Abstract
Background
There are conflicting data regarding a potential survival benefit to adjuvant whole brain radiotherapy (WBRT) among patients with limited brain metastases treated with stereotactic radiosurgery (SRS). We sought to determine if WBRT is associated with improved overall survival among non-small cell lung cancer (NSCLC) patients with favorable prognoses at diagnosis.
Methods
In the N0574 trial, patients with 1–3 brain metastases were randomized to receive SRS or SRS+WBRT with a primary endpoint of cognitive deterioration. We calculated diagnosis-specific graded prognostic assessment (DS-GPA) scores for NSCLC patients and evaluated overall survival according to receipt of WBRT and DS-GPA score using two separate cut-points (≥ 2.0 vs. <2.0 and ≥ 2.5 vs. < 2.5).
Results
A total of 126 NSCLC patients were included for analysis with median follow up of 14.2 months. Data for DS-GPA calculation was available for 86.3% of all enrolled NSCLC patients. Overall, 50.0% of patients had DS-GPA score ≥ 2.0 and 23.0% of patients had DS-GPA scores ≥ 2.5. The SRS and SRS+WBRT groups were well balanced with regard to prognostic factors. The median survival according to receipt of WBRT was 11.3 months (+WBRT) and 17.9 months (−WBRT) for patients with DS-GPA ≥ 2.0 (favorable prognoses, p=0.63; HR, 0.86; 95%CI, 0.47–1.59). Median survival was 3.7 months (+WBRT) and 6.6 months (−WBRT) for patients with DS-GPA < 2.0 patients (unfavorable prognoses, p=0.85; HR, 0.95; 95%CI, 0.56–1.62). Outcomes according to the receipt of WBRT and DS-GPA remained similar utilizing DS-GPA ≥ 2.5 as a cutoff for favorable prognoses. There was no interaction between the continuum of the DS-GPA groups and WBRT on overall survival (p=0.53).
Conclusions
We observed no significant differences in survival according to receipt of WBRT in favorable prognosis NSCLC patients. This study further supports the approach of SRS alone in the majority of patients with limited brain metastases.
INTRODUCTION
Brain metastases are the most common intracranial tumors with an estimated annual incidence exceeding 170,000 in the United States.(1) At least four randomized controlled trials of stereotactic radiosurgery (SRS)+/− adjuvant whole brain radiotherapy (WBRT) among patients with limited (i.e. 1–3) brain metastases have yielded consistent estimates of increased intracranial control with WBRT while overall survival remained similar between treatment arms.(2–5) In addition, SRS alone is associated with improved neurocognitive effects and quality of life despite the increased intracranial relapse rate.(2)
These trials suggest the negative cognitive effects of adjuvant WBRT and efficacy of salvage therapy render SRS alone the preferred initial treatment strategy for patients with limited brain metastases. However, debate exists within the oncology community regarding the role of adjuvant WBRT among select populations.(6, 7) Whereas a secondary analysis of the JROSG-99 trial had demonstrated an overall survival benefit to WBRT among lung cancer patients with favorable diagnosis-specific graded prognostic assessment (DS-GPA) scores(8), an exploratory analysis of the EORTC 22952 trial found no such benefit.(9) In consequence of the persistent, conflicting evidence related to the effect of WBRT on survival in patient sub-populations, we sought to evaluate outcomes of non-small cell lung cancer (NSCLC) patients according to the receipt of WBRT and DS-GPA score within the North Central Cancer Treatment Group N0574 (Alliance) trial.
METHODS
Our study design was an unplanned secondary analysis of the NCCTG N0574 (Alliance) phase III randomized controlled trial.(2) In the original trial, patients with 1–3 brain metastases smaller than 3 cm were randomized to SRS with or without WBRT. The trial was closed on 12/10/2013 after meeting accrual and data for this analysis were frozen on 12/15/2016. We calculated DS-GPA scores(10) among NSCLC patients and utilized two separate cut-points to define favorable prognoses: ≥2.0 and ≥2.5. Modeling after the secondary analysis of JROSG-99 trial, we included only NSCLC patients for homogeneity.
Overall survival was included as a secondary endpoint of the trial and defined from the time of randomization until death. We compared survival distributions according to the receipt of WBRT among patients with favorable and unfavorable DS-GPA scores using the log-rank test. We then tested for interactions between DS-GPA group (0.5–1.0, 1.5–2.0, 2.5–3.0, and 3.5–4.0) and WBRT on overall survival using Cox regression. Median survival times were estimated with 95% confidence intervals. Frequency distributions of categorical variables were assessed via the Fisher’s exact test. Significance was defined at 0.05 level with two sided p values reported.
RESULTS
A total of 126 patients with NSCLC were included in the analysis with a median follow up of 14.2 months. Twenty patients (13.7%) with NSCLC were excluded for missing data as outlined in Supplemental Figure 1. Baseline patient characteristics with regard to DS-GPA are displayed in Supplemental Table 1 and remained well balanced between the SRS and SRS+WBRT groups.
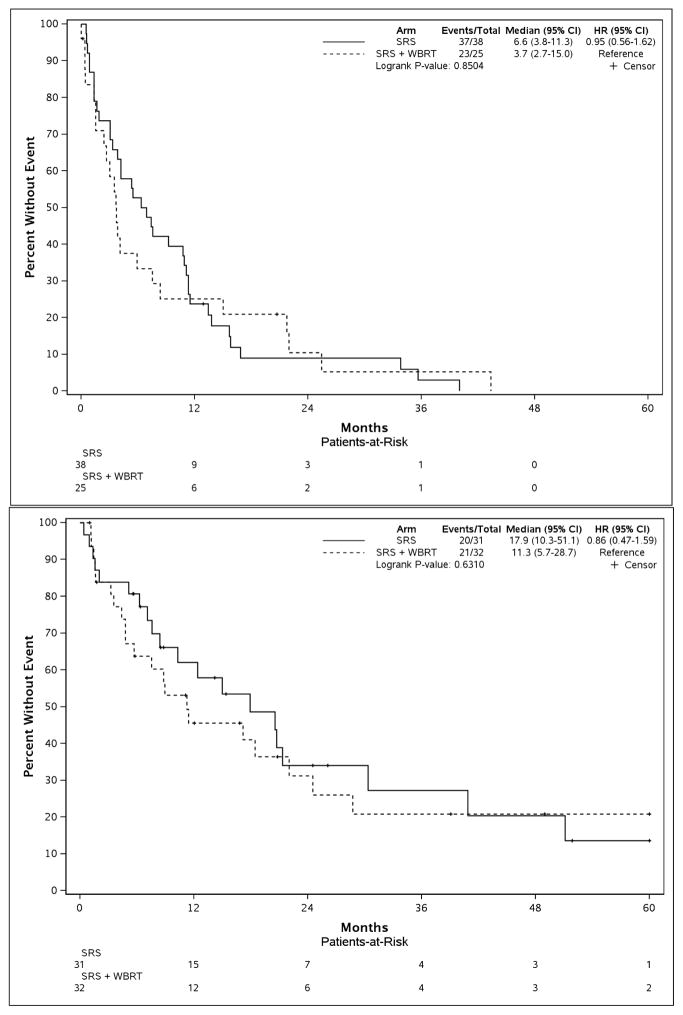
Among eligible patients, 50.0% of patients had DS-GPA scores ≥2.0 and 23.0% had scores ≥2.5 representing favorable sub-groups. Outcomes with regard to overall survival and brain tumor recurrence according to receipt of WBRT and DS-GPA are outlined in Table 1. Survival according to the receipt of WBRT was similar among patients with favorable or unfavorable DS-GPA scores, as illustrated in Figure 1 and Table 1. Outcomes according to the receipt of WBRT and DS-GPA remained similar utilizing DS-GPA≥2.5 as a threshold value for favorable prognoses as seen in Supplemental Table 2 and Supplemental Figure 2.
Table 1.
Outcomes in each treatment group according to diagnosis-specific graded prognostic assessment (DS-GPA) score and receipt of adjuvant whole brain radiotherapy (WBRT). DS-GPA threshold of 2.0 was used to define favorable vs. unfavorable prognostic groups.
| DS-GPA | Treatment Arm, Median (95% CI), mo | WBRT + SRS, HR (95% CI) | P Value | |
|---|---|---|---|---|
| SRS – Alone | SRS + WBRT | |||
| Overall Survival | ||||
| All | 10.8 (7.1–13.8) | 7.5 (3.9–17.1) | 0.98 (0.66–1.46) | 0.92 |
| 0.5–1.5 | 6.6 (3.8–11.3) | 3.7 (2.7–15.0) | 0.95 (0.56–1.62) | 0.85 |
| 2.0–4.0 | 17.9 (10.3–51.1) | 11.3 (5.7–28.7) | 0.86 (0.47–1.59) | 0.63 |
| BTR – Free Time | ||||
| All | 7.4 (5.5–9.2) | 21.6 (14.0-NE) | 4.11 (2.11–8.00) | < 0.001 |
| 0.5–1.5 | 5.3 (2.8–9.9) | NE (14.0-NE) | 9.92 (2.24–44.00) | < 0.001 |
| 2.0–4.0 | 9.1 (7.4-NE) | 20.8 (13.3-NE) | 2.68 (1.17–6.11) | 0.015 |
| Pattern of BTR, Total no. (Local/Distant/Both) | ||||
| All | 33 (12/16/5) | 10 (6/3/1) | --- | --- |
| 0.5–1.5 | 16(4/10/2) | 2 (2/0/0) | --- | --- |
| 2.0–4.0 | 16 (8/6/2) | 8 (4/3/1) | --- | --- |
| Missing | 1 (-/-/1) | ------------ | --- | --- |
| Salvage Brain Treatment for BTR, No. (%) | ||||
| All | 24 (34.3) | 5 (8.8) | ---< 0.001 | |
| 0.5–1.5 | 12 (31.6) | 0 (0.0) | --- | < 0.001 |
| 2.0–4.0 | 11 (35.5) | 5 (15.6) | --- | 0.088 |
| Missing | 1 (100) | ------------ | --- | --- |
| Grade 3 or 4 Late Radiation Toxic Effects, No. (%) | ||||
| All | 4 (5.7) | 5 (8.8) | --- | 0.73 |
| 0.5–1.5 | 2 (5.3) | 1 (4.0) | --- | 1 |
| 2.0–4.0 | 1 (3.2) | 4 (12.5) | --- | 0.35 |
| Missing | 1 (100) | ------------ | --- | --- |
SRS, radiosurgery; BTR, brain tumor recurrence; HR, Hazard Ratio; NE, Non-Estimable.
Figure 1.
Overall survival according to the receipt of adjuvant whole brain radiotherapy (WBRT) stratified by diagnosis-specific graded prognostic assessment (DS-GPA) score. DS-GPA threshold of 2.0 was used to define favorable vs. unfavorable prognostic groups. The top pane displays non-small cell lung cancer (NSCLC) with unfavorable prognoses (DS-GPA 0.5–1.5), while the bottom pane displays NSCLC patients with favorable prognoses (DS-GPA 2.0–4.0). SRS, radiosurgery.
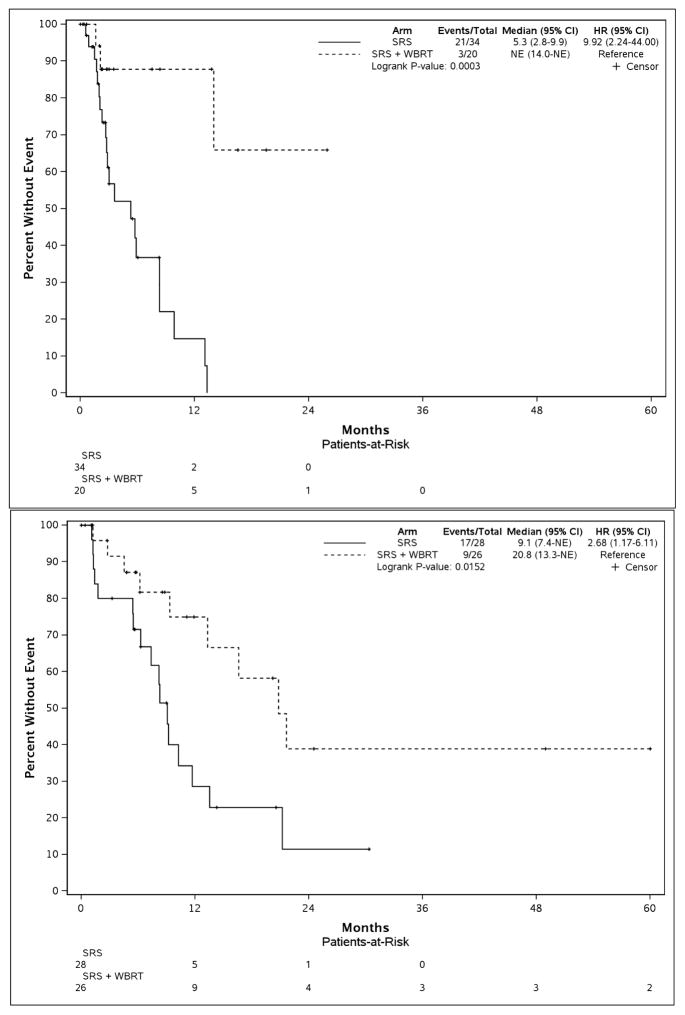
Further, there was no significant interaction between individual DS-GPA groups (0.5–1.0, 1.5–2.0, etc.) and WBRT on overall survival (p=0.53). There remained significant improvement in brain tumor recurrence free survival among all patients receiving WBRT compared to SRS alone (median=21.6 months vs. 7.4, p<0.001; HR, 4.11; 95%CI, 2.11–8.00). This intracranial control benefit persisted among both favorable and unfavorable prognosis patients (Figure 2 and Supplemental Figure 3).
Figure 2.
Brain tumor recurrence free survival according to the receipt of adjuvant whole brain radiotherapy (WBRT) stratified by diagnosis-specific graded prognostic assessment (DS-GPA) score. DS-GPA threshold of 2.0 was used to define favorable vs. unfavorable prognostic groups. The top pane displays non-small cell lung cancer (NSCLC) with unfavorable prognoses (DS-GPA 0.5–1.5), while the bottom pane displays NSCLC patients with favorable prognoses (DS-GPA 2.0–4.0). SRS, radiosurgery.
DISCUSSION
Our secondary analysis showed no difference in overall survival according to the use of WBRT among NSCLC patents with favorable prognoses, despite improved intracranial control associated with WBRT. Although four randomized trials reported similar overall survival among patients receiving SRS or SRS+WBRT(2–5), a post-hoc analysis of the JROSG-99 trial(8) suggested an overall survival benefit to WBRT among NSCLC patients with favorable prognoses (DS-GPA≥2.5). This secondary analysis supported a hypothesis that better prognosis patients, with potentially higher risk of dying from intracranial progression as opposed to extracranial progression, may derive a survival benefit from adjuvant WBRT. However, a second exploratory analysis of the EORTC 22952 trial did not replicate a survival advantage with WBRT among NSCLC patients with favorable DS-GPA scores at baseline or controlled extracranial disease.(9) Our results are similarly consistent with a lack of appreciable survival benefit with WBRT among patients with favorable prognoses, although intracranial control was improved with adjuvant WBRT. Salvage therapy, which was more frequent among the SRS alone group, may be responsible for the lack of a survival advantage in patients who received WBRT.
Important differences exist among these three analyses. The Alliance N0574 trial included fewer patients with relatively favorable prognoses (DS-GPA≥2.5) at 23% compared to the JROSG-99 trial (53%). Moreover, ethnicity varied as a function of accruing trial centers. Mutations in epidermal growth factor receptor are more prevalent among the Japanese population and are associated with delayed onset of brain metastases and longer survival after treatment.(11, 12) Further study of adjuvant WBRT may warrant attention to gene alterations.
Limitations of our study include the post-hoc nature of the analysis, lack of molecular information (i.e. EGFR or ALK gene rearrangements), and the relatively smaller number of patients with favorable prognoses which may have limited power to detect a potential survival difference. However, we observed no significant interaction between DS-GPA groups and WBRT on overall survival among the entire population further arguing against a selective survival benefit of WBRT in the subgroup of patients with a better prognosis.
CONCLUSIONS
Our secondary analysis of the phase III NCCTG N0574 (Alliance) trial failed to detect a survival benefit of adjuvant whole brain radiotherapy among non-small cell lung cancer patients with limited brain metastases and favorable prognoses at baseline. While additional prospective study may be warranted to define sub-populations that might derive benefit from adjuvant whole brain radiotherapy, these results provide further evidence that SRS alone is a preferred management approach for the majority of North American patients with limited brain metastases.
Supplementary Material
SUMMARY.
There are conflicting data regarding whether adjuvant whole brain radiotherapy (WBRT) improves survival among patients with 1–3 brain metastases and favorable prognostic factors. Our post-hoc analysis of the N0574 trial demonstrated that overall survival did not differ according to the use of WBRT among non-small cell lung cancer patients with favorable (p=0.63) or unfavorable (p=0.85) diagnosis-specific graded prognostic assessment scores. In patients with limited (1–3) brain metastases, stereotactic radiosurgery alone remains a preferred treatment approach.
Acknowledgments
FUNDING and ACKNOWLEDGEMENTS
This trial was conducted by the North Central Cancer Treatment Group (NCCTG, Alliance for Clinical Trials in Oncology) in collaboration with other cooperative groups including Radiation Therapy Oncology Group (RTOG), and was supported by Alliance for Clinical Trials in Oncology U10CA180821 and U10CA180882, American College of Surgeons Cancer Treatment Group U10CA076001, NCCTG CA025224, RTOG U10CA21661, NRG U10CA180868, U10CA180790 from the National Cancer Institute (NCI). There were no commercial sponsors of this study. Data confidentiality was governed by NIH policy. The corresponding author and principal investigators of the study (P.D.B. and A.L.A.) had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Role of the Funder/Sponsor: The funding institutions had no role in collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
These data have not been previously published, in full or in part.
Declaration of interests:
The authors have no potential conflicts of interest to disclose.
TRIAL REGISTRATION clinicaltrials.gov Identifier: NCT00377156
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnson JD, Young B. Demographics of brain metastasis. Neurosurg Clin N Am. 1996;7:337–344. [PubMed] [Google Scholar]
- 2.Brown PD, Jaeckle K, Ballman KV, et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA. 2016;316:401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 2295–6001 study. J Clin Oncol Off J Am Soc Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 5.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 6.Mehta MP, Aoyama H, Gondi V. The Changing Role of Whole-Brain Radiotherapy: Demise or Time for Selective Usage? JAMA Oncol. 2017 doi: 10.1001/jamaoncol.2016.5414. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Brown PD. The Diminishing Role of Whole-Brain Radiation Therapy in the Treatment of Brain Metastases. JAMA Oncol. 2017 doi: 10.1001/jamaoncol.2016.5411. [DOI] [PubMed] [Google Scholar]
- 8.Aoyama H, Tago M, Shirato H, et al. Stereotactic Radiosurgery With or Without Whole-Brain Radiotherapy for Brain Metastases: Secondary Analysis of the JROSG 99-1 Randomized Clinical Trial. JAMA Oncol. 2015;1:457–464. doi: 10.1001/jamaoncol.2015.1145. [DOI] [PubMed] [Google Scholar]
- 9.Churilla TM, Handorf E, Collette S, et al. Whole Brain Radiotherapy after Stereotactic Radiosurgery or Surgical Resection among Patients with 1–3 Brain Metastases and Favorable Prognoses: A Secondary Analysis of EORTC 22952–26001. Ann Oncol. doi: 10.1093/annonc/mdx332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII) Am J Cancer Res. 2015;5:2892–2911. [PMC free article] [PubMed] [Google Scholar]
- 12.Sperduto PW, Yang TJ, Beal K, et al. The Effect of Gene Alterations and Tyrosine Kinase Inhibition on Survival and Cause of Death in Patients With Adenocarcinoma of the Lung and Brain Metastases. Int J Radiat Oncol Biol Phys. 2016;96:406–413. doi: 10.1016/j.ijrobp.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.