Abstract
PURPOSE: A better understanding of the molecular basis of urothelial carcinoma (UC) is needed to refine the clinical decision-making process. METHODS AND MATERIALS: We performed next-generation sequencing to investigate the mutational and transcriptional profiles of commonly mutated genes in UC using Ampliseq v2. Copy number variations (CNVs) were detected with nCounter assay. Genetic alterations between upper tract UC (UTUC) and urinary bladder UC (UBUC) were compared. RESULTS: Tumor samples from 31 UTUC and 61 UBUC patients were included in analysis. The two groups showed similar clinicopathologic features including tumor grade and stage. Median survival was longer in UTUC than UBUC patients, though this was statistically nonsignificant (59 vs 41 months, P = .137). In total, we found 982 genetic alterations from 92 samples: single nucleotide variants were the most common type of somatic mutation (479/508, 94.3%). Frequently detected somatic mutations included TP53 (68.5%), KDR (41.3%), and PIK3CA (17.4%). Notably, RB1 mutations were the only mutations significantly different between the UBUC and UTUC groups (19.7% vs. 0%, P = .020). The most common types of CNVs included amplifications (56/62, 90.3%): 17.7% of patients identified amplifications in NOTCH1. We also identified five translocations in the entire study population, including one case with FGFR3-TACC3 (Chr4) fusion. CONCLUSION: Within a small study population, we identified similar genetic alterations in both UTUC and UBUC patients, indicating a basis for similar management strategies.
Introduction
Urothelial carcinoma (UC), a cancer involving the transitional epithelium of the urinary tract, is the seventh most common malignancy in Korea [1]. The majority of UC arises in the urinary bladder (UBUC), whereas only 5% to 10% occurs in the upper urinary tract (UTUC) including the renal pelvis and ureter [2]. Because of the relative rarity of UTUC, clinical decision-making for patients with UTUC is based on treatment data for UBUC [3]. However, prognosis and treatment strategies vary between UTUC and UBUC. UTUC tends to have a poor prognosis with a 5-year cancer-related survival <50% for pT2/pT3 tumors and <10% for pT4 tumors [4]. Although the treatment options for muscle-invasive UBUC have expanded in recent years to include (neo) adjuvant chemotherapy [5], [6], no definitive recommendations exist regarding the use of perioperative chemotherapy in the management of UTUC. In both UTUC and UBUC, cisplatin-containing combination chemotherapy is considered standard treatment for patients with advanced metastatic disease.
There are few data supporting or refuting the assertion that we can apply similar principles to the management of UTUC and UBUC [7], [8]. Although it is recognized that UTUC and UBUC harbor a similar morphology and cytogenetic changes as well as prognostic factors, controversy remains regarding whether UTUC and UBUC have similar biological behavior [9], [10], [11]. Molecular approaches are used extensively to enhance our understanding of cancer biology. The mutation landscape in muscle-invasive bladder cancer from The Cancer Genome Atlas (TCGA) suggests numerous therapeutic opportunities [12]. Gene expression signatures of muscle-invasive UBUC delineate tumor subtypes into luminal and basal types and are associated with efficacy to cisplatin-based chemotherapy [13]. Recently, immune therapy has shown considerable promise for the treatment of invasive UBUC [14]. Emerging immune biology data have revealed an association between response, immune check point inhibition, and survival and have established TCGA subtype and mutation load as potential biomarkers in UC [15], [16]. Molecular studies have demonstrated some biological distinctions between UTUC and UBUC [17], [18].
Understanding of the differences and similarities in the genetic landscapes of UTUC and UBUC is crucial to defining the utility of new diagnostic and treatment strategies. Based on these considerations, this study sought to characterize genetic alterations in UTUC and UBUC in Korean patients via next-generation sequencing (NGS) with Ampliseq.
Material and Methods
Patient Selection
This single-center, biomarker study is a part of the Samsung Medical Center (SMC) Oncology Biomarker study (ClinicalTrials.gov identifier: NCT01831609). We collected tumor samples from samples from 31UTUC and 61 UBUC patients who were referred to our medical oncology department after radical cystectomy or nephroureterectomy between 2012 and 2014. All patients provided written informed consent for the use of tumor tissues as well as their clinical data. This study was performed in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of SMC (Seoul, Korea).
Genomic DNA Extraction
Our dedicated genitourinary pathologist (G.Y.K.) reviewed all pathology specimens to ensure the samples contained >80% tumor cells with <20% necrosis. Genomic DNA was extracted from formalin-fixed, paraffin-embedded (FFPE) samples using a QIAamp DNA Mini Kit (Qiagen, Valencia, CA). After extraction, genomic DNA quality and quantity were analyzed using a NanoDrop 8000 UV-Vis spectrometer (Thermo Scientific Inc., Willington, DE), Qubit 2.0 Fluorometer (Life Technologies Inc., Grand Island, NY), and 2200 TapeStation instrument (Agilent Technologies, Santa Clara, CA). Because this study was retrospective in nature, matched normal tissues were not available.
Sequencing Using an Ion Torrent Ampliseq Cancer Panel V2
We used the Ion Torrent Ampliseq cancer panel v2 to detect frequent somatic mutations that were selected based on a literature review. This panel examines 2855 mutations in 50 commonly mutated oncogenes and tumor suppressor genes (Supplementary Table 1). First, genomic DNA 10 ng from each tumor tissue was used for single-tube, multiplex PCR amplification using the Ion Ampliseq Cancer Primer Pool and the Ion AmpliseqKit reagents (Life Technologies, Carlsbad, CA). The amplicons were then ligated to Ion Xpress Barcode Adapters and purified. Next, massively parallel sequencing was performed on the Ion PGM Sequencing 200 Kit v2 according to the manufacturer's instructions. IonTorrent software was used for automated data analysis. Cluster Kit and TruSeq Rapid SBS Kit (Illumina, San Diego, CA).
Copy Number Variations
For detection of copy number variations (CNVs), nCounter Copy Number Variation CodeSets (NanoString Technologies, Seattle, WA) were used with 300 ng of purified genomic DNA extracted from two to three sections of 4-μm–thick FFPE representative tumor blocks using a QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany). DNA was fragmented via AluI digestion and denatured at 95°C. Fragmented DNA was hybridized with the codeset of 86 genes in the nCounter Cancer CN Assay Kit for 18 hours at 65°C and processed according to the manufacturer's instructions. The nCounter Digital Analyzer counted and tabulated the signals of reporter probes.
Bioinformatic and Statistical Analyses
All synonymous changes were filtered using the Torrent Suite v3.6.0 and the Ion Torrent Variant Caller v3.6 software. We used cutoff values of greater than 6% variant frequency and more than ×100 coverage to detect true mutational changes in accordance with previous reports and our own experience. Variant calls were further analyzed using the ANNOVAR, which included variant filtering and annotation using the Catalogue of Somatic Mutations in Cancer (COSMIC, http://cancer.sanger.ac.uk/cancergenome/projects/cosmic) database, dbSNP build 137, and amino acid change information.
All statistical analyses were performed by the Biostatistics and Clinical Epidemiology Center at our institute. Either the χ2 test or Fisher exact test was used to analyze the differences between the clinicopathologic characteristics of UTUC and UBUC. Overall survival (OS) was defined as the time from surgery until death or the last follow-up visit. Survival estimates were calculated according to the Kaplan-Meier method. A Cox proportional hazards model was used to estimate the hazard ratios for UC survival time under a multivariate model. All P values were two-sided, with P < .05 indicating statistical significance. Statistical analyses were performed using the R for Windows v2.11.1 software (R Core Team, Vienna, Austria; http://www.Rproject.org). We implemented the method found in the R “compound.Cox” package.
Results
Patients and Treatment Characteristics
Baseline characteristics of all patients are listed in Table 1. Median age at the time of surgery of all patients was 65 years (range, 37-83). UC patients were predominantly male (84.8%), but the proportion of female patients was significantly higher in UTUC than in UBUC (29.0% vs 8.2%, P = .02). According to SMC institutional guidelines, radical cystectomy and bilateral lymph node dissection (LND) is the standard treatment for patients with muscle-invasive or locally advanced UBUC. For those with UTUC, unless enlarged lymph nodes are suspected before or during surgery, elective LND is not routinely performed. Therefore, all but one UBUC had undergone LND, whereas it was performed in only 48.4% of UTUC patients. There was significant difference in other clinicopathological features including tumor grade, pT stage, and lymphovascular invasion between UBUC and UTUC.
Table 1.
Baseline Characteristics for All Patients
| All Patients (n = 92) |
UTUC (n = 31) |
UBUC (n = 61) |
||||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Age, years | ||||||
| Median (range) | 65 (37-83) | 65 (50-79) | 65 (37-83) | |||
| Gender | ||||||
| Male | 78 | 84.8 | 22 | 71.0 | 56 | 91.8 |
| Female | 14 | 15.2 | 9 | 29.0 | 5 | 8.2 |
| pT | ||||||
| 1 | 6 | 6.5 | 1 | 3.2 | 5 | 8.2 |
| 2 | 17 | 18.5 | 5 | 16.1 | 12 | 19.7 |
| 3 | 53 | 57.6 | 20 | 64.5 | 33 | 54.1 |
| 4 | 16 | 17.4 | 5 | 16.1 | 11 | 18.0 |
| pN | ||||||
| 0 | 33 | 35.9 | 6 | 19.4 | 27 | 44.3 |
| 1 | 18 | 19.6 | 3 | 9.7 | 15 | 24.6 |
| 2 | 21 | 22.8 | 5 | 16.1 | 16 | 26.2 |
| 3 | 3 | 3.3 | 1 | 3.2 | 2 | 3.3 |
| Not evaluated | 17 | 18.5 | 16 | 51.6 | 1 | 1.6 |
| Grade | ||||||
| 2 | 21 | 22.8 | 10 | 32.3 | 11 | 18.0 |
| 3 | 71 | 77.2 | 21 | 67.7 | 50 | 82.0 |
| Lymphovascular invasion | ||||||
| No | 36 | 39.1 | 14 | 45.2 | 22 | 36.1 |
| Present | 56 | 60.9 | 17 | 54.8 | 39 | 63.9 |
| Perioperative chemotherapy | ||||||
| None | 20 | 21.7 | 10 | 32.3 | 11 | 18.0 |
| Neoadjuvant | 31 | 33.7 | 1 | 3.2 | 30 | 49.2 |
| Adjuvant | 44 | 47.8 | 21 | 67.7 | 23 | 37.7 |
pT, pathological T stage; pN, pathological N stage.
Sixty-eight percent of UTUC patients received adjuvant chemotherapy after surgery. In the UBUC cohort, one-half of patients (49.2%) received neoadjuvant chemotherapy prior to radical cystectomy. In all patients, perioperative chemotherapy was a combination of gemcitabine plus either cisplatin or carboplatin, based on patient renal function.
Survival Outcomes
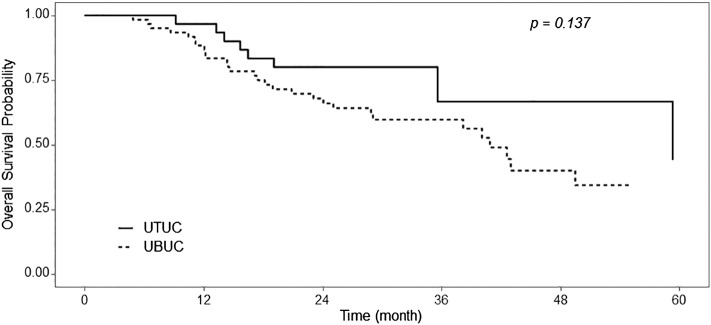
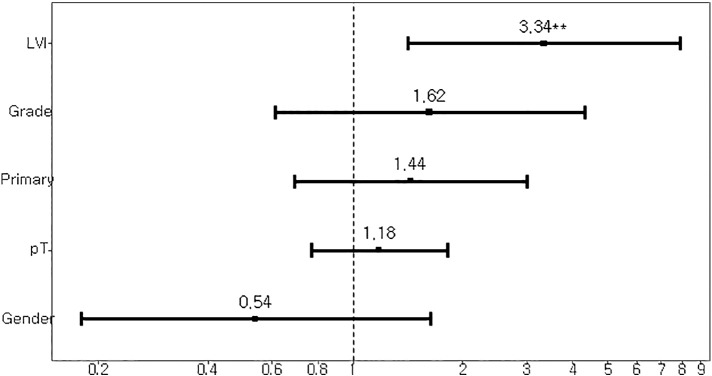
Median follow-up duration was 33 months (range, 28-37). Median overall survival was, although statistically insignificant, longer in the patients with UTUC compared with UBUC (59 months vs 41 months; P = .137; Figure 1). On multivariate analysis, only the presence of lymphovascular invasion was associated with decreased OS (hazard ratio, 3.34; 95% CI, 1.41-7.88; P = .006; Supplementary Figure 1).
Figure 1.
Survival curves according to tumor location: UBUC, dotted line; UTUC, solid line.
Supplementary Figure 1.
Forest plot for overall survival of patients with urothelial carcinoma.
Genomic Alteration Analysis Using the Ampliseq and Copy Number Variations
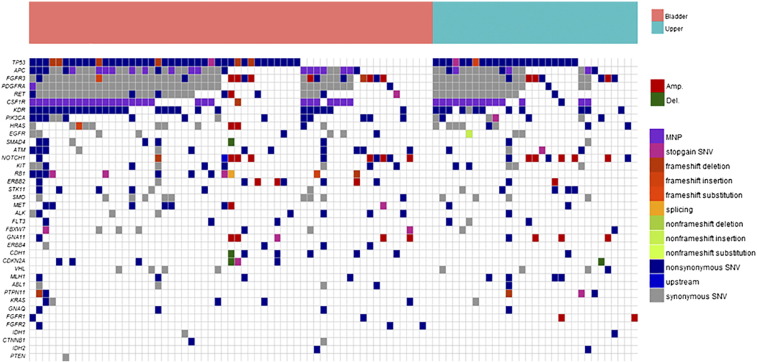
In total, we found 982 genetic alterations from 92 tumor samples (Supplementary Table 2). On average, there were 5.5 somatic mutations and 0.7 genomic copy numbers per sample. Figure 2 describes the landscape of genetic alterations for all patients with UC. Single nucleotide variants were the most common type of somatic mutation (479/508, 94.3%), followed by small insertion-deletions (indels; 29/508, 5.1%). On the other hand, the most common types of CNVs were amplifications (56/62, 90.3%) and deletions (6/62, 9.7%).
Figure 2.
Comparison of the genomic landscape between UBUC and UTUC.
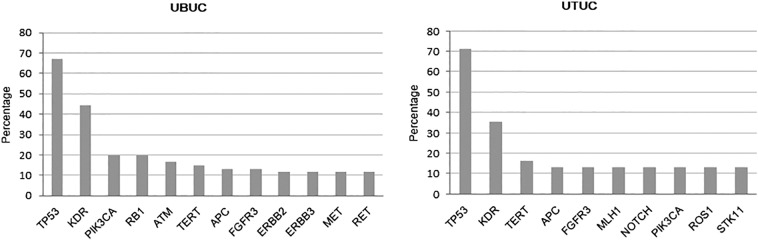
The frequency of mutations was not significantly different between UTUC and UBUC groups (P = .13). The median number of somatic mutations in UBUC and UTUC was 4 (range, 0-25) and 4 (range, 2–20), respectively. The most commonly observed mutation in UBUC patients was TP53 (67.2%) followed by KDR (44.3%), PIK3CA (19.7%), and RB1 (19.7%). In UTUC patients, TP53 (71.0%), KDR (35.5%), and TERT (16.1%) were the most frequently observed somatic mutations. Notably, we detected no RB1 mutations in the UTUC cohort compared with 12.9% frequency in UBUC tumors (P = .020). The most commonly observed CNV in UC patients was NOTCH1 (17.7%), followed by FGFR3 (14.5%) and MDM2 (12.9%). We found that the frequency of CNVs was not significantly different between the UTUC and UBUC groups. (See Fig. 3.)
Figure 3.
Frequency of mutations in UBUC (n = 61) and UTUC (n = 31).
We also identified five translocations in the total UC cohort including one case with FGFR3-TACC3 (Chr4) fusion that was already considered a promising therapeutic target. Other fusions with unknown biological significance included EWSR1-EMID1 (Chr22), ERBB2-PSMD11 (Chr17), ZNF507-AKT2 (Chr19) intrachromosomal translocations, and MLH1-FRY (chr3-chr13) interchromosomal translocations (Table 2).
Table 2.
Translocation Identified in UTUC and UBUC
| No. | Group | Gene A | Gene B | Cover Read A | Chr A | Breakpoint A | Cover Read B | Chr B | Breakpoint B | Direction |
|---|---|---|---|---|---|---|---|---|---|---|
| 7 | UBUC | TACC3 | FGFR3 | 89 | 4p16.3 | 1737129 | 141 | 4p16.3 | 1808709 | B to A |
| 9 | UBUC | EMID1 | EWSR1 | 61 | 22q12.2 | 29639355 | 66 | 22q12.2 | 29695471 | B to A |
| 24 | UBUC | PSMD11 | ERBB2 | 137 | 17q11.2 | 30801183 | 470 | 17q12 | 37855881 | B to A |
| 34 | UTUC | ZNF507 | AKT2 | 64 | 19q13.11 | 32876971 | 119 | 19q13.2 | 40741271 | A to B |
| 39 | UTUC | MLH1 | FRY | 24 | 3p22.2 | 3735154 | 28 | 13q13.1 | 32606865 | A to B |
Discussion
UTUC is a rare subset of UC with a poor prognosis that has not improved in recent decades, as the biological mechanisms of UTUC are still unclear. Whether we can apply similar principles in the treatment of UTUC based on UBUC also remains controversial. In the current study, we revealed that UBUC and UTUC shared common molecular features, while they also have site-specific features. Many observational studies produced conflicting results regarding the significance of anatomical location on the prognosis of UC [19], [20], [21]. In the present study, nonsignificantly longer OS was observed in patients with UTUC compared with UBUC (59 months vs 41 months; P = .137). Also, only the presence of lymphovascular invasion was associated with decreased OS on multivariate analysis (P = .006).
However, it remains unclear whether the clinical behavior of UC originates from innate tumor biology. Recently, Blaveri et al. showed that the fraction genome altered was associated with worse outcome in muscle-invasive UBUC, independent of other clinicopathologic parameters [22]. Based on that study, we speculated that genetic aspects of the disease play an important role in the prognosis of patients with UC. Because of its rarity, comprehensive studies on the molecular basis of UTUC are scarce. Wu et al. identified ALDH2, CCNE1, and SMAD3 as potential prognostic markers in UTUC [23]. Sanford et al. found that UTUC tended to exhibit high expression of genes such as SLITRK6 associated with a luminal subtype [24]. In the current study, the landscape of alterations in UTUC was similar to that of UBUC. Consistent with the genetic alterations by TCGA and others, specific genes including TP53, PIK3CA, and FGFR3 were the main molecular alterations associated with both UBUC and UTUC [12], [17], [18]. TP53 was the most frequently mutated gene (68.5% of all UC cases). On the other hand, FGFR3 mutations (13.0%) occurred less frequently. This variation in genetic alterations can be explained by the higher prevalence of high-grade tumors in this study population. Bakkar et al. demonstrated that FGFR3 mutations were associated with low-stage, low-grade tumors, whereas TP53 mutations were associated with high-stage, high-grade tumors [25]. Surprisingly, KDR mutations were detected in 35.5% of UTUC and 44.3% of UBUC cases in our study. The KDR gene encoding for VEGFR-2 is considered a significant prognostic marker in colorectal carcinoma [26]. However, no prior studies have evaluated the role of KDR mutations in UC. Millis et al. showed that only 2% of UBUC patients harbored KDR mutations [27]. Further study with NGS to analyze both somatic and germline variants in KDR genes is needed. It is noteworthy that the prevalence of mutations differed. The RB1 gene was the only mutated gene in UBUC. Interestingly, RB1 mutations were significantly associated with UBUC. Similarly, comparison of UTUC with UBUC by Sfakianos et al. revealed a lower prevalence of mutations in RB1 [17].
In this study, the most common amplifications were NOTCH1 (17.7%) and FGFR3 (14.5%). The molecular basis for the NOTCH1 amplifications in UC needs to be further elucidated. While FGFR3 has long been considered an attractive therapeutic target in UC, little is known about the role of FGFR1. Tomlinson et al. demonstrated that FGFR1 expression is increased in UBUC tissues and promotes cell proliferation and survival via activation of the MAPK pathway in UBUC cell lines [28]. In the current study, FGFR1 amplification was only observed in the UTUC group (3.2%). Further work will be needed to validate the relative significance of different candidates according to the tumor location and to evaluate their interplay.
Although muscle-invasive UBUCs show many chromosomal rearrangements, the only recurrent gene-gene fusion that has been identified is FGFR3 gene rearrangement [29]; FGFR3 with one of two different fusion partners: TACC3 or BAI1AP2L1 (BAI1-associated protein 2-like 1). Both FGFR3-TACC3 and FGFR3-BAI1AP2L1 translocations generate constitutively activated and oncogenic FGFR3 kinase protein products, and cellular dependence on these drivers confers sensitivity to selective FGFR inhibition [29], [30]. In this study, we identified one case of FGFR3-TACC3 fusion. In addition, we noted the presence of several novel genomic rearrangements including EWSR1-EMID1, ERBB2-PSMD11, SNF507-AKT2, and MLH1-FRY. However, whether this genomic rearrangement produces an in-frame fusion event and the functional significance of these novel rearrangements have yet to be established.
The present study has several limitations. First, the results should be interpreted with caution given the retrospective nature and limited number of samples and mutations analyzed in the current study. Second, accurate identification of somatic mutations was challenged in the absence of normal DNA to distinguish somatic mutations from germline polymorphisms. Third, epigenetic differences and/or differences in gene expression may be more important drivers of disease progression than genomic alterations. Lastly, to extract biologically relevant information from molecular alterations, functional validation is needed.
In conclusion, UC is biologically heterogeneous and has widely variable clinical outcomes and responses to conventional chemotherapy. Our study characterized similarities and differences in the patterns of genetic alteration between UBUC and UTUC. A comprehensive understanding of the biology of UTUC is needed to identify new drug targets in order to improve clinical outcomes.
The following are the supplementary data related to this article.
Genes covered in Ampliseq v2.
Aberrations found in Ampliseq v2.
Disclosure of Potential Conflicts of Interest
There are no conflicts of interest to declare.
References
- 1.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Park EC, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat. 2011;43:1–11. doi: 10.4143/crt.2011.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol. 2000;164:1523–1525. [PubMed] [Google Scholar]
- 3.Roupret M, Zigeuner R, Palou J, Boehle A, Kaasinen E, Sylvester R, Babjuk M, Oosterlinck W. European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. Eur Urol. 2011;59:584–594. doi: 10.1016/j.eururo.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 4.Gandaglia G, Bianchi M, Trinh QD, Becker A, Larouche A, Abdollah F, Roghmann F, Tian Z, Shariat SF, Briganti A. Survival after nephroureterectomy for upper tract urothelial carcinoma: a population-based competing-risks analysis. Int J Urol. 2014;21:249–256. doi: 10.1111/iju.12267. [DOI] [PubMed] [Google Scholar]
- 5.Leow JJ, Martin-Doyle W, Rajagopal PS, Patel CG, Anderson EM, Rothman AT, Cote RJ, Urun Y, Chang SL, Choueiri TK. Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol. 2014;66:42–54. doi: 10.1016/j.eururo.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 6.Sternberg CN, Bellmunt J, Sonpavde G, Siefker-Radtke AO, Stadler WM, Bajorin DF, Dreicer R, George DJ, Milowsky MI, Theodorescu D. ICUD-EAU International Consultation on Bladder Cancer 2012: Chemotherapy for urothelial carcinoma-neoadjuvant and adjuvant settings. Eur Urol. 2013;63:58–66. doi: 10.1016/j.eururo.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Moussa S, Yafi FA, El-Hakim A, Fahmy N, Aprikian A, Tanguay S, Anidjar M, Kassouf W. Outcome of surgical treatment of patients with upper versus lower urinary tract urothelial carcinoma: stage-by-stage comparison. Urol Int. 2010;84:50–55. doi: 10.1159/000273466. [DOI] [PubMed] [Google Scholar]
- 8.Rink M, Ehdaie B, Cha EK, Green DA, Karakiewicz PI, Babjuk M, Margulis V, Raman JD, Svatek RS, Fajkovic H. Stage-specific impact of tumor location on oncologic outcomes in patients with upper and lower tract urothelial carcinoma following radical surgery. Eur Urol. 2012;62:677–684. doi: 10.1016/j.eururo.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Stewart GD, Bariol SV, Grigor KM, Tolley DA, McNeill SA. A comparison of the pathology of transitional cell carcinoma of the bladder and upper urinary tract. BJU Int. 2005;95:791–793. doi: 10.1111/j.1464-410X.2005.05402.x. [DOI] [PubMed] [Google Scholar]
- 10.Yates DR, Catto JW. Distinct patterns and behaviour of urothelial carcinoma with respect to anatomical location: how molecular biomarkers can augment clinico-pathological predictors in upper urinary tract tumours. World J Urol. 2013;31:21–29. doi: 10.1007/s00345-012-0946-6. [DOI] [PubMed] [Google Scholar]
- 11.Lughezzani G, Burger M, Margulis V, Matin SF, Novara G, Roupret M, Shariat SF, Wood CG, Zigeuner R. Prognostic factors in upper urinary tract urothelial carcinomas: a comprehensive review of the current literature. Eur Urol. 2012;62:100–114. doi: 10.1016/j.eururo.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 12.Comprehensive molecular characterization of urothelial bladder carcinomaNature. 2014;507:315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, Roth B, Cheng T, Tran M, Lee IL. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25:152–165. doi: 10.1016/j.ccr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donin NM, Lenis AT, Holden S, Drakaki A, Pantuck A, Belldegrun A, Chamie K. Immunotherapy for the treatment of urothelial carcinoma. J Urol. 2017;197:14–22. doi: 10.1016/j.juro.2016.02.3005. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH, Balmanoukian A, Loriot Y. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J, Perez-Gracia JL. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389:67–76. doi: 10.1016/S0140-6736(16)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sfakianos JP, Cha EK, Iyer G, Scott SN, Zabor EC, Shah RH, Ren Q, Bagrodia A, Kim PH, Hakimi AA. Genomic characterization of upper tract urothelial carcinoma. Eur Urol. 2015;68:970–977. doi: 10.1016/j.eururo.2015.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moss TJ, Qi Y, Xi L, Peng B, Kim TB, Ezzedine NE, Mosqueda ME, Guo CC, Czerniak BA, Ittmann M. Comprehensive genomic characterization of upper tract urothelial carcinoma. Eur Urol. 2017;72:641–649. doi: 10.1016/j.eururo.2017.05.048. [DOI] [PubMed] [Google Scholar]
- 19.Akdogan B, Dogan HS, Eskicorapci SY, Sahin A, Erkan I, Ozen H. Prognostic significance of bladder tumor history and tumor location in upper tract transitional cell carcinoma. J Urol. 2006;176:48–52. doi: 10.1016/S0022-5347(06)00511-8. [DOI] [PubMed] [Google Scholar]
- 20.Catto JW, Yates DR, Rehman I, Azzouzi AR, Patterson J, Sibony M, Cussenot O, Hamdy FC. Behavior of urothelial carcinoma with respect to anatomical location. J Urol. 2007;177:1715–1720. doi: 10.1016/j.juro.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 21.Park S, Hong B, Kim CS, Ahn H. The impact of tumor location on prognosis of transitional cell carcinoma of the upper urinary tract. J Urol. 2004;171:621–625. doi: 10.1097/01.ju.0000107767.56680.f7. [DOI] [PubMed] [Google Scholar]
- 22.Blaveri E, Brewer JL, Roydasgupta R, Fridlyand J, DeVries S, Koppie T, Pejavar S, Mehta K, Carroll P, Simko JP. Bladder cancer stage and outcome by array-based comparative genomic hybridization. Clin Cancer Res. 2005;11:7012–7022. doi: 10.1158/1078-0432.CCR-05-0177. [DOI] [PubMed] [Google Scholar]
- 23.Wu S, Chen J, Dong P, Zhang S, He Y, Sun L, Zhu J, Cheng Y, Li X, Tang A. Global gene expression profiling identifies ALDH2, CCNE1 and SMAD3 as potential prognostic markers in upper tract urothelial carcinoma. BMC Cancer. 2014;14:836. doi: 10.1186/1471-2407-14-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanford T, Porten S, Meng MV. Molecular analysis of upper tract and bladder urothelial carcinoma: results from a microarray comparison. PLoS One. 2015;10 doi: 10.1371/journal.pone.0137141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bakkar AA, Wallerand H, Radvanyi F, Lahaye JB, Pissard S, Lecerf L, Kouyoumdjian JC, Abbou CC, Pairon JC, Jaurand MC. FGFR3 and TP53 gene mutations define two distinct pathways in urothelial cell carcinoma of the bladder. Cancer Res. 2003;63:8108–8112. [PubMed] [Google Scholar]
- 26.Dong G, Guo X, Fu X, Wan S, Zhou F, Myers RE, Bao G, Burkart A, Yang H, Xing J. Potentially functional genetic variants in KDR gene as prognostic markers in patients with resected colorectal cancer. Cancer Sci. 2012;103:561–568. doi: 10.1111/j.1349-7006.2011.02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Millis SZ, Bryant D, Basu G, Bender R, Vranic S, Gatalica Z, Vogelzang NJ. Molecular profiling of infiltrating urothelial carcinoma of bladder and nonbladder origin. Clin Genitourin Cancer. 2015;13:e37–e49. doi: 10.1016/j.clgc.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Tomlinson DC, Lamont FR, Shnyder SD, Knowles MA. Fibroblast growth factor receptor 1 promotes proliferation and survival via activation of the mitogen-activated protein kinase pathway in bladder cancer. Cancer Res. 2009;69:4613–4620. doi: 10.1158/0008-5472.CAN-08-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams SV, Hurst CD, Knowles MA. Oncogenic FGFR3 gene fusions in bladder cancer. Hum Mol Genet. 2013;22:795–803. doi: 10.1093/hmg/dds486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu YM, Su F, Kalyana-Sundaram S, Khazanov N, Ateeq B, Cao X, Lonigro RJ, Vats P, Wang R, Lin SF. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov. 2013;3:636–647. doi: 10.1158/2159-8290.CD-13-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genes covered in Ampliseq v2.
Aberrations found in Ampliseq v2.