Abstract
Objective
That the bone-derived hormone osteocalcin is necessary to promote normal brain development and function, along with its recently described sufficiency in reversing cognitive manifestations of aging, raises novel questions. One of these is to assess whether bone health, which deteriorates rapidly with aging, is a significant determinant of cognition and anxiety-like behavior.
Methods
To begin addressing this question, we used mice haploinsufficient for Runx2, the master gene of osteoblast differentiation and the main regulator of Osteocalcin expression. Control and Runx2+/− mice were evaluated for the expression of osteocalcin's target genes in the brain and for behavioral parameters, using two assays each for cognition and anxiety-like behavior.
Results
We found that adult Runx2+/− mice had defects in bone resorption, reduced circulating levels of bioactive osteocalcin, and reduced expression of osteocalcin's target genes in the brain. Consequently, they had significant impairment in cognitive function and increased anxiety-like behavior.
Conclusions
These results indicate that bone remodeling is a determinant of brain function.
Keywords: Runx2, Osteocalcin, Bone remodeling, Cognition
Highlights
-
•
The transcription factor Runx2 determines circulating osteocalcin levels.
-
•
Runx2 influences cognition through its regulation of osteocalcin expression.
-
•
Runx2 also influences anxiety-like behavior.
1. Introduction
Osteocalcin is a bone-derived hormone that regulates a growing number of physiological functions. In particular, although Osteocalcin is not expressed in any part of the WT brain before or after birth [1], Osteocalcin−/− mice have increased anxiety-like behavior and depression, decreased exploratory behavior, and impaired learning and memory when compared to WT littermates [1]. Remarkably, these phenotypic abnormalities are already severe in young, i.e. 3-month-old, Osteocalcin−/− mice. Osteocalcin is secreted into the general circulation, crosses the blood brain barrier, and binds to discrete parts of the brain including neurons in the brainstem, ventral tegmental area (VTA), and hippocampus, where it signals through Gpr158, a recently-identified second receptor for this hormone [2]. In the brain, osteocalcin promotes the synthesis of monoamine neurotransmitters (i.e., serotonin, dopamine, and norepinephrine) and inhibits synthesis of GABA [1] by regulating the expression of key enzymes implicated in the synthesis of these various neurotransmitters. Together, these properties of osteocalcin contribute, at least in part, to its beneficial influence on cognition and anxiety.
The importance of osteocalcin's regulation of brain function was further underscored recently when it was shown that the presence of osteocalcin is necessary for the ability of plasma from young mice to improve cognition in older mice [2]. Accordingly, the systemic delivery of osteocalcin was shown to be sufficient to reverse age-related cognitive decline in 16-month-old mice [2]. In view of these findings and given that osteocalcin is produced only by osteoblasts, the cell type responsible for bone formation and osteoclast differentiation [3], we asked whether an impairment in osteoblast differentiation and function, as may occur in various skeletal dysplasias or with aging, could affect cognition or anxiety.
Remarkably, we note that cleidocranial dysplasia (CCD), a classical skeletal dysplasia in which cognitive functions are also affected, is an autosomal dominant disease caused by haploinsufficiency at the Runx2 locus. The transcription factor Runx2 is the master regulator of osteoblast differentiation and the main regulator of Osteocalcin expression [4], [5]. Since osteoblasts are responsible for the production of osteocalcin, this raises the question of whether decreased osteocalcin production could be the cause of the cognitive defects that were reported 60 years ago in certain, but not all, CCD patients [6]. To test this hypothesis, we used Runx2+/− mice, which phenocopy, at least in the skeleton, what is observed in Runx2 haploinsufficient humans [4], [7], [8].
2. Methods and materials
2.1. Mice and treatment
Osteocalcin+/− and Runx2+/− mice have been previously described [4], [9]. For all experiments, we used females and littermates as controls unless otherwise stated. Osteocalcin+/− mice were maintained on a pure 129-Sv/Ev genetic background and the Runx2+/− model was maintained on a C57BL/6J background. Runx2+/− mice were generated by breeding WT females with Runx2+/− male mice. Each group described is represented individually in each panel. Mice were housed two to five animals per cage (polycarbonate cages (35.5 × 18 × 12.5 cm)), under a 12 h light/dark cycle with ad libitum access to food and water prior to experimentation, in a pathogen-free animal facility. All experiments involving animals were approved by the Institutional Animal Care and Use Committee of Columbia University Medical Center.
2.2. Behavioral studies
All animals of the same batch were born within an interval of 2 weeks and were kept in mixed genotype per group of 2–5 females in the same cage, at standard laboratory conditions (12 h dark/light cycle, constant room temperature and humidity, and standard lab chow and water ad libitum). For each test, the mice were transported a short distance from the holding mouse facility to the testing room in their home cages or in the transport boxes filled with bedding from their home cages. Behavioral testing of the mice was performed on a battery of functional tests between 3 and 5 months of age, and mouse weight was between 18 g and 28 g. The tests were performed by an experimentalist blind to the genotypes of the mice under study.
2.2.1. Elevated plus maze test (EPMT)
This test takes advantage of the aversion of rodents to open spaces. The EPM apparatus comprises two open and two enclosed arms, each with an open roof, elevated 60 cm from the floor [10], [11]. Testing takes place in bright ambient light conditions. Animals are placed into the central area facing one closed arm and allowed to explore the EPM for 6 min. Time spent in open arms was recorded.
2.2.2. Light to dark transition test
This test is based on the innate aversion of rodents to brightly illuminated areas and on their spontaneous exploratory behavior in response to the stressor that light represents [12]. The test apparatus consists of a dark, safe compartment and an illuminated, aversive one. Mice were tested for 6 min, and time spent in the lit compartment was scored as an index of anxiety-related behavior as well as exploratory activity.
2.2.3. Morris water maze test
Animals are transported to the testing room in their home cages and left undisturbed for at least 30 min prior to the first trial. The maze is comprised of a large swimming pool (150 cm diameter) filled with water (23 C) made opaque with non-toxic white paint. The pool is located in a brightly lit room filled with visual cues, including geometric figures on the walls of the maze demarking the four fixed starting positions of the trials, at (12:00, 3:00, 6:00 and 9:00). A 15 cm round platform is hidden 1 cm beneath the surface of the water at a fixed position. Each daily trial block consisted of four swimming trials, with each mouse starting from the same randomly chosen starting position. The starting position is varied between days. On day 1, mice that fail to find the platform within 2 min are guided to the platform. They must remain on the platform for 15 s before they are returned to their home cage. Mice are not guided to the platform after day 1, and the time it takes them to reach the platform over repeated trials (4 trails/day for the next 10 days) is recorded as a measure of spatial learning.
2.2.4. Novel object recognition test (NOR)
The NOR paradigm assesses the rodent's ability to recognize a novel object in the environment. The NOR task is conducted, as previously described [13], in an opaque plastic box using 2 different objects: (1) a clear plastic funnel (diameter 8.5 cm, maximal height 8.5 cm) and (2) a black plastic box (9.5 cm3). These objects elicit equal levels of exploration as determined in pilot experiments [1], [14]. The NOR paradigm consists of 3 exposures over the course of 3 days. On day 1, the habituation phase, mice are given 5 min to explore the empty arena, without any objects. On day 2, the familiarization phase, mice are given 10 min to explore 2 identical objects, placed at opposite ends of the box. On day 3, the test phase, mice are given 15 min to explore 2 objects, one novel object and a copy of the object from the familiarization phase. The object that serves as the novel object (either the funnel or the box) as well as the left/right starting position of the objects is counterbalanced within each group. Mice are placed in the center of the arena at the start of each exposure. Between exposures, the objects and arenas are cleaned. Discrimination of the novel object is assessed by the following formula: ((time spent with novel object-time spent with old object/total exploration time)). Exploration of each object, as well as number of grid crossings, is scored from video recordings of each exposure and recorded using the Stopwatch program. Climbing or sitting adjancent to the object was not counted as exploration. An equal exploration time for the two objects, or a decreased percentage of time spent with the novel object compared to WT controls indicates impairment in hippocampal memory.
2.3. Hormonal measurement
Circulating levels of osteocalcin in mouse serum were determined with a specific ELISA previously described [15], [16]. Total levels of osteocalcin and carboxylated osteocalcin were estimated using two different specific antibodies. Bioactive osteocalcin was determined by substracting the carboxylated osteocalcin levels from total osteocalcin levels [15], [16].
2.4. Real-Time RNA transcript determination
All brain dissections were performed in ice-cold PBS 1× under a Leica MZ8 dissecting light microscope. The hypothalamus was removed from the midbrain during collection. All parts of the brain isolated were snap frozen in liquid nitrogen and kept at −80 C until use. No contusions were observed in any of the analyzed Runx2+/− brains. Tibia isolated for gene expression were centrifuged for 20 s at 16,100 × g to flush out the bone marrow. All isolated bones were snap frozen in liquid nitrogen and kept at −80 C until use.
RNA was isolated from brain tissue using TRIZOL (Invitrogen). cDNA synthesis was performed following standard protocol. Total RNA was first incubated with DNAse I for 30 min at room temperature to remove any genomic DNA. DNAse I-treated total RNA was converted to cDNA by using M-MLV reverse transcriptase (Thermo: 28025013) and random hexamers (Thermo: N8080127). qPCR was performed on a CFX96 Touch™ Real-Time PCR Detection System (BioRad), and analyses were done using specific quantitative PCR primers and expressed relative to Gapdh levels.
2.5. Biochemistry and molecular biology
For Western blotting, frozen hippocampi from adult mice were lysed and homogenized in 250 μl tissue lysis buffer (25 mM Tris HCl 7.5; 100 mM NaF; 10 mM Na4P2O7; 10 mM EDTA; 1% NP 40). Proteins were transferred to nitrocellulose membranes, and blocked with TBST-5% BSA prior to overnight incubation with primary antibody in TBST–5%BSA. Antibodies: anti-Runx2: M-70 sc-10758, Santa Cruz, anti-BDNF: sc-546, Santa Cruz; anti-tubulin: T6199, Sigma. HRP-coupled secondary antibodies and ECL were used to visualize the signal.
2.6. Quantification and statistical analysis
All values are depicted as mean ± SEM. Statistical parameters including the exact value of n, post hoc tests and statistical significance are reported in each figure and figure legend. The number of mice used for each experiment was estimated to be sufficient based on pilot studies and previously published work [1]. Data are estimated to be statistically significant when p ≤ 0.05 by Student's t-test, one-way ANOVA or repeated-measures ANOVA. In every figure, an asterisk denotes statistical significance (*p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001). Data were analyzed using Graph Pad Prism 6.
3. Results
3.1. Runx2 regulates osteocalcin levels and is not present in the brain
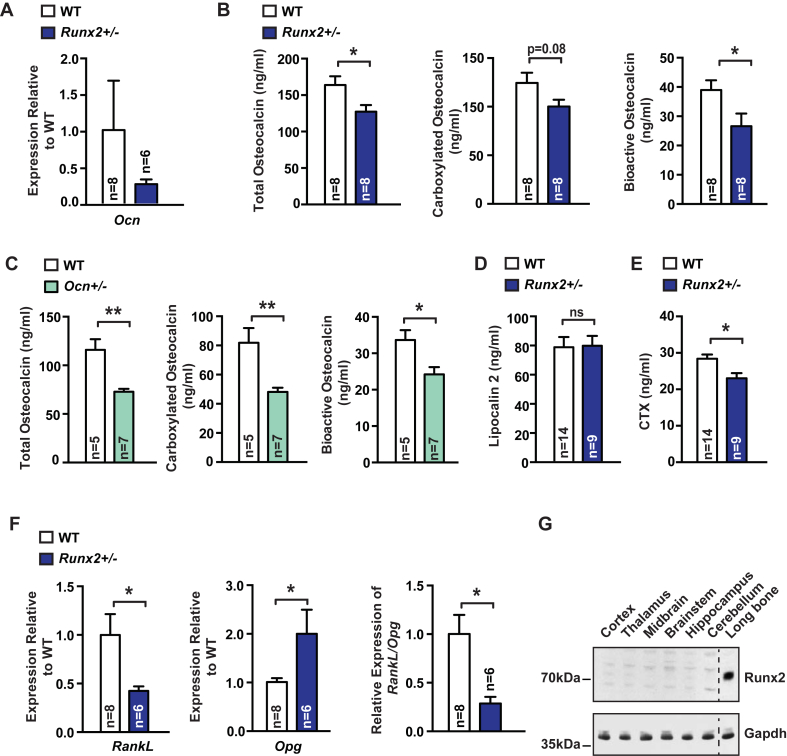
Runx2+/− mice were used to study the influence of bone formation on brain function because they have reduced bone formation [4], [7]. For that reason, we first asked whether circulating bioactive osteocalcin levels are altered in Runx2+/− mice, using an ELISA designed to specifically recognize the bioactive form of osteocalcin [16]. We observed a decrease in osteocalcin expression, as well as a decrease in total and carboxylated circulating osteocalcin levels, and a nearly 40% reduction in circulating levels of uncarboxylated and bioactive osteocalcin in Runx2+/− mice compared to WT littermates (Figure 1A–B). This decrease was of similar magnitude to what was observed in Osteocalcin+/− mice (Figure 1C). As evidence of specificity, circulating levels of Lipocalin 2, another bone-derived hormone signaling in the brain [17], were unchanged in Runx2+/− mice (Figure 1D). In addition, analysis of molecular markers of bone resorption showed that haploinsufficiency at the Runx2 locus results in a significant decrease in bone resorption (Figure 1E–F). Hence, the Runx2+/− mouse is a model of low bone turnover. These results were an incentive to determine if this decrease in circulating osteocalcin levels has any consequences on cognition and anxiety-like behavior. Prior to performing these behavioral studies, we asked whether Runx2 is present in any part of the brain where osteocalcin is known to bind and act. Using an antibody specific to Runx2, we found that while the protein is abundant in the bone, it could not be detected in any region of the brain where osteocalcin is known to bind (Figure 1G).
Figure 1.
Runx2 regulates osteocalcin levels and is not present in the brain. (A) Osteocalcin (Ocn) expression (qPCR) in the tibia of 3-month-old Runx2+/− mice and WT littermates. (B) Circulating levels of total, carboxylated, and bioactive osteocalcin in 3-month-old Runx2+/− and WT littermates. (C) Circulating levels of total, carboxylated, and bioactive osteocalcin in 3-month-old Osteocalcin +/− and WT littermates. (D) Circulating Lipocalin 2 levels in 3-month-old Runx2+/− and WT littermates. (E) Circulating CTX levels in 3-month-old Runx2+/− and WT littermates. (F) Rank ligand (RankL), Osteoprotegerin (Opg), and RankL/Opg expression (qPCR) in the tibia of 3-month-old Runx2+/− mice and WT littermates. (G) Runx2 accumulation (Western blot) in various tissues of 3-month-old WT mouse. Gapdh was used as a loading control. Results are given as mean ± s.e.m. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, n.s., not significant; by Student's t-test (A–F).
3.2. Runx2 haploinsufficiency affects biomarkers regulated by osteocalcin
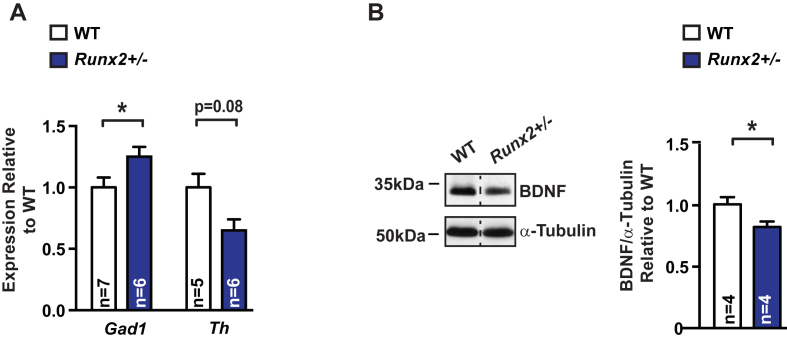
Consistent with the decrease in circulating osteocalcin levels presented in Figure 1, expression of Gad1, a gene down-regulated by osteocalcin signaling [1] and necessary for GABA production, was instead increased, whereas expression of Th, a gene necessary for catecholamine synthesis and the expression of which is up-regulated by osteocalcin signaling [1], was decreased in the Runx2+/− brainstem and midbrain, respectively (Figure 2A). Additionally, accumulation of brain derived neurotrophic factor (BDNF), a growth factor necessary for optimal hippocampal-dependent memory formation [18], [19], [20] with recently demonstrated regulation by osteocalcin signals [2] was significantly decreased in Runx2+/− hippocampi (Figure 2B). These results indicate that haploinsufficiency at the Runx2 locus results in molecular abnormalities in the brain, similar to those caused by osteocalcin deficiency [1].
Figure 2.
Runx2 haploinsufficiency affects biomarkers regulated by osteocalcin. (A) Glutamate decarboxylase-1 (Gad 1) and Tyrosine hydroxylase (Th) expression (qPCR) in the brainstem and midbrain, respectively, of 3-month-old Runx2+/− mice and WT littermates. (B) Brain-derived neurotrophic factor (BDNF) accumulation (representative western blot, left) and quantification of band intensities (right) in hippocampi of 3-month-old Runx2+/− and WT littermates. α-tubulin is used as a loading control. Results are given as mean ± s.e.m. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, n.s., not significant; by Student's t-test (A–B).
3.3. Runx2+/− mice display impaired spatial learning and hippocampal memory
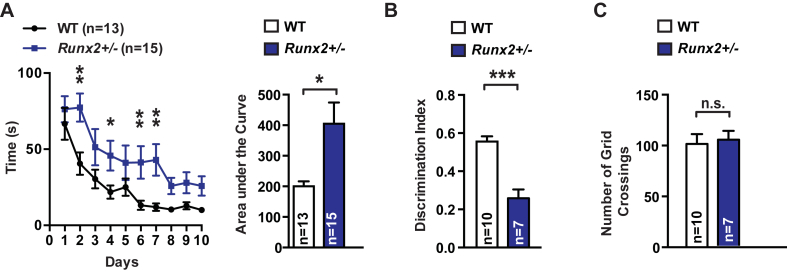
Given the significant decrease in circulating osteocalcin levels and the change in expression of osteocalcin's target genes in Runx2+/− mice (Figure 2) we next asked whether cognition might be affected in these mutant mice. Cognitive functions were assessed by two distinct tests. The first was the Morris water maze test (MWMT), which assesses spatial learning and memory in rodents [2], [21]. In this test, mice are trained to locate a submerged platform using spatial cues surrounding the maze. Runx2+/− mice showed a significant delay in learning the location of the platform over 10 days compared to WT littermates (Figure 3A). A second test, the novel object recognition test (NOR) [1], [14], [22], evaluates hippocampal-dependent memory more specifically [1], [13], [14]. In the NOR, mice are exposed to two identical objects, and after a 24-hour delay, one of these objects is replaced by a novel object. The proportion of time spent exploring the novel object is used as a measure of recognition and memory. Runx2+/− mice spent significantly less time exploring the novel object than WT littermates (Figure 3B), indicating that their hippocampal memory was impaired. Importantly, Runx2+/− mice made a similar number of grid crossings over the NOR testing arena, indicating that they do not have any defects in ambulation or motor activity that would result in decreased exploration (Figure 3C). These observations indicate that impairing bone remodeling suffices to hamper spatial learning and memory.
Figure 3.
Runx2+/− mice display impaired spatial learning and hippocampal memory. (A) Morris water maze test (MWMT) performed over 10 days. The graphic shows the time (s) needed for each group of mice, Runx2+/− and WT littermates, to localize a submerged platform in the swimming area. (B) Novel object recognition (NOR) performed in Runx2+/− and WT littermates. Discrimination index ((time spent with novel object-time spent with old object/total exploration time)) was measured. (C) Number of grid crossings over the NOR arena in Runx2+/− mice and WT littermates. Results are given as mean ± s.e.m. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, n.s., not significant; by two-way repeated measures ANOVA followed by a Fisher's LSD test (A), and Student's t-test (B–C).
3.4. Runx2+/− mice display increased anxiety-like behavior
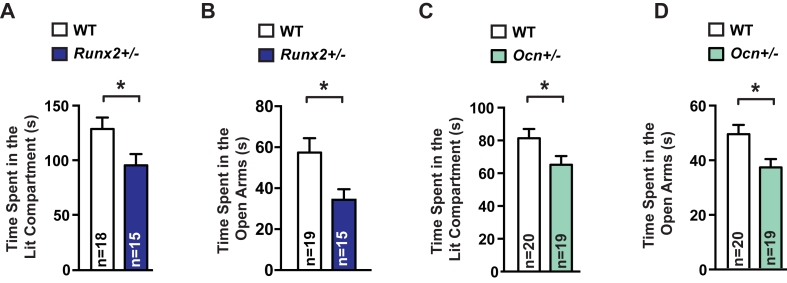
Anxiety and exploratory-like behaviors were analyzed using two tests. The first test, the light to dark transition test, is based on the innate aversion of rodents to brightly illuminated areas and their decreased spontaneous exploratory behavior in response to light [1], [23], [24], [25], [26]. Three-month-old Runx2+/− mice spent less time in the lit compartment than WT littermates (Figure 4A). In a second test, the elevated plus maze test, anxiety results in a decrease in the proportion of time spent in the open arms [10], [11]. Here again, Runx2+/− mice spent less time in the open arms than WT littermates (Figure 4B). Hence, results of these two tests indicate that haploinsufficiency at the Runx2 locus results in greater anxiety-like behavior. That Osteocalcin+/− mice, which have a similar decrease in circulating osteocalcin levels, display the same increase in anxiety-like behavior as Runx2+/− mice, strongly suggests that decreased osteocalcin levels are responsible for this phenotypic abnormality in Runx2+/− mice (Figure 4C and D). More generally, given the abnormalities in both bone formation and bone resorption present in Runx2+/− mice, these results support the notion that Runx2 haploinsufficiency results in anxiety-like behavior.
Figure 4.
Runx2+/− mice display increased anxiety-like behavior. (A) Dark to Light Transition (DLT) test performed in Runx2+/− and WT littermates. Time spent in the lit compartment was measured. (B) Elevated Plus Maze (EPMT) test performed in Runx2+/− and WT littermates. Time spent (s) in the open arms were scored. (C) Dark to Light Transition (DLT) test performed in Ocn+/− and WT littermates. Time spent in the lit compartment was measured. (D) Elevated Plus Maze (EPMT) test performed in Ocn+/− and WT littermates. Time spent (s) in the open arms were scored. Results are given as mean ± s.e.m. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, n.s., not significant; by Student's t-test (A–D).
4. Discussion
This study presents the first direct evidence that bone remodeling, i.e., the processes of constant bone renewal through the alternation of bone resorption followed by bone formation, affects brain functions such as cognition and anxiety-like behavior. Haploinsufficiency of Runx2, a transcription factor exclusively expressed in osteoblast progenitor cells and in bone-forming osteoblasts, regulates circulating levels of bioactive osteocalcin. This is explained by the fact that, on the one hand, Runx2 is the primary regulator of Osteocalcin expression and, on the other hand, a positive regulator of bone resorption, the arm of bone remodeling which activates osteocalcin [4], [27]. Consequently, Runx2 also affects the expression of osteocalcin's target genes in the brain, such as Gad1, Th, and Bdnf, even though Runx2 is undetectable in brain regions where osteocalcin binds. Lastly, and similarly to what is observed in Osteocalcin+/− mice, Runx2+/− mice show impairment in learning and memory, as well as increased anxiety-like behavior. Thus, osteocalcin deficiency in Runx2+/− mice is a major contributor to the deficit in cognitive function and increase in anxiety-like behavior observed in these mice.
Haploinsufficiency of Runx2 in mice and in humans results in cleidocranial dysplasia, CCD, which is characterized by severe skeletal abnormalities including small or missing clavicles, a delay in the closure of the fontanels, short stature, dental defects, and low bone mass [28]. Importantly for this study, a proportion of patients with CCD also present with cognitive impairments [29], [30], suggesting that the novel link between osteoblast endocrine function and brain function, illustrated by the role of osteocalcin in the mouse, may extend to humans, as do other functions of osteocalcin [31]. Our results provide one molecular explanation for this surprising decrease in cognitive function in a skeletal dysplasia that was first reported 60 years ago [6]. More broadly, these data suggest that bone anabolic treatments for degenerative diseases of the skeleton, such as osteoporosis, may also have a beneficial effect on cognitive function and anxiety-like behavior, whereas anti-resorptive agents may potentially be detrimental. Besides this age-related disease, our results also suggest that the high incidence of neuropsychiatric disorders in children born to women who are undernourished during pregnancy, and therefore have hampered bone remodeling [32], could be due at least in part to a deficit in osteocalcin signaling during pregnancy, since maternal osteocalcin is critical for proper brain development and influences cognitive function in the adult offspring [1].
Authors' contributions
L.K. designed experiments, L.K. and A.O. performed experiments, L.K., A.O., and G.K. analyzed data, and L.K. and G.K. wrote the manuscript.
Acknowledgments
This work was supported by 2P01 AG032959-06A1 and the Columbia Aging Center (G.K.), 5T32DK007328-38 Endocrinology Training Grant (L.K.), and Philippe Foundation (A.O.).
Conflict of interest
None declared.
References
- 1.Oury F., Khrimian L., Denny C.A., Gardin A., Chamouni A., Goeden N. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell. 2013;155(1):228–241. doi: 10.1016/j.cell.2013.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khrimian L., Obri A., Ramos-Brossier M., Rousseaud A., Moriceau S., Nicot A.S. Gpr158 mediates osteocalcin's regulation of cognition. The Journal of Experimental Medicine. 2017 doi: 10.1084/jem.20171320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karsenty G., Wagner E.F. Reaching a genetic and molecular understanding of skeletal development. Developmental Cell. 2002;2(4):389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 4.Ducy P., Zhang R., Geoffroy V., Ridall A.L., Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 5.Karsenty G. Transcriptional control of skeletogenesis. Annual Review of Genomics and Human Genetics. 2008;9:183–196. doi: 10.1146/annurev.genom.9.081307.164437. [DOI] [PubMed] [Google Scholar]
- 6.Soule A.B., Jr. Mutational dysostosis (cleidocranial dysostosis) The Journal of Bone and Joint Surgery. American Volume. 1946;28:81–102. [PubMed] [Google Scholar]
- 7.Lee B., Thirunavukkarasu K., Zhou L., Pastore L., Baldini A., Hecht J. Missense mutations abolishing DNA binding of the osteoblast-specific transcription factor OSF2/CBFA1 in cleidocranial dysplasia. Nature Genetics. 1997;16(3):307–310. doi: 10.1038/ng0797-307. [DOI] [PubMed] [Google Scholar]
- 8.Mundlos S., Otto F., Mundlos C., Mulliken J.B., Aylsworth A.S., Albright S. Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell. 1997;89(5):773–779. doi: 10.1016/s0092-8674(00)80260-3. [DOI] [PubMed] [Google Scholar]
- 9.Ducy P., Desbois C., Boyce B., Pinero G., Story B., Dunstan C. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382(6590):448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 10.Holmes A., Parmigiani S., Ferrari P.F., Palanza P., Rodgers R.J. Behavioral profile of wild mice in the elevated plus-maze test for anxiety. Physiology & Behavior. 2000;71(5):509–516. doi: 10.1016/s0031-9384(00)00373-5. [DOI] [PubMed] [Google Scholar]
- 11.Lira A., Zhou M., Castanon N., Ansorge M.S., Gordon J.A., Francis J.H. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biological Psychiatry. 2003;54(10):960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- 12.Takao K., Miyakawa T. Light/dark transition test for mice. Journal of Visualized Experiments. 2006;(1):104. doi: 10.3791/104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broadbent N.J., Gaskin S., Squire L.R., Clark R.E. Object recognition memory and the rodent hippocampus. Learning & Memory. 2010;17(1):5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denny C.A., Burghardt N.S., Schachter D.M., Hen R., Drew M.R. 4- to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning. Hippocampus. 2012;22(5):1188–1201. doi: 10.1002/hipo.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferron M., Wei J., Yoshizawa T., Del Fattore A., DePinho R.A., Teti A. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142(2):296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferron M., Wei J., Yoshizawa T., Ducy P., Karsenty G. An ELISA-based method to quantify osteocalcin carboxylation in mice. Biochemical and Biophysical Research Communications. 2010;397(4):691–696. doi: 10.1016/j.bbrc.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosialou I., Shikhel S., Liu J.M., Maurizi A., Luo N., He Z. MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature. 2017;543(7645):385–390. doi: 10.1038/nature21697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anastasia A., Deinhardt K., Chao M.V., Will N.E., Irmady K., Lee F.S. Val66Met polymorphism of BDNF alters prodomain structure to induce neuronal growth cone retraction. Nature Communications. 2013;4:2490. doi: 10.1038/ncomms3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall J., Thomas K.L., Everitt B.J. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nature Neuroscience. 2000;3(6):533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- 20.Nagahara A.H., Merrill D.A., Coppola G., Tsukada S., Schroeder B.E., Shaked G.M. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nature Medicine. 2009;15(3):331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakazawa K., Quirk M.C., Chitwood R.A., Watanabe M., Yeckel M.F., Sun L.D. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297(5579):211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ennaceur A., Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behavioural Brain Research. 1988;31(1):47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 23.David D.J., Samuels B.A., Rainer Q., Wang J.W., Marsteller D., Mendez I. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62(4):479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crawley J.N. Exploratory behavior models of anxiety in mice. Neuroscience and Biobehavioral Reviews. 1985;9(1):37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- 25.Zernig G., Dietrich H., Maggi C.A., Saria A. The substance P (NK1) receptor antagonist (+/-)-CP-96,345 causes sedation and motor impairment in Swiss albino mice in the black-and-white box behavioral paradigm. Neuroscience Letters. 1992;143(1–2):169–172. doi: 10.1016/0304-3940(92)90258-9. [DOI] [PubMed] [Google Scholar]
- 26.Vicente M.A., Zangrossi H., Jr., dos Santos L., de Macedo C.E., Andrade T.G. Involvement of median raphe nucleus 5-HT1A receptors in the regulation of generalized anxiety-related defensive behaviours in rats. Neurosci Lett. 2008;445(3):204–208. doi: 10.1016/j.neulet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Karsenty G., Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481(7381):314–320. doi: 10.1038/nature10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen M.M., Jr. Biology of RUNX2 and cleidocranial dysplasia. The Journal of Craniofacial Surgery. 2013;24(1):130–133. doi: 10.1097/SCS.0b013e3182636b7e. [DOI] [PubMed] [Google Scholar]
- 29.Izumi K., Yahagi N., Fujii Y., Higuchi M., Kosaki R., Naito Y. Cleidocranial dysplasia plus vascular anomalies with 6p21.2 microdeletion spanning RUNX2 and VEGF. American Journal of Medical Genetics. Part A. 2006;140(4):398–401. doi: 10.1002/ajmg.a.31061. [DOI] [PubMed] [Google Scholar]
- 30.Takenouchi T., Sato W., Torii C., Kosaki K. Progressive cognitive decline in an adult patient with cleidocranial dysplasia. European Journal of Medical Genetics. 2014;57(7):319–321. doi: 10.1016/j.ejmg.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 31.Oury F., Ferron M., Huizhen W., Confavreux C., Xu L., Lacombe J. Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis. The Journal of Clinical Investigation. 2013;123(6):2421–2433. doi: 10.1172/JCI65952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seibel M.J. Nutrition and molecular markers of bone remodelling. Current Opinion in Clinical Nutrition and Metabolic Care. 2002;5(5):525–531. doi: 10.1097/00075197-200209000-00011. [DOI] [PubMed] [Google Scholar]