Abstract
Objective
Excess deposition of fat within and around vital organs and non-adipose tissues is hypothesized to contribute to cardiovascular disease (CVD) risk. We evaluated the association of abdominal intermuscular adipose tissue (IMAT) volume with coronary artery calcification (CAC) in Coronary Artery Risk Development in Young Adults (CARDIA) Study participants.
Approach and Results
We measured IMAT in the abdominal muscles, visceral (VAT) and pericardial (PAT) adipose tissue, and CAC using computed tomography (CT) in 3,051 CARDIA participants (56% women) at the CARDIA year 25 examination (2010–11). Mean IMAT volume and mean IMAT/total muscle volume (IMAT normalized for muscle size) were calculated in a 10-mm block of slices centered at L3–L4. Multivariable analyses included potential confounders and traditional CVD risk factors. Compared to the lowest quartile, the upper quartile of abdominal IMAT volume was associated with higher CAC prevalence [OR (95% CI) 1.6(1.2, 2.1)] after adjusting for CVD risk factors. Results were similar for highest versus lowest quartile of IMAT normalized to total muscle volume [OR (95%CI) 1.5 (1.1, 2.0)]. Significant associations of higher IMAT and normalized IMAT with CAC prevalence persisted when BMI, VAT or PAT were added to the models.
Conclusions
In a large, community-based, cross-sectional study, we found that higher abdominal skeletal muscle adipose tissue volume was associated with subclinical atherosclerosis independent of traditional CVD risk factors and other adipose depots.
Keywords: adipose tissue, muscle, coronary artery calcification, computed tomography
Subject Codes: Obesity, Atherosclerosis, Risk Factors, Epidemiology, Computerized Tomography (CT)
Introduction
Nearly 40% of U.S. adults aged 40 to 59 years have body mass indices ≥30 kg/m2 and are considered to be clinically obese based on recent National Health and Nutrition Examination Survey (NHANES) data1. Obesity, in turn, increases the risk of age-related disability2 and chronic diseases including diabetes, heart disease and cancer3.
Coronary heart disease (CHD) risk related to obesity is partially attributable to expansion of visceral adipose tissue (VAT), the adipose depot most closely associated with cardiometabolic risk factors4. However, excess adipose tissue may also be deposited within and around non-adipose tissues such as skeletal muscle, potentially altering tissue physiology and causing detrimental cardiovascular effects both locally and systemically5–8. Studies that have directly quantified intermuscular adipose tissue (IMAT) using computed tomography (CT) consistently show that higher IMAT is, like VAT, associated with dyslipidemia7, impaired glucose metabolism9, metabolic syndrome10, and inflammation11. Although IMAT is associated with cardiometabolic risk factors for atherosclerosis, to date, the few studies that have evaluated the association of directly measured IMAT with subclinical measures of atherosclerosis such as intima-media thickness or arterial calcification provide conflicting information on a role for IMAT12–16.
The present study directly measured IMAT and other abdominal adipose depots along with coronary artery calcification (CAC) with non-contrast CT in 3,051 participants aged 43–55 in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. We tested the hypothesis that higher abdominal IMAT deposition is directly associated with CAC prevalence independent of traditional CVD risk factors and other measures of adipose deposition.
Materials and Methods
Study population
The Coronary Artery Risk Development in Young Adults (CARDIA) Study began in 1985 with recruitment of 5,115 participants aged 18 to 30 years at field centers located in Birmingham, AL, Chicago, IL, Minneapolis, MN, and Oakland, CA17. The current cross-sectional study includes data from participants who agreed to undergo computed tomography (CT) scans at the year 25 CARDIA examination (3,189 of 3,498 year 25 participants; 91%).
Materials and Methods are available in the online-only Data Supplement
Results
Participant Characteristics
Older age, lower education, lower physical activity, and a history of smoking were associated with higher overall abdominal IMAT volume (p<0.001 for each comparison) whereas race, sex, and alcohol intake did not significantly differ across IMAT quartiles (Table 1). Diabetes prevalence, glucose, HbA1c and HOMA-IR were each directly associated with IMAT volume (p<0.001 for each comparison). CRP, blood pressures and blood pressure treatment, triglycerides, and cholesterol treatment were also associated positively with higher IMAT whereas HDL-c was inversely associated with IMAT (p<0.001 for each comparison). Height, BMI, WC, SAT, VAT and PAT were also associated with higher IMAT (p<0.001 for each comparison).
Table 1.
Participant characteristics [(mean(SD), median(25th, 75th percentile) or %(n)] by sex-specific quartiles overall IMAT volume
| Characteristic | All Participants (n=3051) |
IMAT Volume (cm3), quartiles* | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| <1.27 (n=762) |
1.27 – <1.90 (n=761) |
1.90 – <2.92 (n=764) |
≥2.92 (n=764) |
p | ||
| Age, years | 50.1(3.6) | 49.3(3.6) | 50.2(3.6) | 50.5(3.6) | 50.5(3.6) | <.0001 |
| Female Sex | 56.2%(1716) | 58.7% (447) | 54.3% (413) | 56.5% (432) | 55.5% (424) | 0.241 |
| Black Race | 47.2% (1441) | 45.7% (348) | 46.5% (354) | 46.5% (355) | 50.3% (384) | 0.161 |
| Education, years | 15.6(2.6) | 16.0(2.6) | 15.7(2.6) | 15.4(2.5) | 15.2(2.5) | <.0001 |
| Physical Activity, units | 277 (126–486) | 330 (158–547) | 306 (144–509) | 264 (115–467) | 216 (97–403) | <.0001 |
| Smoking | ||||||
| Never | 60.9% (1859) | 67.3% (513) | 58.6% (446) | 61.1% (467) | 56.7% (433) | |
| Ever | 21.8% (665) | 17.6% (134) | 23.9% (182) | 22.4% (171) | 23.3% (178) | |
| Current | 17.3% (527) | 15.1% (115) | 17.5% (133) | 16.5% (126) | 20.0% (153) | 0.001 |
| Alcohol Intake, ml/day | 2.4 (0–14.7) | 2.7 (0–14.5) | 2.7 (0–17.0) | 2.4 (0–14.3) | 2.4 (0–14.7) | 0.295 |
| Diabetes | 12.3% (375) | 5.2% (40) | 9.3% (71) | 11.6% (89) | 22.9% (175) | <.0001 |
| Glucose | 99.4(28.5) | 92.1(19.4) | 97.2(29.7) | 99.8(25.5) | 108.6(34.9) | <.0001 |
| HbA1c, % | 5.7(1.0) | 5.5(0.7) | 5.7(1.0) | 5.7(0.9) | 6.0(1.2) | <.0001 |
| CRP, mg/l | 1.4 (0.6–3.5) | 0.8 (0.4–1.7) | 1.1 (0.5–2.5) | 1.7 (0.8–3.8) | 3.1 (1.4–6.5) | <.0001 |
| HOMA-IR† | 1.9 (1.1–3.1) | 1.2 (0.8–1.9) | 1.6 (1.0–2.6) | 2.2 (1.4–3.2) | 3.0 (2.0–4.5) | <.0001 |
| Systolic BP, mmHg | 119.8(16.0) | 115.8(15.1) | 119.0(15.1) | 120.5(16.4) | 123.9(16.4) | <.0001 |
| Diastolic BP, mmHg | 75.0(11.2) | 71.1(10.6) | 73.7(11.1) | 75.9(10.7) | 79.3(10.6) | <.0001 |
| BP Treatment | 27.3% (833) | 18.0% (137) | 24.3% (185) | 26.2% (200) | 40.7% (311) | <.0001 |
| Total cholesterol, mg/dl | 192.6(36.9) | 190.3(33.1) | 193.3(38.3) | 194.8(38.0) | 191.9(37.7) | 0.099 |
| LDL-c, mg/dl | 112.1(32.8) | 107.7(29.8) | 112.8(33.1) | 115.0(33.6) | 112.9(34.0) | 0.0001 |
| HDL-c, mg/dl | 58.0(18.0) | 64.9(19.0) | 58.8(18.2) | 55.5(16.3) | 52.8(16.3) | <.0001 |
| Triglycerides, mg/dl | 93 (68–134) | 75 (58–103) | 88 (65–126) | 100 (76–144) | 112 (80–161) | <.0001 |
| Cholesterol Treatment | 15.8% (481) | 10.2% (78) | 13.9% (106) | 17.8% (136) | 21.1% (161) | <.0001 |
| Height, cm | 170.3(9.4) | 168.8(8.8) | 170.5(9.2) | 170.6(9.7) | 171.5(9.7) | <.0001 |
| BMI, kg/m2 | 30.2(7.1) | 24.9(4.1) | 27.9(4.7) | 30.9(5.1) | 37.2(7.4) | <.0001 |
| WC, cm | 94.6(15.8) | 81.0(9.9) | 89.4(10.4) | 96.8(10.8) | 111.1(13.5) | <.0001 |
| SAT, cm3 | 335.0(169.4) | 211.0(115.0) | 289.4(131.4) | 362.2(141.6) | 476.8(160.9) | <.0001 |
| VAT, cm3 | 132.1(73.7) | 74.8(41.4) | 109.7(48.7) | 146.2(59.2) | 197.4(76.8) | <.0001 |
| PAT, cm3 | 56.8(33.7) | 35.6(17.6) | 48.1(23.2) | 60.2(28.6) | 83.1(40.6) | <.0001 |
Quartiles based on overall IMAT volume (cm3) averaged across left and right sides for all abdominal muscles measured in the study (rectus, lateral oblique, psoas and paraspinous);
HOMA-IR is reported for the 2,661 non-diabetics
Significance tests from ANOVA (continuous variables) or chi square analysis (categorical variables)
Muscle Composition
Individual and overall abdominal muscle composition is shown in Supplemental Table I. Compared to other muscle groups, psoas muscles were relatively lean with lower IMAT volume and IMAT normalized to total muscle volume. Lateral oblique and paraspinous muscles had ~2–5 fold greater IMAT volume than either the rectus or psoas muscles. However, normalized IMAT was higher in rectus muscles (~19% fat on average) than either paraspinous or lateral oblique muscles (11–12% fat).
Muscle Correlations with Measures of Adiposity
IMAT volume was correlated with BMI, WC, SAT, VAT, and PAT across all muscle groups (Supplemental Table II) with Pearson correlation coefficients ranging from 0.40 to 0.73 (all paired correlations p<0.001). Normalized IMAT was also significantly associated with anthropometric and CT measures of adipose tissue with correlations ranging from 0.25 to 0.64 (all correlations p<0.001). Total muscle volume was correlated with height, BMI, WC and VAT across all muscle groups (r=0.21–0.66, all correlations p<0.001). Individual muscle IMAT volumes were correlated with each other (r=0.56–0.77, all correlations p<0.001). Also, IMAT volume was correlated with total muscle volume with correlations ranging from r=0.32 for paraspinous to r=0.58 for lateral oblique (all correlations p<0.001).
IMAT and CAC Prevalence
CAC prevalence and scores were associated with overall abdominal IMAT volume (p<0.0001) in univariate analysis (Figure 2). CAC prevalence (CAC>0 Agatston Units) was approximately 20% in the lowest quartile compared to 38% in the highest quartile of IMAT. CAC scores ≥100 Agatston Units were present in 6.6% of participants having IMAT in the lowest quartile compared to 12.5% of participants within the highest quartile for IMAT.
Figure 2.
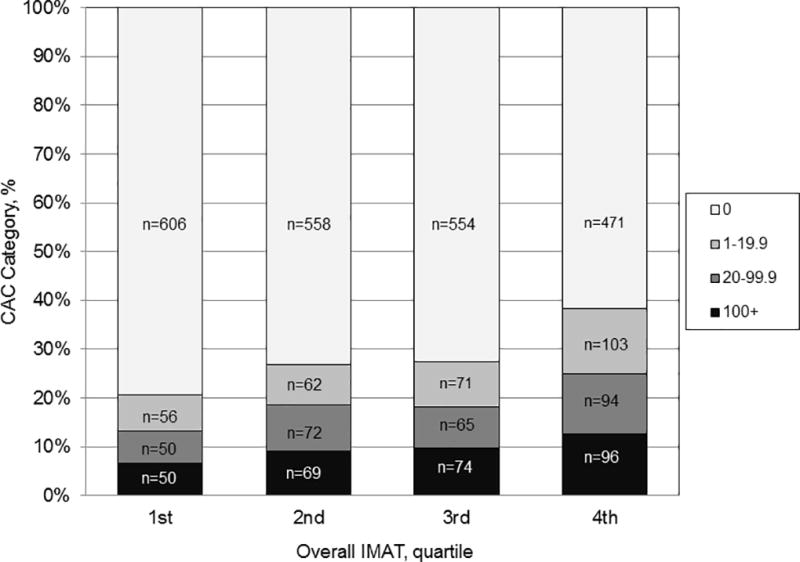
Unadjusted Agatston CAC scores by quartiles of overall IMAT volume (chi square analysis p<.0001).
IMAT volume and normalized IMAT were significantly associated with CAC prevalence after adjusting for potential confounding variables in multivariable logistic regression (model 1, Table 2). In model 1, continuous IMAT volume and normalized IMAT were directly associated with CAC prevalence (ptrend <0.0001), but when quartiles IMAT and normalized IMAT were substituted in the model, CAC prevalence was ~2-fold higher in the 4th quartile compared to the 1st quartile suggesting a threshold effect. Including traditional CVD risk factors (model 2) attenuated these findings, but associations of CAC prevalence with IMAT volume (OR=1.61, 95% CI 1.22–2.13) and normalized IMAT (OR=1.52, 95% CI 1.14–2.03) remained statistically significant. BMI, VAT, and PAT were separately added to model 2 to test the influence of generalized and regional adiposity on the association of IMAT with CAC. Adding BMI, VAT, or PAT to model 2 did not substantially change the association of higher IMAT and normalized IMAT with prevalent CAC (p<0.05 for all comparisons of 4th versus 1st quartile). In 2,661 participants without diabetes, we tested whether insulin resistance might explain associations between IMAT volume and normalized IMAT with prevalent CAC (Supplemental Table III). Comparing models 1 and 2, addition of HOMA-IR, along with other model 2 covariates, attenuated the association of both IMAT and normalized IMAT with prevalent CAC, but the upper quartile of IMAT was still significantly associated with ~1.5-fold more prevalent CAC than the lowest quartile. Adding BMI or VAT to model 2 did not attenuate associations of higher IMAT volume or normalized IMAT with CAC prevalence. Adding PAT to model 2 attenuated the association of the highest quartile of IMAT or normalized IMAT with CAC, but continuous IMAT measures remained significantly associated with higher CAC prevalence (p<0.02).
Table 2.
Logistic regression models for association of overall IMAT volume and normalized overall IMAT with CAC prevalence [Odds Ratio(95% Confidence Interval)]
| Measure | Quartile | CAC (cases/total) |
Model 1 | Model 2 | BMI | Model 2 plus VAT |
PAT |
|---|---|---|---|---|---|---|---|
| IMAT Volume | <1.27 | 156/762 | reference | reference | reference | reference | reference |
| 1.27 – <1.90 | 203/761 | 1.22 (0.95,1.58) | 1.11 (0.85,1.45) | 1.10(0.84,1.44) | 1.14(0.88,1.51) | 1.11(0.85,1.45) | |
| 1.90 – <2.92 | 210/764 | 1.22 (0.95,1.58) | 1.06 (0.81,1.39) | 1.06(0.79,1.41) | 1.13(0.84,1.51) | 1.06(0.80,1.40) | |
| ≥2.92 | 293/764 | 2.10 (1.64,2.69) | 1.61 (1.22,2.13) | 1.60(1.13,2.25) | 1.79(1.29,2.49) | 1.58(1.16,2.17) | |
| Ptrend | <0.0001 | 0.008 | 0.014 | 0.001 | 0.047 | ||
| Continuous | 1.27 (1.17,1.38)* | 1.14 (1.04,1.25) | 1.11 (0.98,1.26) | 1.17 (1.04,1.30) | 1.12(1.00,1.24) | ||
| Normalized IMAT | <0.067 | 201/763 | reference | reference | reference | reference | reference |
| 0.067 – <0.097 | 189/763 | 1.03 (0.80,1.33) | 0.91 (0.70,1.18) | 0.90 (0.70,1.18) | 0.94 (0.72,1.23) | 0.91(0.70,1.18) | |
| 0.097 – <0.144 | 228/761 | 1.47 (1.14,1.89) | 1.26 (0.97,1.65) | 1.24 (0.94,1.63) | 1.34 (1.00,1.78) | 1.25(0.95,1.66) | |
| ≥0.144 | 244/764 | 1.96 (1.51,2.54) | 1.52 (1.14,2.03) | 1.46 (1.04,2.03) | 1.65 (1.18,2.31) | 1.48(1.07,2.05) | |
| Ptrend | <0.0001 | 0.002 | 0.015 | 0.001 | 0.034 | ||
| Continuous | 1.29 (1.18,1.41) | 1.16 (1.05,1.28) | 1.14 (0.99,1.29) | 1.19 (1.06,1.34) | 1.13(1.01,1.27) |
Prevalence based 0 or >0 Agatston Units; normalized IMAT = IMAT volume/total muscle volume ratio Model 1: age, sex, race, sex*race, center, height (except when BMI is included), and education; Model 2: model 1 + physical activity, alcohol consumed, smoking history, diabetes, systolic BP, BP med use, HDL cholesterol, triglycerides, cholesterol med use, and CRP.
estimate per SD IMAT volume (1.63 cm3) or normalized IMAT (0.06 cm3); Bold indicates odds ratio differs significantly (p<.05) from Quartile 1 (reference). Ptrend is based on modeling continuous IMAT.
Associations of CAC prevalence with IMAT in individual muscles are presented in Supplemental Tables IV and V. Findings for individual muscle groups generally reflected those for overall IMAT; however, the associations of paraspinous IMAT with CAC prevalence appeared to be more robust than those in other muscle groups. Higher quartiles of paraspinous IMAT or normalized IMAT remained significantly associated with CAC prevalence in the fully adjusted models including other adipose measures (p<0.05 for comparisons of 4th quartile to 1st quartile in each model).
IMAT and CAC Score
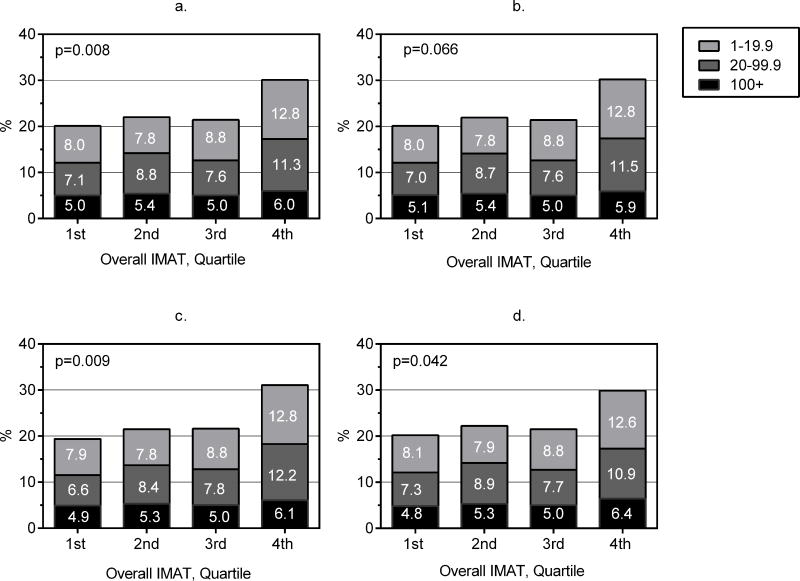
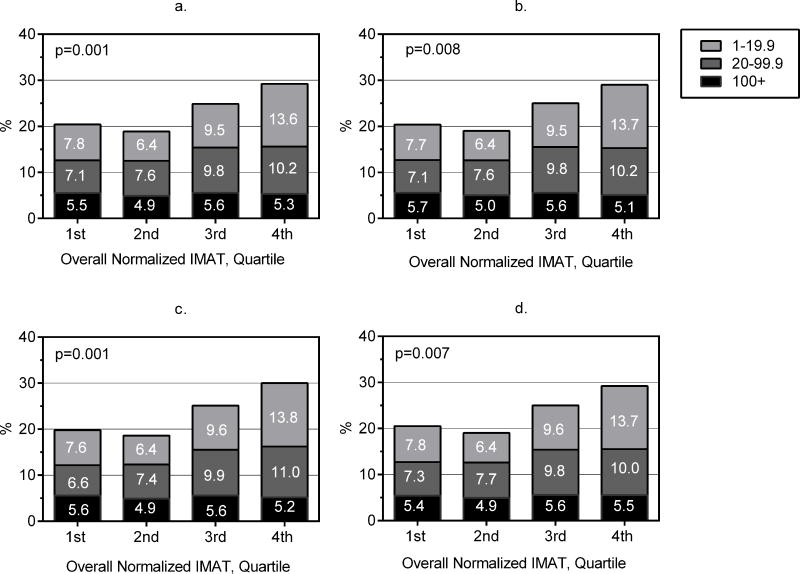
Probabilities of CAC scores across IMAT (Figures 3a–3d) and normalized IMAT (Figures 4a–4d) quartiles were determined using multinomial logistic regression. As shown in panel 3a, CAC scores varied significantly across IMAT quartiles after adjusting for CVD risk factors (p=0.008 for test with 9 degrees of freedom). Adjusting for BMI attenuated associations between IMAT and CAC scores to borderline significance (p=0.066; panel 3b). However, the association of CAC scores with IMAT remained significant when either VAT or PAT was included in the model (both p<0.05; panels 3c and 3d). CAC score probabilities across normalized IMAT quartiles, shown in Figures 4a–4d, were similar in patterning to those for IMAT volume. CAC scores varied significantly across quartiles of normalized IMAT in the CVD risk factor adjusted model (p=0.001; panel 4a), but additional adjustment for BMI, VAT or PAT made little difference in probability distributions (p<0.01 for all models; panels 4b–4d).
Figure 3.
a–d. Probability of positive CAC scores of 1–19.9, 20–99.9, and ≥100 Agatston Units across quartiles of IMAT volume derived using multivariable multinomial logistic regression. Probabilities are adjusted for age, sex, race, sex*race, center, height (except when BMI is included), education, physical activity, alcohol consumed, smoking history, diabetes, systolic BP, BP med use, HDL cholesterol, triglycerides, cholesterol med use, and CRP (panel 3a) with or without BMI (panel 3b) or VAT (panel 3c) or PAT (panel 3d). P-value is based on multinomial logistic regression model.
Figure 4.
a–d. Probability of positive CAC scores of 1–19.9, 20–99.9, and ≥100 Agatston Units across quartiles of normalized IMAT derived using multivariable multinomial logistic regression. Probabilities are adjusted for age, sex, race, sex*race, center, height (except when BMI is included), education, physical activity, alcohol consumed, smoking history, diabetes, systolic BP, BP med use, HDL cholesterol, triglycerides, cholesterol med use, and CRP (panel 4a) with or without BMI (panel 4b) or VAT (panel 4c) or PAT (panel 4d). P-value is based on multinomial logistic regression model.
Discussion
In this community-based, cross-sectional study of more than 3,000 middle-aged participants, we found that higher IMAT within the abdominal musculature was positively associated with CAC, an indicator of subclinical atherosclerotic plaque burden. Moreover, associations of higher IMAT with CAC were consistent across sex and race strata and persisted after adjusting for traditional CVD risk factors and other adipose measures. In our knowledge, this is the first large-scale population-based study to demonstrate that higher abdominal IMAT, directly measured using CT, is associated with subclinical atherosclerosis after adjusting for body composition, including the major ectopic depots of VAT and PAT.
Skeletal muscle is critical to glucose metabolism, accounting for ~80% of glucose utilization in healthy individuals18. Impaired glucose clearance in response to insulin, or insulin resistance, represents a key component in the development of type 2 diabetes and important contributor to cardiovascular risk18. Clinical studies have shown that IMAT accumulation is strongly associated with insulin resistance9,10,13,19. The present data and previous studies show that IMAT is directly correlated with VAT and PAT volume and the latter are strongly associated with adverse CVD risk factor profiles20–23. In CARDIA, we have recently shown that higher PAT is associated with diabetes prevalence even after controlling for BMI and VAT24. Higher VAT is also associated with prevalent subclinical atherosclerosis in the preponderance of studies, though it remains unclear as to whether this relationship is independent of cardiometabolic risk factors and overall obesity15,25–28. PAT is associated with both CAC and incident CVD and these associations appear to be independent from traditional risk factors and other measures of obesity15,16,22,29. Although IMAT contributes a relatively small proportion to the total abdominal adipose tissue stores, it is strongly associated with established CVD risk factors including insulin resistance, dyslipidemia, hypertension, hyperinflammatory states, and type 2 diabetes9–11,13,19. Moreover, a recent report in 1,063 older men demonstrated an association of lower calf muscle attenuation (a marker of higher IMAT levels) with all-cause and CVD mortality that was not explained by traditional risk factors including diabetes and dyslipidemia30.
The present study suggests that higher abdominal IMAT is associated with CAC development which is, in turn, a well-established non-invasive imaging marker for risk of incident CHD and CVD31,32. Previous studies that are directly comparable to the present study are few and may have been limited in power15,16. In a small MESA substudy (n=398) that used the present CT methods, IMAT was associated with CAC in univariate analysis, but this association did not persist in multivariable models15. A second study in African Americans with diabetes (n=422), found no significant associations of IMAT volume with calcified plaque in the coronary, carotid, or aortic arteries16. A number of previous studies should also be discussed in context with the present CARDIA data as those studies suggest that a higher proportion of lean muscle is associated with lower atherosclerosis12–14,33. In a study that included 100 participants, higher thigh muscle attenuation (indicative of leaner tissue) was associated with lower carotid intima-media thickness and the association persisted after adjustment for age, diabetes, insulin resistance, and VAT13. Higher proportion of lean abdominal muscle was associated with lower likelihood of thoracic aorta calcification in 394 participants, but the authors noted there were no significant associations of muscle composition with carotid, coronary, abdominal aorta, or iliac calcifications, nor was IMAT per se associated with higher calcification in any artery bed14. Recently, Ko and colleagues reported that higher total skeletal muscle mass measured using bioelectrical impedance was inversely associated with CAC in more than 31,000 South Korean adults, and these findings remained significant after adjusting for insulin resistance and other potential risk mediators33. In the study by Ko et al., it is important to note that skeletal muscle mass was inversely associated with BMI; in fact, in the highest quartile of muscle mass only 12% of participants were obese compared to 49% of participants in the lowest quartile of muscle mass33. Therefore, the data from Ko and colleagues suggest that it is higher lean muscle mass that is associated with healthier arteries33. The present study shows that higher abdominal IMAT, directly quantified using CT, is associated with the prevalence and extent of subclinical atherosclerosis in midlife. Moreover, these associations persist in multivariable models including CVD risk factors and other adipose measures.
Potential Mechanisms
Although IMAT accumulation is strongly associated with hypertension, diabetes, and dyslipidemia, and these are plausible intermediaries for the association between IMAT and CAC development, adjustment for these and other traditional risk factors did not explain the association of IMAT with CAC in the present study. CARDIA clinical evaluations (e.g. blood pressure, anthropometric measures) and laboratory measures (e.g. lipids, glucose) were thoughtfully designed and have been performed with consistency and precision over the study’s decades-long existence17. Diabetes was carefully defined based on fasting glucose, post-challenge glucose, and HbA1c or use of diabetes medications. Moreover, we performed a sub-analysis in non-diabetics that included adjustment for HOMA-IR, yet none of these traditional CVD risk factors fully explained the association of abdominal IMAT with CAC. Still, the present cross-sectional study relies on measures performed at a single visit. Previous studies in CARDIA have shown that in early life, between 18 and 30 years of age, exposure to even slightly elevated glucose and LDL-cholesterol (below clinical thresholds) is more strongly associated with CAC 15 years later than concurrent measures34. Moreover, CARDIA participants receive feedback on multiple risk factors making it likely that abnormal values are detected earlier in life and possibly treated sooner than in the general population. Though we adjusted for known CVD risk factors and treatment for diabetes, hypertension, and hypercholesterolemia, we cannot be sure that our cross-sectional study captures integrated risk factor exposure over a lifetime.
Excess muscle fat accumulation may be pro-inflammatory as suggested by Beasley and colleagues who found that IMAT was directly associated with IL-6 concentration11. Haam et al. found that CT-quantified IMAT was directly associated with circulating monocyte chemoattractant protein-1 (MCP-1), a pro-inflammatory chemotactic factor for monocytes and T-cells35. These studies, along with other lines of laboratory and clinical evidence, suggest that IMAT has the potential to promote and sustain an inflammatory microenvironment11,18,35. Moreover, as an activator of monocytes, MCP-1 has the potential to promote monocyte adherence to vascular endothelium and subsequent monocyte migration into the vessel wall where they are hypothesized to play a role in atherogenesis via differentiation into macrophages and ultimately foam cells36,37. Although circulating MCP-1 and other cytokines are associated with prevalent and incident CVD, it remains unclear if their roles are independent from traditional risk factors38–40. In the present cross-sectional study, the chronic inflammation marker CRP did not explain the association between IMAT and CAC. We did not measure IL-6 nor any of the myriad other chemokines that may explain the association of IMAT with subclinical atherosclerosis. Regardless, chronic inflammation is a plausible mechanism for development of atherosclerosis that has been linked to IMAT and other ectopic adipose depots4,18. Moreover, based on previous studies11,35, it is possible that the contribution of higher IMAT to risk for atherosclerosis extends beyond its correlation with other ectopic adipose depots. The association of CAC progression with cumulative exposure to central obesity has been documented in CARDIA41, and the present study suggests that excess IMAT may be one component of the risk associated with central obesity.
Limitations
The present study is limited by a cross-sectional design that does not permit us to establish definitively the temporal nature of the associations; however, it seems unlikely that calcified atherosclerosis is driving IMAT accumulation. Though we adjusted for known cardiometabolic risk factors believed to contribute to associations between IMAT and CAC, it is possible that emerging risk factors for CAC, such as circulating calcium-phosphorous product, contribute to associations as well42. The measurement of muscle composition was performed at the L3–L4 vertebral level and VAT volume was measured at the L4–L5 level, but we do not believe this would have a meaningful impact on our results. The composition of the abdominal muscles at these respective lumber levels is similar and highly correlated. Indeed, we chose to measure muscle composition at the L3–L4 level to avoid the pelvic bones that may prevent clear visualization of the lateral oblique muscles in some participants at the L4–L5 level. We primarily focused on overall abdominal IMAT in the present study, but we also measured specific muscle groups. Paraspinous and psoas muscles have often been the focus of prior studies due to their relative ease of measurement. The paraspinous muscles have a relatively high proportion of Type I (slow twitch) fibers and provide postural support along the spine, whereas the other abdominal muscles we measured are relatively enriched in Type II fibers and function in movement43–45. Regardless of these histological and functional differences, trends were similar across all muscle groups in the individual analyses. Since we only measured abdominal muscles in the present study, we cannot address any potential associations of IMAT in peripheral skeletal muscles with CAC. The CARDIA study has many important attributes including its multicenter design with the large number of participants including approximately equal numbers of white and black men and women of varying socioeconomic status and lifestyles. However, these cross-sectional findings require independent confirmation in other populations and we cannot address whether the present CARDIA data are applicable to populations including other age ranges or ethnicities.
Conclusion
Higher abdominal IMAT volume was associated with prevalent CAC and higher CAC scores in this cross-sectional study in middle-age participants. These associations persisted after accounting for traditional CVD risk factors and visceral adiposity. Although prospective studies are needed, these cross-sectional findings raise the possibility that ectopic deposition of fat within abdominal skeletal muscles contributes to the role of adiposity as an adverse CVD risk factor.
Supplementary Material
Examples of paraspinous muscles from participants in lowest and highest IMAT quartiles. Adipose tissue is indicated by lighter blue regions (corresponding to phantom color for pure fat). Participant scans shown are from females with BMI of 32.8 and 31.2 kg/m2 for lowest and highest IMAT quartiles, respectively.
Figure 1.
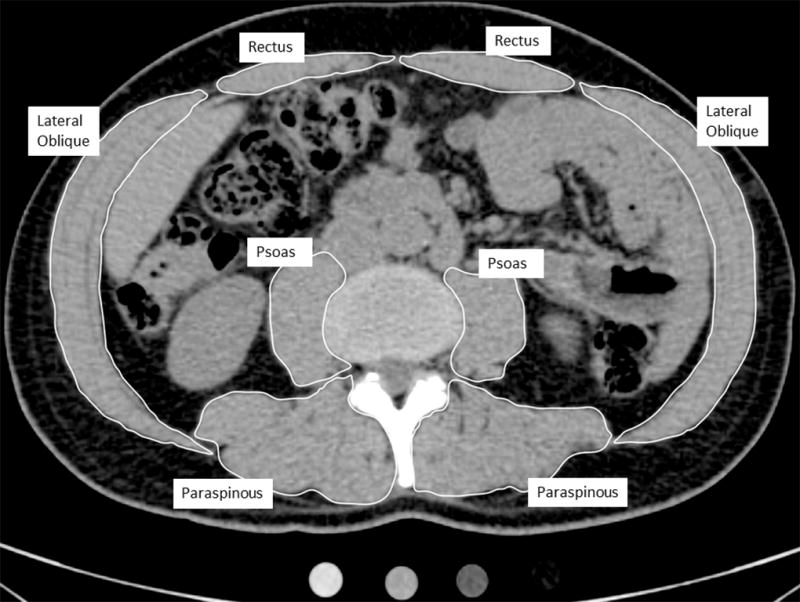
Single CT slice through the abdomen at the L3–L4 lumbar level. Right and left muscle groups are indicated. Left and right sides were averaged for analyses.
Highlights.
Abdominal intermuscular adipose tissue (IMAT) was associated with coronary artery calcification (CAC) prevalence and score in 3,048 community-dwelling participants
The IMAT and CAC association was not explained by traditional cardiovascular disease risk factors
Body mass index and visceral adipose tissue partially attenuated, but did not explain, associations of abdominal IMAT with CAC
Acknowledgments
The Authors would like to thank the investigators, the staff, and the participants of the CARDIA study for their dedication and highly valued contributions. This manuscript has been reviewed by CARDIA for scientific content.
Sources of Funding: The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201300025C & HHSN268201300026C), Northwestern University (HHSN268201300027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C), Johns Hopkins University School of Medicine (HHSN268200900041C) and Vanderbilt School of Medicine (R01-HL098445). CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005).
Abbreviations
- BMI
body mass index
- CAC
coronary artery calcification
- CARDIA
Coronary Artery Risk Development in Young Adults
- CT
computed tomography
- IMAT
intermuscular adipose tissue
- PAT
pericardial adipose tissue
- SAT
subcutaneous adipose tissue
- VAT
visceral adipose tissue
- WC
waist circumference
Footnotes
Disclosures: The authors report no conflicts. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the NHLBI; the National Institutes of Health; or the U.S. Department of Health and Human Services.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. Jama. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. http://dx.doi.org/10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 4.Després J-P. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–1313. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 5.Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 2000;49:467–472. doi: 10.1016/s0026-0495(00)80010-4. http://www.ncbi.nlm.nih.gov/pubmed/10778870. [DOI] [PubMed] [Google Scholar]
- 6.Taira S, Shimabukuro M, Higa M, et al. Lipid Deposition in Various Sites of the Skeletal Muscles and Liver Exhibits a Positive Correlation with Visceral Fat Accumulation in Middle-aged Japanese Men with Metabolic Syndrome. Intern Med. 2013;52:1561–1571. doi: 10.2169/internalmedicine.52.0521. [DOI] [PubMed] [Google Scholar]
- 7.Miljkovic I, Kuipers AL, Kuller LH, Sheu Y, Bunker CH, Patrick AL, Wheeler VW, Evans RW, Zmuda JM. Skeletal muscle adiposity is associated with serum lipid and lipoprotein levels in Afro-Caribbean men. Obesity. 2013;21:1900–1907. doi: 10.1002/oby.20214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124:e837–41. doi: 10.1161/CIRCULATIONAHA.111.077602. [DOI] [PubMed] [Google Scholar]
- 9.Miljkovic I, Cauley JA, Wang PY, Holton KF, Lee CG, Sheu Y, Barrett-Connor E, Hoffman AR, Lewis CB, Orwoll ES, Stefanick ML, Strotmeyer ES, Marshall LM. Abdominal myosteatosis is independently associated with hyperinsulinemia and insulin resistance among older men without diabetes. Obesity (Silver Spring) 2013;21:2118–2125. doi: 10.1002/oby.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Therkelsen KE, Pedley A, Speliotes EK, Massaro JM, Murabito J, Hoffmann U, Fox CS. Intramuscular Fat and Associations With Metabolic Risk Factors in the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2013;33:863–870. doi: 10.1161/ATVBAHA.112.301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beasley LE, Koster A, Newman AB, Javaid MK, Ferrucci L, Kritchevsky SB, Kuller LH, Pahor M, Schaap LA, Visser M, Rubin SM, Goodpaster BH, Harris TB, Study THABC Inflammation and Race and Gender Differences in Computerized Tomography-measured Adipose Depots. Obesity. 2009;17:1062–1069. doi: 10.1038/oby.2008.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexandersen P, Tanko LB, Bagger YZ, Jespersen J, Skouby SO, Christiansen C. Associations between aortic calcification and components of body composition in elderly men. Obesity (Silver Spring) 2006;14:1571–1578. doi: 10.1038/oby.2006.181. [DOI] [PubMed] [Google Scholar]
- 13.Kim S-K, Park S-W, Hwang I-J, Lee Y-K, Cho Y-W. High fat stores in ectopic compartments in men with newly diagnosed type 2 diabetes: an anthropometric determinant of carotid atherosclerosis and insulin resistance. Int J Obes (Lond) 2010;34:105–110. doi: 10.1038/ijo.2009.210. [DOI] [PubMed] [Google Scholar]
- 14.Jensky NE, Criqui MH, Wright CM, Wassel CL, Alcaraz JE, Allison MA. The association between abdominal body composition and vascular calcification. Obesity (Silver Spring) 2011;19:2418–2424. doi: 10.1038/oby.2011.70. [DOI] [PubMed] [Google Scholar]
- 15.Ding J, Kritchevsky SB, Hsu F-C, Harris TB, Burke GL, Detrano RC, Szklo M, Criqui MH, Allison M, Ouyang P, Brown ER, Carr JJ. Association between non-subcutaneous adiposity and calcified coronary plaque: a substudy of the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2008;88:645–650. doi: 10.1093/ajcn/88.3.645. http://ajcn.nutrition.org/cgi/content/long/88/3/645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Divers J, Wagenknecht LE, Bowden DW, Carr JJ, Hightower RC, Ding J, Xu J, Langefeld CD, Freedman BI. Regional adipose tissue associations with calcified atherosclerotic plaque: African American-diabetes heart study. Obesity (Silver Spring) 2010;18:2004–2009. doi: 10.1038/oby.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DRJ, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 18.Miljkovic I, Zmuda JM. Epidemiology of myosteatosis. Curr Opin Clin Nutr Metab Care. 2010;13:260–264. doi: 10.1097/MCO.0b013e328337d826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miljkovic-Gacic I, Wang X, Kammerer CM, Bunker CH, Patrick AL, Wheeler VW, Kuller LH, Evans RW, Zmuda JM. Sex and genetic effects on upper and lower body fat and associations with diabetes in multigenerational families of African heritage. Metabolism. 2008;57:819–823. doi: 10.1016/j.metabol.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Fox CS, Hickson DA, May WD, Hairston KG, Carr JJ, Taylor HA. Impact of Abdominal Visceral and Subcutaneous Adipose Tissue on Cardiometabolic Risk Factors: The Jackson Heart Study. J Clin Endocrinol Metab. 2010;95:5419–5426. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Fox CS, Hickson D, Bidulescu A, Carr JJ, Taylor HA. Fatty Liver, Abdominal Visceral Fat, and Cardiometabolic Risk Factors: The Jackson Heart Study. Arterioscler Thromb Vasc Biol. 2011;31:2715–2722. doi: 10.1161/ATVBAHA.111.234062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Fox CS, Hickson D, Sarpong D, Ekunwe L, May WD, Hundley GW, Carr JJ, Taylor HA. Pericardial adipose tissue, atherosclerosis, and cardiovascular disease risk factors: the Jackson heart study. Diabetes Care. 2010;33:1635–1639. doi: 10.2337/dc10-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim S, Meigs JB. Links Between Ectopic Fat and Vascular Disease in Humans. Arterioscler Thromb Vasc Biol. 2014;34:1820–1826. doi: 10.1161/ATVBAHA.114.303035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alman AC, Jacobs DR, Lewis CE, Snell-Bergeon JK, Carnethon MR, Terry JG, Goff DC, Ding J, Carr JJ. Higher pericardial adiposity is associated with prevalent diabetes: The Coronary Artery Risk Development in Young Adults study. Nutr Metab Cardiovasc Dis. 2016;26:326–332. doi: 10.1016/j.numecd.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu C-Y, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D’Agostino RB, O’Donnell CJ. Abdominal Visceral and Subcutaneous Adipose Tissue Compartments: Association With Metabolic Risk Factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 26.Despres J-P, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodes-Cabau J, Bertrand OF, Poirier P. Abdominal Obesity and the Metabolic Syndrome: Contribution to Global Cardiometabolic Risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. doi: 10.1161/atvbaha.107.159228. [DOI] [PubMed] [Google Scholar]
- 27.Fox CS, Hwang S-J, Massaro JM, Lieb K, Vasan RS, O’Donnell CJ, Hoffmann U. Relation of subcutaneous and visceral adipose tissue to coronary and abdominal aortic calcium (from the Framingham Heart Study) Am J Cardiol. 2009;104:543–547. doi: 10.1016/j.amjcard.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiTomasso D, Carnethon MR, Wright CM, Allison MA. The associations between visceral fat and calcified atherosclerosis are stronger in women than men. Atherosclerosis. 2010;208:531–536. doi: 10.1016/j.atherosclerosis.2009.08.015. doi: http://dx.doi.org/10.1016/j.atherosclerosis.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Ding J, Hsu F-C, Harris TB, Liu Y, Kritchevsky SB, Szklo M, Ouyang P, Espeland MA, Lohman KK, Criqui MH, Allison M, Bluemke DA, Carr JJ. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2009;90:499–504. doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miljkovic I, Kuipers AL, Cauley JA, Prasad T, Lee CG, Ensrud KE, Cawthon PM, Hoffman AR, Dam T-T, Gordon CL, Zmuda JM, Group for the OF in MS Greater Skeletal Muscle Fat Infiltration Is Associated With Higher All-Cause and Cardiovascular Mortality in Older Men. Journals Gerontol Ser A Biol Sci Med Sci. 2015 Apr; doi: 10.1093/gerona/glv027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O’Leary D, Carr JJ, Goff DC, Greenland P, Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. Jama. 2012;308:788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carr JJ, Jacobs DR, Jr, Terry JG, Shay CM, Sidney S, Liu K, Schreiner PJ, Lewis CE, Shikany JM, Reis JP, Goff DC., Jr Coronary artery calcium in adults 32–46 years and incident coronary heart disease and death: The CARDIA Study. JAMA Cardiol. 2017;2:391–399. doi: 10.1001/jamacardio.2016.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko B-J, Chang Y, Jung H-S, Yun KE, Kim C-W, Park HS, Chung EC, Shin H, Ryu S. Relationship Between Low Relative Muscle Mass and Coronary Artery Calcification in Healthy Adults. Arterioscler Thromb Vasc Biol. 2016;36:1016–1021. doi: 10.1161/ATVBAHA.116.307156. [DOI] [PubMed] [Google Scholar]
- 34.Loria CM, Liu K, Lewis CE, Hulley SB, Sidney S, Schreiner PJ, Williams OD, Bild DE, Detrano R. Early Adult Risk Factor Levels and Subsequent Coronary Artery Calcification: The {CARDIA} Study. J Am Coll Cardiol. 2007;49:2013–2020. doi: 10.1016/j.jacc.2007.03.009. doi: http://dx.doi.org/10.1016/j.jacc.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Haam J-H, Kim Y-S, Koo HS, Haam J, Seo NK, Kim HY, Park K-C, Park K-S, Kim MJ. Intermuscular adipose tissue is associated with monocyte chemoattractant protein-1, independent of visceral adipose tissue. Clin Biochem. 2016;49:439–443. doi: 10.1016/j.clinbiochem.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell R. Atherosclerosis — An Inflammatory Disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 37.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. http://dx.doi.org/10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 38.Herder C, Baumert J, Thorand B, Martin S, Löwel H, Kolb H, Koenig W. Chemokines and Incident Coronary Heart Disease. Arterioscler Thromb Vasc Biol. 2006;26:2147 LP–2152. doi: 10.1161/01.ATV.0000235691.84430.86. http://atvb.ahajournals.org/content/26/9/2147.abstract. [DOI] [PubMed] [Google Scholar]
- 39.Hoogeveen RC, Morrison A, Boerwinkle E, Miles JS, Rhodes CE, Sharrett AR, Ballantyne CM. Plasma MCP-1 level and risk for peripheral arterial disease and incident coronary heart disease: Atherosclerosis Risk in Communities study. Atherosclerosis. 2005;183:301–307. doi: 10.1016/j.atherosclerosis.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 40.de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, Gibson CM, Antman EM, McCabe CH, Cannon CP, Braunwald E. Association Between Plasma Levels of Monocyte Chemoattractant Protein-1 and Long-Term Clinical Outcomes in Patients With Acute Coronary Syndromes. Circulation. 2003;107:690 LP–695. doi: 10.1161/01.cir.0000049742.68848.99. http://circ.ahajournals.org/content/107/5/690.abstract. [DOI] [PubMed] [Google Scholar]
- 41.Reis JP, Loria CM, Lewis CE, Powell-Wiley TM, Wei GS, Carr JJ, Terry JG, Liu K. Association between duration of overall and abdominal obesity beginning in young adulthood and coronary artery calcification in middle age. JAMA. 2013;310:280–288. doi: 10.1001/jama.2013.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwak SM, Kim JS, Choi Y, Chang Y, Kwon M-J, Jung J-G, Jeong C, Ahn J, Kim HS, Shin H, Ryu S. Dietary Intake of Calcium and Phosphorus and Serum Concentration in Relation to the Risk of Coronary Artery Calcification in Asymptomatic Adults. Arterioscler Thromb Vasc Biol. 2014;34:1763–1769. doi: 10.1161/ATVBAHA.114.303440. [DOI] [PubMed] [Google Scholar]
- 43.Arbanas J, Klasan GS, Nikolic M, Jerkovic R, Miljanovic I, Malnar D. Fibre type composition of the human psoas major muscle with regard to the level of its origin. J Anat. 2009;215:636–641. doi: 10.1111/j.1469-7580.2009.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles: An autopsy study. J Neurol Sci. 1973;18:111–129. doi: 10.1016/0022-510x(73)90023-3. doi: http://dx.doi.org/10.1016/0022-510X(73)90023-3. [DOI] [PubMed] [Google Scholar]
- 45.Mannion AF, Dumas GA, Cooper RG, Espinosa FJ, Faris MW, Stevenson JM. Muscle fibre size and type distribution in thoracic and lumbar regions of erector spinae in healthy subjects without low back pain: normal values and sex differences. J Anat. 1997;190(Pt 4):505–513. doi: 10.1046/j.1469-7580.1997.19040505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Examples of paraspinous muscles from participants in lowest and highest IMAT quartiles. Adipose tissue is indicated by lighter blue regions (corresponding to phantom color for pure fat). Participant scans shown are from females with BMI of 32.8 and 31.2 kg/m2 for lowest and highest IMAT quartiles, respectively.