Abstract
INTRODUCTION
Extracts of the plant Passiflora incarnata L. (Passifloraceae) were administered intraperitoneally in order to test its effects on sleep.
METHOD
Experiments were carried out on chronically implanted male adult wistar rats to obtain cerebral (EEG), ocular (EOG) and muscular (EMG) activities throughout their states of vigilance. Polygraphic recordings were taken during 9 continuous hours before and after the extract administration (500 mg/kg).
RESULTS
Passiflora incarnata induced a significant increment in the total sleep time (p<0.05). This increment was due to an increase in the time spent by animals in slow wave sleep (SWS). Concomitantly, a significant decrement in wakefulness (W) was observed (p<0.05). In contrast, time spent in rapid eye movement (REM) sleep showed a decreasing tendency, since both its frequency and mean duration were reduced.
CONCLUSIONS
The extracts obtained from Passiflora incarnata can be considered as appropriated sleep inducers.
Keywords: Passifloraceae; Sleep Aids, Pharmaceutical; Insomnia; Sleep; Sleep, REM
INTRODUCTION
Sleep is a complex phenomenon constituted by two electrophysiologically and behaviorally different stages: Non-REM or Slow wave sleep (SWS) and paradoxical or rapid eye movement (REM) sleep. SWS is characterized by presenting a high voltage slow wave electroencephalographic pattern, while REM sleep exhibits a low voltage fast wave electroencephalographic pattern and ocular movements. Both sleep phases may be disturbed both by external and internal factors; therefore, the normal sleep patterns may be frequently affected.
Insomnia is a sleep disturbance that is increasing in the general population inducing serious troubles of public health; this situation has led many researchers to seek alternative therapies and test sleep inducing substances of diverse chemical nature to cope with this problem1.
Substances regarded as appropriated hypnotics are those which prevent continuous awakenings, shorten the period of latency for sleep initiation and increase sleep duration, besides displaying low toxicity.
The chemical structure of hypnotic drugs is diverse. Barbiturates, introduced more than 50 years ago, are considered to be the prototype for these drugs. However, since they exhibit serious undesired side effects their use has declined2.
Benzodiazepines are currently the most well-known and most frequently prescribed hypnotic medications, although their use in recent years is being replaced by newer non-benzodiazepine hypnotic drugs3, and the hormone melatonin4.
The most recently discovered class of hypnotics are the non-benzodiazepine drugs which were introduced in the 1980s with the development of the so-called "Z-drugs" such as Zopiclone, Zolpidem, Zaleplon and Ezopiclone5. The non-benzodiazepine drugs have similar properties to the benzodiazepines, but different chemical make-ups. They are considered relatively safer than benzodiazepines because they are less likely to cause addiction or fatal overdose.
However, they have been known to cause amnesia, hallucinations, sleepwalking problems and an increased risk of depression.These substances are usually prescribed for reducing sleep latency and increasing the total sleeping time. Among benzodiazepines, the most commonly utilized are flurazepam, triazolam, temazepam and nitrazepam. AIl these drugs increase the total sleeping period, but their effect on the sleep latency differs and some of them show undesired side effects, such as oversedation, excessive somnolence and different degrees of ataxia6.
Variations in the specific effects caused by hypnotic drugs basically depend on their physico-chemical characteristics, especially their liposolubility as well as their intensity and duration7. It is also frequent for intermediate metabolites to be pharmacologically active, which implies a longer duration of the effects. Therefore, when considering the pharmacokinetics of a hypnotic substance it is necessary to contemplate the original compound and its active metabolites, since the effect of a given substance depends on both factors8.
As mentioned previously, treatment of insomnia by means of synthesized substances originates different types of undesirable side effects. This has motivated the searching of alternative approaches such as the employment of phythotherapeutic agents9. In this context, there is ethnomedical information concerning medicinal plants which possess sedative and hypnotic properties. Among these plants are Valeriana officinalis L. (Valerianaceae)10 and Galphimia glauca Cav (Malpighiaceae)11 which have shown important sedative effects.
Hypnotic effects have also been reported for Passiflora incarnata L.12, a medicinal plant native from tropical areas of America. However, this assumption has been originated from no quantifiable subjective information13 and consequently it may lead to imprecise interpretations. Therefore the aim of this work was to analyze by means of electrophysiological methods, the effect of administration of an extract of P. incarnata L. on sleep.
METHODS
Animals
Six male adult wistar rats were used to perform this study according to human criteria and institutional approval (Bioethical Committee and the Mexican Standard for the production care and use of laboratory animals NOM-062-Z00-1999). Under general anesthesia (Sodium pentobarbital, 50 mg/kg ip) two pairs of stainless steel electrodes were epidurally implanted to obtain the electrical activity of the frontal (2 mm anterior to bregma) and occipital (5 mm posterior to bregma) regions of the brain (EEG). Ocular activity (EOG) was also obtained by means of similar electrodes placed on the supraorbital bone of each eye. Another pair of electrodes was placed on the nape muscles to obtain the electromyogram (EMG).
Animals were left to recover from surgery during a minimal period of one week. Subsequently, they were placed for their habituation to experimental conditions during 7 days in a sound attenuated chamber under a 12-hr light (7 AM-7 PM), 12-hr dark cycle (7 PM-7AM). Temperature inside the chamber oscillated between 23 and 25º C. Food and water were continuously available.
Instrumentation
Under saline injection, control polygraphic recordings were made with a Model 7 Grass electroencephalograph during 9 continuous hours from 09:00 to 18:00 hr. On the next day, an extract from Passiflora incarnata was injected intraperitoneally (500 mg/kg) at 09:00 hr and polygraphic recordings were immediately taken during another period of 9 continuous hours, as for control conditions.
Preparation of the extract
Shade-dried powdered aerials parts (3.5 kg), fruits being included, were macerated. Afterwards, the plant material was extracted exhaustively by percolation with an ethanol-water (6:4) mixture. The resulting hydro-ethanol extract was evaporated and concentrated under reduced pressure at a temperature of 40ºC in order to eliminate the ethanol. Water was then added to reach the original volume of the fluid extract. The resulting solution was considered as the aqueous extract used for pharmacological tests.
Data analysis
Recordings were visually analyzed. Quantification of states of vigilance was carried out second by second and the total time spent by animals in each state of vigilance per 9 hour periods was measured. Average duration, frequency and latency of the REM sleep phase were obtained, as well as the latency of SWS. A statistical analysis by means paired sample t-tests followed by power analysis was used to determine significant differences among the variables induced by the studied substance.
RESULTS
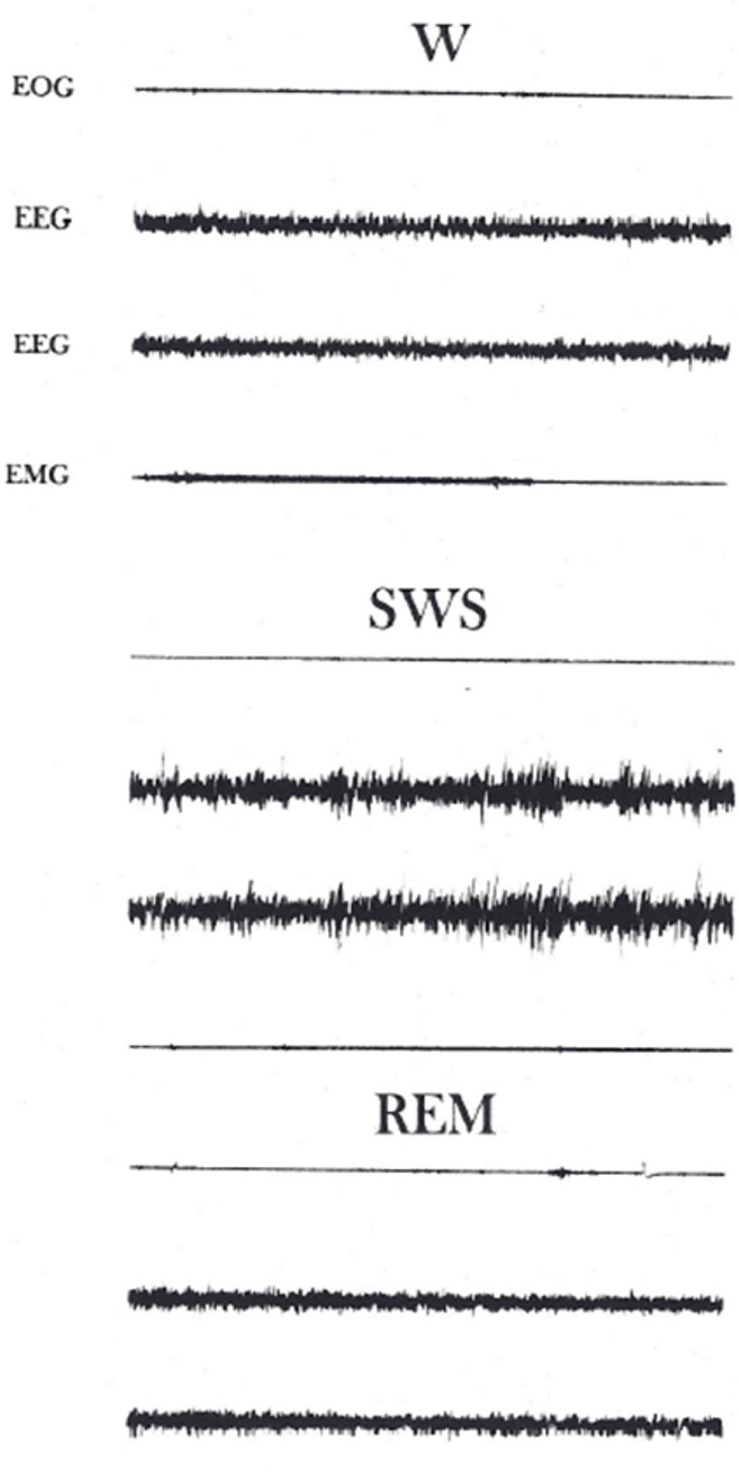
Animals displayed three different states of vigilance: Wakefulness (W), slow wave sleep (SWS) and rapid eye movement (REM) sleep (Figure 1).
Figure 1.
States of vigilance. EOG, Electro-oculogram; EEG, cerebral activity from the left and right hemisphere respectively; EMG, Electromyogram. Note the electroencephalographic patterns during each state of vigilance. Certain ocular activity is present during W, it is absent in SWS and reappears during REM sleep. Muscular tonic activity is intense in W, reduces in SWS and is almost absent during REM sleep. Calibration: 5 sec, 50 µv.
During W animals showed diverse types of behaviors such as eating, drinking and grooming. Brain activity was characterized by presenting a low voltage, high frequency pattern, the EOG showed artifacts produced by ocular movements and blinking. Muscular tone was high with numerous bursts originated by movements of the animal.
The onset of sleep coincided with quietness of the animals. They remained motionless across the SWS phase, eyes were closed and ocular movements were absent. A high voltage, slow frequency brain activity pattern was present during this sleep phase. Muscular activity showed a slight reduction and the EOG was silent.
After animals spent several minutes in SWS, they were into REM sleep. This sleep phase was characterized by the intermittent apparition of motor activity involving myoclonic jerks and ocular movements. EEG patterns were similar to those observed in W. Muscular activity was abolished, however, there were sporadic discharges coinciding with the animal jerks.
Administration of the extracts of Passiflora incarnata induced a significant decrease in the total time spent by animals in W (p<0.05; statistical power=0.85); concomitantly, an important increase in the amount of SWS (p<0.05; statistical power=0.98) was observed. Table 1 displays the individual and mean values of each vigilance state under control and experimental conditions. As observed, all the tested animals showed a decrease in the time spent in W after administration of the extract. At the same time, the individual values of SWS increased. Latency of SWS shortened from 44.38 to 25.92 minutes (p<0.05).
Table 1.
Total time spent by animals in each state of vigilance.
| ANIMAL | W | SWS | REM | |||
|---|---|---|---|---|---|---|
| C | E | C | E | C | E | |
| 1 | 234.26 | 205.07 | 276.03 | 320.24 | 29.71 | 14.69 |
| 2 | 185.65 . | 91.55 | 319.95 | 442.19 | 34.40 | 6.26 |
| 3 | 285.22 | 280.19 | 216.23 | 253.06 | 38.55 | 6.75 |
| 4 | 244.99 | 191.41 | 263.72 | 322.21 | 31.29 | 26.38 |
| 5 | 259.45 | 152.18 | 243.75 | 335.14 | 36.80 | 52.68 |
| 6 | 243.68 | 175.19 | 253.36 | 316.79 | 42.95 | 48.02 |
| MEAN | 242.21 | 182.60* | 262.17 | 331.60* | 35.62 | 25.80 |
| SD | 33.92 | 62.25 | 34.81 | 61.40 | 4.87 | 20.42 |
Time in minutes.
p<0.05
In contrast to SWS, the effect of the extract on REM sleep was not consistent. Although the average of total time spent by animals in REM sleep decreased from 35.62±4.87 to 30.81±3.92 min. (Mean±SD), this decrement was not significant (p>0.5, Statistical power=0.18). Furthermore, individual values indicate that while the administration of the extract reduced the amount of REM sleep in four animals, this parameter increased in other two.
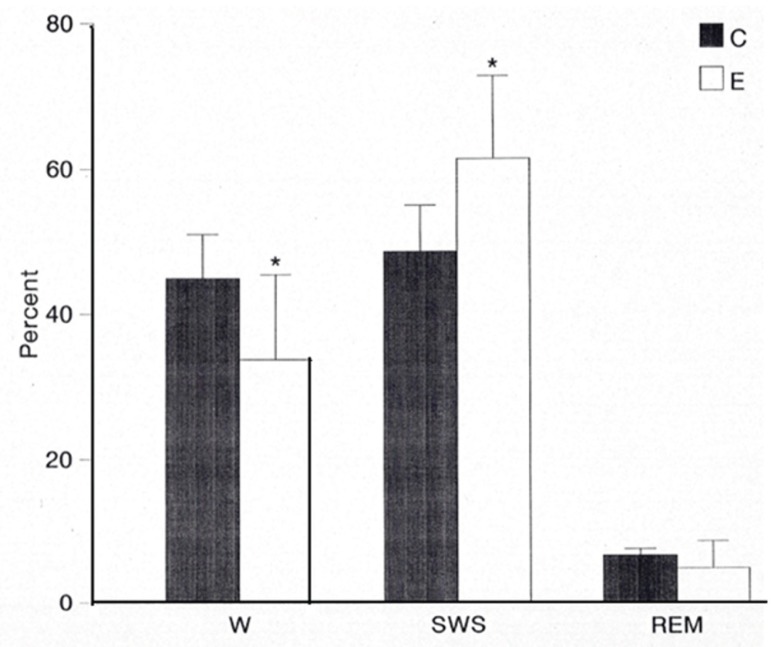
Figure 2 displays the amount of time, expressed in percentages spent by animals in each state of vigilance during the total recording period, before and after the tested extract was administered. It is evident the inhibitory effect exerted by the extract on W coinciding with a significant increment in SWS.
Figure 2.
Percentages of total recording time occupied by each state of vigilance under control (C) and experimental (E) conditions. A significant increment (p<0.05) in SWS is observed concomitantly with a significant decrement in W (p<0.05) and a slight one in REM sleep after the extract administration.
Occurrence of REM sleep phases showed a decreasing tendency throughout the recording periods. Excepting only one of the experimental subjects, the number of REM sleep phases decreased in all of them after administering the extract (Table 2). Mean duration of this sleep phase was similarly affected, since it decreased from 87.91±52.48 (Mean±SD) to 76.34±43.91 seconds. Thus, the decrement in the total REM sleep time was due to both frequency and mean duration reduction of this sleep phase. Latency of REM sleep was not significantly affected, decreasing only slightly, from 129.49±81.79 (Mean±SD) to 123.97±73.51 minutes under control and experimental conditions respectively.
Table 2.
Number of REM sleep phases during the recording periods
| ANIMAL | 1 | 2 | 3 | 4 | 5 | 6 | MEAN ± SD |
|---|---|---|---|---|---|---|---|
| CONTROL | 34 | 24 | 21 | 24 | 18 | 26 | 24.50 ±5.43 |
| EXPERIMENTAL | 26 | 16 | 13 | 16 | 25 | 26 | 20.33 ± 5.95 |
DISCUSSION
The employment of vegetable products may be an adequate approach for insomnia treatment. Previous studies indicate that the plant Valeriana officinalis L. has been utilized for treatment of insomniac patients. Subjective analysis indicates a shortening of the sleep latency, a decrease in number of awakenings through the night and feelings of having a repairing sleep after administration of the extracts of this plant14.
Behavioral studies using other plants indicate that Tilia tormentosa and Passiflora officinalis are able to induce sleep in a way similar to that under physiological conditions15.
Extracts from passion flower (Passiflora incarnata) have been used in patients to reduce anxiety and insomnia13. This has been corroborated by mean numerous behavioral studies in rodents which demonstrate a reduced anxiety and stress with passion flower treatment16. However, slight effects were observed on total times of non-REM sleep and REM sleep when administered in sleep-disturbed model rats17.
The results obtained in this experimental work by means of electrophysiological techniques support the behavioral observations related to the hypnotic effects induced by the administration of extracts from Passiflora incarnata18. The used experimental approach allowed us to determine the precise state of vigilance affected by this extract. Similarly, we were able to quantify the intensity of the effect.
In this way, it was evidenced that Passiflora incarnata increases significantly the time spent by animals in SWS while that of REM sleep showed a tendency to diminish. The decrement of REM sleep after administering the extract of Passiflora incarnata was due to a decrease in the occurrence of this sleep phase, as well as to a reduction in its average duration. These effects may indicate that the tested extract exerts a facilitatory action on the regulating mechanisms of SWS and, at the same time, an inhibitory action on those related to REM sleep.
Although the anxiolytic and hypnotic activities of Passiflora species has been repeatedly evaluated in the past few years, there is only limited information on the mechanism of action. Some reports have speculated about its effects on the GABA system, but investigations concerning the mechanism are still lacking.
It has been described that numerous pharmacological effects of Passiflora incarnata are mediated via modulation of the GABA system including affinity to GABAA and GABAB receptors, and effects on GABA uptake19.
It is very likely that binding to the GABA-site of the GABAA receptor is one of the clinically relevant modes of action of Passiflora incarnata extract.
Because preadministration of flumazenil (Ro 15-1788), an antagonist of the benzodiazepine binding site of the GABAA receptor, attenuates the effects of Passiflora incarnata in vivo.
Another possible target might be the binding of Passiflora incarnata extract to the GABAB receptor. There is accumulating evidence that modulators of the GABAB receptor might act as an anxiolytic, although the compounds responsible for the hypnotic activity of Passiflora incarnata are yet to be identified. However, it is probable that the hypnotic activity could be exerted by the flavonoids chrysin and/ or homoorientin, orientin, vitexin, and isovitexin20, compounds isolated from Passiflora incarnata extracts which have shown a significant anxiolityc effect mediated via the GABAergic system.
CONCLUSION
The obtained results suggest that Passiflora incarnata contains components that facilitate the presence of the SWS and inhibit that of REM sleep. Additional experiments should be carried out in order to isolate the active principles and evaluate their effects on each sleep phase. Thus, it could be possible to induce SWS, without affecting REM sleep, after administration of some specific component.
Shortening of sleep latency and increasing in the amount of SWS produced by the extracts of Passiflora incarnata suggest that this plant possesses adequate properties to be considered as sleep inducer. These characteristics could be profited in the treatment of insomniac patients complaining about problems related to sleep onset.
ACKNOWLEDGMENTS
We thank Dr. Erik Mateos Salgado for editorial support.
REFERENCES
- 1.Palaniappan K, Thenappan A. Physiological Basis of Alternative Therapies to Alleviate Sleep Disturbances. J Sleep Disord Ther. 2015;5:221–221. doi: 10.4172/2167-0277.1000221. [DOI] [Google Scholar]
- 2.Johns MW. Sleep and hypnotic drugs. Drugs. 1975;9(6):448–478. doi: 10.2165/00003495-197509060-00004. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann F. Benefits and risks of benzodiazepines and Z-drugs: comparison of perceptions of GPs and community pharmacists in Germany. Ger Med Sci. 2013;11:Doc10–Doc10. doi: 10.3205/000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costello RB, Lentino CV, Boyd CC, O'Connell ML, Crawford CC, Sprengel ML, et al. The effectiveness of melatonin for promoting healthy sleep: a rapid evidence assessment of the literature. Nutr J. 2014;13:106–106. doi: 10.1186/1475-2891-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang MP, Radadia K, Macone BW, Auerbach SH, Datta S. Effects of eszopiclone and zolpidem on sleep-wake behavior, anxiety-like behavior and contextual memory in rats. Behav Brain Res. 2010;210(1):54–66. doi: 10.1016/j.bbr.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermeeren A. Residual effects of hypnotics: epidemiology and clinical implications. CNS Drugs. 2004;18(5):297–328. doi: 10.2165/00023210-200418050-00003. [DOI] [PubMed] [Google Scholar]
- 7.Ashton H. Guidelines for the rational use of benzodiazepines. When and what to use. Drugs. 1994;48(1):25–40. doi: 10.2165/00003495-199448010-00004. [DOI] [PubMed] [Google Scholar]
- 8.Obach RS. Pharmacologically active drugs metabolites: impact on drug discovery and pharmacotherapy. Pharmacol Rev. 2013;65(2):578–640. doi: 10.1124/pr.111.005439. [DOI] [PubMed] [Google Scholar]
- 9.Tortoriello J, Romero O. Plants used by Mexican traditional medicine with presumable sedative properties: an ethnobotanical approach. Arch Med Res. 1992;23(3):111–116. [PubMed] [Google Scholar]
- 10.Houghton PJ. The biological activity of Valerian and related plants. J Ethnopharmacol. 1988;22(2):121–142. doi: 10.1016/0378-8741(88)90123-7. [DOI] [PubMed] [Google Scholar]
- 11.Tortoriello J, Ortega A. Sedative effect of Galphimine B, a Nor-seco-triterpenoid from Galphimia glauca. Planta Med. 1993;59(5):398–400. doi: 10.1055/s-2006-959717. [DOI] [PubMed] [Google Scholar]
- 12.Speroni E, Minghetti A. Neuropharmacological activity of extracts from Passiflora incarnata. Planta Med. 1988;54(6):488–491. doi: 10.1055/s-2006-962525. [DOI] [PubMed] [Google Scholar]
- 13.Ngan A, Conduit R. A double-blind, placebo-controlled Investigation of the effects of Passiflora incarnate (passionflower) herbal tea on subjective sleep quality. Phytother Res. 2011;25(8):1153–1159. doi: 10.1002/ptr.3400. [DOI] [PubMed] [Google Scholar]
- 14.Leathwood PD, Chauffard F, Heck E, Muñoz-Box R. Aqueous extract of valerian root (Valeriana officinalis L.) improves sleep quality in man. Pharmacol Biochem Behav. 1982;17(1):65–71. doi: 10.1016/0091-3057(82)90264-7. [DOI] [PubMed] [Google Scholar]
- 15.Gugliada R, Raggi A. Insomnia. Erboristeria Domani. 1991;10:46–54. [Google Scholar]
- 16.Miyasaka LS, Atallah AN, Soares BG. Passiflora for anxiety disorder. Cochrane Database Syst Rev. 2007;(1):CD004518–CD004518. doi: 10.1002/14651858.CD004518.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Shinomiya K, Inoue T, Utsu Y, Tokunaga S, Masuoka T, Ohmori A, et al. Hypnotic activities of chamomile and passiflora extracts in sleep-disturbed rats. Biol Pharm Bull. 2005;28(5):808–810. doi: 10.1248/bpb.28.808. [DOI] [PubMed] [Google Scholar]
- 18.Soulimani R, Younos C, Jarmouni S, Bousta D, Misslin R, Mortier F. Behavioural effects of Passiflora incarnata L. and its indole alkaloid and flavonoid derivatives and maltol in the mouse. J Ethnopharmacol. 1997;57(1):11–20. doi: 10.1016/s0378-8741(97)00042-1. [DOI] [PubMed] [Google Scholar]
- 19.Appel K, Rose T, Fiebich B, Kammler T, Hoffmann C, Weiss G. Modulation of the γ-aminobutyric acid (GABA) system by Passiflora incarnata L. Phytother Res. 2011;25(6):838–843. doi: 10.1002/ptr.3352. [DOI] [PubMed] [Google Scholar]
- 20.Brown E, Hurd NS, McCall S, Ceremuga TE. Evaluation of the anxiolytic effects of chrysin, a Passiflora incarnata extract, in the laboratory rat. AANA J. 2007;75(5):333–337. [PubMed] [Google Scholar]