Abstract
Purpose
To identify clinical and treatment variables associated with a higher risk of local failure in Ewing sarcoma patients treated on recent Children's Oncology Group protocols.
Methods and Materials
Data for 956 patients treated with ifosfamide and etoposide–based chemotherapy on INT-0091, INT-0154, and AEWS0031 were analyzed. Local treatment modalities were defined as surgery, definitive radiation therapy (RT), or surgery plus radiation (S+RT). Five-year cumulative incidence of local failure was determined.
Results
The local failure rate for the entire cohort was 7.3%, with a 3.9% rate for surgery, 15.3% for RT (P<.01), and 6.6% for S+RT (P = .12). The local failure incidence was 5.4% for extremity tumors, 13.2% for pelvis tumors (P<.01), 5.3% for axial non-spine tumors (P = .90), 9.1% for extraskeletal tumors (P=.08), and 3.6% for spine tumors (P = .49). The incidence of local failure was 14.8% for extremity tumors and 22.4% for pelvis tumors treated with RT, compared with 3.7% for extremity tumors and 3.9% for pelvis tumors treated with surgery (P≤01). There was no difference in local failure incidence by local treatment modality for axial non-spine, spine, and extraskeletal tumors. The local failure incidence was 11.9% in patients aged ≥18 years versus 6.7% in patients aged <18 years (P = .02). Age ≥18 years (hazard ratio 1.9, P = .04) and treatment with RT (hazard ratio 2.40, P<.01) remained independent prognostic factors for higher local failure incidence on multivariate analysis. Tumor size (</≥ 8 cm) was available in 40% of patients and did not correlate with local failure incidence.
Conclusions
Local tumor control is excellent and similar between surgery and RT for axial non-spine, spine, and extraskeletal tumors. Age ≥18 years and use of RT, primarily for pelvis and extremity tumors, are associated with the highest risk of local failure. Further efforts should focus on improving outcomes for these patients.
Introduction
Modern era local failure rates in Ewing sarcoma (ES) range from 5% to 25% (1-5). The choice of local therapy is an individual decision based on patient and tumor characteristics. In North America, definitive surgery is typically used for dispensable bones, whereas definitive radiation therapy (RT) is reserved for pelvis and axial tumors that cannot be resected with acceptable morbidity. Radiation is added to surgery (S+RT) in cases of incomplete resection. Studies demonstrate inferior local tumor control for all tumors treated with RT (1-6); however, it is unclear whether there are subsets of patients treated with RT who have differential rates of local tumor control.
Given the vague associations between clinical and treatment variables and local tumor control outcomes, a comprehensive local failure analysis in a large cohort of patients treated with modern chemotherapy is needed. Such an analysis will help identify optimal local treatment modalities for certain patients, while highlighting cohorts that may benefit from alternate strategies. The purpose of this study was therefore to identify clinical and treatment variables associated with a higher risk of local failure on recent Children's Oncology Group (COG) ES protocols.
Methods and Materials
Patients and treatment
Patients with localized skeletal or extraskeletal ES treated on INT-0091, INT-0154, and AEWS0031 were eligible for inclusion in this retrospective cohort study. The final cohort consisted of 956 patients after excluding patients with cranial primaries (n = 62), unknown primaries (n = 11), incomplete local treatment data (n = 164), local treatment administration <2 or >6 months after randomization at study entry to standard versus experimental chemotherapy (n = 95), and/or administration of non–ifosfamide and etoposide (IE)–based chemotherapy (n = 156). Cranial tumors were excluded owing to many deviations from protocol local treatment guidelines. Only patients who received IE-based chemotherapy were analyzed because this regimen is associated with improved local tumor control (3). Local treatment modalities were defined as definitive surgery, RT, or S+RT. Treating physicians determined the method of local treatment for each case. Local therapy details are published in the primary manuscripts and in the Supplementary Text (available online at www.redjournal.org) (2, 3, 5).
Statistical analysis
Designation of skeletal versus extraskeletal tumors was determined by the enrolling facility. Skeletal tumors consisted of tumors with any degree of bone involvement. Skeletal tumors were classified as extremity, axial non-spine (rib, clavicle, sternum, and scapula), spine, and pelvis. Extraskeletal tumors consisted of tumors without any degree of bone involvement, and included all anatomic sites. Tumor size was available in 383 patients (40%) from the INT-0091 and INT-0154 cohorts, and classified as </≥ 8 cm in maximum dimension. Tumor size was not collected in AEWS0031.
The primary outcome was local failure incidence, considering other events (distant failure, death, or secondary malignancy) as competing risks. The 5-year cumulative incidence of local failure from time of local treatment is reported, and the association of clinical and treatment variables with local failure incidence was assessed using the Fine and Gray method extending the Cox model. Categorical patient and tumor characteristics were compared among the 3 local treatment modalities using χ2 tests. Multiple variable models considered those variables with a univariate significance of <.2 and used a backward selection method. Tumor size was not included in multivariate analysis owing to the high percentage of missing data. A P value <.05 was considered statistically significant.
Results
Patient, tumor, and local treatment characteristics are listed in Table 1. The median age at diagnosis was 13 years (range, 0.5-45 years). Definitive surgery was utilized in 52.51% of patients, RT in 23.64% of patients, and S+RT in 23.85% of patients. This proportion did change by study (Table 1). For the 184 extremity and 78 pelvis tumors with information on size, 123 extremity (67%) and 56 pelvis (72%) tumors were ≥8 cm (Table E1; available online at www.redjournal.org). There was no obvious difference in local treatment modality used according to tumor size for extremity or pelvis tumors (Table E2; available online at www.redjournal.org).
Table 1. Patient, tumor, and treatment characteristics for the entire cohort and by local treatment modality.
| Characteristic | All patients (N=956) | Surgery (n=502; 52.51%) | Radiation (n=226; 23.64%) | Surgery + radiation (n=228; 23.85%) | P |
|---|---|---|---|---|---|
| Sex | .83 | ||||
| Male | 524 (55) | 278 (53) | 125 (24) | 121 (23) | |
| Female | 432 (45) | 224 (52) | 101 (23) | 107 (25) | |
| Age (y) | .49 | ||||
| <18 | 842 (88) | 446 (53) | 194 (23) | 202 (24) | |
| ≥18 | 114 (12) | 56 (49) | 32 (28) | 26 (23) | |
| Tumor site | <.01* | ||||
| Extremity | 419 (43.8) | 310 (74) | 54 (13) | 55 (13) | |
| Pelvis | 176 (18.4) | 51 (29) | 86 (49) | 39 (22) | |
| Axial non-spine | 156 (16.3) | 82 (53) | 21 (13) | 53 (34) | |
| Extraskeletal | 146 (15.3) | 55 (38) | 28 (19) | 63 (43) | |
| Spine | 59 (6.2) | 4 (6.8) | 37 (62.7) | 18 (30.5) | |
| Maximum tumor size (cm)† | .21 | ||||
| <8 | 136 (36) | 73 (54) | 42 (31) | 21 (15) | |
| ≥8 | 247 (64) | 134 (54.25) | 60 (24.29) | 53 (21.46) | |
| Study | <.01* | ||||
| INT-0091 | 164 (17.2) | 65 (40) | 64 (39) | 35 (21) | |
| INT-0154 | 333 (34.8) | 208 (62) | 69 (21) | 56 (17) | |
| AEWS0031 | 459 (48.0) | 229 (50) | 93 (20) | 137 (30) |
Values are number (percentage). Categorical patient and tumor characteristics were compared among the 3 local treatment modalities using χ2 tests.
Statistically significant.
Tumor size available in 383 of 956 patients (40%).
Seventy local failures were documented: 53 local only and 17 combined local and distant failures. The 5-year cumulative incidence of local failure for the entire cohort was 7.3% (95% confidence interval [CI] 5.6%-8.9%). The 5-year cumulative incidence of local failure by local treatment modality was 3.9% for surgery (95% CI 2.2%-5.7%), 15.3% for RT (95% CI 10.4%-19.9%), and 6.6% for S+RT (95% CI 3.3%-9.8%) (Fig. 1a). The 5-year estimate of local failure by tumor site was 5.4% for extremities (95% CI 3.2%-7.6%), 13.2% for pelvis (95% CI 8.0%-18.0%), 5.3% for axial non-spine (95% CI 1.7%-8.8%), 9.1% for extraskeletal (95% CI 4.3%-13.8%), and 3.6% for spine (95% CI 0.0%-8.3%) (Fig. 1b, Table 2). There was no difference in local failure incidence by study (Table 2).
Fig. 1.
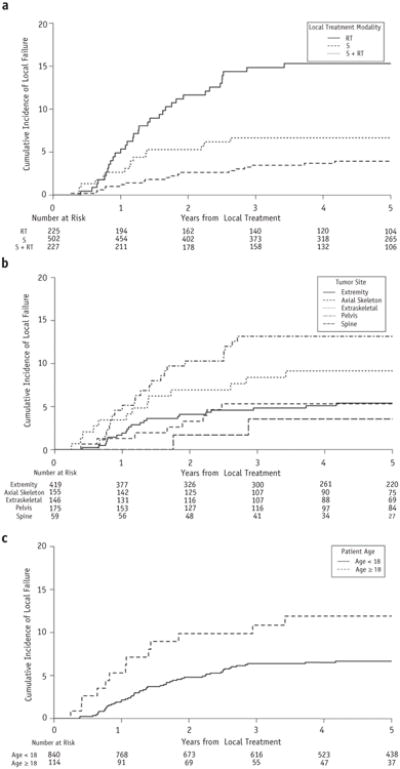
(a) Five-year cumulative incidence of local failure by local treatment modality. (b) Five-year cumulative incidence of local failure by tumor site. (c) Five-year cumulative incidence of local failure by age. Note all y axes range from 0% to 20%. Abbreviations: RT = radiation therapy; S = surgery.
Table 2. Five-year cumulative incidence of local failure for the entire cohort and by patient, tumor, and treatment characteristics.
| Characteristic | Local failure (%) | Hazard ratio (95% CI) | P |
|---|---|---|---|
| All patients | 7.3 | - | - |
| Sex | |||
| Male | 6.7 | 1.0 | - |
| Female | 7.3 | 1.29 (0.81-2.06) | .28 |
| Age (y) | |||
| <18 | 6.7 | 1.0 | - |
| ≥18 | 11.9 | 1.97 (1.09-3.54) | .02* |
| Tumor site | |||
| Extremity | 5.4 | 1.0 | - |
| Pelvis | 13.2 | 2.47 (1.38-4.40) | <.01* |
| Axial non-spine | 5.3 | 0.95 (0.43-2.12) | .90 |
| Extraskeletal | 9.1 | 1.82 (0.94-3.54) | .08 |
| Spine | 3.6 | 0.60 (0.14-2.53) | .49 |
| Maximum tumor size (cm) | |||
| <8 | 8.3 | 1.0 | - |
| ≥8 | 7.9 | 0.92 (0.45-1.88) | .83 |
| Local treatment modality | |||
| Surgery | 3.9 | 1.0 | - |
| Radiation | 15.3 | 4.12 (2.39-7.12) | <.01* |
| Surgery + radiation | 6.6 | 1.69 (0.87-3.31) | .12 |
| Study | |||
| INT-0091 | 6.2 | 1.0 | - |
| INT-0154 | 8.0 | 1.38 (0.67-2.84) | .38 |
| AEWS0031 | 7.1 | 1.18 (0.58-2.39) | .66 |
Abbreviation: CI = confidence interval.
Statistically significant.
Significant variables associated with an increased risk of local failure included age ≥18 years (Fig. 1c, Table 2), use of RT (compared with surgery; Table 2), and pelvis tumors (compared with extremity tumors; Table 2). Tumor size did not correlate with local failure risk in the 40% of patients with size information (P = .83; Table 2). In a multiple variable model, age ≥18 years (hazard ratio 1.9; 95% CI 1.0-3.4; P = .04) and use of RT (hazard ratio 2.4; 95% CI 1.3-4.4; P<.01) remained statistically significant variables for increased risk of local failure.
Because tumor site can influence choice of local therapy (Table 1), outcomes for each tumor site by local treatment modality were evaluated (Table 3). There was no statistically significant difference in local failure incidence by local treatment modality for axial non-spine, spine, and extraskeletal tumors. Extremity and pelvis tumors treated with RT were associated with a higher local failure incidence. The local failure rate was 14.8% for extremity tumors and 22.4% for pelvis tumors treated with RT, compared with 3.7% for extremity tumors (P<.01) and 3.9% for pelvis tumors treated with surgery (P = .01). There was no statistically significant difference in local failure incidence by tumor size for pelvis or extremity tumors treated with RT (Table E3; available online at www.redjournal.org). Similarly, there was no difference in outcomes by tumor size for axial non-spine, extraskeletal, and spine tumors (data not shown).
Table 3. Association of local treatment modality with 5-year cumulative incidence of local failure according to primary tumor site.
| Tumor site | n | Local failure (%) | Hazard ratio (95% CI) | P |
|---|---|---|---|---|
| Extremity tumors (n=419) | ||||
| Surgery | 310 | 3.7 | 1.0 | - |
| Radiation | 54 | 14.8 | 3.99 (1.62-9.80) | <.01* |
| Surgery + radiation | 55 | 5.4 | 1.42 (0.40-5.00) | .59 |
| Pelvis tumors (n=176) | ||||
| Surgery | 51 | 3.9 | 1.0 | - |
| Radiation | 86 | 22.4 | 6.31 (1.48-26.96) | .01* |
| Surgery + radiation | 39 | 5.1 | 1.31 (0.19-9.28) | .78 |
| Axial non-spine tumors (n=156) | ||||
| Surgery | 82 | 2.5 | 1.0 | - |
| Radiation | 21 | 10.6 | 4.05 (0.59-27.61) | .15 |
| Surgery + radiation | 53 | 7.8 | 3.28 (0.60-17.82) | .17 |
| Spine tumors (n=59) | ||||
| Surgery | 4 | 0.0 | 1.0 | - |
| Radiation | 18 | 5.6 | 0.41 (0.00-23.13) | .67 |
| Surgery+ radiation | 37 | 0.0 | 0.19 (0.00-34.29) | .53 |
| Extraskeletal tumors (n=146) | ||||
| Surgery | 55 | 7.7 | 1.0 | - |
| Radiation | 28 | 10.9 | 1.97 (0.51-7.62) | .33 |
| Surgery + radiation | 63 | 9.5 | 1.30 (0.37-4.58) | .69 |
Abbreviation: CI = confidence interval.
Statistically significant.
In the surgery-only cohort, age correlated with local failure risk (Table 4). No clinical characteristics were associated with increased local failure risk in the RT or S+RT cohorts (Table 4). Tumor site was not included in these analyses because outcomes for each site by local treatment modality were evaluated in Table 3. Multivariate analysis was not performed for the surgery or S+RT cohorts owing to too few events for analysis (surgery, 20 events; S+RT, 15 events). Patient age and tumor site were included in a multiple variable model for the RT cohort. Neither variable was statistically significant (Table E4; available online at www.redjournal.org).
Table 4. Univariate analysis of clinical characteristics associated with 5-year cumulative incidence of local failure by local treatment modality.
| Parameter | Surgery (n=502) | Radiation (n=226) | Surgery + radiation (n=228) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| LF (%) | HR (95% CI) | P | LF (%) | HR (95% CI) | P | LF (%) | HR (95% CI) | P | |
| All patients | 3.9 | - | - | 15.3 | - | - | 6.6 | - | - |
| Sex | |||||||||
| Male | 4.2 | 1.0 | - | 13.9 | 1.0 | - | 5.0 | 1.0 | - |
| Female | 3.6 | 1.01 (0.42-2.45) | .98 | 17.0 | 1.30 (0.67-2.51) | .44 | 8.5 | 1.76 (0.63-4.92) | .28 |
| Age (y) | |||||||||
| <18 | 3.5 | 1.0 | - | 14.0 | 1.0 | - | 6.5 | 1.0 | - |
| ≥18 | 7.4 | 2.81 (1.03-7.68) | .04* | 23.2 | 1.72 (0.75-3.98) | .20 | 7.7 | 1.22 (0.27-5.46) | .80 |
| Tumor size (cm) | |||||||||
| <8 | 7.2 | 1.0 | - | 12.2 | 1.0 | - | 4.8 | 1.0 | - |
| ≥8 | 3.1 | 0.53 (0.15-1.80) | .31 | 20.0 | 1.46 (0.56-3.81) | .44 | 5.9 | 1.23 (0.13-11.68) | .86 |
Abbreviations: CI = confidence interval; HR = hazard ratio; LF = local failure.
Statistically significant.
Quality control data were available in 196 patients treated with RT. Radiation therapy protocol deviations were documented in 63 patients: 25 with major protocol deviations and 39 with minor protocol deviations. Of the 10 patients with protocol deviations and a local recurrence, 2 were documented to have dose deviations, and 8 were documented to have volume/field deviations. The local failure incidence was 17.7% for patients without a protocol deviation and 16.0% for patients with a protocol deviation (P = .68). There was no statistically significant difference in local failure incidence by the presence or absence of a protocol deviation among patients with extremity tumors treated with RT, among patients with pelvis tumors treated with RT, by study, or by subcategorization of protocol deviations into major and minor categories (data not shown).
Discussion
Local therapy is a critical component of the multimodal treatment strategy employed in ES. DuBois et al (1) demonstrated that although there is no significant difference in survival between local treatment modalities for ES patients, RT is associated with a higher risk of local failure. In this study we aimed to further characterize cohorts at highest risk for local failure to help guide future local treatment intensification efforts because patients who experience a local failure have a postrelapse survival of <25% (7, 8). Our analysis of 956 patients treated on the most recent COG protocols demonstrates that older patients and patients treated with RT, especially for extremity and pelvis tumors, comprise the highest risk groups for experiencing a local failure.
Radiation therapy was associated with a higher local failure incidence of 15.3%, compared with 3.9% for surgery and 6.6% for S+RT Analysis by tumor site, however, revealed that the higher local failure rate with RT was primarily seen for extremity and pelvis tumors. There was no statistically significant difference in local failure incidence by local treatment modality for axial non-spine, extraskeletal, or spine tumors; and local failure rates in these locations were ≤10.9%. The local failure incidence for extremity tumors and pelvis tumors treated with RT was 14.8% and 22.4%, respectively. This is in contrast to surgical patients, in whom there was no difference in local failure rate by tumor site. The local failure incidence for patients with pelvis and extremity tumors able to undergo surgery was ≤3.9%.
Although extremity location was a site associated with poorer local tumor control, only a small number of extremity tumors (n = 54) were treated with RT in our cohort. Even so, the EURO-EWING99 local failure analysis similarly demonstrated poorer local tumor control for extremity tumors treated with RT compared with surgery or S+RT (9). A retrospective review of 158 extremity ES tumors also echoed this finding (10). Extremity and pelvis ES tumors treated with RT are by and large anatomically unfavorable cases in which tumor spans the entire bone, joint spaces, multiple adjacent bones (eg, sacrum, ilium, acetabulum, and pubic bones), and/or neurovasculature (Fig. 2). The alternative oncologic resection in these cases is often amputation or hip disarticulations, which are associated with significant morbidity. Thus, RT will remain the preferred treatment for a minority of extremity tumors, and further efforts to characterize those at highest risk for relapse are needed. Moreover, investigative efforts to determine methods of intensifying local therapy for these cases are warranted.
Fig. 2.
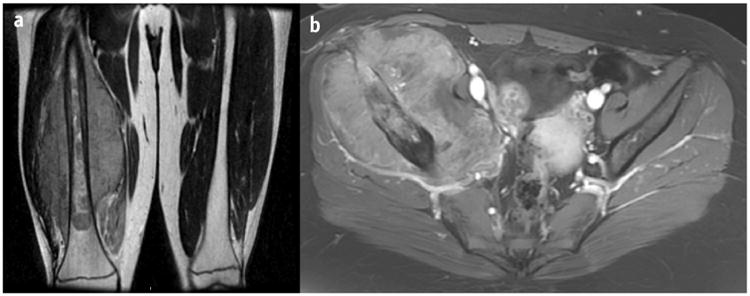
(a) Coronal T2 magnetic resonance image of a right lower extremity Ewing sarcoma tumor treated with radiation therapy. The tumor extended 30.0 cm along the right femur and was associated with a soft-tissue mass measuring 23.0 × 22.0 × 12.6 cm. Surgery would involve a non–limb-sparing approach. (b) Axial contrast T1 spoiled gradient magnetic resonance image of a right pelvis Ewing sarcoma tumor treated with radiation therapy. The tumor measured 15.0 × 13.2 × 9.3 cm and extended from the ilium to the superior pubic ramus. Surgery would require a hemipelvectomy.
Our results suggesting tumor size is not significantly associated with local failure incidence contradicts many other reports demonstrating inferior local tumor control outcomes for large tumors (4, 9, 11, 12) and may be attributed to lack of size information for 60% of our sample. The local failure analysis of patients treated on the EURO-EWING99 trial found tumor volume ≥200 mL was associated with inferior local tumor control (9). The 8-cm size cutoff used in this analysis corresponds to a 268-mL spherical tumor (13). Given that 8 cm estimates a tumor larger than 200 mL but is not associated with inferior local tumor control may indicate maximum tumor dimension measurements are an inadequate prognostic variable with contemporary treatment. In fact, Aghighi et al (14) demonstrated that 3-dimensional tumor measurements for ES significantly correlated with clinical outcomes compared with 1- or 2-dimensional measurements, in which the correlation was weaker or not significant.
It is also possible the lack of statistically significant size correlation with local failure incidence, especially for surgical cases, suggests size is becoming less significant as a prognostic variable with improvements in systemic therapy. For example, a 16-cm tumor with 90% volumetric response may be more favorable than a 7-cm tumor with 10% response. It is important to remember, however, previous COG studies analyzed the association between tumor size and event-free survival (EFS), not local failure incidence, and demonstrated that larger tumors predict for poorer EFS (13). This suggests tumor size is still a prognostic variable for biologically aggressive disease and distant failure. Again, the high percentage of missing size data in the cohort significantly limits our inferences. The COG study AEWS1031 (closed to study entry but not yet ready for analysis) collected baseline volumetric size to further determine the importance of size as a prognostic variable for EFS and local tumor control with modern COG chemotherapy.
Age ≥18 years was associated with a higher local failure rate of 11.9%, versus 6.7% for age <18 years. The same finding was observed in the surgery-only cases, but the absolute number was small. Data regarding outcomes for adult ES patients in the literature are conflicting. Some studies report equivalent outcomes compared with children, whereas others report inferior outcomes (15-19). On AEWS0031, the 5-year EFS was 72% for children versus 47% for adults (5). Although these results suggest the presence of a biologically different tumor in adults, there is currently no pathologic- or molecular-based data to support this notion. Older patients are less sensitive to the long-term effects of RT (16), and consideration should be given to dose escalation for RT cases and combined-modality therapy for cases amenable to surgery.
Additional clinical and tumor variables are needed to help stratify high-risk ES patients. Histologic response to neoadjuvant chemotherapy for European regimens is a known prognostic factor for local tumor control and helps guide treatment intensification decisions (9, 12, 20). However, histologic response to neoadjuvant chemotherapy can only be assessed in surgical cases and thus can influence a decision for adjuvant RT; but it is not helpful for RT cases. A surrogate metric to determine whether treatment intensification is warranted in patients undergoing RT is needed. Many studies demonstrate tumors with minimal metabolic changes on [18F]fluorodeoxyglucose positron emission tomography (18F-FDG PET) following neo-adjuvant therapy are associated with a higher risk of progression (21-23). None of these reports discuss the association of local tumor control with 18F-FDG PET, so it is unclear whether 18F-FDG PET has a role in determining high-risk RT cases. For pelvis tumors, recent data suggest anatomic localization within the pelvis may correlate with outcomes and could be exploited to help determine high-risk pelvis cases (24, 25). Pelvis subsite (eg, sacral, acetabular, pubic ramus) was not available in our data set, so we are unable to evaluate whether location within the pelvis is a prognostic factor for local control in our series.
Surgery plus RT is standard of care in the majority of high-risk extremity soft-tissue sarcomas (26) and has been suggested as a method to improve local tumor control in pelvis ES on the EURO-EWING99 study (12). Surgery plus RT outcomes were equivalent to surgery and superior to RT in our series. Several studies have also illustrated a local tumor control advantage for S+RT even in cases of acceptable histologic response to induction chemotherapy or negative margins (4, 27-29). Concern with S+RT resulting in increased risk of long-term treatment-associated toxicities, especially in the absence of a survival benefit (1), has resulted in limiting use of combined-modality treatment in North America to postoperative RT after positive surgical margins. An analysis of long-term functional outcomes and physical activity in 618 ES survivors treated on 4 European studies concluded that most ES survivors return to normal life with minor limitations (30). Notably, 56% of these patients were treated with S+RT (30). Similarly, a long-term functional outcomes analysis of ES patients treated in the United States suggested local treatment modality does not significantly affect musculoskeletal outcomes or quality of life (31). On the basis of these results, consideration for S+RT may be justified in high-risk pelvis or extremity tumors. Low-dose (36.0 Gy) preoperative RT was encouraged on AEWS1031 as a method to improve local tumor control for large pelvis tumors. The results of this study are still pending.
Dose escalation is another strategy that has been utilized to improve local tumor control. For example, the current COG intermediate-risk rhabdomyosarcoma trial (ARST1431) will determine whether RT dose escalation to 59.4 Gy after induction chemotherapy for tumors >5 cm at diagnosis is beneficial because larger tumors are associated with higher local failure rates (32, 33). Dose escalation has been discouraged in ES owing to older data demonstrating an increased secondary malignancy rate with doses ≥60.0 Gy (34). However, single-institution ES series have suggested higher RT doses may be associated with improved local tumor control (15, 25, 35). Furthermore, a phase 2 study in 45 patients with ES did not document any local failures in tumors ≥8 cm receiving 64.8 Gy (36). Therefore, the benefit of improved local tumor control may outweigh the risks of second malignancy for patients at high risk for local failure. It is also hypothesized that the risk of radiation-induced secondary malignancy in the contemporary era is lower with more conformal treatment volumes (treatment of tumor plus margin vs entire bone) and more conformal planning techniques (intensity modulated RT and proton therapy). Extremity tumors are especially amenable to dose escalation, given the absence of dose-limiting adjacent normal tissues. Dose escalation must be studied more cautiously in the pelvis owing to the numerous adjacent radiosensitive organs. Proton RT has safely been used for the treatment of osteosarcomas, chordomas, and chondrosarcomas in children and adults (37-39) to higher doses than are likely necessary in ES, suggesting dose escalation for pelvis ES using highly conformal techniques is currently feasible (40).
The retrospective nature of our local failure analysis, incomplete tumor size information, and exclusion of tumor size from multivariate analyses are limitations of this report. Moreover, there are inherent biases in selecting local treatment reflective of tumor extent at diagnosis and response to neoadjuvant chemotherapy. For instance, surgery is likely to be used for tumors amenable to an oncologic resection with acceptable morbidity, whereas RT is reserved for tumors that cannot be resected with acceptable morbidity and/or for tumors with minimal response to neoadjuvant chemotherapy. However, randomized studies comparing local treatments in ES do not exist and will likely never transpire. Therefore, we must rely on retrospective analyses to help determine optimal local tumor control strategies. The key strength of our analysis is that it was performed in a large cohort of prospectively collected patients treated with IE-based chemotherapy and with recommended local treatment guidelines. Our results demonstrate older age and use of RT, primarily in pelvis and extremity tumors, comprise the highest-risk cohorts for local failure. Further efforts should focus on identification and incorporation of additional prognostic variables and improving outcomes for these high-risk patients with intensification of local therapy.
Supplementary Material
Summary.
Our analysis of local tumor control outcomes in 956 Ewing sarcoma patients demonstrates age ≥18 years and use of definitive radiation therapy, primarily for pelvis and extremity tumors, are associated with the highest risk of local failure. Local tumor control is excellent and similar between surgery and definitive radiation therapy for axial non-spine, spine, and extra-skeletal tumors.
Acknowledgments
Research reported in this publication was supported by the Children's Oncology Group.
Footnotes
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.DuBois SG, Krailo MD, Gebhardt MC, et al. Comparative evaluation of local control strategies in localized Ewing sarcoma of bone: A report from the Children's Oncology Group. Cancer. 2015;121:467–475. doi: 10.1002/cncr.29065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Granowetter L, Womer R, Devidas M, et al. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: A Children's Oncology Group Study. J Clin Oncol. 2009;27:2536–2541. doi: 10.1200/JCO.2008.19.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 4.Schuck A, Ahrens S, Paulussen M, et al. Local therapy in localized Ewing tumors: Results of 1058 patients treated in the CESS 81, CESS 86, and EICESS 92 trials. Int J Radiat Oncol Biol Phys. 2003;55:168–177. doi: 10.1016/s0360-3016(02)03797-5. [DOI] [PubMed] [Google Scholar]
- 5.Womer RB, West DC, Krailo MD, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: A report from the Children's Oncology Group. J Clin Oncol. 2012;30:4148–4154. doi: 10.1200/JCO.2011.41.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuck A, Hofmann J, Rube C, et al. Radiotherapy in Ewing's sarcoma and PNET of the chest wall: Results of the trials CESS 81, CESS 86 and EICESS 92. Int J Radiat Oncol Biol Phys. 1998;42:1001–1006. doi: 10.1016/s0360-3016(98)00294-6. [DOI] [PubMed] [Google Scholar]
- 7.Stahl M, Ranft A, Paulussen M, et al. Risk of recurrence and survival after relapse in patients with Ewing sarcoma. Pediatr Blood Cancer. 2011;57:549–553. doi: 10.1002/pbc.23040. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Galindo C, Billups CA, Kun LE, et al. Survival after recurrence of Ewing tumors: The St Jude Children's Research Hospital experience, 1979-1999. Cancer. 2002;94:561–569. doi: 10.1002/cncr.10192. [DOI] [PubMed] [Google Scholar]
- 9.Andreou D, Ranft A, Dijkstra S, et al. Factors influencing local control in Ewing sarcoma (EWS) patients: An analysis of the data of the EURO-EWING99 trial. Connective Tissue Oncology Society Annual Meeting; November 9-12, 2016; Lisbon, Portugal. Abstract 015, 2016. [Google Scholar]
- 10.Biswas B, Rastogi S, Khan SA, et al. Outcomes and prognostic factors for Ewing-family tumors of the extremities. J Bone Joint Surg. 2014;96:841–849. doi: 10.2106/JBJS.M.00411. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Galindo C, Navid F, Liu T, et al. Prognostic factors for local and distant control in Ewing sarcoma family of tumors. Ann Oncol. 2008;19:814–820. doi: 10.1093/annonc/mdm521. [DOI] [PubMed] [Google Scholar]
- 12.Dirksen U, Jurgens H, Ranft A, et al. Local control and survival in pelvic Ewing sarcoma (EwS) in the Euro-Ewing99 Trial. Pediatr Blood Cancer. 2016;63:S20. [Google Scholar]
- 13.Marina N, Granowetter L, Grier HE, et al. Age, tumor characteristics, and treatment regimen as event predictors in Ewing: A Children's Oncology Group report. Sarcoma. 2015;2015:8. doi: 10.1155/2015/927123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aghighi M, Boe J, Rosenberg J, et al. Three-dimensional radiologic assessment of chemotherapy response in Ewing sarcoma can be used to predict clinical outcome. Radiology. 2016;280:905–915. doi: 10.1148/radiol.2016151301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed SK, Robinson SI, Okuno SH, et al. Adult Ewing sarcoma: Survival and local control outcomes in 102 patients with localized disease. Sarcoma. 2013;2013:7. doi: 10.1155/2013/681425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casey DL, Meyers PA, Alektiar KM, et al. Ewing sarcoma in adults treated with modern radiotherapy techniques. Radiother Oncol. 2014;113:248–253. doi: 10.1016/j.radonc.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Cotterill SJ, Ahrens S, Paulussen M, et al. Prognostic factors in Ewing's tumor of bone: Analysis of 975 patients from the European Intergroup Cooperative Ewing's Sarcoma Study Group. J Clin Oncol. 2000;18:3108–3114. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 18.Baldini EH, Demetri GD, Fletcher CD, et al. Adults with Ewing's sarcoma/primitive neuroectodermal tumor: Adverse effect of older age and primary extraosseous disease on outcome. Ann Surg. 1999;230:79–86. doi: 10.1097/00000658-199907000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pretz JL, Barysauskas CM, George S, et al. Localized adult Ewing sarcoma: Favorable outcomes with alternating vincristine, doxorubicin, cyclophosphamide, and ifosfamide, etoposide (VDC/IE)-based multimodality therapy. Oncologist. 2017 doi: 10.1634/theoncologist.2016-0463. http://dx.doi.org/10.1634/theoncologist.2016-0463 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 20.Gaspar N, Rey A, Berard PM, et al. Risk adapted chemotherapy for localised Ewing's sarcoma of bone: The French EW93 study. Eur J Cancer. 2012;48:1376–1385. doi: 10.1016/j.ejca.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins DS, Schuetze SM, Butrynski JE, et al. [18F]Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol. 2005;23:8828–8834. doi: 10.1200/JCO.2005.01.7079. [DOI] [PubMed] [Google Scholar]
- 22.Koshkin VS, Bolejack V, Schwartz LH, et al. Assessment of imaging modalities and response metrics in Ewing sarcoma: Correlation with survival. J Clin Oncol. 2016;34:3680–3685. doi: 10.1200/JCO.2016.68.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raciborska A, Bilska K, Drabko K, et al. Response to chemotherapy estimates by FDG PET is an important prognostic factor in patients with Ewing sarcoma. Clin Transl Oncol. 2016;18:189–195. doi: 10.1007/s12094-015-1351-6. [DOI] [PubMed] [Google Scholar]
- 24.Hesla AC, Tsagozis P, Jebsen N, et al. Improved prognosis for patients with Ewing sarcoma in the sacrum compared with the innominate bones: The Scandinavian Sarcoma Group experience. J Bone Joint Surg. 2016;98:199–210. doi: 10.2106/JBJS.O.00362. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed SK, Robinson SI, Arndt CA, et al. Pelvis Ewing sarcoma: Local control and survival in the modern era. Pediatr Blood Cancer. 2017;64:e26504. doi: 10.1002/pbc.26504. [DOI] [PubMed] [Google Scholar]
- 26.O'Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet. 2002;359:2235–2241. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 27.Foulon S, BrennanB, Gaspar N, et al. Can postoperative radiotherapy be omitted in localised standard-risk Ewing sarcoma? An observational study of the Euro-E.W.I.N.G group. Eur J Cancer. 2016;61:128–136. doi: 10.1016/j.ejca.2016.03.075. [DOI] [PubMed] [Google Scholar]
- 28.Shankar AG, Pinkerton CR, Atra A, et al. United Kingdom Children's Cancer Study Group (UKCCSG) Local therapy and other factors influencing site of relapse in patients with localised Ewing's sarcoma. Eur J Cancer. 1999;35:1698–1704. doi: 10.1016/s0959-8049(99)00144-6. [DOI] [PubMed] [Google Scholar]
- 29.Bacci G, Forni C, Longhi A, et al. Long-term outcome for patients with non-metastatic Ewing's sarcoma treated with adjuvant and neo-adjuvant chemotherapies. 402 patients treated at Rizzoli between 1972 and 1992. Eur J Cancer. 2004;40:73–83. doi: 10.1016/j.ejca.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 30.Ranft A, Seidel C, Hoffmann C, et al. Quality of survivorship in a rare disease: Clinicofunctional outcome and physical activity in an observational cohort study of 618 long-term survivors of Ewing sarcoma. J Clin Oncol. 2017;35:1704–1712. doi: 10.1200/JCO.2016.70.6226. [DOI] [PubMed] [Google Scholar]
- 31.Stish BJ, Ahmed SK, Rose PS, et al. Patient-reported functional and quality of life outcomes in a large cohort of long-term survivors of Ewing sarcoma. Pediatr Blood Cancer. 2015;62:2189–2196. doi: 10.1002/pbc.25710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Children's Oncology Group. ARST1431: A Randomized Phase 3 Study of Vincristine, Dactinomycin, Cyclophosphamide (VAC) Alternating with Vincristine and Irinotecan (VI) Versus VAC/VI Plus Temsirolimus (TORI, Torisel, NSC# 683864, IND# 122782) in Patients with Intermediate Risk (IR) Rhabdomyosarcoma (RMS) [Accessed April 11, 2016]; Available at: https://cogmembers.org/Site/Prot/ProtInfo.aspx?ProtocolNum=1856.
- 33.Wolden SL, Lyden ER, Arndt CA, et al. Local control for intermediate-risk rhabdomyosarcoma: Results from D9803 according to histology, group, site, and size: A report from the Children's Oncology Group. Int J Radiat Oncol Biol Phys. 2015;93:1071–1076. doi: 10.1016/j.ijrobp.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tucker MA, D'Angio GJ, Boice JD, Jr, et al. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med. 1987;317:588–593. doi: 10.1056/NEJM198709033171002. [DOI] [PubMed] [Google Scholar]
- 35.Indelicato DJ, Keole SR, Shahlaee AH, et al. Definitive radiotherapy for Ewing tumors of extremities and pelvis: Long-term disease control, limb function, and treatment toxicity. Int J Radiat Oncol Biol Phys. 2008;72:871–877. doi: 10.1016/j.ijrobp.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 36.Talleur AC, Navid F, Spunt SL, et al. Limited margin radiation therapy for children and young adults with Ewing sarcoma achieves high rates of local tumor control. Int J Radiat Oncol Biol Phys. 2016;96:119–126. doi: 10.1016/j.ijrobp.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciernik IF, Niemierko A, Harmon DC, et al. Proton-based radiotherapy for unresectable or incompletely resected osteosarcoma. Cancer. 2011;117:4522–4530. doi: 10.1002/cncr.26037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Indelicato DJ, Rotondo RL, Begosh-Mayne D, et al. A prospective outcomes study of proton therapy for chordomas and chondrosarcomas of the spine. Int J Radiat Oncol Biol Phys. 2016;95:297–303. doi: 10.1016/j.ijrobp.2016.01.057. [DOI] [PubMed] [Google Scholar]
- 39.DeLaney TF, Liebsch NJ, Pedlow FX, et al. Phase II study of high-dose photon/proton radiotherapy in the management of spine sarcomas. Int J Radiat Oncol Biol Phys. 2009;74:732–739. doi: 10.1016/j.ijrobp.2008.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rombi B, DeLaney TF, MacDonald SM, et al. Proton radiotherapy for pediatric Ewing's sarcoma: Initial clinical outcomes. Int J Radiat Oncol Biol Phys. 2012;82:1142–1148. doi: 10.1016/j.ijrobp.2011.03.038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


