Abstract
Introduction
The retina may reflect Alzheimer's disease (AD) neuropathological changes and is easily visualized with optical coherence tomography (OCT). Retinal thickness decrease has been correlated to AD, however, without information on amyloid status. We correlated retinal (layer) thickness to AD biomarkers in amyloid-positive early-onset AD (EOAD) patients and amyloid-negative controls.
Methods
We measured macular thickness and peripapillary retinal nerve fiber layer thickness with OCT in 15 EOAD patients and 15 controls and correlated retinal thickness to visual rating scores for atrophy on magnetic resonance imaging.
Results
Total macular thickness correlated to parietal cortical atrophy in both groups (Spearman ρ −0.603, P = .001). Macular and peripapillary retinal nerve fiber layer thicknesses were not significantly decreased in EOAD compared to controls.
Discussion
Retinal thickness does not discriminate EOAD from controls but is correlated to parietal cortical atrophy in both groups. These findings may suggest reflection of cerebral cortical changes in the retina, independent of amyloid.
Keywords: Optical coherence tomography (OCT), Retinal thickness, Biomarker, Alzheimer's disease, Retina, Cortical atrophy
Highlights
-
•
Retinal thickness correlates with parietal cortical atrophy in amyloid-positive early-onset Alzheimer's disease patients and amyloid-negative controls.
-
•
Macular and retinal nerve fiber layer thicknesses do not discriminate amyloid-positive early-onset AD patients from amyloid-negative controls.
-
•
Reflection of cerebral cortical changes may be present in the retina, independent of amyloid.
1. Introduction
With the currently increasing amount of clinical trials for Alzheimer's disease (AD) and its prodromal stage, a patient-friendly and sensitive diagnostic method for an early diagnosis is urgently needed. The retina is embryologically derived from the neural tube, and as a protrusion from the brain, it shares many similarities with brain tissue. Through the pupil, the retina and its neurons are easily examined with optical coherence tomography (OCT) thus serving as a potential noninvasive diagnostic target in neurodegenerative diseases such as AD, Parkinson's disease (PD), and dementia with Lewy bodies [1], [2], [3], [4].
Currently, cortical atrophy on magnetic resonance imaging (MRI), as a biomarker for neurodegeneration, can be assessed with visual rating scores for global cortical atrophy, medial temporal lobe atrophy, and parietal cortical atrophy [5], [6], [7]. Typically, late-onset Alzheimer's disease (LOAD) shows medial temporal lobe atrophy, whereas early-onset Alzheimer's disease (EOAD) shows a diffuse pattern including parietal cortical atrophy [8]. Retinal thickness decrease measured with OCT might serve as a noninvasive proxy of cortical atrophy on MRI. Previous research showed both total macular and peripapillary retinal nerve fiber layer (RNFL) thinning in AD measured with OCT; however, this was not consistent in all studies. In a recent meta-analysis, we found an absolute decrease of peripapillary RNFL of 10 μm and of total macular thickness of 16 μm in AD compared to controls [9].
Similar to AD, glaucoma is a complex neurodegenerative disease and shows considerable overlap of neuroretinal changes with AD [10], [11], [12], [13], [14]. Previous studies did not account for the possible confounding effect of glaucoma on OCT measurements in AD patients, nor did they include established AD biomarkers as part of AD clinical diagnosis (NIA-AA) [10], [11], [15].
The objective of this study was to assess retinal layer thickness in amyloid-positive early-onset AD patients compared to amyloid-negative healthy controls, with the exclusion of glaucoma. In addition, correlation of retinal measures with established biomarkers in AD, such as cortical atrophy on MRI, was assessed.
2. Methods
2.1. Subjects
Fifteen subjects with EOAD and 17 controls (age < 70 years, Mini–Mental State Examination ≥17, thus capable of giving informed consent) were included from the screening program of the Alzheimer Center of the VU University Medical Center embodying the basis of the Alzheimer Dementia Cohort (ADC) [16]. Controls comprised subjects with subjective cognitive decline, defined as subjective cognitive complaints without objective cognitive impairment on neuropsychological assessment, no signs of neurodegeneration on neuroimaging, and absence of amyloid pathology based on cerebrospinal fluid (CSF) and/or amyloid positron emission tomography (PET). Patients and controls underwent a standardized ADC screening program including MMSE, MRI, and lumbar puncture for amyloid-β(1–42), tau181, and phosphorylated tau (pTau) levels. MRI visual rating scores for cortical atrophy were used for medial temporal lobe atrophy (MTA), global cortical atrophy (GCA), and parietal cortical atrophy (PCA) [6], [7]. MRI scans were scored by a blinded rater before a multidisciplinary consensus meeting where a clinical diagnosis was made by consensus. All AD patients fulfilled NIA-AA criteria and had evidence of amyloid pathology in CSF and/or amyloid PET (florbetaben n = 13, florbetapir n = 9) [15]. CSF tau181/amyloid β(1–42) ratio >0.52 was considered indicative for AD [17]. Parametric images of amyloid-PET scans were assessed by experienced raters and visually interpreted as amyloid positive or amyloid negative following FDA guidelines for florbetaben and florbetapir. Exclusion criteria were (ophthalmological) conditions interfering with OCT quality/retinal thickness: severe cataract, age-related macular degeneration, and glaucoma, and neurological or systemic chronic conditions known to interfere with retinal thickness (e.g., multiple sclerosis, PD, diabetes mellitus, rheumatoid arthritis, sarcoidosis, Crohn's disease, and colitis ulcerosa). In addition, we excluded subjects with ischemic stroke and/or mild-to-severe white-matter hyperintensities on MRI, operationalized as a Fazekas score >1 [18].
2.2. Eye examinations
Subjects were included within a year after the ADC screening program and underwent the following eye examinations: best corrected visual acuity, intraocular pressure (IOP) using noncontact tonometry (if IOP > 20 mm Hg, we used contact applanation tonometry), slit-lamp examination of the anterior and posterior segment and fundus photography (Topcon TRC 50DX type IA), Heidelberg Retinal Tomography (HRT) optic nerve head analysis, and Frequency Doubling Technology for visual fields. Tropicamide 0.5% was administrated for pupil dilation to facilitate optimal ophthalmic examination.
We followed the fourth European Glaucoma Guideline criteria: glaucoma was diagnosed when two of the three following measurements were abnormal: ocular pressure (>21 mm Hg), structural glaucomatous changes (examined with HRT using the Moorfields Regression Analysis), and functional changes (examined with Frequency Doubling Technology) [19]. All examinations were interpreted by an ophthalmologist and resident in ophthalmology (F.D.V. and S.F.J.). This study was designed and conducted according to the Declaration of Helsinki, and the study protocol was approved by the Ethical Committee of the VU University Medical Center. All patients gave their written informed consent in the presence of their caregiver.
2.3. Optical coherence tomography
Two protocols for both eyes were performed in each subject with Heidelberg Spectralis Spectral-Domain OCT: (1) central retina (macula) dense horizontal scanning; central 20° × 20° area; 49 B-scans (averaging 16 frames per B-scan); 512 A-scans per B-scan and (2) axonal ring scan around the optic nerve head for RNFL.
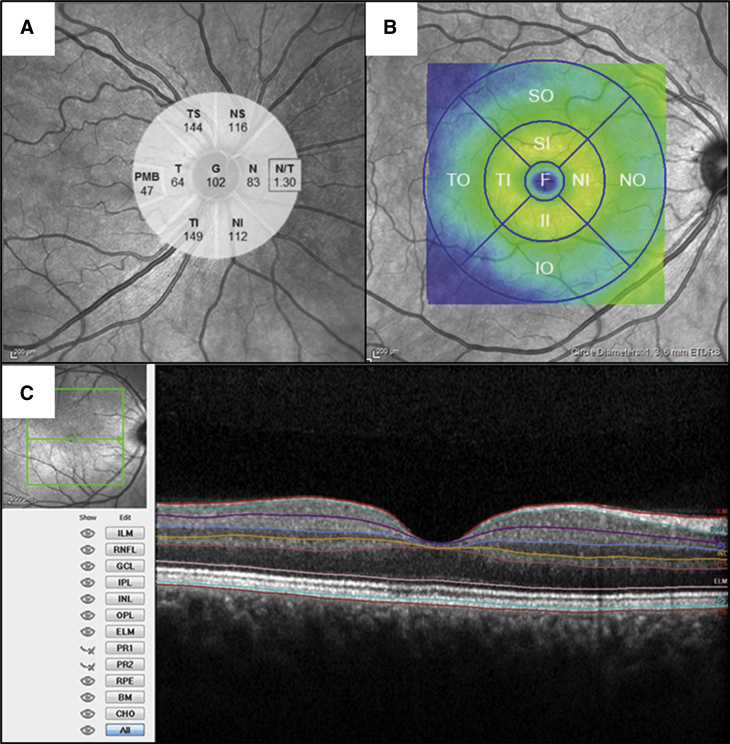
Peripapillary RNFL was measured in six sectors provided by Heidelberg software. Macular thickness was measured in the Early Treatment of Diabetic Retinopathy Study (ETDRS) map (fovea [Ø 1 mm], and the mean of four quadrants of both the inner ring [Ø 3 mm], area 2 to 5, and the outer ring [Ø 6 mm], area 6 to 9) (Fig. 1). In the fovea, the inner and the outer ring segmentation analysis was performed with Heidelberg segmentation software (version 1.9.204.0) to calculate thickness of the following retinal layers: RNFL, ganglion cell layer, inner plexiform layer, inner nuclear layer, outer plexiform layer, outer nuclear layer, and retinal pigment epithelium (Fig. 1).
Fig. 1.
Optical coherence tomography (OCT) images. (A) Around the optic nerve head (peripapillary): retinal nerve fiber layer (RNFL) thickness is measured in the following sectors: temporal superior (TS), nasal superior (NS), nasal (N), nasal inferior (NI), temporal inferior (TI), temporal (T). Mean RNFL thickness (G), nasal/temporal ratio (N/T), and papillomacular bundle (PMB) are also calculated. (B) In the macula, retinal thickness is measured in the fovea (F) (Ø 1 mm), inner ring (Ø 3 mm), and outer ring (Ø 6 mm) following the ETDRS grid. The inner ring and outer ring are subdivided into four sectors (superior, nasal, inferior, and temporal). (C) OCT scan (horizontal B-scan) through the macula shows segmentation of individual retinal layers: RNFL, retinal nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; and RPE, retinal pigment epithelium. Displayed are also ILM, inner limiting membrane; ELM, external limiting membrane; and BM, basal membrane.
2.4. Statistical analysis
2.4.1. Sample size calculation
In our previous meta-analysis with 887 AD patients and 864 controls, we found a mean RNFL difference of 10 μm with a standard deviation of 9 μm [9]. If the true effect is 10 μm with a standard deviation of 9 μm, 13 subjects in each group are needed to reject the null hypothesis of no difference between the AD and control group with a power of 0.80. We therefore included 15 subjects in each group.
2.4.2. Data analysis
Means of retinal layer thickness in both eyes and visual cortical atrophy scores in both hemispheres were calculated. Data were (visually) tested for a normal distribution using histograms and Q-Q plots. We tested measures that were normally distributed with an independent-sample t-test, nonnormally distributed measures with a Mann-Whitney U test, and binary variables with a Fisher exact test. We used Pearson to correlate retinal layer thickness with CSF and MMSE and Spearman's ρ to correlate with MRI visual rating scores. We considered but did not choose to correct with a post hoc Bonferroni correction for multiple testing because of the explorative nature of the study. Data analysis was performed with IBM SPSS Statistics (version 22.0), and GraphPad Prism (version 6.0) was used for graphs.
3. Results
3.1. Baseline characteristics
Characteristics of patients and controls are listed in Table 1. We found no differences for sex, age, BCVA, and IOP. MMSE, atrophy scores on MRI, CSF levels for amyloid β1–42, tau181, and pTau, and amyloid PET were indicative for an AD diagnosis in patients and were normal in controls. Two controls were excluded because of epiretinal membrane in one and glaucoma in the other. None of the other participants fulfilled criteria for glaucoma. Two patients and two controls however could be considered glaucoma suspect due to suspicious but not abnormal optic nerve head topography and subtle visual field defects. Excluding these participants did not alter the results.
Table 1.
Cohort characteristics
| Variables | EOAD | Controls | P-value |
|---|---|---|---|
| Demographics | |||
| Number | 15 | 15 | |
| Sex (m/f) | 8/7 | 8/7 | 1.000∗ |
| Age | 62.20 (±3.67) | 62.00 (±6.27) | .916† |
| MMSE | 21.10 (±3.49) | 28.53 (±1.68) | .000† |
| CSF‡ | |||
| Amyloid1–42 | 527.21 (±110.18) | 1095.27 (±143.17) | .000† |
| Tau181 | 739.21 (±397.64) | 220.27 (±81.35) | .000† |
| pTau | 104.57 (±72.78) | 37.45 (±10.92) | .006† |
| PET§ | |||
| Amyloid PET (n positive/n total) | 7/7 | 0/13 | .000∗ |
| MRI | |||
| MTA | 1.5 (0, 2.5) | 0 (0, 2.0) | .003‖ |
| GCA | 1.0 (0, 2.0) | 0 (0) | .001‖ |
| PCA | 1.5 (0, 2.0) | 0 (0, 1.0) | .002‖ |
| Ophthalmologic | |||
| IOP (mm Hg) | 15.97 (±2.65) | 15.83 (±2.79) | .894† |
| Visual acuity (logmar) | −0.04 (±.06) | −0.04 (±0.11) | .919† |
| HRT: Moorfield | 1.43 (0.95, 2.13) | 1.51 (1.14, 1.89) | 0.106‖ |
| FDT: MD | −1.86 (−20.99, 4.39) | 0.15 (−3.82, 5.74) | 0.021‖ |
| FDT: PSD | 5.05 (2.12, 14.64) | 3.99 (2.74, 6.09) | 0.202‖ |
Abbreviations: AD, Alzheimer's disease; m, male; f, female; MMSE, Mini–Mental State Examination; CSF, cerebrospinal fluid; pTau, phosphorylated tau; MTA, medial temporal lobe atrophy; GCA, global cortical atrophy; PCA, parietal cortical atrophy; IOP, intraocular pressure; HRT, Heidelberg Retinal Tomography; FDT, frequency doubling technology; MD, mean defect; PSD, pattern standard deviation; SCD, subjective cognitive decline.
NOTE. For normal distributed measures, means and standard deviations are shown. For nonnormal distributed measures, median, minimum, and maximum are shown. MMSE, CSF biomarkers, amyloid-PET, and visual rating scores for atrophy (in bold) significantly differed between EOAD and controls as expected. In addition an increased mean defect on visual field testing was observed in EOAD patients.
Fisher exact test.
Independent-sample t-test.
CSF was missing in one AD patient and four controls.
Amyloid PET was performed in 7 AD patients and 13 SCD subjects.
Mann-Whitney U test.
3.2. Retinal (layer) thickness
Peripapillary RNFL (AD 95.9 μm [±9.0], HC 97.5 μm [±6.96], P = .479) and total retinal layer thicknesses in the ETDRS-defined areas (AD 316.1 μm [±11.0], HC 320.5 μm [±7.5], P = .136) were not significantly different between groups (Fig. 2, Table 2). Segmentation of the ETDRS-defined foveal, inner, and outer ring areas showed no significant decrease of the individual retinal layers: RNFL, ganglion cell layer, inner plexiform layer, inner nuclear layer, outer plexiform layer, outer nuclear layer, and retinal pigment epithelium in AD patients (Table 2).
Fig. 2.
Peripapillary RNFL (in μm). Peripapillary retinal nerve fiber layer thickness (RNFL) presented as mean and in six sectors do not significantly differ between AD and controls. Abbreviation: AD, Alzheimer's disease.
Table 2.
Macular thickness: segmentation analysis
| Retinal measure | AD | Controls | P value∗ |
|---|---|---|---|
| Retinal thickness | |||
| Fovea | 280.47 (18.00) | 281.867 (17.19) | .853 |
| Inner ring | 339.63 (11.33) | 343.69 (9.00) | .286 |
| Outer ring | 292.67 (11.46) | 297.30 (8.34) | .216 |
| RNFL | |||
| Fovea | 12.70 (2.07) | 13.17 (1.68) | .503 |
| Inner ring | 21.92 (1.91) | 21.81 (1.73) | .872 |
| Outer ring | 34.17 (3.33) | 35.57 (3.92) | .303 |
| GCL | |||
| Fovea | 15.47 (3.90) | 15.67 (3.36) | .881 |
| Inner ring | 50.73 (2.99) | 51.26 (2.83) | .620 |
| Outer ring | 34.47 (2.16) | 35.05 (2.02) | .456 |
| IPL | |||
| Fovea | 21.67 (3.72) | 22.00 (3.39) | .799 |
| Inner ring | 41.55 (1.90) | 41.88 (2.05) | .648 |
| Outer ring | 28.57 (1.62) | 28.94 (1.47) | .512 |
| INL | |||
| Fovea | 22.33 (5.74) | 21.57 (3.57) | .664 |
| Inner ring | 41.18 (2.87) | 40.91 (2.63) | .786 |
| Outer ring | 32.47 (1.62) | 32.54 (1.56) | .902 |
| OPL | |||
| Fovea | 26.93 (4.66) | 25.87 (3.40) | .480 |
| Inner ring | 32.39 (4.30) | 33.49 (3.30) | .439 |
| Outer ring | 27.28 (1.97) | 27.78 (2.23) | .515 |
| ONL | |||
| Fovea | 94.97 (11.05) | 97.13 (8.48) | .552 |
| Inner ring | 70.21 (8.66) | 72.71 (6.78) | .386 |
| Outer ring | 56.54 (6.81) | 58.68 (5.92) | .367 |
| RPE | |||
| Fovea | 16.50 (1.31) | 16.30 (1.10) | .654 |
| Inner ring | 15.18 (0.60) | 15.58 (1.39) | .323 |
| Outer ring | 13.50 (0.70) | 13.78 (1.30) | .476 |
| Macular volume (mm3) | 8.51 (0.40) | 8.69 (0.21) | .146 |
Abbreviations: RNFL, retinal nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium.
NOTE. Retinal thickness in μm (SD) in the macula of all individual layers and macular volume do not significantly differ between AD and controls.
Independent-sample t-test.
3.3. Correlation with AD biomarkers
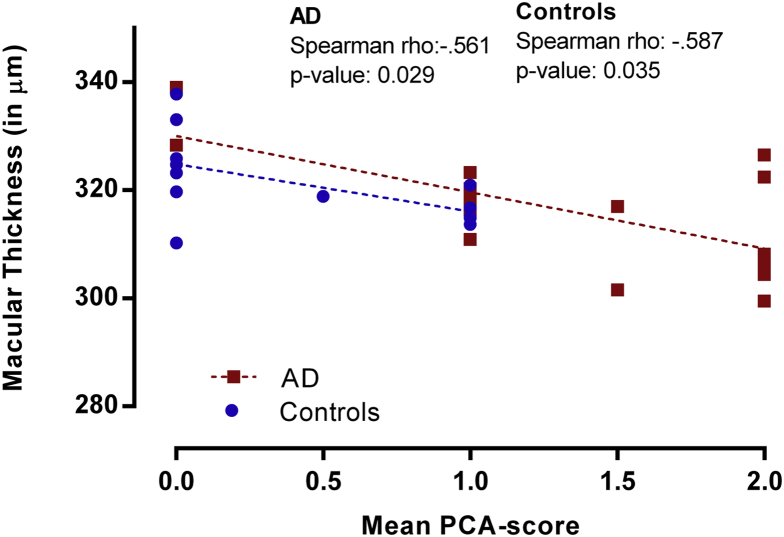
We found a correlation between mean total macular thickness in the inner ring and outer ring with PCA (Spearman's ρ = −0.603, P = .001) and GCA (Spearman's ρ = −0.443, P = .018) on MRI in the total group, but not with MTA (Fig. 3). Correlations with PCA in separate diagnostic groups were comparable (AD: Spearman ρ = −0.561, P = .029, controls: Spearman ρ = −0.587 P = .035). The correlation with GCA in the AD subgroup was significant (Spearman ρ = −0.592, P = .020). All controls were scored GCA 0, and as a result, no correlation could be calculated. Total macular thickness in the fovea, the inner ring, and the outer ring were not correlated with CSF amyloid β1–42, tau181, and p-tau levels. Mean RNFL was not correlated with CSF amyloid β1–42, tau181, and p-tau levels or any of the cortical atrophy scores on MRI. No correlation with MMSE as measure for disease severity was found.
Fig. 3.
Correlation between macular thickness and parietal cortical atrophy (PCA). Scatterplot visualizing the inverse correlation between macular thickness (mean of inner ring and outer ring of ETDRS grid) and mean visual rating score for parietal cortical atrophy on MRI. Abbreviation: AD, Alzheimer's disease; MRI, magnetic resonance imaging.
4. Discussion
We showed that total macular thickness does not differ between AD and controls but does correlate with posterior cortical atrophy in both EOAD and controls, suggesting a relationship between the eye and brain that is independent of amyloid. The PCA visual rating score incorporates atrophy of the posterior cingulate, parietal-occipital sulcus (including the cuneus), and parietal lobe, covering cortices involved in optical processing [7]. Our findings suggest a direct correlation between macular thinning and focal neuronal loss of these cortical areas.
Only few studies described the relation between retinal layers and MRI features in cognitive impairment and/or controls. One study found an association between decreased brain gray-matter volume and disrupted white-matter microstructure and retinal layer thickness that was present only in controls and not in cognitively impaired patients [20]. Others described a relation between medial temporal lobe volume with RNFL thickness and total macular thickness in cognitively healthy [21]. This confirms the relation we found between retinal thinning and cortical atrophy. Remarkably, no study assessed this relationship specifically in a group of AD patients. We added to this literature in relating cortical atrophy measurements of AD patients to retinal thickness, also taking parietal cortical atrophy in account.
In contrast to previous studies, we did not find retinal thickness to be decreased in AD in this clearly defined and biomarker-proven cohort [3], [9], [22]. The absence of retinal thickness thinning in the current EOAD cohort, compared to previous studies with OCT in predominantly LOAD, may be a reflection of differences between EOAD and LOAD. Neuroradiologically, for example, MTA is often more prominent in LOAD compared to EOAD [8]. MTA is however also affected by age and should therefore be taken into account when assessing atrophy [8], [23]. Like MTA, retinal thickness decreases with age, and therefore, retinal thinning may be more prominent in LOAD compared to EOAD [24], [25] explaining the lack of RNFL thinning in this study.
On the other hand, EOAD often shows a diffuse pattern of posterior atrophy on MRI (posterior cingulate cortex, temporo-parietal areas, and precuneus), while hippocampal atrophy may be absent [26], [27]. As these regions contain cortices involved in visual processing, retrograde atrophy of the retina might be expected [28], [29]. Despite the presence of posterior atrophy in our EOAD cohort, no decrease in retinal thickness was observed. Thus, contraintuitively, our data showed no support for retrograde atrophy resulting from atrophy in cortices involved in visual processing in EOAD.
One of the key strengths of this study is the use of amyloid biomarkers. To assess the retinal changes as a result of AD, without the contribution of age-related (neurodegenerative) factors, we deliberately designed this study in an EOAD cohort with confirmed amyloid pathology in CSF and/or amyloid PET. Previous studies with OCT in AD lacking amyloid biomarker conformation might have included a heterogeneous patient cohort with different dementia types. Clinical amyloid-PET studies show that a clinical diagnosis of AD is not confirmed by amyloid PET in 5%–38% of the cases [30], [31], [32], [33] and neuropathological research reported a mismatch of clinical and neuropathological diagnosis of at least 17% [34]. Other studies in neurodegenerative diseases showed that retinal thinning is also present in PD and dementia with Lewy bodies [1], [4], [35]. This stresses the need to use disease-specific biomarkers as part of inclusion criteria to assess cohorts based on their underlying neuropathology.
Another strength is the assessment of glaucoma. Because both AD and glaucoma are neurodegenerative diseases with progressive loss of the neuronal tissue and overlapping pathological molecular pathways, glaucoma should be considered as a possible confounder in the relationship between AD and retinal thickness [10], [11], [12], [13], [14]. We therefore strictly assessed glaucoma with both structural and functional tests and found that visual field defects were more often present in AD. This may be explained by the fact that visual field testing is not only a retinal but also a cognitive task and might thus be challenging for AD patients to perform. Nevertheless, none of the patients fulfilled clinical criteria for glaucoma, ruling it out as a cause of retinal thinning in both the EOAD and control groups.
Limitations of this study are its small number of patients and its cross-sectional design. The presented cohort is powered to detect a true effect of ≥10 μm of mean RNFL thickness as reported in previous studies [9]. To detect a smaller true effect, a larger cohort is needed. Second, comparing retinal thickness decrease over time may be more sensitive for a neurodegenerative disease process, independent of amyloid load, and thus may be suitable as an alternative to MRI for showing progression over time.
Future studies should include more subjects including a LOAD group, longitudinal measurements, and disease-specific imaging aiming to detect retinal amyloid. Correlation with volumetric MRI data (e.g., hippocampus, optical tract, cortical thickness) may add to the understanding of the relation between retinal and cerebral neuronal loss.
5. Conclusion
In this amyloid-proven cohort without glaucoma, retinal layer thickness is correlated to parietal cortical atrophy in both EOAD and controls. This suggests reflections of cortical thickness in the retina. Longitudinal studies including a larger number of EOAD and LOAD patients correlating retinal thickness with biomarkers for amyloid/neuronal injury as well as disease specific biomarkers (e.g., retinal amyloid) are needed to elucidate the possible role of the retina as a source of diagnostic and/or prognostic biomarkers in AD.
Research in Context.
-
1.
Systematic review: Previous studies showed that retinal thinning is present in Alzheimer's disease and may be used as a noninvasive biomarker for neurodegeneration. However, no information on amyloid status to support a clinical Alzheimer's disease diagnosis was present in these studies.
-
2.
Interpretation: Our study shows that retinal thickness does not discriminate amyloid positive early-onset Alzheimer's disease from amyloid negative controls but is correlated to parietal cortical atrophy in both groups. This finding may suggest reflection of cerebral cortical changes in the retina, independent of amyloid.
-
3.
Future directions: Larger and longitudinal cohorts comparing retinal thickness with biomarkers for amyloid and neuronal injury are needed to elucidate the possible role of retinal thickness as a diagnostic and/or prognostic biomarker in (early-onset)Alzheimer's disease.
Acknowledgments
J.d.H. performed statistical analysis. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
The authors have no conflict of interest to report.
References
- 1.Yu J.G., Feng Y.F., Xiang Y., Huang J.H., Savini G., Parisi V. Retinal nerve fiber layer thickness changes in Parkinson disease: a meta-analysis. PLoS One. 2014;9:e85718. doi: 10.1371/journal.pone.0085718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang M., Zhu Y., Shi Z., Li C., Shen Y. Meta-analysis of the relationship of peripheral retinal nerve fiber layer thickness to Alzheimer's disease and mild cognitive impairment. Shanghai Arch Psychiatry. 2015;27:263–279. doi: 10.11919/j.issn.1002-0829.215100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomson K.L., Yeo J.M., Waddell B., Cameron J.R., Pal S. A systematic review and meta-analysis of retinal nerve fiber layer change in dementia, using optical coherence tomography. Alzheimers Dement (Amst) 2015;1:136–143. doi: 10.1016/j.dadm.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreno-Ramos T., Benito-Leon J., Villarejo A., Bermejo-Pareja F. Retinal nerve fiber layer thinning in dementia associated with Parkinson's disease, dementia with Lewy bodies, and Alzheimer's disease. J Alzheimers Dis. 2013;34:659–664. doi: 10.3233/JAD-121975. [DOI] [PubMed] [Google Scholar]
- 5.Frisoni G.B., Fox N.C., Jack C.R., Jr., Scheltens P., Thompson P.M. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol. 2010;6:67–77. doi: 10.1038/nrneurol.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frisoni G.B., Scheltens P., Galluzzi S., Nobili F.M., Fox N.C., Robert P.H. Neuroimaging tools to rate regional atrophy, subcortical cerebrovascular disease, and regional cerebral blood flow and metabolism: consensus paper of the EADC. J Neurol Neurosurg Psychiatry. 2003;74:1371–1381. doi: 10.1136/jnnp.74.10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koedam E.L., Lehmann M., van der Flier W.M., Scheltens P., Pijnenburg Y.A., Fox N. Visual assessment of posterior atrophy development of a MRI rating scale. Eur Radiol. 2011;21:2618–2625. doi: 10.1007/s00330-011-2205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Flier W.M., Pijnenburg Y.A., Fox N.C., Scheltens P. Early-onset versus late-onset Alzheimer's disease: the case of the missing APOE ɛ4 allele. Lancet Neurol. 2011;10:280–288. doi: 10.1016/S1474-4422(10)70306-9. [DOI] [PubMed] [Google Scholar]
- 9.den Haan J., Verbraak F.D., Visser P.J., Bouwman F.H. Retinal thickness in Alzheimer's disease: a systematic review and meta-analysis. Alzheimers Dement (Amst) 2017;6:162–170. doi: 10.1016/j.dadm.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta V., Gupta V.B., Chitranshi N., Gangoda S., Vander Wall R., Abbasi M. One protein, multiple pathologies: multifaceted involvement of amyloid beta in neurodegenerative disorders of the brain and retina. Cell Mol Life Sci. 2016;73:4279–4297. doi: 10.1007/s00018-016-2295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wostyn P., De Groot V., Van Dam D., Audenaert K., Killer H.E., De Deyn P.P. Age-related macular degeneration, glaucoma and Alzheimer's disease: amyloidogenic diseases with the same glymphatic background? Cell Mol Life Sci. 2016;73:4299–4301. doi: 10.1007/s00018-016-2348-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cordeiro M.F. Eyeing the brain. Acta Neuropathol. 2016;132:765–766. doi: 10.1007/s00401-016-1628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis B.M., Crawley L., Pahlitzsch M., Javaid F., Cordeiro M.F. Glaucoma: the retina and beyond. Acta Neuropathol. 2016;132:807–826. doi: 10.1007/s00401-016-1609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janssen S.F., Gorgels T.G., Ramdas W.D., Klaver C.C., van Duijn C.M., Jansonius N.M. The vast complexity of primary open angle glaucoma: disease genes, risks, molecular mechanisms and pathobiology. Prog Retin Eye Res. 2013;37:31–67. doi: 10.1016/j.preteyeres.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 15.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Flier W.M., Pijnenburg Y.A., Prins N., Lemstra A.W., Bouwman F.H., Teunissen C.E. Optimizing patient care and research: the Amsterdam Dementia Cohort. J Alzheimers Dis. 2014;41:313–327. doi: 10.3233/JAD-132306. [DOI] [PubMed] [Google Scholar]
- 17.Duits F.H., Teunissen C.E., Bouwman F.H., Visser P.J., Mattsson N., Zetterberg H. The cerebrospinal fluid “Alzheimer profile”: easily said, but what does it mean? Alzheimers Dement. 2014;10:713–723.e2. doi: 10.1016/j.jalz.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 18.Fazekas F., Chawluk J.B., Alavi A., Hurtig H.I., Zimmerman R.A. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 19.European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th Edition - Chapter 2: Classification and terminology Br J Ophthalmol 2017;101:73-127. [DOI] [PMC free article] [PubMed]
- 20.Liu S., Ong Y.T., Hilal S., Loke Y.M., Wong T.Y., Chen C.L. The association between retinal neuronal layer and brain structure is disrupted in patients with cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2016;54:585–595. doi: 10.3233/JAD-160067. [DOI] [PubMed] [Google Scholar]
- 21.Casaletto K.B., Ward M.E., Baker N.S., Bettcher B.M., Gelfand J.M., Li Y. Retinal thinning is uniquely associated with medial temporal lobe atrophy in neurologically normal older adults. Neurobiol Aging. 2016;51:141–147. doi: 10.1016/j.neurobiolaging.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coppola G., Di Renzo A., Ziccardi L., Martelli F., Fadda A., Manni G. Optical coherence tomography in Alzheimer's disease: a meta-analysis. Eye (Lond) 2015;10:e0134750. doi: 10.1371/journal.pone.0134750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhodius-Meester H.F.M., Benedictus M.R., Wattjes M.P., Barkhof F., Scheltens P., Muller M. MRI visual ratings of brain atrophy and white matter hyperintensities across the spectrum of cognitive decline are differently affected by age and diagnosis. Front Aging Neurosci. 2017;9:117. doi: 10.3389/fnagi.2017.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alasil T., Wang K., Keane P.A., Lee H., Baniasadi N., de Boer J.F. Analysis of normal retinal nerve fiber layer thickness by age, sex, and race using spectral domain optical coherence tomography. J Glaucoma. 2013;22:532–541. doi: 10.1097/IJG.0b013e318255bb4a. [DOI] [PubMed] [Google Scholar]
- 25.Demirkaya N., van Dijk H.W., van Schuppen S.M., Abramoff M.D., Garvin M.K., Sonka M. Effect of age on individual retinal layer thickness in normal eyes as measured with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:4934–4940. doi: 10.1167/iovs.13-11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karas G., Scheltens P., Rombouts S., van Schijndel R., Klein M., Jones B. Precuneus atrophy in early-onset Alzheimer's disease: a morphometric structural MRI study. Neuroradiology. 2007;49:967–976. doi: 10.1007/s00234-007-0269-2. [DOI] [PubMed] [Google Scholar]
- 27.Frisoni G.B., Testa C., Sabattoli F., Beltramello A., Soininen H., Laakso M.P. Structural correlates of early and late onset Alzheimer’s disease: voxel based morphometric study. J Neurol Neurosurg Psychiatry. 2004;76:112–114. doi: 10.1136/jnnp.2003.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prins D, Hanekamp S, Cornelissen FW. Structural brain MRI studies in eye diseases: are they clinically relevant? A review of current findings. [DOI] [PubMed]
- 29.Cowan W.M. Anterograde and retrograde transneuronal degeneration in the central and peripheral nervous system. In: Nauta W.J., Ebbesson S.O., editors. Contemporary Research Methods in Neuroanatomy. Springer Berlin Heidelberg; Berlin, Heidelberg: 1970. pp. 217–251. [Google Scholar]
- 30.Ossenkoppele R., Jansen W.J., Rabinovici G.D., Knol D.L., van der Flier W.M., van Berckel B.N. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA. 2015;313:1939–1949. doi: 10.1001/jama.2015.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zwan M.D., Bouwman F.H., Konijnenburg E., van der Flier W.M., Lammertsma A.A., van Berckel B.N.M. Diagnostic impact of [18F]flutemetamol amyloid imaging in young-onset dementia. Alzheimers Res Ther. 2017;9:2. doi: 10.1186/s13195-016-0228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schipke C.G., Peters O., Heuser I., Grimmer T., Sabbagh M.N., Sabri O. Impact of beta-amyloid-specific florbetaben PET imaging on confidence in early diagnosis of Alzheimer's disease. Dement Geriatr Cogn Disord. 2012;33:416–422. doi: 10.1159/000339367. [DOI] [PubMed] [Google Scholar]
- 33.Grundman M., Pontecorvo M.J., Salloway S.P., Doraiswamy P.M., Fleisher A.S., Sadowsky C.H. Potential impact of amyloid imaging on diagnosis and intended management in patients with progressive cognitive decline. Alzheimer Dis Assoc Disord. 2013;27:4–15. doi: 10.1097/WAD.0b013e318279d02a. [DOI] [PubMed] [Google Scholar]
- 34.Beach T.G., Monsell S.E., Phillips L.E., Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol. 2012;71:266–273. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pillai J.A., Bermel R., Bonner-Jackson A., Rae-Grant A., Fernandez H., Bena J. Retinal nerve fiber layer thinning in Alzheimer's disease: a case-control study in comparison to normal aging, Parkinson's disease, and non-Alzheimer's dementia. Am J Alzheimers Dis Other Demen. 2016;31:430–436. doi: 10.1177/1533317515628053. [DOI] [PMC free article] [PubMed] [Google Scholar]