Abstract
This study was conducted to assess the influence of dietary protein content in poultry when using the 15N-leucine single-injection method to determine endogenous amino acid losses (EAALs) in poultry. Forty-eight cecectomized roosters (2.39 ± 0.23 kg) were randomly allocated to eight dietary treatments containing protein levels of 0, 3%, 6%, 9%, 12%, 15%, 18% and 21%. Each bird was precisely fed an experimental diet of 25 g/kg of body weight. After feeding, all roosters were subcutaneously injected with a 15N-leucine solution at a dose of 20 mg/kg of body weight. Blood was sampled 23 h after the injection, and excreta samples were continuously collected during the course of the 48-h experiment. The ratio of 15N-enrichment of leucine in crude mucin to free leucine in plasma ranged from 0.664 to 0.763 and remained relatively consistent (P > 0.05) across all treatments. The amino acid (AA) profiles of total endogenous AAs, except isoleucine, alanine, aspartic acid, cysteine, proline and serine, were not influenced (P > 0.05) by dietary protein contents. The predominant endogenous AAs in the excreta were glutamic acid, aspartic acid, threonine, serine and proline. The order of the relative proportions of these predominant AAs also remained relatively constant (P > 0.05). The endogenous losses of total AAs determined with the 15N-leucine single-injection method increased curvilinearly with the dietary protein contents. The true digestibility of most AAs and total AAs was independent of their respective dietary protein levels. Collectively, the 15N-leucine single-injection method is appropriate for determining EAALs and the true digestibility of AAs in poultry fed varying levels of protein-containing ingredients.
Introduction
The accurate quantification of endogenous amino acids losses (EAALs) in the intestines of animals is crucial for determining amino acid (AA) requirements and for calculating the true AA digestibility of feedstuffs. In poultry, EAALs have traditionally been determined by the fasted cecectomized rooster method, linear regression and the nitrogen-free diet method [1]. However, these methods can only be used to determine basal (or diet-independent) EAALs. Some dietary ingredients associated with “diet-specific” losses induce higher than basal losses [2]. To determine the total EAALs (basal + specific) in poultry fed various types of diets, our group proposed a method with a single injection of 15N-leucine (i.e., the 15N-leucine single-injection method) [3], which allowed us to estimate the true AA digestibility of feedstuffs. Compared with the traditional 15N-isotope infusion method that involves the continuous infusion of a15N-labeled AA [4–6], our method has advantages such as low cost and simplicity. This method mainly relies on the assumption that the ratio of 15N-enrichment of endogenous leucine in excreta (NLe) to leucine in deproteinized plasma (NLp) remains relatively constant (i.e., the NLe/NLp ratio is a constant value) after the injection of a single bolus of 15N-leucine into birds fed different protein-containing diets. Our previous results supported this method with dietary crude protein (CP) levels ranging from 0 to 5% [3]. However, further data are needed to assess the influence of higher dietary CP levels before practical application. Because the value of NLe cannot be determined directly due to interference from undigested dietary leucine, obtaining an alternative technique that reflects the value of NLe is the key to evaluating the method.
The gut mucosa is the biggest source of EAALs in the intestinal lumen [5, 7, 8]. Moreover, findings by Selle et al. [9] and Miner-Williams et al. [10] indicated that the mucin content was highest in all components of endogenous protein in the digesta. Mucin protein accounted for more than half of the non-bacterial protein components in the digesta [11]. Based on the above findings, we assume that mucin excretion can be taken individually as a reference for the whole endogenous protein content in the excreta. Therefore, we can investigate the effect of dietary CP levels on the NLcm (15N-enrichment of leucin in crude mucin)/NLp ratio to evaluate the 15N-leucine single-injection method.
Consistent with the 15N-isotope dilution method, the 15N-leucine single-injection method can only be used to estimate the endogenous losses of the labeled AA and not the levels of each AA [12]. A relatively constant AA profile of total endogenous protein is assumed to calculate the endogenous loss of each AA [4, 13], but few data are available in support of this hypothesis. In the present study, we proposed a gradient protein method to obtain the AA profile of total endogenous AAs in poultry fed protein-containing diets. The gradient protein method depends on the theory that the true AA digestibility is independent of the dietary CP level when the ingredient composition of the diet is constant [2, 14, 15].
The objectives of this study were: 1) to investigate the effect of dietary CP levels on the NLcm/NLp ratio using crude mucin in excreta as a reference for the whole endogenous protein in the excreta and 2) to obtain the AA profiles of total endogenous AAs using the gradient protein method and 3) to obtain estimates of the true digestibility of AAs in cecectomized roosters.
Materials and methods
Ethics statement
The experimental proposal and surgical procedures were approved by the Northwest A&F University Animal Care and Use Committee.
Animals and diets
The animal feeding experiment was performed at the Experimental Center of Animal Science at Northwest A&F University. A total of 48 cecectomized Lohmann Brown roosters (35 wks old), with an average body weight of 2.39 ± 0.23 kg, were obtained from the Da Cheng Poultry Industry (Xianyang, Shaanxi Province, China). Cecectomy and postoperative care were performed as described by Payne et al. [16]. All birds were individually raised in a single cage under a 16 h light and 8 h dark photoperiod. Drinking water was offered ad libitum. The birds were randomly divided among 8 dietary treatments. The experimental diets included a nitrogen-free diet and seven protein-containing diets (Table 1). Soybean meal was the only source of dietary CP. The AA composition of the diets is shown in S1 Table. All other nutrients and energy met or exceeded the estimated requirements for roosters [17]. A fiber source was used to equalize the crude fiber levels in all experimental diets. The electrolyte balance was constant across all the diets recommended by Adedokun et al. [1].
Table 1. Ingredient composition of the experimental diets (g/kg as-fed basis).
| Item | Dietary CP level (%) | Pooled SEM | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | |||
| Ingredient | ||||||||||
| Corn starch | 650.4 | 587.7 | 523.9 | 462.2 | 401.4 | 335.1 | 275.5 | 215.4 | - | - |
| Soybean meal | 0.0 | 69.0 | 138.0 | 207.0 | 273.0 | 345.0 | 414.0 | 483.0 | - | - |
| Sucrose | 210.0 | 215.0 | 220.0 | 225.0 | 230.0 | 235.0 | 238.0 | 240.0 | - | - |
| Cellulose | 60.0 | 51.2 | 43.7 | 33.9 | 26.3 | 18.3 | 8.5 | 0.0 | - | - |
| Soybean oil | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | - | - |
| Premix1 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | - | - |
| CaHPO4 | 19.0 | 18.5 | 17.9 | 17.4 | 16.8 | 16.3 | 15.7 | 15.3 | - | - |
| CaCO3 | 10.0 | 9.8 | 9.6 | 9.4 | 9.2 | 9.0 | 8.8 | 8.6 | - | - |
| K2CO32 | 11.7 | 10.1 | 8.4 | 6.7 | 5.1 | 3.3 | 1.6 | 0.0 | - | - |
| KCl2 | 2.2 | 2.1 | 2.0 | 1.9 | 1.9 | 1.8 | 1.7 | 1.6 | - | - |
| NaHCO32 | 3.7 | 3.6 | 3.5 | 3.4 | 3.4 | 3.3 | 3.2 | 3.1 | - | - |
| Choline chloride | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | - | - |
| Chemical composition3 | ||||||||||
| ME (kJ/kg) | 2969 | 2965 | 2958 | 2957 | 2952 | 2945 | 2943 | 2936 | 2.3 | 0.05 |
| CP | 2.2h | 32.4g | 62.7f | 92.9e | 121.9d | 153.4c | 183.7b | 213.9a | 11.1 | <0.001 |
| CF | 29.4 | 29.1 | 29.5 | 28.8 | 29 | 29.3 | 28.6 | 28.5 | 0.27 | 0.971 |
| NDF | 55.2 | 54.5 | 55.5 | 54.4 | 55.1 | 55.9 | 54.9 | 55.1 | 0.33 | 0.963 |
| Ca | 9.5 | 9.6 | 9.4 | 9.4 | 9.4 | 9.5 | 9.4 | 9.4 | 0.09 | 0.999 |
| Available P | 4.3 | 4.2 | 4.4 | 4.6 | 4.4 | 4.3 | 4.4 | 4.5 | 0.09 | 0.967 |
CP = crude protein; CF = crude fiber; ME = metabolizable energy; NDF = neutral-detergent fiber.
1Supplied (per kg of diet): vitamin A 5000 IU, vitamin D3 1500 IU, vitamin E 30 IU, vitamin K 3 mg, vitamin B1 4 mg, vitamin B2 6 mg, calcium pantothenate 15 mg, niacin 35 mg, vitamin B6 4 mg, folic acid 5 mg, cyanocobalamin 30 μg, biotin 0.3 mg, iron 60 mg, copper 10 mg, manganese 80 mg, zinc oxide 80 mg, selenium 0.3 mg, potassium iodate 0.9 mg, antioxidant 100 mg.
2Na+ + K+ − Cl− − milliequivalent value of 203.
3Values are means with pooled SEM (n = 5). The degrees of freedom between groups are 7 and those within groups are 32. The F-ratios for ME, CP, CF, NDF, Ca and available P are 3.704, 3611.58, 0.243, 0.264, 0.068 and 0.253, respectively.
a-hMeans with the same superscript letters in the row are not significantly different (P > 0.05), and those with different letters are significantly different (P < 0.05).
15N-Leucine injection and sampling
The general outline of the 15N-leucine injection and sampling protocol is summarized in S2 Table. The experiment included three periods. In the first period, the birds were adapted to the experimental diets for 5 consecutive days. On day 6, the roosters were deprived of the diet for 24 h to empty the digestive tract of all dietary residues. Then, the birds were precision-fed 25.0 g of dry matter/kg of body weight of each experimental diet using the method presented by Kim et al. [18]. Following the precision feeding, excreta were collected quantitatively for 48 h. A bag (250 mL) was attached to a hollowed-out plastic bottle cap fitted to the rooster’s cloaca. Then, 10 mL of a 10% formic acid solution was added to the bags to inhibit enzyme and microbial activity in the excreta. The excreta in the bag was collected once every two hours, and frozen (−40°C) immediately. The 48-h excreta samples were pooled for each bird when the experiment was over. Blood samples (5 mL) were sampled 23 h after precision feeding [3]. The above blood and excreta samples were used to determine the basal 15N-enrichment of leucine in the birds. In the second period, all birds were given one week to recover from the first period. In the third period, all roosters were subcutaneously injected with 15N-L-leucine (98% 15N-enrichment; Cambridge Isotope Labs. Inc., Andover, MA, USA) at a dose of 20 mg/kg of body weight after precision feeding. The other procedures were the same as those described for the first period.
Sample preparation
The sample preparation protocol was adapted from the study by Steendam et al. [19]. Briefly, blood samples were centrifuged (10 min, 2000×g, 4°C) to recover plasma and then treated twice with a 10% (W/V) trichloroacetic acid solution to obtain deproteinized plasma. The AAs in the deproteinized plasma were purified though passage on AG 50W-X8 cation-exchange resin (hydrogen form, 200 μm, Sigma, St. Louis, MO, USA). The AAs were eluted with 4 mol/L fresh NH4OH. After NH4OH evaporation, the samples were re-dissolved with 5 mL of ultrapure water for the 15N analysis. The excreta samples were thawed, homogenized, freeze-dried, weighed and then carefully ground to pass through a 1 mm screen for further analysis. Crude mucin in the excreta was isolated with the ethanol precipitation method described by Lien et al. [5] and Leterme et al. [20]. Hydrolyzed excreta or crude mucin samples that were obtained using hydrochloric acid method (10 mL of 6 mol/L HCl, 110°C, 24 h) were purified similarly to the plasma samples for 15N analysis.
Chemical and isotope analyses
Dry matter, CP (N × 6.25) and neutral detergent fiber were analyzed in the ingredients and diets [21]. The AA concentrations of the ingredients, diets and excreta samples were analyzed using the Biochrom 30 amino acid analyzer (Pharmacia Biotech, Cambridge, UK). The 15N-enrichment of leucine was determined with a gas chromatograph coupled with a combustion oven and an isotope ratio mass spectrometer (GC-C-IRMS, Thermo-Finngan, USA) system according to a previously presented study [4]. The AAs were derivatized with thionyl chloride and pivaloyl chloride [22], which led to the formation of N-pivaloyl-isopropyl derivatives. The samples (1.0 μL, split mode = 10:1) were injected onto an HP ULTRA 2 column (50 m × 0.32 mm × 0.52 μm) at an injector temperature of 250°C. The column oven was initially set at 70°C (for 1 min), rise at a rate of 3°C/min to 220°C, and then 10°C/min to 300°C with a holding time of 8 min. The temperatures of the CuO/Pt combustion reactor and reduction oven were 850°C and 650°C, respectively.
Principles of calculations
15N-enrichment in leucine
The 15N-enrichment (atom percent excess, APE) in leucine was obtained according to formulas (1) and (2), which were adapted from Wolfe et al. [23]:
| (1) |
| (2) |
where the 15N/14N ratios originate from the ratios of the m/z 29 to m/z 28 ion current signals in a labeled sample (sam) or in basal sample (bas) [22]. The value of A is the natural abundance of 15N.
The 15N-leucine single-injection method
The calculation principle of this method, which has been shown previously [3], is expressed as the formula (3):
| (3) |
where i denotes the number of experimental protein-containing diets, and NLe0/NLp0 and NLei/NLpi are the 15N-enrichment ratios of endogenous leucine in the excreta and free leucine in the deproteinized plasma from the birds fed either nitrogen-free diet or the ith protein-containing diet, respectively.
The 15N-leucine content in the excreta was derived from endogenous 15N-leucine; therefore, we obtained formula (4). By combining formulas (3) and (4), we generated the formula (5):
| (4) |
| (5) |
where NLex represents 15N-enrichment of total leucine in the excreta; We and Wt (mg/kg of dry matter intake (DMI)) are the losses of endogenous leucine and total leucine in the excreta, respectively.
The AA profile of total endogenous AAs was assumed to be relatively stable [4, 13]. Therefore, the AA profile obtained from the nitrogen-free diet could be used to calculate the endogenous losses of other AAs in birds fed protein-containing diets.
The gradient protein method for estimating the total endogenous AAs profile
Apparent digestibility (AD) and true digestibility (TD) of AAs in diets were calculated according to formulas (6) and (7):
| (6) |
| (7) |
where TI, TF and E denote the total AA input (mg/kg of DMI), total fecal AA output (mg/kg of DMI) and EAALs (mg/kg of DMI) from the experimental diets, respectively.
When the source of dietary protein and the contents of the other dietary components, including fiber and anti-nutritive factors, are similar among the experimental diets, then the TD will be the same at varying dietary CP levels [2, 15]. Therefore,
| (8) |
Based on several previous studies [24–26], we assumed that the dietary CP levels, which varied within a small range (3%), had little effect on the EAALs.
| (9) |
According to formulas (6), (7), (8) and (9), we obtained formula (10):
| (10) |
where (mg/kg of DMI) represents the mean endogenous losses of AAs between the ith and (i+1)th protein-containing diets.
The profile of the total endogenous AAs, expressed as a proportion of total AAs, was calculated as follows:
| (11) |
where and (mg/kg of DMI) represented mean proportion of each individual AA and the total endogenous losses of AAs between the ith and (i+1)th protein-containing diets, respectively.
Statistical analysis
Statistical analysis was performed using SPSS 21.0 (IBM-SPSS Inc., Chicago, Il, USA). The data are presented as the means and pooled standard error (SEM) (n = 6). Differences between the means of all groups were compared by one-way ANOVA, followed by Bonferroni corrections test. Significant differences were declared at P<0.05.
Results
The values of 15N-enrichment of leucine in the deproteinized plasma, crude mucin and excreta are presented in Table 2. The values of 15N-enrichment were significantly different in the different samples for all treatments. The highest 15N-enrichment was observed in the deproteinized plasma, followed by the crude mucin and then the excreta. All the values of 15N-enrichment significantly decreased (P < 0.05) when the dietary CP level was increased to 15%. The ratios of 15N-enrichment in the different samples are also shown in Table 2. The value for NLcm/NLp ratios ranged from 0.664 to 0.763, whereas the NLex/NLp ratios ranged from 0.262 to 0.744. The dietary CP levels had no influence (P > 0.05) on the NLcm/NLp ratios.
Table 2. 15N-enrichment of leucine in the deproteinized plasma, crude mucin and excreta sampled from precision-fed cecectomized roosters after a single-injection of 15N-leucine1.
| Item | Dietary CP level (%) | Pooled SEM |
P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | |||
| 15N-enrichment of leucine (atom % excess, APE) | ||||||||||
| Plasma2 (NLp) |
0.256a | 0.254a | 0.233ab | 0.209ab | 0.182ab | 0.165b | 0.147b | 0.135b | 0.008 | <0.001 |
| Crude mucin (NLcm) | 0.183a | 0.176a | 0.158ab | 0.147ab | 0.131ab | 0.122b | 0.110b | 0.091b | 0.006 | <0.001 |
| Excreta3 (NLex) |
0.188a | 0.169ab | 0.144ab | 0.116b | 0.092bc | 0.076bc | 0.050c | 0.034c | 0.008 | <0.001 |
| Ratio of 15N-enrichment of leucine in different samples | ||||||||||
| NLcm/NLp | 0.720 | 0.698 | 0.682 | 0.702 | 0.728 | 0.705 | 0.763 | 0.664 | 0.014 | 0.778 |
| NLex/NLp | 0.744a | 0.670ab | 0.613ab | 0.559b | 0.496b | 0.427bc | 0.338c | 0.262c | 0.025 | <0.001 |
CP = crude protein; NLp = 15N-enrichment of leucine in the deproteinized plasma; NLcm = 15N-enrichment of leucine in crude mucin; NLex = 15N-enrichment of total leucine in excreta; NLcm/NLp = the ratio of NLcm to NLp; NLex/NLp = the ratio of NLex to NLp.
1Values are means with pooled SEM (n = 6). The degrees of freedom between groups are 7 and those within groups are 40. The F-ratios for NLp, NLcm, NLex, NLcm/NLp and NLex/NLp are 9.011, 8.490, 30.043, 0.567 and 30.570, respectively.
2Plasma samples were sampled 23.0 h after subcutaneous single-injection of 15N-leucine in precision-fed cecectomized roosters; the NLp at this time point represents the weighted mean of the definite integral of 15N-enrichment in leucine during the course of the 48.0-h experiment [3].
3Excreta samples were continuously sampled during the course of the 48.0-h experiment after the precision-feeding and pooled at the end of the experiment. NLex represents the weighted mean of 15N-enrichment in pooled excreta samples (Xu et al. 2011).
a, b, cMeans with the same superscript letters in the row are not significantly different (P > 0.05), and those with different letters are significantly different (P < 0.05).
The contribution of endogenous leucine to total leucine and the endogenous losses of leucine in the excreta are presented in Table 3. No significant differences (P > 0.05) were found between contributions calculated with NLe0/NLp0 and NLcm/NLp. Endogenous leucine losses calculated with NLe0/NLp0 increased significantly (P < 0.05) when the dietary CP level increased to 12%.
Table 3. Contribution of endogenous leucine to total leucine and endogenous losses of leucine in excreta sampled from precision-fed cecectomized roosters1.
| Item | Dietary CP level (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 | 6 | 9 | 12 | 15 | 18 | 21 | |||
| Contribution (%) of endogenous leucine to total leucine | |||||||||
| Contributione0/p0 | 90.4 | 83.5 | 75.4 | 68.3 | 58.3 | 46.6 | 35.8 | ||
| Contributioncm/p | 95.2 | 90.9 | 81.2 | 69.1 | 61.5 | 45.5 | 39.3 | ||
| Pooled SEM | 2.72 | 3.72 | 3.33 | 3.18 | 2.38 | 3.10 | 2.92 | ||
| P-value | 0.269 | 0.117 | 0.089 | 0.833 | 0.506 | 0.805 | 0.553 | ||
| Total excretion or endogenous losses of leucine (mg/kg of DMI) | Pooled SEM |
P-value | |||||||
| Total leucine excretion | 590.6d | 784.1cd | 1136.3cd | 1492.8c | 1688.7b | 2295.3bc | 3073.6a | 135.3 | <0.001 |
| Endogenous leucine losses2 | 533.4b | 654.5b | 856.9a | 1019.6a | 984.5ab | 1069.6a | 1100.4a | 36.8 | <0.001 |
CP = crude protein; DMI = dry matter intake; Contributione0/p0 = contribution (%) of endogenous leucine to total leucine calculated with NLe0/NLp0 (NLe0 = 15N-enrichments of leucine in excreta for nitrogen-free diet, NLp0 = 15N-enrichments of leucine in deproteinized plasma for nitrogen-free diet); Contributioncm/p = contribution (%) of endogenous leucine to total leucine calculated with NLcm/NLp (NLcm = 15N-enrichments of leucine in crude mucin for protein-containing diets, NLp = 15N-enrichments of leucine in deproteinized plasma for protein-containing diets).
1Values are means with pooled SEM (n = 6). The degrees of freedom between groups of Contributione0/p0 or Contributioncm/p are 1 and those within groups are 10. The degrees of freedom between groups of total excretion or endogenous losses of leucine are 6 and those within groups are 35. The F-ratios for 3%, 6%, 9%, 12%, 15%, 18% and 21% CP are 1.370, 2.934, 3.557, 0.047, 0.476, 0.064 and 0.376, respectively. The F-ratios for total excretion and endogenous losses of leucine are 41.476 and 9.780, respectively.
2Determined with the 15N-leucine single-injection method when Contributione0/p0 was used.
a, b, c, dMeans with the same superscript letters in the row are not significantly different (P>0.05), and those with different letters are significantly different (P<0.05).
The AA profile of the total endogenous AAs (expressed as a proportion of total endogenous AAs) determined with the gradient protein method is shown in Table 4. The ratios of most of the indispensable AAs (except isoleucine) and glutamic acid were not influenced (P > 0.05) by the dietary CP levels. The proportions of isoleucine, aspartic acid and serine in the total endogenous AAs were greater (P < 0.05) in diets with increasing CP levels from 12% to 21% than those in the nitrogen-free diet, whereas the proportions of alanine, cysteine and proline were lower (P < 0.05). No significant differences (P > 0.05) were observed in the ratios of most AAs in the total endogenous AAs when the dietary CP levels were increased from 3% to 12% or from 12% to 21%. The order of the relative proportions of these predominant AAs was similar across all dietary treatments.
Table 4. Amino acid profile (%) of total endogenous amino acids calculated with the gradient protein method in precision-fed cecectomized roosters fed the nitrogen-free diet and soybean meal diets at varying crude protein ranges1.
| Item | Dietary CP level (%) | Pooled SEM |
P-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 3~6 | 6~9 | 9~12 | 12~15 | 15~18 | 18~21 | |||
| Indispensable amino acids | |||||||||
| Arginine | 5.40 | 4.87 | 4.76 | 5.04 | 4.53 | 3.96 | 4.31 | 0.15 | 0.175 |
| Histidine | 4.89 | 4.26 | 3.91 | 3.54 | 3.59 | 4.04 | 4.05 | 0.18 | 0.503 |
| Isoleucine | 3.07b | 4.13ab | 4.16ab | 4.19ab | 4.63ab | 4.67ab | 4.81a | 0.15 | 0.035 |
| Leucine | 4.95 | 5.35 | 5.58 | 5.16 | 5.22 | 4.71 | 4.93 | 0.14 | 0.727 |
| Lysine | 6.08 | 5.85 | 5.54 | 5.82 | 5.84 | 5.00 | 4.76 | 0.19 | 0.438 |
| Methionine | 1.01 | 1.50 | 1.59 | 1.78 | 1.53 | 1.47 | 1.57 | 0.07 | 0.178 |
| Phenylalanine | 4.30 | 3.90 | 4.97 | 5.01 | 4.89 | 3.93 | 3.94 | 0.16 | 0.180 |
| Threonine | 8.46 | 8.30 | 8.22 | 8.27 | 8.18 | 9.08 | 9.24 | 0.20 | 0.711 |
| Valine | 6.81 | 7.18 | 7.48 | 7.08 | 6.74 | 7.03 | 7.36 | 0.21 | 0.969 |
| Dispensable amino acids | |||||||||
| Alanine | 6.24ab | 6.36a | 5.58a | 4.58b | 4.62a | 5.23a | 4.25b | 0.17 | <0.001 |
| Aspartic acid | 10.50b | 10.77b | 10.94b | 12.59ab | 13.83a | 14.38a | 14.62a | 0.32 | <0.001 |
| Cysteine | 5.97a | 5.64a | 5.24a | 4.71ab | 4.02b | 3.97b | 4.06b | 0.21 | 0.021 |
| Glutamic acid | 14.42 | 14.39 | 14.68 | 15.17 | 15.12 | 15.49 | 15.54 | 0.33 | 0.948 |
| Proline | 11.79a | 10.19ab | 9.31ab | 8.74ab | 8.50b | 7.05b | 6.43b | 0.36 | <0.001 |
| Serine | 7.12b | 7.32b | 8.04ab | 8.34ab | 9.31ab | 10.51a | 10.13a | 0.32 | 0.009 |
CP = crude protein.
1Values are means with pooled SEM (n = 6). The degrees of freedom between groups are 6 and those within groups are 35. The F-ratios are 1.596, 2.896, 1.004, 0.623, 1.605, 2.595, 0.602, 0.216, 0.905, 1.588, 3.410, 6.652, 5.585, 10.664 and 0.268 according to the order of amino acids in the table, respectively.
a, bMeans with the same superscript letters in the row are not significantly different (P > 0.05), and those with different letters are significantly different (P < 0.05).
The endogenous losses of other AAs calculated with the AA profile of total endogenous AAs in the nitrogen-free diet are presented in Table 5. The dietary CP levels had a significant effect (P < 0.05) on the endogenous loss of most AAs (except histidine). The endogenous loss of total AAs in roosters fed the 21% CP diet was approximately 2.2 times higher than the endogenous losses determined with birds fed the nitrogen-free diet. Similar endogenous losses (P > 0.05) of all individual AAs were observed when increasing the dietary CP levels from 0 to 6%. The endogenous losses of most individual AAs and the total AAs increased slightly (P > 0.05) when the dietary CP levels were increased from 0 to 6% or from 12% to 21%. The endogenous losses of most individual AAs and the total AAs increased dramatically when the dietary CP levels were increased from 6% to 12% (especially from 6% to 9%).
Table 5. Endogenous amino acid losses (mg/kg of DMI) determined with the 15N-leucine single-injection method in precision-fed cecectomized roosters fed the nitrogen-free diet and soybean meal diets at varying crude protein levels1.
| Item | Dietary CP level (%) | Pooled SEM |
P-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | |||
| Indispensable amino acids | ||||||||||
| Arginine | 550.8b | 588.1b | 715.7b | 941.3b | 1109.6ab | 1080.5ab | 1176.1ab | 1207.9ab | 42.4 | <0.001 |
| Histidine | 479.9 | 506.7 | 634.0 | 834.9 | 998.9 | 1008.0 | 1081.4 | 1088.0 | 68.0 | 0.093 |
| Isoleucine | 309.5c | 332.9c | 405.6bc | 530.4b | 632.0ab | 607.1ab | 660.3ab | 681.8ab | 22.6 | <0.001 |
| Leucine | 498.4c | 533.4c | 654.5bc | 856.9b | 1019.6ab | 984.4ab | 1069.6ab | 1100.4a | 36.8 | <0.001 |
| Lysine | 609.0c | 654.5bc | 803.6bc | 1051.9b | 1253.8ab | 1207.9ab | 1308.9ab | 1346.4a | 47.2 | <0.001 |
| Methionine | 101.3b | 109.7b | 133.3b | 175.0ab | 208.8ab | 199.1ab | 219.5ab | 222.3a | 8.6 | <0.001 |
| Phenylalanine | 435.1c | 465.2c | 570.0bc | 746.4b | 885.0ab | 853.2ab | 926.2ab | 956.9ab | 32.3 | <0.001 |
| Threonine | 840.8c | 896.0c | 1116.8bc | 1453.6b | 1742.0ab | 1672.2ab | 1801.3ab | 1871.1a | 65.3 | <0.001 |
| Valine | 687.6c | 741.2bc | 900.9bc | 1177.4b | 1403.8ab | 1348.1ab | 1462.9ab | 1514.8a | 50.9 | <0.001 |
| Dispensable amino acids | ||||||||||
| Alanine | 634.2c | 680.1bc | 827.4bc | 1084.3b | 1283.7ab | 1242.5ab | 1344.8ab | 1393.9a | 47.4 | <0.001 |
| Aspartic acid | 1058.0c | 1134.1c | 1386.1bc | 1813.3b | 2160.1ab | 2090.5ab | 2264.3ab | 2338.8a | 76.8 | <0.001 |
| Cystine | 604.3c | 651.4bc | 788.4bc | 1031.4b | 1228.0ab | 1186.4ab | 1285.1ab | 1331.7a | 45.3 | <0.001 |
| Glutamic acid | 1458.0c | 1558.4c | 1905.3bc | 2496.2b | 2962.9ab | 2883.0ab | 3117.0ab | 3220.8a | 107.4 | <0.001 |
| Proline | 1194.9c | 1274.6bc | 1558.2bc | 2039.0b | 2415.6ab | 2356.1ab | 2532.2ab | 2639.2ab | 91.6 | <0.001 |
| Serine | 708.4c | 756.7c | 938.7bc | 1223.7b | 1466.6ab | 1410.8ab | 1524.8ab | 1575.5ab | 54.1 | <0.001 |
| Total | 10210.9c | 10956.6bc | 13181.2bc | 17341.2b | 20512.6ab | 20111.6ab | 21900.6ab | 22456.2a | 757.8 | <0.001 |
CP = crude protein; DMI = dry matter intake.
1Values are means with pooled SEM (n = 6). The degrees of freedom between groups are 7 and those within groups are 40. The F-ratios are 7.176, 14.121, 12.692, 15.375, 11.777, 17.333, 17.560, 15.413, 1.910, 15.857, 16.749, 13.967, 14.433, 20.233, 18.504 and 13.196 according to the order of amino acids in the table, respectively.
a, b, cMeans with the same superscript letters in the row are not significantly different (P > 0.05), and those with different letters are significantly different (P < 0.05).
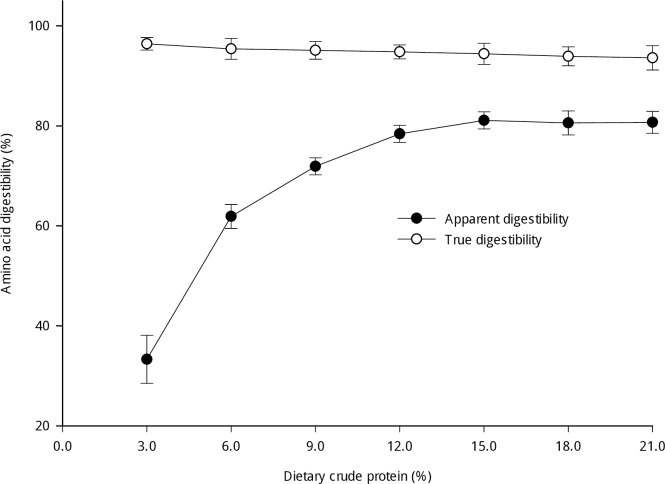
The true AA digestibility calculated with the EAALs determined by the 15N-leucine single-injection method (total losses) was relatively constant except for a few AAs that had lower digestibility at dietary CP levels of 18% or 21% (Table 6). The apparent digestibility of the total AAs increased nonlinearly with the dietary CP levels, whereas the true digestibility of total AAs was independent of the dietary CP levels (Fig 1).
Table 6. True amino acid digestibility calculated from the endogenous losses estimated by the 15N-leucine single-injection method in cecectomized roosters fed soybean meal diets at varying crude protein levels1.
| Item | Dietary CP level (%) | Pooled SEM |
P-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 | 6 | 6 | 12 | 15 | 18 | 21 | |||
| Indispensable amino acids | |||||||||
| Arginine | 97.4 | 97.2 | 97.1 | 97.1 | 96.9 | 95.3 | 95.6 | 1.78 | 0.087 |
| Histidine | 96.7a | 95.5ab | 95.6ab | 94.8ab | 95.4ab | 93.1b | 92.6b | 1.63 | 0.005 |
| Isoleucine | 97.2 | 96.9 | 96.6 | 96.1 | 95.7 | 93.9 | 94.0 | 1.25 | 0.091 |
| Leucine | 97.2 | 97.0 | 96.7 | 96.4 | 96.1 | 95.5 | 95.1 | 0.97 | 0.081 |
| Lysine | 96.8 | 96.3 | 95.7 | 95.5 | 95.2 | 93.7 | 94.5 | 1.96 | 0.061 |
| Methionine | 98.1 | 97.0 | 96.3 | 96.1 | 96.8 | 94.5 | 94.3 | 2.02 | 0.057 |
| Phenylalanine | 97.1a | 96.3ab | 96.4ab | 95.8ab | 95.7ab | 93.2b | 93.6b | 1.12 | 0.002 |
| Threonine | 94.9a | 93.7ab | 93.1ab | 92.4ab | 92.3ab | 90.2ab | 90.0b | 1.62 | 0.014 |
| Valine | 96.9 | 94.8 | 94.5 | 94.2 | 94.0 | 92.8 | 92.5 | 2.42 | 0.121 |
| Dispensable amino acids | |||||||||
| Alanine | 96.2 | 95.2 | 94.6 | 94.3 | 93.7 | 92.4 | 92.8 | 2.25 | 0.104 |
| Aspartic acid | 97.8a | 96.2ab | 95.6ab | 95.2ab | 94.8ab | 92.7b | 92.9b | 1.07 | <0.001 |
| Cystine | 92.5 | 89.7 | 86.6 | 88.7 | 86.6 | 86.9 | 87.5 | 2.40 | 0.074 |
| Glutamic acid | 97.2a | 96.8ab | 96.6ab | 96.4ab | 96.1b | 94.3b | 94.7b | 1.91 | 0.003 |
| Proline | 95.9 | 93.0 | 92.4 | 92.3 | 92.1 | 90.3 | 91.4 | 1.86 | 0.152 |
| Serine | 96.2a | 95.5ab | 95.1ab | 94.5ab | 94.2ab | 92.1b | 92.1b | 1.19 | 0.001 |
| Total | 96.4 | 95.4 | 95.1 | 94.8 | 94.4 | 93.9 | 93.6 | 2.19 | 0.085 |
CP = crude protein.
1Values are means with pooled SEM (n = 6). The degrees of freedom between groups are 6 and within groups are 35. The F-ratios are 3.581, 2.132, 4.958, 3.158, 3.594, 5.351, 5.373, 3.301, 3.816, 4.571, 4.636, 1.694, 4.039, 5.713,4.188 and 3.787 according to the order of amino acids in the table, respectively.
a, bMeans with same superscript letters in the row are not significantly different (P>0.05), and those with different letters are significantly different (P<0.05).
Fig 1. Influence of the dietary crude protein level on the apparent and true digestibility of total AAs in cecectomized roosters fed soybean meal diets.
The true digestibility was calculated from the endogenous losses determined by the 15N-leucine single-injection method.
Discussion
One of the aims of the present study was to evaluate the 15N-leucine single-injection method within a wide range of dietary CP levels. The results showed that the NLcm/NLp ratios were not influenced by dietary CP levels. Meanwhile, the contributions of endogenous leucine to total leucine in the excreta calculated as NLe0/NLp0 and NLcm/NLp were similar at the same dietary CP level. Despite some changes in individual AA proportions (mainly dispensable AAs) when the dietary CP levels were increased from 0 to 21%, the AA profile of total endogenous AAs was relatively constant in the present study. These findings support our assumptions.
The tracer can be given as either a single bolus or continuously when using the isotope labeling technique to trace endogenous AAs [23]. Continuous 15N-leucine infusion is the most commonly used technique to determine EAALs in pigs and rats [4–6]. This method assumes that the 15N-enrichment of endogenous leucine in excreta is approximately equal to that in plasma (i.e., NLe = NLp) after continuous 15N-infusion for 7 to 8 d. Instead of using continuous 15N-leucine infusion to obtain a steady state, we proposed the NLcm/NLp ratio remained constant when testing another way of using a single bolus. By regression analysis of the NLp value and time in our previous study, we found the optimum time of blood sampling in our method was 23 h after the isotope injection [3]. A single blood sample taken at this time could be used to reflect the weighted mean value of definite integral NLp during the sampling period. Additionally, excreta samples from one bird were pooled to obtained the NLcm value, which was more representative than the 15N-enrichment at a single time point. Therefore, the 15N-leucine injection and sample preparation in our method are more economical and simpler than the 15N-isotope infusion method.
After a single injection, 15N-leucine becomes a part of the pool of the total blood leucine and has the same metabolic rate as unlabeled leucine [3]. Thus, the ratio of labeled to unlabeled endogenous leucine in the excreta has the same tendency to vary as the ratio of labeled to unlabeled leucine in the different sources of endogenous AAs (e.g., plasma, mucosa, or desquamated cells). In other words, the NLcm/NLp ratio indicates the NLe/NLp ratio, which was also confirmed by our present data. Moreover, we proposed a gradient protein method to obtain the AA profiles of total endogenous AAs in birds fed protein-containing diets. The gradient protein method depends on the theory that true AA digestibility is independent of the dietary CP level [2, 14]. Based on several previous studies [24–26], we assumed that variation in the dietary CP levels within a small range had little effect on the EAALs. A study in pigs fed diets with equal graded protein levels of 4% found no significant differences in the EAALs among the adjacent CP levels. In the present study, the EAALs were assumed to be relatively constant or to vary little when the dietary CP levels varied within 3%.
Many studies have generally assumed that the AA profiles of endogenous protein are constant [4, 6, 27]. No significant differences were observed in the ratios of most AAs in the endogenous protein in broiler chickens when increasing the dietary enzyme-hydrolyzed casein concentration from 5% to 20% [24]. This result was almost in agreement with the present data in our study. Although increasing the dietary CP levels stimulates the secretion of digestive enzymes, sloughed cells and mucin [24], the degree of this stimulation source is similar for each secretion. Therefore, the proportion of each excretion source in the total endogenous excretion will not change. Meanwhile, although the relative contribution of each source may vary, the AA composition of a single source tends to be constant [28]. Thus, we infer that the AA profiles of total endogenous AAs are not influenced by dietary CP levels. Moreover, glutamic acid, aspartic acid, threonine, proline and serine dominated the AA profiles of total endogenous AAs, which was consistent with results from chickens [29, 30] and pigs [5, 27]. Additionally, a summary by Boisen et al. [31] indicated that the AA profiles of endogenous protein, which were obtained from different diets and different methods of determination, remained relatively stable. Therefore, the AA profiles of total endogenous AAs in birds fed a nitrogen-free diet were generally acceptable for use in the calculations. Notably, endogenous losses of alanine, cysteine and proline may be underestimated at high dietary CP levels due to the lower proportions of these AAs when the AA profiles of total endogenous AA in the nitrogen-free diet treatment are used.
Another purpose of this study was to estimate total EAALs using the 15N-leucine single-injection method and to obtain the true digestibility of AAs in cecectomized roosters fed soybean meal diets with varying CP levels. The results showed that endogenous losses of total AAs increased in response to increases in dietary CP levels, and reached relative equilibrium above 12% dietary CP. Similar changes were found in previous studies [24, 32]. The dietary protein or AA content is one of the main factors affecting EAALs [1]. An increasing dietary CP content likely results in higher specific endogenous losses of AAs, which may be responsible for this result. Moreover, although the apparent AA digestibility nonlinearly increased with the dietary CP level, the true AA digestibility was independent of the respective dietary CP. This finding is in accordance with a well-known conclusion that has been widely demonstrated [2, 15]. This finding also indirectly proves that the 15N-leucine single-injection method can be an effective means for determining total EAALs in poultry fed varying levels of protein-containing ingredients.
Conclusions
The present data support the assumptions of the 15N-leucine single-injection method within a wide range of dietary CP levels. The results show that this method allows for the determination the total EAALs and true AAs digestibility in poultry fed protein-containing diets. Notably, endogenous losses of alanine, cysteine and proline may be underestimated at high dietary CP levels. Further studies are also necessary to investigate the accuracy and repeatability of this method, especially using other ingredients as the source of dietary protein.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
We thank Dr. Yangcun Cao of Northwest A&F University, Yangling, China, for comments on the manuscript.
Abbreviations
- EAALs
endogenous amino acid losses
- AA
amino acid
- CP
crude protein
- NLe
15N-enrichment of endogenous leucine in excreta
- NLp
15N-enrichment of endogenous leucine in deproteinized plasma
- NLcm
15N-enrichment of leucine in crude mucin
- APE
atom percent excess
- DMI
dry matter intake
- AD
apparent digestibility of amino acid
- TD
true digestibility of amino acid
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Adedokun SA, Adeola O, Parsons CM, Lilburn MS, Applegate TJ. Factors affecting endogenous amino acid flow in chickens and the need for consistency in methodology. Poult Sci. 2011;90(8):1737–48. doi: 10.3382/ps.2010-01245 . [DOI] [PubMed] [Google Scholar]
- 2.Stein HH, Seve B, Fuller MF, Moughan PJ, de Lange CFM. Invited review: Amino acid bioavailability and digestibility in pig feed ingredients: Terminology and application. J Anim Sci. 2007;85(1):172–80. doi: 10.2527/jas.2005-742 . [DOI] [PubMed] [Google Scholar]
- 3.Xu M, Yao JH, Wang KN, Meng DL, Luo DY, Wu XB, et al. 3H-leucine single-injection method for determining endogenous amino acid losses of broilers. Nutrition. 2011;27(7–8):829–32. doi: 10.1016/j.nut.2010.08.014 . [DOI] [PubMed] [Google Scholar]
- 4.Hess V, Thibault JN, Seve B. The 15N-amino acid dilution method allows the determination of the real digestibility and of the ileal endogenous losses of the respective amino acid in pigs. J Nutr. 1998;128(11):1969–77. . [DOI] [PubMed] [Google Scholar]
- 5.Lien KA, Sauer WC, Dugan MER. Evaluation of the 15N-isotope dilution technique for determining the recovery of endogenous protein in ileal digesta of pigs: Effect of the pattern of blood sampling, precursor pools, and isotope dilution technique. J Anim Sci. 1997;75(1):159–69. doi: 10.2527/1997.751159x . [DOI] [PubMed] [Google Scholar]
- 6.Moughan PJ, Buttery PJ, Essex CP, Soar JB. Evaluation of the isotope dilution technique for determining ileal endogenous nitrogen excretion in the rat. J Sci Food Agr. 1992;58(2):165–72. doi: 10.1002/jsfa.2740580202 [Google Scholar]
- 7.Souffrant W, Darcy-Vrillon B, Corring T, Laplace J, Köhler R, Gebhardt G, et al. Recycling of endogenous nitrogen in the pig (preliminary results of a collaborative study). Arch Anim Nutr. 1986;36(2–3):269–74. doi: 10.1080/17450398609425271 . [DOI] [PubMed] [Google Scholar]
- 8.Moughan PJ, Rutherfurd SM. Gut luminal endogenous protein: Implications for the determination of ileal amino acid digestibility in humans. Brit J Nutr. 2012;108:S258–S63. doi: 10.1017/S0007114512002474 . [DOI] [PubMed] [Google Scholar]
- 9.Selle PH, Cowieson AJ, Cowieson NP, Ravindran V. Protein-phytate interactions in pig and poultry nutrition: a reappraisal. Nutr Res Rev. 2012;25(1):1–17. doi: 10.1017/S0954422411000151 . [DOI] [PubMed] [Google Scholar]
- 10.Miner-Williams W, Deglaire A, Benamouzig R, Fuller MF, Tome D, Moughan PJ. Endogenous proteins in the ileal digesta of adult humans given casein-, enzyme-hydrolyzed casein- or crystalline amino-acid-based diets in an acute feeding study. Eur J Clin Nutr. 2014;68(3):363–9. doi: 10.1038/ejcn.2013.270 . [DOI] [PubMed] [Google Scholar]
- 11.Miner-Williams W, Deglaire A, Benamouzig R, Fuller MF, Tome D, Moughan PJ. Endogenous proteins in terminal ileal digesta of adult subjects fed a casein-based diet. Am J Clin Nutr. 2012;96(3):508–15. doi: 10.3945/ajcn.111.033472 . [DOI] [PubMed] [Google Scholar]
- 12.De Lange C, Souffrant W, Sauer W. Real ileal protein and amino acid digestibilities in feedstuffs for growing pigs as determined with the 15N-isotope dilution technique. J Anim Sci. 1990;68(2):409–18. doi: 10.2527/1990.682409x [DOI] [PubMed] [Google Scholar]
- 13.Schulze H, Butts CA, Moughan PJ, Verstegen MWA. The 15N-isotope dilution method for determining ileal endogenous nitrogen-excretion in the young (10 Kg liveweight) pig. J Sci Food Agr. 1995;69(1):41–50. doi: 10.1002/jsfa.2740690108 [Google Scholar]
- 14.Nyachoti CM, deLange CFM, McBride BW, Schulze H. Significance of endogenous gut nitrogen losses in the nutrition of growing pigs: A review. Can J Anim Sci. 1997;77(1):149–63. doi: 10.4141/A96-044 [Google Scholar]
- 15.Fan MZ, Sauer WC, McBurney MI. Estimation by regression analysis of endogenous amino acid levels in digesta collected from the distal ileum of pigs. J Anim Sci. 1995;73(8):2319–28. doi: 10.2527/1995.7382319x . [DOI] [PubMed] [Google Scholar]
- 16.Payne W, Kifer R, Snyder D, Combs G. Studies of protein digestion in the chicken 1. investigation of apparent amino acid digestibility of fish meal protein using cecectomized, adult male chickens. Poult Sci. 1971;50(1):143–50. doi: 10.3382/ps.0500143 [Google Scholar]
- 17.NRC. Nutrient Requirements of Poultry. Washington CD: National Academy Press; 1994. [Google Scholar]
- 18.Kim EJ, Utterback PL, Parsons CM. Development of a precision-fed ileal amino acid digestibility assay using 3-week-old broiler chicks. Poult Sci. 2011;90(2):396–401. doi: 10.3382/ps.2010-01088 . [DOI] [PubMed] [Google Scholar]
- 19.Steendam CA, Verstegen MWA, Tamminga S, Boer H, van 't End M, Verstappen B, et al. Route of tracer administration does not affect real endogenous nitrogen recovery measured with the 15N-isotope dilution technique in pigs fed rapidly digestible diets. J Nutr. 2004;134(11):3068–75. [DOI] [PubMed] [Google Scholar]
- 20.Leterme P, Seve B, Thewis A. The current 15N-Leucine infusion technique is not suitable for quantitative measurements of ileal endogenous amino acid flows in pigs. J Nutr. 1998;128(11):1961–8. . [DOI] [PubMed] [Google Scholar]
- 21.AOAC. Official Methods of Analysis. 18th ed. Gaithersburg, MD, USA: AOAC Int; 2007. [Google Scholar]
- 22.Metges CC, Petzke KJ, Hennig U. Gas chromatography/combustion/isotope Ratio mass Sspectrometric comparison of N-Acetyl- and N-pivaloyl amino acid esters to measure 15N isotopic abundances in physiological samples: a pilot study on amino acid synthesis in the upper gastro-intestinal tract of minipigs. J Mass Spectrom. 1996;31(4):367–76. doi: 10.1002/(SICI)1096-9888(199604)31:4<367::AID-JMS310>3.0.CO;2-V [DOI] [PubMed] [Google Scholar]
- 23.Wolfe RR, Chinkes DL. Isotope tracers in metabolic research: principles and practice of kinetic analysis. 2nd ed. New York: John Wiley & Sons; 2004. [Google Scholar]
- 24.Ravindran V, Morel PCH, Rutherfurd SM, Thomas DV. Endogenous flow of amino acids in the avian ileum as influenced by increasing dietary peptide concentrations. Brit J Nutr. 2009;101(6):822–8. doi: 10.1017/S0007114508039974 . [DOI] [PubMed] [Google Scholar]
- 25.Eklund M, Mosenthin R, Piepho HP, Rademacher M. Effect of dietary crude protein level on basal ileal endogenous losses and standardized ileal digestibilities of crude protein and amino acids in newly weaned pigs. J Anim Physiol an Nutri. 2008;92(5):578–90. doi: 10.1111/j.1439-0396.2007.00751.x . [DOI] [PubMed] [Google Scholar]
- 26.Hodgkinson SM, Moughan PJ. An effect of dietary protein content on endogenous ileal lysine flow in the growing rat. J Sci Food Agr. 2007;87(2):233–38. doi: 10.1002/jsfa.2701 [Google Scholar]
- 27.Pedersen C, Boisen S. Establishment of tabulated values for standardized ileal digestibility of crude protein and essential amino acids in common feedstuffs for pigs. Acta Agr Scand A-Anim Sci. 2002;52(3):121–40. doi: 10.1080/090647002320229374 [Google Scholar]
- 28.De Lange C, Sauer W, Souffrant W. The effect of protein status of the pig on the recovery and amino acid composition of endogenous protein in digesta collected from the distal ileum. J Anim Sci. 1989;67(3):755–62. doi: 10.2527/jas1989.673755x . [DOI] [PubMed] [Google Scholar]
- 29.Ravindran V, Hendriks W. Recovery and composition of endogenous protein collected at the terminal ileum as influenced by the age of broiler chickens. Aust J Agr Res. 2004;55(6):705–09. doi: 0.1071/AR04008. [Google Scholar]
- 30.Ravindran V, Hew LI, Ravindran G, Bryden WL. Endogenous amino acid flow in the avian ileum: quantification using three techniques. Brit J Nutr. 2004;92(2):217–23. doi: 10.1079/BJN20041202 . [DOI] [PubMed] [Google Scholar]
- 31.Boisen S, Moughan PJ. Dietary influences on endogenous ileal protein and amino acid loss in the pig—a review. Acta Agr Scand A-Anim Sci. 1996;46(3):154–64. doi: 10.1080/09064709609415866 [Google Scholar]
- 32.Zhang H, Qiao S, Chen X, Wang X, Xing J, Yin Y. Effects of graded levels of soya-bean protein on endogenous ileal lysine loss and amino acid digestibility in growing pigs. Anim Sci. 2005;81(02):257–64. doi: 10.1079/ASC50240257 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.