Summary
We have demonstrated the feasibility of an endoscopic ultrasound-guided injectable hydrogel separation technique using a cadaveric model to increase the space between the head of the pancreas and duodenum. Using modeling studies, we identified the minimum distance of this separation for optimal sparing of the duodenum, setting the foundation for future clinical trials using this technique to enable dose escalation with either stereotactic or intensity-modulated radiation therapy for patients with unresectable pancreatic cancer.
Purpose
We assessed the feasibility and theoretical dosimetric advantages of an injectable hydrogel to increase the space between the head of the pancreas (HOP) and duodenum in a human cadaveric model.
Methods and Materials
Using 3 human cadaveric specimens, an absorbable radiopaque hydrogel was injected between the HOP and duodenum by way of open laparotomy in 1 case and endoscopic ultrasound (EUS) guidance in 2 cases. The cadavers were subsequently imaged using computed tomography and dissected for histologic confirmation of hydrogel placement. The duodenal dose reduction and planning target volume (PTV) coverage were characterized using pre- and postspacer injection stereotactic body radiation therapy (SBRT) plans for the 2 cadavers with EUS-guided placement, the delivery method that appeared the most clinically desirable. Modeling studies were performed using 60 SBRT plans consisting of 10 previously treated patients with unresectable pancreatic cancer, each with 6 different HOP–duodenum separation distances. The duodenal volume receiving 15 Gy (V15), 20 Gy (V20), and 33 Gy (V33) was assessed for each iteration.
Results
In the 3 cadaveric studies, an average of 0.9 cm, 1.1 cm, and 0.9 cm HOP–duodenum separation was achieved. In the 2 EUS cases, the V20 decreased from 3.86 cm3 to 0.36 cm3 and 3.75 cm3 to 1.08 cm3 (treatment constraint <3 cm3), and the V15 decreased from 7.07 cm3 to 2.02 cm3 and 9.12 cm3 to 3.91 cm3 (treatment constraint <9 cm3). The PTV coverage improved or was comparable between the pre- and postinjection studies. Modeling studies demonstrated that a separation of 8 mm was sufficient to consistently reduce the V15, V20, and V33 to acceptable clinical constraints.
Conclusions
Currently, dose escalation has been limited owing to radiosensitive structures adjacent to the pancreas. We demonstrated the feasibility of hydrogel separation of the HOP and duodenum. Future studies will evaluate the safety and efficacy of this technique with the potential for more effective dose escalation using SBRT or intensity-modulated radiation therapy to improve the outcomes in patients with unresectable pancreatic cancer.
Introduction
Pancreatic ductal adenocarcinoma is now the third leading cause of cancer-related deaths, with a devastating 5-year overall survival rate of nearly 8%, despite having the 12th most common incidence of all malignancies in the United States (1). One third of patients will present with borderline resectable or unresectable, locally advanced pancreatic cancer (BR/LAPC) (2-5). In the case of LAPC, chemotherapy with or without radiation therapy (RT) can be recommended to improve quality of life by relieving symptoms and extending survival (6-11). Despite aggressive combined modality therapy, the median survival has remained 9 to 15 months (12, 13).
The current guidelines for the management of BR/LAPC include single- or multiagent chemotherapy or chemoradiation (CRT) in sequence with chemotherapy (14). The results of studies comparing chemotherapy alone to CRT for patients with BR/LAPC have been mixed (10-12, 15). The importance of local control or delaying local progression to improve morbidity and possibly mortality in patients with pancreatic cancer is supported by autopsy data demonstrating that 30% of patients die of locally destructive disease (16). It follows that in cases of LAPC, advanced RT techniques using dose escalation with intensity modulated RT (IMRT) and stereotactic body RT (SBRT) are potential strategies to improve local control. The feasibility of dose escalation, however, has been limited owing to the sensitivity of the surrounding gastrointestinal organs, in particular, the small bowel, which is directly adjacent to the head of the pancreas (HOP). Advances in image guidance have provided the opportunity to safely deliver greater biologically effective doses (BEDs) of RT using IMRT of >70 Gy (57.25 Gy in 25 fractions; BED 70.36 Gy) compared with standard fractionation regimens (50.40 Gy in 28 fractions or 50 Gy in 25 fractions; BED of 59.47 Gy and 60 Gy, respectively) resulting in improved overall survival (17). However, candidates for dose escalation were restricted to those with >1 cm of separation between the pancreas and the closest gastrointestinal mucosa, restricting the therapy to <25% of patients in reported series of LAPC patients (17).
SBRT involves a short course of RT, ≤5 fractions, and has demonstrated greater rates of local control compared with CRT at other disease sites (18). Early studies evaluating SBRT for pancreatic cancer used single fractions of 25 Gy, resulting in local control rates of 100% at 1 year but unacceptably high rates of gastrointestinal toxicity (19-22). More recently, hypofractionated SBRT (33 Gy total; 6.6-Gy daily fractions) has been evaluated and used by our group in an effort to reduce the toxicity of therapy, with results demonstrating a nearly 80% rate of freedom from local progression at 1 year and an acceptable 11% rate of longterm gastrointestinal toxicity (23). The outcomes with SBRT have thus been promising. Higher local control rates with dose escalation might be achievable; however, current practice has been limited owing to the risks of toxicity (24).
Previously, hydrogel injection for spacing organs at risk (OARs) from the radiation target to increase the deliverable dose or improve the safety of RT has been evaluated most extensively in the treatment of prostate cancer (25-28), with some experience in gynecologic cancers as well (29). Hydrogel spacer injection between the rectum and prostate and seminal vesicles has resulted in a reduced rectal dose and toxicity and improved quality of life (25). A smaller report of patients with gynecologic malignancies demonstrated the utility of hydrogel injection in patients requiring repeat irradiation to increase the space between the vagina and rectum to allow for a reduction in the dose delivered to the rectum (29). A similar hydrogel is available that has been approved as a tissue marker (TraceIT). The product consists of a hydrogel paste that creates a bleb of particles at the needle tip on injection. This bleb remains dimensionally stable for 3 months and is fully absorbed after 7 months. This product has been reported in several studies for marking purposes, demonstrating its stability in the esophagus (30), bladder (31), and cervix (32); however, it has not yet been evaluated for its functionality in organ spacing.
The aim of the present study was to assess the feasibility of using this novel injectable absorbable radiopaque hydrogel to facilitate dose escalation by increasing the space between the HOP and duodenum. Human cadaveric models were used to evaluate the spacer technique and to assess its theoretical dosimetric advantages using simulated SBRT plans. Our results will thus set the stage for further investigations using the technique to separate the HOP–duodenum interface in patients, enabling further dose intensification with SBRT or IMRT to improve the clinical outcomes in patients with BR/LAPC.
Methods and Materials
Preparation and imaging of cadaveric specimens
After institutional review board approval, 3 refrigerated, unfixed, unfrozen, cadaveric specimens were obtained within 3 postmortem days. Before injection of the hydrogel, computed tomography (CT) simulation (Philips Brilliance Big Bore CT; 2-mm slice thickness, 120 kVp, 200 mA, 60-cm field of view) was performed with the cadaveric specimen supine. Laparotomy was performed by a pancreatic surgeon (J. H) on 1 cadaveric specimen to expose the HOP–duodenum interface. An absorbable hydrogel synthesized as iodinated polyethylene glycol microparticles (TraceIT Tissue Marker; Augmenix, Bedford, MA) was injected by an experienced gastroenterologist (E. S) in the space between the HOP and the third portion of the duodenal loop using an 18-gauge needle. To assess the feasibility of endoscopic ultrasound (EUS)-guided hydrogel injection, a linear EUS scope (Pentax EG-3870UTK) coupled to an ultrasound workstation (Hitachi Preirus) was used to identify the duodenum and HOP interface in 2 cadaveric models, followed by hydrogel injection in this peripancreatic space using a 19-gauge fine needle aspiration needle in increments of 1 mL, delivering as many milliliters as needed in a particular region to generate the desired space. The EUS scope was then adjusted (slightly advanced or retracted) around the target region to provide shape and conformity around the tumor to generate the desired space, with the total injection volume ranging from 10.0 to 27.5 mL. A visible separation between the HOP and duodenum was created as shown in Figure 1 and Figure E1 (available online at www.redjournal.org). CT simulation was subsequently repeated on all 3 cadaveric specimens, 1 after laparotomy and 2 after EUS guidance, to confirm the location of the hydrogel and to measure the distance created between the duodenum and HOP. The mean distance of separation by hydrogel placement was measured by averaging the measured thickness of the gel on each CT slice on which gel was visualized on the postinjection simulation CT scan obtained with a 2-mm slice thickness (Fig. E1; available online at www.redjournal.org).
Fig. 1.
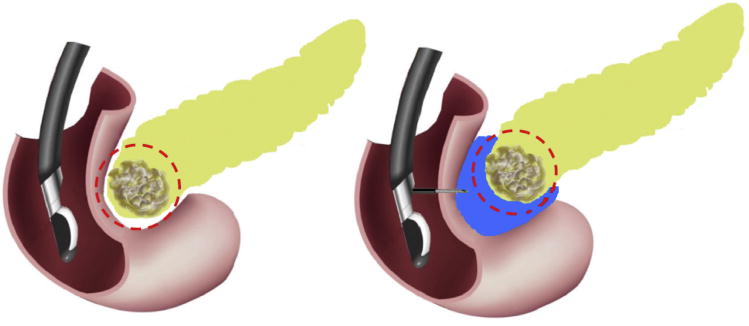
Diagram of absorbable radiopaque hydrogel spacer implanted under endoscopic ultrasound imaging.
Histologic analysis of gel placement
After injection, a pancreatic surgeon (J. H) dissected each cadaveric specimen by performing an en bloc resection of the pancreas and duodenum, preserving the injection site for histologic examination. The tissue was serially sectioned to grossly visualize the injection cavity, and the sections were then fixed in formalin, embedded in paraffin and stained with hematoxylin and eosin for microscopic examination of gel placement.
Planning methods
On the pre- and posthydrogel spacer placement CT scans, the duodenum, stomach, liver, kidneys, and a mock spherical tumor with a diameter of 2 cm were contoured (Pinnacle; Philips Radiation Oncology Systems, Milpitas, CA). On the prehydrogel spacer injection CT scans, the mock 2-cm spherical tumor was placed within the HOP, with the tumor edge at the interface of the HOP–duodenum. On the posthydrogel spacer injection CT scans, the mock tumor was placed in the same axial level in the HOP, with the tumor edge abutting the edge of the gel spacer. The target volumes for the mock tumor were created as previously described for our previous SBRT trial (23), with the final planning target volume (PTV) involving a 2-mm margin expansion of the mock tumor, unless this resulted in direct expansion into within 2 mm of the duodenum (our current institutional practice). A total of 4 SBRT plans were created, 1 for each of the pre- and posthydrogel spacer injection CT scans of the 2 cadaver specimens with EUS-guided spacer placement.
The planning objectives and parameters were identical for all pre- and postspacer treatment plans (ie, the same number of beams, number of iterations, and objective functions were used) and were as follows: 10 or 11 coplanar step-and-shoot beams, direct machine parameter optimization, 50 iterations, PTV receiving ≥33 Gy of ≥95% (weight 20), PTV maximum dose 33 Gy (weight 20), liver volume receiving ≥9 Gy of <50% (weight 1), kidney volume receiving ≥9 Gy of <25% (weight 1), proximal stomach volume receiving ≥8 Gy of <3% (weight 3), proximal duodenum volume receiving ≥15 Gy (V15) of <30% (weight 3), proximal duodenum maximum dose 20 Gy (weight 3), and PTV 1 to 2-cm ring maximum dose of 14 Gy (weight 1). The protocol passing criteria for OARs were as follows: volume receiving ≥15 Gy (V15) <9 cm3, ≥20 Gy (V20) <3 cm3, and ≥33 Gy (V33) <1 cm3 to the proximal duodenum, stomach, and small bowel, defined as within 1 cm above and below the PTV; liver volume receiving ≥12 Gy (V12) <50%; combined kidney volume receiving ≥12 Gy (V12) <75%; and spinal cord volume receiving ≥8 Gy (V8) <1 cm3 (23).
Radiation planning and dose modeling studies using clinical treatment plans
Ten patients with BR/LAPC who underwent SBRT at our institution were randomly selected after institutional review board approval. The V15, V20, and V33 of the proximal duodenum were evaluated from the original clinically treated plans prescribed to 33 Gy in 6.6-Gy fractions. HOP–duodenum displacement was simulated in these cases by shifting the proximal duodenum a series of distinct distances away from the original PTV, without overlap of the stomach; thus, modeling the separation distance and the resultant effect on the dose to the OARs of a theoretical spacer placement of various thicknesses using identical planning parameters and objectives as stated. The V15, V20, and V33 and the respective relative reductions of the dose to the duodenum were evaluated in each plan with serially increasing separation distances. The relative reduction of the dose volume for comparison was calculated as follows:
Statistical analysis was performed using the paired t test between the original plans and simulation plans (separation of 2, 3, 5, 8, and 15 mm, respectively). P<.01 was considered statistically significant.
Results
Technical feasibility of spacer placement
To first assess the feasibility of expanding the space between the HOP and the duodenum, hydrogel injection after laparotomy and exposure of the retroperitoneum of the cadaveric specimen was attempted. Compared with the preinjection CT scans (Fig. 2A), the postinjection axial CT scans demonstrated successful injection of the radiopaque hydrogel as a contrast-enhancing region between the HOP and duodenum (Fig. 2B) after laparotomy, direct visualization, and spacer injection. Gross histologic sections confirmed hydrogel (dyed blue) placement in the HOP–duodenum interface (Fig. 2C). The mean thickness of the spacer was 0.9 cm (range 0.7-1.2) on the post-injection CT scans.
Fig. 2.
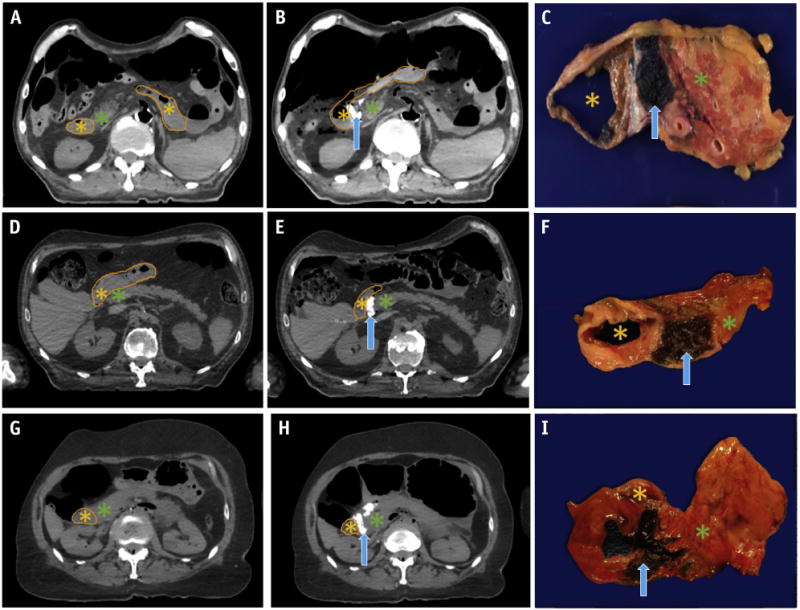
Computed tomography scans before and after hydrogel spacer injection between the head of the pancreas and duodenum, with postinjection gross histologic specimens confirming location of the spacer. (A-C) Gel placed using laparotomy. Gel placed endoscopically in (D-F) EUS cadaveric specimen 1 and (G-I) EUS cadaveric specimen 2. Duodenal lumen (orange outline and asterisk), hydrogel spacer (blue arrow), and head of the pancreas (green asterisk) are denoted.
After demonstration of successful hydrogel cavity formation and separation of the HOP and duodenum with spacer injection by laparotomy and needle injection, endoscopic spacer injection was performed on 2 cadaveric specimens using EUS guidance. The preinjection axial CT scan, postinjection axial CT scan, and gross histologic sections of EUS cadaveric specimen 1 (Fig. 2D-F) and EUS cadaveric specimen 2 (Fig. 2G-I) showed successful spacing of the duodenum and pancreas after hydrogel injection. The mean thickness of the spacer was 1.1 cm (range 0.9-1.2) and 0.9 cm (range 0.8-1.1) for EUS cadaveric specimens 1 and 2 on the postinjection CT scans, respectively.
Microscopic examination of gel placement
Microscopic examination of the formalin-fixed, paraffin-embedded sections after hematoxylin and eosin staining revealed complete separation of the full duodenal mucosa from the pancreatic tissue in most regions without injection into the muscularis propia (Figs. 3A and 3B). Because of its particulated nature, most of the gel washed away from the tissue during the formalin-fixation process; however, small fragments of remnant gel were microscopically visible in the injection cavity. A few sections, however, revealed the presence of the gel as a small collection within the muscularis propia (Fig. 3C), with longitudinal areas of separation of the muscularis propia by strands of gel (Fig. 3D). One section of the cadaveric specimen injected by way of open laparotomy revealed possible fragments of gel within the lumen of a vein within the peripancreatic connective tissue (Fig. 3E). However, this was not seen on sections of the 2 cadaveric specimens with spacer placement performed with EUS guidance.
Fig. 3.
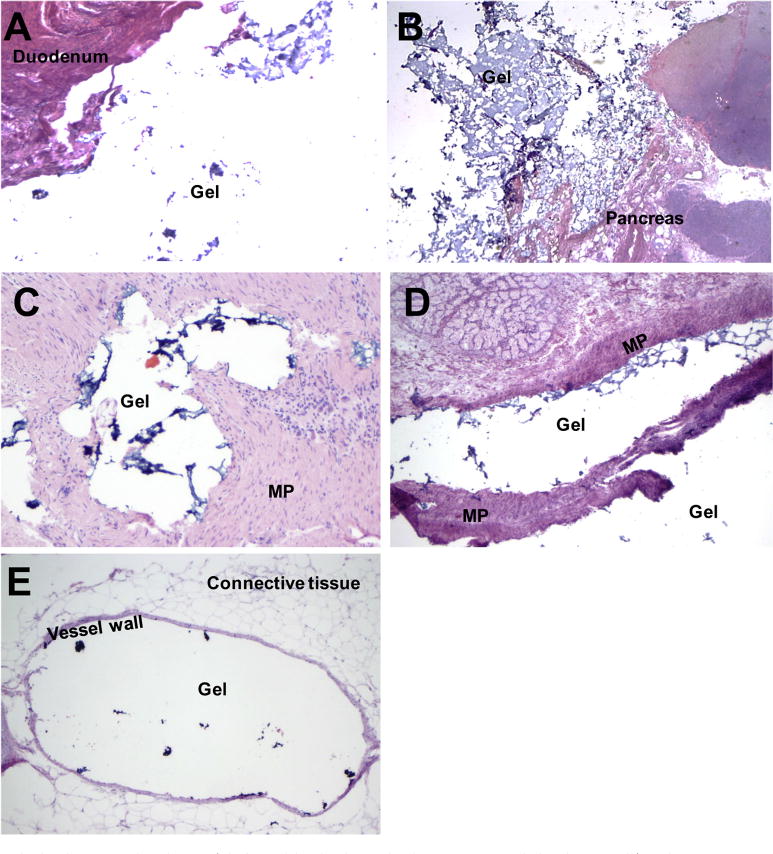
Histopathologic examination of injectable hydrogel placement. Original magnification (A,B,D) ×20 (2 × 10); (C) ×100 (10 × 10); and (E) ×40 (4 × 10). Abbreviation: MP = muscularis propria.
Dosimetric advantages of increased pancreas–duodenum interface spacing
Because the ideal placement of the hydrogel spacer would be by the less-invasive EUS-guided approach, the dosimetric advantage of the increased space between the HOP and the duodenum was evaluated on the 2 cadaveric specimens with hydrogel spacer injection using EUS guidance to most closely replicate the achievable separation in future clinical practice. SBRT plans were created for both pre- and postinjection simulation CT scans, targeting a spherical simulated tumor measuring 2 cm in diameter as described.
The pre- and postinjection plans for EUS cadaveric specimens 1 and 2 are shown in Figure 4. In both cases, a clinically acceptable plan achieving the predefined dose constraints for the proximal duodenum could not be achieved using the baseline preinjection simulation CT scan. To achieve PTV coverage of ≥95%, the optimal plans exceeded the predefined dose constraints to the proximal duodenum, estimating unacceptable doses of V20 of 3.86 cm3 in EUS cadaveric specimen 1 (Fig. 4A) and V15 of 9.12 cm3 and V20 of 3.75 cm3 in EUS cadaveric specimen 2 (Fig. 4C; treatment constraint V15 <9 cm3 and V20 <3 cm3). Spacer injection enabled the generation of clinically acceptable plans for both specimens, achieving all dose constraints with a significant margin and improved PTV coverage, as demonstrated by the postinjection plans for EUS cadaveric specimen 1 (Fig. 4B) and specimen 2 (Fig. 4D).
Fig. 4.
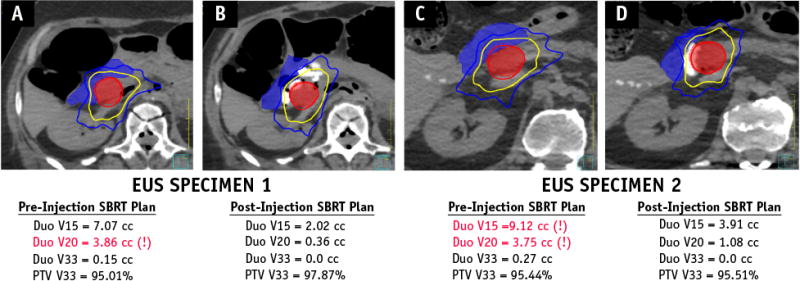
Comparison of stereotactic body radiation therapy plans before and after hydrogel spacer placement. Computed tomography scans of EUS Specimen 1 and 2 are shown before (A and C) and after (B and D) hydrogel spacer placement. The planning target volume (red shadowed) was generated by a 2-mm uniform expansion of the spherical mock tumor placed at the boundary of the head of the pancreas-duodenum interface in the pre-injection trials and abutting the boundary of the spacer in the head of the pancreas in the post-injection trials. The duodenum (blue shadowed) and isodose lines for 33 Gy (red), 20 Gy (yellow), and 15 Gy (blue) are depicted for each plan.
Modeling on sample patients
An example of the modeling technique used to assess the dosimetric effect of discrete, interval increases in spacing of the duodenum from the pancreas is shown in Figure 5. With simulated serial separations of 2, 3, 5, 8, and 15 mm between the duodenum and pancreas, the volumes of the proximal duodenum covered by 15 Gy, 20 Gy, and 33 Gy decreased (Fig. 5B-F). Furthermore, the low-dose region to the liver and chest wall was reduced and coverage of the PTV improved with an increasing distance of separation.
Fig. 5.
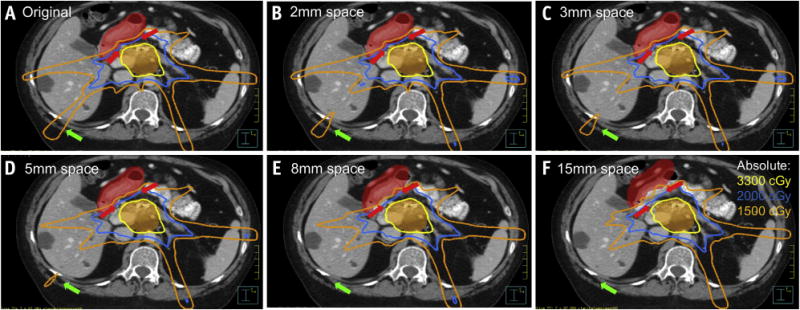
The effect of serially increased spacing (red arrowheads) of the proximal duodenum (red shaded) from the planning target volume (orange shaded). (A) Baseline scan and plan shown, with subsequently increased spacing of proximal duodenum from PTV of (B) 2 mm, (C) 3 mm, (D) 5 mm, (E) 8 mm, and (F) 15 mm. Isodose lines of 33 Gy (yellow), 20 Gy (blue), and 15 Gy (orange) are displayed for each of the simulated plans, with serially increased conformality of the low-dose region (green arrow) with a reduced dose to the liver and chest wall.
Absolute (Fig. 6A1-A3) and relative percent reduction (Fig. 6B1-B3) of the proximal duodenum V15, V20, and V33 were plotted from the 6 treatment plans generated to model the increasing space between the pancreas and duodenum in each of the 10 patients. Considering the duodenum dose constraints in our institute (Figs. 6A1 and 6A2, blue dash line, which represent V15 <9 cm3 and V20 <3 cm3, respectively), all sample patient plans achieved clinically acceptable plans with a minimum separation of 8 mm, although the objectives were achievable with even less separation in certain sample cases.
Fig. 6.
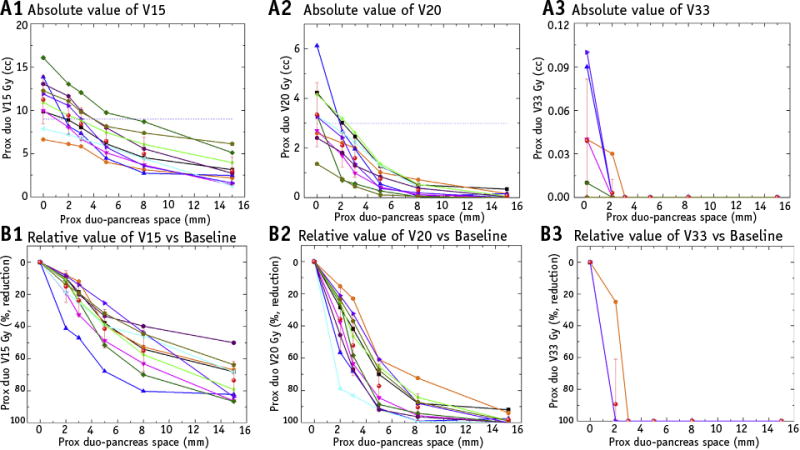
The comparison of absolute and relative values of duodenum in volume receiving 15 Gy (V15), volume receiving 20 Gy (V20), and volume receiving 33 Gy (V33) from 10 sample patient stereotactic body radiation therapy plans, each with serially increasing space between the proximal duodenum and pancreas. The blue dash line represents the clinical limits of endpoints in our institution (duodenum V15 <9 cm3,V20 <3 cm3,V33 <1 cm3). The red star dots with error bar indicate the mean value of corresponding endpoints at each separation distance. As the simulated separation from gel injection increased, both the duodenum V15, V20, and V33 absolute and relative volumes decreased.
The mean V15 of the proximal duodenum decreased from 11.24 cm3 in the original plan to 9.41 cm3 (P<.01), 8.42 cm3 (P<.01), 6.46 cm3 (P<.01), 4.98 cm3 (P<.01), and 3.00 cm3 (P<.01) in the simulation plans with a separation of 2, 3, 5, 8, and 15 mm, respectively. The mean V20 of the proximal duodenum decreased from 3.35 cm3 in the original plans to 2.10 cm3 (P<.01), 1.59 cm3 (P<.01), 0.75 cm3 (P<.01), 0.30 cm3 (P<.01), and 0.07 cm3 (P<.01) in the simulation plans with a separation of 2, 3, 5, 8, and 15 mm, respectively. The mean proximal duodenum V33 was 0 cm3 when the separation was ≥3 mm. Further details are provided in Tables E1-E4 (available online at www.redjournal.org).
As shown in Figs. 6B1 and 6B2, the mean proximal duodenum V15 and V20 decreased by 73.51% and 98.08% with 15 mm of gel injection, respectively. Compared with the steady decrease of the mean value of V15, the mean value of V20 showed large decreases of 35.79%, 52.06%, 77.19%, and 90.38% with 2, 3, 5, and 8 mm of separation, respectively, with a relatively small additional dose reduction after 8 mm of separation.
Discussion
The results of the present study have demonstrated the feasibility of injecting an absorbable radiopaque hydrogel spacer between the HOP and duodenum in a human cadaveric model to create sufficient separation of these 2 structures to enhance the potential for dose escalation. We further conducted a series of simulation studies to understand the dosimetric effects of serial spacing of the HOP–duodenum interface. We found a minimum separation distance of 8 mm would achieve significant dose reduction to the duodenum across all modeled cases–a distance of separation that was achievable with the minimally invasive EUS-guided injection technique. The clinical utility of this spacer placement is promising for the facilitation of safe dose escalation for improving clinical outcomes with RT for BR/LAPC.
Hydrogel injection for spacing of the bowel and rectum from other RT target structures in the abdomen has been shown to reduce toxicity and, in some cases, enable repeat RT when it otherwise might not have been feasible. Most experience with hydrogel injection has been in the region between the prostate and rectum for men receiving dose-escalated prostate RT (25-28). Recently reported results of a phase III study of dose-escalated IMRT for prostate cancer in 222 men randomized 2:1, with and without spacer placement, respectively, demonstrated a reduced rectal dose and toxicity and improved quality of life (25).
Smaller, but still promising, case reports of hydrogel, saline, or mesh spacing have also demonstrated the utility of organ spacing in RT for gynecologic (29), paraspinal (33), and liver (34) tumors. Three cases of recurrent gynecologic tumors, one uterine and two endometrial cancers, requiring repeat irradiation underwent hydrogel spacer placement (29). All 3 patients underwent transrectal ultrasound-guided insertion of the gel spacer in the fat plane between the vagina and rectum. With hydrogel spacer placement, the average reduction in the dose to the rectum for all 3 patients was 11%. For the 2 patients with sigmoid colon in the RT field, the average dose reduction to the sigmoid after spacer placement was 45%. A slightly more invasive technique of placement of an all-purpose drainage catheter, followed by infusion of normal saline containing 5% to 10% iohexol has been explored to separate para-spinal tumors from bowel or kidneys in 10 patients (33). Patients were treated in 1 to 3 fractions with repeat injection of the normal saline with iohexol before each fraction, with confirmation of localization using cone beam CT imaging. A mean separation of 17.5 mm was achieved between the PTV and the OAR of interest. The feasibility of a biologic mesh spacer placement was investigated in patients with liver cancer with close proximity to radio-sensitive bowel in 7 patients treated with a mean dose of 76 Gy over 13 to 25 fractions, with the exception of 1 patient who received 4 fractions of SBRT to 40 Gy (34). Even with such dose-intensive therapy, no patients experienced significant gastrointestinal toxicity, supporting the benefit of organ-spacing treatment of liver tumors.
TraceIT hydrogel has been used previously for marking purposes in the esophagus (30), bladder (31), and cervix (32). These studies reported that the hydrogel remained dimensionally stable throughout the RT course, without migrating through the tissue. These organs, admittedly, have many different properties than the interface between the pancreas and duodenum, but TraceIT hydrogel has so far been stable, even in highly mobile organs such as the bladder and esophagus.
The possible risks of hydrogel spacer placement must be considered. Although no significant adverse events of spacer injection have been reported to date in the prostateerectum hydrogel spacer (25) nor for TraceIT hydrogel as a tissue marker (30-32), the unique anatomy and technical challenges of spacer placement in the potential space between the pancreas and duodenum could potentially pose new risks. Microscopic analysis of the duodenal mucosa and pancreatic parenchyma after hydrogel injection in our cadaveric specimens revealed a few regions of injection within the muscularis propia of the duodenum. This could have resulted from the injection technique or, possibly, was specific to our cadaveric model, which had undergone some degree of postmortem autolysis of tissue. The possible side effects of injection in the muscularis propria should be better understood before expanding this technique to human subjects.
From a tumor control perspective, an important consideration with the spacing technique is the concern of disrupting and disseminating microscopic disease at the HOP–duodenum interface. The procedure of injecting the hydrogel spacer from the perspective of the duodenal lumen into the peripancreatic region will theoretically reduce this risk of pushing disease away from the treatment volume. Still, to definitively investigate this possibility, our upcoming clinical trial evaluating this technique will be performed in patients with borderline resectable disease. A significant proportion of these patients will undergo surgical resection; thus, histopathologic analysis of the resected HOP-spacer-duodenum interface from the pancreaticoduodenectomy specimen will be able to identify whether the spacer is interrupting the microscopic disease extent. Because the dose to the pancreas and duodenum interface would be reduced, this separation technique would be contraindicated for any patient with imaging or examination findings on EUS suggestive of duodenal invasion.
The major limitation of the present study was the small sample numbers, owing to the availability of resources, including the cadaveric specimens and access to a cadaver/animal research endoscopic EUS unit. Although we have discussed the potential risks to be considered with hydrogel injection, the small numbers in our study might have limited our ability to encounter or hypothesize all the potential risks of this approach. Upcoming studies will focus on further understanding the stability of hydrogel placement and the safety profile of this separation technique, starting initially in animal models. A porcine study is being planned to understand the stability of the hydrogel placement and side effects of injection into the duodenal mucosa to assess for immediate perforation or ulcer formation. Additionally, the risk of pancreatitis with injection directly into the pancreas will be assessed. The study will also evaluate the persistent radiopacity, which could provide valuable information regarding patient positioning by showing the HOP–duodenum interface using cone beam CT. If animal studies have demonstrated acceptability, clinical trials will be conducted to assess the effect of unique patient anatomy on the success of EUS-guided injection and to explore the true dosimetric and subsequent clinical advantages of this novel absorbable radiopaque hydrogel spacer for patients receiving RT for pancreatic cancer. An additional minor limitation was our lack of ability to simulate nearby organ motion (eg, stomach or other bowel loops) affected by the serial spacing of the duodenum in our modeling study. It is likely that the movement of the duodenum would alter the positioning of nearby structures, which would likely alter the dose distribution to those OARs; however, we were unable to model this alternation in our current simulation strategy.
Conclusions
We have demonstrated the feasibility of hydrogel separation of the HOP and duodenum. Ongoing studies will evaluate the stability, safety, and efficacy of this technique in animal studies with the intent to follow with planned human trials to evaluate the potential for more effective dose escalation using SBRT or IMRT to improve the outcomes in patients with unresectable pancreatic cancer using this novel hydrogel spacer placement.
Supplementary Material
Acknowledgments
The present study was supported by a Radiation Oncology Discovery grant and National Institutes of Health grant CA62924. Augmenix, creator of the hydrogel spacer, funded the purchasing of the cadaveric specimens but had no role in the analysis or reporting of the results. None of the authors have a financial interest in Augmenix.
Footnotes
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Rudra S, Narang AK, Pawlik TM, et al. Evaluation of predictive variables in locally advanced pancreatic adenocarcinoma patients receiving definitive chemoradiation. Pract Radiat Oncol. 2012;2:77–85. doi: 10.1016/j.prro.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dholakia AS, Hacker-Prietz A, Wild AT, et al. Resection of borderline resectable pancreatic cancer after neoadjuvant chemoradiation does not depend on improved radiographic appearance of tumor–vessel relationships. J Radiat Oncol. 2013;2:413–425. doi: 10.1007/s13566-013-0115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: A report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 5.Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol. 2008;26:3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman JM, Wild AT, Wang H, et al. Randomized phase III multi-institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: Final results. J Clin Oncol. 2013;31:886–894. doi: 10.1200/JCO.2012.44.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Josef E, Schipper M, Francis IR, et al. A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2012;84:1166–1171. doi: 10.1016/j.ijrobp.2012.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gastrointestinal Study Group. Treatment of locally unresectable carcinoma of the pancreas: Comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst. 1988;80:751–755. [PubMed] [Google Scholar]
- 9.Li J, Ng J, Allendorf J, Saif MW. Locally advanced pancreatic adenocarcinoma: Are we making progress? J Pancreas. 2011;12:347–350. [PubMed] [Google Scholar]
- 10.Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer: Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19:1592–1599. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 11.Huguet F, Andre T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 12.Loehrer PJ, Sr, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: An Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben-Josef E, Shields AF, Vaishampayan U, et al. Intensity-modulated radiotherapy (IMRT) and concurrent capecitabine for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;59:454–459. doi: 10.1016/j.ijrobp.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 14.NCCN guidelines version 1. Pancreatic adenocarcinoma. [Accessed March 12, 2017];2017 Available at: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf.
- 15.Hammel P, Huguet F, van Laethem JL, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: The LAP07 randomized clinical trial. JAMA. 2016;315:1844–1853. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 16.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishnan S, Chadha AS, Suh Y, et al. Focal radiation therapy dose escalation improves overall survival in locally advanced pancreatic cancer patients receiving induction chemotherapy and consolidative chemoradiation. Int J Radiat Oncol Biol Phys. 2016;94:755–765. doi: 10.1016/j.ijrobp.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timmerman RD, Kavanagh BD, Cho LC, et al. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol. 2007;25:947–952. doi: 10.1200/JCO.2006.09.7469. [DOI] [PubMed] [Google Scholar]
- 19.Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radio-surgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017–1021. doi: 10.1016/j.ijrobp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Schellenberg D, Goodman KA, Lee F, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72:678–686. doi: 10.1016/j.ijrobp.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 21.Schellenberg D, Kim J, Christman-Skieller C, et al. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;81:181–188. doi: 10.1016/j.ijrobp.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115:665–672. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]
- 23.Herman JM, Chang DT, Goodman KA, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 20157:121. 1128–1137. doi: 10.1002/cncr.29161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldsmith C, Price P, Cross T, et al. Dose-volume histogram analysis of sterotactic body radiotherapy treatment of pancreatic cancer: A focus on duodenal dose constraints. Semin Radiat Oncol. 2016;26:149–156. doi: 10.1016/j.semradonc.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Hamstra D, Mariados N, Sylvester J, et al. Continued benefit to rectal separation for prostate radiation therapy: Final results of a phase III trial. Int J Radiat Oncol Biol Phys. 2017;97:976–985. doi: 10.1016/j.ijrobp.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 26.Hatiboglu G, Pinkawa M, Vallee JP, et al. Application technique: Placement of a prostate-rectum spacer in men undergoing prostate radiation therapy. BJU Int. 2012;110:E647–E652. doi: 10.1111/j.1464-410X.2012.11373.x. [DOI] [PubMed] [Google Scholar]
- 27.Pinkawa M, Piroth MD, Holy R, et al. Spacer stability and prostate position variability during radiotherapy for prostate cancer applying a hydrogel to protect the rectal wall. Radiother Oncol. 2013;106:220–224. doi: 10.1016/j.radonc.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Pinkawa M, Berneking V, Konig L, et al. Hydrogel injection reduces rectal toxicity after radiotherapy for localized prostate cancer. Strahlenther Onkol. 2017;193:22–28. doi: 10.1007/s00066-016-1040-6. [DOI] [PubMed] [Google Scholar]
- 29.Viswanathan AN, Damato AL, Nguyen PL. Novel use of a hydrogel spacer permits reirradiation in otherwise incurable recurrent gynecologic cancers. J Clin Oncol. 2013;31:e446–e447. doi: 10.1200/JCO.2012.47.9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin P, Hulshof MC, De Jong R, et al. Quantification of respiration-induced esophageal tumor motion using fiducial markers and four-dimensional computed tomography. Radiother Oncol. 2016;118:492–497. doi: 10.1016/j.radonc.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Chao M, Ho H, Liodakis P, et al. The use of TraceIT® as a fiducial marker in bladder radiotherapy. Int J Urol. 2016;23:47. [Google Scholar]
- 32.Bair RJ, Bair E, Viswanathan AN. Radiopaque polymer hydrogel used as a fiducial marker in gynecologic brachytherapy. Brachytherapy. 2014;13:S73. doi: 10.1016/j.brachy.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katsoulakis E, Solomon SB, Maybody M, et al. Temporary organ displacement coupled with image-guided, intensity-modulated radiotherapy for paraspinal tumors. Radiat Oncol. 2013;8:150. doi: 10.1186/1748-717X-8-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ismael HN, Denbo J, Cox S, et al. Biologic mesh spacer placement facilitates safe delivery of dose-intense radiation therapy: A novel treatment option for unresectable liver tumors. Eur J Surg Oncol. 2016;42:1591–1596. doi: 10.1016/j.ejso.2016.05.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


