Abstract
Objective
To investigate the association of cartilage degeneration with previous knee injuries not undergoing surgery, determined by morphological and quantitative 3 Tesla (3T) magnetic resonance imaging (MRI).
Materials and Methods
We performed a nested cross-sectional study of right knee MRIs from participants in the Osteoarthritis Initiative (OAI) aged 45–79 with baseline Kellgren-Lawrence score of 0–2. Cases were 142 right knees with self-reported history of injury limiting ability to walk for at least two days. Controls were 426 right knees without history of injury, frequency-matched to cases on age, BMI, gender, KL-scores and race (1:3 ratio). Cases and controls were compared using covariate-adjusted linear regression analysis, with the outcomes of region-specific T2 mean, laminar analysis and heterogeneity measured by texture analysis to investigate early cartilage matrix abnormalities and Whole-Organ Magnetic Resonance Imaging Score (WORMS) to investigate morphological knee lesions.
Results
Compared to control subjects we found significantly higher mean T2 values in the injury (lateral tibia (28.10ms vs. 29.11ms, p=0.001), medial tibia (29.70ms vs. 30.40ms, p=0.014) and global knee cartilage (32.73ms vs. 33.29ms, p=0.005)). Injury subjects also had more heterogeneous cartilage as measured by GLCM texture contrast, variance and entropy (p<0.05 in 14 out of 18 texture parameters). WORMS gradings were not significantly different between both groups (p>0.05).
Conclusion
A history of knee injury not treated surgically is associated with higher and more heterogeneous T2 values, but not with morphological knee abnormalities. Our findings suggest that significant, conservatively treated knee injuries are associated with permanent cartilage matrix abnormalities.
Keywords: Cartilage Imaging, Knee Injury, Magnetic Resonance Imaging, Osteoarthritis, Cartilage, Conservative Treatment of Knee Injury
INTRODUCTION
Osteoarthritis (OA) is a slowly progressive joint disease manifested by morphologic, biomechanical, biochemical and molecular changes and is the most prevalent type of arthritis which is expected to affect 18% of the US population by 2020 [1]. The prevalence of OA in different joints and clinical manifestation varies by gender, ethnic groups and age [2] with a broad range of risk factors impacting the course of OA. The systemic risk factors age, female gender, family history, overweight as well as local risk factors such as increased biomechanical loading on part of the joint, physical activity and previous knee surgery have already been established in the incidence, progression and disease burden of knee OA[3–6].
Knee injuries are a local biomechanical insult that may damage joint structures and have been recognized to increase the risk for clinically significant symptomatic knee OA involving pain and impaired quality of life [7–9]. However, it is not well known how knee injuries that are conservatively treated in patients that have never undergone knee surgery affect the knee internal structures and the cartilage matrix. Insights into cartilage matrix breakdown associated with this type of knee injury could provide relevant information to patients in preventing knee OA by instituting life style changes and adhering to injury prevention regimens.
Magnetic resonance imaging (MRI) is a well-established technique to measure knee OA structural tissue changes [10] and also allows quantitative assessment of early cartilage matrix abnormalities. In previous studies, T2 relaxation time measurements [11–13], laminar and T2 grey-level co-occurrence matrix analysis (GLCM texture analysis) [14–16] have shown to be sensitive biomarkers that can identify patients at risk for knee OA by characterizing collagen integrity and water content [12, 13].
The purpose of this cross-sectional nested study was to determine whether individuals with a history of right knee injury that was not treated surgically but with limited function for at least 2 days show differences in their biochemical cartilage composition and joint structure compared with control subjects without previous injury history. We used 3 Tesla (3T) MRI-based T2 relaxation time measurements as well as laminar analysis and texture parameters of the knee cartilage to investigate biochemical cartilage composition and the Whole-Organ Magnetic Resonance Imaging Score (WORMS) to investigate morphological joint structure. Our hypothesis was that subjects with a history of knee injury are more likely to show differences in their biochemical cartilage composition and joint structure as compared to control subjects without a history of knee injury and are therefore at greater risk to develop OA.
METHODS
Subjects
Subjects included in this study were participants from the Osteoarthritis Initiative (OAI), a longitudinal, prospective multi-center cohort study (http://www.oai.ucsf.edu/). The OAI was launched to improve prevention, diagnosis and treatment of OA by evaluating new biomarkers for assessment of onset and progression of knee OA. The 4796 participants that were enrolled in the study were aged between 45 and 79 years and were recruited at four clinical sites across the US. All subjects from our initial pool either had symptomatic knee OA at baseline, a high risk of developing OA in the following years (with risk factors such as previous knee surgery, overweight or family history of total knee replacement) or no risk factors for OA and no clinical symptoms. The OAI study is HIPAA compliant and approved by the institutional review boards at each clinical site. All study participants signed informed consent forms prior to enrollment.
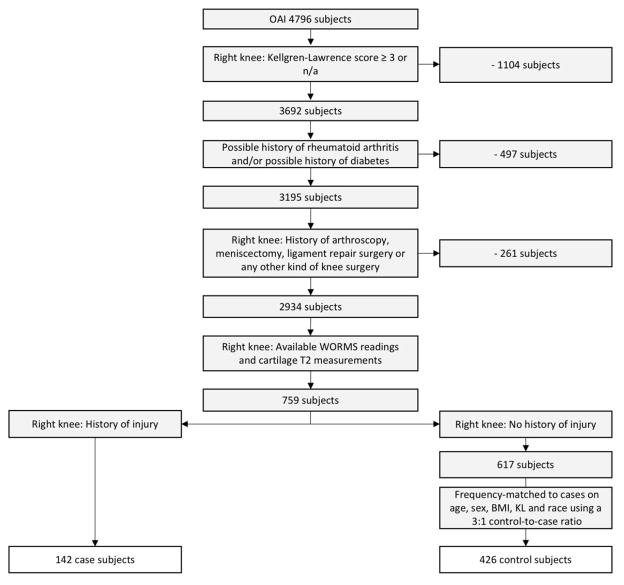
We performed a nested cross-sectional study of right knees with, and without, a history of injury at baseline. We included right knees with a Kellgren-Lawrence (KL) grade of 0–2 at baseline. We excluded subjects with rheumatoid arthritis or inflammatory arthritis and with self-reported diabetes, since we have previously found this to be associated with early degenerative cartilage alterations [17]. We also excluded those with a self-reported history of surgery in the right knee, based on yes/no questions asking about arthroscopy, replacement surgery, ligament repair surgery, meniscectomy or any other kind of surgery of the right knee. Of the remaining 2,934 right knees, cartilage T2 measurements and WORMS data were available in 759 right knees from previous and current studies by our group. Of these, we identified 142 case knees with a history of one or more right knee injuries that limited the ability to walk for at least two days. The selection of injured subjects was performed using the variable “P01INJR” from the Screening Visit workbook of the OAI. The variable is based on a self-reported history of having a right knee injury serious enough to limit ability to walk for at least two days. This variable is well validated and has been used in both the Framingham OA study and the “Multicenter Osteoarthritis Study” (MOST). Two recent studies have used this variable in their study selection [18, 19]. From the remaining knees that did not have a history of injury, we randomly selected 426 knees (3 for each case), frequency-matched on age, BMI, gender, KL-scores and race. A larger number of controls was included as increases in the control-to-case ratio have been shown to improve statistical power [20]. This resulted in a final total of 568 subjects. KL scores lower than 3 were chosen to assure that enough knee cartilage remained to assess MRI-based T2 relaxation time measurements. Fig. 1 shows a flowchart of patient selection.
Fig. 1.
Flow-chart shows selected subjects from the OAI database.
Imaging
All participants in this study had bilateral standing posterior-anterior fixed flexion radiographs of the right knee taken at baseline. One musculoskeletal radiologist and two rheumatologists graded the radiographs using KL Scores [21] with disagreements settled by adjudication [22, 23]. More information concerning the grading is available on http://www.oai.epi.org.
Using the OAI MRI Imaging Protocol in each patient, a right knee MRI was obtained at baseline [24]. All sequences were obtained at the 4 OAI clinical sites (Memorial Hospital of Rhode Island, Pawtucket, RI; University of Pittsburgh, Pittsburgh, PA; The Ohio State University, Columbus, OH; University of Maryland, School of Medicine, Baltimore, MD) using identical 3T MRI scanners (Siemens Magnetom Trio; Siemens, Erlangen, Germany) and quadrature transmit-receive knee coils (USA Instruments, Aurora, OH). For the T2 measurements the following sequence was used: A sagittal, 2D, multislice, multiecho (MSME) sequence (echo times (TE) = 10, 20, 30, 40, 50, 60 and 70 milliseconds (ms), spatial resolution = 0.313 × 0.446 × 3.0 mm, and 0.5 mm gap, pulse repetition time (TR) = 2700 ms; field-of-view =12 cm; bandwidth = 250 Hz/pixel). For morphological gradings the following additional sequences were used: 1) coronal proton density-weighted fast spin-echo (FSE) (TE= 29 ms; TR= 3700 ms), 2) sagittal 3D dual-echo in steady state (DESS) with selective water excitation (TE= 4.7 ms; TR= 16.3 ms; flip angle= 250), and 3) sagittal intermediate-weighted FSE with fat suppression (FS) (TE= 30 ms; TR= 3200 ms). More information on the OAI MRI sequence parameters is available in Peterfy. et al.[24].
WORMS Grading
Morphological MR sequences were reviewed on a picture archiving communication system (PACS) workstation using the semi-quantitative modified Whole-Organ Magnetic Resonance Imaging Score (WORMS) grading system [25, 26]. The MR images were assessed for location and severity of meniscal and cartilage lesions as well as bone marrow edema pattern (BMEP). Areas of elevated signal in the fatty bone marrow on fluid sensitive, fat suppressed FSE images with vague demarcation were considered as BMEP lesions. They were graded in the subchondral zone of the lateral femur (LF), lateral tibia (LT), medial femur (MF), medial tibia (MT) and patella (PAT) with a scale ranging from 0 to 3 by lesion size (0 = none, 1 = smaller than 5 mm in diameter, 2 = 5–20 mm in diameter, 3 = larger than 20 mm in diameter).
Cartilage lesions were graded in the LF, LT, MF, MT, PAT using an 8 point scoring system, as detailed before [27]: 0 = normal, 1 = abnormal signal on fluidsensitive sequences with and without swelling, 2 = focal defect smaller than 1 cm in width not reaching the subchondral bone, 2.5 = focal defect reaching the subchondral bone smaller than 1 cm in width, 3 = combination of normal thickness and multiple grade 2 lesions or grade 2 lesion wider than 1 cm but smaller than three quarters of the cartilage region, 4 = scattered cartilage loss of partial-thickness covering ≥ 75% of the region, 5 = multiple areas of grade 2.5 lesions covering <75% of the region and 6 = diffuse full thickness cartilage loss in more or at least three quarters of the region. Cartilage signal abnormalities are scored as WORMS grade 1 and are considered as early degenerative changes and a previous study by Schwaiger et al [28] has shown that signal abnormalities have a high probability to turn into focal cartilage defects.
For the evaluation of meniscal lesions we defined the six separate regions were reviewed: the anterior, body and posterior part of the medial and lateral meniscus, respectively. We used a score from 0 to 4 (0 = regular, 1 = abnormality within the substance, 2 = non-misplaced tear, 3 = displaced or complex tear and 4 = fully destructed/macerated meniscus).
A maximum BMEP (BMEP Max), cartilage (Cart Max) and meniscal (Men Max) value was computed for each knee for the respective greatest WORMS Score in any compartment similar to previous studies [5, 29, 30]. A Max>0 in any joint structure was interpreted as a lesion. Readers were blinded to the group status, risk profile and demographic characteristics of the corresponding patients.
MRI Knee Cartilage Segmentation
For T2 cartilage segmentation, five cartilage compartments (LF, LT, MF, MT and PAT) were segmented by trained researchers under the supervision of a radiologist. Segmentations were performed creating regions of interest (ROIs) [31] that enclosed the entirety of the cartilage tissue of the compartment [27]. For this semi-automated, spline-based segmentation the researchers were using proprietary software designed as a MATLAB (The Mathworks Inc., Natick, MA) application [16, 32]. Segmentations were done on a slice-by-slice basis and spanned all slices that contained the cartilage tissue. Single slices were excluded if the quality of the image was poor because of artifacts in the MRI or if the image exhibited full thickness cartilage loss or severely damaged cartilage tissue or overlapping fluid, as described before [33] [34]. Segmentators were blinded to the group status, risk profile and demographic characteristics of the corresponding patients.
T2 relaxation time measurements
For all 568 subjects, T2 maps were computed on a pixel-by-pixel basis using three parameter fittings to account for noise. As it has been shown that sparing the first echo time (10ms) maximizes the signal-to-noise ratio [35], T2 calculations were performed using the second (20ms) to the last (70ms) echo images (echo times = 20–70ms) [35–37]. Averaging the mean T2 values of all 5 compartments provided a global T2 value for the entire joint. We did not use the trochlea compartment in our study due to pulsation artifacts originating from the popliteal artery, which limited segmentation and T2 measurement.
Laminar and GLCM texture analysis
To obtain more detailed information on the spatial distribution of T2 values within the respective knee cartilage compartment (LF, LT, MF, MT or PAT), we performed laminar and texture analysis, latter with grey-level co-occurrence matrix (GLCM) algorithms [14, 15]. Laminar T2 analysis splits the cartilage into two layers of approximately the same width, a deep layer adjacent to the bone-cartilage interface (referred to as the bone layer) and a superficial layer along the articular surface (referred to as the articular layer) [38]. Furthermore the spatial distribution of cartilage T2 relaxation times in the respective compartment was calculated by GLCM texture analysis. Occurrence of similarity in neighboring grey-level values in a specific compartment was calculated using the approach of Haralick et al [15, 39]. GLCM parameters can be used as non-invasive imaging biomarkers for early cartilage matrix breakdown since they provide information on the extent of heterogeneity within the cartilage matrix going beyond information provided by regular T2 measurements. Three GLCM parameters were chosen in this study that have been used and validated in previous studies [14, 40]: 1) Contrast, with elevated T2 contrast demonstrating higher differences in neighboring pixel values, 2) Variance assessing the distribution of pixels about the mean and 3) Entropy measures the disorder in an image with high entropy values signifying less uniform distribution of probabilities of T2 relaxation time co-occurrences. There is strong evidence that GLCM texture parameters by measuring heterogeneity within the cartilage provide additional information compared to regular T2 measurements in characterizing matrix collagen matrix breakdown [40, 41]
Reproducibility
Intra- and interreader reproducibility for the semi-quantitative analyses of the WORMS score by our group has previously been described [26, 42]. For intra-observer agreement the intra-class correlation coefficients were reported to be 0.86 (0.80–0.93) for cartilage WORMS and 0.87 (0.80–0.93) for meniscus WORMS [42]. For inter-observer agreement intra-class correlation coefficients were reported to be 0.79 (0.72–0.87) for cartilage WORMS and 0.84 (0.77–0.91) for meniscus WORMS [42]. Averaged over all compartments, mean inter-observer and intra-observer reproducibilities for T2 measurements in our group have been previously reported with a root mean square error of 1.57% for inter-observer reproducibility [43] and of 1.17% for mean intra-reader reproducibility [29].
Statistical analysis
The statistical analysis for this study was performed with both STATA Version 13 (StataCorp, College Station, TX) and SPSS 23 (SPSS Inc., Chicago, IL, USA). A 2-sided 0.05 level of significance was used. The differences in subject characteristics between injured and uninjured subjects were assessed using T-tests for continuous variables and Chi-square tests for categorical variables. Linear regression models were used to determine the differences in T2 parameters (mean, laminar, and GLCM texture) and WORMS scores between injured and uninjured subjects in each cartilage region (LF, LT, MF, MT, PAT). Our main analyses were adjusted for the matching variables age, BMI, gender, KL-scores and race as well as the potentially important covariate physical activity assessed with the Physical Activity in the Elderly Scale (PASE) to account for remaining imbalances. We decided to adjust and thereby control for matching variables in order to control for confounding by the residual covariate imbalances of the matching factors in our main analysis as previously described [44]. Furthermore we performed sensitivity analysis adjusting for Western Ontario and McMaster Universities Arthritis Index (WOMAC) Pain which we treated as an outcome variable in our main analyses. In our subanalysis comparing remote versus more recent injuries we compared both patients with one injury less than or exactly 5 years ago versus patients with one injury more than 5 years ago and patients with one injury less than or exactly 20 years ago versus patients with one injury more than 20 years ago. We used these cut-off points to generate statistically meaningful analyses with sufficient power in the individual groups. In addition to these adjusted models, we performed analysis without adjustments. A two-sided p-value smaller than 0.05 was considered statistically significant.
RESULTS
Subject Characteristics
Subject characteristics are shown in Table 1. The case and control group were comparable in age (58.80 ± 9.26 years and 59.61 ± 9.50 years, respectively (p=0.38)) and mean BMIs (mean ± SD) (29.40 ± 4.68 kg/m2 and 28.75 ± 4.04 kg/m2, respectively (p=0.12)). There were also no significant differences in gender, KL grade, and race distributions between cases and controls (p > 0.05). However, WOMAC Pain, Stiffness, Disability and WOMAC Total Score were significantly higher in the case subjects (p < 0.001). For the Physical Activity Scale for the Elderly (PASE), we approached but did not reach clinical significance (p = 0.082) with the case subjects demonstrating a higher level of activity.
Table 1.
Subject characteristics and differences by case (n=142) and control (n=426) status. Subjects in the two groups are matched on age, BMI, gender, KL-scores and race. Differences were assessed using independent t-tests or Pearson’s chi-squared test as appropriate. Data are expressed as unadjusted means ± SD. Significant p-values (p<0.05) are highlighted in bold.
| Controls (n=426) | Injury cases (n=142) | P-value Injury vs controls | |
|---|---|---|---|
| Age (years) | 59.61 ± 9.50 | 58.80 ± 9.26 | 0.376 |
| Body mass index (kg/m2) | 28.75 ± 4.04 | 29.40 ± 4.68 | 0.115 |
| Gender – Male [n (%)] | 168 (39.4%) | 55 (38.7%) | 0.882 |
| PASE score | 159.4 ± 85.6 | 173.9 ± 84.8 | 0.082 |
| WOMAC Pain | 2.29 ± 3.17 | 3.72 ± 3.57 | <0.001 |
| WOMAC Stiffness | 1.37 ± 1.54 | 2.18 ± 1.77 | <0.001 |
| WOMAC Disability | 6.81 ± 9.90 | 13.04 ± 12.71 | <0.001 |
| WOMAC Total Score | 10.44 ± 13.78 | 18.90 ± 17.12 | <0.001 |
|
| |||
| Baseline KL grade | 0.671 | ||
| KL 0 [n (%)] | 177 (40.8%) | 54 (38.7%) | |
| KL 1 [n (%)] | 69 (16.2%) | 22 (15.5 %) | |
| KL 2 [n (%)] | 180 (43.0%) | 66 (45.8%) | |
|
| |||
| Racial composition | 0.775 | ||
| Caucasian [n (%)] | 337 (79.1%) | 110 (76.8%) | |
| Other Non-white [n (%)] | 8 (1.9 %) | 4 (2.8%) | |
| African American [n (%)] | 81 (19.0%) | 28 (20.4%) | |
|
| |||
| OAI Risk factors | <0.001 | ||
| History of 1 knee injury [n (%)] | 0 (0.0%) | 105 (73.9%) | |
| History of 2 knee injuries [n (%)] | 0 (0.0%) | 24 (16.9%) | |
| History of 3 knee injuries [n (%)] | 0 (0.0%) | 13 (9.2%) | |
|
| |||
| Years since first injury | <0.001 | ||
| 0 – 5 years ago [n (%)] | - | 24 (16.9%) | |
| 6 – 10 years ago [n (%)] | - | 17 (12.0%) | |
| 11 – 20 years ago [n (%)] | - | 23 (16.2%) | |
| 21 – 30 years ago [n (%)] | - | 24 (16.9%) | |
| 31 – 40 years ago [n (%)] | - | 28 (19.7%) | |
| More than 40 years ago [n (%)] | - | 26 (18.3%) | |
T2 measurements
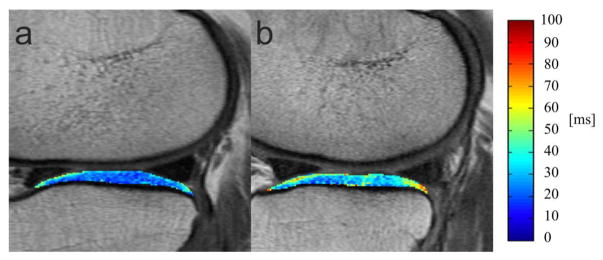
The 142 case subjects demonstrated higher T2 values (mean T2, bone layer, articular layer) in all compartments and globally compared to the 426 uninjured control subjects as shown in Table 2. Regarding mean T2 values a significant difference was found for 2 compartments and globally (LT: p = 0.001; MT: p = 0.014; Global: p = 0.005). Fig. 2 shows a lower T2 relaxation time in the LT in a control subject without previous injury compared to an injured case subject. Similar results were found separately for the bone (LT, MT, PAT and global values significantly higher in case subjects; p<0.05) and articular layer (LF, LT and global values significantly higher in case subjects; p<0.05).
Table 2.
Comparison of cartilage T2 values (95% confidence intervals) between case (n=142) and control (n=426) cohorts. Differences were assessed using linear regression adjusting for age, BMI, gender, KL-scores, race and PASE in the main analysis and adjusting for BMI, gender, race and PASE in the sensitivity analysis by KL-score. Significant P-values (p < 0.05) are highlighted in bold.
| Parameter | Non-injured (n = 426) |
Injured (n = 142) |
Adjusted P-value |
Non-injured KL 0&1 (n = 246) |
Injured KL0&1 (n = 76) |
Adjusted P-value |
Non-injured KL 2 (n= 180) |
Injured KL2 (n= 66) |
Adjusted P-value |
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| predicted mean values [95% CI] (ms) | predicted mean values [95% CI] (ms) | predicted mean values [95% CI] (ms) | |||||||
| Cartilage T2 | |||||||||
| Global knee T2 | 32.73 [32.54, 32.93] | 33.29 [32.96, 33.63] | 0.005 | 32.48 [32.23, 32.73] | 33.25 [32.80, 33.70] | 0.004 | 33.07 [32.77, 33.37] | 33.40 [32.90, 33.90] | 0.256 |
| LF T2 | 35.57 [35.32, 35.81] | 36.05 [35.62, 36.48] | 0.058 | 35.16 [34.86, 35.46] | 35.69 [35.14, 36.24] | 0.098 | 36.09 [35.67, 36.50] | 36.56 [35.86, 37.25] | 0.257 |
| LT T2 | 28.10 [27.82, 28.39] | 29.11 [28.62, 29.61] | 0.001 | 28.09 [27.72, 28.47] | 29.48 [28.81, 30.14] | <0.001 | 28.11 [27.66, 28.56] | 28.70 [27.95, 29.45] | 0.188 |
| MF T2 | 38.95 [38.69, 39.21] | 39.07 [38.61, 39.53] | 0.654 | 38.38 [38.04, 38.72] | 38.98 [38.36, 39.60] | 0.096 | 39.70 [39.30, 40.10] | 39.27 [38.59, 39.94] | 0.281 |
| MT T2 | 29.70 [29.43, 29.98] | 30.40 [29.92, 30.88] | 0.014 | 29.45 [29.09, 29.80] | 30.39 [29.76, 31.02] | 0.011 | 30.04 [29.62, 30.46] | 30.43 [29.70, 31.17] | 0.365 |
| PAT T2 | 31.57 [31.27, 31.86] | 32.13 [31.61, 32.65] | 0.065 | 31.50 [31.13, 31.86] | 32.29 [31.64, 32.94] | 0.038 | 31.68 [31.17, 32.18] | 31.90 [31.03, 32.78] | 0.661 |
|
| |||||||||
| Deep layer T2 | |||||||||
| Global knee deep layer T2 | 30.24 [29.92, 30.56] | 31.19 [30.64, 31.75] | 0.004 | 29.85 [29.55, 30.16] | 30.89 [30.34, 31.44] | 0.001 | 30.74 [30.12, 31.37] | 31.62 [30.59, 32.66] | 0.153 |
| LF deep layer T2 | 33.27 [32.90, 33.64] | 33.93 [33.28, 34.57] | 0.087 | 32.72 [32.37, 33.06] | 33.10 [32.48, 33.73] | 0.290 | 34.00 [33.26, 34.75] | 35.00 [33.76, 36.25] | 0.176 |
| LT deep layer T2 | 24.87 [24.46, 25.28] | 26.37 [25.65, 27.08] | <0.001 | 24.78 [24.29, 25.27] | 26.62 [25.74, 27.50] | <0.001 | 24.98 [24.29, 25.68] | 26.08 [24.90, 27.26] | 0.118 |
| MF deep layer T2 | 36.79 [36.39, 37.19] | 37.47 [36.77, 38.16] | 0.098 | 36.12 [35.73, 36.51] | 36.93 [36.24, 37.63] | 0.045 | 37.68 [36.91, 38.46] | 38.23 [36.91, 39.55] | 0.486 |
| MT deep layer T2 | 27.52 [27.10, 27.94] | 28.66 [27.91, 29.40] | 0.009 | 27.32 [26.78, 27.85] | 28.47 [27.53, 29.42] | 0.037 | 27.78 [27.09, 28.47] | 28.96 [27.75, 30.16] | 0.098 |
| PAT deep layer T2 | 29.02 [28.61, 29.43] | 30.11 [29.38, 30.83] | 0.011 | 28.57 [28.18, 28.96] | 29.84 [29.15, 30.54] | 0.002 | 29.70 [28.84, 30.55] | 30.50 [29.05, 31.95] | 0.349 |
|
| |||||||||
| Superficial layer T2 | |||||||||
| Global knee superficial T2 | 35.69 [35.33, 36.05] | 36.54 [35.91, 37.16] | 0.021 | 35.69 [35.33, 36.05] | 36.54 [35.91, 37.16] | 0.011 | 36.20 [35.50, 36.90] | 37.01 [35.84, 38.17] | 0.248 |
| LF superficial T2 | 38.06 [37.65, 38.48] | 39.08 [38.35, 39.80] | 0.018 | 37.55 [37.13, 37.96] | 38.48 [37.73, 39.23] | 0.034 | 38.73 [37.93, 39.53] | 39.91 [38.57, 41.25] | 0.141 |
| LT superficial T2 | 31.77 [31.35, 32.19] | 32.98 [32.25, 33.72] | 0.005 | 31.66 [31.22, 32.11] | 32.93 [32.13, 33.73] | 0.007 | 31.91 [31.11, 32.70] | 33.07 [31.73, 34.40] | 0.144 |
| MF superficial T2 | 41.41 [40.99, 41.84] | 41.85 [41.10, 42.60] | 0.326 | 40.65 [40.20, 41.09] | 41.53 [40.73, 42.33] | 0.060 | 42.42 [41.62, 43.23] | 42.38 [41.00, 43.76] | 0.959 |
| MT superficial T2 | 32.79 [32.35, 33.23] | 33.52 [32.74, 34.30] | 0.109 | 32.33 [31.81, 32.85] | 33.03 [32.10, 33.95] | 0.201 | 33.38 [32.61, 34.15] | 34.24 [32.90, 35.59] | 0.275 |
| PAT superficial T2 | 34.74 [34.22, 35.25] | 35.57 [34.66, 36.47] | 0.118 | 34.53 [34.02, 35.04] | 35.45 [34.54, 36.36] | 0.085 | 35.05 [34.01, 36.10] | 35.74 [33.96, 37.52] | 0.511 |
Fig. 2.
Two representative sagittal T2 color maps of the lateral tibia cartilage of the right knee overlaid with the first-echo images of the MSME sequence. (A) 61-year old woman without injury (BMI 31.7, KL 1) from the control group and (B) 67-year old woman with injury (BMI 31.5, KL 1) from the case group. Blue color demonstrates low, while red color demonstrates high cartilage T2 values. Lateral tibia cartilage of uninjured subject showed lower T2 relaxation time (22.18 ms) compared to the subject with injury (37.31 ms).
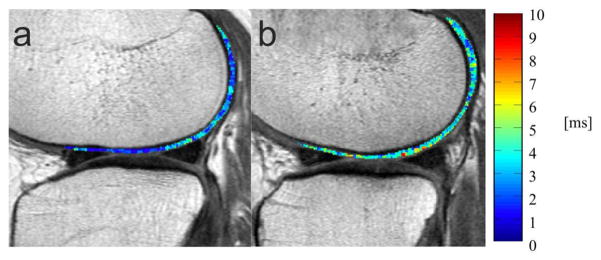
Differences in GLCM texture measures were even greater as shown in Table 3; GLCM contrast and GLCM variance were significantly higher globally and in the LF, LT, MT and PAT (p<0.05). GLCM entropy was significantly higher for LF, LT, MT and globally (p<0.05). Fig. 3 demonstrates lower GLCM contrast in the LF in a control subject without previous injury compared to an injured case subject.
Table 3.
Comparison of mean Grey Level Co-occurrence Matrix (GLCM) parameters (95% confidence intervals) between case (n=142) and control (n=426) cohorts. Differences were assessed using linear regression models adjusting for age, BMI, gender, KL-scores, race and PASE in the main analysis and adjusting for BMI, gender, race and PASE in the sensitivity analysis by KL-score. Significant P-values (p < 0.05) are highlighted in bold.
| Parameter | Non-injured (n = 426) |
Injured (n = 142) |
Adjusted P-value |
Non-injured KL 0&1 (n = 246) |
Injured KL0&1 (n = 76) |
Adjusted P-value |
Non-injured KL 2 (n= 180) |
Injured KL2 (n= 66) |
Adjusted P-value |
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| predicted mean values [95% CI] | predicted mean values [95% CI] | predicted mean values [95% CI] | |||||||
|
|
|
||||||||
| Contrast | |||||||||
| Global knee contrast | 276.16 [269.89, 282.43] | 298.84 [287.97, 309.72] | <0.001 | 262.62 [255.10, 270.13] | 288.87 [275.34, 302.40] | 0.001 | 293.62 [282.87, 304.37] | 312.97 [295.12, 330.81] | 0.070 |
| LF contrast | 258.04 [252.97, 263.12] | 275.47 [266.64, 284.31] | <0.001 | 245.27 [239.30, 251.25] | 261.72 [250.95, 272.48] | 0.009 | 274.77 [265.93, 283.61] | 294.13 [279.37, 308.88] | 0.028 |
| LT contrast | 174.44 [168.09, 180.80] | 201.13 [190.04, 212.22] | <0.001 | 170.79 [162.58, 179.01] | 201.34 [186.55, 216.12] | 0.000 | 179.04 [168.93, 189.16] | 202.06 [185.05, 219.07] | 0.023 |
| MF contrast | 410.34 [400.14, 420.55] | 426.49 [408.60, 444.39] | 0.125 | 389.72 [376.65, 402.80] | 415.60 [392.16, 439.04] | 0.059 | 437.46 [421.08, 453.84] | 443.71 [415.76, 471.67] | 0.706 |
| MT contrast | 295.51 [284.78, 306.23] | 322.95 [304.06, 341.85] | 0.014 | 282.51 [268.66, 296.36] | 316.67 [292.12, 341.23] | 0.018 | 312.92 [295.78, 330.06] | 331.94 [301.92, 361.96] | 0.282 |
| PAT contrast | 243.30 [232.21, 254.39] | 275.61 [256.16, 295.07] | 0.005 | 227.78 [218.37, 237.20] | 251.36 [234.49, 268.23] | 0.017 | 266.12 [242.14, 290.10] | 311.28 [270.45, 352.11] | 0.062 |
|
| |||||||||
| Entropy | |||||||||
| Global knee entropy | 6.08 [6.06, 6.11] | 6.14 [6.11, 6.18] | 0.007 | 6.07 [6.04, 6.10] | 6.13 [6.08, 6.18] | 0.029 | 6.10 [6.07, 6.14] | 6.16 [6.10, 6.22] | 0.095 |
| LF entropy | 6.58 [6.56, 6.60] | 6.64 [6.60, 6.67] | 0.013 | 6.55 [6.52, 6.57] | 6.61 [6.56, 6.66] | 0.032 | 6.62 [6.59, 6.66] | 6.67 [6.61, 6.72] | 0.196 |
| LT entropy | 5.56 [5.53, 5.60] | 5.68 [5.61, 5.74] | 0.003 | 5.56 [5.53, 5.60] | 5.68 [5.61, 5.74] | 0.037 | 5.57 [5.51, 5.63] | 5.69 [5.59, 5.79] | 0.037 |
| MF entropy | 6.78 [6.75, 6.80] | 6.80 [6.76, 6.84] | 0.325 | 6.74 [6.71, 6.77] | 6.78 [6.73, 6.83] | 0.195 | 6.82 [6.78, 6.86] | 6.83 [6.76, 6.90] | 0.810 |
| MT entropy | 5.69 [5.66, 5.72] | 5.77 [5.71, 5.82] | 0.020 | 5.66 [5.62, 5.71] | 5.71 [5.63, 5.78] | 0.336 | 5.73 [5.68, 5.78] | 5.85 [5.77, 5.94] | 0.016 |
| PAT entropy | 5.84 [5.80, 5.88] | 5.83 [5.77, 5.90] | 0.822 | 5.88 [5.83, 5.92] | 5.94 [5.86, 6.02] | 0.154 | 5.80 [5.72, 5.87] | 5.68 [5.56, 5.81] | 0.114 |
|
| |||||||||
| Variance | |||||||||
| Global knee variance | 199.58 [195.37, 203.80] | 215.71 [208.39, 223.03] | <0.001 | 190.14 [185.06, 195.23] | 209.15 [200.00, 218.30] | <0.001 | 211.69 [204.52, 218.85] | 225.28 [213.39, 237.18] | 0.056 |
| LF variance | 191.10 [187.47, 194.72] | 203.99 [197.68, 210.30] | <0.001 | 181.07 [176.69, 185.46] | 193.80 [185.90, 201.70] | 0.006 | 204.19 [198.03, 210.35] | 218.05 [207.78, 228.33] | 0.024 |
| LT variance | 140.84 [136.19, 145.48] | 162.43 [154.32, 170.54] | <0.001 | 138.48 [132.13, 144.83] | 164.19 [152.77, 175.61] | <0.001 | 143.74 [136.94, 150.54] | 161.28 [149.84, 172.71] | 0.010 |
| MF variance | 283.41 [277.13, 289.69] | 291.05 [280.04, 302.06] | 0.239 | 268.19 [260.13, 276.24] | 283.39 [268.95, 297.83] | 0.072 | 303.39 [293.37, 313.41] | 303.43 [286.32, 320.53] | 0.997 |
| MT variance | 200.57 [193.77, 207.37] | 219.60 [207.61, 231.58] | 0.007 | 191.74 [182.85, 200.63] | 215.61 [199.84, 231.38] | 0.010 | 212.35 [201.68, 223.02] | 225.53 [206.85, 244.21] | 0.231 |
| PAT variance | 183.97 [175.76, 192.18] | 207.07 [192.67, 221.47] | 0.006 | 174.25 [167.42, 181.08] | 191.59 [179.36, 203.82] | 0.016 | 198.29 [180.43, 216.16] | 229.72 [199.30, 260.13] | 0.081 |
Fig. 3.
Two representative sagittal T2 texture color maps of the lateral femur cartilage of the right knee overlaid with the first-echo images of the MSME sequence. (A) 61-year old woman without injury (BMI 31.7, KL 1) from the control group and (B) 67-year old woman with injury (BMI 31.5, KL 1) from the case group. Blue color demonstrates low, while red color demonstrates high differences in neighboring pixel values to visualize GLCM contrast texture measures. The calculated GLCM contrast of the lateral femur cartilage of the uninjured subject was lower (182.37) compared to the subject with injury (388.49).
Impact of multiple injuries on T2 measurements
While mean T2 values in subjects with two and more injuries (n=37) were higher for LT, MF, MT and globally compared to subjects who reported only one injury (n=105) differences were not significant (P>0.05). GLCM texture parameters were also higher for LF, LT, MF, MT, Global (variance), LF, LT, MF, MT, PAT, Global (contrast) LF, LT, MF, MT, Global (entropy) compared to subjects who reported only one injury, but differences were not significant (p>0.05). Comparison was performed both without adjustments and adjusting for age, BMI, gender, KL-scores, race and PASE.
Impact of years since injury on T2 measurement
We also analyzed the association of years since injury and T2 measurements in case knees with only one previous injury (n = 105). In these patients with one previous injury we did not find a statistically significant relationship between the years since injury and the T2 parameters (mean T2, superficial and deep layer T2, GLCM contrast, GLCM entropy and GLCM variance) for any compartment or globally.
Separating subjects into two groups with a previous trauma less or exactly 20 years ago (n = 54) and more than 20 years ago (n = 51) we found higher T2 values for LF, LT, MF, PAT and global joint in knees with injury more than 20 years ago compared to subjects with injury less than 20 years ago, but only the difference in mean T2 of the LF approached significance (P=0.069) in our comparison without adjustments. Similarly a number of texture measure were higher in the knees with injury more than 20 years ago compared to subjects with injury less than or exactly 20 years ago but differences were not significant (p>0.05).
In a sensitivity analysis comparing two groups with a previous trauma less or exactly 5 years ago (n = 22) and more than 5 years ago (n = 83) we did not find significant differences in T2 parameters (mean T2, superficial and deep layer T2, GLCM contrast, GLCM entropy and GLCM variance) for any compartment or globally. All comparisons were performed both without adjustments and adjusting for age, BMI, gender, KL-scores, race and PASE.
WORMS Grading
None of the WORMS subscores (BMEP, cartilage and meniscus lesions) showed significant differences between the injured and the uninjured groups (P>0.05; Table 4) in the adjusted and unadjusted comparisons. The two groups showed a similar number and severity of morphological cartilage, meniscus and subchondral bone marrow abnormalities. Moreover, we did not detect significant differences between the subgroups with multiple injuries compared to a single injury. WORMS scores of subjects with trauma more and less than 20 years ago and more and less than 5 years ago were comparable and there was no significant difference for any parameter (P > 0.05).
Table 4.
Comparison of WORMS Grading for meniscal and cartilage lesions and bone marrow edema pattern (95% confidence intervals) between case (n=142) and control (N=426) cohorts. Differences were assessed using linear regression models adjusting for age, BMI, gender, KL-scores, race and PASE in the main analysis and adjusting for BMI, gender, race and PASE in the sensitivity analysis by KL-score. None of the P-values was significant in the main analysis (p > 0.05). Significant P-Values in the sensitivity analysis by KL score are highlighted in bold
| WORMS scores | Non-Injured (n = 426) | Injured (n = 142) | Adjusted P-value | Non-injured KL 0&1 (n = 246) | Injured KL0&1 (n = 76) | Adjusted P-value | Non-injured KL 2 (n= 180) | Injured KL2 (n= 66) | Adjusted P-value |
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| predicted mean [95% CI] | predicted mean [95% CI] | predicted mean [95% CI] | |||||||
| Cartilage lesions | |||||||||
| Sum Score | 4.23 [3.92, 4.53] | 4.12 [3.58, 4.65] | 0.732 | 2.96 [2.61, 3.31] | 2.92 [2.29, 3.55] | 0.913 | 5.87 [5.32, 6.43] | 5.71 [4.79, 6.63] | 0.768 |
| Max Score | 2.58 [2.43, 2.74] | 2.43 [2.16, 2.70] | 0.330 | 1.94 [1.75, 2.13] | 1.86 [1.52, 2.21] | 0.717 | 3.42 [3.16, 3.68] | 3.18 [2.75, 3.61] | 0.357 |
| LF | 0.37 [0.28, 0.45] | 0.45 [0.30, 0.60] | 0.333 | 0.19 [0.09, 0.28] | 0.40 [0.23, 0.58] | 0.033 | 0.60 [0.45, 0.76] | 0.54 [0.29, 0.80] | 0.700 |
| LT | 0.65 [0.54, 0.76] | 0.75 [0.56, 0.93] | 0.393 | 0.49 [0.37, 0.61] | 0.70 [0.48, 0.93] | 0.101 | 0.86 [0.67, 1.05] | 0.83 [0.51, 1.15] | 0.881 |
| MF | 0.76 [0.65, 0.87] | 0.64 [0.45, 0.83] | 0.296 | 0.51 [0.38, 0.63] | 0.37 [0.15, 0.60] | 0.305 | 1.09 [0.89, 1.29] | 0.99 [0.66, 1.32] | 0.602 |
| MT | 0.23 [0.15, 0.30] | 0.35 [0.22, 0.48] | 0.122 | 0.15 [0.07, 0.23] | 0.12 [-0.02, 0.26] | 0.776 | 0.33 [0.19, 0.48] | 0.62 [0.38, 0.87] | 0.048 |
| PAT | 2.22 [2.05, 2.38] | 1.93 [1.64, 2.21] | 0.085 | 1.63 [1.44, 1.82] | 1.32 [0.97, 1.66] | 0.120 | 2.98 [2.69, 3.27] | 2.72 [2.24, 3.20] | 0.362 |
|
| |||||||||
| Meniscus lesions | |||||||||
| Sum Bilateral | 1.62 [1.39, 1.85] | 1.77 [1.37, 2.17] | 0.536 | 1.26 [0.97, 1.54] | 1.80 [1.28, 2.31] | 0.072 | 0.91 [0.81, 1.01] | 0.92 [0.75, 1.09] | 0.926 |
| Sum Medial | 0.88 [0.74, 1.02] | 0.93 [0.68, 1.17] | 0.750 | 0.69 [0.53, 0.86] | 0.78 [0.48, 1.07] | 0.635 | 1.12 [0.87, 1.37] | 1.14 [0.73, 1.55] | 0.937 |
| Sum Lateral | 0.74 [0.58, 0.91] | 0.84 [0.56, 1.13] | 0.553 | 0.56 [0.36, 0.77] | 1.02 [0.66, 1.38] | 0.031 | 0.97 [0.69, 1.24] | 0.68 [0.22, 1.14] | 0.288 |
| Max Bilateral | 0.91 [0.81, 1.01] | 0.92 [0.75, 1.09] | 0.926 | 0.73 [0.61, 0.85] | 0.87 [0.66, 1.09] | 0.275 | 1.14 [0.97, 1.30] | 1.01 [0.75, 1.29] | 0.461 |
| Max Medial | 0.63 [0.55, 0.72] | 0.66 [0.52, 0.81] | 0.720 | 0.52 [0.42, 0.62] | 0.52 [0.34, 0.70] | 0.948 | 0.78 [0.64, 0.93] | 0.85 [0.61, 1.09] | 0.640 |
| Max Lateral | 0.45 [0.37, 0.54] | 0.49 [0.35, 0.64] | 0.629 | 0.36 [0.25, 0.46] | 0.60 [0.42, 0.79] | 0.024 | 0.57 [0.43, 0.71] | 0.39 [0.16, 0.62] | 0.180 |
|
| |||||||||
| Bone marrow edema pattern | |||||||||
| Sum Score | 1.29 [1.14, 1.44] | 1.26 [1.00, 1.52] | 0.848 | 0.88 [0.72, 1.05] | 0.93 [0.64, 1.22] | 0.791 | 1.83 [1.55, 2.11] | 1.72 [1.25, 2.18] | 0.692 |
| Max Score | 0.97 [0.88, 1.06] | 0.84 [0.68, 0.99] | 0.135 | 0.97 [0.88, 1.06] | 0.84 [0.68, 0.99] | 0.135 | 0.97 [0.88, 1.06] | 0.84 [0.68, 0.99] | 0.135 |
| LF | 0.08 [0.05, 0.12] | 0.10 [0.03, 0.16] | 0.734 | 0.04 [0.00, 0.08] | 0.12 [0.05, 0.19] | 0.050 | 0.15 [0.08, 0.21] | 0.07 [-0.04, 0.18] | 0.296 |
| LT | 0.14 [0.09, 0.18] | 0.22 [0.14, 0.30] | 0.083 | 0.06 [0.01, 0.10] | 0.24 [0.15, 0.32] | 0.000 | 0.24 [0.14, 0.33] | 0.22 [0.06, 0.37] | 0.858 |
| MF | 0.19 [0.14, 0.25] | 0.15 [0.06, 0.23] | 0.357 | 0.12 [0.07, 0.18] | 0.08 [-0.02, 0.18] | 0.463 | 0.29 [0.19, 0.38] | 0.24 [0.07, 0.40] | 0.600 |
| MT | 0.11 [0.07, 0.16] | 0.17 [0.10, 0.25] | 0.198 | 0.04 [0.00, 0.07] | 0.07 [0.00, 0.14] | 0.388 | 0.22 [0.13, 0.31] | 0.30 [0.15, 0.45] | 0.337 |
| PAT | 0.77 [0.68, 0.85] | 0.63 [0.48, 0.78] | 0.115 | 0.63 [0.53, 0.74] | 0.42 [0.23, 0.61] | 0.060 | 0.94 [0.80, 1.08] | 0.88 [0.65, 1.12] | 0.678 |
| WORMS overall | 8.96 [8.33, 9.58] | 9.13 [8.04, 10.21] | 0.794 | 6.14 [5.40, 6.88] | 6.97 [5.65, 8.30] | 0.283 | 12.63 [11.53, 13.73] | 12.04 [10.22, 13.87] | 0.590 |
Sensitivity Analysis by KL grade
Finally, we evaluated if any observed cartilage abnormalities were associated with more severe radiological evidence of OA, suggesting OA and not trauma as the source of abnormality. We separately compared injured versus non-injured subjects by KL score included in Table 2, 3 and 4. We divided all study subjects into subjects with KL scores 0/1 (no OA) and subjects with a KL score of 2 (mild OA). Our analysis resulted in 246 case subjects versus 76 control subjects with KL0/1 and 180 case subjects versus 66 control subjects with KL 2. All significantly elevated T2 parameters from the main analysis were significant for the 76 injured versus 246 control subjects with KL0/1 as well except for MT entropy. Moreover, in this subanalysis we found significantly elevated mean T2 for the PAT (p = 0.038) and deep layer T2 for the MF (p = 0.045). Also, we found significantly higher grades of WORMS cartilage lesions in the LF (p = 0.033), WORMS sum of lateral meniscus lesions (p = 0.031), WORMS maximal lateral meniscus lesions (p = 0.024), WORMS BME in the LF (p = 0.050) and WORMS BME in the LT (p = 0.000). When comparing the 66 injured subjects versus 180 control subjects with KL2 we did not find any significant differences for mean T2 measurements or laminar analysis. However, both mean T2 and laminar analysis did still show a slight but non significant elevation for all parameters except MF mean T2 and MF superficial T2 in the injured subjects. However, in this subanalysis we found significantly elevated GLCM texture parameters in the injury group for LF contrast (p = 0.028), LT contrast (p = 0.023), LT entropy (p = 0.037), MT entropy (p = 0.016), LF variance (p = 0.024) and LT variance (p = 0.010). In this subanalysis the injured subjects also showed significantly elevated WORMS cartilage lesions in the MT (p = 0.048).
DISCUSSION
This study demonstrated a mild, but significant elevation of cartilage T2 mean and texture parameters (mean T2, superficial and deep layer T2, GLCM contrast, GLCM entropy and GLCM variance) in subjects with previous knee injury compared to uninjured controls. However, the prevalence of morphological lesions such as cartilage lesions, meniscal abnormalities and BMEP assessed with WORMS was not associated with previous knee injury in this study population.
Cartilage 3T MRI-based T2 relaxation time mapping detects increase in water content and disruption of the organization of the anisotropic arrangement of collagen fibrils in the extracellular cartilage matrix [45, 46]. Our findings suggest impairment of the cartilage matrix in subjects who have formerly experienced a knee injury that was not treated surgically, interestingly at the same time there were no differences in morphological cartilage abnormalities between injured and uninjured subjects with an overall mild degree of degeneration in both groups. The altered spatial distribution of T2 values found with the GLCM texture analysis is consistent with these findings and also suggests early cartilage matrix breakdown before irreversible cartilage loss occurs in these trauma subjects [40, 47, 48]. Especially local variance has been previously shown to serve as a sensitive biomarker for detection of early extracellular matrix changes in patients at risk for OA underlining that texture analysis is a promising tool to investigate early changes of the biochemical cartilage composition [49].
The relationship between surgically treated acute joint trauma and development of post-traumatic OA has been extensively studied [50]. Meniscal subluxation, meniscal tears, ACL tears and residual joint instability have been identified to elevate the risk for future OA [51–54]. T2 mapping has been used for post-operative evaluation of cartilage degeneration [49] and it has been proven to be a powerful tool to evaluate early changes of the cartilage matrix for example after acute ACL injury and reconstruction [55]. In our study, however, we focused on injuries that were not acute and did not require surgery (or at least the subjects did not undergo surgery of any kind) even though the injury limited function for 2 or more days. While T2 values were higher in the injury cohort, interestingly subjects did not show differences in WORMS scores, that would have reflected more severe focal morphological damage. Accordinlgy, our findings suggest that the mild differences of the cartilage T2 matrix place an individual at increased risk for future cartilage damage. This hypothesis is supported by a previous study that has shown that higher T2 values predict development of radiographic OA over 4 years [12]. This study indicates that it may take several years for these abnormalities to manifest themselves morphologically. The mild elevations of T2 values we found may take a longer time or need potentially to be combined with other risk factors.
In addition to that all of our superficial layer T2 times were higher than the corresponding deep layer T2 values and in that sense our results are in line with existent literature on layer-specific cartilage T2 relaxation times in knees [56]. However, while the lateral femur demonstrated statistically more pronounced differences in the superficial layer, for the other compartments and globally we found statistically more distinct differences for the deep layer. The deep cartilage layer or tidemark (calcified) zone has previously been found to play a significant role for cartilage degeneration, especially in the evolution of cartilage delamination; therefore we hypothesize that the deep layer may have been potentially more injured through trauma [51].
Interestingly, one study found that acute Anterior Cruciate Ligament (ACL) disruption resulting in a chondral injury that is detectable at the time of injury using MRI may be associated with cartilage degradation in compartments unaffected by the initial trauma at follow-up MR-based assessment [57]. Considering that our results suggest altered T2 measurements for all compartments our study supports these findings of global, post-traumatic degradation. It is important to note, however, that our study was not limited to ACL tears and may indicate that several kinds of knee trauma not requiring knee surgery trigger a whole joint response. These global, posttraumatic cartilage matrix abnormalities may be explained through damage of the cartilage homeostasis, which is associated with a stress response of chondrocytes and the subsequent release of cartilage matrix degeneration products [58]. In addition to that recent data indicate an increased level of inflammatory mediators resulting from joint injury for a long-lasting period after trauma [59]. Inflammation affects both quality and quantity of the extracellular matrix in cartilage and inflammatory factors lead to an elevation of matrix degrading proteins [60]. Cellular stress response and inflammation might both explain our findings of abnormal cartilage matrix.
Another important finding of our study was that persons with a previous injury also had significantly more knee pain. While the injured subjects in this study reported significantly higher pain, the results for neither T2 measurements nor WORMS scores did substantially change when we adjusted for pain. On the other hand a previous study showed that higher T2 values were associated with pain in subjects with risk factors for OA [26]. One may hypothesize that cartilage matrix changes related to injury and associated with higher T2 values may make the joint more susceptible to pain sensations. This could have a potentially protective effect on the knee joint and may explain why the subjects with previous injury have no increased morphological damage compared to subjects without knee injury. Furthermore, the increased pain and an inflammatory reaction could make pharmacological, analgesic treatment necessary. For this kind of injury without any abnormalities that require surgery a broad range of treatments used in the clinical practice is available [59]. Our results provide more insights into the potential need for anti-inflammatory pharmacological treatment to avoid harmful side effects of the injury on the biochemical composition of cartilage as well as the consideration of therapeutic options that slow down or reverse matrix degeneration.
Finally, our study showed more pronounced differences in T2 measurements when comparing subjects in the two groups without OA (KL0/1). This may in part be due to the smaller number of KL grade 2 subjects. However, when focusing on KL2 we have to take into account confounding variables that may have lead to mild OA in these subjects. We hypothesize that in a cohort of KL2 subjects confounders may act stronger than an injury that did not require a surgical intervention. Furthermore injuries that could have contributed to an observable difference in KL grade might already have progressed to KL3 and 4. This might explain that we did not find significant morphological damage in our case cohort.
The prevalence of knee abnormalities has previously been shown to be associated with the level of physical activity measured using the PASE [5]. Our injured subjects demonstrated higher PASE scores with the differences between the two groups approaching statistical significance. However, our main analysis adjusting for the matching variables and PASE on the one hand and our analysis without adjustments on the other hand did not substantially differ and we therefore do not believe that physical activity may have been a significant driver of our results.
Nevertheless, this study has several limitations. Firstly, our definition of knee trauma may include a broad range of different injuries since the only criterion was the inability to walk for at least two days. However, we excluded all injuries that resulted in knee surgery, which means that our case knees were unlikely to have suffered a major knee injury; still we were able to demonstrate a strong link between knee trauma and alteration of the cartilage extracellular matrix. Secondly, there is a considerable potential for confounding in a cross-sectional study. Alterations in the cartilage matrix may have developed both before and after the injury. Furthermore we did not include possible confounders like medication, non-surgical treatment of the injury and other treatment for OA. Thirdly, gait analysis was not included in our study as gait data were not provided by the OAI and beyond the scope of this study. Post-traumatic gait mechanics have been found to shift the loading patterns to cartilage regions that are not suited for this load [4]. Fourth, following the OAI MRI Procedure Manual for Examinations of the knee sagittal T2 maps are only acquired in the right knee. Only patients with a knee replacement or implants or foreign bodies identified on the right knee localizer had this sequence performed on the left knee. Therefore we were not able to compare T2 measurements in the same patient. Fifth, our compartment-specific T2 analyses are suited to assess cartilage matrix damage and identify the affected compartment. However, we did not pinpoint the exact location of altered cartilage composition as correlation with the site of the injury is not possible retrospectively. Sixth, our retrospective design bears a higher risk of error due to confounding than a prospective study design and we could not stratify our data according to a specific type of conservatively treated injury.
In conclusion, our study shows that elevated cartilage T2 average and texture measurements were associated with conservatively treated knee injuries. Our study suggests that cartilage T2 measurements are able to detect cartilage composition abnormalities in knees with a history of injury not treated with surgery before significant morphological differences appear. Our findings underline both the need to treat post-traumatic cartilage matrix alterations and strongly encourage ongoing investigations on treatment that might help best protect the cartilage matrix after conservatively treated significant knee trauma.
Acknowledgments
This study was performed with support by the OAI which is a public-private partnership comprising five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2- 2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health which constitutes a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. The private funding partners involved are Novartis Pharmaceuticals Corporation; Pfizer, Inc.; Merck Research Laboratories; and GlaxoSmithKline. The private sector funding for the OAI is administered by the Foundation for the National Institutes of Health. Analyses in this study were also funded through grants awarded by the NIH NIAMS (National Institute of Arthritis and Musculoskeletal and Skin Diseases) grants R01AR064771 and P50-AR060752).
Footnotes
Conflict of interest disclosure
The authors declare that they have no conflict of interest.
Ethical approval: The OAI study is HIPAA compliant and approved by the institutional review boards at each clinical site. All study participants signed informed consent forms prior to enrolment.
References
- 1.Sharma L, Kapoor D, Issa S. Epidemiology of osteoarthritis: an update. Curr Opin Rheumatol. 2006;18(2):147–156. doi: 10.1097/01.bor.0000209426.84775.f8. [DOI] [PubMed] [Google Scholar]
- 2.Wise BL, Niu J, Yang M, Lane NE, Harvey W, Felson DT, et al. Patterns of compartment involvement in tibiofemoral osteoarthritis in men and women and in whites and African Americans. Arthritis Care Res (Hoboken) 2012;64(6):847–852. doi: 10.1002/acr.21606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gersing AS, Link TM. Imaging of Osteoarthritis in Geriatric Patients. Curr Radiol Rep. 2016;4(1) doi: 10.1007/s40134-015-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent KR, Conrad BP, Fregly BJ, Vincent HK. The pathophysiology of osteoarthritis: a mechanical perspective on the knee joint. PM R. 2012;4(5 Suppl):S3–9. doi: 10.1016/j.pmrj.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage. 2010;18(6):776–786. doi: 10.1016/j.joca.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–199. doi: 10.1093/bmb/lds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133(5):321–328. doi: 10.7326/0003-4819-133-5-200009050-00007. [DOI] [PubMed] [Google Scholar]
- 8.Toivanen AT, Heliovaara M, Impivaara O, Arokoski JP, Knekt P, Lauren H, et al. Obesity, physically demanding work and traumatic knee injury are major risk factors for knee osteoarthritis--a population-based study with a follow-up of 22 years. Rheumatology (Oxford) 2010;49(2):308–314. doi: 10.1093/rheumatology/kep388. [DOI] [PubMed] [Google Scholar]
- 9.Muthuri SG, McWilliams DF, Doherty M, Zhang W. History of knee injuries and knee osteoarthritis: a meta-analysis of observational studies. Osteoarthritis Cartilage. 2011;19(11):1286–1293. doi: 10.1016/j.joca.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Hunter DJ, Zhang W, Conaghan PG, Hirko K, Menashe L, Reichmann WM, et al. Responsiveness and reliability of MRI in knee osteoarthritis: a meta-analysis of published evidence. Osteoarthritis Cartilage. 2011;19(5):589–605. doi: 10.1016/j.joca.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8(4):355–368. doi: 10.1055/s-2004-861764. [DOI] [PubMed] [Google Scholar]
- 12.Liebl H, Joseph G, Nevitt MC, Singh N, Heilmeier U, Subburaj K, et al. Early T2 changes predict onset of radiographic knee osteoarthritis: data from the osteoarthritis initiative. Ann Rheum Dis. 2015;74(7):1353–1359. doi: 10.1136/annrheumdis-2013-204157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baum T, Joseph GB, Karampinos DC, Jungmann PM, Link TM, Bauer JS. Cartilage and meniscal T2 relaxation time as non-invasive biomarker for knee osteoarthritis and cartilage repair procedures. Osteoarthritis Cartilage. 2013;21(10):1474–1484. doi: 10.1016/j.joca.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carballido-Gamio J, Joseph GB, Lynch JA, Link TM, Majumdar S. Longitudinal analysis of MRI T2 knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative: a texture approach. Magn Reson Med. 2011;65(4):1184–1194. doi: 10.1002/mrm.22693. [DOI] [PubMed] [Google Scholar]
- 15.Haralick R. Statistical and Structured Approaches to Texture. Proceedings of the IEEE. 1979;61(5):786–803. [Google Scholar]
- 16.Yu A, Heilmeier U, Kretzschmar M, Joseph GB, Liu F, Liebl H, et al. Racial differences in biochemical knee cartilage composition between African-American and Caucasian-American women with 3 T MR-based T2 relaxation time measurements--data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2015;23(9):1595–1604. doi: 10.1016/j.joca.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jungmann PM, Kraus MS, Alizai H, Nardo L, Baum T, Nevitt MC, et al. Association of metabolic risk factors with cartilage degradation assessed by T2 relaxation time at the knee: data from the osteoarthritis initiative. Arthritis Care Res (Hoboken) 2013;65(12):1942–1950. doi: 10.1002/acr.22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Driban JB, Eaton CB, Lo GH, Ward RJ, Lu B, McAlindon TE. Association of knee injuries with accelerated knee osteoarthritis progression: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2014;66(11):1673–1679. doi: 10.1002/acr.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Driban JB, Lo GH, Eaton CB, Price LL, Lu B, McAlindon TE. Knee Pain and a Prior Injury Are Associated with Increased Risk of a New Knee Injury: Data from the Osteoarthritis Initiative. J Rheumatol. 2015;42(8):1463–1469. doi: 10.3899/jrheum.150016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hennessy S, Bilker WB, Berlin JA, Strom BL. Factors influencing the optimal control-to-case ratio in matched case-control studies. Am J Epidemiol. 1999;149(2):195–197. doi: 10.1093/oxfordjournals.aje.a009786. [DOI] [PubMed] [Google Scholar]
- 21.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felson DT, Nevitt MC, Yang M, Clancy M, Niu J, Torner JC, et al. A new approach yields high rates of radiographic progression in knee osteoarthritis. J Rheumatol. 2008;35(10):2047–2054. [PMC free article] [PubMed] [Google Scholar]
- 23.Felson DT, Niu J, Guermazi A, Sack B, Aliabadi P. Defining radiographic incidence and progression of knee osteoarthritis: suggested modifications of the Kellgren and Lawrence scale. Ann Rheum Dis. 2011;70(11):1884–1886. doi: 10.1136/ard.2011.155119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Baum T, Joseph GB, Arulanandan A, Nardo L, Virayavanich W, Carballido-Gamio J, et al. Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2012;64(2):248–255. doi: 10.1002/acr.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kretzschmar M, Heilmeier U, Yu A, Joseph GB, Liu F, Solka M, et al. Longitudinal analysis of cartilage T2 relaxation times and joint degeneration in African American and Caucasian American women over an observation period of 6 years -data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2016;24(8):1384–1391. doi: 10.1016/j.joca.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwaiger BJ, Gersing AS, Mbapte Wamba J, Nevitt MC, McCulloch CE, Link TM. Can Signal Abnormalities Detected with MR Imaging in Knee Articular Cartilage Be Used to Predict Development of Morphologic Cartilage Defects? 48-Month Data from the Osteoarthritis Initiative. Radiology. 2016;281(1):158–167. doi: 10.1148/radiol.2016152308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan J, Stehling C, Muller-Hocker C, Schwaiger BJ, Lynch J, McCulloch CE, et al. Vastus lateralis/vastus medialis cross-sectional area ratio impacts presence and degree of knee joint abnormalities and cartilage T2 determined with 3T MRI - an analysis from the incidence cohort of the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2011;19(1):65–73. doi: 10.1016/j.joca.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stehling C, Liebl H, Krug R, Lane NE, Nevitt MC, Lynch J, et al. Patellar cartilage: T2 values and morphologic abnormalities at 3.0-T MR imaging in relation to physical activity in asymptomatic subjects from the osteoarthritis initiative. Radiology. 2010;254(2):509–520. doi: 10.1148/radiol.09090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teichtahl AJ, Wluka AE, Tanamas SK, Wang Y, Strauss BJ, Proietto J, et al. Weight change and change in tibial cartilage volume and symptoms in obese adults. Ann Rheum Dis. 2015;74(6):1024–1029. doi: 10.1136/annrheumdis-2013-204488. [DOI] [PubMed] [Google Scholar]
- 32.Honkonen SE. Degenerative arthritis after tibial plateau fractures. J Orthop Trauma. 1995;9(4):273–277. doi: 10.1097/00005131-199509040-00001. [DOI] [PubMed] [Google Scholar]
- 33.Joseph GB, McCulloch CE, Nevitt MC, Heilmeier U, Nardo L, Lynch JA, et al. A reference database of cartilage 3 T MRI T2 values in knees without diagnostic evidence of cartilage degeneration: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2015;23(6):897–905. doi: 10.1016/j.joca.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wise BL, Niu J, Guermazi A, Liu F, Heilmeier U, Ku E, et al. Magnetic resonance imaging lesions are more severe and cartilage T2 relaxation time measurements are higher in isolated lateral compartment radiographic knee osteoarthritis than in isolated medial compartment disease - data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2016 doi: 10.1016/j.joca.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raya JG, Dietrich O, Horng A, Weber J, Reiser MF, Glaser C. T2 measurement in articular cartilage: impact of the fitting method on accuracy and precision at low SNR. Magn Reson Med. 2010;63(1):181–193. doi: 10.1002/mrm.22178. [DOI] [PubMed] [Google Scholar]
- 36.Smith HE, Mosher TJ, Dardzinski BJ, Collins BG, Collins CM, Yang QX, et al. Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging. 2001;14(1):50–55. doi: 10.1002/jmri.1150. [DOI] [PubMed] [Google Scholar]
- 37.Maier CF, Tan SG, Hariharan H, Potter HG. T2 quantitation of articular cartilage at 1.5 T. J Magn Reson Imaging. 2003;17(3):358–364. doi: 10.1002/jmri.10263. [DOI] [PubMed] [Google Scholar]
- 38.Carballido-Gamio J, Blumenkrantz G, Lynch JA, Link TM, Majumdar S. Longitudinal analysis of MRI T(2) knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative. Magn Reson Med. 2010;63(2):465–472. doi: 10.1002/mrm.22201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haralick R, Shanmugam K, Dinstein I. Textural Features for Image Classification. IEEE Transactions on Systems, Man, and Cybernetics. 1973;SMC-1:610–618. [Google Scholar]
- 40.Blumenkrantz G, Stahl R, Carballido-Gamio J, Zhao S, Lu Y, Munoz T, et al. The feasibility of characterizing the spatial distribution of cartilage T(2) using texture analysis. Osteoarthritis Cartilage. 2008;16(5):584–590. doi: 10.1016/j.joca.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carballido-Gamio J, Joseph GB, Lynch JA, Link TM, Majumdar S. Longitudinal analysis of MRI T(2) knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative: A texture approach. Magn Reson Med. 2010 doi: 10.1002/mrm.22693. [DOI] [PubMed] [Google Scholar]
- 42.Bucknor MD, Nardo L, Joseph GB, Alizai H, Srikhum W, Nevitt MC, et al. Association of cartilage degeneration with four year weight gain--3T MRI data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2015;23(4):525–531. doi: 10.1016/j.joca.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stehling C, Baum T, Mueller-Hoecker C, Liebl H, Carballido-Gamio J, Joseph GB, et al. A novel fast knee cartilage segmentation technique for T2 measurements at MR imaging--data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2011;19(8):984–989. doi: 10.1016/j.joca.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pearce N. Analysis of matched case-control studies. BMJ. 2016;352:i969. doi: 10.1136/bmj.i969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liess C, Lusse S, Karger N, Heller M, Gluer CC. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage. 2002;10(12):907–913. doi: 10.1053/joca.2002.0847. [DOI] [PubMed] [Google Scholar]
- 46.Crema MD, Roemer FW, Marra MD, Burstein D, Gold GE, Eckstein F, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 2011;31(1):37–61. doi: 10.1148/rg.311105084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X, Pai A, Blumenkrantz G, Carballido-Gamio J, Link T, Ma B, et al. Spatial distribution and relationship of T1rho and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson Med. 2009;61(6):1310–1318. doi: 10.1002/mrm.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carballido-Gamio J, Stahl R, Blumenkrantz G, Romero A, Majumdar S, Link TM. Spatial analysis of magnetic resonance T1rho and T2 relaxation times improves classification between subjects with and without osteoarthritis. Med Phys. 2009;36(9):4059–4067. doi: 10.1118/1.3187228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eagle S, Potter HG, Koff MF. Morphologic and quantitative magnetic resonance imaging of knee articular cartilage for the assessment of post-traumatic osteoarthritis. J Orthop Res. 2016 doi: 10.1002/jor.23345. [DOI] [PubMed] [Google Scholar]
- 50.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 51.Buckwalter JA, Lane NE. Athletics and osteoarthritis. Am J Sports Med. 1997;25(6):873–881. doi: 10.1177/036354659702500624. [DOI] [PubMed] [Google Scholar]
- 52.Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. [PubMed] [Google Scholar]
- 53.Gale DR, Chaisson CE, Totterman SM, Schwartz RK, Gale ME, Felson D. Meniscal subluxation: association with osteoarthritis and joint space narrowing. Osteoarthritis Cartilage. 1999;7(6):526–532. doi: 10.1053/joca.1999.0256. [DOI] [PubMed] [Google Scholar]
- 54.Johnson VL, Guermazi A, Roemer FW, Hunter DJ. Comparison in knee osteoarthritis joint damage patterns among individuals with an intact, complete and partial anterior cruciate ligament rupture. Int J Rheum Dis. 2016 doi: 10.1111/1756-185X.13003. [DOI] [PubMed] [Google Scholar]
- 55.Su F, Hilton JF, Nardo L, Wu S, Liang F, Link TM, et al. Cartilage morphology and T1rho and T2 quantification in ACL-reconstructed knees: a 2-year follow-up. Osteoarthritis Cartilage. 2013;21(8):1058–1067. doi: 10.1016/j.joca.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wirth W, Maschek S, Roemer FW, Eckstein F. Layer-specific femorotibial cartilage T2 relaxation time in knees with and without early knee osteoarthritis: Data from the Osteoarthritis Initiative (OAI) Sci Rep. 2016;6:34202. doi: 10.1038/srep34202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Potter HG, Jain SK, Ma Y, Black BR, Fung S, Lyman S. Cartilage injury after acute, isolated anterior cruciate ligament tear: immediate and longitudinal effect with clinical/MRI follow-up. Am J Sports Med. 2012;40(2):276–285. doi: 10.1177/0363546511423380. [DOI] [PubMed] [Google Scholar]
- 58.Houard X, Goldring MB, Berenbaum F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr Rheumatol Rep. 2013;15(11):375. doi: 10.1007/s11926-013-0375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Filardo G, Kon E, Longo UG, Madry H, Marchettini P, Marmotti A, et al. Non-surgical treatments for the management of early osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1775–1785. doi: 10.1007/s00167-016-4089-y. [DOI] [PubMed] [Google Scholar]
- 60.Maldonado M, Nam J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed Res Int. 2013;2013:284873. doi: 10.1155/2013/284873. [DOI] [PMC free article] [PubMed] [Google Scholar]