Abstract
OBJECTIVE
In a device based on midsagittal jaw movements analysis, we assessed a sleep-wake automatic detector as an objective method to measure sleep in healthy adults by comparison with wrist actigraphy against polysomnography (PSG).
METHODS
Simultaneous and synchronized in-lab PSG, wrist actigraphy and jaw movements were carried out in 38 healthy participants. Epoch by epoch analysis was realized to assess the ability to sleep-wake distinction. Sleep parameters as measured by the three devices were compared. This included three regularly reported parameters: total sleep time, sleep onset latency, and wake after sleep onset. Also, two supplementary parameters, wake during sleep period and latency time, were added to measure quiet wakefulness state.
RESULTS
The jaw movements showed sensitivity level equal to actigraphy 96% and higher specificity level (64% and 48% respectively). The level of agreement between the two devices was high (87%). The analysis of their disagreement by discrepant resolution analysis used PSG as resolver revealed that jaw movements was right (58.9%) more often than actigraphy (41%). In sleep parameters comparison, the coefficient correlation of jaw movements was higher than actigraphy in all parameters. Moreover, its ability to distinct sleep-wake state allowed for a more effective estimation of the parameters that measured the quiet wakefulness state.
CONCLUSIONS
Midsagittal jaw movements analysis is a reliable method to measure sleep. In healthy adults, this device proved to be superior to actigraphy in terms of estimation of all sleep parameters and distinction of sleep-wake status.
Keywords: Actigraphy, Movement, Sleep Wake Disorders, Sleep
INTRODUCTION
Obstructive sleep apnea is an increasingly common condition and, when untreated, leads to significant social, cardiac and cerebrovascular morbidity and mortality1.
Full attended polysomnography (PSG) is considered as gold standard test to diagnose obstructive sleep apnea. Its capacity to measure apnea-hypopnea index is an essential parameter in the diagnosis2. PSG requires hospital environment, attended technologist and expert interpretation of data3 which may delay the management. Therefore, the development of portable monitor (PM) devices has been proposed as an alternative strategy4.
The evolution of American Academy of Sleep Medicine (AASM) practice parameters confirmed the growing role of PM in the management of obstructive sleep apnea patients5-8. The lack of sleep measures has been a major limitation of PM, because it makes the calculation of apnea-hypopnea index impossible, hence, the use of total recording time (TRT) to calculate respiratory event index9. A more ambitious solution was the use of alternative methods to PSG in order to measure sleep. The new classification of PM showed the wide popularity of wrist-actigraphy (WAC) with multiple setting and software9.
New technologies were developed recently for home-based sleep assessment10,11, notably based on autonomic signals analysis. Despite the variety in their technologies, little was proposed in the context of PM. Most devices able describing sleep pattern, e.g. Watch- PAT12 and M1-Sleepimage13 integrated the actigraphy signal to develop their sleep estimate algorithm rather than the use of autonomic signals analysis.
The midsagittal jaw movement based device (JAWAC), was previously shown to detect and classify abnormal respiratory events reliably14-17. Several studies described specific changes in jaw posture during sleep in healthy and patient population18-20. A new sleep-wake automatic detector based on jaw posture has been proposed to measure sleep objectively in order to replace the TRT by an estimated total sleep time (TST)21,22.
In the current study we validated the ability of this jaw posture-based software to analyse sleep pattern in healthy adult population. Synchronized and simultaneous recording of JAWAC, WAC and PSG were performed in healthy volunteers. Epoch by epoch analysis was realized to compare the sleep-wake distinction ability of the JAWAC and WAC. Their sleep parameters estimates were assessed and compared to PSG measurements as reference standard.
METHODS
The present study was conducted at the Sleep Center of the University Hospital of Liege, Belgium, between October 2014 and May 2016. In accordance with the principles of Declaration of Helsinki for Human Experimentation; all the participants agreed to participate after being fully informed of the aims and consequences of the study23.
Subjects
Subjects were healthy volunteers without sleep complaints. Epworth sleepiness scale score less than 8, and Hospital anxiety and depression scale less than 10 for each axis were required. The exclusion criteria were the presence of one or more of the following: body mass index over 26 kg/m2, known chronic disorder, acute illness in the study day, taking of any drugs, consumption of more than 4 units of alcohol/day or more than 6 cigarettes /day, and pathological PSG results.
Polysomnography
PSG was carried out using randomly one of three EMBLA N7000 systems running the Somnologica software. The PSG montage included three EEG channels, left and right EOG, Chin EMG, bilateral tibialis anterior EMG, EKG, nasal cannula/pressure transducer, chest and abdominal inductance plethysmography belts, fingertip pulse Oximetry, snoring sensor, body position sensor, and light sensor. Manual scoring according to AASM scoring rules was realized by qualified technologists blinded to the results of automatic analysis of the other devices24.
Wrist-Actigraphy (WAC)
Data were collected using randomly one of two Actiwatch monitors (Actiwatch 2; Philips - Respironics, Murrysville PA, USA) attached to the non-dominant wrist. Data were collected in 30-seconds epochs and analyzed thereafter by Philips ActiWare software version 6.0.1. The "default" settings provided by the manufacturer was selected for automatic analysis. The Actiwatch and the "default" settings have been widely used to measure sleep objectively25.
Jaw movements device (JAWAC)
The JAWAC (Nomics -Liege- Belgium) is a distance-meter, non-invasive motion sensor, based on the principle of electromagnetic self-induction. The output voltage at the receiver coil is a monotonic cubic function of the distance between the transmitter and the receiver coils. Therefore, when the two coils are placed parallel to each other on the median-line of forehead and chin, the distance between them, which represent the jaw vertical movement, can be calculated from the properties of the received signal. The output was amplified, digitalized at a rate of 10 Hz and available both on line with the PSG channels. The data were stored for subsequent retrieval and analysis.
The first software based on jaw movement analysis to detect and classify the ventilatory effort has been developed and validated14-17. A second software has then been added to recognize sleep and wake using a wavelet-based complexity measure of the jaw movement signal. A multi-layer perceptrons as decision organs has been elaborated and validated against PSG in patient population21,22. Three devices were used randomly. Only the results of the sleep-wake analysis software were included in the current study.
Procedures
Each participant was admitted to the sleep laboratory between 14:00 and 17:00 for a time of adaptation. She or he was equipped first with WAC and JAWAC sensors and thereafter with PSG equipments. The acquisition time of each device was the period between the installation and the removal of the equipment; thus the acquisition time was different due to practical constraints. To ensure a reliable temporal synchronization between the three devices, we used the Network Time Protocol. Before each sleep study, the computer of each device was connected to Internet and its clock was synchronized manually with Internet time server. The participants were asked to maintain their habitual rhythm of wake-sleep. They were free to choose the time devoted to sleep, reported by light turn off /on.
Results of scoring of each device were available in 30s epoch. PSG epochs labeled as one of sleep stages (N1, N2, N3, and R) were modified to "sleep". WAC and JAWAC results were labeled directly "sleep" or "wake".
For analysis, we selected the time devoted to sleep as identified by the PSG light sensor.
Periods where the signal from one of the devices was deficient or interrupted were excluded. The remained periods defined as the "analysis time" were used for comparative analysis.
Analysis
Data analysis
The primary aim of the study was to compare the absolute performance of JAWAC and WAC as regards the analysis of sleep. The second aim was to investigate the potential differences in the relative performance of the two devices. For this purpose, two sets of analysis were performed, an epoch by epoch analysis, and the sleep parameters analysis. The statistical approach was based on the statistical guidance of the Food and Drug Administration26.
In epoch by epoch analysis, in pooled-epoch basis combined all scored epochs from all subjects, we measured the sensitivity, specificity, likelihood ratios, and Cohen's Kappa correlation. The sensitivity was defined as the proportion of epochs scored as sleep by the PSG analysis, in which the label given by the device was "sleep". The specificity was defined as the proportion of epochs scored wake by PSG analysis, in which the label given by the device was "wake". Positive likelihood ratio was calculated by the formula [LR + = sensitivity/ 1-specificity]. Negative likelihood ratio, was calculated by the formula [LR- = 1-sensitivity/specificity].
To investigate the differences in the relative performance between WAC and JAWAC, three-way presentation of results was used. Thus, we considered PSG as reference standard, WAC as non-reference standard and JAWAC as new test.
Three agreement levels between WAC and JAWAC were calculated. They were defined as the proportion of epochs labeled identically by the two devices in epochs scored consequently by the PSG as sleep, wake and the overall of two states.
In epochs where the two devices were disagreed, a discrepant resolution test used PSG as resolver to determine the "right" device.
In the sleep parameters analysis, we used the same definitions for the three devices. These included three AASM recommended parameters: 1) the total sleep time (TST) defined as the duration of all epochs labeled as sleep. 2) The sleep onset latency (SOL) measured as the time from Light-off to the first epoch off sleep. 3) The wake after sleep onset (WASO) which is the time scored as wake from first sleep epoch to Light-on. Two additional parameters were added: 1) the wake during sleep period (WDSP), calculated as the time of wake between the first and the last epoch of sleep and 2) the latency to arising (LTA) measured as the elapsed time from last sleep epoch to Light-on. Sleep efficiency, was not included, because the "analysis time" was the same for the three devices; therefore the interpretation of TST and sleep efficiency results became univocal.
The Pearson product-moment correlation coefficient and paired t-test were realized first to determine the absolute performance of JAWAC and WAC compared to PSG analysis, and secondly to determine the relative performance between JAWAC and WAC.
For each subject, the difference between PSG measures, JAWAC and WAC estimates were computed for each sleep parameter. The mean differences (bias) and standard deviation of the differences were calculated. Bias represents the discrepancies between the two devices, and standard deviation provides an estimation of the variation of mean difference between the two devices. Positive bias indicates an overestimation relatively to PSG analysis, and negative bias indicates an underestimation by the JAWAC or WAC. A t-test was used to evaluate the significance of the bias of JAWAC and WAC.
Results were reported both as fraction and percentage with two sided 95 percent confidence intervals. Times and latencies were expressed in minutes. Significance level was set at 0.05.
RESULTS
Participants
Fifty one subjects were included in the study. Four subjects were excluded on the basis of clinical evaluation; 47 sleep studies were conducted. Among them, 5 were excluded because of pathologic PSG results, 3 because of loss of JAWAC signal during sleep and 1 because of WAC software problem. The failure rate was 6.3% for the JAWAC and 2.1% for the WAC.
Thirty-eight remaining participants were submitted to the whole analysis process. Table 1 described their demographic characteristics and Table 2 their essentials results as measured by the three devices.
Table 1.
Demographic of the participants.
| Subjects (M/F) | 38 (20/18) |
|---|---|
| Age | 23,5±1,5 years |
| Body Mass Index | 22,2±1,8 Kg/m2 |
| Epworth Sleepiness Scale | 3,7±1,7 Points |
| Hospital Anxiety and Depression Scale (Anxiety) | 4,3±2,7 Points |
| Hospital Anxiety and Depression Scale (Depression) | 2±1,4 Points |
| Apnea-Hypopnea Index | 3,2±2 /h |
Table 2.
Sleep parameters measured by Polysomnography, Jaw movements and Actigraphy.
| PSG | JAWAC | WAC | |
|---|---|---|---|
| 416.1±(75.7) | 425,9±(78.4) | 438.2±(65.6) | |
| Sleep Onset Latency | 25.2±(21.8) | 25.9±(22.9) | 3.0±(2.1) |
| Wake After Sleep Onset | 46.9±(49.3) | 36.5±(52.5) | 47.0±(25.5) |
| Wake During Sleep Period | 34.4±(45.1) | 26.2±(27.6) | 43.5±(24.8) |
| Latency Time to Arising | 12.5±(22.2) | 10.2±(12.1) | 3.5±( 4.7) |
Sleep parameters presented in minutes (Mean ± Standard Deviation), PSG = Polysomnography, JAWAC = Jaw movements, WAC = Actigraphy
Epoch-by-Epoch analysis results
Table 3 showed the results of comparative performance of JAWAC and WAC. No discernible differences were observed in terms of sensitivity, specificity and Cohen's kappa values. The two devices had a high ability to recognize sleep as showed by their high sensitivity level, while their moderate specificity level indicate moderate ability to recognize wake. Likelihood ratio results showed a higher level of accuracy to recognize sleep correctly by the JAWAC device.
Table 3.
Comparative performance of Jaw movements and Actigraphy against Polysomnography in Pooled-epoch basis.
| JAWAC | WAC | |
|---|---|---|
| Sensitivity | 96% | 96% |
| Specificity | 64% | 48% |
| Positive Likelihood Ratio | 2.70 | 1.87 |
| Negative Likelihood Ratio | 0.05 | 0.53 |
| Cohen's Kappa | 0.64 | 0.51 |
JAWAC=Jaw movements, WAC=Actigraphy
The raw data of the three devices, derived from pooled-epoch basis were reported in threeway presentation (Table 4).
Table 4.
Three-way presentation, comparing the raw data Polysomnography, Jaw movements and Actigraphy in Pooled-epoch basis.
| JAWAC | WAC | Total epochs | PSG (Sleep) |
PSG (Wake) |
|---|---|---|---|---|
| Sleep | Sleep | 30491 | 29450 | 1041 |
| Sleep | Wake | 1851 | 972 | 879 |
| Wake | Sleep | 2817 | 1035 | 1782 |
| Wake | Wake | 1959 | 170 | 1789 |
| Total | 37118 | 31627 | 5491 |
PSG=Polysomnography, JAWAC=Jaw movements, WAC=Actigraphy
The overall agreement level between the JAWAC and WAC raw pooled-epoch data was high (87.4%), due to a very good agreement in epochs scored as sleep by the PSG analysis (93.6%) and a moderate agreement level in epochs scored as wake by the PSG (51.5%) (Table 5).
Table 5.
Agreements levels between Jaw movements and Actigraphy in Pooled-epoch basis.
| Fraction | Percentage | |
|---|---|---|
| Agreement in PSG sleep epochs | (29450+170)/(31627) | 93.6% |
| Agreement in PSG wake epochs | (1041+1789)/(5491) | 51.5% |
| Agreement in overall PSG epochs | (30491+1959)/(37118) | 87.4% |
Data extracted from the three way presentation table
In epochs with disagreement between JAWAC and WAC, the discrepant resolution, revealed that globally, JAWAC was "right" in 58.9% and WAC in 41.1% of these epochs. This accuracy was more pronounced in wake epochs (JAWAC 66.9%, WAC 33.1%), while in sleep epochs the accuracy was approximately equal (JAWAC 48.4%, WAC 51.6%) (Table 6).
Table 6.
Discrepant resolution analysis to determine the 'right' device in epochs with disagreement between Jaw movements and Actigraphy.
| PSG | JAWAC | WAC | ||
|---|---|---|---|---|
| Epochs | Fraction | Percentage | Fraction | ercentage |
| Sleep | (972)/(972+1035) | 48.4% | (1035)/ (972+1035) | 51.6% |
| Wake | (1782)/ (879+1782) | 66.9% | (879)/ (879+1782) | 33.1% |
| Overall | (972+1782)/ (972+1035+ 879+1782) | 58.9% | (1035+879)/ (972+1035+ 879+1782) | 41.1% |
PSG =Polysomnography, JAWAC=Jaw movements, WAC=Actigraphy, Data extracted from the three way presentation table
Sleep parameters analysis results
Sleep parameters as obtained from PSG, JAWAC and WAC were depicted in Table 7. The Pearson product-moment correlation coefficients between PSG and JAWAC were strong for TST, SOL, WASO and WDSP (0.94, 0.82, 0.89 and 0.83 respectively) and moderate for LTA (0.57). Between PSG and WAC, the correlation coefficients were strong for TST only (0.88), moderate for WASO and WDSP (0.77 and 0.62) and poor for SOL and LTA (0.01 and -0.04).
Table 7.
Comparison of sleep parameters as measured by Polysomnography, Jaw movements and Actigraphy.
| PSG | JAWAC | WAC | PSG versus JAWAC | PSG versus WAC | JAWAC versus WAC | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean±(SD) | Mean±(SD) | Mean±(SD) | |||||||
| r | p | r | p | r | p | ||||
| TST | 416.1±(75.7) | 0.94 | <0.05 | 0.88 | <0.05 | 0.86 | 0.06 | ||
| SOL | 25.2±(21.8) | 25.9±(22.9) | 3.0±(2.1) | 0.82 | 0.77 | 0.01 | <0.05 | -0.03 | <0.05 |
| WASO | 46.9±(49.3) | 36.5±(52.5) | 47.0±(25.5) | 0.89 | <0.05 | 0.77 | 0.89 | 0.73 | 0.09 |
| WDSP | 34.4±(45.1) | 26.2±(27.6) | 43.5±(24.8) | 0.83 | 0.06 | 0.62 | 0.07 | 0.67 | <0.05 |
| LTA | 12.5±(22.2) | 10.2±(12.1) | 3.5±(4.7) | 0.57 | 0.45 | -0.04 | <0.05 | 0.03 | <0.05 |
PSG=Polysomnography, JAWAC=Jaw movements, WAC=Actigraphy, Sleep parameters presented in minutes; SD=Standard Deviation, r=Pearson's correlation coefficient, p=P values from paired t-test, TST=total sleep time, SOL=sleep onset latency, WASO=wake after sleep onset, WDSP=wake during sleep period, LTA=latency time to arising.
The correlation coefficient between JAWAC and WAC were strong for TST (0.86), moderate for WASO and WDSP (0.73 and 0.67) and poor for SOL and LTA (-0.03 and 0.03). No significant difference was founded between PSG measures-JAWAC estimates for SOL, WDSP and LTA, and between PSG measures-WAC estimates for WASO and WDSP.
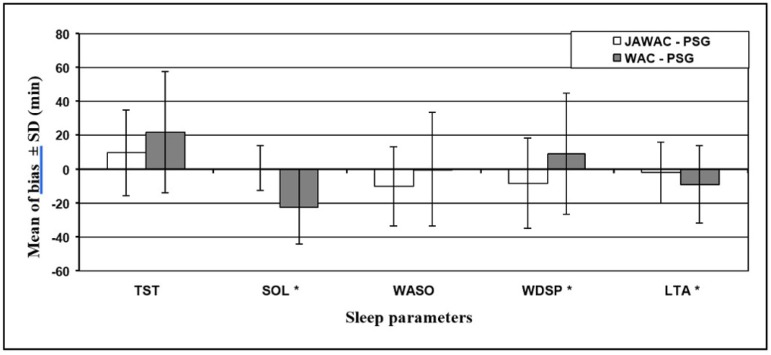
Figure 1 shows that mean bias of all sleep parameters except WASO were smaller for JAWAC than WAC estimates. The nearly zero of mean bias of WASO as estimated by WAC was the result of opposition in the direction of mean bias between WDSP and LTA who compose it.
Figure 1.
Mean of bias and standard deviation of mean bias of sleep parameters. SD = Standard Deviation, TST = total sleep time, SOL = sleep onset latency, WASO = wake after sleep onset, WDSP = wake during sleep period, LTA = latency time to arising,* = p-values from paired t-test = 0.05 for the mean bias (JAWAC-PSG Vs WAC-PSG) PSG = Polysomnography, JAWAC = Jaw movements, WAC = Actigraphy.
DISCUSSION
Our study showed that estimation of sleep parameters and sleep-wake detection by midsagittal jaw movements analysis was superior to WAC in healthy subjects. Midsagittal jaw movements analysis proved excellent ability to overcome the well-known weakness of actigraphy in differentiating quiet wakefulness from sleep27. The JAWAC superiority might result from what each device measures precisely. The JAWAC provides information on both the posture of the mandible and its vertical movements14,17,20, whereas actigraphy simply measures movements of a limb28. Therefore the difference in the estimation of quiet wakefulness might be explained by the concept of sleep onset spectrum, where the sleep onset is a gradual rather than a discrete process29.
It begins by some quiescence and inactivity identified by actigraphy as sleep onset and not detected as such by the JAWAC system. Then the muscle tone decreases, followed quickly by the EEG change that constitutes the PSG criteria to sleep onset24. This decrease in muscle tone induces a slight opening of the mandible18 identifiable on the JAWAC tracing and considered by its software as sleep onset22. The same concept might explain the excellent ability of JAWAC to identify sleep offset.
This postulate was confirmed by the comparison of sleep parameters. The SOL, WASO, WDSP and LTA which were related to "wake epochs"; showed better correlation with PSG measures for the JAWAC estimates (0.82, 0.89, 0.83 and 0.57 respectively) than the WAC estimates (0.01, 0.77, 0.62 and -0.04). Interestingly, we noted that the excellent estimation of the WASO by WAC was the result of the difference in the direction of bias of its two components, the WDSP and LTA, rather than a real correct estimation by the device. The TST, as the sole parameter related to "sleep" epochs, showed very good correlation between JAWAC and WAC estimates (0.86) and also between each estimate and the PSG measure (0.94 for the JAWAC and 0.88 for the WAC).
The epoch-by-epoch comparison pointed a better ability of JAWAC to identify "wake" epochs correctly. Thus, beyond the estimation of sensitivity (96% for both devices) and specificity (JAWAC 64%, WAC 48%); the discrepant resolution of the disagreement between JAWAC and WAC allowed an elaborated analysis of their performance. It showed higher level of agreement for the JAWAC than the WAC in the identification of the "wake" epochs of PSG (66.9% and 33.1% respectively). This explained a higher level of overall agreement for the JAWAC (58.9% and 41.1%) despite similar level of agreement in "sleep" epochs (48.4% and 51.6%).
Several elements strengthen this study. First, we compared JAWAC data simultaneously with standard reference -PSG and the non standard reference-WAC rather than comparing with PSG and published data of WAC. This choice was motivated by the few number of validation reports of actigraphy systems in the context of PM. Moreover, these reports showed large variability in accuracy results depending on the evaluated sleep parameters, the producers of actigraphy, the algorithms and the thresholds used11. Second, the inclusion of WDSP and LTA in the analysis allowed better understanding of the performance of each device beyond the simple comparison of WASO. Third, by the discrepant resolution we were able to compare precisely the accuracy of JAWAC and WAC during the "wake" and "sleep" epochs of PSG.
However, the current study had several limitations. The included subjects were young (23.5±1.5y) while it would be important to include oldest ones. The use of other settings for the algorithm of WAC and other brands of actigraphy could allow better evaluation of the real value of JAWAC.
CONCLUSION
To conclude, an algorithm based on vertical jaw movement analysis is a simple and reliable way to measure sleep parameters in healthy young adults. In the context of PM, a clear advantage of JAWAC is its ease of use as it could measure, by a unique sensor, both sleep parameters and respiratory events in order to calculate apnea-hypopnea index accurately. Although JAWAC performance in sleep parameters analysis need to be evaluated in subjects with sleep disorders, it is a promising tool to facilitate sleep-disordered breathing diagnosis and quantification.
REFERENCES
- 1.Jennum P, Riha RL. Epidemiology of sleep apnoea/hypopnoea syndrome and sleep-disordered breathing. Eur Respir J. 2009;33(4):907–914. doi: 10.1183/09031936.00180108. [DOI] [PubMed] [Google Scholar]
- 2.Qaseem A, Dallas P, Owens DK, Starkey M, Holty JE, Shekelle P, Clinical Guidelines Committee of the American College of Physicians Diagnosis of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014;161(3):210–220. doi: 10.7326/M12-3187. [DOI] [PubMed] [Google Scholar]
- 3.Fischer J, Dogas Z, Bassetti CL, Berg S, Grote L, Jennum P, et al. Executive Committee of the Assembly of the National Sleep SocietiesBoard of the European Sleep Research Society (ESRS), Regensburg, Germany Standard procedures for adults in accredited sleep medicine centres in Europe. J Sleep Res. 2012;21(4):357–368. doi: 10.1111/j.1365-2869.2011.00987.x. [DOI] [PubMed] [Google Scholar]
- 4.Kuna ST. Portable-monitor testing: an alternative strategy for managing patients with obstructive sleep apnea. Respir Care. 2010;55(9):1196–1215. [PubMed] [Google Scholar]
- 5.Practice parameters for the use of portable recording in the assessment of obstructive sleep apnea. Standards of Practice Committee of the American Sleep Disorders Association. Sleep. 1994;17(4):372–377. [PubMed] [Google Scholar]
- 6.Practice parameters for the indications for polysomnography and related procedures. Polysomnography Task Force, American Sleep Disorders Association Standards of Practice Committee. Sleep. 1997;20(6):406–422. [PubMed] [Google Scholar]
- 7.Chesson AL, Jr, Berry RB, Pack A, American Academy of Sleep Medicine. American Thoracic Society. American College of Chest Physicians Practice parameters for the use of portable monitoring devices in the investigation of suspected obstructive sleep apnea in adults. Sleep. 2003;26(7):907–913. doi: 10.1093/sleep/26.7.907. [DOI] [PubMed] [Google Scholar]
- 8.Kushida CA, Littner MR, Morgenthaler T, Alessi CA, Bailey D, Coleman J, Jr, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28(4):499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 9.Collop NA, Tracy SL, Kapur V, Mehra R, Kuhlmann D, Fleishman SA, et al. Obstructive sleep apnea devices for out-of-center (OOC) testing: technology evaluation. J Clin Sleep Med. 2011;7(5):531–548. doi: 10.5664/JCSM.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly JM, Strecker RE, Bianchi MT. Recent developments in home sleep-monitoring devices. ISRN Neurol. 2012;2012:768794–768794. doi: 10.5402/2012/768794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van de Water AT, Holmes A, Hurley DA. Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography--a systematic review. Pt 2J Sleep Res. 2011;20(1):183–200. doi: 10.1111/j.1365-2869.2009.00814.x. [DOI] [PubMed] [Google Scholar]
- 12.Pittman SD, Ayas NT, MacDonald MM, Malhotra A, Fogel RB, White DP. Using a wrist-worn device based on peripheral arterial tonometry to diagnose obstructive sleep apnea: in-laboratory and ambulatory validation. Sleep. 2004;27(5):923–933. doi: 10.1093/sleep/27.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas RJ, Mietus JE, Peng CK, Goldberger AL. An electrocardiogram-based technique to assess cardiopulmonary coupling during sleep. Sleep. 2005;28(9):1151–1161. doi: 10.1093/sleep/28.9.1151. [DOI] [PubMed] [Google Scholar]
- 14.Senny F, Destiné J, Poirrier R. Midsagittal jaw movement analysis for the scoring of sleep apneas and hypopneas. IEEE Trans Biomed Eng. 2008;55(1):87–95. doi: 10.1109/TBME.2007.899351. [DOI] [PubMed] [Google Scholar]
- 15.Maury G, Cambron L, Jamart J, Marchand E, Senny F, Poirrier R. Added value of a mandible movement automated analysis in the screening of obstructive sleep apnea. J Sleep Res. 2013;22(1):96–103. doi: 10.1111/j.1365-2869.2012.01035.x. [DOI] [PubMed] [Google Scholar]
- 16.Cheliout-Heraut F, Senny F, Djouadi F, Ouayoun M, Bour F. Obstructive sleep apnoea syndrome: comparison between polysomnography and portable sleep monitoring based on jaw recordings. Neurophysiol Clin. 2011;41(4):191–198. doi: 10.1016/j.neucli.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Maury G, Senny F, Cambron L, Albert A, Seidel L, Poirrier R. Mandible behaviour interpretation during wakefulness, sleep and sleep-disordered breathing. J Sleep Res. 2014;23(6):709–716. doi: 10.1111/jsr.12180. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto K, Ozbek MM, Lowe AA, Sjöholm TT, Love LL, Fleetham JA, et al. Mandibular posture during sleep in healthy adults. Arch Oral Biol. 1998;43(4):269–275. doi: 10.1016/s0003-9969(97)00122-2. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto K, Ozbek MM, Lowe AA, Sjöholm TT, Love LL, Fleetham JA, et al. Mandibular posture during sleep in patients with obstructive sleep apnoea. Arch Oral Biol. 1999;44(8):657–664. doi: 10.1016/s0003-9969(99)00057-6. [DOI] [PubMed] [Google Scholar]
- 20.Tsuda H, Lowe AA, Chen H, Fleetham JA, Ayas NT, Almeida FR. The relationship between mouth opening and sleep stage-related sleep disordered breathing. J Clin Sleep Med. 2011;7(2):181–186. [PMC free article] [PubMed] [Google Scholar]
- 21.Senny F, Maury G, Cambron L, Leroux A, Destiné J, Poirrier R. The sleep/wake state scoring from mandible movement signal. Sleep Breath. 2012;16(2):535–542. doi: 10.1007/s11325-011-0539-4. [DOI] [PubMed] [Google Scholar]
- 22.Senny F, Destiné J, Poirrier R. Midsagittal jaw movements as a sleep/wake marker. IEEE Trans Biomed Eng. 2009;56(2):303–309. doi: 10.1109/TBME.2008.2003264. [DOI] [PubMed] [Google Scholar]
- 23.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 24.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. 1st ed. Westchester: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 25.O'Hare E, Flanagan D, Penzel T, Garcia C, Frohberg D, Heneghan C. A comparison of radio-frequency biomotion sensors and actigraphy versus polysomnography for the assessment of sleep in normal subjects. Sleep Breath. 2015;19(1):91–98. doi: 10.1007/s11325-014-0967-z. [DOI] [PubMed] [Google Scholar]
- 26.Statistical Guidance on Reporting Results from Studies Evaluating Diagnostic Tests. 2007. [2017 Aug 22]. Available from: www.fda.gov/RegulatoryInformation/Guidances/ucm071148.htm. [Google Scholar]
- 27.Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139(6):1514–1527. doi: 10.1378/chest.10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, et al. Standards of Practice Committee. American Academy of Sleep Medicine Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30(4):519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 29.Tryon WW. Issues of validity in actigraphic sleep assessment. Sleep. 2004;27(1):158–165. doi: 10.1093/sleep/27.1.158. [DOI] [PubMed] [Google Scholar]