Abstract
Background
BMP-5 is expressed in the nervous system throughout development and into adulthood. However its effects on neural tissues are not well defined. BMP-5 is a member of the 60A subgroup of BMPs, other members of which have been shown to stimulate dendritic growth in central and peripheral neurons. We therefore examined the possibility that BMP-5 similarly enhances dendritic growth in cultured sympathetic neurons.
Results
Sympathetic neurons cultured in the absence of serum or glial cells do not form dendrites; however, addition of BMP-5 causes these neurons to extend multiple dendritic processes, which is preceded by an increase in phosphorylation of the Smad-1 transcription factor. The dendrite-promoting activity of BMP-5 is significantly inhibited by the BMP antagonists noggin and follistatin and by a BMPR-IA-Fc chimeric protein. RT-PCR and immunocytochemical analyses indicate that BMP-5 mRNA and protein are expressed in the superior cervical ganglia (SCG) during times of initial growth and rapid expansion of the dendritic arbor.
Conclusions
These data suggest a role for BMP-5 in regulating dendritic growth in sympathetic neurons. The signaling pathway that mediates the dendrite-promoting activity of BMP-5 may involve binding to BMPR-IA and activation of Smad-1, and relative levels of BMP antagonists such as noggin and follistatin may modulate BMP-5 signaling. Since BMP-5 is expressed at relatively high levels not only in the developing but also the adult nervous system, these findings suggest the possibility that BMP-5 regulates dendritic morphology not only in the developing, but also the adult nervous system.
Background
Bone morphogenetic proteins (BMPs) are secreted signaling molecules of the TGF-β superfamily that have been implicated in the control of a host of critical developmental phenomena in the central and peripheral nervous systems [1-3]. BMP-5, one of the more prominently expressed BMPs in the nervous system, has been detected in multiple regions of the nervous system throughout development and into adulthood [3-6], yet its biological activities in the nervous system are not well defined. A role for BMP-5 in dorsal forebrain patterning has been proposed based on its expression in the dorsal midline of the developing forebrain and observations that ectopic expression of BMP-5 in the developing neural tube of chicks markedly downregulates ventral markers while maintaining dorsal markers [5,7]. Further support for BMP-5 regulation of early forebrain development has been provided by studies of Bmp5/Bmp7 double mutants [6]. However, reports that BMP-5 in the mouse brain exhibits peak expression levels in the adult striatum and brainstem and that maximal expression in the hippocampus and cerebellum occurs at E18 through PN1 and again in the mature nervous system [3], suggest additional roles for BMP-5 during later stages of neural development and into adulthood.
BMPs have been divided into subgroups based on structural and evolutionary considerations [8]. Although closely related BMPs have been shown to elicit distinct cellular responses [5,9-13], members within a subgroup often display conservation of not only structure, but also function [4-6,14]. BMP-5 belongs to the 60A subgroup of BMPs, which also includes BMP-6/Vgr-1, BMP-7/OP-1, BMP-8a/OP-2, BMP-8b and Drosophila 60A [3,8]. Other members of the 60A subgroup have been shown to modulate neuronal morphogenesis through selective effects on dendrites. Thus, BMPs 6, 7, and 60A stimulate dendritic growth in cultured sympathetic neurons derived from either perinatal or adult ganglia in the absence of effects on cell survival or axonal growth [15-17]. BMP-7 has also been shown to enhance dendritic growth in hippocampal, cortical and spinal motor neurons [18-20].
Whether BMP-5 similarly promotes dendritic growth has not been previously addressed. Since dendrites are the primary site of synapse formation, we felt it was important to examine this possibility. Moreover, since dendritic remodeling occurs throughout the life of the animal, such studies could suggest a function for BMP-5 in the adult nervous system. In this report, we demonstrate that like other members of the 60A subgroup, BMP-5 triggers robust dendritic growth in sympathetic neurons in vitro coincident with activation of Smad-1. Noggin and follistatin, soluble proteins known to function as physiological antagonists for BMP-7 [21], also inhibit the dendrite-promoting activity of BMP-5. Furthermore, BMP-5 mRNA and protein are detected in intact sympathetic ganglia and neuron/glia cocultures, respectively, consistent with a proposed role for BMP-5 in regulating dendritic growth in sympathetic neurons in vivo.
Results
BMP-5 induces dendritic growth in cultured sympathetic neurons
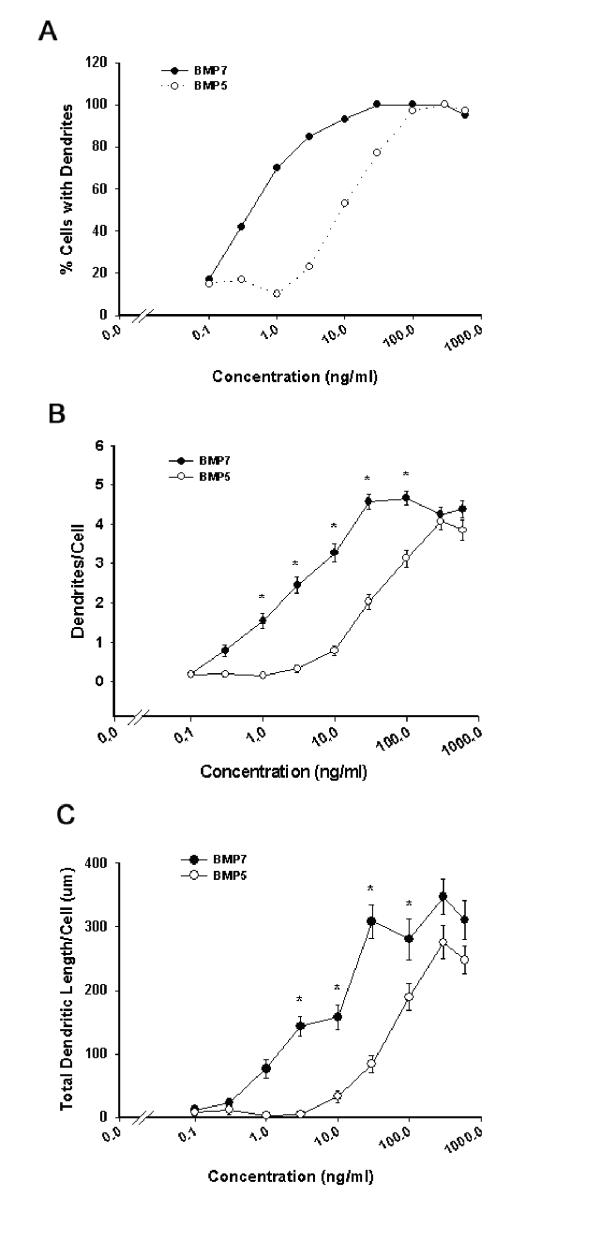
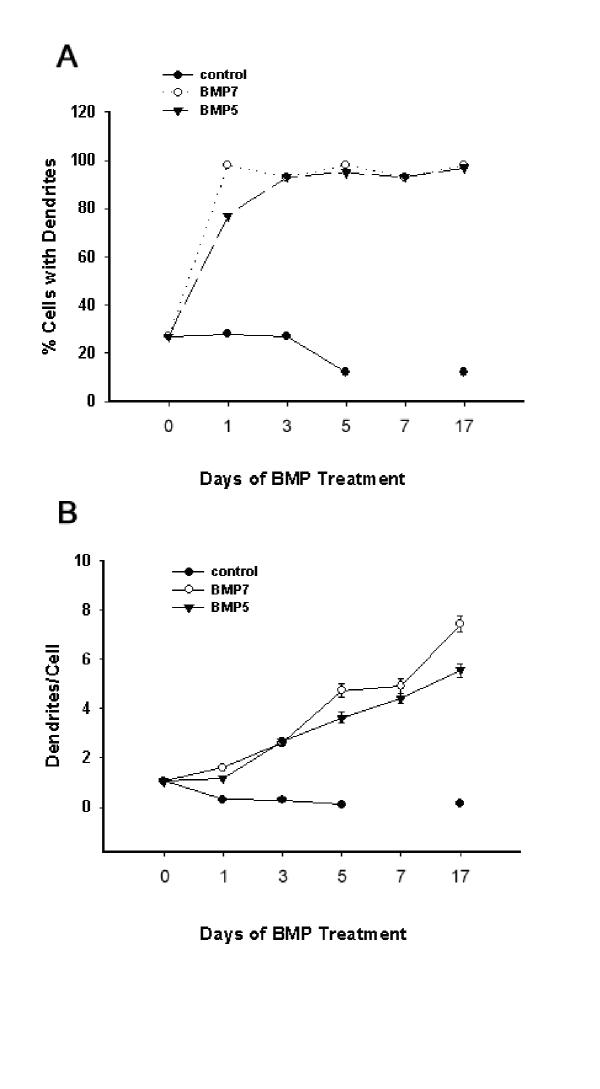
As previously reported [16], sympathetic neurons grown in the absence of serum and glial cells fail to form dendrites (Figure 1A); however when grown in the presence of BMP-7, these neurons extend multiple dendrites (Figure 1B). Similarly, addition of recombinant human BMP-5 to the culture medium elicits dendritic growth in sympathetic neurons as evidenced by the presence of tapered processes that are immunoreactive for MAP2 (Figure 1C). Comparative analyses of concentration-effect relationships for BMP-7 and BMP-5 (Figure 2), indicate EC50 values for BMP-5 approximately 10-fold higher than those of BMP-7; however maximally effective concentrations of these two growth factors have comparable effects on sympathetic neurons as assessed by the percentage of neurons with dendritic growth, the number of dendrites per cell, and the total dendritic length per cell. Time course studies of dendritic growth in neurons exposed to maximally effective concentrations of BMP-5 and BMP-7 reveal comparable temporal responses as well (Figure 3). Both BMP-5 and BMP-7 elicit dendrite formation in 90–100% of the neuronal cell population within 24 to 48 hr (Figure 3A). The gradual increase in the number of dendrites per cell throughout a 17-day treatment with BMP-5 parallels that observed in sister cultures exposed to BMP-7 during the same period (Figure 3B). Experiments in which cultured sympathetic neurons were treated simultaneously with maximally effective concentrations of BMP-5 and BMP-7 indicate that the effects of BMP-5 and BMP-7 on dendritic growth are not additive (data not shown).
Figure 1.
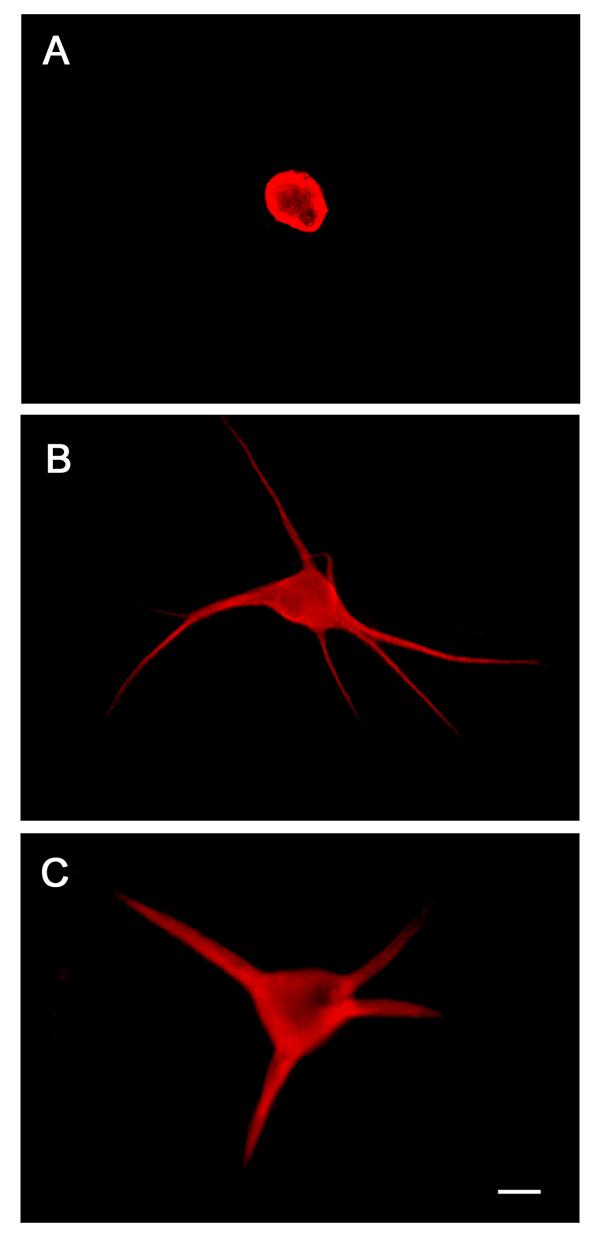
BMP-5 promotes dendritic growth in cultured sympathetic neurons. Non-neuronal cells were eliminated from SCG cultures by treatment with anti-mitotic agent during the 2nd and 3rd days in vitro. Beginning on day 5, sympathetic neurons were treated with either control medium (A), medium supplemented with 50 ng/ml BMP-7 (B), or medium containing 300 ng/ml BMP-5 (C). Six days later, cultures were immunostained with mAb against MAP2, a protein found primarily in dendrites and neuronal somata. Neurons grown under control conditions lack dendrites (A). In contrast, neurons exposed to BMP-7 (B) or BMP-5 (C) typically have several tapered dendrites. Bar, 50 μm.
Figure 2.
BMP-5 is less potent than BMP-7 but equally efficacious in promoting dendritic growth in cultured sympathetic neurons. Beginning on the fifth day in vitro after elimination of glial cells, SCG neurons were exposed to varying concentrations of BMP-5 or BMP-7. After 6 days of exposure to BMPs, cultures were immunostained with a mAb to the dendritic marker MAP2. Dendritic growth was quantified with respect to the percent of cells with dendrites (A), number of dendrites per neuron (B) and total dendritic length (C). Data in panels B and C are presented as the mean ± S.E.M. (n = 60 per experimental condition). * p < 0.05.
Figure 3.
The time course of dendritic growth is comparable between maximally effective concentrations of BMP-5 and BMP-7. Beginning on the fifth day in vitro, SCG neurons grown in the absence of glial cells were treated with control medium or medium supplemented with maximally effective concentrations of BMP-5 (300 ng/ml) or BMP-7 (50 ng/ml). After varying exposure times, cultures were fixed and immunostained with mAb to the dendritic marker MAP2. Dendritic growth was quantified with respect to the percent of cells with dendrites (A) and the number of dendrites per neuron (B). Data in panel B are expressed as the mean ± S.E.M. (n = 60 per experimental condition). There were no significant differences between BMP-7 and BMP-5 at any time point at p < 0.05.
Treatment of sympathetic neurons with BMP-5 induces phosphorylation of Smad1
In most cell systems that have been examined, the primary signal transduction pathway for BMPs involves phosphorylation of Smad-1 [22], and recent evidence suggests that this is also true for BMP-7 induced dendritic growth in sympathetic neurons [23]. To determine if BMP-5 initiates similar signaling events in sympathetic neurons, cultured sympathetic neurons grown in the absence of glial cells were treated with BMP-5 for 30 or 60 min then analyzed by Western blotting using Abs specific for total Smad-1 or for phosphorylated Smad-1. Phosphorylation of Smad-1 is evident within 30 min after exposure to BMP-5 (Figure 4A) with significantly increased pSmad-1 levels apparent at 60 min. In contrast, treatment with BMP-5 did not alter levels of total Smad-1 (Figure 4B).
Figure 4.
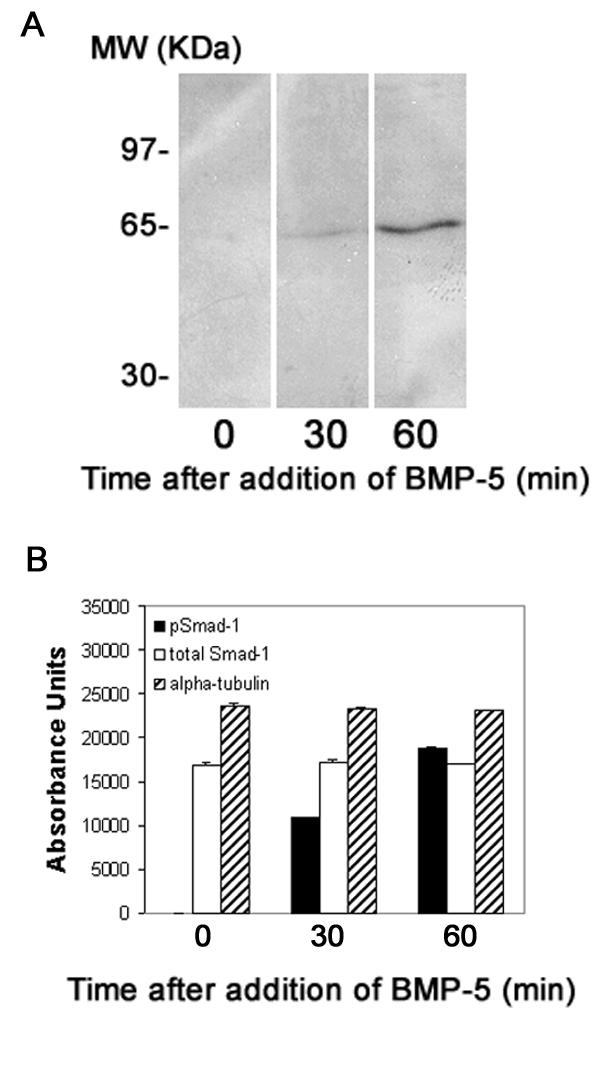
BMP-5 induces phosphorylation of Smad-1 in cultured sympathetic neurons. (A) Blots of cell lysates from SCG cultures consisting solely of neuronal cells were probed using Ab that specifically recognizes the phosphorylated form of Smad-1. Under control conditions (time = 0 min), phosphorylated Smad-1 is not detected. Treatment with BMP-5 (100 ng/ml) causes a time-dependent increase in the band density of phosphorylated Smad-1. (B) Densitometric analyses of Western blots of cell lysates from purified neuronal cell cultures at varying times after exposure to BMP-5. Equal amounts of protein were loaded into all wells and each blot was probed initially for p-Smad, then stripped and successively probed for total Smad and tubulin. Data are presented as the mean ± S.E.M. (n = 2 per experimental condition).
Antagonists of BMP- function inhibit BMP-5-induced dendritic growth
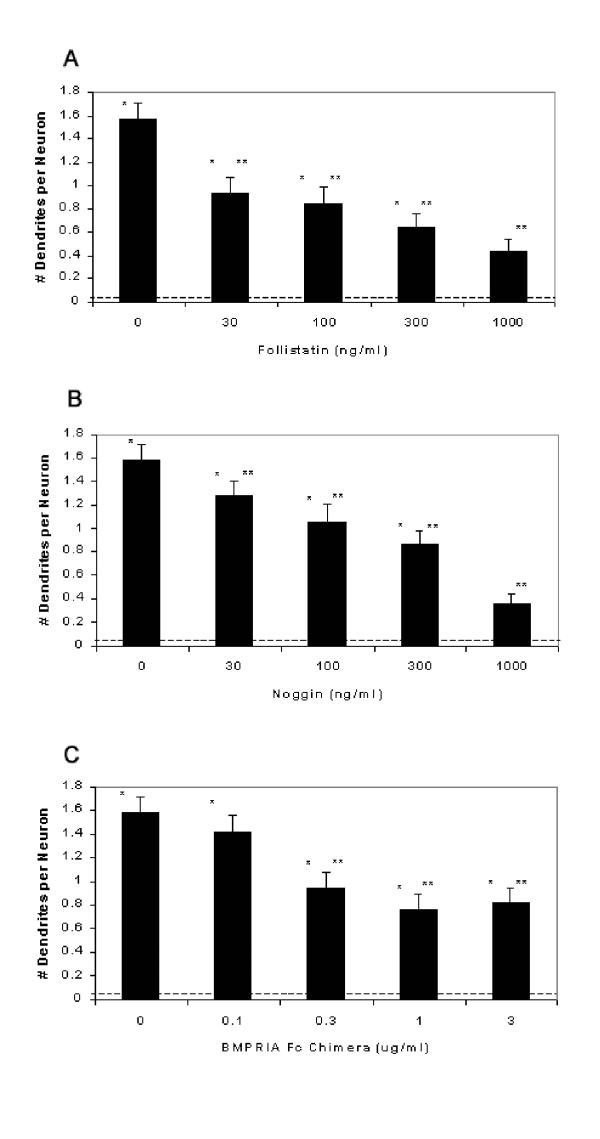
Recent data suggest that in the developing nervous system, signaling by at least BMPs 2, 4 and 7 is determined by the spatiotemporal expression patterns of BMPs and their receptors, and by relative levels of the soluble BMP antagonists such as follistatin and noggin that directly bind BMPs in the extracellular compartment and prevent functional receptor/ligand interaction [1,24-28]. The extent to which these factors may regulate BMP-5 signaling is difficult to determine because of the paucity of data regarding either antagonism of BMP-5 signaling by follistatin and noggin or the interaction between known BMP receptors and BMP-5. To address the former question, we tested the ability of follistatin and noggin to block the dendrite-promoting activity of BMP-5. The latter was evaluated indirectly using a recombinant chimeric protein containing the extracellular domain of the BMP receptor type IA fused to the immunoglobulin Fc domain (BMPR-IA-Fc). The chimeric protein containing BMPR-IA was chosen because it has been previously reported that this is the predominant BMP receptor type expressed in embryonic and postnatal superior cervical ganglia (SCG) [29]. As indicated in Figure 5, follistatin and noggin significantly inhibit the dendrite promoting activity of BMP-5 in a concentration-dependent manner. Maximally effective concentrations of noggin (1000 ng/ml) and follistatin (1000 ng/ml) reduce BMP-5-induced dendritic growth by 77% and 72% respectively. The BMPR-IA-Fc chimera also significantly inhibited BMP-5-induced dendritic growth. Increasing the concentration of any of these antagonists to levels greater than those expressed in Figure 5 did not cause greater inhibition of BMP-5-induced dendritic growth (data not shown).
Figure 5.
Soluble BMP antagonists inhibit BMP-5-induced dendritic growth. Beginning on the fifth day in vitro, SCG neurons were exposed to BMP-5 (100 ng/ml) in the absence or presence of varying concentrations of follistatin (A), noggin (B) or the BMP-RIA-Fc chimera (C). On the tenth day in vitro, cultures were immunostained with mAb to nonphosphorylated forms of the M and H neurofilaments to visualize dendrites. Dendritic growth was quantified with respect to the number of dendrites per neuron expressed as the mean ± S.E.M. (n = 50 per experimental condition). * Indicates a significant difference from negative control values (- BMP-5/- antagonist) indicated by the dashed line in each bar graph at p < 0.01 and ** from positive control values (+ BMP-5/- antagonist) at p < 0.01.
Expression of BMP-5 mRNA and protein in SCG cells
If BMP-5 is a physiological regulator of dendritic morphogenesis in sympathetic neurons, then its spatiotemporal patterns of expression should be coincident with dendritic growth in this neuronal cell type. Potential sources of BMP-5 include neuronal and glial cells within the ganglia. To examine this possibility, antibody (Ab) specific for BMP-5 was used to immunostain cocultures of SCG neurons and glial cell that had been cultured for 2 weeks to allow for significant expansion of the glial cell population. The specificity of the BMP-5 Ab used in these experiments is indicated by demonstrations that it recognizes purified recombinant BMP-5, but not purified recombinant BMP-6 or BMP-7 in Western blots (Figure 6). Moreover, the reaction with BMP-5 protein is significantly blocked by preincubation of the BMP-5 Ab with blocking peptide (Figure 6). In SCG cultures immunostained with this BMP-5 Ab, immunoreactivity is present in most if not all ganglionic glial and neuronal cells (Figure 7A and 7B). In glial cells, fluorescence is present throughout the cell but excluded from the nucleus; in neurons, immunoreactivity is evident in both the soma and processes. Preincubation of the BMP-5 Ab with specific blocking peptide (Figure 7C and 7D) reduced BMP-5 immunoreactivity to levels comparable to those observed in control cultures reacted with the secondary Ab only (Figure 7E and 7F).
Figure 6.
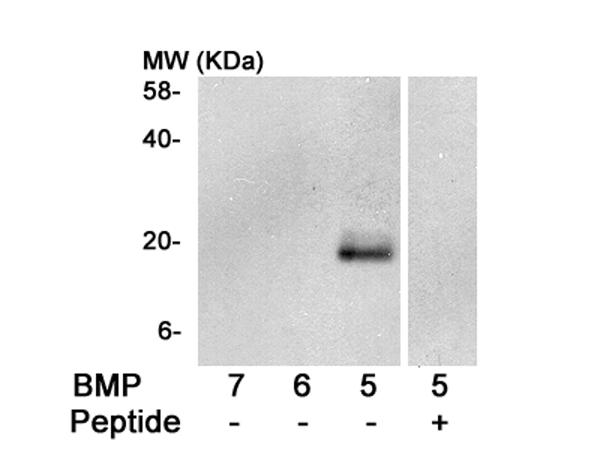
The specificity of the BMP-5 Ab used in these studies as assessed by Western blot analysis. BMP-5 Ab (0.5 μg/ml) reacts with purified recombinant BMP-5 (50 ng), but not with equal amounts of purified recombinant BMP-6 or -7. Preincubation of BMP-5 Ab with specific blocking peptide (2.5 μg/ml) significantly reduces binding to BMP-5 protein. Data represented in this figure were obtained from the same immunoblot.
Figure 7.
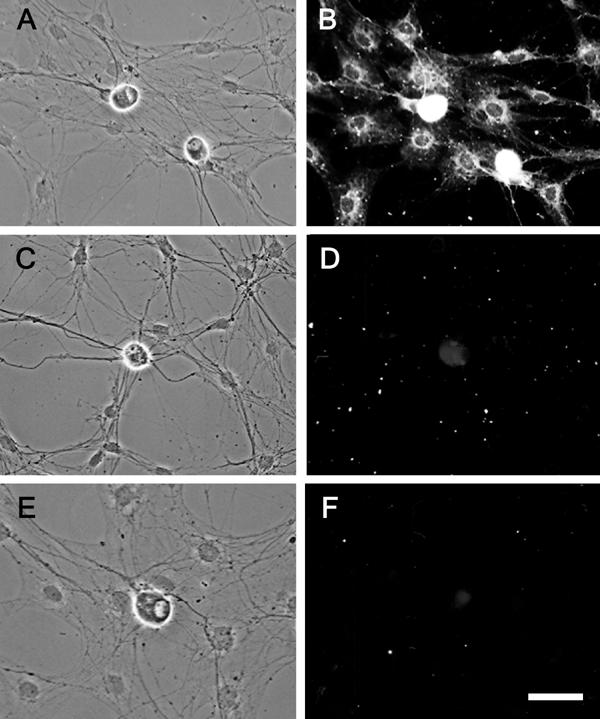
BMP-5 immunoreactivity is detected in co-cultures of SCG neurons and glia. Phase contrast (A,C,E) and fluorescence (B,D,F) micrographs of SCG cultures immunostained for BMP-5 after 10 days in vitro. Both glial cells and neurons express significant BMP-5 immuno-reactivity, and in neurons, the processes as well as the soma are brightly stained (A,B). Preincubation of BMP-5 Ab with blocking peptide prior to reaction with SCG cultures (C,D) reduces the intensity of immunostaining to levels comparable to the background fluorescence observed in SCG cultures reacted only with secondary Ab (E,F).
BMP-5 expression in vivo was assessed by RT-PCR analyses of SCG harvested from rats of different ages corresponding to various stages of dendritic growth in sympathetic neurons. As indicated in Figure 8, BMP-5 mRNA is present in SCG at embryonic day 20 (E20), which corresponds to the period of initial extension of primary dendrites, and at postnatal days 3 and 7 (PN3 and PN7), which corresponds to the period of maximal dendritic growth. In contrast, BMP-5 mRNA was not detected in adult SCG.
Figure 8.
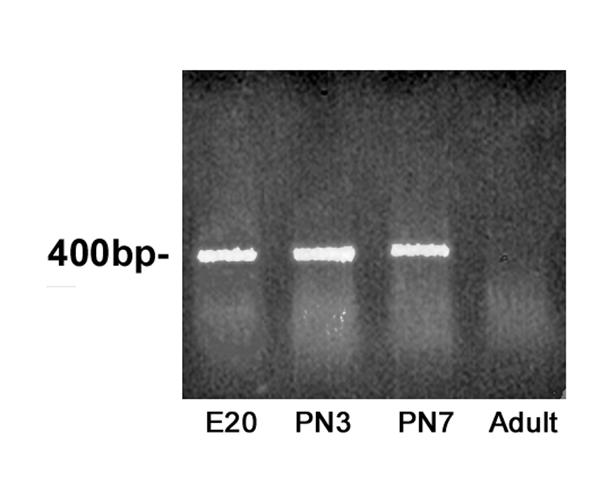
BMP-5 mRNA is expressed in SCG at times corresponding to maximal dendritic growth in SCG neurons. BMP-5 mRNA was detected by RT-PCR in total RNA extracted from rats SCG at E20, PN3 and PN7, which correspond to times of initial dendrite extension (E20) and maximal expansion of the dendritic arbor (PN3 and PN7). In contrast, PCR product was not detected in equal amounts of total RNA isolated from adult SCG.
Discussion
BMP-5 is widely expressed in the nervous system throughout development and into adulthood [3-6], yet the only function described for this growth factor thus far is dorsal patterning of the developing forebrain [5-7]. Our data suggest that BMP-5 may also regulate later stages of neural development, specifically dendritic morphogenesis. The most direct support for this hypothesis is the finding that addition of BMP-5 to sympathetic neurons in culture causes these cells to extend multiple dendritic processes. These data are consistent with conclusions from previous studies indicating that dendrite-promoting activity is restricted to BMPs from the 60A or dpp (BMPs 2 and 4) subgroups and is not observed with BMPs from other subgroups such as BMP-3, BMP-13 or dorsalin, or with other members of the TGF-β superfamily such as activin, TGF-β1, -β2 or -β3 [16,17]. Functional redundancy between BMPs of the 60A subgroup has been previously reported with respect to other developmental endpoints [6], of which some, such as upregulation of cell adhesion molecules [30], may be directly relevant to effects on dendritic growth. BMP-6 and BMP-7 as well as dpp subgroup members, BMP-2 and BMP-4, have been shown to influence other aspects of sympathetic neuron development, such as differentiation of adrenergic sympathetic neurons from neural crest [13,31-38] and neuropeptide phenotype [39]. It will be of interest to determine if BMP-5 also exhibits functional redundancy with respect to these effects.
Pharmacological studies of BMP-5 indicate that relative to BMP-7, BMP-5 is less potent but equally efficacious in promoting dendritic growth in cultured sympathetic neurons. These data, together with observations that maximally effective concentrations of BMP-5 and BMP-7 are not additive, suggest that the two ligands share aspects of a common signaling pathway. It has been shown that phosphorylation of Smad-1 precedes dendritic extension in cultured sympathetic neurons exposed to BMP-7; moreover, expression of a dominant negative construct of Smad-1 in cultured sympathetic neurons significantly inhibits BMP-7-induced dendritic growth in these neurons [23]. These data suggest that activation of Smad-1 is a necessary component of the signal transduction pathway by which BMP-7 induces dendritic growth. In this report we demonstrate that BMP-5 similarly induces Smad-1 phosphorylation in sympathetic neurons as detected by Western blot analyses using antibodies specific for the phosphorylated form of Smad-1. These data suggest conserved mechanisms of signaling within the 60A subgroup. The molecular mechanism(s) of BMP-induced dendritic growth downstream of Smad-1 activation are not well characterized. Previous studies have demonstrated that transcriptional and translational events are required for dendritic growth in response to BMPs [15], but the gene expression profile responsible for BMP-induced dendritic growth has yet to be determined. Thus, it is not clear if BMP-5 or -7 induces dendritic growth directly, or if some other factor made by the cells in response to BMPs is responsible for initiating dendritic growth.
BMPs activate Smad-1 by binding to type I and type II serine-threonine kinase receptors [40,41]. Specific receptor subunits shown to bind BMPs include BMP receptor type IA (BMPR-IA), BMPR-IB, BMR-II, activin receptor type I (ActR-I), and ActR-II [42-44]. BMP ligands can bind to either type I or type II receptor subunits independently, but both receptor types are required for high-affinity binding and signaling [40]. The finding that the soluble BMPR-IA-Fc chimera significantly inhibits BMP-5 induced dendritic growth suggests that BMP-5 may be activating the Smad-1 signaling pathway via interactions with BMPR-IA. Although the physiological relevance of this finding has yet to be confirmed by ligand binding studies using endogenous neuronal BMPR-IA, these data are consistent with reports that BMPR-IA is the predominant BMP receptor type expressed in embryonic and postnatal superior cervical ganglia (SCG) [29].
It is becoming increasingly evident that BMP signaling is regulated not only by the spatiotemporal expression of BMP ligands and receptors, but also by relative levels of soluble BMP antagonists, which directly bind BMPs and prevent functional receptor/ligand interaction [1,24-28]. The different BMP antagonists bind to BMPs and other TGF-β family members with varying degrees of specificity. For example, follistatin binds both activin and BMP-7 avidly but competes weakly or not at all with the type I receptor for BMP-4 binding [25,45], whereas noggin binds to BMPs -2 and -4 with greater affinity than BMP-7 [46]. Whether BMP-5 function can be antagonized by noggin or follistatin has not been previously reported, but our results suggest that simultaneous addition of either antagonist with BMP-5 significantly inhibits the dendrite-promoting activity of BMP-5 in a concentration-dependent manner. Thus, profiling the BMP binding affinities as well as the expression patterns of these BMP antagonists will be critical to understanding the regulation of BMP-5 signaling in the nervous system.
If BMP-5 is important in regulating dendritic growth in intact ganglia, its expression should correlate with periods of dendritic growth in vivo. In sympathetic ganglia, dendritic growth begins prenatally and maximal expansion of the dendritic arbor occurs during postnatal weeks 1 and 2 [47,48]. RT-PCR analyses of intact SCG indicate that cells of the SCG express BMP-5 transcripts from E20 through P7. Preliminary observations indicate that these BMP-5 transcripts are translated into protein in SCG in vivo as assessed by Western blot analyses using BMP-5 Ab (P. Lein, unpublished observations). Earlier studies have demonstrated the expression of BMP-4 transcripts in developing avian sympathetic ganglia [49], suggesting the presence of multiple BMPs in sympathetic ganglia throughout development. These data, in conjunction with observations that mRNA for BMP type IA and type II receptors are expressed in the developing sympathetic ganglia [29], are consistent with a potential role for BMP-5 in regulating the initiation and rapid expansion of the dendritic arbor in sympathetic ganglia of perinatal animals.
The cellular distribution of BMP-5 was determined by immunocytochemistry in sympathetic neurons cocultured with ganglionic glial cells. Both neurons and glial cells express BMP-5 protein. In situ hybridization analyses of BMP-6 and BMP-7 indicate that both cell types also express BMP transcripts (P. Lein, unpublished observations). These findings are consistent with previous reports that dendritic growth can be induced in sympathetic neurons in vitro when cultured at high neuronal cell density [50] or in the presence of ganglionic glial cells [51].
Dendritic growth continues, albeit to a lesser extent, into adulthood, and dendritic remodeling occurs throughout the animal's life. Mature sympathetic neurons cultured from adult animals respond to BMP-7 with enhanced dendritic growth [15], and treatment with BMP-7 enhances recovery in animal models of stroke [52-56]. Although BMP-5 mRNA was not detected in adult SCG, Western blot analyses indicate that BMP-5 protein is present in adult SCG (P. Lein, unpublished observations), presumably derived from nonganglionic sources such as serum or target tissues. These observations together with reports that BMP-5 is expressed at significant levels in the adult nervous system [3] suggest a potential role for BMP-5 in modulating dendritic morphology not only during development, but also in adult nervous systems.
Conclusions
These data suggest that BMP-5 regulates dendritic growth. Addition of BMP-5 to sympathetic neurons in culture triggers significant dendritic growth that is concentration-dependent. Data from western blot analyses using Ab specific for phosphorylated epitopes of Smad-1 as well as analyses of dendritic growth in cultures exposed to BMP-5 in the presence of a soluble BMPR-IA-Fc chimeric protein are consistent with a signaling pathway that involves binding to the BMPR-IA and activation of Smad-1. BMP-5 signaling may be modulated by noggin and follistatin since these BMP antagonists were observed to inhibit the dendrite-promoting activity of BMP-5. Spatiotemporal patterns of BMP-5 expression at the mRNA level, as assessed by RT-PCR, and the protein level, as determined by immunocytochemistry correspond to periods of initial dendritic growth and rapid expansion of the dendritic arbor. These observations, together with previously published reports from other laboratories indicating significant levels of BMP-5 expression in the developing and adult nervous system [3] suggest a potential role for BMP-5 in modulating dendritic morphology not only during development, but also in adult nervous systems.
Materials and Methods
Materials
Purified human recombinant BMPs (5, 6 and 7) were prepared using previously published methods [57] and provided by Creative Biomolecules (Hopkinton, MA). Affinity-purified polyclonal antibody (Ab) specific for BMP-5, the blocking peptide for the BMP-5 Ab, and the recombinant human BMP-RIA-Fc chimera were purchased from Research Diagnostics (Flanders, NJ). Ab specific for the phosphorylated form of Smad-1 (Ser 463/465) as well as Ab that recognizes both phosphorylated and nonphosphorylated Smad-1 (e.g., total Smad-1) was purchased from Upstate Biological (Lake Placid, NY). Xenopus noggin protein [58] was the generous gift of Drs. Josè de Jesús and Richard Harland (UC at Berkeley). Recombinant human follistatin (B4384) was obtained through Dr. A.F. Parlow at the NHPP, NIDDK (Torrance, CA).
Tissue culture
Sympathetic neurons were dissociated from the SCG of perinatal (E21 to PN1) Holtzmann rats (Harlan Sprague-Dawley, Rockford, IL) according to previously described methods [59]. Cells were plated onto glass coverslips (for immunocytochemical and morphological studies) or 35 mm plastic culture dishes (for Western blot analyses) precoated with 100 μg/ml poly-D-lysine (Sigma, St. Louis, MO). Cultures were maintained in serum-free medium supplemented with β-NGF (100 ng/ml), bovine serum albumin (500 μg/ml), insulin (10 μg/ml), and transferrin (20 μg/ml). In most experiments, endogenous non-neuronal cells were eliminated from cultures by adding cytosine-β-D-arabinofuranoside (Sigma, St. Louis, MO) to the culture medium at 1 μM for 48 hr beginning on day 2. In some experiments this antimitotic was not added to cultures but rather endogenous non-neuronal cells were allowed to proliferate. Previous studies have demonstrated that under these culture conditions, the non-neuronal cells are primarily ganglionic glia [51].
Morphological analyses
Dendritic growth was assessed in cultures immunostained with monoclonal antibodies (mAb) previously shown to react selectively with the somatodendritic compartment of cultured sympathetic neurons [16]. These mAb's include SMI-52, which is specific for the cytoskeletal protein MAP2, and SMI-32, which reacts with the non-phosphorylated forms of the M and H neurofilament subunits (Sternberger Immunocytochemicals, Baltimore, MD). Antigens were localized by indirect immunofluorescence as previously described [16]. Dendritic growth was quantified using SPOT image analysis system. Data in the text are presented as the mean ± S.E.M. and statistical significance was determined using ANOVA followed by Tukey's test.
Western blot analyses
Western blot analyses were performed on purified recombinant BMPs to assess the specificity of the BMP-5 Ab, and on cell lysates of cultured sympathetic neurons to assess the effects of BMP-5 on phosphorylation of Smad1 as well as levels of total Smad (nonphosphorylated and phosphorylated). Cell lysates were obtained by rinsing 12-day old neuronal cell cultures with ice-cold phosphate-buffered saline prior to trituration in ice-cold lysis buffer (PBS supplemented with 1% Igepal, 0.5% sodium deoxycholate, 0.1% SDS, 100 μg/ml PMSF and 300 μg/ml aprotinin). Cell lysates were microfuged at maximum speed for 5 min and the resultant supernatant collected. Protein concentration was determined using the Bradford assay (BioRad, Hercules, CA). Samples containing equivalent amounts of protein were resolved by 12% polyacrylamide SDS PAGE under reducing conditions and then electroblotted onto PVDF membranes. Blots were blocked at room temperature for 1 hour in TBS-T (10 mM Tris, pH 8.0, 150 mM NaCl, 0.1% Tween-20) containing 5% dried fat-free milk, then incubated overnight at 4°C in TBS-T containing 0.5% milk and primary Ab (0.5 μg/ml for BMP-5 Ab; 10 μg/ml for Smad-1 Ab). Blots were washed twice with TBS-T containing 0.5% milk, then incubated at room temperature for 2 hours in TBS-T containing 0.5% milk and 1:5000 dilutions of secondary Ab conjugated to peroxidase (for BMP-5, anti-goat IgG-peroxidase from Chemicon, Temecula CA; for Smad1, anti-rabbit Ig-peroxidase from Amersham, Piscataway, NJ). Subsequently, blots were washed three times as described above, and visualized using an enhanced chemiluminescence detection method (ECL, Amersham). Blots of cell lysates were stripped and reprobed using antibodies specific for α-tubulin (1:10,000, Sigma). To quantify data, films were scanned using an HP ScanJet ADF scanner and HP Precision ScanPro software, and band density determined as arbitrary absorption units using the MacBas software program (version 2.31, Fuji Film).
Immunocytochemistry
BMP-5 Ab was used to localize BMP-5 protein in SCG cultures containing sympathetic neurons and ganglionic glial cells. After 2 weeks in culture, cells were fixed in 4% paraformaldehyde, permeabilized with methanol at -20°C (Sigma, St. Louis, MO), and then reacted with anti-BMP-5 Ab (10 μg/ml). Immunoreactivity was visualized by indirect immunofluorescence as previously described [16]. The specificity of the immunoreaction was determined by preincubating the BMP-5 Ab with its specific blocking peptide (100 μg/ml) prior to reaction with the cultures.
RNA Isolation and Analyses
Total RNA was extracted from freshly harvested superior cervical ganglia (SCG) using Trizol (Life Technology, Carlsbad, CA). RNA samples (5 μg) were reverse transcribed using random primers at annealing temperatures of 65°C (You-Prime-the-First-Strand kit, Amersham, Piscataway, NJ). Resultant cDNA was amplified by PCR for 35 cycles using an annealing temperature of 55°C for 30 sec and denaturing temperature of 95°C for 30 sec; the Mg++ concentration in these reactions was 1.5 mM. As a negative control, each sample was run through PCR without prior reverse-transcription. Primers used for amplification of BMP-5 cDNA were designed to unique sequences of rat BMP-5 using the Primer3 program http://www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi. The specific primer sequences were BMP-5 sense, 5'-TTATGCAAAAGGAGGCTTGG-3' and BMP-5 antisense, 5'-TCATGACCATGTCAGCATCA-3'. After synthesis, PCR products were subjected to 1% agarose gel electrophoresis and found to have the expected size of 420 base pairs.
List of abbreviations
Ab, polyclonal antibody
BMP, bone morphogenetic protein
BMPR-IA, BMP receptor type IA
E20 or E21, embryonic day 20 or 21
mAb, monoclonal antibody
PN1, PN3 or PN7, postnatal day 1, 3 or 7
SCG, superior cervical ganglia
Acknowledgments
Acknowledgements
We thank Creative Biomolecules (Hopkinton, MA), now known as Curis (Cambridge, MA), for supplying the purified recombinant BMPs. This work was supported by a Johns Hopkins University School of Public Health Faculty Innovation Award (PJL), NSF grant #IBN 01–21210 (DH) and NIH grants HL54659 (DBJ) and HL61013 (DBJ).
Contributor Information
Hiroko N Beck, Email: hbeck@jhsph.edu.
Karen Drahushuk, Email: drahushu@buffalo.edu.
David B Jacoby, Email: djacoby@jhmi.edu.
Dennis Higgins, Email: higginsd@acsu.buffalo.edu.
Pamela J Lein, Email: plein@jhsph.edu.
References
- Lein P, Drahushak K, Higgins D. Effects of Bone Morphogenetic Proteins on Neural Tissue. The Biology of Bone Morphogenetic Proteins (Edited by Vukicevic S) Switzerland: Birkhauser Verlag. 2001.
- Ebendal T, Bengtsson H, Soderstrom S. Bone morphogenetic proteins and their receptors: potential functions in the brain. J Neurosci Res. 1998;51:139–46. doi: 10.1002/(SICI)1097-4547(19980115)51:2<139::AID-JNR2>3.3.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Mehler MF, Mabie PC, Zhang D, Kessler JA. Bone morphogenetic proteins in the nervous system. Trends Neurosci. 1997;20:309–17. doi: 10.1016/S0166-2236(96)01046-6. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Robertson EJ. Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev Dyn. 1997;208:349–62. doi: 10.1002/(SICI)1097-0177(199703)208:3<349::AID-AJA6>3.3.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Piston DW, Hogan BL. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124:2203–12. doi: 10.1242/dev.124.11.2203. [DOI] [PubMed] [Google Scholar]
- Solloway MJ, Robertson EJ. Early embryonic lethality in Bmp5;Bmp7 double mutant mice suggests functional redundancy within the 60A subgroup. Development. 1999;126:1753–68. doi: 10.1242/dev.126.8.1753. [DOI] [PubMed] [Google Scholar]
- Golden JA, Bracilovic A, McFadden KA, Beesley JS, JL RR, Grinspan JB. Ectopic bone morphogenetic proteins 5 and 4 in the chicken forebrain lead to cyclopia and holoprosencephaly. Proc Natl Acad Sci U S A. 1999;96:2439–44. doi: 10.1073/pnas.96.5.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins in development. Curr Opin Genet Dev. 1996;6:432–8. doi: 10.1016/S0959-437X(96)80064-5. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Rubenstein JL. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–18. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- Song Q, Mehler MF, Kessler JA. Bone morphogenetic proteins induce apoptosis and growth factor dependence of cultured sympathoadrenal progenitor cells. Dev Biol. 1998;196:119–27. doi: 10.1006/dbio.1998.8847. [DOI] [PubMed] [Google Scholar]
- Augsburger A, Schuchardt A, Hoskins S, Dodd J, Butler S. BMPs as mediators of roof plate repulsion of commissural neurons. Neuron. 1999;24:127–41. doi: 10.1016/s0896-6273(00)80827-2. [DOI] [PubMed] [Google Scholar]
- Farkas LM, Jaszai J, Unsicker K, Krieglstein K. Characterization of bone morphogenetic protein family members as neurotrophic factors for cultured sensory neurons. Neuroscience. 1999;92:227–35. doi: 10.1016/S0306-4522(98)00735-0. [DOI] [PubMed] [Google Scholar]
- Varley JE, Maxwell GD. BMP-2 and BMP-4, but not BMP-6, increase the number of adrenergic cells which develop in quail trunk neural crest cultures. Exp Neurol. 1996;140:84–94. doi: 10.1006/exnr.1996.0118. [DOI] [PubMed] [Google Scholar]
- Galter D, Bottner M, Krieglstein K, Schomig E, Unsicker K. Differential regulation of distinct phenotypic features of serotonergic neurons by bone morphogenetic proteins. Eur J Neurosci. 1999;11:2444–52. doi: 10.1046/j.1460-9568.1999.00667.x. [DOI] [PubMed] [Google Scholar]
- Lein P, Guo X, Hedges AM, Rueger D, Johnson M, Higgins D. The effects of extracellular matrix and osteogenic protein-1 on the morphological differentiation of rat sympathetic neurons. Int J Dev Neurosci. 1996;14:203–15. doi: 10.1016/0736-5748(96)00008-1. [DOI] [PubMed] [Google Scholar]
- Lein P, Johnson M, Guo X, Rueger D, Higgins D. Osteogenic protein-1 induces dendritic growth in rat sympathetic neurons. Neuron. 1995;15:597–605. doi: 10.1016/0896-6273(95)90148-5. [DOI] [PubMed] [Google Scholar]
- Guo X, Rueger D, Higgins D. Osteogenic protein-1 and related bone morphogenetic proteins regulate dendritic growth and the expression of microtubule-associated protein-2 in rat sympathetic neurons. Neurosci Lett. 1998;245:131–4. doi: 10.1016/S0304-3940(98)00192-X. [DOI] [PubMed] [Google Scholar]
- Withers GS, Higgins D, Charette M, Banker G. Bone morphogenetic protein-7 enhances dendritic growth and receptivity to innervation in cultured hippocampal neurons. Eur J Neurosci. 2000;12:106–16. doi: 10.1046/j.1460-9568.2000.00889.x. [DOI] [PubMed] [Google Scholar]
- Le Roux P, Behar S, Higgins D, Charette M. OP-1 enhances dendritic growth from cerebral cortical neurons in vitro. Exp Neurol. 1999;160:151–63. doi: 10.1006/exnr.1999.7194. [DOI] [PubMed] [Google Scholar]
- Granholm AC, Sanders LA, Ickes B, Albeck D, Hoffer BJ, Young DA, Kaplan PL. Effects of osteogenic protein-1 (OP-1) treatment on fetal spinal cord transplants to the anterior chamber of the eye. Cell Transplant. 1999;8:75–85. doi: 10.1177/096368979900800116. [DOI] [PubMed] [Google Scholar]
- Harland R. Neural induction. Curr Opin Genet Dev. 2000;10:357–62. doi: 10.1016/S0959-437X(00)00096-4. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–91. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Guo X, Lin Y, Horbinski C, Drahushuk K, Kim I, Kaplan P, Lein P, Wang T, Higgins D. Dendritic growth induced by BMP-7 requires Smad1 and proteasome activity. J Neurobiol. [PubMed]
- Smith A, Graham A. Restricting Bmp-4 mediated apoptosis in hindbrain neural crest. Dev Dyn. 2001;220:276–83. doi: 10.1002/1097-0177(20010301)220:3<276::AID-DVDY1110>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Iemura S, Yamamoto TS, Takagi C, Uchiyama H, Natsume T, Shimasaki S, Sugino H, Ueno N. Direct binding of follistatin to a complex of bone-morphogenetic protein and its receptor inhibits ventral and epidermal cell fates in early Xenopus embryo. Proc Natl Acad Sci U S A. 1998;95:9337–42. doi: 10.1073/pnas.95.16.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12:1438–52. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, LoTurco JJ. Noggin is a negative regulator of neuronal differentiation in developing neocortex. Dev Neurosci. 2000;22:68–73. doi: 10.1159/000017428. [DOI] [PubMed] [Google Scholar]
- Sela-Donenfeld D, Kalcheim C. Regulation of the onset of neural crest migration by coordinated activity of BMP4 and Noggin in the dorsal neural tube. Development. 1999;126:4749–62. doi: 10.1242/dev.126.21.4749. [DOI] [PubMed] [Google Scholar]
- Zhang D, Mehler MF, Song Q, Kessler JA. Development of bone morphogenetic protein receptors in the nervous system and possible roles in regulating trkC expression. J Neurosci. 1998;18:3314–26. doi: 10.1523/JNEUROSCI.18-09-03314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkemeyer MF, Pajerski M, Charness ME. Alcohol inhibition of cell adhesion in BMP-treated NG108-15 cells. Alcohol Clin Exp Res. 1999;23:1711–20. [PubMed] [Google Scholar]
- Lo L, Sommer L, Anderson DJ. MASH1 maintains competence for BMP2-induced neuronal differentiation in post-migratory neural crest cells. Curr Biol. 1997;7:440–50. doi: 10.1016/s0960-9822(06)00191-6. [DOI] [PubMed] [Google Scholar]
- Lo L, Tiveron MC, Anderson DJ. MASH1 activates expression of the paired homeodomain transcription factor Phox2a, and couples pan-neuronal and subtype-specific components of autonomic neuronal identity. Development. 1998;125:609–20. doi: 10.1242/dev.125.4.609. [DOI] [PubMed] [Google Scholar]
- Lo L, Morin X, Brunet JF, Anderson DJ. Specification of neurotransmitter identity by Phox2 proteins in neural crest stem cells. Neuron. 1999;22:693–705. doi: 10.1016/s0896-6273(00)80729-1. [DOI] [PubMed] [Google Scholar]
- Shah NM, Groves AK, Anderson DJ. Alternative neural crest cell fates are instructively promoted by TGFbeta superfamily members. Cell. 1996;85:331–43. doi: 10.1016/s0092-8674(00)81112-5. [DOI] [PubMed] [Google Scholar]
- White PM, Morrison SJ, Orimoto K, Kubu CJ, Verdi JM, Anderson DJ. Neural crest stem cells undergo cell-intrinsic developmental changes in sensitivity to instructive differentiation signals. Neuron. 2001;29:57–71. doi: 10.1016/s0896-6273(01)00180-5. [DOI] [PubMed] [Google Scholar]
- Varley JE, Wehby RG, Rueger DC, Maxwell GD. Number of adrenergic and islet-1 immunoreactive cells is increased in avian trunk neural crest cultures in the presence of human recombinant osteogenic protein-1. Dev Dyn. 1995;203:434–47. doi: 10.1002/aja.1002030406. [DOI] [PubMed] [Google Scholar]
- Schneider C, Wicht H, Enderich J, Wegner M, Rohrer H. Bone morphogenetic proteins are required in vivo for the generation of sympathetic neurons. Neuron. 1999;24:861–70. doi: 10.1016/s0896-6273(00)81033-8. [DOI] [PubMed] [Google Scholar]
- Reissmann E, Ernsberger U, Francis-West PH, Rueger D, Brickell PM, Rohrer H. Involvement of bone morphogenetic protein-4 and bone morphogenetic protein-7 in the differentiation of the adrenergic phenotype in developing sympathetic neurons. Development. 1996;122:2079–88. doi: 10.1242/dev.122.7.2079. [DOI] [PubMed] [Google Scholar]
- Fann MJ, Patterson PH. Depolarization differentially regulates the effects of bone morphogenetic protein (BMP)-2, BMP-6, and activin A on sympathetic neuronal phenotype. J Neurochem. 1994;63:2074–9. doi: 10.1046/j.1471-4159.1994.63062074.x. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Miyazono K, C-H H. Signaling via hetero-oligomeric complexes of type I and type II serine/threonine kinase receptors. Curr Opin Cell Biol. 1996;8:139–145. doi: 10.1016/S0955-0674(96)80058-5. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-beta signaling: receptors, transducers, and Mad proteins. Cell. 1996;85:947–950. doi: 10.1016/s0092-8674(00)81296-9. [DOI] [PubMed] [Google Scholar]
- Liu F, Ventura F, Doody J, Massague J. Human type II receptor for bone morphogenetic proteins (BMPs): extension of the two-kinase receptor model to the BMPs. Mol Cell Biol. 1995;15:3479–3486. doi: 10.1128/mcb.15.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke P, Yamashita H, Sampath TK, Reddi AH, Estevez M, Riddle DL, Ichijo H, Heldin CH, Miyazono K. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J Biol Chem. 1994;269:16985–8. [PubMed] [Google Scholar]
- Rosenzweig BL, Imamura T, Okadome T, Cox GN, Yamashita H, ten Dijke P, Heldin CH, Miyazono K. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci U S A. 1995;92:7632–6. doi: 10.1073/pnas.92.17.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainsod A, Deissler K, Yelin R, Marom K, Epstein M, Pillemer G, Steinbeisser H, Blum M. The dorsalizing and neural inducing gene follistatin is an antagonist of BMP-4. Mech Dev. 1997;63:39–50. doi: 10.1016/S0925-4773(97)00673-4. [DOI] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- Rubin E. Development of the rat superior cervical ganglion: ganglion cell maturation. J Neurosci. 1985;5:673–84. doi: 10.1523/JNEUROSCI.05-03-00673.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyvodic JT. Development and regulation of dendrites in the rat superior cervical ganglion. J Neurosci. 1987;7:904–12. doi: 10.1523/JNEUROSCI.07-03-00904.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson CE, Varley JE, Maxwell GD. Expression and regulation of type I BMP receptors during early avian sympathetic ganglion development. Dev Biol. 2000;221:220–32. doi: 10.1006/dbio.2000.9684. [DOI] [PubMed] [Google Scholar]
- Bruckenstein DA, Higgins D. Morphological differentiation of embryonic rat sympathetic neurons in tissue culture. I. Conditions under which neurons form axons but not dendrites. Dev Biol. 1988;128:324–36. doi: 10.1016/0012-1606(88)90295-3. [DOI] [PubMed] [Google Scholar]
- Tropea M, Johnson MI, Higgins D. Glial cells promote dendritic development in rat sympathetic neurons in vitro. Glia. 1988;1:380–92. doi: 10.1002/glia.440010605. [DOI] [PubMed] [Google Scholar]
- Liu Y, Belayev L, Zhao W, Busto R, Saul I, Alonso O, Ginsberg MD. The effect of bone morphogenetic protein-7 (BMP-7) on functional recovery, local cerebral glucose utilization and blood flow after transient focal cerebral ischemia in rats. Brain Res. 2001;905:81–90. doi: 10.1016/S0006-8993(01)02502-1. [DOI] [PubMed] [Google Scholar]
- Ren J, Kaplan PL, Charette MF, Speller H, Finklestein SP. Time window ofintracisternal osteogenic protein-1 in enhancing functional recovery after stroke. Neuropharmacology. 2000;39:860–5. doi: 10.1016/S0028-3908(99)00261-0. [DOI] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–87. doi: 10.1016/S0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Lin SZ, Hoffer BJ, Kaplan P, Wang Y. Osteogenic protein-1 protects against cerebral infarction induced by MCA ligation in adult rats. Stroke. 1999;30:126–33. doi: 10.1161/01.str.30.1.126. [DOI] [PubMed] [Google Scholar]
- Kawamata T, Ren J, Chan TC, Charette M, Finklestein SP. Intracisternal osteogenic protein-1 enhances functional recovery following focal stroke. Neuroreport. 1998;9:1441–5. doi: 10.1097/00001756-199805110-00035. [DOI] [PubMed] [Google Scholar]
- Sampath TK, Maliakal JC, Hauschka PV, Jones WK, Sasak H, Tucker RF, White KH, Coughlin JE, Tucker MM, Pang RH, et al. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J Biol Chem. 1992;267:20352–62. [PubMed] [Google Scholar]
- Lamb TM, Knecht AK, Smith WC, Stachel SE, Economides AN, Stahl N, Yancopolous GD, Harland RM. Neural induction by the secreted polypeptide noggin. Science. 1993;262:713–8. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- Higgins D, Lein P, Osterhout D, Johnson MI. Tissue culture of autonomic neurons. Culturing Nerve Cells (Edited by Banker G, Goslin K) Cambridge, MA: MIT Press. 1991. pp. 177–205.