Abstract
Background and Objectives
We assessed the safety, tolerability, and preliminary efficacy of nabilone, a cannabinoid agonist, to treat cannabis dependence.
Methods
Eighteen adults with DSM-IV cannabis dependence were randomized to receive either 2 mg/day of nabilone (n=10) or placebo (n=8) for 10 weeks in addition to medication management. Twelve participants, 6 in each group, completed treatment. The safety and tolerability of nabilone was assessed at each visit. Any side effects from nabilone or the placebo were documented. Cannabis use outcomes were assessed via self-report of days of use and twice-weekly urine cannabinoid tests; secondary outcomes included cannabis craving and anxiety.
Results
We assessed safety and tolerability at each study visit. A total of 8 adverse events, all mild or moderate, were reported in 2 participants in the nabilone group and 6 events were reported in 4 participants in the placebo group during study treatment. A total of 8 adverse events were reported in 2 participants in the nabilone group and 6 events were reported in 4 participants in the placebo group during study treatment. All reported adverse events were rated mild-to-moderate. There were no side effects deemed serious enough to be classified as an FDA-defined serious adverse event. In general, participants in both groups reported reduced cannabis use according to self-report over the course of the study, although these reductions were not statistically discernible. Moreover, there was no difference in cannabis use between the nabilone group and the placebo group as measured by self-report.
Discussion and Conclusions
Nabilone pharmacotherapy was safe and well-tolerated in participants with cannabis dependence. Future studies might evaluate a higher dose of nabilone to determine its effects on cannabis use outcomes in participants with cannabis dependence.
Scientific Significance
There remains a clear need for additional pharmacotherapy trials for cannabis dependence, and nabilone remains a candidate for such trials.
INTRODUCTION
Cannabis is the most frequently used illicit substance in the United States.1 Twenty-two million Americans reported using cannabis in the past month, with overall use doubling within the past ten years.2 It has been estimated that approximately 9% of people who use cannabis will become dependent upon it, with approximately 2 million Americans meeting the DSM-IV criteria for dependence on cannabis in 2012.3,4 Despite the critical need for treatment for these individuals, there are currently no FDA-approved medications for cannabis use disorder.
Clinical trials of cannabis dependence pharmacotherapies have yielded varied outcomes and the side effect profile of these medications continues to be studied. Dronabinol, a synthetic form of delta-9-tetrahydrocannabinol (THC), has been used to treat cannabis-dependent adults in combination with motivational enhancement and relapse prevention therapy without significant effect.5 Although there was no significant difference between dronabinol and placebo, dronabinol was well-tolerated, led to a reduction of cannabis use over time, and improved treatment retention as well as withdrawal symptoms. Gabapentin, a gamma butyric acid analog, significantly reduced cannabis use as measured by urine toxicology and self-report while also decreasing cannabis withdrawal symptoms in adults with cannabis dependence.6 Similarly, N-acetylcysteine, an over-the-counter antioxidant and glutamate modulator, has been studied as a potential pharmacotherapy for cannabis dependence in adolescents. N-acetylcysteine was well tolerated, and participants taking active medication were twice as likely to submit negative urine cannabinoid tests during treatment when compared with participants taking placebo, although no difference was found between N-acetylcysteine and placebo on secondary cannabis outcomes.7 Treatment with N-acetylcysteine was not, however, more efficacious in producing abstinence from cannabis use in adults with cannabis use disorder.8
Previous clinical trials of agonist treatments, such as dronabinol, for other substance use disorders have been shown to be effective by reducing withdrawal symptoms, improving treatment retention, reducing the reinforcing effects of the addictive drug, and promoting abstinence.9, 10, 11 For example, the agonist methadone and the partial agonist buprenorphine (especially when combined with the opioid antagonist naloxone as in Suboxone™) have proven to be effective pharmacotherapies for opioid-dependent patients, and nicotine replacement therapy is effective as a treatment for nicotine dependence .12,13,14,15 Agonist treatment may also provide a viable approach for patients with cannabis dependence, potentially decreasing the desire to use cannabis while eliminating the anxiety, sleep difficulties, and craving associated with withdrawal from cannabis. Nabiximols, a cannabinoid agonist containing delta-9-tetrahydrocannabinol (THC) and cannabidiol, mitigated cannabis withdrawal symptoms and improved treatment retention is a double-blind, placebo-controlled inpatient clinical trial.16 Given these trends, we sought to examine the treatment potential of nabilone, a synthetic cannabinoid that is an FDA-approved treatment of side effects caused by some cancer chemotherapies at a usual dose of 2–4 mg daily.17 Like dronabinol, nabilone is an agonist at both CB1 and CB2 receptors. However, it has some characteristics that may make it a more effective pharmacotherapy for cannabis dependence than dronabinol: it is more bioavailable, it has a longer time to peak effect, and it is not cross-reactive in urine assays, meaning that its presence does not produce a positive for THC in standard urine drug screens.18 In two human laboratory studies, nabilone has been shown to significantly reduce cannabis withdrawal symptoms in non-treatment seeking cannabis users.19,20 We conducted a small pilot trial comparing nabilone to placebo in order to test the safety and tolerability of using this agent in cannabis-dependent patients.
METHODS
Study Design
In a randomized prospective pilot study aimed at testing the safety, tolerability, and promise of using nabilone (up to 2 mg/day) as pharmacotherapy to treat cannabis dependence, potential participants were recruited from the Boston area via online and newspaper advertisements, as well as word-of-mouth. Enrollment began in September 2010 and was completed in April 2013. There was a 9-month period during that time when arrangements were made for the supply of study medication once funding was secured. The study protocol included three functional magnetic resonance brain scans that required 12 hours of abstinence from cannabis. All procedures were conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki, approved by the McLean Hospital Institutional Review Board, and registered on ClinicalTrials.gov (identifier NCT01347762). All participants gave written, informed consent prior to study participation. As part of the aims of the grant that funded this study, participants also consented to neuroimaging and this assessment was part of their baseline and follow-up visits.
Screening Assessment
To be eligible for participation, individuals had to be between 18 and 45 years of age and meet DSM-IV criteria for current cannabis dependence.21 Exclusion criteria included a current diagnosis of other drug or alcohol dependence (excluding caffeine and nicotine); current serious psychiatric illness or history of psychosis, schizophrenia, or bipolar I disorder; current suicidal or homicidal risk; current treatment with opioid analgesics, sedative-hypnotics, or other known central nervous system depressants; major medical illness; a history of seizures, head trauma, or other history of central nervous system injury; pregnancy, lactation, or inadequate contraception. Those agreeing to participate had a comprehensive evaluation that included medical, psychiatric, and drug use histories as well as physical, psychiatric, and laboratory examinations. All psychiatric and substance use disorder diagnoses were made using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV (SCID). All diagnostic interviews were conducted at study enrollment.
Treatment
Behavioral Intervention
Participants were seen weekly during treatment by a study physician for medical visits. In addition to dispensing study medication, the physician utilized an adaptation of Medical Management (MM)22, a manualized treatment that was used in the NIAAA COMBINE Study.23 MM was derived from empirically-tested manualized therapies24,25 that were originally designed to approximate a primary care approach to the treatment of alcohol dependence. The treatment was delivered by a medical professional who monitored medication side effects, provided strategies to increase medication adherence26, and supported abstinence. The initial 45-minute session included reviewing the cannabis dependence diagnosis and negative consequences from smoking cannabis, recommending abstinence, providing medication information, and offering strategies to enhance medication adherence. In subsequent 15–25 minute visits, recent substance use, overall functioning, medication adherence, and side effects were discussed.
Medication
Participants were randomized to receive either placebo or nabilone for 10 weeks and received 7-day prescriptions in blister packs containing one week of medication or placebo, separated into daily doses. Nabilone was provided by Meda (now Mylan) Pharmaceuticals and the McLean Research Pharmacy prepared capsules that contained either nabilone or placebo, and riboflavin 25 mg (to confirm medication adherence). Participants were monitored in our laboratory for 3 hours following the initial dose of the medication and the first dose that represented a dose increase as a safety precaution. This monitoring, coupled with the close surveillance resulting from twice weekly visits, yielded few adverse events from treatment with nabilone or placebo. Participants in the nabilone group were initiated at a dose of 0.5 mg daily for 7 days, then increased to 1 mg daily for 7 days, then 1.5 mg daily for 7 days, then 2 mg daily for 4 weeks before tapering the medication over the final 3 weeks by reversing the titration schedule.
Biochemical Monitoring
Quantitative urine cannabinoid tests (UCTs) were obtained at screening and twice-weekly during the study. The urine samples were screened by immunoassay for the tetrahydrocannabinol (THC) metabolite 11-nor-carboxy-Δ 9-tetrahydrocannabinol (THC-COOH) as well as other drugs of abuse. This standard quantitative UCT did not detect the presence of nabilone. The threshold for detection of THC-COOH was 50 ng/ml. Samples positive for this metabolite underwent further analysis by gas chromatography-mass spectroscopy to obtain a quantitative THC-COOH concentration. Levels of THC-COOH were normalized to creatinine and reported as a ratio to mitigate variability in drug measurement due to urine dilution.27
Measures
We used the Timeline Follow Back interview (TLFB)28 to obtain retrospective estimates of cannabis use in the week prior to study entry and weekly use of cannabis during the study. Use was recorded as days of use, use sessions per day, and inhalations per day in order to obtain a comprehensive assessment. Cannabis craving was assessed at screening and weekly using the Marijuana Craving Questionnaire (MCQ).29 The Beck Anxiety Inventory (BAI)30 and the Quick Inventory for Depressive Symptoms (QIDS)31 were administered at screening and during weeks 4, 10, and 14. Three different methods were used to assess medication adherence pill counts, daily diaries, and urinary riboflavin levels. Participants received compensation of $100 for their completed diaries as well as a cash bonus of $40 for completing all four medication monitoring visits. Participants earned up to a total of $955 for their participation in the study.
Statistical Analysis
The primary hypothesis was that cannabis-dependent participants receiving nabilone would experience no difference in adverse events during study treatment versus those receiving placebo during a 10-week randomized clinical trial. A secondary preliminary hypothesis was that cannabis-dependent participants receiving nabilone would use less marijuana as measured by self-report and UCTs during study treatment versus those receiving placebo. An intent-to-treat (ITT) approach including all randomized participants was used as the primary efficacy analysis. For the ITT analysis, all available data on those lost to follow-up or missing study visits were included in the analyses of all outcome measures.
At baseline descriptive statistics were calculated for all participants and compared between treatment groups. T-tests and chi-squared tests were used to compare baseline demographic and clinical measures between treatment groups. A repeated measures linear mixed effects regression model was utilized to estimate the overall effect of nabilone (versus placebo) on changes in quantitative test results (e.g., creatinine-adjusted cannabinoid levels) during active treatment. Secondary analyses using linear mixed effects models were developed to assess craving (Marijuana Craving Questionnaire; MCQ) and anxiety (Beck Anxiety Inventory, BAI). For these calculations, three post-baseline time periods were used: weeks 1–5 of treatment, weeks 6–10 of treatment, and the 4-week follow-up period.
Data were analyzed using Stata version 13 and the SPSS statistical package (IBM, Somers, NY). Results are presented as mean changes (from baseline) with 95% confidence intervals (CI). Significance was set at a two-sided α-level of .05 and no corrections have been applied to presented p-values.
RESULTS
Participant Characteristics and Study Retention
The CONSORT diagram showing participant flow through the study is provided in Figure 1. A total of 32 participants (10 female, 22 male) attended an in-person screening, 18 of whom were randomized to receive either nabilone of placebo. Five participants were tobacco smokers, all of whom smoked 5 or fewer cigarettes per day. Three participants withdrew from the study due to time constraints or moving out of the area, while two participants were withdrawn by study staff due to repeated missed visits. Participants were withdrawn if they missed and medication management visit and went more than 4 days without taking the medication. One participant was removed from the study due to a positive pregnancy test at week 5; she completed all remaining study visits for monitoring purposes after the medication was stopped in week 5. A total of 12 participants (3 female, 9 male) were enrolled and completed the treatment phase. These 12 participants were all Caucasian and employed with a mean age of 25.1 ± 6.8 years. Aside from investigator-approved contraception, no participants were on prescribed daily medications.
Figure 1. Consort Diagram.
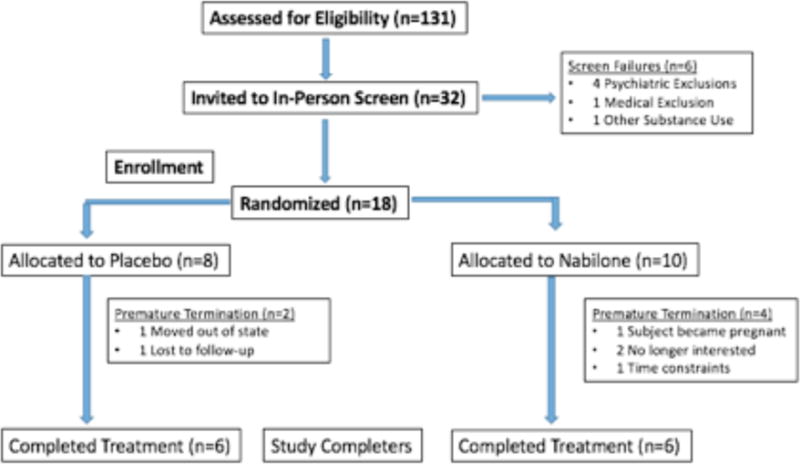
Consort diagram depicting the flow of participants through the recruitment process including the reasons for premature termination. 131 potential subjects were screened over the phone and 32 of those subjects underwent an in-person screening visit. 8 subjects withdrew from the study after the screening visit due to no longer wanting to continue with the study.
Using weekly pill counts to assess medication adherence, 96.1% (1743/1813) of possible doses were taken by participants; 97.0% (923/952) in the group randomized to receive nabilone and 95.6% (890/931) in the placebo group. Measuring adherence using daily diaries, 95.1% (1725/1813) of possible doses were taken by participants, 93.9% (894/952) in the nabilone group and 96.8% (901/931) in the placebo group. Finally, using qualitative riboflavin tests to assess adherence, 88.3% (241/273) of tests demonstrated adherence; 85.9% (122/142) in the group randomized to receive nabilone and 90.1% (127/141) in the placebo group.
Safety and Tolerability
We assessed safety and tolerability at each study visit. A clinician evaluated patients for possible adverse events with an open-ended interview and a comprehensive review of clinical measurements. A total of 8 adverse events were reported in 2 participants in the nabilone group and 6 events were reported in 4 participants in the placebo group during study treatment. All reported adverse events were rated mild-to-moderate. Nausea, vomiting, and sedation were the most commonly-reported adverse events; nausea was reported by 1 participant in both the nabilone and the placebo groups, vomiting was reported by 2 participants in the placebo group, and sedation was reported by 1 participant in both groups. No FDA-defined serious adverse events were documented during the study. No other participants in either group discontinued medication due to adverse events.
Efficacy
In the ITT analysis, treatment with nabilone had no effect on changes in cannabis use patterns over time as measured by TLFB or daily diaries (Table 2). Study participants reported daily cannabis use frequency (sessions per day) and inhalations per day via TLFB taken at each visit (Figure 2). The nabilone-treated group reported an average of 2.55 ±0.86 use sessions per day during the ten-week treatment period; the placebo group reported 3.14 ± 1.91 sessions per day. There was no significant treatment effect on changes in cannabis use sessions between the participants randomized to nabilone compared to placebo at the end of treatment (z=−1.05, p=0.29) or the end of follow-up (z=0.63, p=0.53). The nabilone-treated group reported using cannabis an average of 91.7± 12.6% of days during the 70 days of treatment while the placebo-treated group used a reported 89.0 ± 10.7% days during the treatment phase. There was no treatment effect on changes in the percent of days of use between either group the placebo group at the end of treatment (z=−1.23, p=0.22) or the end of follow-up (z=0.24, p=0.81). The nabilone-treated group reported an average of 42.5 ± 34.6 inhalations per day during treatment while the placebo-treated group reported 28.0 ± 20.5 inhalations per day. There was no treatment effect on changes in the number of inhalations per day between the nabilone-treated group and the placebo-treated group at the end of treatment (z=−1.09, p=0.28) or the end of follow-up (z=−0.12, 0.90). As shown in Table 2, self-report from the Daily Diaries also showed no difference in inhalations or days of use between the nabilone- and placebo-treated groups.
Table 2.
Cannabis Use Outcome Measures
| 2a. Baseline to End of Treatment (EOT) | |||||
|---|---|---|---|---|---|
| Change In Placebo Group | Change In Nabilone Group | Difference | |||
| Coefficient 95% C.I. | Coefficient 95% C.I. | z p-value | |||
| TLFB | |||||
| Inhalations | 2.74 (−17.9, 23.4) | −10.9 (−24.0, 2.26) | −1.09 | 0.26 | |
| Sessions/day | 0.51 (−1.26, 2.27) | −0.50 (−1.12, .116) | −1.05 | 0.29 | |
| Days of use | 4.70 (−11.5, 20.9) | −9.26 (−24.5, 5.97) | −1.23 | 0.22 | |
| Daily Diaries | |||||
| Inhalations | −23.2 (−39.9, −6.99) | −12.0 (−25.7, 1.77) | 1.04 | 0.30 | |
| Days of use | −8.61 (−13.1, −4.11) | −12.0 (−25.6, −1.71) | −0.46 | 0.65 | |
| UCT | |||||
| Ratio | 66.7 (−52.6, 186.0) | −121.4 (−368.9, 126.1) | −1.34 | 0.16 | |
| 2b. Baseline to End of Follow-Up (EOF) | |||||
| TLFB | |||||
| Inhalations | −10.7 (−21.3, −.124) | −12.0 (−28.6, 4.64) | −0.12 | 0.90 | |
| Sessions/day | −1.10 (−2.22, .022) | −.619 (−1.61, .368) | 0.63 | 0.54 | |
| Days use | −14.2 (30.5, 2.03) | −10.7 (−35.2, 13.9) | 0.24 | 0.81 | |
| Daily Diaries | |||||
| Inhalations | −37.5 (−62.7, −12.2) | −14.6 (−30.3, 1.25) | 1.51 | 0.13 | |
| Days of use | −23.5 (−40.4, −6.64) | −12.5 (−30.6, 5.02) | 0.89 | 0.38 | |
| UCT | |||||
| Ratio | −34.3 (−149.7, 81.1) | −146.2 (−414.0, 121.5 | −0.75 | 0.45 | |
Figure 2. Cannabis Use During Study.
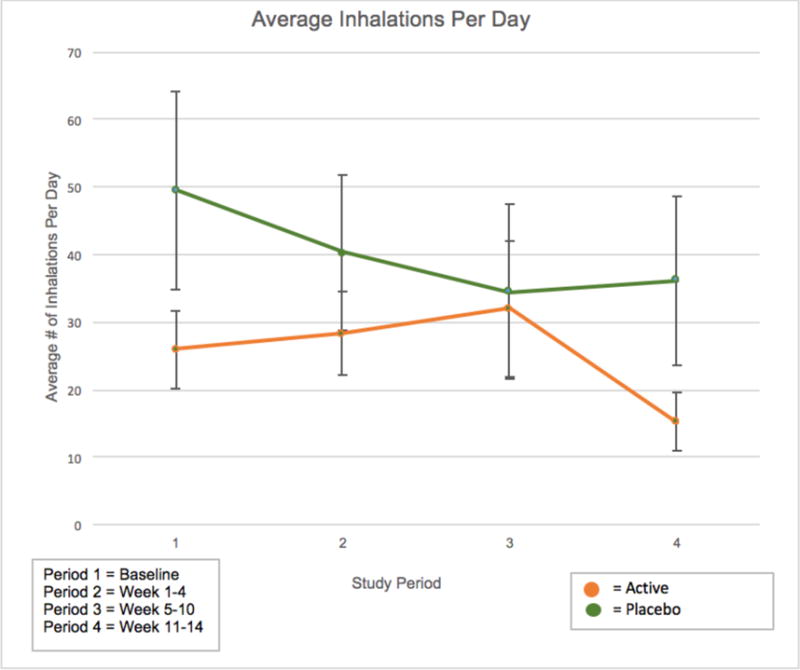
Reported cannabis use in nabilone- and placebo-treated participants as recorded by self-reported inhalations per day as a function of study period. Study periods were defined as: 1) Baseline; 2) weeks 1–4; 3) weeks 5–10; 4) weeks 11–14.
In addition, there was no significant treatment effect on changes in the urine cannabinoid levels between participants randomized to nabilone as compared to placebo at the end of treatment (z=−1.39, p=0.17) or the end of follow-up (z=−0.96, p=0.34).
Other Outcomes
Craving was assessed using the MCQ total score as well as the subscale scores (purposefulness, emotionality, expectancy, and compulsion). Although both the nabilone-treated and placebo-treated groups showed a reduction in MCQ total scores, there were no significant treatment group differences at either the end of treatment or the end of follow-up (z= −0.34, p=0.74 and z=−0.40, p=0.69). However, within each group, there was a statistically significant decrease in MCQ total scores in both the nabilone (z=−2.34, p=0.02) and placebo groups (z=−2.06, p=0.04). The nabilone-treated group exhibited a significant decrease during the entire study in both the MCQ Factor 1: Compulsivity (z=−2.89, p=0.004) and MCQ Factor 2: Emotionality (z=−2.63, p=0.008). The placebo group demonstrated a significant decrease during the study in MCQ Factor 3: Expectancy (z=−3.02, p=0.002). Both groups experienced a significant decrease in MCQ Factor 4: Purposefulness (z=−2.57, p=0.01 and z=−2.01, p=0.05) but this was only during the treatment period. There were no significant changes in BAI scores between the two groups both at the end of treatment (z=0.68, p=0.50) and at the end of the follow-up period (z=−0.10, p=0.92). Similarly, there was no significant difference in QIDS scores between groups at the end of treatment (z=0.76, p=0.46) and at the end of the follow-up period (z=0.91, p=0.36).
DISCUSSION
The results from the present pilot study demonstrated that nabilone pharmacotherapy was safe and tolerable. Adverse events were rare and did not occur more commonly in participants receiving nabilone. In addition, participants adhered to the medication regimen well while in the study protocol. These findings, taken together with human laboratory studies utilizing higher doses, encourage expanding its evaluation using higher doses.19,20
Nabilone pharmacotherapy did not demonstrate an advantage over placebo in cannabis use outcomes. There was no significant difference in use, as measured by both self-report and UCTs, between treatment groups. These findings were similar to results demonstrated in other clinical trials for cannabis dependence, thus underscoring the need to develop an effective medication for this patient population.32,33,34,35,36
There are several limitations to this pilot study. We randomized participants more slowly than anticipated, perhaps due the considerable number of study visits and imaging visits at baseline and follow-up that were long in duration and required 12 hours of abstinence prior to the imaging visits. Several potential participants did not feel that they could be abstinent for 12 hours prior to these visits and opted not to participate. In addition to a small sample size conducted at a single site, we experienced high drop-out rates similar to those described in other randomized controlled trials of medications for cannabis dependence.36,37 Those with cannabis dependence often do not seek treatment and when they do, they have difficulty adhering to a program. In this era of medical cannabis and legalized recreational cannabis, perception of risk has declined, making it harder for some to accept their cannabis use disorder as a significant problem requiring treatment.5 The small sample size also resulted in relatively low statistical power in our between-group comparisons. Although necessary in the progression to study higher doses, the maximum dose of nabilone, 2 mg daily, in this study, decided upon during the FDA Investigational Drug application process, was modest in order to assess for the safety and tolerability of this medication in participants with cannabis dependence. Previous studies on nabilone in cannabis users had been conducted in a human laboratory setting with continuous medical monitoring.19,20 Combining pharmacotherapy with an effective behavioral intervention more intensive than the medical management we utilized may result in increased reductions in cannabis use.
This pilot study demonstrates that nabilone pharmacotherapy for cannabis dependence is safe and tolerable at a maximum dose of 2 mg daily. Future studies should test higher doses of nabilone as pharmacotherapy for cannabis dependence or combine nabilone with more intensive behavioral interventions. As more states in the United States consider medical cannabis and recreational cannabis legalization, the development of medications such as nabilone for cannabis use disorders remains an urgent clinical and public health need.
Table 1.
Demographics and Clinical Characteristics
| Variable | Total (n=18) | Nabilone (n=10) | Placebo (n=8) | P Value |
|---|---|---|---|---|
| Age | 26.4 ± 6.5 | 24.4 ± 5.2 | 28.9 ± 7.5 | 0.154 |
| Male | 12 (67%) | 7 (70%) | 5 (63%) | 0.755 |
| Caucasian | 12 (67%) | 6 (60%) | 6 (75%) | 0.531 |
| Body Mass Index (BMI) | 24.0 ± 3.7 | 23.5 ± 3.0 | 24.6 ± 4.5 | 0.526 |
| Years of Education | 14.4 ± 3.4 | 13.7 ± 1.8 | 15.4 ± 4.7 | 0.297 |
| # of Days Used in Past 30 Days | 28.1 ± 2.9 | 27.8 ± 3.2 | 28.7 ± 2.4 | 0.533 |
| THC/Creatinine Ratio | 4.2 ± 4.8 | 4.5 ± 3.5 | 4.2 ± 7.0 | 0.915 |
| MCQ Total Score | 51.9 ± 12.3 | 51.7 ± 14.0 | 52.3 ± 10.4 | 0.933 |
| MCQ Compulsivity Score | 7.5 ± 4.6 | 8.1 ± 5.3 | 6.7 ± 3.7 | 0.572 |
| MCQ Emotionality Score | 11.7 ± 4.6 | 12.0 ± 3.7 | 11.2 ± 4.9 | 0.714 |
| MCQ Expectancy Score | 14.9 ± 4.0 | 14.2 ± 4.5 | 15.8 ± 3.4 | 0.467 |
| MCQ Purposefulness Score | 17.9 ± 3.0 | 17.3 ± 3.43 | 18.7 ± 2.4 | 0.426 |
Acknowledgments
This study was supported by NIDA K99/R00 DA029115 (Kevin P. Hill, MD, MHS, PI), NIDA K24 DA019855 (Shelly F. Greenfield, MD, MPH, PI), NIDA K24 DA022288 (Roger D. Weiss, MD, PI).
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. Dr. Weiss has consulted in the past for Indivior and GW Pharmaceuticals. Nabilone was provided by Meda (now Mylan) Pharmaceuticals.
References
- 1.NSDUH. Substance Abuse and Mental Health Services Administration, 2009 Results from the 2008 National Survey on Drug Use and Health: National Findings. Rockville, MD: http://oas.samhsa.gov/nsduh/2k8results.pdf. [Google Scholar]
- 2.Center for Behavioral Health Statistics and Quality (CBHSQ) Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015. (HHS Publication No. SMA 15-4927, NSDUH Series H-50). [Google Scholar]
- 3.Lopez-Quintero C, Perez de los Cobos K, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the national epidemiologic survey on alcohol and related conditions (NESARC) Drug Alcohol Depend. 2011;115(1–2):120–30. doi: 10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volkow ND, Baier RD, Compton WM, Wise SRB. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219–2227. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug and Alcohol Depend. 2011;116(1–3):142–150. doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689–1698. doi: 10.1038/npp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of n -acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805–812. doi: 10.1176/appi.ajp.2012.12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray KM, Sonne SC, McClure EA, et al. A randomized placebo-controlled trial of n-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Depend. doi: 10.1016/j.drugalcdep.2017.04.020. In Press. NIHMSIDL NIHMS883959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comer SD, Collins ED, Fischman MW. Buprenorphine sublingual tablets: effects on IV heroin self-administration by humans. Psychopharmacology (Berl) 2001;154:28–37. doi: 10.1007/s002130000623. [DOI] [PubMed] [Google Scholar]
- 10.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;8(3) doi: 10.1002/14651858.CD002209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008;(1) doi: 10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]
- 12.Dole VP, Nyswander M. A medical treatment of diacetylmorphine (herom) addiction. JAMA. 1965;195:646–650. doi: 10.1001/jama.1965.03090080008002. [DOI] [PubMed] [Google Scholar]
- 13.Kleber HD. Pharmacologic treatments for opioid dependence: detoxification and maintenance options. Dialogues Clin Neurosci. 2007;9(4):455–470. doi: 10.31887/DCNS.2007.9.2/hkleber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss RD. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence. Arch Gen Psychiatry. 2011;68(12):1238. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews Reviews. 2012 doi: 10.1002/14651858.cd000146.pub4. [DOI] [PubMed] [Google Scholar]
- 16.Allsop DJ, Copeland J, Lintzeris N, et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiatry. 2014;71(3):281–91. doi: 10.1001/jamapsychiatry.2013.3947. [DOI] [PubMed] [Google Scholar]
- 17.Cesamet® [product information] Quebec, Canada: Meda Pharmaceuticals; 2013. [Google Scholar]
- 18.Lile JA, Kelly TH, Hays LR. Separate and combined effects of the cannabinoid agonists nabilone and Δ9-THC in humans discriminating Δ9-THC. Drug Alcohol Depend. 2011;116(1–3):86–92. doi: 10.1016/j.drugalcdep.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haney M, Cooper ZD, Bedi G, et al. Nabilone decreases marijuana withdrawal and a laboratory measure of marijuana relapse. Neuropsych. 2013;38(8):1557–65. doi: 10.1038/npp.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrmann ES, Cooper ZD, Bedi G, et al. Effects if zolpidem alone and in combination with nabilone on cannabis withdrawal and a laboratory model of relapse in cannabis users. Psychopharmacology (Berl) 2016;233(13):2469–78. doi: 10.1007/s00213-016-4298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis 1 Disorders, Research Version, Patient Edition (SCID-I/P) New York, NY: Biometrics Research, New York State Institute; 2012. [Google Scholar]
- 22.Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;335:365–74. doi: 10.1056/NEJMoa055255. [DOI] [PubMed] [Google Scholar]
- 23.Anton RF, O’Malley SS, Ciraulo DA, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: The combine study: A randomized controlled trial. JAMA. 2006;295(17):2003–17. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 24.Carroll KM, O’Malley SS. Compliance Enhancement: A Manual for the Psychopharmacotherapy of Alcohol Dependence. 1996 Unpublished manuscript. [Google Scholar]
- 25.Mason BJ, Goodman AM. Brief Intervention and Medication Compliance Procedures—Therapist’s Manual. 1997 http://www.alcohol-free.com.
- 26.Volpicelli JR, Rhines KC, Rhines JS, Volpicelli LA, Alterman AI, O’Brien CP. Naltrexone and alcohol dependence. Role of subject compliance. Arch Gen Psychiatry. 1997;54(8):737–742. doi: 10.1001/archpsyc.1997.01830200071010. [DOI] [PubMed] [Google Scholar]
- 27.Fraser AD, Worth D. Urinary excretion profiles of 11-nor-9-carboxy-delta9 tetrahydrocannabinol: a delta9-THCCOOH to creatinine ratio study. J Anal Toxicol. 1999;23:531–534. doi: 10.1093/jat/23.6.531. [DOI] [PubMed] [Google Scholar]
- 28.Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the timeline followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav. 2009;28(1):154–162. doi: 10.1037/a0030992. Retrieved from http://ezproxy.neu.edu/login?url=http://search.proquest.com.ezproxy.neu.edu/docview/1266150234?accountid=12826. [DOI] [PubMed] [Google Scholar]
- 29.Heishman SJ, Evans R, Singleton EG, Levin KH, Copersino ML, Gorelick DA. Reliability and validity of a short form of the marijuana craving questionnaire. Drug Alcohol Depend. 2008;102(1–3):35–40. doi: 10.1016/j.drugalcdep.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fydrich T, Dowdall D, Chambless DL. Reliability and validity of the beck anxiety inventory. J Anxiety Disord. 1992;6(1):55–61. [Google Scholar]
- 31.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QID-C), and self-report (QIDS_SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 32.Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116(1–3):142–150. doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carpenter KM, McDowell D, Brooks DJ, Cheng W, Levin FR. A preliminary trial: double-blind comparison of nefazodone, bupropion-SR and placebo in the treatment of cannabis dependence. Am J Addict. 2009;18(1):53–64. doi: 10.1080/10550490802408936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levin FR, McDowell D, Evans SM, et al. Pharmacotherapy for marijuana dependence: a double-blind, placebo-controlled pilot study of divalproex sodium. Am J Addict. 2011;13(1):21–32. doi: 10.1080/10550490490265280. [DOI] [PubMed] [Google Scholar]
- 35.McRae-Clark AL, Carter RE, Killeen TK, et al. A placebo-controlled trial of buspirone for the treatment of marijuana dependence. Drug Alcohol Depend. 2009;105(1–2):132–138. doi: 10.1016/j.drugalcdep.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McRae-Clark AL, Baker NL, Gray KM, Killeen T, Hartwell KJ, Simonian SJ. Vilazodone for cannabis dependence: a randomized, controlled pilot trial. Am J Addict. 2012;25(1):69–75. doi: 10.1111/ajad.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Compton WM, Han B, Jones CM, Blanco C, Hughes A. Marijuana use and use disorders in adults in the USA, 2002–14:analysis of annual cross-sectional surveys. Lancet Psychiatry. 2016 doi: 10.1016/S2215-0366(16)30208-5. [DOI] [PubMed] [Google Scholar]


