Abstract
An altered intestinal microbiota composition has been implicated in the pathogenesis of metabolic disease including obesity and type 2 diabetes mellitus (T2DM). Low grade inflammation, potentially initiated by the intestinal microbiota, has been suggested to be a driving force in the development of insulin resistance in obesity. Here, we report that bacterial DNA is present in mesenteric adipose tissue of obese but otherwise healthy human subjects. Pyrosequencing of bacterial 16S rRNA genes revealed that DNA from the Gram-negative species Ralstonia was most prevalent. Interestingly, fecal abundance of Ralstonia pickettii was increased in obese subjects with pre-diabetes and T2DM. To assess if R. pickettii was causally involved in development of obesity and T2DM, we performed a proof-of-concept study in diet-induced obese (DIO) mice. Compared to vehicle-treated control mice, R. pickettii-treated DIO mice had reduced glucose tolerance. In addition, circulating levels of endotoxin were increased in R. pickettii-treated mice. In conclusion, this study suggests that intestinal Ralstonia is increased in obese human subjects with T2DM and reciprocally worsens glucose tolerance in DIO mice.
Introduction
The worldwide epidemic of obesity, which is a major risk factor for insulin resistance, drives the development of common medical conditions such as type 2 diabetes mellitus (T2DM), dyslipidaemia and cardiovascular disease [1]. The development of obesity and T2DM is complex and is driven by both environmental and genetic factors [2]. Obesity-induced inflammatory changes in white adipose tissue have been postulated to play a crucial part in the pathophysiology of obesity and T2DM. Although the majority of our fat depot is located in subcutaneous adipose tissue, approximately 10–20% of the total adipose tissue mass is located intra-abdominally [3]. Especially mesenteric visceral adipose tissue inflammation is linked to insulin resistance reflected in reduced plasma adiponectin levels, which are associated with development of insulin resistance [4] and macrophage influx [5]. In turn, insulin resistance correlates with upregulation of visceral adipose genes involved in innate immunity and inflammation [5].
An increasing body of evidence suggests that the composition of the intestinal microbiota is related to energy intake and obesity [6] and to the development of chronic low-grade inflammation and insulin resistance [7, 8]. This is further supported by data suggesting that (postprandial) endotoxins derived from Gram-negative intestinal bacteria are involved in chronic low-grade inflammation and insulin resistance [9–11]. Indeed, the degree of endotoxemia was found to predict insulin resistance and development of T2DM in otherwise healthy obese subjects [12] through a process that is thought to stem from impaired gut barrier function [13]. Murine studies showed that macrophages in mesenteric adipose tissue indeed contain bacterial DNA that originates from the intestine [14]. However, it remains to be proven that specific intestinal bacteria are indeed causative in the pathogenesis of insulin resistance [15].
Here, we report that bacterial 16S rDNA, including that of the Gram-negative species Ralstonia, can be identified in mesenteric visceral adipose tissue of human obese subjects that were otherwise healthy. Interestingly, in a separate cohort of obese subjects with T2DM, fecal abundance of Ralstonia pickettii was increased compared to non-diabetic obese controls. To assess a potential causal role of R. pickettii in development of a diabetes-like phenotype in an obese model system, we treated diet-induced obese (DIO) mice with R. pickettii for four weeks. Interestingly, R. pickettii-treated DIO mice had reduced glucose tolerance compared to glycerol treated controls.
Results
Identification of Ralstonia bacterial DNA in mesenteric visceral adipose tissue from obese individuals
Bacterial 16S rDNA was PCR amplified from DNA isolated from human mesenteric visceral adipose tissue biopsies, whereas PCR amplification from omental or subcutaneous adipose tissue biopsies barely yielded any 16S rDNA amplicons. Amplicons from DNA isolated from mesenteric adipose tissue were subjected to denaturing gradient gel electrophoresis (DGGE) profiling (Fig 1A). Subsequent Sanger sequencing identified that the dominant band showed highest similarity to Ralstonia spp. Pyrosequencing analysis of bar-coded 16S rDNA amplicons obtained from the same DNA, identified seven bacterial genera in the mesenteric visceral adipose tissue from obese humans. Actinobacteria was the most prevalent Gram-positive and Ralstonia the most prevalent Gram-negative bacteria (Fig 1B). Considering emerging data in the field suggesting that endotoxin derived from Gram-negative bacteria is involved in metabolic endotoxemia and reduced glucose tolerance [9, 14, 16], for this project we focused on the role of Ralstonia in glucose homeostasis.
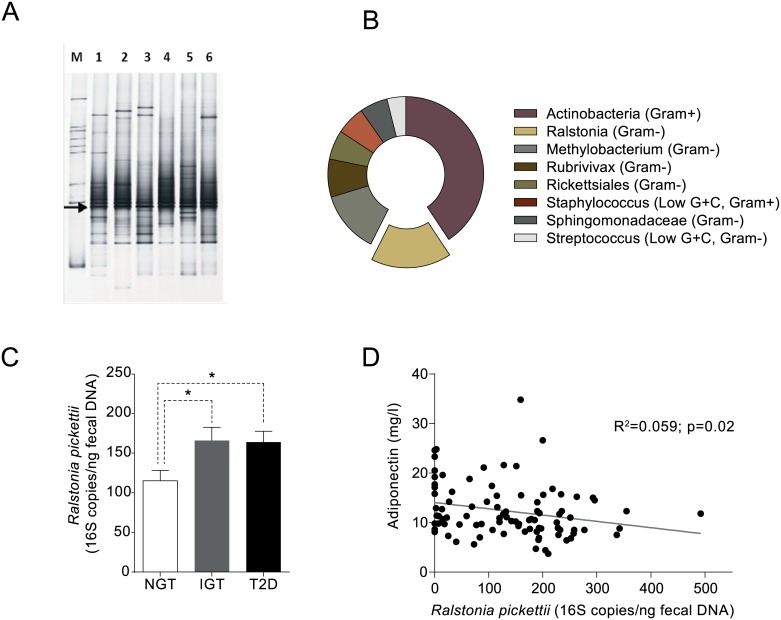
Fig 1. Ralstonia pickettii levels correlate with insulin resistance and T2DM in obese subjects.
(A) Bacterial DNA is present in mesenteric-visceral adipose tissue from otherwise healthy obese subjects that underwent laparoscopic surgery. Each lane depicts bacterial amplicons in a single mesenteric adipose tissue specimen of a subset of 6 patients. Arrow depicts the dominant amplicon of Ralstonia spp. as identified by Sanger sequencing of isolated bands. M = standard. (B) Pyrosequencing revealed presence of different species (percentage of total bacterial DNA) in human mesenteric visceral adipose tissue specimen (n = 12 subjects) with Ralstonia spp. being the most abundant Gram-negative bacteria. (C) Fecal 16S rRNA R. pickettii levels in obese postmenopausal women with normal glucose tolerance (NGT) (n = 42), impaired glucose tolerance (IGT) (n = 45) and type 2 diabetes mellitus (T2DM) (n = 47). (D) Correlation between fecal R. pickettii and plasma adiponectin in obese postmenopausal women with NGT, IGT and T2DM (population mixed in this figure). Error bars are represented as mean ± SEM. Mann-Whitney U testing (two sided) was performed to analyze the difference between clinical groups (C) and Spearman rank test (two sided) was used to calculate correlation coefficients (D). P-values < 0.05 (indicated by *) were considered statistically significant (using GraphPad Prism 5.1 and SPSS).
The Ralstonia genus belongs to the Proteobacteria phylum and Burkholderiales order, and comprises flagellated facultative anaerobic Gram-negative rod-shaped bacteria that are predominantly found in soil and water. Four species of Ralstonia (R. insidiosa, R. eutropha, R. mannitolilytica and R. pickettii) are known to reside in the human intestinal tract, with R. pickettii being most frequently associated with human infections [15].
Increased levels of fecal R. pickettii in patients with IGT or T2DM
Based on previous data regarding the association between altered intestinal microbiota and insulin resistance, we thus tested the hypothesis whether fecal R. pickettii levels could classify subjects with normal glucose tolerance (NGT), impaired glucose tolerance (IGT) and T2DM in a cohort of otherwise healthy subjects [7, 17]. Interestingly, fecal R. pickettii levels were significantly increased in IGT and T2DM subjects compared to NGT controls (Fig 1C). Furthermore, fecal R. pickettii levels correlated significantly (r = 0.059, p = 0.02) with plasma adiponectin in IGT and T2DM subjects (Fig 1D). Based on these associative data in obese T2DM human subjects, we questioned if R. pickettii could place a causal driving role in development of insulin resistance in a rodent model for obesity and insulin resistance.
Metabolic effects of R. pickettii gavage in diet induced obesity (DIO) mice
To examine potential causality of R. pickettii in development of obesity and insulin resistance, mice were fed a high-fat diet (HFD) (60% Kcal) for eight weeks. DIO-mice were then gavaged daily with heat-inactivated (HI) or living R. pickettii (106 CFU in 10% glycerol in PBS, final volume 100ul) for four weeks. 10% glycerol in PBS (glycerol) was used as control treatment.
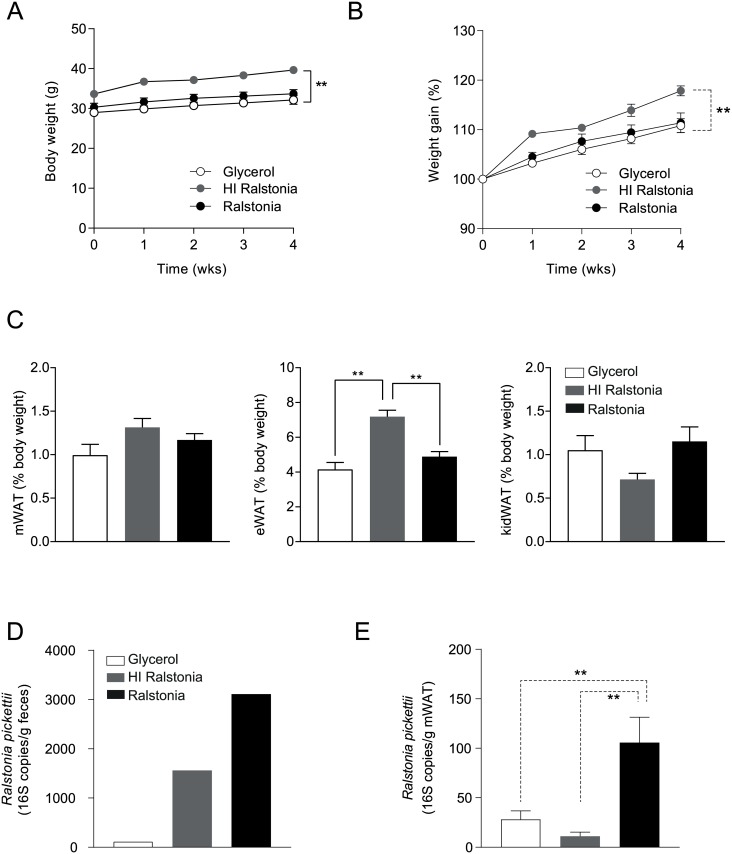
During the four-week treatment period, weight gain in HI-R. pickettii-treated DIO mice was increased compared to glycerol- and R. pickettii treated controls (Fig 2A). It is important to point out though, that HI-R. pickettii-treated DIO mice had slightly higher body weight at the start of the treatment period. Although we cannot fully explain this discrepancy, it might in part contribute to the increased relative weight gain (Fig 2B) upon HI R. pickettii treatment. The epididymal white adipose tissue (eWAT) compartment was significantly increased in HI-R. pickettii-treated mice compared to glycerol and R. pickettii-treated mice whereas mesenteric and kidney WAT compartments did not differ between groups (Fig 2C). R. pickettii content was increased in feces of HI-R. pickettii and R. pickettii-treated mice compared to glycerol-treated controls (Fig 2D). In contrast, R. pickettii DNA was not increased in mesenteric white adipose tissue (mWAT) of HI-R. pickettii treated mice compared to glycerol controls, whereas R. pickettii treated mice had increased levels of R. pickettii DNA in this adipose tissue compartment (Fig 2E). This suggests that live bacteria may be required for translocation from the gut consistent with a previous finding (14).
Fig 2. Ralstonia pickettii augments weight gain in DIO mice.
Diet-induced obese (DIO) C57Bl6 mice received 10E6 CFU heat-inactivated (HI)- or R. pickettii daily by means of oral gavage for four weeks. Glycerol was used as control. (A) Absolute body weight (g) during intervention time. HI R. pickettii group had higher starting body weight compared to glycerol and R. pickettii treated mice and gained more weight throughout the gavage experiment. (B) Relative (%) weight gain of glycerol, heat-inactivated (HI)- and R. pickettii-treated mice. HI-R. pickettii-treated mice gained more weight compared to glycerol- and R. pickettii-treated counterparts. (C) Relative weight (as % of body weight at time of termination) of mesenteric white adipose tissue (mWAT); epididymal white adipose tissue (eWAT) and kidney white adipose tissue (kWAT). eWAT weight was higher in HI R. pickettii-treated mice compared to glycerol and R.pickettii-treated mice. (D) qPCR analysis of R. pickettii DNA abundance per gram feces (per cage of mice) treated with glycerol, HI R. pickettii and R. pickettii. (E) qPCR analysis of R. pickettii DNA abundance per gram mesenteric white adipose tissue (mWAT) of mice treated with glycerol, HI R. pickettii and R. pickettii. N = 10 mice per group. Error bars are represented as mean ± SEM; p values were determined by Mann-Whitney U test or two-way ANOVA testing with Bonferroni post-test for multiple-comparison analysis (for weight gain). P-values < 0.05 (indicated by *) or < 0.01 (indicated by **) were considered statistically significant (using GraphPad Prism 5.1 and SPSS).
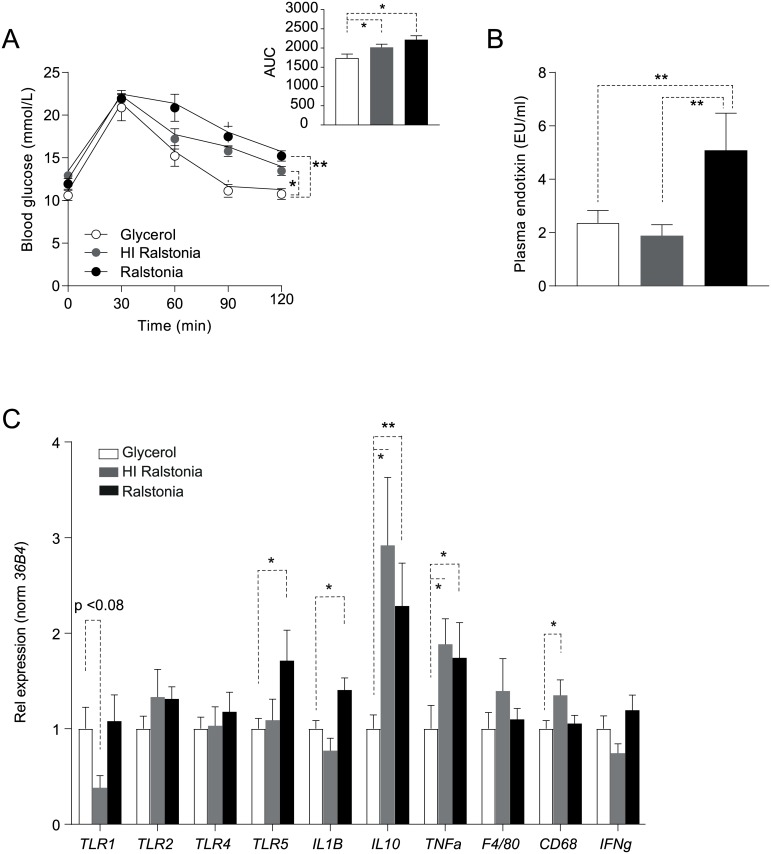
Oral glucose tolerance testing (OGTT) in week three of the treatment period revealed that clearance of glucose from the circulation was reduced in HI- and R. pickettii-treated DIO mice (Fig 3A). To assess if HFD feeding was a prerequisite to reduce glucose tolerance following four weeks of R. pickettii treatment, lean, chow-fed mice (age-matched with DIO mice) were gavaged daily with R. pickettii (106 CFU in 10% glycerol in PBS, final volume 100ul) for four weeks. Importantly, lean mice were not susceptible to weight gain and reduced glucose tolerance during the four week treatment period (S1A and S1B Fig).
Fig 3. Ralstonia pickettii supplementation reduces glucose tolerance and augments inflammatory tone in DIO mice.
(A) Oral glucose tolerance testing (OGTT) revealed that (HI)-R. pickettii treatment decreased glucose tolerance compared to glycerol treatment in DIO-mice. Area under the curve (AUC) is represented in the insert. (B) Plasma endotoxin levels (EU/ml) were increased in R. pickettii-treated mice compared with glycerol and HI R. pickettii-treated counterparts. (C) Relative mRNA expression of Tlr1, Tlr2, Tlr4, Tlr5, IL1B, IL10, TNFα, F4/80, CD68 and IFNγ in mesenteric white adipose tissue (mWAT) of mice treated with glycerol, HI R. pickettii and R. pickettii. Gene expression was normalized using 36B4 as a housekeeping gene. N = 10 mice per group. Error bars are represented as mean ± SEM; p values were determined by Mann-Whitney U test or two-way ANOVA testing with Bonferroni post-test for multiple-comparison analysis (for OGTT). P-values < 0.05 (indicated by *) or < 0.01 (indicated by **) were considered statistically significant (using GraphPad Prism 5.1 and SPSS).
Metabolic endotoxemia, a process resulting from translocation of endotoxic compounds (e.g., LPS) of Gram-negative intestinal bacteria, was first described based on the association between alterations in intestinal microbiota composition, circulating levels of the bacterial cell membrane component LPS and onset of T2DM (9). To assess if R. pickettii administration affected circulating endotoxin levels, we analyzed endotoxin levels in (HI) R. pickettii-treated DIO (Fig 3B) and lean (S1C Fig) mice. In DIO mice, endotoxin levels were significantly increased in the live R. pickettii-treated group compared to glycerol treated controls. Interestingly, HI-R. pickettii-treatment did not increase circulating endotoxin levels: endotoxin levels were comparable to levels in glycerol-treated DIO mice (Fig 3B). Although treatment with live R. pickettii increased endotoxin levels compared to treatment with HI-R. pickettii in lean mice, this difference did not reach statistical significance (S1C Fig). In line, fecal R. pickettii DNA content (S1D Fig) was higher only in live R. pickettii-treated lean mice compared to the HI R. pickettii and glycerol treated controls. Despite similar daily CFU doses, HI-R. Pickettii-treated DIO mice had reduced levels of circulating endotoxin compared to live R. pickettii-treated mice and this group gained more weight and had reduced glucose tolerance compared to glycerol-treated mice. This indicates that potential translocation and endotoxemia are not a prerequisite to develop reduced glucose tolerance upon Ralstonia administration.
Toll-like receptors (TLR) are pattern recognition receptors (PPRs) that interact with bacterial cell wall components and can subsequently induce an inflammatory response. To investigate the effect of R. pickettii administration on mWAT inflammation, gene expression levels of Tlr1, Tlr2, Tlr4 and Tlr5 were measured using qPCR (Fig 3C). Tlr5 expression was significantly upregulated in mWAT of DIO-mice treated with R. pickettii compared to HI- R. pickettii or glycerol-treated controls. In addition, expression of interleukins (IL) 1B and Il10 were significantly enhanced and indicative of activation of inflammatory pathways in mWAT of R. pickettii-treated DIO-mice. TNFa, F4/80, CD68 and CD14a were unaffected by (HI) R. pickettii-treatment. As control, gene expression of Tlr1, Tlr2, Tlr4, Tlr5, IL1B, IL10, TNFα, F4/80, CD68 and IFNγ was assessed in epididymal white adipose tissue (eWAT, S1E Fig). In contrast to mWAT, expression levels of assessed genes were mainly unaffected in eWAT. TNFα expression, however, like TNFα expression in mWAT, was significantly increased in (HI)-R. pickettii- treated mice compared to glycerol-treated controls. To assess if circulating TNFα levels were affected, we performed ELISA analysis in plasma. Circulating TNFα levels were below detection limit in all study groups.
Error bars are represented as mean ± SEM; p values were determined by Mann-Whitney U test or two-way ANOVA testing with Bonferroni post-test for multiple-comparison analysis (for weight gain and OGTT). P-values < 0.05 (indicated by *) or < 0.01 (indicated by **) were considered statistically significant (using GraphPad Prism 5.1 and SPSS).
Discussion
The development of insulin resistance and type 2 diabetes mellitus is associated with low-grade inflammation, or metabolic endotoxemia [10, 12, 16]. Although there is much debate as to whether intestinal microbiota composition is a causal factor or just a bystander in development of T2DM in humans [7, 8, 18, 19], mesenteric visceral adipose tissue inflammation is a well-known pathophysiological driver of insulin resistance [2]. Others have shown that Gram-negative flagellin-bearing pathogens, including Ralstonia are present in feces of human T2DM patients [16, 20]. In agreement with these observations we here demonstrate that DNA from Gram-negative Ralstonia species resides in human intestine but also in mesenteric visceral adipose tissue. Moreover, R. pickettii fecal concentrations were associated with impaired glucose tolerance and T2DM in human obese subjects. Furthermore, fecal R. pickettii levels correlated with plasma adiponectin levels as marker for impaired metabolic control.
Our proof-of-concept studies in mice suggested that intestinal bacterial strains like R. pickettii may indeed be causally linked to the pathophysiology of insulin resistance in obesity. R. pickettii inoculation reduced glucose tolerance and increased markers of mesenteric visceral adipose tissue inflammation in DIO mice. Interestingly, heat-inactivated bacteria generated similar effects as viable bacteria. Lean, chow-fed mice did not develop these pathologies when challenged with this bacterium. A HFD diet therefore seems to be a prerequisite for R. pickettii-mediated augmentation of metabolic derangements.
Our findings align with recent studies using murine conditional knockout models that suggest a distinct role for the intestinal bacterial pathogens and epithelial pattern recognition receptors in the development of insulin resistance [21–25]. The underlying mechanisms, however, remain to be studied but could be mediated by intestinal adaptive immune cells such as Innate Lymphoid Cells (ILC) [26, 27] or via enhanced B cell-mediated IgA antibody production against pathogens such as Ralstonia [28]. A potential relation between inflammatory tone, the innate immune system and TLRs has been implicated in the development of murine obesity and T2DM [5, 29, 30]. On the other hand, intestinal pathogens catabolize mucosal carbohydrates during their expansion that could subsequently enhance bacterial translocation [31]. Bacterial translocation has been defined as the passage of viable bacteria from the gastrointestinal tract to otherwise sterile peripheral tissues. This translocation potentially occurs via mesenteric lymph nodes and then to peripheral organs [32]. Recent data obtained from studies in humans have suggested that bacteria might be able to directly translocate from the intestinal wall to mesenteric adipose tissue via the circulation [33].
Our study has certain limitations. First, as Ralstonia spp. can be found in different environments including soil and (drinking) water [34], we cannot fully exclude potential contamination [35]. However, as we could not identify bacterial (Ralstonia) DNA in human omental and subcutaneous adipose tissue or in our controls, this seems to be less plausible. Moreover, the fact that R. pickettii inoculation reduced glucose tolerance in mice reduces the likelihood that R. pickettii effects are merely due to contamination. Second, it is currently unknown whether bacterial DNA detected in mesenteric adipose tissue is derived from alive or dead bacteria and studies using labelled bacteria are needed to study in vivo bacterial translocation. In addition, it remains to be determined whether bacterial DNA is equally present in all mesenteric adipose tissue depots along the human gastrointestinal tract and what mechanism underlies potential Ralstonia translocation. Our findings that HI-R. pickettii-treated mice have reduced circulating endotoxin levels and reduced 16S rDNA content in mesenteric adipose tissue suggests that in part alive bacteria are required in order to breach the gut-epithelial lining and reach the extra-intestinal compartment. Inflammatory markers in mesenteric adipose tissue of HI- or live R. pickettii-groups, however, were increased to similar extend for some (i.e., IL10, TNFα and CD68) but not all (i.e., TLR5 and IL1ββ) genes. If and how these differences in inflammatory expression patterns are related to potential bacterial translocation remains to be determined. Nevertheless, both HI-R. pickettii and active R. pickettii-treated mice had reduced glucose tolerance compared to glycerol-treated controls. Although we have not addressed this option, we speculate that increased levels of HI- R. pickettii might be sensed by the local intestinal immune system, thereby affecting inflammatory tone and augmenting glucose intolerance [36].
In conclusion, this proof-of-concept study shows that specific gram negative intestinal bacterial like R. pickettii are associated with the pathophysiology of insulin resistance in obesity. Our data support the preliminary hypothesis that bacterial translocation of these bacterial strains might be involved in the development of insulin resistance. Disentangling such a specific signature of intestinal microbiota involved in insulin resistance shifts might help to apply approaches aiming to better predict loss of insulin sensitivity and design targeted microbiota-based interventions in obese humans.
Materials and methods
Participants
Caucasian subjects (Fig 1A and 1B) (male/postmenopausal females, scheduled for elective laparoscopic cholecystectomy) were screened by the attending surgeon (n = 12 individuals included). Inclusion criteria were: age between 18–75 years and body-mass index (BMI) between 25–40 kg/m2. Exclusion criteria were: malignancy, diagnosed T2DM, chronic inflammatory disease and use of probiotics and/or antibiotics in the past three months. Written informed consent was obtained from all subjects. The study was approved by the AMC Ethics committee and conducted at the Flevo hospital (Almere, The Netherlands), Sint Lucas Andreas hospital (Amsterdam, The Netherlands) and Academic Medical Center (Amsterdam, The Netherlands), in accordance with the Declaration of Helsinki. Participants could continue their own diet, but were asked to fill out a week-long online nutritional diary (www.dieetinzicht.nl) to monitor caloric intake. Prior to surgery, anthropometric measurements were taken and a fasted blood sample was taken to determine levels of metabolic parameters in plasma. (see S1 Table).
The DIWA study (Fig 1C and 1D) included overweight women (average age 70 years old, BMI 25.8 to 28 kg/m2) with either normal glucose tolerance (NGT), impaired glucose tolerance (IGT) or type 2 diabetes mellitus (T2DM). Exclusion criteria were chronic inflammatory disease and treatment with antibiotics during the preceding three months. Further details about this cohort have been described elsewhere [7, 17]. All subjects gave informed consent and provided a fresh morning stool sample.
Animals
Male C56BL6/J mice were obtained from Charles River Laboratories. Mice were randomly allocated in treatment groups. Mice were fed a standard laboratory chow diet (Research Diets Inc., USA) or a high-fat diet (HFD) (60% Kcal fat, D12492, Research Diets Inc., USA) as indicated in the manuscript. Dietary components of the HFD are depicted in Table 1.
Table 1. Dietary components of the HFD.
| gm% | kcal% | |
| Protein | 26.2 | 20 |
| Carbohydrate | 26.3 | 20 |
| Fat | 34.9 | 60 |
| Total kcal/gm | 5.24 | |
| gm | kcal | |
| Casein, 30 Mesh | 200 | 800 |
| L-Cysteine | 3 | 12 |
| Corn Starch | 0 | 0 |
| Maltodextrin 10 | 125 | 500 |
| Sucrose | 68.8 | 275.2 |
| Cellulose, BW200 | 50 | 0 |
| Soybean Oil | 25 | 225 |
| Lard | 245 | 2205 |
| Mineral Mix S10026 | 10 | 0 |
| DiCalcium Phosphate | 13 | 0 |
| Calcium Carbonate | 5.5 | 0 |
| Potassium Citrate, 1 H2O | 16.5 | 0 |
| Vitamin Mix V10001 | 10 | 40 |
| Choline Bitartrate | 2 | 0 |
| FD&C Blue Dye #1 | 0.05 | 0 |
| Total | 773.85 | 4057 |
Diet-induced obesity (DIO) was realised by feeding mice a HFD for eight weeks starting at four weeks of age. Mice were housed in a constant 12-hour light-dark cycle with controlled temperature and humidity and were given access to food and water ad libitum.
Starting at the age of 13 weeks, Ralstonia pickettii (DSM 6297, Deutsche Sammlung von Mikroorganismen und Zellkulturen) was administered daily for four weeks by oral gavage (106 CFU in 10% glycerol in PBS, final volume 100ul). Heat-inactivated (10 min at 70°C) R. picketti (106 CFU in 10% glycerol- PBS, final volume 100ul) and glycerol (10% in PBS, final volume 100ul) were used as controls. Heat-inactivation fully impaired the ability of R. pickettii to grow (S2 Fig). Viability and purity of all R. pickettii stored at -80°C was tested up to 12 months by culture and sequencing. Weight gain and food intake were monitored throughout the treatment period. Feces was collected per cage (n = 5 mice per cage) in week three of treatment.
Oral glucose tolerance tests (OGTT) were performed in week three of the treatment period. Mice were fasted for 4 hours and blood glucose levels were measured from the tip of the tail. Mice received an oral bolus of D-glucose (2 g/kg bodyweight in 200ul sterile saline) and blood glucose levels were subsequently measured at t = 30, 60, 90, and 120 minutes.
After four weeks of R. pickettii- or control-treatment, mice were terminated by cardiac puncture under sodium pentobarbital anesthesia. Blood was collected in EDTA-coated tubes and was kept on ice until centrifugation (8,000xg, 4°C, 20min). Plasma was aliquoted and used for analysis immediately or stored at -80°C. Organs and tissues including mesenteric adipose tissue (aligning the colon transversum) were quickly excised under sterile conditions, snap-frozen in liquid nitrogen and stored at -80°C until further analysis.
All animal experiments were conducted in accordance with the principles of the “Guide to the Care and Use of Experimental Animals” and were prospectively approved by the Institutional Animal Care and Use Committee ("Dierexperimentencommissie (DEC) of the Academic Medical Center (AMC) in Amsterdam).
Bacterial DNA isolation and sequencing
Genomic DNA of both prokaryotic and eukaryotic origin was isolated from biopsies according to the phenol-choloform method as described by Zoetendal et al. [37]. In short, a standardized amount of fat tissue was treated with a mix of SDS and proteinase K at 55°C and homogenized by mechanical disruption using zirconium glass beads (1mm) in the FAST Prep-24 (MP Biomedical) in the presence of phenol. The genomic DNA was extracted using a series of phenol/chloroform extractions and precipitated in the presence of absolute ethanol.
The prokaryotic fraction was studied using a range of 16S rRNA specific primers and assays. Full-length 16S rDNA amplicons were generated using PCR by using primers Bact-27F (5’GTTTGATCCTGGCTCAG-3’) and Prok-1392R (5’GCCCGGGAACGTATTCACCG-3’). The PCR conditions have been described by Rajilic-Stojanovic et al. [38]. The resulting amplicons were purified and used as input for a nested PCR using primers 968-GC-F and 1392, generating fragments fit for a diversity analysis by denaturing gradient gel electrophoresis (DGGE) profiling using conditions described by Heilig et al. [39]. Importantly, no amplicons were obtained from control PCRs with water and buffers. The dominant band appearing in the DGGE analyses was subcloned from the DGGE amplicon in a pGEM-T easy vector (Promega, Leiden, The Netherlands) and transformed into Stratagene E. coli XL-1 Blue competent cells (Agilent Technologies, Amstelveen, The Netherlands) according to the manufacturers’ specifications. Clones containing the correct insert that migrated to the same position as the dominant band in the DGGE gel were subjected to Sanger Next Generation Sequence analysis (GATC Biotech, Konstanz, Germany). Sequences were identified by performing a BLAST search (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Sequence analysis of the dominant band appearing in DGGE showed highest similarity to sequences of Ralstonia species.
Moreover, the genomic DNA was subjected to 454-pyrosequencing of the V4-V6 region of the 16S rRNA (using primers 520F 5'- AYT GGG YDT AAA GNG -3' and 1100R 5'- GGG TTN CGN TCG TTG -3'). The quality-controlled reads (normalized to at least 1000 reads per sample) were processed through the QIIME pipeline [40]. To quantify the Ralstonia-spp. bacteria, we performed a qPCR in the fecal DNA and mWAT DNA samples [41]. Samples were analyzed in a 25-μl reaction mix consisting of 12.5 μl 1xSYBR Green Master Mix buffer (Thermo Scientific, Waltham, Massachusetts, USA), water, 0.2 μM of each primer and 5 μl of template of genomic DNA extracted from feces or mWAT. Standard curve of 16S rRNA PCR product of Ralstonia pickettii was created using serial 10-fold dilution of purified full length 16S rDNA PCR product. The qPCR primers were based on R. pickettii (F’: ATGATCTAGC-TTGCTAGATTGAT; R’: ACTGATCGTCGCCTTGGTG). Data are expressed as copies of 16S rDNA Ralstonia compared to total bacterial DNA [42].
Plasma endotoxin measurement
Blood LPS endotoxin activity was measured using Endosafe-MCS (Charles River Laboratories, Lyon, France) based on the Limulus amaebocyte Lysate (LAL) kinetic chromogenic methodology that measures color intensity directly related to the endotoxin concentration in a sample. Plasma was diluted 1/10 with endotoxin free buffer (Charles River Laboratories) to minimize interferences in the reaction and heated for 15 min at 70°C. Each sample was diluted with endotoxin-free LAL reagent water (Charles River Laboratories) and treated in duplicate. Two spikes for each sample were included in the determination. All samples have been validated for the recovery and the coefficient variation. The lower limit of detection was 0.005 EU/ml [43].
Quantitative Real time PCR
Mouse (mesenteric and epidydimal) adipose tissue sections were homogenized using tissue-magnaLyzer (Roche, Switzerland). Total RNA was extracted using Tri-pure reagent (Roche). cDNA was prepared by reverse transcription of 1μg total RNA using a reverse transcription kit (BioRad, USA). Real-time qPCR was performed using Sensifast SYBR master mix (GC biotech). Gene-specific intron-exon boundary spanning primers were used and all the results were normalized to the house keeping gene 36B4. All samples were analyzed in duplicate and data were analyzed according to the 2ΔΔCT method.
Statistical analysis
Mann Whitney tests (two sided) were used to analyze the difference between (clinical) groups. Spearman rank test (two sided) was used to calculate correlation coefficients. P-values < 0.05 (indicated by *) or < 0.01 (indicated by **) were considered statistically significant (using GraphPad Prism 5.1 and SPSS).
Supporting information
Lean, chow-fed C57Bl6 mice received 10E6 CFU (HI) R. pickettii daily by means of oral gavage for four weeks. Glycerol was used as control. (A) Relative (%) weight gain during four weeks of glycerol, HI R. pickettii or R. pickettii administration. (B) Oral glucose tolerance tests (OGTT) in glycerol, HI R. pickettii or R. pickettii-treated mice. (C) Plasma endotoxin levels (EU/ml) in 4-hr fasted mice treated with glycerol, HI R. pickettii and R. pickettii. (D) qPCR analysis of R. pickettii DNA abundance per gram feces (per cage of mice) treated with glycerol, HI R. pickettii and R. pickettii. (E) Diet-induced obese (DIO) C57Bl6 mice received 10E6 CFU heat-inactivated (HI)- or R. pickettii daily by means of oral gavage for four weeks. Glycerol was used as control. Relative mRNA expression of Tlr1, Tlr2, Tlr4, Tlr5, IL1B, IL10, TNFα, F4/80, CD68 and IFNγ in epididymal white adipose tissue (eWAT) of DIO mice treated with glycerol, HI R. pickettii and R. pickettii. Gene expression was normalized using 36B4 as a housekeeping gene. N = 10 mice per group. Error bars are represented as mean ± SEM; p values were determined by Mann-Whitney U test or two-way ANOVA testing with Bonferroni post-test for multiple-comparison analysis (for weight gain). P-values < 0.05 (indicated by *) or < 0.01 (indicated by **) were considered statistically significant (using GraphPad Prism 5.1 and SPSS).
(EPS)
R. pickettii was heat-inactivated at 70°C for 10min. HI- or live R. pickettii were streaked onto blood agar plates and grown aerobically at 37°C overnight. Arrows indicate example colonies or R. pickettii.
(EPS)
Data are presented as mean±standard deviation.
(EPS)
Data Availability
All relevant data are included in the paper, its Supporting Information files, and from Dryad Digital Repository at https://doi.org/10.5061/dryad.q5s51.
Funding Statement
F. Bäckhed is supported by Swedish Research Council, Swedish Diabetes Foundation, Swedish Heart Lung Foundation, Swedish Foundation for Strategic Research, Knut and Alice Wallenberg foundation, Göran Gustafsson Foundation, Ingbritt and Arne Lundberg's foundation, Swedish Heart Lung Foundation, Torsten Söderberg's Foundation, Ragnar Söderberg's Foundation, NovoNordisk Foundation, AFA insurances, and LUA-ALF grants from Västra Götalandsregionen and Stockholm County Council. P.D.Cani is a research associate from the FRS-FNRS in Belgium. P.D. Cani is the recipient of FRFS-WELBIO under grant: WELBIO-CR-2012S-02R, the Funds Baillet Latour (Grant for Medical Research 2015), and ERC Starting Grant 2013 (Starting grant 336452-ENIGMO). FB is a recipient of ERC Consolidator Grant (European Research Council, Consolidator grant 615362 - METABASE). WM de Vos is supported by the Finland Academy of Sciences (grants 137389, 141140 and 1272870), the Netherlands Organization for Scientific Research (Spinoza Award and SIAM Gravity Grant) and the European Research Council (ERC Advanced Grant 250172 MicrobesInside and a POC Grant 632241) M. Nieuwdorp is supported by a ZONMW-VIDI grant 2013 (016.146.327). F.B. and G.B. received support in the form of equity from MetaboGen AB, Sweden. M.N. and W.M.deV. received support in the form of equity from Scientific Advisory Board of Caelus Pharmaceutical. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Cecchini M, Sassi F, Lauer JA, Lee YY, Guajardo-Barron V, Chisholm D. Tackling of unhealthy diets, physical inactivity, and obesity: health effects and cost-effectiveness. Lancet. 2010;376(9754):1775–84. doi: 10.1016/S0140-6736(10)61514-0 . [DOI] [PubMed] [Google Scholar]
- 2.Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Magi R, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518(7538):187–96. doi: 10.1038/nature14132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kredel LI, Siegmund B. Adipose-tissue and intestinal inflammation—visceral obesity and creeping fat. Front Immunol. 2014;5:462 doi: 10.3389/fimmu.2014.00462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fagerberg B, Kellis D, Bergstrom G, Behre CJ. Adiponectin in relation to insulin sensitivity and insulin secretion in the development of type 2 diabetes: a prospective study in 64-year-old women. J Intern Med. 2011;269(6):636–43. doi: 10.1111/j.1365-2796.2010.02336.x . [DOI] [PubMed] [Google Scholar]
- 5.Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59(7):1648–56. doi: 10.2337/db09-0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049–57. doi: 10.2337/db10-0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karlsson FH, Tremaroli V, Nookaew I, Bergstrom G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103. doi: 10.1038/nature12198 . [DOI] [PubMed] [Google Scholar]
- 8.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–6. doi: 10.1038/nature12506 . [DOI] [PubMed] [Google Scholar]
- 9.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–81. doi: 10.2337/db07-1403 . [DOI] [PubMed] [Google Scholar]
- 10.Clemente-Postigo M, Queipo-Ortuno MI, Murri M, Boto-Ordonez M, Perez-Martinez P, Andres-Lacueva C, et al. Endotoxin increase after fat overload is related to postprandial hypertriglyceridemia in morbidly obese patients. J Lipid Res. 2012;53(5):973–8. doi: 10.1194/jlr.P020909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harte AL, Varma MC, Tripathi G, McGee KC, Al-Daghri NM, Al-Attas OS, et al. High fat intake leads to acute postprandial exposure to circulating endotoxin in type 2 diabetic subjects. Diabetes Care. 2012;35(2):375–82. doi: 10.2337/dc11-1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun L, Yu Z, Ye X, Zou S, Li H, Yu D, et al. A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes Care. 2010;33(9):1925–32. doi: 10.2337/dc10-0340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gummesson A, Carlsson LM, Storlien LH, Backhed F, Lundin P, Lofgren L, et al. Intestinal permeability is associated with visceral adiposity in healthy women. Obesity (Silver Spring). 2011;19(11):2280–2. doi: 10.1038/oby.2011.251 . [DOI] [PubMed] [Google Scholar]
- 14.Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermudez-Humaran LG, et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med. 2011;3(9):559–72. doi: 10.1002/emmm.201100159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan MP, Adley CC. Ralstonia spp.: emerging global opportunistic pathogens. Eur J Clin Microbiol Infect Dis. 2014;33(3):291–304. doi: 10.1007/s10096-013-1975-9 . [DOI] [PubMed] [Google Scholar]
- 16.Amar J, Serino M, Lange C, Chabo C, Iacovoni J, Mondot S, et al. Involvement of tissue bacteria in the onset of diabetes in humans: evidence for a concept. Diabetologia. 2011;54(12):3055–61. doi: 10.1007/s00125-011-2329-8 . [DOI] [PubMed] [Google Scholar]
- 17.Brohall G, Behre CJ, Hulthe J, Wikstrand J, Fagerberg B. Prevalence of diabetes and impaired glucose tolerance in 64-year-old Swedish women: experiences of using repeated oral glucose tolerance tests. Diabetes Care. 2006;29(2):363–7. . [DOI] [PubMed] [Google Scholar]
- 18.Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308(11):1150–9. doi: 10.1001/2012.jama.11132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vrieze A, Van Nood E, Holleman F, Salojarvi J, Kootte RS, Bartelsman JF, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–6 e7. doi: 10.1053/j.gastro.2012.06.031 . [DOI] [PubMed] [Google Scholar]
- 20.Burcelin R, Serino M, Chabo C, Garidou L, Pomie C, Courtney M, et al. Metagenome and metabolism: the tissue microbiota hypothesis. Diabetes Obes Metab. 2013;15 Suppl 3:61–70. doi: 10.1111/dom.12157 . [DOI] [PubMed] [Google Scholar]
- 21.Carvalho FA, Koren O, Goodrich JK, Johansson ME, Nalbantoglu I, Aitken JD, et al. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe. 2012;12(2):139–52. doi: 10.1016/j.chom.2012.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chassaing B, Ley RE, Gewirtz AT. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology. 2014;147(6):1363–77 e17. doi: 10.1053/j.gastro.2014.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cullender TC, Chassaing B, Janzon A, Kumar K, Muller CE, Werner JJ, et al. Innate and adaptive immunity interact to quench microbiome flagellar motility in the gut. Cell Host Microbe. 2013;14(5):571–81. doi: 10.1016/j.chom.2013.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denou E, Lolmede K, Garidou L, Pomie C, Chabo C, Lau TC, et al. Defective NOD2 peptidoglycan sensing promotes diet-induced inflammation, dysbiosis, and insulin resistance. EMBO Mol Med. 2015;7(3):259–74. doi: 10.15252/emmm.201404169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Everard A, Geurts L, Caesar R, Van Hul M, Matamoros S, Duparc T, et al. Intestinal epithelial MyD88 is a sensor switching host metabolism towards obesity according to nutritional status. Nat Commun. 2014;5:5648 doi: 10.1038/ncomms6648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519(7542):242–6. doi: 10.1038/nature14115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geiger TL, Abt MC, Gasteiger G, Firth MA, O'Connor MH, Geary CD, et al. Nfil3 is crucial for development of innate lymphoid cells and host protection against intestinal pathogens. J Exp Med. 2014;211(9):1723–31. doi: 10.1084/jem.20140212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shulzhenko N, Morgun A, Hsiao W, Battle M, Yao M, Gavrilova O, et al. Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nat Med. 2011;17(12):1585–93. doi: 10.1038/nm.2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–25. doi: 10.1172/JCI28898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328(5975):228–31. doi: 10.1126/science.1179721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng KM, Ferreyra JA, Higginbottom SK, Lynch JB, Kashyap PC, Gopinath S, et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature. 2013;502(7469):96–9. doi: 10.1038/nature12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berg RD, Garlington AW. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979;23(2):403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peyrin-Biroulet L, Gonzalez F, Dubuquoy L, Rousseaux C, Dubuquoy C, Decourcelle C, et al. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn's disease. Gut. 2012;61(1):78–85. doi: 10.1136/gutjnl-2011-300370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan MP, Pembroke JT, Adley CC. Genotypic and phenotypic diversity of Ralstonia pickettii and Ralstonia insidiosa isolates from clinical and environmental sources including High-purity Water. Diversity in Ralstonia pickettii. BMC Microbiol. 2011;11:194 doi: 10.1186/1471-2180-11-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glassing A, Dowd SE, Galandiuk S, Davis B, Chiodini RJ. Inherent bacterial DNA contamination of extraction and sequencing reagents may affect interpretation of microbiota in low bacterial biomass samples. Gut Pathog. 2016;8:24 doi: 10.1186/s13099-016-0103-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi L, Li M, Miyazawa K, Li Y, Hiramatsu M, Xu J, et al. Effects of heat-inactivated Lactobacillus gasseri TMC0356 on metabolic characteristics and immunity of rats with the metabolic syndrome. Br J Nutr. 2013;109(2):263–72. doi: 10.1017/S000711451200116X . [DOI] [PubMed] [Google Scholar]
- 37.Zoetendal EG, von Wright A, Vilpponen-Salmela T, Ben-Amor K, Akkermans AD, de Vos WM. Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl Environ Microbiol. 2002;68(7):3401–7. doi: 10.1128/AEM.68.7.3401-3407.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajilic-Stojanovic M, Biagi E, Heilig HG, Kajander K, Kekkonen RA, Tims S, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141(5):1792–801. doi: 10.1053/j.gastro.2011.07.043 . [DOI] [PubMed] [Google Scholar]
- 39.Heilig HG, Zoetendal EG, Vaughan EE, Marteau P, Akkermans AD, de Vos WM. Molecular diversity of Lactobacillus spp. and other lactic acid bacteria in the human intestine as determined by specific amplification of 16S ribosomal DNA. Appl Environ Microbiol. 2002;68(1):114–23. doi: 10.1128/AEM.68.1.114-123.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–6. doi: 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zwielehner J, Lassl C, Hippe B, Pointner A, Switzeny OJ, Remely M, et al. Changes in human fecal microbiota due to chemotherapy analyzed by TaqMan-PCR, 454 sequencing and PCR-DGGE fingerprinting. PLoS One. 2011;6(12):e28654 doi: 10.1371/journal.pone.0028654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergmark L, Poulsen PH, Al-Soud WA, Norman A, Hansen LH, Sorensen SJ. Assessment of the specificity of Burkholderia and Pseudomonas qPCR assays for detection of these genera in soil using 454 pyrosequencing. FEMS Microbiol Lett. 2012;333(1):77–84. doi: 10.1111/j.1574-6968.2012.02601.x . [DOI] [PubMed] [Google Scholar]
- 43.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–71. doi: 10.1073/pnas.1219451110 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lean, chow-fed C57Bl6 mice received 10E6 CFU (HI) R. pickettii daily by means of oral gavage for four weeks. Glycerol was used as control. (A) Relative (%) weight gain during four weeks of glycerol, HI R. pickettii or R. pickettii administration. (B) Oral glucose tolerance tests (OGTT) in glycerol, HI R. pickettii or R. pickettii-treated mice. (C) Plasma endotoxin levels (EU/ml) in 4-hr fasted mice treated with glycerol, HI R. pickettii and R. pickettii. (D) qPCR analysis of R. pickettii DNA abundance per gram feces (per cage of mice) treated with glycerol, HI R. pickettii and R. pickettii. (E) Diet-induced obese (DIO) C57Bl6 mice received 10E6 CFU heat-inactivated (HI)- or R. pickettii daily by means of oral gavage for four weeks. Glycerol was used as control. Relative mRNA expression of Tlr1, Tlr2, Tlr4, Tlr5, IL1B, IL10, TNFα, F4/80, CD68 and IFNγ in epididymal white adipose tissue (eWAT) of DIO mice treated with glycerol, HI R. pickettii and R. pickettii. Gene expression was normalized using 36B4 as a housekeeping gene. N = 10 mice per group. Error bars are represented as mean ± SEM; p values were determined by Mann-Whitney U test or two-way ANOVA testing with Bonferroni post-test for multiple-comparison analysis (for weight gain). P-values < 0.05 (indicated by *) or < 0.01 (indicated by **) were considered statistically significant (using GraphPad Prism 5.1 and SPSS).
(EPS)
R. pickettii was heat-inactivated at 70°C for 10min. HI- or live R. pickettii were streaked onto blood agar plates and grown aerobically at 37°C overnight. Arrows indicate example colonies or R. pickettii.
(EPS)
Data are presented as mean±standard deviation.
(EPS)
Data Availability Statement
All relevant data are included in the paper, its Supporting Information files, and from Dryad Digital Repository at https://doi.org/10.5061/dryad.q5s51.