Abstract
Patients with schizophrenia show a deficit in cognitive ability compared to estimated premorbid and familial intellectual abilities. However, the degree to which this pattern holds across psychotic disorders and is familial is unclear. The present study examined deviation from expected cognitive level in schizophrenia, schizoaffective disorder, and psychotic bipolar disorder probands and their first-degree relatives. Using a norm-based regression approach, parental education and WRAT-IV Reading scores (both significant predictors of cognitive level in the healthy control group) were used to predict global neuropsychological function as measured by the composite score from the Brief Assessment of Cognition in Schizophrenia (BACS) test in probands and relatives. When compared to healthy control group, psychotic probands showed a significant gap between observed and predicted BACS composite scores and a greater likelihood of robust cognitive decline. This effect was not seen in unaffected relatives. While BACS and WRAT-IV Reading scores were themselves highly familial, the decline in cognitive function from expectation had lower estimates of familiality. Thus, illness-related factors such as epigenetic, treatment, or pathophysiological factors may be important causes of illness related decline in cognitive abilities across psychotic disorders. This is consistent with the markedly greater level of cognitive impairment seen in affected individuals compared to their unaffected family members.
Keywords: psychotic disorders, brief assessment of cognition in schizophrenia, first-degree relatives, cognitive decline, premorbid cognition
1. Introduction
Cognitive deficits are well established in psychotic disorders (Bilder et al., 2000;Dickinson et al., 2008;Hill et al., 2004;Keefe et al., 2006;Reilly and Sweeney, 2014). Typically, cognitive impairments are determined by evaluating neuropsychological performance in patient groups compared to normative data or a psychiatrically healthy control sample. However, performance on neuropsychological tests in patients may be related to both heritable familial intellectual ability (Bouchard, 2004) and illness-related factors that lead to a decline from that expected ability. Following this reasoning, the deviation score (decline from expected ability) is of potential interest as it may reflect the impact of disease on cognitive function more closely than level of performance. Indeed, many schizophrenia patients display substantial discrepancy between neuropsychological performance and that predicted by factors such as parental education and personal premorbid ability as estimated by single-word reading (Keefe et al., 2005;Bryson et al., 1993;Palmer et al., 1997;Woodward and Heckers, 2015). However, some important issues have not been systematically investigated, including the extent of deviation from premorbid expectation in psychotic disorders other than schizophrenia and their unaffected family members, and the degree to which decline from expectation is itself a familial characteristic.
Though not investigated systematically, unaffected relatives of schizophrenia probands may show a degree of decline from expectation. Indeed, a recent population based investigation using a national registry indicated that familial cognitive aptitude (general intellectual ability) was distinct from neurodevelopmental factors that predispose one to schizophrenia (Kendler et al., 2016). This provides some indirect support for the notion that familial factors that determine intellectual ability may be distinct from disease-related factors impacting cognitive function.
Thus, direct assessment of familial patterns of deviation in neuropsychological competence from expectation across psychotic disorders may be helpful for evaluating the extent to which significant decline from expected cognitive levels can be attributed to disease-related or shared familial factors impacting intellectual ability. Therefore, the present study evaluated the discrepancy of expected and current neuropsychological ability across psychotic disorders and in unaffected relatives, and the familiality of these indices using a norm-based regression approach.
2. Methods
2.1. Participants
Cognitive testing and assessment of parental SES was performed as part of the Bipolar and Schizophrenia Network for Intermediate Phenotypes (B-SNIP) study. Recruitment strategy, procedures, and clinical characterization of the study sample have been reported previously (Tamminga et al., 2013), as were observed BACS composite scores in the sample (Hill et al., 2013). Probands were required to have a consensus diagnosis based on SCID interviews (First et al., 1995) of schizophrenia, schizoaffective disorder, or bipolar disorder with psychotic features (see Table 1). All participants had 1) no history of seizures or head injury with sustained loss of consciousness, 2) no diagnosis of substance abuse in the preceding 30 days or substance dependence in the prior 6 months, and a negative urine drug screen for drugs of abuse on the day of testing, 3) a stable medication regimen and clinical status over the prior month, 4) no history of systemic medical or neurological disorder known to affect cognitive abilities, and 5) age-corrected Wide Range Achievement Test-IV Reading standard score (SS) ≥65. First-degree relatives with a lifetime history of psychosis were excluded from the present analyses in order to assess cognitive function in relatives unaffected by the presence of a psychotic disorder.
Table 1.
| a. Demographic and clinical data for probands with a history of psychosis and healthy controls | |||||
|---|---|---|---|---|---|
|
| |||||
| Healthy Controls |
Schizophrenia Probands |
Schizoaffective Probands |
Bipolar Probands |
||
|
| |||||
| n=391 | n=321 | n=200 | n=258 | Findings | |
| Age in Years Mean(SD) | 37.62(12.57) | 35.70(12.87) | 36.85(11.79) | 36.04(12.76) | F=1.61ns |
| Years of Education Mean (SD) a | 14.92(2.52) | 12.76(2.28) | 13.04(2.14) | 14.16(2.35) | F=58.96*** |
| WRAT-IV: Reading Mean(SD) b | 103.29(13.76) | 94.47(15.45) | 97.00(14.73) | 101.56(14.09) | F=25.41*** |
| Gender c | |||||
| Male | 45.0 % | 67.6 % | 40.5% | 36.4 % | χ2 = 68.95*** |
| Female | 55.0% | 32.4% | 59.5% | 63.6% | |
| Raced | |||||
| Caucasian | 62.7 % | 46.4% | 55.0 % | 75.2 % | |
| African-American | 28.1% | 44.9% | 40.0% | 19.4% | χ2 = 60.61*** |
| Other | 9.2% | 8.7% | 5.0% | 5.4% | |
|
| |||||
| Clinical Data | |||||
|
| |||||
| MADRS e | 8.22(7.92) | 14.79(10.32) | 10.09(9.08) | F=31.76*** | |
| PANSS Positive f | 16.69(5.72) | 17.97(5.26) | 12.78(4.63) | F=63.12*** | |
| PANSS Negative g | 16.50(5.82) | 16.04(5.15) | 11.82(3.94) | F=67.22*** | |
| YMRS Total h | 5.56(5.82) | 7.56 (6.68) | 5.87(6.80) | F= 6.24** | |
| b. Demographic data, history of psychosis, and psychosis spectrum personality traits for first degree relatives | |||||
|---|---|---|---|---|---|
|
| |||||
| Healthy Controls |
Relatives of Schizophrenia Probands |
Relatives of Schizoaffective Probands |
Relatives of Bipolar Probands |
||
|
| |||||
| n=391 | n=298 | n=193 | n=271 | Findings | |
| Age in Years Mean(SD) a | 37.62(12.57) | 44.10(15.12) | 41.69(16.17) | 40.70(16.04) | F=11.28*** |
| Years of Education Mean (SD) b | 14.92(2.52) | 14.22(2.42) | 14.09(2.93) | 14.58(2.71) | F= 6.09*** |
| WRAT-IV: Reading Mean(SD) c | 103.29(13.76) | 98.88(14.64) | 100.45(16.40) | 103.82(14.12) | F= 7.60*** |
| Gender d | |||||
| Male | 45.0% | 28.5% | 26. 9% | 34.7% | χ2 = 28.09*** |
| Female | 55.0% | 71.5% | 73.1% | 65.3% | |
| Race e | |||||
| Caucasian | 62.7% | 56.4% | 63. 3% | 81.2% | |
| African-American | 28.1% | 38.9% | 32.6% | 14.4% | χ2 = 55.77*** |
| Other | 9.2% | 4.7% | 4.1% | 4.4% | |
WRAT-IV: Wide Range Achievement Test: Fourth Edition
YMRS: Young Mania Rating Scale
PANSS: Positive and Negative Syndrome Scale
MADRS: Montgomery Asberg Depression Rating Scale
p < 0.05;
p < 0.01;
p < 0.001
Healthy Control > Schizophrenia, Schizoaffective, & Bipolar; Bipolar > Schizophrenia & Schizoaffective
Healthy Control > Schizophrenia and Schizoaffective; Bipolar > Schizophrenia and Schizoaffective
Males over-represented in Schizophrenia group
African-Americans over-represented in Schizophrenia group
Schizoaffective > Bipolar > Schizophrenia
Schizoaffective > Schizophrenia > Bipolar
Schizophrenia & Schizoaffective > Bipolar
Schizoaffective > Schizophrenia & Bipolar
Healthy Controls < Schizophrenia Relatives, Schizoaffective Relatives, & Bipolar Relatives; Schizophrenia Relatives > Bipolar Relatives
Healthy Control > Schizophrenia and Schizoaffective
Healthy Control > Schizophrenia; Bipolar > Schizophrenia
Females over-represented in Schizophrenia & Schizoaffective Relative groups
Caucasians over-represented in Bipolar Relatives
2.2. General Cognitive Function
The Brief Assessment of Cognition in Schizophrenia (BACS) is a neuropsychological battery designed to evaluate global neuropsychological function (Keefe et al., 2004). The BACS consists of six subtests covering four domains (Verbal Memory, Processing Speed, Reasoning, and Problem Solving, and Working Memory). Subtest scores were converted to z-scores using published norms (Keefe et al., 2008). To limit the impact of extreme values on group means, subtest scores were winsorized to a maximum absolute value of 4.0.
2.3. Estimating decline in cognitive ability compared to intellectual potential
2.3.1. Predictor variables
Deviation-based approaches for evaluating decline in cognitive function have used a mixture of norm-based and premorbid indices to estimate familial intellectual potential. This approach has been used successfully in broad sample of patients with schizophrenia (Keefe et al., 2005;Woodward and Heckers, 2015), high-functioning schizophrenia (Vaskinn et al., 2014), and traumatic brain injury (Johnstone et al., 1995) using single word reading scores and parental education. A variety of parental and patient demographic variables (parental education and occupation, patient educational achievement) have been used to estimate intellectual potential in the schizophrenia and dementia literatures, and are used in the clinical practice of neuropsychology. Single-word reading (e.g., NART, WRAT reading) (Bright et al., 2002;Gladsjo et al., 1999) combined with parental education (Kareken et al., 1995;Kremen et al., 2000) provides a reliable estimate of familial or premorbid intellectual potential (Keefe et al., 2005). These parameters were utilized in the present study based on the prior findings and a failure of other factors such as personal years of education and parental income estimates.
We used a norm-based hierarchical regression approach to predict BACS composite score. To account for demographic differences among groups (see Table 1a & 1b), age, race, and sex were entered first followed by parental education (highest level of either parent) and the participants WRAT-IV Reading standard score. In healthy controls, BACS composite scores from these selected predictors were significantly, and independently, predicted from both parental education [step 1: R2 = 0.07, F(1,381) = 28.48, p < 0.001], and personal single word reading scores as measured by the WRAT-IV Reading subtest [step 2: R2 = 0.28, F(1,381) = 74.11, p < 0.001; F-change (1, 379) = 111.34, p < 0.001]. This model was then applied to all proband and relative groups to compute a predicted BACS composite score. The difference between observed and predicted BACS scores was computed to provide a continuous variable reflecting the degree of deviation from expectation for each participant.
2.3.2 Group Comparisons
First, an ANOVA was conducted to compare groups in terms of deviation scores. Second, the proportion of cases with robust deviation from expectation was computed (defined as deviation scores below the 95% confidence interval (CI) of predicted performance). Whereas population-based (mean) confidence intervals were previously used to determine the cut score for significant decline from expectation (Keefe et al., 2005), the present study used a more conservative approach based on individual (prediction) intervals to estimate the likely range of predicted performance (Tabachnick and Fidell, 2007). Just 5.4% of controls fell below the 95% CI cutoff. Odds ratios for rates of significant deviation from expectation compared to controls were computed for each proband and relative group.
As a complimentary descriptive analysis, the relationship between observed BACS composite scores and deviation from expected BACS was computed. To assess their clinical relevance, deviation scores were evaluated in relation to functional status, based on the Birchwood Social Functioning Scale (Birchwood et al., 1990), and severity of clinical symptoms, using the Positive and Negative Symptom Scale (Kay et al., 1989).
2.3.3 Familiality
A heritability analysis to calculate familiality estimates for deviation from expectation was performed using Sequential Oligogenic Linkage Analysis Routine software (SOLAR). In a design such as ours, an estimate of familiality (h2) represents the portion of phenotypic variance accounted for by family membership. To test for the significance of familiality, a maximum likelihood ratio test compared phenotypic variation explained by family membership to a model assuming that no variation is explained by familial factors. Age, sex, and race were included in the model as covariates. A correction was applied to account for ascertainment bias as families were recruited through the identification of a psychotic proband and not a representative community sample. We have previously reported the familiality of the BACS composite, which was 0.50 (90% CI = 0.37–0.63) in schizophrenia pedigrees and 0.61 (90% CI = 0.42–0.79) in bipolar pedigrees (Hill et al., 2013).
3. Results
3.1. Group differences in deviation from cognitive expectation
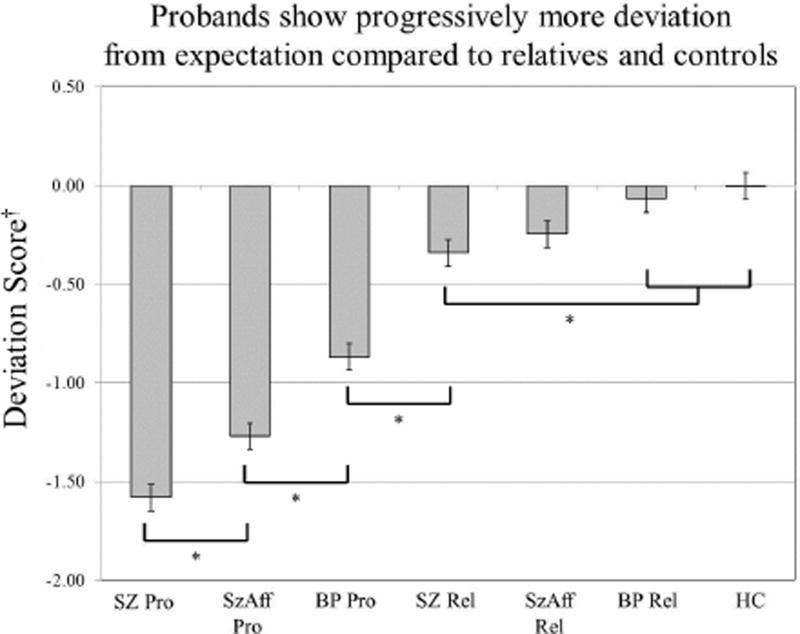
ANOVA comparing deviation from expected BACS performance indicated significant differences across groups [F(7,1951)=62.42, p<0.001]. Simple contrasts were used to clarify significant omnibus findings using a Hochberg correction for multiple comparisons. All patient groups showed greater deviation from expected BACS performance compared to controls with schizophrenia probands showing significantly greater decline than schizoaffective and psychotic bipolar groups. The relative groups did not differ from each other or controls.
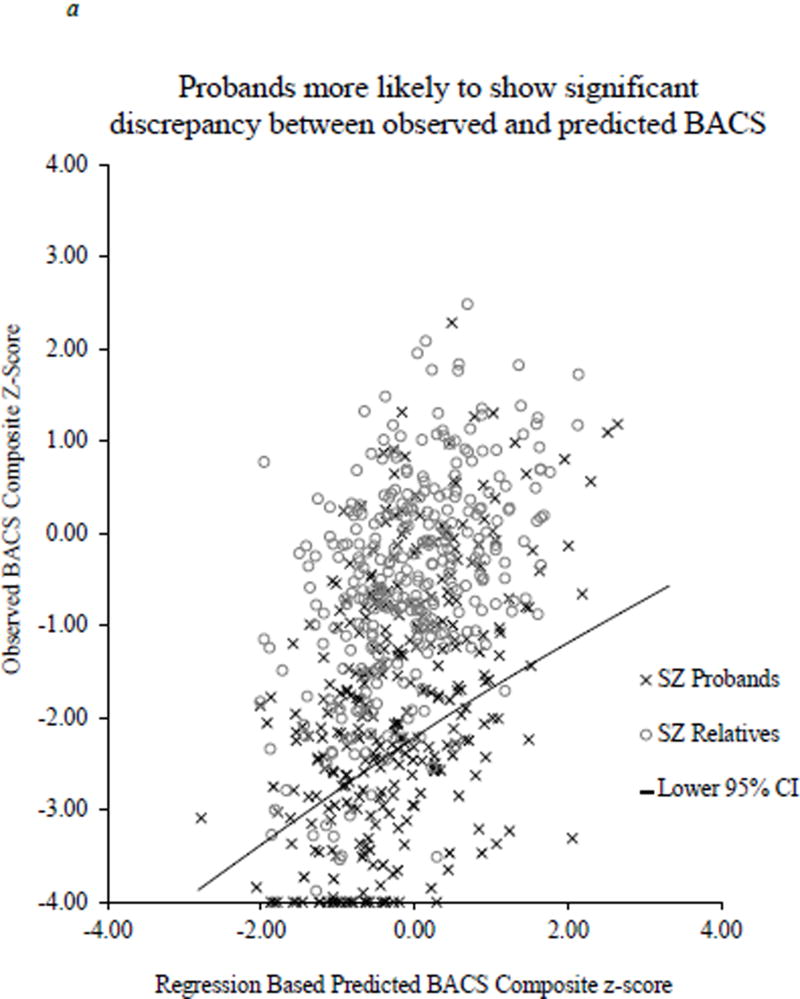
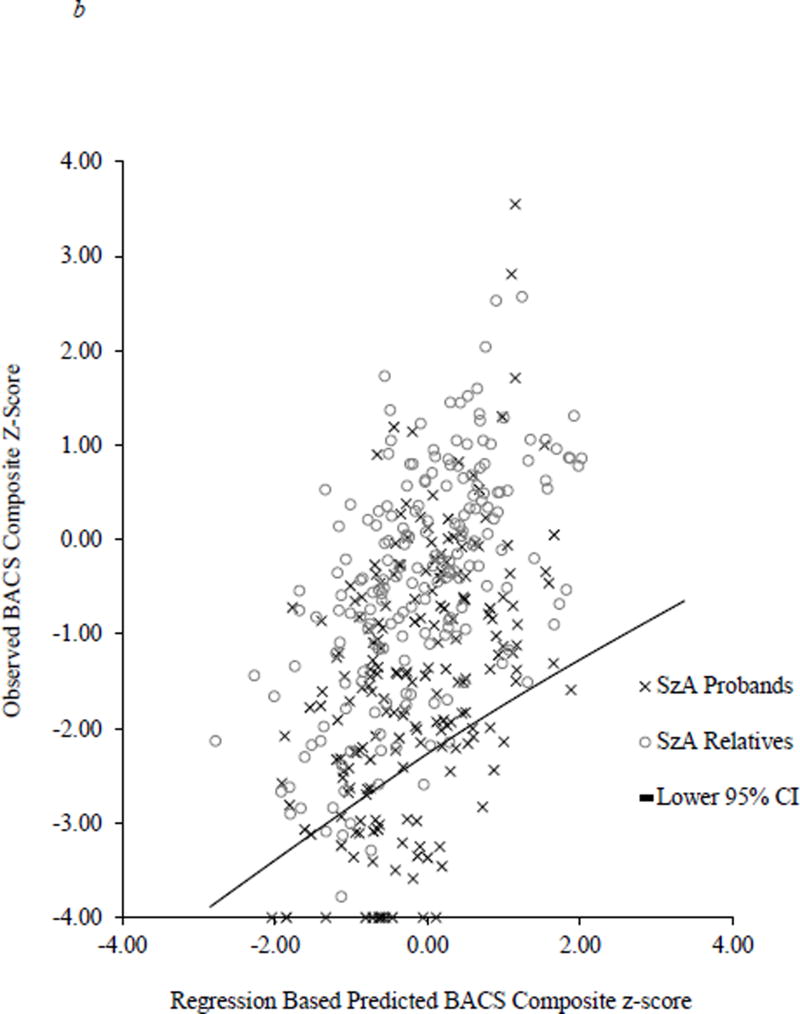
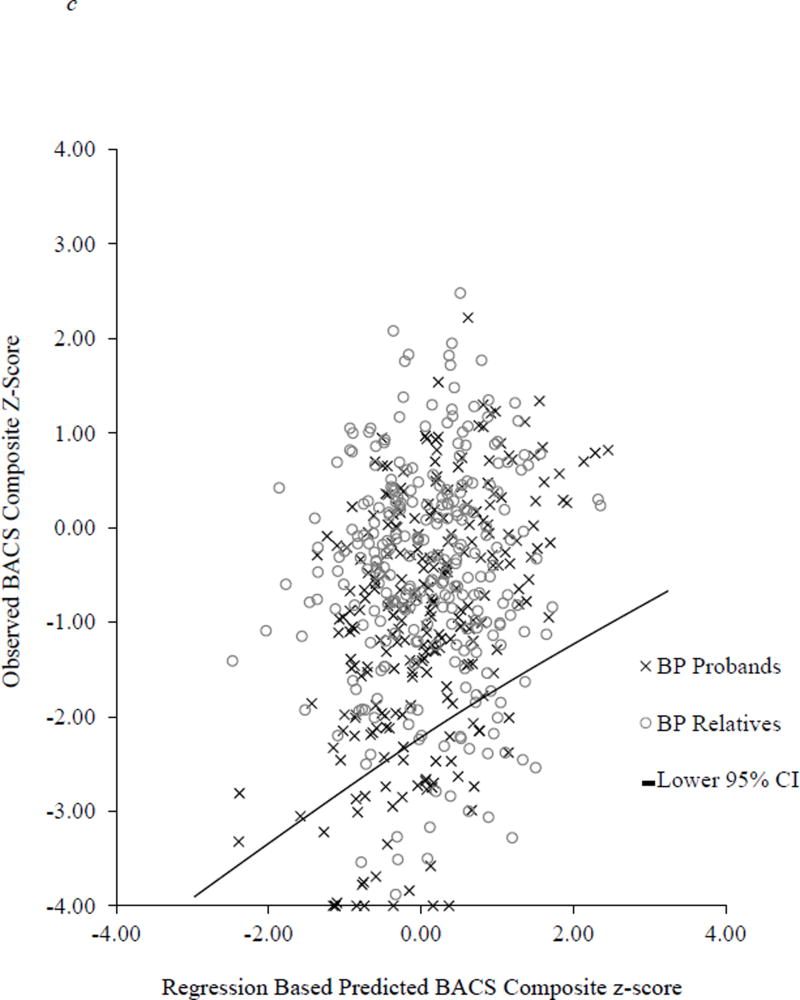
A significant omnibus logistic regression indicated that the likelihood of robust deviation from expectation (performing below the lower 95 % CI cutoff) varied significantly by group [Wald χ2(6) = 180.58, p < 0.0001]. Consistent with mean comparisons, there was a higher rate of robust deviation from expectation in all proband groups compared to controls [Schizophrenia: 25.5% below expectation, OR = 13.10, 95% CI 7.45–23.06, p < 0.0001 (Figure 2a); Schizoaffective: 23.1% below expectation, OR = 7.72, 95% CI 4.19–14.22, p < 0.0001 (Figure 2b); Bipolar: 19.8% below expectation, OR = 4.59, 95% CI 2.48–8.50, p < 0.0001 (Figure 2c)]. A higher proportion of schizophrenia probands showed robust deviation from expectation compared to schizoaffective probands [Wald χ2(1) = 6.72, p < 0.01] who, in turn, had a higher proportion of cases below this 95% CI cutoff than bipolar probands [Wald χ2(1) = 4.71, p < 0.05]. In contrast, none of the relative groups showed greater rates of deviation from expectation compared to controls (range of 4.9 to 6.7% abnormality; OR = 0.77–1.24; see Figure 1).
Figure 2.
Scatterplot of observed BACS composite score and predicted BACS based on WRAT and parent education. The solid line represents the lower end of the 95% confidence interval derived from the healthy control regression equation.
Figure 1.
All proband groups show a lower deviation scores compared to both the relative groups and controls. Mean deviation from expectation scores differed significantly among proband groups from bipolar to schizoaffective to schizophrenia. This pattern suggests disruption of premorbid cognitive trajectories across psychotic disorders.
†Deviation Score = Difference between Observed and Predicted BACS Composite z-score.
3.2 Comparing of probands with extreme deviation scores
Probands with deviation scores below the 95% CI threshold were compared to the remaining probands in terms of parent education level and WRAT – IV Reading scores to assess for group differences prior to computing predicted BACS scores. Findings indicated significant differences for both WRAT IV - Reading [F(1,774) = 54.40, p< 0.001] and parent education [F(1,721) = 21.82, p < 0.001] in which probands below 95% CI threshold had lower single word reading scores and parents with 1.29 fewer years of education. Thus, cognitive decline was stronger among probands with lower word reading ability and less educated parents.
To further characterize probands displaying robust deviation from cognitive expectation, we evaluated select clinical measures of illness severity, medication status, and daily living skills. Findings indicated that neither positive nor negative symptoms were significantly correlated with deviation scores for probands above or below the cutoff. However, probands below the 95% CI endorsed more negative symptoms [F(1,760) = 7.54, p < 0.01], had higher doses (CPZ equivalent) of antipsychotics [F(1,490) = 7.70, p < 0.01], and had a higher anticholinergic burden (Eum et al., 2017) [F(1,738) =5.99, p < 0.01] than probands with BACS scores above this threshold. As CPZ levels were not associated with deviation scores, these findings suggest that participants with cognitive decline may be less responsive to treatment. However, the relationship to functional status, as indicated by SFS total scores, was only marginal [F(1,617) = 2.26, p = 0.13].
Finally, we evaluated whether robust deviation scores were related to psychosis biotypes described by Clements and colleagues (Clementz et al., 2016). Findings indicated an uneven distribution across biotypes (χ2 [2] = 38.20, p < 0.001) in which patients below the 95% CI cutoff were significantly over-represented in Biotypes 1 and 2, and significantly under-represented in Biotype 3.
3.3. Level of performance and deviation from expectation
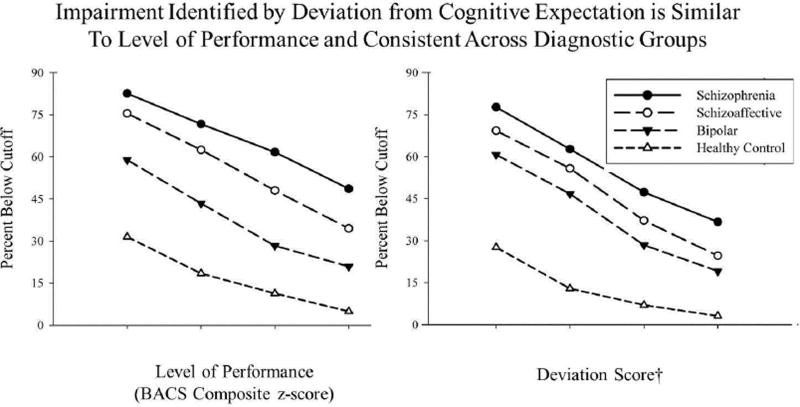
Level of neuropsychological performance is commonly classified as impaired based on a threshold of 0.5 to 2.0 standard deviations below the mean compared to either normative data or a control group. From a level of performance perspective, more than70% of probands showed at least mild overall neuropsychological impairment (BACS composite z-score ≤ −0.5) while over a third showed more severe overall impairments (BACS composite z-scores ≤ −2.0) (see Figure 2 & 3). To assess the potential overlap for identifying impairment on a case-wise basis, the degree to which level of performance and deviation from expectation identified similar cases was evaluated. The corresponding thresholds for deviation from cognitive expectation were similar for schizophrenia and schizoaffective probands and somewhat more inclusive for the psychotic bipolar group. Overall, there appears to be considerable overlap among the level of performance and deviation from expectation approaches for indexing cognitive impairment.
Figure 3.
Rates of cognitive impairment based on cut-scores for level of performance (left figure) and deviation from cognitive expectation (right figure).
†Deviation Score = Difference between Observed and Predicted BACS Composite z-score.
3.4. Evaluating familial effects
As noted in a previous B-SNIP report, familiality estimates for the BACS composite (h2 = 0.50 to 0.61) were strong across proband groups (Hill et al., 2013). Although similar computations for deviation of BACS scores from expectation indicated slightly lower values (schizophrenia: h2=0.32, 90% CI = 0.12–0.48; bipolar: h2=0.37, CI = 0.18–0.57), the confident intervals did not cross zero. Thus, familiality estimates were interpreted as statistically significant, but low to moderate in magnitude.
4. Discussion
The present study was designed to evaluate decline from expected cognitive ability based on parental and personal premorbid indicators across psychotic disorders, and familiality compared to neuropsychological test performance. Findings indicated greater decline from expected BACS performance in all proband groups compared to both controls and first-degree relatives. This effect was evident in all proband groups, but increased progressively from bipolar disorder with psychosis to schizoaffective disorder to schizophrenia. Although robust deviation from expectation was evenly distributed across DSM diagnostic groups, probands with profiles of cognitive decline were over-represented among the more severely affected biotypes (Clementz et al., 2016). The reduced familiality of decline from expectation in comparison to the familiality for the raw BACS composite scores (Hill et al., 2013) suggests that severe cognitive decline may be more related to the presence of psychotic illness than familial genetic factors, though the possibility of a highly nonlinear effect of incremental levels of risk genes on cognition remains a possibility.
4.1. Normative heritability of cognition
Genetic factors are well known to account for level of cognitive ability (Lyons et al., 2009); (Plomin and Craig, 1997;Haworth et al., 2010) and increased risk for intellectual decline (Burdick et al., 2007). The present findings of lower familiality estimates for deviation from expected cognitive ability against a background of strong heritability for BACS performance suggests that factors related to the presence of psychotic illness rather than shared familial factors are the primary cause of decline from cognitive expectation. This pattern is supported by observations that probands with robust deviation from expectation (below the 95% CI) had higher levels of persistent negative symptoms. Furthermore, probands with a robust decline from expectation had greater decrements than their relatives whereas probands above the cutoff had similar deviation scores compared to their corresponding relatives. These findings suggest that cognitive decline related to illness is clinically relevant and present in some, but not all, patients with psychotic disorders.
4.2. Implications for etiology of cognitive deficits
Cognitive abilities are highly heritable both in psychiatric and non-psychiatric populations (Blokland et al., 2016), therefore cognitive ability has a strong familial component both in the general population and in individuals with a history of psychosis. While significant, estimates of familiality or heritability of general cognitive abilities in schizophrenia (Egan et al., 2001;Chen et al., 2009;Greenwood et al., 2007) and across the psychosis spectrum (Hill et al., 2013) have consistently been lower than heritability estimates for intellectual ability in the general population (Bouchard, Jr. and McGue, 1981;Plomin and Deary, 2015). Against a background of significant heritability for both intelligence (69%) and history of a psychotic disorder (56%), the shared variance for intelligence and psychosis is about 7% (Fowler et al., 2012). These findings, along with the markedly different level of cognitive deficit in affected individuals compared to unaffected relatives was consistent with recent findings indicating that family cognitive aptitude is distinct from risk for schizophrenia (Kendler et al., 2016).
These findings provide indirect support for the view that disease related processes might disrupt cognition variably and, to a large degree, independently from familial factors determining intellectual abilities. It thus appears that genetic factors determining general cognitive abilities have a reduced penetrance in psychotic patients, with non-familial environmental or pathophysiological factors playing a greater role in causing the prominent cognitive and related functional disability in psychotic disorders. A number of potential non-familial factors related to disease might account for the greater cognitive decline seen in psychotic disorders, and their expression in schizophrenia, including acute and chronic treatments effects (Sweeney et al., 1991;Bishop et al., 2015;Reilly et al., 2006), impact of prodromal illness and neurodevelopmental alterations on school learning, progressive brain changes (Zhang et al., 2015), and other neurobiological factors.
4.3. Utility of evaluating deviation from expectation
Deviation from cognitive expectation may provide a useful complementary approach to level of performance for assessing cognitive deficits in patients with psychotic disorders. Generalized cognitive deficits are well established in the majority of psychosis patients (Bilder et al., 2000;Dickinson et al., 2008;Hill et al., 2004;Keefe et al., 2006;Reilly and Sweeney, 2014). Assessing the decline from estimated intellectual potential as an alternative or complement to level of performance may have utility in several important ways. First, a meaningful discrepancy between observed and expected neuropsychological performance may be useful as a more direct indicator of the degree of illness-related disruption of cognitive abilities. Second, the presence of neuropsychological decline might be a useful way to stratify patients for trials or evaluate outcomes of treatments targeting cognitive deficits associated with psychotic illness. The deviation from expectation approach may hold an advantage that stems from providing an outcome measure less influenced by inter-individual variability in familial intelligence. For example, an individual with a deviation score significantly below expectation might be more responsive to potential cognitive benefits of treatment if level of performance is in the normal range and their relatives have much smaller deviation scores. Similarly, a reduction of deviation from expectation may be more informative as a cognitive endophenotype in contrast to traditional indicators such as mean level of neuropsychological performance.
4.4. Limitations
The proportion of psychosis patients with a profile of robust deviation from expectation was much lower than previous reports (Keefe et al., 2005;Woodward and Heckers, 2015) . This may reflect different methodology for the threshold of determining significant impairment. For example, the first study to assess deviation from cognitive expectation used population based (mean) confidence intervals (Keefe et al., 2005) while the present investigation used a more conservative approach based on individual (prediction) confidence intervals. More recently, Woodward & Heckers used a cutoff of .80 standard deviations below the predicted cognitive score (Keefe et al., 2005;Woodward and Heckers, 2015). Thus, the present methodology resulted in a more conservative cutoff with fewer participants below the cutoff potentially representing a lower functioning subsample. Although single word reading is well established as an objective measure of premorbid intellectual ability and is used for that purpose in clinical neuropsychology and predictors of premorbid ability were selected based on their reliability and relative resistance to disease-related decline (Bright et al., 2002;Gladsjo et al., 1999;Kareken et al., 1995;Kremen et al., 2000;Keefe et al., 2005), these scores could potentially be impacted by early illness manifestations and other early life events that disrupt neurologic development and educational attainment. In this manner, single word reading could underestimate the degree of decline from potential. Similarly, parental education has strong associations with educational attainment, IQ, and neuropsychological test performance, but is limited by the validity of the data available (recall/knowledge of probands and relatives). Variability in type or medication, combinations of medications, and/or dosing may impact cognition and cognitive trajectories differently. Although correlational analyses indicated that antipsychotics did not impact deviation scores, anticholinergic burden, medication history, and other related factors that may be related to cognitive impairment or decline and were investigated in more depth in a separate report (Eum et al., 2017). Finally, the B-SNIP recruitment strategy required that patients have at least one relative willing and able to participate. Thus, sparse and potentially biased assessment of relatives may have reduced the precision of estimated familiality of cognitive abilities. To better assess the familiality/heritability of these traits it would be optimal to assess a larger family pedigree (e.g. more family member to assess concordance regarding the extent of deviation from cognitive expectation among affected siblings and relatives).
4.5. Concluding remarks
The current study demonstrated that a significant proportion of probands with a psychotic disorder display a profile of meaningful cognitive decline from expectation based primarily on estimated premorbid abilities. Moreover, robust deviation from expectation, which was more prominent in schizophrenia than bipolar disorder, impacted cognition significantly and diminished familial or heritable effects on cognition, presumably via the sequelae of disease processes. Furthermore, this approach may have broader applications for subtyping probands with psychotic disorders, and as a complementary way for evaluating treatment outcomes or a novel approach to separating familial and non-familial illness risk factors.
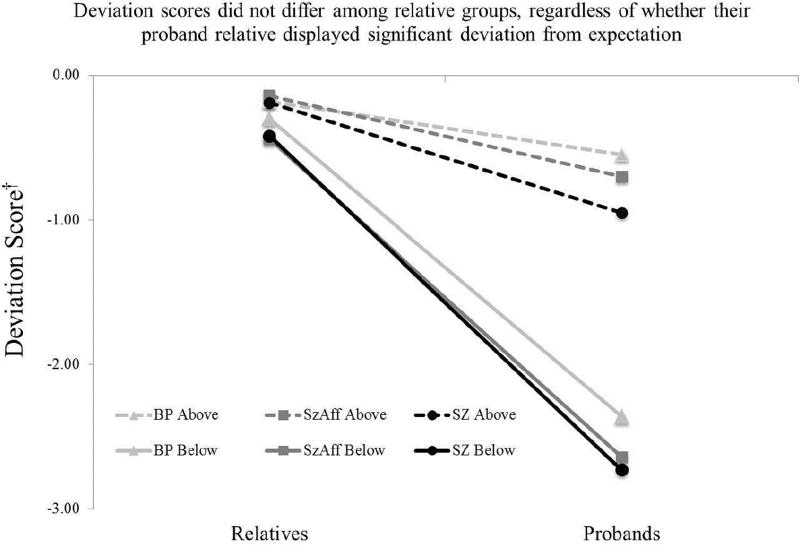
Figure 4.
Cases were classified based on significant deviation from expectation (below the 95% CI cutoff) in probands. The magnitude of deviation between probands and relatives increased dramatically, regardless of diagnosis, as a function the proband deviation status. Thus, the cognitive decline seen in probands below the cut score does not stem from a familial pattern of cognitive vulnerability.
†Deviation Score = Difference between Observed and Predicted BACS Composite z-score.
Acknowledgments
This study was supported in part by NIMH grants MH078113, MH077945, MH077852, MH077851, MH077862, MH072767, and MH083888. We thank Gunvant K. Thaker for his collaboration, design, and implementation of this study.
Role of the Funding Source
The funding agency had no role in the study design, data collection, data management, statistical analysis, interpretation of findings, manuscript preparation, or review and approval of the manuscript.
Dr. Tamminga has received support from Intracellular Therapies (ITI, Inc.), PureTech Ventrues, Eli Lilly Pharmaceuticles, Sunovion, Astellas, Merck (ad hoc consulting), International Congress on Schizophrenia Research (unpaid volunteer), NAMI (unpaid volunteer), American Psychiatric Association (Deputy Editor), and Finnegan Henderson Farabow Garrett & Dunner, LLP. Dr. Keefe has received investigator initiated support from the Department of Veteran’s Affair, Feinstein Institute for Medical Research, GlaxoSmithKline, National Institute of Mental Health, Novartis, Psychogenics, Research Foundation for Mental Hygiene, Inc., and the Singapore National Medical Research Council. Dr. Keefe has received honoraria, served as a consultant, or advisory board member for Abbvie, Akebia, Amgen, Astellas, Asubio, AviNeuro/ChemRar, BiolineRx, Biomarin, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, EnVivo, Helicon, Lundbeck, Merck, Mitsubishi, Otsuka, Pfizer, Roche, Shire, Sunovion, Takeda, Targacept. Dr. Keefe is a shareholder in Sengenix and NeuroCog Trials, Inc. and receives royalties from the BACS testing battery and the MATRICS Battery (BACS Symbol Coding). Dr. Keshavan has received support from Sunovion and GlaxoSmithKline. Dr. Sweeney has received support from Takeda, BMS, Roche, and Eli Lilly and research funding from Janssen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Dr. Hochberger is the lead author and was responsible for data analysis and manuscript preparation. Dr. Hill is the corresponding author and was involved in all aspects of the report. Dr. Sweeney was a site PI and was involved in all aspects of the report. Dr. Carrathers was involved in data analysis, literature review, and writing portions of the report. Dr. Reilly has been involved in all aspects of the project including data collection, clinical characterization, data processing and quality control, conducted the heritability analysis, and advised on further data analysis and interpretation. Dr. Keefe provided the BACS battery, training for the BACS, quality control of the BACS throughout the study, and consultation regarding the statistical analytic approach and interpretation. Drs. Godfrey, Keshavan, Clementz, and Tamminga are part of the Bipolar and Schizophrenia Network on Intermediate Phenotype (B-SNIP) consortium and were PIs for B-SNIP grants. All authors have approved the final version.
Conflicts & Disclosures
The other authors have no disclosers at this time.
References
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, Pappadopulos E, Willson DF, Alvir JM, Woerner MG, Geisler S, Kane JM, Lieberman JA. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry. 2000;157:549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The Social Functioning Scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br J Psychiatry. 1990;157:853–9. 853–859. doi: 10.1192/bjp.157.6.853. [DOI] [PubMed] [Google Scholar]
- Bishop JR, Reilly JL, Harris MS, Patel SR, Kittles R, Badner JA, Prasad KM, Nimgaonkar VL, Keshavan MS, Sweeney JA. Pharmacogenetic associations of the type-3 metabotropic glutamate receptor (GRM3) gene with working memory and clinical symptom response to antipsychotics in first-episode schizophrenia. Psychopharmacology (Berl) 2015;232:145–154. doi: 10.1007/s00213-014-3649-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokland GA, Mesholam-Gately RI, Toulopoulou T, Del Re EC, Lam M, DeLisi LE, Donohoe G, Walters JT, Seidman LJ, Petryshen TL. Heritability of Neuropsychological Measures in Schizophrenia and Nonpsychiatric Populations: A Systematic Review and Meta-analysis. Schizophr Bull. 2016:sbw146. doi: 10.1093/schbul/sbw146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard TJ. Genetic incluence on human psychological traits: A survey. Current Directions in Psychological Science. 2004;13:148–151. [Google Scholar]
- Bouchard TJ, Jr, McGue M. Familial studies of intelligence: a review. Science. 1981;212:1055–1059. doi: 10.1126/science.7195071. [DOI] [PubMed] [Google Scholar]
- Bright P, Jaldow E, Kopelman MD. The National Adult Reading Test as a measure of premorbid intelligence: a comparison with estimates derived from demographic variables. J Int Neuropsychol Soc. 2002;8:847–854. doi: 10.1017/s1355617702860131. [DOI] [PubMed] [Google Scholar]
- Bryson GJ, Silverstein ML, Nathan A, Stephen L. Differential rate of neuropsychological dysfunction in psychiatric disorders: comparison between the Halstead-Reitan and Luria-Nebraska batteries. Percept Mot Skills. 1993;76:305–306. [PubMed] [Google Scholar]
- Burdick KE, Goldberg TE, Funke B, Bates JA, Lencz T, Kucherlapati R, Malhotra AK. DTNBP1 genotype influences cognitive decline in schizophrenia. Schizophr Res. 2007;89:169–172. doi: 10.1016/j.schres.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LS, Rice TK, Thompson PA, Barch DM, Csernansky JG. Familial aggregation of clinical and neurocognitive features in sibling pairs with and without schizophrenia. Schizophr Res. 2009;111:159–166. doi: 10.1016/j.schres.2009.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, Keshavan MS, Tamminga CA. Identification of Distinct Psychosis Biotypes Using Brain-Based Biomarkers. Am J Psychiatry. 2016;173:373–384. doi: 10.1176/appi.ajp.2015.14091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Ragland JD, Gold JM, Gur RC. General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol Psychiatry. 2008;64:823–827. doi: 10.1016/j.biopsych.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Gscheidle T, Weirich M, Rawlings R, Hyde TM, Bigelow L, Weinberger DR. Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biol Psychiatry. 2001;50:98–107. doi: 10.1016/s0006-3223(01)01133-7. [DOI] [PubMed] [Google Scholar]
- Eum S, Hill SK, Rubin LH, Carnaham RM, Reilly JL, Ivleva EI, Keedy SK, Tamminga CA, Pearlson GD, Clementz BA, Gershon ES, Keshavan MS, Keefe RSE, Sweeney JA, Bishop JR. Cognitive burden of anticholinergeic medications in psychotic disorders. Schizophr Res. 2017 doi: 10.1016/j.schres.2017.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MD, Gibbon GE, Spitzer RL, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders-patient edition Biometrics Research Department. NYSPI; New York: 1995. [Google Scholar]
- Fowler T, Zammit S, Owen MJ, Rasmussen F. A population-based study of shared genetic variation between premorbid IQ and psychosis among male twin pairs and sibling pairs from Sweden. Arch Gen Psychiatry. 2012;69:460–466. doi: 10.1001/archgenpsychiatry.2011.1370. [DOI] [PubMed] [Google Scholar]
- Gladsjo JA, Heaton RK, Palmer BW, Taylor MJ, Jeste DV. Use of oral reading to estimate premorbid intellectual and neuropsychological functioning. J Int Neuropsychol Soc. 1999;5:247–254. doi: 10.1017/s1355617799533079. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Braff DL, Light GA, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Green MF, Gur RE, Gur RC, Mintz J, Nuechterlein KH, Olincy A, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Schork NJ. Initial heritability analyses of endophenotypic measures for schizophrenia: the consortium on the genetics of schizophrenia. Arch Gen Psychiatry. 2007;64:1242–1250. doi: 10.1001/archpsyc.64.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth CM, Wright MJ, Luciano M, Martin NG, de Geus EJ, van Beijsterveldt CE, Bartels M, Posthuma D, Boomsma DI, Davis OS, Kovas Y, Corley RP, Defries JC, Hewitt JK, Olson RK, Rhea SA, Wadsworth SJ, Iacono WG, McGue M, Thompson LA, Hart SA, Petrill SA, Lubinski D, Plomin R. The heritability of general cognitive ability increases linearly from childhood to young adulthood. Mol Psychiatry. 2010;15:1112–1120. doi: 10.1038/mp.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Keefe RS, Gold JM, Bishop JR, Gershon ES, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA. Neuropsychological Impairments in Schizophrenia and Psychotic Bipolar Disorder: Findings from the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Study. Am J Psychiatry. 2013;11:1275–84. doi: 10.1176/appi.ajp.2013.12101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Schuepbach D, Herbener ES, Keshavan MS, Sweeney JA. Pretreatment and longitudinal studies of neuropsychological deficits in antipsychotic-naive patients with schizophrenia. Schizophr Res. 2004;68:49–63. doi: 10.1016/S0920-9964(03)00213-5. [DOI] [PubMed] [Google Scholar]
- Johnstone B, Hexum CL, Ashkanazi G. Extent of cognitive decline in traumatic brain injury based on estimates of premorbid intelligence. Brain Inj. 1995;9:377–384. doi: 10.3109/02699059509005777. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Gur RC, Saykin AJ. Reading on the Wide Range Achievement Test-Revised and parental education as predictors of IQ: comparison with the Barona formula. Arch Clin Neuropsychol. 1995;10:147–157. [PubMed] [Google Scholar]
- Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br J Psychiatry Suppl. 1989:59–67. [PubMed] [Google Scholar]
- Keefe RS, Eesley CE, Poe MP. Defining a cognitive function decrement in schizophrenia. Biol Psychiatry. 2005;57:688–691. doi: 10.1016/j.biopsych.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Harvey PD, Goldberg TE, Gold JM, Walker TM, Kennel C, Hawkins K. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS) Schizophr Res. 2008;102:108–115. doi: 10.1016/j.schres.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Perkins DO, Gu H, Zipursky RB, Christensen BK, Lieberman JA. A longitudinal study of neurocognitive function in individuals at-risk for psychosis. Schizophr Res. 2006;88:26–35. doi: 10.1016/j.schres.2006.06.041. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Mezuk B, Sundquist JO, Sundquist K. Observed Cognitive Performance and Deviation From Familial Cognitive Aptitude at Age 16 Years and Ages 18 to 20 Years and Risk for Schizophrenia and Bipolar Illness in a Swedish National Sample. JAMA Psychiatry. 2016;73:465–71. doi: 10.1001/jamapsychiatry.2016.0053. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Seidman LJ, Faraone SV, Toomey R, Tsuang MT. The paradox of normal neuropsychological function in schizophrenia. J Abnorm Psychol. 2000;109:743–752. doi: 10.1037//0021-843x.109.4.743. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, York TP, Franz CE, Grant MD, Eaves LJ, Jacobson KC, Schaie KW, Panizzon MS, Boake C, Xian H, Toomey R, Eisen SA, Kremen WS. Genes determine stability and the environment determines change in cognitive ability during 35 years of adulthood. Psychol Sci. 2009;20:1146–1152. doi: 10.1111/j.1467-9280.2009.02425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer BW, Heaton RK, Paulsen JS, Kuck J, Braff D, Harris MJ, Zisook S, Jeste DV. Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology. 1997;11:437–446. doi: 10.1037//0894-4105.11.3.437. [DOI] [PubMed] [Google Scholar]
- Plomin R, Craig I. Human behavioural genetics of cognitive abilities and disabilities. Bioessays. 1997;19:1117–1124. doi: 10.1002/bies.950191211. [DOI] [PubMed] [Google Scholar]
- Plomin R, Deary IJ. Genetics and intelligence differences: five special findings. Mol Psychiatry. 2015;20:98–108. doi: 10.1038/mp.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly JL, Harris MS, Keshavan MS, Sweeney JA. Adverse effects of risperidone on spatial working memory in first-episode schizophrenia. Arch Gen Psychiatry. 2006;63:1189–1197. doi: 10.1001/archpsyc.63.11.1189. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Sweeney JA. Generalized and specific neurocognitive deficits in psychotic disorders: utility for evaluating pharmacological treatment effects and as intermediate phenotypes for gene discovery. Schizophr Bull. 2014;40:516–522. doi: 10.1093/schbul/sbu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney JA, Keilp JG, Haas GL, Hill J, Weiden PJ. Relationships between medication treatments and neuropsychological test performance in schizophrenia. Psychiatry Res. 1991;37:297–308. doi: 10.1016/0165-1781(91)90065-w. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5. Pearson: 2007. [Google Scholar]
- Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, Morris DW, Bishop J, Thaker GK, Sweeney JA. Clinical Phenotypes of Psychosis in the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am J Psychiatry. 2013;170:1263–74. doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PubMed] [Google Scholar]
- Vaskinn A, Ueland T, Melle I, Agartz I, Andreassen OA, Sundet K. Neurocognitive Decrements are Present in Intellectually Superior Schizophrenia. Front Psychiatry. 2014;5:45. doi: 10.3389/fpsyt.2014.00045. eCollection;%2014., 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Heckers S. Brain Structure in Neuropsychologically Defined Subgroups of Schizophrenia and Psychotic Bipolar Disorder. Schizophr Bull. 2015;41:1349–1359. doi: 10.1093/schbul/sbv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Deng W, Yao L, Xiao Y, Li F, Liu J, Sweeney JA, Lui S, Gong Q. Brain Structural Abnormalities in a Group of Never-Medicated Patients With Long-Term Schizophrenia. Am J Psychiatry. 2015;172:995–1003. doi: 10.1176/appi.ajp.2015.14091108. [DOI] [PubMed] [Google Scholar]