Abstract
Background and Objectives
Moderators of treatment response to serotonin reuptake inhibitor sertraline (SRT) for cocaine dependence were assessed in two randomized, double blind, placebo-controlled clinical trials.
Methods
Generalized estimating equation modeling was performed on data from cocaine-dependent volunteers randomized to receive SRT or placebo (N=126) who completed ≥ 2-week drug-free residential portions of the 12-week trials, in which subsequent outpatient treatment (weeks 3–12) included weekly cognitive behavioral therapy and thrice-weekly supervised urine toxicology.
Primary outcome measure: relapse (2 consecutive cocaine-positive or missing urines) following residential stay. Potential moderators included treatment, sex, age, race, depression measures, baseline cocaine urine result, and alcohol dependence diagnosis (ADDx).
Results
Odds ratios (OR) for relapse showed placebo-treated participants were significantly more likely to relapse than SRT participants. Regardless of treatment condition, participants more likely to relapse were male, and those with lower Hamilton depression ratings, or baseline cocaine-negative urines. Older subjects or those with current ADDx had higher relapse risk than those without ADDx; however, treating older or ADDx participants with SRT reduced cocaine relapse more than placebo.
Discussion and Conclusions
Women or those with more severe cocaine use or depressive symptoms may have fewer cocaine relapses regardless of medication treatment. SRT at 200 mg reduced cocaine relapse more than placebo, especially in older participants or in those with comorbid ADDx.
Scientific Significance
SRT may be efficacious to support relapse prevention among cocaine-dependent patients in the context of brief residential followed by outpatient treatment, especially in older participants or those with comorbid alcohol/cocaine dependence.
Keywords: cocaine dependence, randomized clinical trial, placebo control, sertraline, relapse prevention, cognitive behavioral therapy
Background/Objectives
Cocaine dependence remains a major US public health challenge with relatively stable prevalence of approximately 1.5 million individuals in the US.1,2 Problems associated with cocaine use include psychiatric issues,3 cognitive effects,4 high-risk sexual behavior, HIV and HCV transmission,5 cardiotoxicity,6 cerebrovascular consequences,7 crime, and fetal drug exposure.8 Despite these compelling factors, no robustly effective pharmacotherapy for cocaine dependence has been developed.9 Long-term cocaine use is associated with alterations in serotonergic (5-HT) and dopaminergic (DA) functioning, with deficits in 5-HT and DA function following abrupt termination of chronic cocaine use.10 These deficits are thought to underlie withdrawal symptoms following cessation of chronic use, including depressive symptoms and suicidal ideation.11 Indeed, similar 5-HT and DA dysfunctions occur during depression, 12,13 symptoms of which are highly prevalent in cocaine users, especially during early abstinence.14–16 Depressive symptoms among cocaine-dependent patients have been associated with greater severity of cocaine dependence and impairment17 as well as poor treatment outcome.18 Although most well controlled trials with antidepressants in unselected cocaine-dependent patients have had disappointing results (e.g., 19,20), antidepressants have shown some efficacy in treating depressed subgroups of cocaine-dependent patients (e.g., 21,22). Consequently, administration of antidepressants that inhibit both 5-HT and DA reuptake would theoretically address dysfunction in those systems associated with cocaine abuse and/or depression,23 particularly if pharmacotherapy begins during early abstinence.
Two clinical trials with similar designs examined efficacy of the selective serotonin reuptake inhibitor (SSRI) sertraline (SRT), which also has DA reuptake activity24 to prolong abstinence in recently abstinent cocaine-dependent patients with depressive symptoms. Results of each study indicated that, significantly greater proportions of SRT-treated participants did not lapse (i.e., have one cocaine-positive urine) and/or relapse (i.e., two consecutive cocaine-positive urines) relative to those who were placebo-treated.25,26 These findings suggest that SRT may have utility as a relapse prevention agent for cocaine dependence. Nevertheless, response to SRT was not complete, indicating the need to identify potential prognosticators in order to optimize treatment outcomes.
Thus, the present study was undertaken to: 1) examine relative efficacy of SRT with a larger, more diverse sample comprised of data from the two smaller studies cited above, 2) explore potential prognosticators of treatment response regardless of treatment group and 3) determine potential predictors of response to SRT versus placebo. To this end, we combined and analyzed data from 126 participants in the two prior clinical trials, all of whom were randomized to receive either placebo or SRT and completed at least the residential stay to confirm two weeks of abstinence.25,26 Several baseline/demographic variables were chosen based on prior reports that they may impact treatment outcomes (e.g., sex,27 baseline cocaine use,28,29 comorbid alcohol dependence,30 and depressive symptoms28,29).
Methods
Participants
Data from 126 treatment seeking cocaine-dependent individuals randomized to receive either placebo or SRT in two previously reported studies25,26 were analyzed. Male and female participants were recruited from the areas of greater New Haven during 2000–2004 (N=76)25 and Little Rock during 2005–2009 (N=50) 26,31 after giving informed consent to participate in the studies approved by the Yale Human Investigations Committee/VA Connecticut Human Studies Subcommittee and University of Arkansas for Medical Sciences Institutional Review Boards, respectively. In both studies, participants had to meet Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for cocaine dependence, as determined by the Structured Clinical Interview for DSM-IV,32 had urine toxicological examination results positive for cocaine/metabolite (benzoylecgonine) during the month before study entry, and/or had a history of cocaine use of at least 1 gram during the previous 3 months. Exclusion criteria were: medical conditions contraindicating SRT use; current use of psychiatric medication; current diagnosis of drug (nicotine excluded) or alcohol physiological dependence; history of major psychiatric disorder (psychosis, schizophrenia, or bipolar); ill health; current suicidal tendency; and/or inability to read and understand the consent form. Women of childbearing age were included provided they had a negative urine pregnancy test result, agreed to use adequate contraception during the study, and consented to monthly pregnancy tests.25,26 Although the New Haven study had excluded 17 subjects from final analyses due to HAM-D scores of less than 16 (N=8) or due to drop out almost immediately after the residential portion (N=9), data from these participants were included in the present study as HAM-D score is one of the factors of interest, thus providing a greater range of scores with which to examine significance of this factor. Completing the residential stay is the minimum requirement for inclusion in present analyses, given relapse at any point after the residential stay is our primary outcome.
Design and Procedure
Detailed study procedures have been described previously.25,26 In these 12-week, randomized, double blind, placebo-controlled clinical trials, participants were randomized to receive either placebo or SRT (200 mg/day) for 12 weeks, starting at a local residential facility for the first two weeks, and then followed as outpatients for 10 weeks. [Note that the Little Rock trial included a SRT plus gabapentin arm, 26 but only SRT- and placebo-treated participants’ data were included in these analyses.] During the outpatient treatment phase participants at both sites received the same weekly, individual, manual-guided Cognitive Behavioral Therapy (CBT) designed for cocaine-focused treatment.33 Compliance with study requirements was promoted during outpatient weeks (3–12 inclusive) through use of contingency management procedures, such that participants were given fixed monetary compensation for each clinic attendance and take-home blister pack return (up to $280.00 total). Supervised urine samples, self-reported adverse effects and vital signs were obtained thrice weekly; mood and drug use self-reports were obtained once weekly. At the end of 12 weeks, participants were tapered off study medication and referred to appropriate treatment programs, if desired. Due to higher than expected drop out in the UAMS study, incentives were added for residing at the residential program for approximately half of the participants (up to $70.00 total).
Because cocaine abstinence had to be initiated and maintained during weeks 1–2, participants had to submit urine samples negative for cocaine by the beginning of week 2 and were administratively discharged from the study if a urine toxicology screen indicated resumed cocaine or other drug use. Participants were discharged from the outpatient portion of the trials if they missed attending clinic to receive weekly medication, missed 3 consecutive supervised urine samples, or a subject’s health or well-being was threatened by continuation in the study as per study physicians. Participants administratively discharged from the study (New Haven study: N=46; UAMS study: N=33) were offered referrals to local treatment programs.25,26
Assessments
Analytic factors used in the present study were obtained from a demographics form, the Structured Clinical Interview for DSM-IV,32 the Addiction Severity Index,34 and the Hamilton Depression Scale (HAM-D),35 all of which were completed prior to admission to the residential facility. For the outcome measure of interest (see below), results from thrice-weekly supervised urine toxicology screens for cocaine metabolite (benzoylecgonine) were utilized.
Data Analyses
As in our prior studies,25,26 the primary outcome measure was cocaine relapse. Overall, 16.6% of individual urine cocaine results were missing. Missing urine results were treated as informative missing, with cocaine relapse defined as two positive or two missing urine cocaine results in a row. Participants who dropped out during weeks 3–12 were considered treatment failures, with date of second missed urine sample collection defined as relapse in these individuals.
Differences in baseline participant characteristics between treatment groups by site were determined using t-tests or nonparametric analogs, Wilcoxon or Kruskal-Wallis tests for continuous variables, or Pearson χ2 tests for categorical variables 25,26. Missing rates of potential moderators used in the models were low (i.e., sex and race had 0.8% missing (n=1); lifetime depression, current depression, lifetime alcohol dependence and current alcohol dependence each had 1.6% missing (n=2) and thus were ignored.
Modeling was performed using generalized estimating equation (GEE) and Quasi-Likelihood Information Criteria (QIC) model fit statistics, with model-building analyses as follows.
Model 1
Univariate GEE models, clustered on site, were performed with the following 10 possible predictors: Treatment Group (sertraline vs. placebo), Sex (male vs. female), Age (centered on median of 39 years), Race (African-American vs. Non-African-American), HAM-D score (median split of 18), Current Depression (yes vs. no), Lifetime Depression (yes vs. no), Current ADDx (yes vs. no), Lifetime ADDx (yes vs. no) and baseline cocaine urine result (positive vs. negative). This analysis tested whether treatment group itself predicted outcome, and identified prognostic indicators of treatment response regardless of treatment group. Those with p values >0.1 in univariate models were eliminated from further consideration.
Of the initially retained predictors, three were measures of depression. To avoid multicollinearity, two measures were eliminated from further consideration by entering each into a multivariate model with the other predictors. The depression measure with the lowest p-value and best QIC was retained.
Model 2
The GEE model was clustered on site, and built considering all remaining predictors and examining predictors’ interactions with treatment group. An iterative stepwise process based on p-values and QIC analysis was performed to eliminate or retain the predictor variables and interactions. All predictors and interactions with p-values <0.05 were retained in the final model using the Quasi-Likelihood Information Criteria (QIC) model fit statistic.
Because this type of modeling does not include multiple comparisons of pairwise means, family-wise error rate adjustments were not done. The variable selection and model building process depends partly on testing many p-values for including or excluding variables; however, p-value adjustment was not used there as those are not main outcomes of hypothesis testing. SAS software (SAS System for Windows Version 9.2, SAS Institute Inc., Cary, NC) was used for all analyses.
Results
Baseline and demographic characteristics
Baseline and demographic characteristics of the 126 participants based on site (New Haven or Little Rock), as well as treatment group (placebo or SRT) are shown in Table 1. Several baseline measures differed by site; those at the New Haven site were more likely than those at the Little Rock site to have: no more than a high school education, lifetime or current diagnosis of depression, higher scores on the HAM-D scale, and higher frequency of alcohol and cocaine use. However, the percentage of those with lifetime or current alcohol dependence diagnosis (ADDx) did not differ between sites. Participants at the New Haven site were also more likely to be retained in treatment relative to those at the Little Rock site.
TABLE 1.
Sample baseline and demographic characteristics by site and medication group
| Parameter | New Haven (SD) | Little Rock (SD) | Statistic | df | p-value | Placebo (SD) | SRT (SD) | Statistic | df | p-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (Years) | 38.24 (6.77) | 39.50 (7.50) | t-test | 0.331 | 38.89 (7.12) | 38.60 (7.08) | t-test | 0.823 | ||
| Race (%African- American) | 66.7 | 74 | χ2= 0.76 | 1 | 0.383 | 74.2 | 65.1 | χ2=1.23 | 1 | 0.268 |
| Gender (%Female) | 36 | 24 | χ2= 2.01 | 1 | 0.156 | 30.6 | 31.7 | χ2=0.02 | 1 | 0.894 |
| Education (%≤High School) | 78.7 | 50 | χ2=11.18 | 1 | 0.0008* | 69.4 | 65.1 | χ2=0.26 | 1 | 0.611 |
| Employment (%Not Working) | 57.3 | 66 | χ2=0.95 | 1 | 0.331 | 59.7 | 61.9 | χ2=0.07 | 1 | 0.799 |
| Marital status (%Cohabiting) | 6.7 | 10 | χ2=0.45 | 1 | 0.501 | 9.7 | 6.3 | χ2=0.47 | 1 | 0.493 |
| Current Alcohol Dependence (% “Yes”) | 50 | 37.5 | χ2=1.86 | 1 | 0.173 | 47.5 | 42.9 | χ2=0.27 | 1 | 0.600 |
| Lifetime Alcohol Dependence (% “Yes”) | 39.5 | 52.1 | χ2=2.62 | 2 | 0.270 | 41 | 47.6 | χ2=2.02 | 1 | 0.365 |
| Current Depression (%”Yes”) | 73.7 | 50 | χ2=7.21 | 1 | 0.007* | 60.7 | 68.3 | χ2=0.78 | 1 | 0.377 |
| Lifetime Depression (%”Yes”) | 76.3 | 43.8 | χ2=13.49 | 1 | 0.0002* | 60.7 | 66.7 | χ2=0.48 | 1 | 0.486 |
| HAM-D Score (baseline) | 20.32 (6.49) | 16.18 (7.65) | t-test | 0.002* | 19.35 (6.95) | 18.05 (7.49) | t-test | 0.330 | ||
| Alcohol use (days/past 30) | 9.29 (9.70) | 5.32 (7.36) | Rank sum | 0.019* | 7.80 (8.93) | 7.83 (9.29) | Rank sum | 0.760 | ||
| Cocaine use (days/past 30) | 20.17 (8.13) | 11.50 (11.77) | Rank sum | 0.0001* | 14.81 (10.71) | 18.59 (10.22) | Rank sum | 0.045* | ||
| Retention (Weeks in study) | 9.37 (4.59) | 7.16 (4.13) | Rank sum | 0.0004* | 8.02 (4.56) | 8.95 (4.48) | Rank sum | 0.238 |
Regardless of site, self-reported baseline frequency of cocaine use differed by treatment group such that, compared to placebo, the SRT group reported a higher frequency of cocaine use in the preceding 30 days. No other potential prognosticators of interest differed by treatment group regardless of site, and treatment groups did not differ in terms of retention.
Model 1 (Univariate) Results
Figure 1 shows the impact of chosen factors on relapse. SRT-treated participants were significantly less likely to relapse relative to those receiving placebo (OR=0.430; 95% CI 0.280, 0.660, p<0.0001). Participants also less likely to relapse were younger (OR=0.992; 95% CI 0.987, 0.998, p<0.005), were female (OR=0.554; 95% CI 0.329, 0.932, p=0.026), had either a current (OR=0.509; 95% CI 0.480, 0.541, p<0.0001) or lifetime (OR=0.391; 95% CI 0.381, 0.401, p<0.0001) depression diagnosis, or had higher baseline HAM-D scores (OR=0.393; 95% CI 0.254, 0.606, p<0.0001), compared to those who were older, male, had a baseline negative cocaine urine result, no depression diagnosis/history, or lower HAM-D scores. Participants whose urine drug screens were negative for cocaine at baseline were more likely to relapse (OR=2.493; 95% CI 2.047, 3.037, p<0.0001) than those who tested cocaine-positive at baseline. Compared to participants who had no ADDx history, participants with current (OR=1.560; 95% CI 1.092, 2.230, p=0.0147), but not lifetime (OR=1.171; 95% CI 0.544, 2.521, p=0.686) ADDx were more likely to relapse. Race also did not predict relapse (OR=1.023; 95% CI 0.844, 1.239, p=0.82).
Figure 1. Model 1 (Univariate) Results.
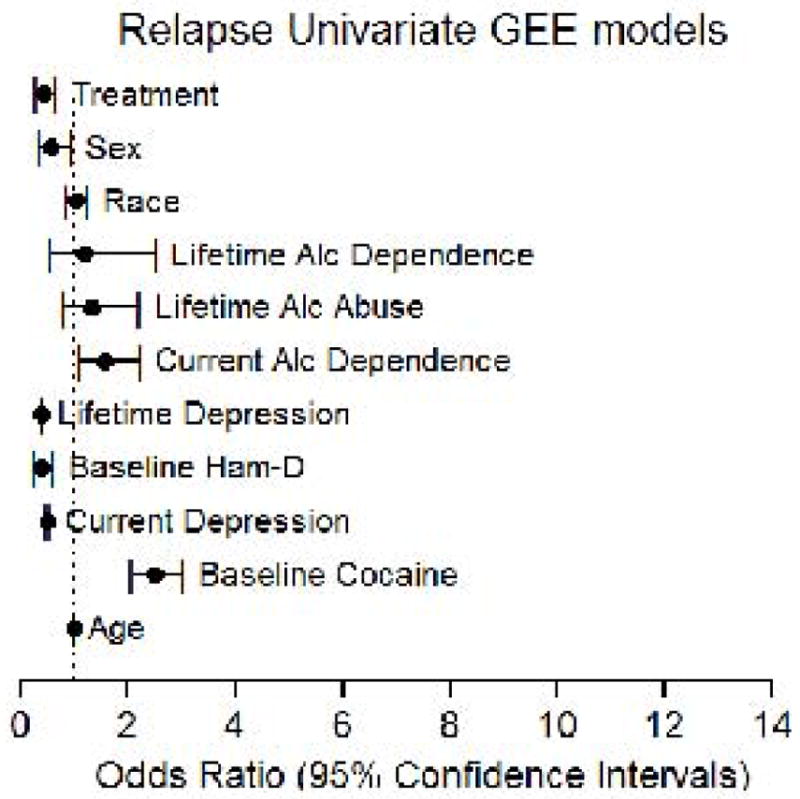
Forest plot of the Odds Ratios (points) and 95% Confidence Intervals (bars) for all predictor variables in the univariate model for relapse. X-axis: scale for odds ratios with a dotted line at 1.0 for reference. Y-axis: predictor variables. [Note: “Age” factor appears to be plotted at/near “1.0”, while results indicated significant (p<0.005) influence of age; this is an effect of scaling of graphic elements in the figure.]
Model 2 (Multivariate) Results
Table 2 shows the results of those factors that were eliminated from the final QIC-chosen model. As noted above, two of three depressions measures, current depression diagnosis and HAM-D scores, were eliminated due to having higher p values than lifetime depression diagnosis. Factors showing no significant predictor by treatment interactions were baseline cocaine urine result, lifetime depression diagnosis and sex.
Table 2.
GEE Logistic Regression Model for RELAPSE (Clustered on site, age centered at the median: 39) Covariates and Interactions ELIMINATED from final QIC chosen model
| Model Effect | Effect 1 Category | Estimate | Odds Ratio (95% Confidence Limits) | p-value |
|---|---|---|---|---|
| BL Current Depression | Yes | 0.1043 | 1.110 (0.961, 1.282) | 0.1572 |
| No | 0.0000 | . | ||
| BL Ham-D >/< Median | High | −0.3795 | 0.684 (0.367, 1.274) | 0.2315 |
| Low | 0.0000 | . | ||
| TX x Cocaine Positive at Baseline | Sertraline | −0.1780 | 0.837 (0.515, 1.360) | 0.4727 |
| Placebo | 0.0000 | . | ||
| TX x Lifetime Depression (Yes) | Sertraline | −0.4363 | 0.646 (0.060, 6.917) | 0.7183 |
| Placebo | 0.0000 | . | ||
| TX x Sex (Female) | Sertraline | 0.1918 | 1.211 (0.194, 7.552) | 0.8372 |
| Placebo | 0.0000 | . |
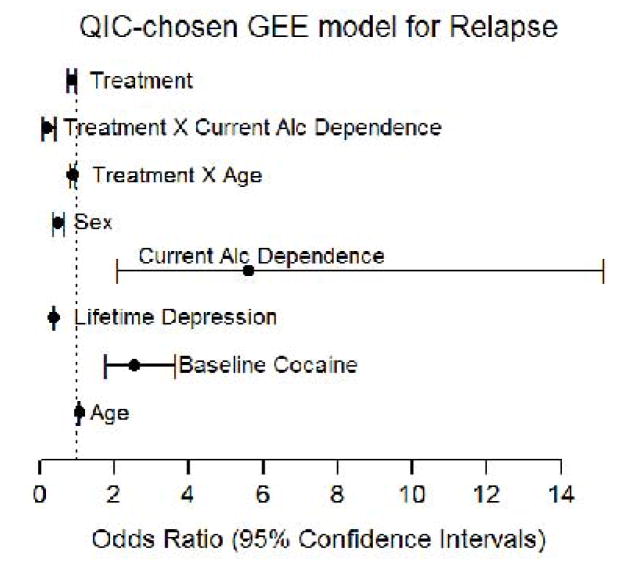
Factors found to have significant impacts on relapse and retained in the final QIC-chosen model are shown in Figure 2. SRT-treated participants were still significantly less likely to relapse relative to those receiving placebo (OR=0.862; 95% CI 0.761, 0.976, p=0.0194). Participants less likely to relapse were female (OR=0.478; 95% CI 0.348, 0.657, p<0.0001), or had lifetime depression diagnosis (OR=0.367; 95% CI 0.346, 0.3889, p<0.0001), compared to those who were male or had no history of depression. Participants with baseline urine drug screens negative for cocaine were more likely to relapse than those with baseline positive cocaine screens (OR=2.536; 95% CI 1.761, 3.652, p<.0001). Older participants were at higher risk for relapse than younger participants (OR=1.062; 95% CI 1.060, 1.064, p<0.0001). However, older participants receiving SRT were less likely to relapse than those on placebo (OR=0.886; 95% CI 0.826, 0.950, p=0.0006). Similarly, although participants with current ADDx were at higher risk for relapse than those without ADDx (OR=5.613; 95% CI 2.078, 15.164, p=0.0007), participants with current ADDx in the SRT group were less likely to relapse than those on placebo (OR=0.186; 95% CI 0.083, 0.414, p<0.0001).
Figure 2. Model 2 (Multivariate) Results.
Forest plot of the Odds Ratios (points) and 95% Confidence Intervals (bars) for all those predictor variables and interactions that were retained in the final chosen multivariate model for relapse. X-axis: scale for odds ratios with a dotted line at 1.0 for reference. Y-axis: predictor variables.
Discussion
These findings indicate that cocaine dependent participants with at least two weeks of initial abstinence in residential treatment may be more likely to avoid relapse when treated with SRT (200 mg/day) than with placebo. Therapeutic effects of SRT appear clinically significant, since those in the SRT group avoided relapse at a rate (34%) nearly twofold greater than those on placebo (18%). These findings add to growing literature about potential efficacy of more “selective” SSRIs to treat cocaine dependence.36,37 Previous outcomes with first-generation SSRI fluoxetine were mixed; a meta-analysis of RCTs found that antidepressants (including some SSRIs) did not demonstrate efficacy in maintaining abstinence from cocaine.38 Nevertheless, only one trial in the review had examined SRT and concluded that SRT was unlikely to be an effective treatment for cocaine dependence; however, the SRT dose tested was one-half that used in our trials and its setting was exclusively outpatient,39 with no requirement for establishing cocaine abstinence at the start of the treatment protocol.
Although the dose of SRT clearly needs to be adequate, the 2-week residential stay with objectively verified abstinence used in our trials is a methodological distinction that may make a critical difference in demonstrating SRT’s efficacy. While SRT administered to non-abstinent cocaine-dependent participants appears to lack remarkable abstinence promoting effects (as in 39), SRT may have more potential utility in sustaining abstinence, even if the initial abstinence period is relatively brief. Indeed, chronic fluoxetine administration has been shown to suppress cocaine-primed reinstatement, while having no effect on cocaine self-administration. These findings suggest that SSRIs may be more effective for relapse prevention than for initiating cocaine abstinence.40
Subject characteristics identified as being associated with relapse avoidance in this context regardless of treatment condition included being female, having depressive symptomatology, being younger, or having a baseline cocaine-positive urine result. The widespread belief historically has been that treatment outcomes of women generally are worse than men. However, this assumption is thought to be based on “older literature”,27 with more recent findings indicating that gender per se, may not be a major influence on addiction treatment outcomes. Indeed, other factors (social, attitudinal, cognitive/behavioral, etc.) may be more relevant prognostic correlates.41,42,43,43,43,44 Nevertheless, the results of the present study are consistent with the report that, when gender differences have been found, adult women generally have had treatment outcomes better than men.27
Although depressive symptomatology was associated with relapse avoidance, evidence in the literature is mixed with respect to depression as a predictor of cocaine treatment outcomes.28 Unfortunately, methodological differences between our studies and those employed previously, including differing cocaine use severities, treatments, and settings, make any definitive comparisons impossible. Similarly, the lack of interaction between depressive symptomatology and treatment conditions in the present study add to mixed findings of other studies employing antidepressants28 and may have been due to the relatively homogeneous sample, with the vast majority of participants having clinically relevant depressive symptoms during screening; however, to inform a more definitive conclusion on whether depression interacts with SRT in this context, prospective research in samples with a greater range in depressive symptom severity and history is necessary.
The finding that those with baseline cocaine-positive urine drug screens were less likely to relapse than baseline-negative participants may seem somewhat paradoxical, particularly due to prior reports in exclusively outpatient trials that positive baseline cocaine use predicted poorer treatment outcomes.45,28 Nevertheless, Tiet, et al.46 reported an interaction between baseline substance use severity and treatment setting, such that patients with greater baseline substance use severity showed better outcomes after receiving inpatient/residential than outpatient treatment, whereas those with less baseline severity had better outcomes after outpatient treatment. Together these findings may suggest that those with greater baseline cocaine use severity would benefit more from residential stays than those with less severe cocaine use. In contrast, that older individuals were more likely to relapse than younger participants is inconsistent with prior findings in cocaine dependent patients who have undergone intensive outpatient treatment,29 as well as individuals who have undergone treatment for substance-related disorders in a variety of treatment settings.47 The reason for this difference is unclear, although SRT did ameliorate the negative impact of age on cocaine relapse avoidance in the present study. Unfortunately, due to methodological differences with these prior reports, including the lack of prior studies’ controlling for prescribed use of psychoactive medications, definitive conclusions about age as a moderator of treatment outcome cannot be made at this time.
The finding that those with current alcohol use disorder overall were more likely to relapse is consistent with prior reports of generally poorer treatment prognosis of dually cocaine- and alcohol-use disordered patients (e.g.,30). Noteworthy, however, is that SRT reduced cocaine relapse relative to placebo in this group, suggesting that SRT may be particularly effective in ameliorating worse outcomes in recently abstinent, dually cocaine- and alcohol-use-disordered patients. Although the reason for this is unclear, 5-HT and DA involvement in a variety of addiction-related behaviors and their reciprocal interactions with addictive substances including alcohol and cocaine have been well established.23, 48–50 This includes growing evidence of genetic variations in 5-HT systems implicated in addictive phenomena, as well as various behavioral/affective conditions closely associated with using—and abstaining from—addictive substances.51
Limitations of the study include that almost one-third of the intent-to-treat sample dropped out during the residential stay across the two trials 25,26 so the lack of outcome data from those individuals cannot inform overall results of analyses presented here. Methodological aspects of the studies, including use of an efficacy versus effectiveness study design in both trials, application of inclusion/exclusion criteria that resulted in a more homogeneous, 2-week verified abstinent study sample, and use of monetary compensation to facilitate retention, limits generalizability of these findings to more “real world” conditions.25,26 Moreover, some baseline and demographic characteristics differed by site and, although site differences were controlled for by clustering analyses on site, whether any specific site difference may have impacted our findings cannot be ruled out. Data were also compiled from studies conducted several years prior to this secondary analysis, so there may be unknown factors limiting the generalizability of these findings to the current cocaine-using population.
Overall, the findings of this study suggest that SRT may increase the likelihood of relapse avoidance among cocaine dependent patients attaining initial abstinence during a 2-week residential stay followed by outpatient CBT. Moreover, several other factors; that is, women, those with more severe cocaine use or those with depressive symptomatology, have been found to be associated with relapse avoidance in this context. Finally, SRT reduced cocaine relapse more than placebo, especially in older participants or in those with comorbid ADDx. These findings suggest a complex interplay among subject characteristics and study methodologies and highlight the need for more trials with study designs that include an initial verified abstinence period in order to elucidate drug efficacy within different contexts. Further prospective investigations to confirm the efficacy of SRT, particularly in older and/or comorbid cocaine- and alcohol-use-disordered patients, as well as to investigate putative mechanisms underlying treatment response, are warranted in order to optimize this treatment strategy.
Acknowledgments
This work was supported by grants P50-DA12762 and K05-DA00454 (TRK) from the National Institute on Drug Abuse and, in part, by the Arkansas Biosciences Institute, a partnership of scientists from Arkansas Children’s Hospital Research Institute, Arkansas State University, the University of Arkansas-Division of Agriculture, the University of Arkansas, Fayetteville, and the University of Arkansas for Medical Sciences. The Arkansas Biosciences Institute is the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000. Preliminary reports of this work were presented at the 2013 Annual Meeting of the College on Problems of Drug Dependence in San Diego, CA, June 20, 2013.
Footnotes
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
Clinical Trial Registration: Data are from trials that began recruitment in 1999 and 2005, prior to the NIH requirement that clinical trials had to be registered.
References
- 1.DASIS. Drug and Alcohol Services Information System; Substance Abuse and Mental Health Services Administration CfBHSaQ, editor. National Admissions to Substance Abuse Treatment Services. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. Treatment Episode Data Set (TEDS) : 2002–2012. BHSIS Series S-71. [Google Scholar]
- 2.DHHS, SAMHSA; Department of Health and Human Services, editor. Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health. Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2015. [Google Scholar]
- 3.USDHHS. Mental Health: A report from the surgeon general. US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Mental Health Services, NIH, NIMH; 1999. [Google Scholar]
- 4.Spronk D, van Wel J, Ramaekers J, Verkes R. Characterizing the cognitive effects of cocaine: a comprehensive review. Neurosci Biobehav Rev. 2013;37(8):1838–1859. doi: 10.1016/j.neubiorev.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Perez CM, Suarez E, Torres EA. Epidemiology of hepatitis C infection and its public health implications in Puerto Rico. P R Health Sci J. 2004;23(2 Suppl):11–28. [PubMed] [Google Scholar]
- 6.Maraj S, Figueredo VM, Lynn Morris D. Cocaine and the heart. Clin Cardiol. 2010;33(5):264–269. doi: 10.1002/clc.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fonseca A, Ferro J. Drug abuse and stroke. Curr Neurol Neurosci Rep. 2013;13(2):325. doi: 10.1007/s11910-012-0325-0. [DOI] [PubMed] [Google Scholar]
- 8.Sorenson JLWL, Gibson DR, Choi K, Guydish JR. Preventing AIDS in drug users and their sexual partners / James L. Sorensen … [et al.] New York: Guilford Press; 1991. [Google Scholar]
- 9.Vocci F, Ling W. Medications development: successes and challenges. Pharmacol Ther. 2005;108(1):94–108. doi: 10.1016/j.pharmthera.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Rothman RB, Blough BE, Baumann MH. Dopamine/serotonin releasers as medications for stimulant addictions. Prog Brain Res. 2008;172:385–406. doi: 10.1016/S0079-6123(08)00919-9. [DOI] [PubMed] [Google Scholar]
- 11.Baumann MH, Rothman RB. Serotonergic dysfunction during cocaine withdrawal: implications for cocaine-induced depression. In: Karch SB, editor. Drug abuse handbook. Boca Raton, FL: CRC Press; 1998. pp. 463–484. [Google Scholar]
- 12.Michelsen KA, Prickaerts J, Steinbusch HW. The dorsal raphe nucleus and serotonin: implications for neuroplasticity linked to major depression and Alzheimer’s disease. Prog Brain Res. 2008;172:233–264. doi: 10.1016/S0079-6123(08)00912-6. [DOI] [PubMed] [Google Scholar]
- 13.Yadid G, Friedman A. Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog Brain Res. 2008;172:265–286. doi: 10.1016/S0079-6123(08)00913-8. [DOI] [PubMed] [Google Scholar]
- 14.Kleinman PH, Miller AB, Millman RB, et al. Psychopathology among cocaine abusers entering treatment. J Nerv Ment Dis. 1990;178(7):442–447. doi: 10.1097/00005053-199007000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Satel SL, Price LH, Palumbo JM, et al. Clinical phenomenology and neurobiology of cocaine abstinence: a prospective inpatient study. Am J Psychiatry. 1991;148(12):1712–1716. doi: 10.1176/ajp.148.12.1712. [DOI] [PubMed] [Google Scholar]
- 16.Brown RA, Monti PM, Myers MG, et al. Depression among cocaine abusers in treatment: relation to cocaine and alcohol use and treatment outcome. Am J Psychiatry. 1998;155(2):220–225. doi: 10.1176/ajp.155.2.220. [DOI] [PubMed] [Google Scholar]
- 17.Booth BM, Weber JE, Walton MA, et al. Characteristics of cocaine users presenting to an emergency department chest pain observation unit. Acad Emerg Med. 2005;12(4):329–337. doi: 10.1197/j.aem.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 18.McKay JR, Pettinati HM, Morrison R, Feeley M, Mulvaney FD, Gallop R. Relation of depression diagnoses to 2-year outcomes in cocaine-dependent patients in a randomized continuing care study. Psychol Addict Behav. 2002;16(3):225–235. [PubMed] [Google Scholar]
- 19.Covi L, Hess JM, Kreiter NA, Haertzen CA. Effects of combined fluoxetine and counseling in the outpatient treatment of cocaine abusers. Am J Drug Alcohol Abuse. 1995;21(3):327–344. doi: 10.3109/00952999509002701. [DOI] [PubMed] [Google Scholar]
- 20.Batki SL, Washburn AM, Delucchi K, Jones RT. A controlled trial of fluoxetine in crack cocaine dependence. Drug and Alcohol Dependence. 1996;41(2):137–142. doi: 10.1016/0376-8716(96)01233-1. [DOI] [PubMed] [Google Scholar]
- 21.Ziedonis DM, Kosten TR. Depression as a prognostic factor for pharmacological treatment of cocaine dependence. Psychopharmacol Bull. 1991;27(3):337–343. [PubMed] [Google Scholar]
- 22.Carroll KM, Nich C, Rounsaville BJ. Differential symptom reduction in depressed cocaine abusers treated with psychotherapy and pharmacotherapy. J Nerv Ment Dis. 1995;183(4):251–259. doi: 10.1097/00005053-199504000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Rothman RB, Blough BE, Baumann MH. Dual dopamine/serotonin releasers: potential treatment agents for stimulant addiction. Exp Clin Psychopharmacol. 2008;16(6):458–474. doi: 10.1037/a0014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodnick PJ, Goldstein BJ. Selective serotonin reuptake inhibitors in affective disorders--I. Basic pharmacology. J Psychopharmacol. 1998;12(3 Suppl B):S5–20. doi: 10.1177/0269881198012003021. [DOI] [PubMed] [Google Scholar]
- 25.Oliveto A, Poling J, Mancino MJ, et al. Sertraline delays relapse in recently abstinent cocaine-dependent patients with depressive symptoms. Addiction. 2012;107(1):131–141. doi: 10.1111/j.1360-0443.2011.03552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mancino MJ, McGaugh J, Chopra MP, et al. Clinical Efficacy of Sertraline Alone and Augmented With Gabapentin in Recently Abstinent Cocaine-Dependent Patients With Depressive Symptoms. Journal of clinical psychopharmacology. 2014;34(2):234–239. doi: 10.1097/JCP.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenfield SF, Brooks AJ, Gordon SM, et al. Substance Abuse Treatment Entry, Retention, and Outcome in Women: A Review of the Literature. Drug and alcohol dependence. 2007;86(1):1–21. doi: 10.1016/j.drugalcdep.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poling J, Kosten TR, Sofuoglu M. Treatment outcome predictors for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33(2):191–206. doi: 10.1080/00952990701199416. [DOI] [PubMed] [Google Scholar]
- 29.McKay JR, Van Horn D, Rennert L, Drapkin M, Ivey M, Koppenhaver J. Factors in sustained recovery from cocaine dependence. J Subst Abuse Treat. 2013;45(2):163–172. doi: 10.1016/j.jsat.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carroll KM, Rounsaville BJ, Bryant KJ. Alcoholism in treatment-seeking cocaine abusers: clinical and prognostic significance. J Stud Alcohol. 1993;54(2):199–208. doi: 10.15288/jsa.1993.54.199. [DOI] [PubMed] [Google Scholar]
- 31.Oliveto A, Thostenson J, Kosten TR, Mancino MJ. Mediators of response to sertraline vs placebo among recently abstinent, cocaine dependent patients. Presented at the Annual Meeting of the College on Problems of Drug Dependence; San Diego, CA. June 20, 2013. [Google Scholar]
- 32.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV, patient ed. Washington DC: American Psychiatric Press; 1995. [Google Scholar]
- 33.Carroll KM. Therapy manuals for drug addiction, manual 1: a cognitive-behavioral approach: treating cocaine addiction. NIH publication. 1998:98–4308. [Google Scholar]
- 34.McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. Journal of Nervous and Mental Disease. 1980;168(1):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton MA. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–82. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moeller FG, Schmitz JM, Steinberg JL, et al. Citalopram combined with behavioral therapy reduces cocaine use: a double-blind, placebo-controlled trial. Am J Drug Alcohol Abuse. 2007;33(3):367–378. doi: 10.1080/00952990701313686. [DOI] [PubMed] [Google Scholar]
- 37.Vayalapalli S, Vaughn M, Salles-Shahid K, Byrd-Sellers J, Drexler K. High-Dose Citalopram for Cocaine Dependence in Veteran Population-A Pilot Project. Am J Addict. 2011;20(5):485–486. doi: 10.1111/j.1521-0391.2011.00162.x. [DOI] [PubMed] [Google Scholar]
- 38.Pani PPTE, Vecchi S, Amato L. Antidepressants for cocaine dependence and problematic cocaine use. Cochrane Database Syst Rev. 2011:CD002950. doi: 10.1002/14651858.CD002950.pub3. [DOI] [PubMed] [Google Scholar]
- 39.Winhusen TMSE, Harrer JM, Mezinskis JP, Montgomery MA, Goldsmith RJ, et al. A placebo-controlled screening trial of tiagabine, sertraline and donepezil as cocaine dependence treatments. Addiction. 2005;100(S):68–77. doi: 10.1111/j.1360-0443.2005.00992.x. [DOI] [PubMed] [Google Scholar]
- 40.Winstanley EL, Bigelow GE, Silverman K, Johnson RE, Strain EC. A randomized controlled trial of fluoxetine in the treatment of cocaine dependence among methadone-maintained patients. J Subst Abus Treat. 2011;40(3):255–264. doi: 10.1016/j.jsat.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss RD, Martinez-Raga J, Griffin ML, Greenfield SF, Hufford C. Gender differences in cocaine dependent patients: a 6 month follow-up study. Drug and Alcohol Dependence. 1997;44(1):35–40. doi: 10.1016/s0376-8716(96)01319-1. [DOI] [PubMed] [Google Scholar]
- 42.Hser Y-I, Huang D, Teruya C, Anglin MD. Gender comparisons of drug abuse treatment outcomes and predictors. Drug & Alcohol Dependence. 72(3):255–264. doi: 10.1016/j.drugalcdep.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Greenfield SF, Hufford MR, Vagge LM, Muenz LR, Costello ME, Weiss RD. The relationship of self-efficacy expectancies to relapse among alcohol dependent men and women: a prospective study. Journal of Studies on Alcohol. 2000;61(2):345–351. doi: 10.15288/jsa.2000.61.345. [DOI] [PubMed] [Google Scholar]
- 44.Carroll ME, Smethells JR. Sex Differences in Behavioral Dyscontrol: Role in Drug Addiction and Novel Treatments. Frontiers in Psychiatry. 2016;6(175) doi: 10.3389/fpsyt.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmadi J, Kampman KM, Oslin DM, Pettinati HM, Dackis C, Sparkman T. Predictors of Treatment Outcome in Outpatient Cocaine and Alcohol Dependence Treatment. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2009;18(1):81–86. doi: 10.1080/10550490802545174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tiet QQ, Ilgen MA, Byrnes HF, Harris AH, Finney JW. Treatment setting and baseline substance use severity interact to predict patients’ outcomes. Addiction. 2007;102(3):432–440. doi: 10.1111/j.1360-0443.2006.01717.x. [DOI] [PubMed] [Google Scholar]
- 47.Stahler GJ, Mennis J, DuCette JP. Residential and outpatient treatment completion for substance use disorders in the U.S.: Moderation analysis by demographics and drug of choice. Addict Behav. 2016;58:129–135. doi: 10.1016/j.addbeh.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 48.Tupala E, Hall H, Mantere T, Rasanen P, Sarkioja T, Tiihonen J. Dopamine receptors and transporters in the brain reward circuits of type 1 and 2 alcoholics measured with human whole hemisphere autoradiography. Neuroimage. 2003;19(1):145–155. doi: 10.1016/s1053-8119(03)00060-0. [DOI] [PubMed] [Google Scholar]
- 49.Müller CP, Homberg JR. The role of serotonin in drug use and addiction. Behavioural Brain Research. 2015;277:146–192. doi: 10.1016/j.bbr.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 50.Marcinkiewcz CA, Lowery-Gionta EG, Kash TL. Serotonin’s Complex Role in Alcoholism: Implications for Treatment and Future Research. Alcoholism: Clinical and Experimental Research. 2016;40(6):1192–1201. doi: 10.1111/acer.13076. [DOI] [PubMed] [Google Scholar]
- 51.Rubens M, Ramamoorthy V, Attonito J, Saxena A, Appunni S, Shehadeh N, Dévieux JG. A review of 5-HT transporter linked promoter region (5-HTTLPR) polymorphism and associations with alcohol use problems and sexual risk behaviors. Journal of Community Genetics. 2016;7(1):1–10. doi: 10.1007/s12687-015-0253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]