles of sub-lethal aBL inactivation. Interestingly, we observed an increased aBL sensitivity of A. baumanii with the increased number of cycles of sub-lethal aBL inactivation (P = 0.04; Figure 2).
Figure 2.
Reduction of CFU (log10 CFU) of A. baumannii in different cycles of sub-lethal bacterial inactivation by aBL at 415 nm followed by bacterial regrowth (Zhang et al., 2014).
In a study performed by Guffey et al. (Guffey et al., 2013b), the investigators found that the sensitivity to sub-lethal aBL (405 nm) of S. aureus increased with the number of cycles of aBL inactivation through the first 4 cycles (post-aBL survival from 32.9% to 59.5%). However, as of the 4th cycle, the sensitivity to aBL decreased with the cycles of aBL inactivation (post-aBL survival from 59.5% in the 4th cycle to 18.0% in the 7th cycle), suggesting the development of aBL resistance by S. aureus.
In a later study, Guffey et al. tested the approach to retard the development of aBL-resistance by S. aureus via manipulating the light energy (Guffey et al., 2014a). Bacterial suspensions were exposed, in each cycle, to 405 nm, 464 nm or the combinations of 464 nm with 850 nm light at the exposures of 9 and 30 J/cm2 and the irradiance of 10 to 125 mW/cm2. Results showed that varying the radiant exposure of aBL over successive cycles between 30 J/cm2 and 9 J/cm2 is a possible technique for delaying aBL-resistance formation. Combination of infrared wavelengths with aBL may assist in delaying resistance formation. Lower rates of light delivery (i.e., lower irradiance) may be more effective when attempting to decontaminate tissues colonized by S. aureus.
7. A novel approach for interstitial delivery of antimicrobial blue light
One limitation to the application of light-based therapy including aBL therapy for infections is the limited penetration depth of light in living tissue, which is <1 mm for aBL (ICNIRP, 2013). As a result, for deeply seated infections, topical application is usually not able to deliver sufficient aBL energy to the infection sites.
To overcome this limitation, in recent years, microneedles have been investigated as a useful approach for interstitial light or drug delivery. Kosoglu et al. developed several types of fiberoptic microneedles for red or near infrared light delivery in photothermal therapy (Kosoglu et al., 2010; Kosoglu et al., 2011). The ex vivo results showed that the sharp tip microneedles penetrated skin more smoothly with a lower maximum force compared with the flat tip microneedles. However, there was a risk of breakage of small silica particles from the microneedle tips inside or underneath the skin.
Our group developed a novel optical lens-microneedle array (OMNA) that can be used to deliver aBL interstitially in tissue (Kim et al., 2016). The OMNA device consists of a microneedle array and a micro-lens array (Fig. 3a). The micro-lens array focuses incoming light into each microneedle at appropriate converging angles to reduce insertion loss and enable propagation of incoming light along each microneedle (Fig. 3b). The light propagating inside each microneedle is extracted into the surrounding tissue (Fig. 3c). The design of OMNA was optimized to enable the delivery of aBL to the depths up to 2.5 mm below the tissue surface.
Fig. 3.
Design principle of the OMNA (Kim et al., 2016). (a) Schematics of a microneedle array and microlens array. (b) Illustration of an assembled OMNA. The microlens array focuses incident light through the microneedles. (c) Illustration of light delivery into a porcine muscle tissue slice. The design has been optimized to achieve uniform and maximal light intensity at a target depth.
We used a CCD camera to measure the transmission of a laser beam (491 nm) through tissues without and with an OMNA. Figure 4a shows the transmitted light through a bovine muscle slice with a thickness of 3.1 mm. The total transmission over the central area of 10 mm × 10 mm was only 0.85% when the laser beam was directly applied to the back tissue (Fig. 4b), but the transmission was increased to 8.5% with the OMNA (Fig. 4c). Figure 4d shows a typical transmission image of a 2.7-mm-thick porcine muscle tissue slice. The transmission was 7.6% without (Fig. 4e) and 32% with the OMNA (Fig. 4f).
Fig. 4.
Enhanced light penetration into tissues by OMNA (Kim et al., 2016). (a–c) Light penetration through a 3.1-mm-thick bovine tissue slice: (a) Camera image of the tissue; Transmission intensity maps without (b) and with the OMNA (c). (d–f) Light transmission through a 2.7-mm-thick porcine tissue slice: (d) Camera image; Transmission intensity maps without (e) and with (f) the OMNA. The magnitudes of transmission are indicated in (c), (d), (e), and (f).
The OMNA can be fabricated into a bandage-like patch to cover up burns and wounds. The microneedles could be formed by aqueous polymer gels (hydrogels), which can serve as perfect light diffusers. In addition, as hydrogel is stretchable and can be shaped into a wide range of geometries, the OMNA can be in close contact with the human body.
8. Potential new applications of antimicrobial blue light
In addition to the treatment of infections, many new applications of aBL have been explored recently.
8.1. Decontamination of biological fluids
Bacterial contamination of biological fluids is now the greatest residual source of transfusion-transmitted disease (Brecher and Hay, 2005). aBL was explored for the decontamination of plasma (Maclean et al., 2016). At a typical “natural” contamination level (≤103 CFU/mL) in pre-bagged rabbit and human plasma, 3-log10 CFU reduction of bacteria (S. aureus, S. epidermidis, and E. coli) was achieved after an exposure of 144 J/cm2 aBL at 405 nm was delivered.
In another study, Srimagal et al (Srimagal et al., 2016) investigated the effectiveness of aBL (406 nm) from LEDs in inactivating E. coli in milk. After 38 min illumination (the irradiance of aBL was not reported in this study) at 13.8 °C, a 5-log10 reduction of bacterial CFU was achieved with no significant change in the physicochemical properties of the milk, including its overall color. In addition, the shelf-life of the aBL-treated milk under refrigerated storage increased almost twofold.
aBL was also exploited for the disinfection of orange juice contaminated by Salmonella (Ghate et al., 2016). In the study, pasteurized orange juice contaminated by 106 CFU/mL of Salmonella enterica serovars Gaminara, Montevideo, Newport, Typhimurium, and Saintpaul was exposed to aBL at 460 nm from LED. After a 13.58 h irradiation at the irradiance of 92 mW/cm2 (i.e., an exposure of 4.5 kJ/cm2), approximately 3.3-, 3.6-, and 4.8-log10 reductions in bacterial CFU were observed at the set temperatures of 4, 12, and 20 °C, respectively.
8.2. Disinfection of contact lenses
Wearing contact lenses offers many advantages for patients with visual impairments, but may also result in serious complications. Problems such as infectious and inflammatory diseases, which are associated with contact lens wearing, could not be fully resolved until today. Hoenes et al. determined the feasibility of aBL for disinfection of contact lens (Hoenes et al., 2016). Representative eye pathogens S. auricularis, B. subtilis, and E. coli in suspensions were exposed to aBL at 405 nm. After an exposure of 75.6 J/cm2, 2-log10 reduction in CFU was observed in B. subtilis, and after an exposure of 151.2 J/cm2, > 3-log10 and > 5-log10 CFU reduction was achieved in E. coli and S. auricularis, respectively.
8.3. Postharvest preservation
aBL has also been explored to treat plant postharvest disease. It was found that aBL (410–540 nm) suppressed the growth of blue mold (Penicillium italicum), green mold (Penicillium digitatum), gray mold (Botrytis cinerea), and stem end rot (Phomopsis citri), and significantly reduced the symptom of postharvest decays (Liao et al., 2013). In addition, aBL reduced cell wall digest enzyme activity of P. digitatum, induce octanal production in citrus fruits, which is lethal to the molds (Liao et al., 2013), and increased the scoparone concentration and ethylene production in citrus fruits (Ballester and Lafuente, 2017), which elicit the resistance to mold infections.
To examine whether aBL at 405-nm suppresses B. cinerea development in tomato plants, Imada et al. irradiated the detached leaves inoculated with B. cinerea spores using 405-nm aBL (Imada et al., 2014). Results demonstrated that the greatest inhibitory effect of 405-nm aBL on the development of B. cinerea on the detached leaves was obtained when the leaves were exposed to aBL for 15 min per h at 50 mW/cm2 (or 45 J/cm2 per h) during the aBL exposure. The aBL exposure resulted in a statistically significant reduction of lesion diameter at day 4 after bacterial inoculation compared with that of non-irradiated controls. The above findings were reinforced by the results from a study of Xu et al. (Xu et al., 2017), which showed that 400–410 nm and 450–460 nm aBL significantly inhibited the growth of B. cinerea mycelium. The lesion development of B. cinerea on tomato leaves was significantly inhibited upon irradiation with 400–410 nm aBL.
Kim and colleagues probed the antibacterial effect of 405 ± 5 nm LED on E. coli O157:H7, L. monocytogenes, and Salmonella spp. on the surface of fresh-cut mango or papaya and its effect on fruit quality at different storage temperatures (Kim et al., 2017a; Kim et al., 2017b). At chilling temperatures, approximately 0.3–1.3 log10 CFU inactivation of Salmonella spp. on papaya after aBL exposures of 1.3–1.7 kJ/cm2, and 1.0–1.6 log10 CFU inactivation of E. coli O157:H7, L. monocytogenes, and Salmonella spp on mango after aBL exposures of 2.6–3.5 kJ/cm2 were observed. However, at room temperature, rapid regrowth of Salmonella spp on papaya and L. monocytogenes and Salmonella spp on mango after aBL exposures of 0.9 and 1.7 kJ/cm2 occurred. It was also shown that aBL did not adversely affect the physicochemical and nutritional qualities of the fruits, regardless of storage temperature.
aBL was also shown to be effective in disinfecting cucumbers and process meat products. In a study carried out by Guffey et al. (Guffey et al., 2016), Salmonella and E. coli on actual food stuffs were significantly inactivated when exposed to 464 nm aBL at 18 J/cm2 (inactivation rates 80.2–100%) and 405 nm aBL at 60 J/cm2 (inactivation rates 75.6–96.3%), respectively.
Lacombe et al. investigated the disinfecting properties of 405-nm aBL against E. coli O157:H7, Salmonella, and nonpathogenic bacteria (E. coli K-12 and an avirulent strain of S. Typhimurium) inoculated onto the surface of almonds at higher or lower inoculation levels (8 or 5 CFU/g) (Lacombe et al., 2016). For E. coli K-12, reductions of up to 1.85- or 1.63-log10 CFU were observed for higher and lower inoculum levels, respectively; reductions up to 2.44- and 1.44-log10 CFU were seen for E. coli O157:H7 (higher and lower inoculation levels, respectively). Attenuated Salmonella was reduced by up to 0.54- and 0.97-log10 CFU, whereas pathogenic Salmonella was reduced by up to 0.70- and 0.55-log10 CFU (higher and lower inoculation levels, respectively). Inoculation level did not significantly affect minimum effective treatment times, which ranged from 1 to 4 min. The nonpathogenic strains of E. coli and Salmonella each responded to aBL in a comparable manner to their pathogenic counterparts. These results suggest that these nonpathogenic strains may be useful in experiments with aBL in which a surrogate is required.
8.4. Control of insect pests
Hori and colleagues revealed that blue light can kill some household pests and field crop pests (Hori et al., 2014; Hori and Suzuki, 2017). Insect species Drosophila melanogaster, Culex pipiens molestus, Tribolium confusum, and Galerucella grisescens, and wavelengths 407, 417, 438, and 465 nm were studied. It was shown that the effective wavelengths of blue light were species-dependent and growth-stage-dependent (e.g., egg stage, pupal stage, etc.). The lethal effect of blue light on insects was thought to be attributed to the ROS produced by the blue light excitation of endogenous photosensitizers in the insect tissues (Hori and Suzuki, 2017). The ROS damaged the tissues and killed the insects.
9. Discussion
Since 2012, considerable studies in the area of aBL have been carried out to further investigate the efficacy of aBL, its mechanism of action, synergism of aBL with other antimicrobials, its effect on host cells and the potential development of aBL resistance by microbes. A wide range of microbial species were studied, including Gram-positive bacteria, Gram-negative bacteria, mycobacteria, molds, yeasts, and dermatophytes. Although varying results of the aBL efficacy were observed in different studies due to the varied conditions (Decarli et al., 2017; Fyrestam et al., 2016), the majority of the microbes studied were found to be sensitive to aBL inactivation. In general, the tolerance of molds to aBL was higher than that of bacteria and other fungal species, and the endospores of microbes were more tolerant to aBL compared to their vegetative counterparts.
One of the significant advances since 2012 has been the preclinical in vivo studies of aBL therapy for various infections in murine models and pilot clinical studies. Animal studies demonstrated that aBL significantly reduced microbial load in infected lesions, indicating the potency of aBL for infectious diseases. Two pilot clinical studies showed the effectiveness of aBL in treating dental pathogens. In several ongoing studies of our laboratory, we are testing aBL therapy for urinary tract infections, genital tract infections, implant-related infections, and open fracture infections in murine models.
Since 2012, great efforts have also been exerted in further elucidating the mechanism of action of aBL. The identification of the endogenous photosensitizing chromophores in some important microbial cells, including S. aureus, P. aeruginosa, A. baumanii, C. albicans, A. actinomycetemcomitans, P. gingivalis, and Salmonella was performed for the first time using fluorescence spectroscopy or HPLC. Both the results of fluorescence spectroscopy and HPLC supported the hypothesis of the presence of endogenous porphyrins, flavins, and/or NADH within microbial cells as well as the mechanism underlying aBL. The main species of endogenous porphyrinic were found to be coproporphyrin I, coproporphyrin III, and PpIX (Battisti et al., 2017b; Fyrestam et al., 2016).
The hypothesis regarding the mechanism of action of aBL was further endorsed by DNA manipulation to knockout the porphyrin synthesis pathway of a microbe sensitive to aBL and then the comparison of the aBL susceptibilities between the knockout mutant and its parental strain (Galbis-Martinez et al., 2012; Grinholc et al., 2015). It was also reported that the endogenous porphyrins patterns in vitro (e.g., porphyrin quantity and porphyrin species) are highly affected by the culturing conditions (e.g., time of culturing, passaging, culture media) (Fyrestam et al., 2016). As such, to make the results from different studies comparable, there is a need for more standardized methods to be employed when evaluating the efficacy of aBL in vitro (Decarli et al., 2017).
However, conflicting observations were reported by different studies regarding the role of endogenous coproporphyrins in the efficacy of aBL. While Nitzan et al. reported that the total amount of coproporphyrin determined the efficacy of aBL, irrespective of total amount of porphyrins (including the amount of other porphyrinic species) (Nitzan et al., 2004), Kim and Yuk observed that the total amount of coproporphyrins was not a contributing factor to the efficacy of aBL (Kim and Yuk, 2017).
Evidences were reported that aBL inactivation of microbes was attributed to ROS-induced cell membrane damage (Biener et al., 2017; McKenzie et al., 2016), inactivation of virulence factors (Fila et al., 2016), DNA-oxidation (Kim and Yuk, 2017; Yoshida et al., 2017), genetic changes (Adair and Drum, 2016; Kim and Yuk, 2017; Yang et al., 2017), etc. However, some other studies showed that only slight damage to membrane integrity after aBL inactivation (Kim and Yuk, 2017).
As a safety study of aBL application, the effects of aBL on host cells and tissues have been further investigated in the past five years, including cytotoxicity and genotoxicity. Several studies showed that under certain exposures, aBL exerted both cytotoxic and genotoxic effects on relevant host cells. However, most of the studies are not the “side-by-side” comparison between aBL effect on microbes and that on host cells. Due to the different experimental conditions of different studies, it is not feasible to directly compare the effect of aBL exposures in those safety studies with that in different studies of antimicrobial efficacy. There are only few “side-by-side” studies evaluating the safety of the therapeutic exposure for inactivating pathogenic microbes, and those studies suggested that there exists a therapeutic window where microbes are selectively inactivated while host cells are preserved (Dai et al., 2013a; Dai et al., 2013b; Ramakrishnan et al., 2014; Zhang et al., 2016). In addition, no evidence of genotoxicity of aBL was observed in mouse skin in vivo when exposed to the therapeutic aBL exposure for inactivating mature biofilms (Wang et al., 2016).
Contradictory results were reported in different studies regarding the aBL-resistance developed by pathogenic microbes. While no evidence of aBL-resistance development by P. aeruginosa, A. baumanii, and C. albicans was observed in the studies of our group (Amin et al., 2016; Zhang et al., 2016; Zhang et al., 2014), a study from another group of investigators reported the development of aBL-resistance by MRSA (Guffey et al., 2013b). Our group is now further exploring this issue by exposing microbes to increased number of cycles of sub-lethal aBL irradiation, e.g., 20 cycles. If aBL resistance develops, we will determine the mutation(s) associated with it and confirm their contribution as well as generating functional interpretations based on known gene functions.
It is interesting that several studies reported the synergistic antimicrobial effect of aBL and other types of antimicrobials, such as antibiotics, disinfectants, extracts from medicinal plants, nanoparticles, ultrasound, etc. It is commonly accepted that synergistic combinations of two or more agents can overcome toxicity and other side effects associated with high doses of single drugs by countering biological compensation, allowing reduced dose of each compound or accessing context-specific multi-target mechanisms (Keith et al., 2005). For improved therapeutic outcome, this approach of combination therapy of aBL with other antimicrobials is worthy of further extensive investigation, e.g., the mechanism behind the synergistic effect, the optimization of the doses of aBL and other antimicrobials, etc.
Acknowledgments
Research in TD group has been supported by the National Institute of Health (R21AI109172 and R01AI123312), Department of Defenses (FA9550-16-1-0479 and FA9550-17-1-0277), Center for Integration of Medicine and Innovative Technology (#13-1021, #13-1033, and #14-1894), Airlift Research Foundation (#109421), American Society for Laser Medicine & Surgery (BS.F04.14), Orthopaedic Trauma Association (#16 and #181), Executive Committee On Research at Massachusetts General Hospital, and the Smith Infectious Diseases Foundation. Yucheng Wang was supported by an ASLMS Student Research Grant (BS.S02.15). We are grateful to Tayyaba Hasan, PhD, at the Wellman Center for her co-mentorship for Yuecheng Wang.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abana CM, Brannon JR, Ebbott RA, Dunigan TL, Guckes KR, Fuseini H, Powers J, Rogers BR, Hadjifrangiskou M. Characterization of blue light irradiation effects on pathogenic and nonpathogenic Escherichia coli. Microbiologyopen. 2017 doi: 10.1002/mbo3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adair TL, Drum BE. RNA-Seq reveals changes in the Staphylococcus aureus transcriptome following blue light illumination. Genomics Data. 2016;9:4–6. doi: 10.1016/j.gdata.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamskaya N, Dungel P, Mittermayr R, Hartinger J, Feichtinger G, Wassermann K, Redl H, van Griensven M. Light therapy by blue LED improves wound healing in an excision model in rats. Injury. 2011;42:917–921. doi: 10.1016/j.injury.2010.03.023. [DOI] [PubMed] [Google Scholar]
- Akram FE, El-Tayeb T, Abou-Aisha K, El-Azizi M. A combination of silver nanoparticles and visible blue light enhances the antibacterial efficacy of ineffective antibiotics against methicillin-resistant Staphylococcus aureus (MRSA) Ann Clin Microbiol Antimicrob. 2016;15:48. doi: 10.1186/s12941-016-0164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin RM, Bhayana B, Hamblin MR, Dai T. Antimicrobial blue light inactivation of Pseudomonas aeruginosa by photo-excitation of endogenous porphyrins: In vitro and in vivo studies. Lasers Surg Med. 2016;48:562–568. doi: 10.1002/lsm.22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astuti SD, Wibowo RA, Triyana K. Antimicrobial photodynamic effects of polychromatic light activated by magnetic fields to bacterial viability. J Int Dent Med. 2017;10:111–117. [Google Scholar]
- Ballester AR, Lafuente MT. LED Blue Light-induced changes in phenolics and ethylene in citrus fruit: Implication in elicited resistance against Penicillium digitatum infection. Food Chem. 2017;218:575–583. doi: 10.1016/j.foodchem.2016.09.089. [DOI] [PubMed] [Google Scholar]
- Barneck MD, Rhodes NL, de la Presa M, Allen JP, Poursaid AE, Nourian MM, Firpo MA, Langell JT. Violet 405-nm light: a novel therapeutic agent against common pathogenic bacteria. J Surg Res. 2016;206:316–324. doi: 10.1016/j.jss.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Battisti A, Morici P, Ghetti F, Sgarbossa A. Spectroscopic characterization and fluorescence imaging of Helicobacter pylori endogenous porphyrins. Biophysical chemistry. 2017a doi: 10.1016/j.bpc.2017.05.010. [DOI] [PubMed] [Google Scholar]
- Battisti A, Morici P, Signore G, Ghetti F, Sgarbossa A. Compositional analysis of endogenous porphyrins from Helicobacter pylori. Biophysical chemistry. 2017b doi: 10.1016/j.bpc.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Biener G, Masson-Meyers DS, Bumah VV, Hussey G, Stoneman MR, Enwemeka CS, Raicu V. Blue/violet laser inactivates methicillin-resistant Staphylococcus aureus by altering its transmembrane potential. J Photochem Photobiol B. 2017;170:118–124. doi: 10.1016/j.jphotobiol.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Bouchard P, Carra MC, Boillot A, Mora F, Range H. Risk factors in periodontology: a conceptual framework. Journal of clinical periodontology. 2017;44:125–131. doi: 10.1111/jcpe.12650. [DOI] [PubMed] [Google Scholar]
- Brecher ME, Hay SN. Bacterial contamination of blood components. Clinical microbiology reviews. 2005;18:195–204. doi: 10.1128/CMR.18.1.195-204.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumah VV, Aboualizadeh E, Masson-Meyers DS, Eells JT, Enwemeka CS, Hirschmugl CJ. Spectrally resolved infrared microscopy and chemometric tools to reveal the interaction between blue light (470nm) and methicillin-resistant Staphylococcus aureus. J Photochem Photobiol B. 2016;167:150–157. doi: 10.1016/j.jphotobiol.2016.12.030. [DOI] [PubMed] [Google Scholar]
- Bumah VV, Masson-Meyers DS, Cashin S, Enwemeka CS. Optimization of the antimicrobial effect of blue light on methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers Surg Med. 2015a;47:266–272. doi: 10.1002/lsm.22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumah VV, Masson-Meyers DS, Enwemeka CS. Blue 470 nm light suppresses the growth of Salmonella enterica and methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers Surg Med. 2015b doi: 10.1002/lsm.22385. [DOI] [PubMed] [Google Scholar]
- Bumah VV, Whelan HT, Masson-Meyers DS, Quirk B, Buchmann E, Enwemeka CS. The bactericidal effect of 470-nm light and hyperbaric oxygen on methicillin-resistant Staphylococcus aureus (MRSA) Lasers Med Sci. 2015c;30:1153–1159. doi: 10.1007/s10103-015-1722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K, Courvalin P, Dantas G, Davies J, Eisenstein B, Huovinen P, Jacoby GA, Kishony R, Kreiswirth BN, Kutter E, Lerner SA, Levy S, Lewis K, Lomovskaya O, Miller JH, Mobashery S, Piddock LJ, Projan S, Thomas CM, Tomasz A, Tulkens PM, Walsh TR, Watson JD, Witkowski J, Witte W, Wright G, Yeh P, Zgurskaya HI. Tackling antibiotic resistance. Nat Rev Microbiol. 2011;9:894–896. doi: 10.1038/nrmicro2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Burden of foodborne illness: findings. CDC; Atlanta, GA: 2016. [Google Scholar]
- Chebath-Taub D, Steinberg D, Featherstone JD, Feuerstein O. Influence of blue light on Streptococcus mutans re-organization in biofilm. J Photochem Photobiol B. 2012;116:75–78. doi: 10.1016/j.jphotobiol.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Chen F, Reheman A, Cao J, Wang Z, Dong Y, Zhang Y, Chen Y. Effect of melatonin on monochromatic light-induced T-lymphocyte proliferation in the thymus of chickens. J Photochem Photobiol B. 2016;161:9–16. doi: 10.1016/j.jphotobiol.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Cheong KK, Strub C, Montet D, Durand N, Alter P, Meile JC, Schorr Galindo S, Fontana A. Effect of different light wavelengths on the growth and ochratoxin A production in Aspergillus carbonarius and Aspergillus westerdijkiae. Fungal Biol. 2016;120:745–751. doi: 10.1016/j.funbio.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Chui C, Hiratsuka K, Aoki A, Takeuchi Y, Abiko Y, Izumi Y. Blue LED inhibits the growth of Porphyromonas gingivalis by suppressing the expression of genes associated with DNA replication and cell division. Lasers Surg Med. 2012;44:856–864. doi: 10.1002/lsm.22090. [DOI] [PubMed] [Google Scholar]
- Cieplik F, Spath A, Leibl C, Gollmer A, Regensburger J, Tabenski L, Hiller KA, Maisch T, Schmalz G. Blue light kills Aggregatibacter actinomycetemcomitans due to its endogenous photosensitizers. Clin Oral Investig. 2014;18:1763–1769. doi: 10.1007/s00784-013-1151-8. [DOI] [PubMed] [Google Scholar]
- Coenye T, Nelis HJ. In vitro and in vivo model systems to study microbial biofilm formation. Journal of microbiological methods. 2010;83:89–105. doi: 10.1016/j.mimet.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Cohen-Berneron J, Steinberg D, Featherstone JD, Feuerstein O. Sustained effects of blue light on Streptococcus mutans in regrown biofilm. Lasers Med Sci. 2016;31:445–452. doi: 10.1007/s10103-016-1873-3. [DOI] [PubMed] [Google Scholar]
- Cokol M, Chua HN, Tasan M, Mutlu B, Weinstein ZB, Suzuki Y, Nergiz ME, Costanzo M, Baryshnikova A, Giaever G, Nislow C, Myers CL, Andrews BJ, Boone C, Roth FP. Systematic exploration of synergistic drug pairs. Mol Syst Biol. 2011;7:544. doi: 10.1038/msb.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen LE, Sanglard D, Howard SJ, Rogers PD, Perlin DS. Mechanisms of Antifungal Drug Resistance. Cold Spring Harb Perspect Med. 2014 doi: 10.1101/cshperspect.a019752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai T, Gupta A, Huang YY, Sherwood ME, Murray CK, Vrahas MS, Kielian T, Hamblin MR. Blue light eliminates community-acquired methicillin-resistant Staphylococcus aureus in infected mouse skin abrasions. Photomed Laser Surg. 2013a;31:531–538. doi: 10.1089/pho.2012.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai T, Gupta A, Huang YY, Yin R, Murray CK, Vrahas MS, Sherwood ME, Tegos GP, Hamblin MR. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: efficacy, safety, and mechanism of action. Antimicrob Agents Chemother. 2013b;57:1238–1245. doi: 10.1128/AAC.01652-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai T, Gupta A, Murray CK, Vrahas MS, Tegos GP, Hamblin MR. Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist Updat. 2012;15:223–236. doi: 10.1016/j.drup.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lucca AJ, Carter-Wientjes C, Williams KA, Bhatnagar D. Blue light (470 nm) effectively inhibits bacterial and fungal growth. Lett Appl Microbiol. 2012;55:460–466. doi: 10.1111/lam.12002. [DOI] [PubMed] [Google Scholar]
- de Sousa DL, Lima RA, Zanin IC, Klein MI, Janal MN, Duarte S. Effect of Twice-Daily Blue Light Treatment on Matrix-Rich Biofilm Development. PLoS One. 2015a;10:e0131941. doi: 10.1371/journal.pone.0131941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa NT, Santos MF, Gomes RC, Brandino HE, Martinez R, de Jesus Guirro RR. Blue Laser Inhibits Bacterial Growth of Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa. Photomed Laser Surg. 2015b;33:278–282. doi: 10.1089/pho.2014.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decarli MC, Correa TQ, Vollet-Filho JD, Bagnato VS, Souza CWO. The influence of experimental conditions on the final result of photoinhibition of Staphylococcus aureus. Photodiagnosis Photodyn Ther. 2017 doi: 10.1016/j.pdpdt.2017.06.013. [DOI] [PubMed] [Google Scholar]
- Del Olmo-Aguado S, Nunez-Alvarez C, Osborne NN. Blue Light Action on Mitochondria Leads to Cell Death by Necroptosis. Neurochem Res. 2016;41:2324–2335. doi: 10.1007/s11064-016-1946-5. [DOI] [PubMed] [Google Scholar]
- Dereci O, Sindel A, Serap Toru H, Yuce E, Ay S, Tozoglu S. The Comparison of the Efficacy of Blue Light-Emitting Diode Light and 980-nm Low-Level Laser Light on Bone Regeneration. The Journal of craniofacial surgery. 2016;27:2185–2189. doi: 10.1097/SCS.0000000000003068. [DOI] [PubMed] [Google Scholar]
- Diao WR, Hu QP, Feng SS, Li WQ, Xu JG. Chemical composition and antibacterial activity of the essential oil from green huajiao (Zanthoxylum schinifolium) against selected foodborne pathogens. J Agric Food Chem. 2013;61:6044–6049. doi: 10.1021/jf4007856. [DOI] [PubMed] [Google Scholar]
- Fang J, Xing J, Gao L, Shen B, Kang H, Jie L, Peng C. LED array designing and its bactericidal effect researching on Pseudomonas aeruginosa in vitro. In: Lee B, Su Y, Gu M, Yuan X, JD, editors. Proc SPIE 9672, AOPC; 2015; Beijing, China. 2015. pp. 96720C–96726. [Google Scholar]
- Fantin B, Leggett J, Ebert S, Craig WA. Correlation between in vitro and in vivo activity of antimicrobial agents against gram-negative bacilli in a murine infection model. Antimicrob Agents Chemother. 1991;35:1413–1422. doi: 10.1128/aac.35.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein O, Moreinos D, Steinberg D. Synergic antibacterial effect between visible light and hydrogen peroxide on Streptococcus mutans. J Antimicrob Chemother. 2006;57:872–876. doi: 10.1093/jac/dkl070. [DOI] [PubMed] [Google Scholar]
- Fila G, Kawiak A, Grinholc MS. Blue light treatment of Pseudomonas aeruginosa: Strong bactericidal activity, synergism with antibiotics and inactivation of virulence factors. Virulence. 2016:1–21. doi: 10.1080/21505594.2016.1250995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer MR, Abel M, Lopez Kostka S, Rudolph B, Becker D, von Stebut E. Blue light irradiation suppresses dendritic cells activation in vitro. Exp Dermatol. 2013;22:558–560. doi: 10.1111/exd.12193. [DOI] [PubMed] [Google Scholar]
- Fonseca BM, Tien M, Rivera M, Shi L, Louro RO. Efficient and selective isotopic labeling of hemes to facilitate the study of multiheme proteins. BioTechniques. 2012;52 doi: 10.2144/000113859. [DOI] [PubMed] [Google Scholar]
- Fontana CR, Song X, Polymeri A, Goodson JM, Wang X, Soukos NS. The effect of blue light on periodontal biofilm growth in vitro. Lasers Med Sci. 2015;30:2077–2086. doi: 10.1007/s10103-015-1724-7. [DOI] [PubMed] [Google Scholar]
- Fyrestam J, Bjurshammar N, Paulsson E, Johannsen A, Ostman C. Determination of porphyrins in oral bacteria by liquid chromatography electrospray ionization tandem mass spectrometry. Analytical and bioanalytical chemistry. 2015;407:7013–7023. doi: 10.1007/s00216-015-8864-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyrestam J, Bjurshammar N, Paulsson E, Mansouri N, Johannsen A, Ostman C. Influence of Culture Conditions on Porphyrin Production in Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis. Photodiagnosis Photodyn Ther. 2016 doi: 10.1016/j.pdpdt.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Galbis-Martinez M, Padmanabhan S, Murillo FJ, Elias-Arnanz M. CarF mediates signaling by singlet oxygen, generated via photoexcited protoporphyrin IX, in Myxococcus xanthus light-induced carotenogenesis. J Bacteriol. 2012;194:1427–1436. doi: 10.1128/JB.06662-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J, Wood DK, Weingeist DM, Prasongtanakij S, Navasumrit P, Ruchirawat M, Engelward BP. Standard fluorescent imaging of live cells is highly genotoxic. Cytometry A. 2013;83:552–560. doi: 10.1002/cyto.a.22291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genina EA, Titorenko VA, Belikov AV, Bashkatov AN, Tuchin VV. Adjunctive dental therapy via tooth plaque reduction and gingivitis treatment by blue light-emitting diodes tooth brushing. J Biomed Opt. 2015;20:128004. doi: 10.1117/1.JBO.20.12.128004. [DOI] [PubMed] [Google Scholar]
- Ghate V, Kumar A, Zhou W, Yuk HG. Irradiance and Temperature Influence the Bactericidal Effect of 460-Nanometer Light-Emitting Diodes on Salmonella in Orange Juice. Journal of food protection. 2016;79:553–560. doi: 10.4315/0362-028X.JFP-15-394. [DOI] [PubMed] [Google Scholar]
- Ghate V, Leong AL, Kumar A, Bang WS, Zhou W, Yuk HG. Enhancing the antibacterial effect of 461 and 521 nm light emitting diodes on selected foodborne pathogens in trypticase soy broth by acidic and alkaline pH conditions. Food Microbiol. 2015;48:49–57. doi: 10.1016/j.fm.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Ghate VS, Ng KS, Zhou W, Yang H, Khoo GH, Yoon WB, Yuk HG. Antibacterial effect of light emitting diodes of visible wavelengths on selected foodborne pathogens at different illumination temperatures. Int J Food Microbiol. 2013;166:399–406. doi: 10.1016/j.ijfoodmicro.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Giannelli M, Landini G, Materassi F, Chellini F, Antonelli A, Tani A, Nosi D, Zecchi-Orlandini S, Rossolini GM, Bani D. Effects of photodynamic laser and violet-blue led irradiation on Staphylococcus aureus biofilm and Escherichia coli lipopolysaccharide attached to moderately rough titanium surface: in vitro study. Lasers Med Sci. 2017 doi: 10.1007/s10103-017-2185-y. [DOI] [PubMed] [Google Scholar]
- Gillespie JB, Maclean M, Given MJ, Wilson MP, Judd MD, Timoshkin IV, MacGregor SJ. Efficacy of Pulsed 405-nm Light-Emitting Diodes for Antimicrobial Photodynamic Inactivation: Effects of Intensity, Frequency, and Duty Cycle. Photomed Laser Surg. 2016 doi: 10.1089/pho.2016.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godley BF, Shamsi FA, Liang FQ, Jarrett SG, Davies S, Boulton M. Blue light induces mitochondrial DNA damage and free radical production in epithelial cells. J Biol Chem. 2005;280:21061–21066. doi: 10.1074/jbc.M502194200. [DOI] [PubMed] [Google Scholar]
- Gomez GF, Huang R, MacPherson M, Ferreira Zandona AG, Gregory RL. Photo Inactivation of Streptococcus mutans Biofilm by Violet-Blue light. Current microbiology. 2016;73:426–433. doi: 10.1007/s00284-016-1075-z. [DOI] [PubMed] [Google Scholar]
- Grinholc M, Rodziewicz A, Forys K, Rapacka-Zdonczyk A, Kawiak A, Domachowska A, Golunski G, Wolz C, Mesak L, Becker K, Bielawski KP. Fine-tuning recA expression in Staphylococcus aureus for antimicrobial photoinactivation: importance of photo-induced DNA damage in the photoinactivation mechanism. Appl Microbiol Biotechnol. 2015;99:9161–9176. doi: 10.1007/s00253-015-6863-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guffey JS, Payne W, Buchanan B, Daugherty J, Meurer L, Hensley P. Susceptibility of Trichopyton mentagrophytes to Visible Light Wavelengths. Advances in skin & wound care. 2017;30:218–222. doi: 10.1097/01.ASW.0000512271.19164.ef. [DOI] [PubMed] [Google Scholar]
- Guffey JS, Payne W, James L. Inactivation of mycobacterium smegmatis following exposure to 405-nanometer light from a supraluminous diode array. Wounds. 2013a;25:131–135. [PubMed] [Google Scholar]
- Guffey JS, Payne W, Jones T, Martin K. Evidence of resistance development by Staphylococcus aureus to an in vitro, multiple stage application of 405 nm light from a supraluminous diode array. Photomed Laser Surg. 2013b;31:179–182. doi: 10.1089/pho.2012.3450. [DOI] [PubMed] [Google Scholar]
- Guffey JS, Payne W, Martin K, Dodson C. Delaying the Onset of Resistance Formation: Effect of Manipulating Dose, Wavelength, and Rate of Energy Delivery of 405-, 464-, and 850-Nanometer Light for Staphylococcus aureus. Wounds. 2014a;26:95–100. [PubMed] [Google Scholar]
- Guffey JS, Payne WC, Greenway J, Buchanan B, Dodson CM, Lancaster K. Inhibition of Klebseilla pneumoniae by visible and near-IR radiation. Eur J Acad Essays. 2014b;1:1–5. [Google Scholar]
- Guffey JS, Payne WC, Motts SD, Towery P, Hobson T, Harrell G, Meurer L, Lancaster K. Inactivation of Salmonella on tainted foods: using blue light to disinfect cucumbers and processed meat products. Food Sci Nutr. 2016;4:878–887. doi: 10.1002/fsn3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther NWt, Phillips JG, Sommers C. The Effects of 405-nm Visible Light on the Survival of Campylobacter on Chicken Skin and Stainless Steel. Foodborne pathogens and disease. 2016;13:245–250. doi: 10.1089/fpd.2015.2084. [DOI] [PubMed] [Google Scholar]
- Gupta S, Maclean M, Anderson JG, MacGregor SJ, Meek RM, Grant MH. Inactivation of micro-organisms isolated from infected lower limb arthroplasties using high-intensity narrow-spectrum (HINS) light. Bone Joint J. 2015;97-B:283–288. doi: 10.1302/0301-620X.97B2.35154. [DOI] [PubMed] [Google Scholar]
- Halstead FD, Thwaite JE, Burt R, Laws TR, Raguse M, Moeller R, Webber MA, Oppenheim BA. Antibacterial Activity of Blue Light against Nosocomial Wound Pathogens Growing Planktonically and as Mature Biofilms. Appl Environ Microbiol. 2016;82:4006–4016. doi: 10.1128/AEM.00756-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin MR, Viveiros J, Yang C, Ahmadi A, Ganz RA, Tolkoff MJ. Helicobacter pylori accumulates photoactive porphyrins and is killed by visible light. Antimicrob Agents Chemother. 2005;49:2822–2827. doi: 10.1128/AAC.49.7.2822-2827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton T. Report reveals scope of US antibiotic resistance threat. JAMA. 2013;310:1661–1663. doi: 10.1001/jama.2013.280695. [DOI] [PubMed] [Google Scholar]
- Hessling M, Spellerberg B, Hoenes K. Photoinactivation of bacteria by endogenous photosensitizers and exposure to visible light of different wavelengths - a review on existing data. FEMS microbiology letters. 2017;364 doi: 10.1093/femsle/fnw270. [DOI] [PubMed] [Google Scholar]
- Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- Hoenes K, Stangl F, Gross A, Hessling M. Improved contact lens disinfection by exposure to violet radiation. Technol Health Care. 2016;24:145–151. doi: 10.3233/THC-151104. [DOI] [PubMed] [Google Scholar]
- Hope CK, Hindley JA, Khan Z, de de Jong EJ, Higham SM. Lethal photosensitization of Porphyromonas gingivalis by their endogenous porphyrins under anaerobic conditions: an in vitro study. Photodiagnosis Photodyn Ther. 2013;10:677–682. doi: 10.1016/j.pdpdt.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Hori M, Shibuya K, Sato M, Saito Y. Lethal effects of short-wavelength visible light on insects. Scientific reports. 2014;4:7383. doi: 10.1038/srep07383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori M, Suzuki A. Lethal effect of blue light on strawberry leaf beetle, Galerucella grisescens (Coleoptera: Chrysomelidae) Scientific reports. 2017;7:2694. doi: 10.1038/s41598-017-03017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICNIRP. ICNIRP Guidelines on Limits of Exposure to Laser Radiation of Wavelengths between 180 nm and 1,000 μm. Health Physics. 2013;105:271–295. doi: 10.1097/HP.0b013e3182983fd4. [DOI] [PubMed] [Google Scholar]
- Imada K, Tanaka S, Ibaraki Y, Yoshimura K, Ito S. Antifungal effect of 405-nm light on Botrytis cinerea. Lett Appl Microbiol. 2014;59:670–676. doi: 10.1111/lam.12330. [DOI] [PubMed] [Google Scholar]
- Keith CT, Borisy AA, Stockwell BR. Multicomponent therapeutics for networked systems. Nat Rev Drug Discov. 2005;4:71–78. doi: 10.1038/nrd1609. [DOI] [PubMed] [Google Scholar]
- Keshishyan ES, Zaporozhtseva ZV, Zenina OM, Zrodnikov VS. Photodynamic inactivation of bacteria in vitro under the effect of blue light. Bull Exp Biol Med. 2015;158:475–477. doi: 10.1007/s10517-015-2788-x. [DOI] [PubMed] [Google Scholar]
- Kim M, An J, Kim KS, Choi M, Humar M, Kwok SJ, Dai T, Yun SH. Optical lens-microneedle array for percutaneous light delivery. Biomed Opt Express. 2016;7:4220–4227. doi: 10.1364/BOE.7.004220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Bang WS, Yuk HG. 405 +/− 5 nm light emitting diode illumination causes photodynamic inactivation of Salmonella spp. on fresh-cut papaya without deterioration. Food Microbiol. 2017a;62:124–132. doi: 10.1016/j.fm.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Miks-Krajnik M, Kumar A, Ghate V, Yuk HG. Antibacterial effect and mechanism of high-intensity 405 +/− 5 nm light emitting diode on Bacillus cereus, Listeria monocytogenes, and Staphylococcus aureus under refrigerated condition. J Photochem Photobiol B. 2015;153:33–39. doi: 10.1016/j.jphotobiol.2015.08.032. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Tang CH, Bang WS, Yuk HG. Antibacterial effect of 405+/−5nm light emitting diode illumination against Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella on the surface of fresh-cut mango and its influence on fruit quality. Int J Food Microbiol. 2017b;244:82–89. doi: 10.1016/j.ijfoodmicro.2016.12.023. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Yuk HG. Antibacterial Mechanism of 405-Nanometer Light-Emitting Diode against Salmonella at Refrigeration Temperature. Appl Environ Microbiol. 2017;83 doi: 10.1128/AEM.02582-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim J, Lim W, Jeon S, Kim O, Koh JT, Kim CS, Choi H. In vitro bactericidal effects of 625, 525, and 425 nm wavelength (red, green, and blue) light-emitting diode irradiation. Photomed Laser Surg. 2013;31:554–562. doi: 10.1089/pho.2012.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinpenning MM, Smits T, Frunt MH, van Erp PE, van de Kerkhof PC, Gerritsen RM. Clinical and histological effects of blue light on normal skin. Photodermatology, photoimmunology & photomedicine. 2010;26:16–21. doi: 10.1111/j.1600-0781.2009.00474.x. [DOI] [PubMed] [Google Scholar]
- Kosoglu MA, Hood RL, Chen Y, Xu Y, Rylander MN, Rylander CG. Fiber optic microneedles for transdermal light delivery: ex vivo porcine skin penetration experiments. Journal of biomechanical engineering. 2010;132:091014. doi: 10.1115/1.4002192. [DOI] [PubMed] [Google Scholar]
- Kosoglu MA, Hood RL, Rossmeisl JH, Jr, Grant DC, Xu Y, Robertson JL, Rylander MN, Rylander CG. Fiberoptic microneedles: novel optical diffusers for interstitial delivery of therapeutic light. Lasers Surg Med. 2011;43:914–920. doi: 10.1002/lsm.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Ghate V, Kim MJ, Zhou W, Khoo GH, Yuk HG. Antibacterial efficacy of 405, 460 and 520 nm light emitting diodes on Lactobacillus plantarum, Staphylococcus aureus and Vibrio parahaemolyticus. Journal of applied microbiology. 2016;120:49–56. doi: 10.1111/jam.12975. [DOI] [PubMed] [Google Scholar]
- Kumar A, Ghate V, Kim MJ, Zhou W, Khoo GH, Yuk HG. Kinetics of bacterial inactivation by 405nm and 520nm light emitting diodes and the role of endogenous coproporphyrin on bacterial susceptibility. J Photochem Photobiol B. 2015;149:37–44. doi: 10.1016/j.jphotobiol.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Kuse Y, Ogawa K, Tsuruma K, Shimazawa M, Hara H. Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light. Scientific reports. 2014;4:5223. doi: 10.1038/srep05223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushibiki T, Hirasawa T, Okawa S, Ishihara M. Blue laser irradiation generates intracellular reactive oxygen species in various types of cells. Photomed Laser Surg. 2013;31:95–104. doi: 10.1089/pho.2012.3361. [DOI] [PubMed] [Google Scholar]
- Lacombe A, Niemira BA, Sites J, Boyd G, Gurtler JB, Tyrell B, Fleck M. Reduction of Bacterial Pathogens and Potential Surrogates on the Surface of Almonds Using High-Intensity 405-Nanometer Light. Journal of food protection. 2016;79:1840–1845. doi: 10.4315/0362-028X.JFP-15-418. [DOI] [PubMed] [Google Scholar]
- Lagori G, Fornaini C, Rocca JP, Merigo E. Use of photo-Fenton’s reaction by 400-nm LED light for endodontic disinfection: A preliminary in vitro study on Enterococcus faecalis. J Photochem Photobiol B. 2017;171:85–89. doi: 10.1016/j.jphotobiol.2017.04.033. [DOI] [PubMed] [Google Scholar]
- Lee JB, Kim SH, Lee SC, Kim HG, Ahn HG, Li Z, Yoon KC. Blue light-induced oxidative stress in human corneal epithelial cells: protective effects of ethanol extracts of various medicinal plant mixtures. Invest Ophthalmol Vis Sci. 2014;55:4119–4127. doi: 10.1167/iovs.13-13441. [DOI] [PubMed] [Google Scholar]
- Li J, Cao J, Wang Z, Dong Y, Chen Y. Melatonin plays a critical role in inducing B lymphocyte proliferation of the bursa of Fabricius in broilers via monochromatic lights. J Photochem Photobiol B. 2015;142:29–34. doi: 10.1016/j.jphotobiol.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Liao H-L, Alferez F, Burns JK. Assessment of blue light treatments on citrus postharvest diseases. Postharvest Biology and Technology. 2013;81:81–88. [Google Scholar]
- Liebmann J, Born M, Kolb-Bachofen V. Blue-light irradiation regulates proliferation and differentiation in human skin cells. J Invest Dermatol. 2010;130:259–269. doi: 10.1038/jid.2009.194. [DOI] [PubMed] [Google Scholar]
- Lipovsky A, Gedanken A, Nitzan Y, Lubart R. Enhanced inactivation of bacteria by metal-oxide nanoparticles combined with visible light irradiation. Lasers Surg Med. 2011;43:236–240. doi: 10.1002/lsm.21033. [DOI] [PubMed] [Google Scholar]
- Losi A. Flavin-based Blue-Light photosensors: a photobiophysics update. Photochem Photobiol. 2007;83:1283–1300. doi: 10.1111/j.1751-1097.2007.00196.x. [DOI] [PubMed] [Google Scholar]
- Maclean M, Anderson JG, MacGregor SJ, White T, Atreya CD. A new proof of concept in bacterial reduction: antimicrobial action of violet-blue Light (405 nm) in ex vivo stored plasma. J Blood Transfus. 2016;2016:2920514. doi: 10.1155/2016/2920514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean M, Murdoch LE, MacGregor SJ, Anderson JG. Sporicidal effects of high-intensity 405 nm visible light on endospore-forming bacteria. Photochem Photobiol. 2013;89:120–126. doi: 10.1111/j.1751-1097.2012.01202.x. [DOI] [PubMed] [Google Scholar]
- Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makdoumi K, Goodrich R, Backman A. Photochemical eradication of methicillin-resistant Staphylococcus aureus by blue light activation of riboflavin. Acta Ophthalmol. 2017 doi: 10.1111/aos.13409. [DOI] [PubMed] [Google Scholar]
- Mamalis A, Garcha M, Jagdeo J. Light emitting diode-generated blue light modulates fibrosis characteristics: fibroblast proliferation, migration speed, and reactive oxygen species generation. Lasers Surg Med. 2015;47:210–215. doi: 10.1002/lsm.22293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Calvo MS, Codony F, Agusti G, Lahera C. Visible light enhances the antimicrobial effect of some essential oils. Photodiagnosis Photodyn Ther. 2016 doi: 10.1016/j.pdpdt.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Masson-Meyers DS, Bumah VV, Biener G, Raicu V, Enwemeka CS. The relative antimicrobial effect of blue 405 nm LED and blue 405 nm laser on methicillin-resistant Staphylococcus aureus in vitro. Lasers Med Sci. 2015;30:2265–2271. doi: 10.1007/s10103-015-1799-1. [DOI] [PubMed] [Google Scholar]
- Masson-Meyers DS, Bumah VV, Enwemeka CS. Blue light does not impair wound healing in vitro. J Photochem Photobiol B. 2016a;160:53–60. doi: 10.1016/j.jphotobiol.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Masson-Meyers DS, Bumah VV, Enwemeka CS. A comparison of four methods for determining viability in human dermal fibroblasts irradiated with blue light. J Pharmacol Toxicol Methods. 2016b;79:15–22. doi: 10.1016/j.vascn.2016.01.001. [DOI] [PubMed] [Google Scholar]
- McFee RB. Nosocomial or hospital-acquired infections: an overview. Dis Mon. 2009;55:422–438. doi: 10.1016/j.disamonth.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie K, Maclean M, Grant MH, Ramakrishnan P, MacGregor SJ, Anderson JG. The effects of 405 nm light on bacterial membrane integrity determined by salt and bile tolerance assays, leakage of UV-absorbing material and SYTOX green labelling. Microbiology (Reading, England) 2016;162:1680–1688. doi: 10.1099/mic.0.000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie K, Maclean M, Timoshkin IV, Endarko E, MacGregor SJ, Anderson JG. Photoinactivation of bacteria attached to glass and acrylic surfaces by 405 nm light: potential application for biofilm decontamination. Photochem Photobiol. 2013;89:927–935. doi: 10.1111/php.12077. [DOI] [PubMed] [Google Scholar]
- McKenzie K, Maclean M, Timoshkin IV, MacGregor SJ, Anderson JG. Enhanced inactivation of Escherichia coli and Listeria monocytogenes by exposure to 405 nm light under sub-lethal temperature, salt and acid stress conditions. Int J Food Microbiol. 2014;170:91–98. doi: 10.1016/j.ijfoodmicro.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Menezes PFC, Requena MB, Lizarelli RFZ, Bagnato VS. Blue LED irradiation to hydration of skin. 2015:95311W-95311W–95317. [Google Scholar]
- Mira A, Simon-Soro A, Curtis MA. Role of microbial communities in the pathogenesis of periodontal diseases and caries. Journal of clinical periodontology. 2017;44(Suppl 18):S23–s38. doi: 10.1111/jcpe.12671. [DOI] [PubMed] [Google Scholar]
- Monfrecola G, Lembo S, Cantelli M, Ciaglia E, Scarpato L, Fabbrocini G, Balato A. The effect of visible blue light on the differentiation of dendritic cells in vitro. Biochimie. 2014;101:252–255. doi: 10.1016/j.biochi.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Moorhead S, Maclean M, Coia JE, MacGregor SJ, Anderson JG. Synergistic efficacy of 405 nm light and chlorinated disinfectants for the enhanced decontamination of Clostridium difficile spores. Anaerobe. 2016a;37:72–77. doi: 10.1016/j.anaerobe.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Moorhead S, Maclean M, MacGregor SJ, Anderson JG. Comparative Sensitivity of Trichophyton and Aspergillus Conidia to Inactivation by Violet-Blue Light Exposure. Photomed Laser Surg. 2016b;34:36–41. doi: 10.1089/pho.2015.3922. [DOI] [PubMed] [Google Scholar]
- Motts SD, Guffey JS, Payne WC, Towery P, Hobson T, Harrell G, Meurer L, Lancaster K. The use of 405nm and 464nm blue light to inhibit Listeria monocytogenes in Ready-to-Eat (RTE) meat. Eur J Acad Essays. 2016;3:76–80. [Google Scholar]
- Murdoch LE, Maclean M, Endarko E, MacGregor SJ, Anderson JG. Bactericidal effects of 405 nm light exposure demonstrated by inactivation of Escherichia, Salmonella, Shigella, Listeria, and Mycobacterium species in liquid suspensions and on exposed surfaces. TheScientificWorldJournal. 2012;2012:137805. doi: 10.1100/2012/137805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch LE, McKenzie K, Maclean M, Macgregor SJ, Anderson JG. Lethal effects of high-intensity violet 405-nm light on Saccharomyces cerevisiae, Candida albicans, and on dormant and germinating spores of Aspergillus niger. Fungal Biol. 2013;117:519–527. doi: 10.1016/j.funbio.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Ishiyama K, Sheng H, Ikai H, Kanno T, Niwano Y. Bactericidal Activity and Mechanism of Photoirradiated Polyphenols against Gram-Positive and -Negative Bacteria. J Agric Food Chem. 2015;63:7707–7713. doi: 10.1021/jf5058588. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Yamada Y, Ikai H, Kanno T, Sasaki K, Niwano Y. Bactericidal action of photoirradiated gallic acid via reactive oxygen species formation. J Agric Food Chem. 2012;60:10048–10054. doi: 10.1021/jf303177p. [DOI] [PubMed] [Google Scholar]
- Neelakantan P, Cheng CQ, Ravichandran V, Mao T, Sriraman P, Sridharan S, Subbarao C, Sharma S, Kishen A. Photoactivation of curcumin and sodium hypochlorite to enhance antibiofilm efficacy in root canal dentin. Photodiagnosis Photodyn Ther. 2015;12:108–114. doi: 10.1016/j.pdpdt.2014.10.011. [DOI] [PubMed] [Google Scholar]
- NIH. Large Research Projects for Prevention and Management of Healthcare-Associated Infections. 2015 [Google Scholar]
- Nitzan Y, Salmon-Divon M, Shporen E, Malik Z. ALA induced photodynamic effects on gram positive and negative bacteria. Photochemical & photobiological sciences: Official journal of the European Photochemistry Association and the European Society for Photobiology. 2004;3:430–435. doi: 10.1039/b315633h. [DOI] [PubMed] [Google Scholar]
- Nour El Din S, El-Tayeb TA, Abou-Aisha K, El-Azizi M. In vitro and in vivo antimicrobial activity of combined therapy of silver nanoparticles and visible blue light against Pseudomonas aeruginosa. Int J Nanomedicine. 2016;11:1749–1758. doi: 10.2147/IJN.S102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donoghue B, NicAogain K, Bennett C, Conneely A, Tiensuu T, Johansson J, O’Byrne C. Blue-light inhibition of Listeria monocytogenes growth Is mediated by reactive oxygen species and is influenced by sigmaB and the blue-Light sensor Lmo0799. Appl Environ Microbiol. 2016;82:4017–4027. doi: 10.1128/AEM.00685-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panhoca VH, Esteban Florez FL, Correa TQ, Paolillo FR, de Souza CW, Bagnato VS. Oral Decontamination of Orthodontic Patients Using Photodynamic Therapy Mediated by Blue-Light Irradiation and Curcumin Associated with Sodium Dodecyl Sulfate. Photomed Laser Surg. 2016;34:411–417. doi: 10.1089/pho.2015.4080. [DOI] [PubMed] [Google Scholar]
- Pereira NLF, Aquino PEA, Junior J, Cristo JS, Vieira Filho MA, Moura FF, Ferreira NMN, Silva MKN, Nascimento EM, Correia FMA, Cunha FAB, Boligon AA, Coutinho HDM, Matias EFF, Guedes MIF. In vitro evaluation of the antibacterial potential and modification of antibiotic activity of the Eugenia uniflora L. essential oil in association with led lights. Microbial pathogenesis. 2017a;110:512–518. doi: 10.1016/j.micpath.2017.07.048. [DOI] [PubMed] [Google Scholar]
- Pereira NLF, Aquino PEA, Junior J, Cristo JS, Vieira Filho MA, Moura FF, Ferreira NMN, Silva MKN, Nascimento EM, Correia FMA, Cunha FAB, Boligon AA, Coutinho HDM, Ribeiro-Filho J, Matias EFF, Guedes MIF. Antibacterial activity and antibiotic modulating potential of the essential oil obtained from Eugenia jambolana in association with led lights. J Photochem Photobiol B. 2017b;174:144–149. doi: 10.1016/j.jphotobiol.2017.07.027. [DOI] [PubMed] [Google Scholar]
- Phan TX, Jaruga B, Pingle SC, Bandyopadhyay BC, Ahern GP. Intrinsic Photosensitivity Enhances Motility of T Lymphocytes. Scientific reports. 2016;6:39479. doi: 10.1038/srep39479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan P, Maclean M, MacGregor SJ, Anderson JG, Grant MH. Cytotoxic responses to 405nm light exposure in mammalian and bacterial cells: Involvement of reactive oxygen species. Toxicology in vitro: an international journal published in association with BIBRA. 2016;33:54–62. doi: 10.1016/j.tiv.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan P, Maclean M, MacGregor SJ, Anderson JG, Grant MH. Differential sensitivity of osteoblasts and bacterial pathogens to 405-nm light highlighting potential for decontamination applications in orthopedic surgery. J Biomed Opt. 2014;19:105001. doi: 10.1117/1.JBO.19.10.105001. [DOI] [PubMed] [Google Scholar]
- Ramirez-Castillo FY, Loera-Muro A, Jacques M, Garneau P, Avelar-Gonzalez FJ, Harel J, Guerrero-Barrera AL. Waterborne pathogens: detection methods and challenges. Pathogens. 2015;4:307–334. doi: 10.3390/pathogens4020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez MS, Traglia GM, Perez JF, Muller GL, Martinez MF, Golic AE, Mussi MA. White and blue light induce reduction in susceptibility to minocycline and tigecycline in Acinetobacter spp. and other bacteria of clinical importance. J Med Microbiol. 2015;64:525–537. doi: 10.1099/jmm.0.000048. [DOI] [PubMed] [Google Scholar]
- Randazzo W, Aznar R, Sanchez G. Curcumin-Mediated Photodynamic Inactivation of Norovirus Surrogates. Food and environmental virology. 2016;8:244–250. doi: 10.1007/s12560-016-9255-3. [DOI] [PubMed] [Google Scholar]
- Ray AC, Eakin RE. Studies on the biosynthesis of aspergillin by Aspergillus niger. Appl Microbiol. 1975;30:909–915. doi: 10.1128/am.30.6.909-915.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes NL, de la Presa M, Barneck MD, Poursaid A, Firpo MA, Langell JT. Violet 405 nm light: A novel therapeutic agent against beta-lactam-resistant Escherichia coli. Lasers Surg Med. 2016;48:311–317. doi: 10.1002/lsm.22457. [DOI] [PubMed] [Google Scholar]
- Rice LB. Do we really need new anti-infective drugs? Curr Opin Pharmacol. 2003;3:459–463. doi: 10.1016/j.coph.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Roh HJ, Kim A, Kang GS, Kim D-H. Photoinactivation of major bacterial pathogens in aquaculture. Fisheries and Aquatic Sciences. 2016;19:28. [Google Scholar]
- Romling U, Balsalobre C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J Intern Med. 2012;272:541–561. doi: 10.1111/joim.12004. [DOI] [PubMed] [Google Scholar]
- Rosa LP, da Silva FC, Viana MS, Meira GA. In vitro effectiveness of 455-nm blue LED to reduce the load of Staphylococcus aureus and Candida albicans biofilms in compact bone tissue. Lasers Med Sci. 2016;31:27–32. doi: 10.1007/s10103-015-1826-2. [DOI] [PubMed] [Google Scholar]
- Schafer ME, McNeely T. Coincident Light/ultrasound therapy to treat bacterial biofilms. 2015 IEEE International Ultrasonics Symposium (IUS) 2015:1–4. [Google Scholar]
- Schmid J, Hoenes K, Rath M, Vatter P, Spellerberg B, Hessling M. Photoinactivation of Legionella rubrilucens by visible light. Eur J Microbiol Immunol (Bp) 2017;7:146–149. doi: 10.1556/1886.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnedeker AH, Cole LK, Lorch G, Diaz SF, Bonagura J, Daniels JB. In vitro bactericidal activity of blue light (465 nm) phototherapy on meticillin-susceptible and meticillin-resistant Staphylococcus pseudintermedius. Veterinary dermatology. 2017 doi: 10.1111/vde.12451. [DOI] [PubMed] [Google Scholar]
- Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol. 2013;34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- Simkovitch R, Gajst O, Zelinger E, Yarden O, Huppert D. Irradiation by blue light in the presence of a photoacid confers changes to colony morphology of the plant pathogen Colletotrichum gloeosporioides. J Photochem Photobiol B. 2017;174:1–9. doi: 10.1016/j.jphotobiol.2017.06.041. [DOI] [PubMed] [Google Scholar]
- Sommers C, Gunther NWt, Sheen S. Inactivation of Salmonella spp., pathogenic Escherichia coli, Staphylococcus spp., or Listeria monocytogenes in chicken purge or skin using a 405-nm LED array. Food Microbiol. 2017;64:135–138. doi: 10.1016/j.fm.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Song HH, Lee JK, Um HS, Chang BS, Lee SY, Lee MK. Phototoxic effect of blue light on the planktonic and biofilm state of anaerobic periodontal pathogens. J Periodontal Implant Sci. 2013;43:72–78. doi: 10.5051/jpis.2013.43.2.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukos NS, Stultz J, Abernethy AD, Goodson JM. Phototargeting human periodontal pathogens in vivo. Lasers Med Sci. 2015;30:943–952. doi: 10.1007/s10103-013-1497-9. [DOI] [PubMed] [Google Scholar]
- Srimagal A, Ramesh T, Sahu JK. Effect of light emitting diode treatment on inactivation of Escherichia coli in milk. LWT - Food Science and Technology. 2016;71:378–385. [Google Scholar]
- Sterer N, Jeffet U, Dadoun A, Greenstein RB, Kohavi D. Zinc enhances the phototoxic effect of blue light against malodour-producing bacteria in an experimental oral biofilm. J Med Microbiol. 2014;63:1071–1075. doi: 10.1099/jmm.0.075044-0. [DOI] [PubMed] [Google Scholar]
- Tomb RM, Maclean M, Coia JE, Graham E, McDonald M, Atreya CD, MacGregor SJ, Anderson JG. New Proof-of-Concept in Viral Inactivation: Virucidal Efficacy of 405 nm Light Against Feline Calicivirus as a Model for Norovirus Decontamination. Food and environmental virology. 2016 doi: 10.1007/s12560-016-9275-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter LA, Patel D, Dubin S, Guerra C, McCloud V, Lockwood P, Messer R, Wataha JC, Lewis JB. Violet/blue light activates Nrf2 signaling and modulates the inflammatory response of THP-1 monocytes. Photochemical & photobiological sciences: Official journal of the European Photochemistry Association and the European Society for Photobiology. 2017;16:883–889. doi: 10.1039/c6pp00299d. [DOI] [PubMed] [Google Scholar]
- Trzaska WJ, Wrigley HE, Thwaite JE, May RC. Species-specific antifungal activity of blue light. Scientific reports. 2017;7:4605. doi: 10.1038/s41598-017-05000-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada M, Sheng H, Kamachi T, Niwano Y. Microbicidal action of photoirradiated aqueous extracts from wine lees. Journal of food science and technology. 2016a;53:3020–3027. doi: 10.1007/s13197-016-2273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada M, Sheng H, Tada M, Mokudai T, Oizumi S, Kamachi T, Niwano Y. Bactericidal Action of Photo-Irradiated Aqueous Extracts from the Residue of Crushed Grapes from Winemaking. Biocontrol science. 2016b;21:113–121. doi: 10.4265/bio.21.113. [DOI] [PubMed] [Google Scholar]
- Vandersee S, Beyer M, Lademann J, Darvin ME. Blue-violet light irradiation dose dependently decreases carotenoids in human skin, which indicates the generation of free radicals. Oxid Med Cell Longev. 2015;2015:579675. doi: 10.1155/2015/579675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmerhausen TL, Conneely A, Bennett C, Wagner VE, Victor JC, O’Byrne CP. Visible and UVA light as a potential means of preventing Escherichia coli biofilm formation in urine and on materials used in urethral catheters. J Photochem Photobiol B. 2017;170:295–303. doi: 10.1016/j.jphotobiol.2017.04.018. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wu X, Chen J, Amin R, Lu M, Bhayana B, Zhao J, Murray CK, Hamblin MR, Hooper DC, Dai T. Antimicrobial Blue Light Inactivation of Gram-Negative Pathogens in Biofilms: In Vitro and In Vivo Studies. J Infect Dis. 2016;213:1380–1387. doi: 10.1093/infdis/jiw070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasson CJ, Zourelias JL, Aardsma NA, Eells JT, Ganger MT, Schober JM, Skwor TA. Inhibitory effects of 405 nm irradiation on Chlamydia trachomatis growth and characterization of the ensuing inflammatory response in HeLa cells. BMC Microbiol. 2012;12:176. doi: 10.1186/1471-2180-12-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong R, Xie G, Edmondson AE, Sheard MA. A mathematical model for bacterial inactivation. Int J Food Microbiol. 1999;46:45–55. doi: 10.1016/s0168-1605(98)00172-x. [DOI] [PubMed] [Google Scholar]
- Xu H, Fu Y-n, Li T-l, Wang R. Effects of different LED light wavelengths on the resistance of tomato against Botrytis cinerea and the corresponding physiological mechanisms. Journal of Integrative Agriculture. 2017;16:106–114. [Google Scholar]
- Yang P, Wang N, Wang C, Yao Y, Fu X, Yu W, Cai R, Yao M. 460-nm visible light irradiation eradicates MRSA via inducing prophage activation. J Photochem Photobiol B. 2017;166:311–322. doi: 10.1016/j.jphotobiol.2016.12.001. [DOI] [PubMed] [Google Scholar]
- Yasukawa H, Konno N, Haneda Y, Yamamori B, Iseki M, Shibusawa M, Ono Y, Kodaira K, Funada H, Watanabe M. Photomanipulation of antibiotic susceptibility and biofilm formation of Escherichia coli heterologously expressing photoactivated adenylyl cyclase. The Journal of general and applied microbiology. 2012;58:183–190. doi: 10.2323/jgam.58.183. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Sasaki H, Toyama T, Araki M, Fujioka J, Tsukiyama K, Hamada N, Yoshino F. Antimicrobial effect of blue light using Porphyromonas gingivalis pigment. Scientific reports. 2017;7:5225. doi: 10.1038/s41598-017-05706-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Shiotsu-Ogura Y, Wada-Takahashi S, Takahashi SS, Toyama T, Yoshino F. Blue light irradiation-induced oxidative stress in vivo via ROS generation in rat gingival tissue. J Photochem Photobiol B. 2015;151:48–53. doi: 10.1016/j.jphotobiol.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Yoshino F, Makita T, Maehata Y, Higashi K, Miyamoto C, Wada-Takahashi S, Takahashi SS, Takahashi O, Lee MC. Reactive oxygen species production in mitochondria of human gingival fibroblast induced by blue light irradiation. J Photochem Photobiol B. 2013;129:1–5. doi: 10.1016/j.jphotobiol.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Yuan D, Collage RD, Huang H, Zhang X, Kautza BC, Lewis AJ, Zuckerbraun BS, Tsung A, Angus DC, Rosengart MR. Blue light reduces organ injury from ischemia and reperfusion. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:5239–5244. doi: 10.1073/pnas.1515296113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhu Y, Chen J, Wang Y, Sherwood ME, Murray CK, Vrahas MS, Hooper DC, Hamblin MR, Dai T. Antimicrobial blue light inactivation of Candida albicans: In vitro and in vivo studies. Virulence. 2016;7:536–545. doi: 10.1080/21505594.2016.1155015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhu Y, Gupta A, Huang Y, Murray CK, Vrahas MS, Sherwood ME, Baer DG, Hamblin MR, Dai T. Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: implications for prophylaxis and treatment of combat-related wound infections. J Infect Dis. 2014;209:1963–1971. doi: 10.1093/infdis/jit842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Kochevar IE, Behlau I, Zhao J, Wang F, Wang Y, Sun X, Hamblin MR, Dai T. Antimicrobial blue light therapy for infectious keratitis: ex vivo and in vivo studies. Invest Ophthalmol Vis Sci. 2017;58:586–593. doi: 10.1167/iovs.16-20272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, Keohane C, Denham CR, Bates DW. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173:2039–2046. doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]