Abstract
Tumor masses are deprived of oxygen and characterized by enhanced glucose uptake followed by glycolysis. Elevated glucose levels induce non-enzymatic glycosylation or glycation of proteins which leads to accumulation of advanced glycation end products (AGE). These AGE molecules bind to their respective receptors called the receptor for advanced glycation end products (RAGE) and initiate several aberrant signaling pathways leading to onset of diseases such as diabetes, Alzheimer’s, atherosclerosis, heart failure and cancer. The role of AGE in cancer progression is being extensively studied in recent years. As cancer cells are hypoxic in nature and adapted to glycolysis, which induces glycation, its effects need to be understood in greater detail. Since AGE-RAGE signaling is involved in cancer progression, inhibition of AGE-RAGE interaction could be a potential therapeutic target. The purpose of this review is to highlight the role of AGE-RAGE interaction in hypoxic cancer cells.
Keywords: Glycation, Advanced Glycation End Products, RAGE, Cancer, Hypoxia, Hif1α
1. Introduction
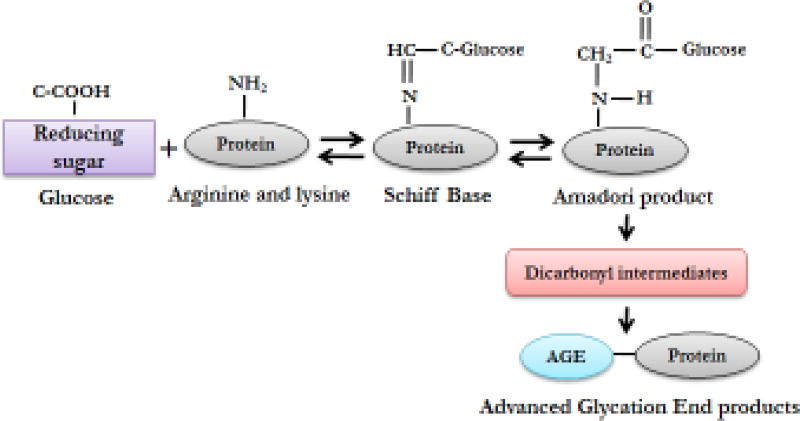
Cancer is rated as one of the most prevalent diseases around the globe and several factors are responsible for its progression. Over the past few decades, glycation has emerged as one of the important factors whose role has been discussed extensively in cancer progression [1]. Glycation is the non-enzymatic reaction between the reducing sugars and amino groups of proteins, lipids and nucleic acids [2]. This reaction is known as Maillard reaction [3, 4] and is distinct from glycosylation which is an enzymatic process. The final products generated during glycation are known as Advanced Glycation End Products (AGE) which are mainly derived from arginine and lysine groups of proteins after reaction with the carbonyl group of the sugar moiety [5]. Detailed mechanism of Maillard reaction is schematically presented in figure 1. Involvement of AGE in cancer progression is yet to be fully understood, however, a few reports explain AGE-mediated cancer progression in different types of cancer. One recent finding showed involvement of AGE in enhancing proliferation, migration and invasion during breast cancer progression [6] while another report explained its crucial role in prostate cancer progression through inducing invasiveness [7]. Jiao et al. [8] observed that dietary consumption of AGE was associated with a modest increased risk of pancreatic cancer in men. Apart from cancer, accumulation of AGE has been linked to multiple diseases including diabetes, cardiovascular disease, renal failure, arthritis and neurodegenerative disorders [9].
Figure 1. Schematic presentation of the Maillard reaction.
Amino group of protein (arginine and lysine derivatives) react with carbonyl moiety of reducing sugars to form reversible Schiff base which further rearranges to form Amadori products. These products finally form Advanced Glycation End Products (AGE) through dicarbonyl intermediates.
The inner mass of solid tumors is hypoxic in nature and this creates a pre-metastatic niche which further enhances the aggressiveness of cancer cells [10]. AGE accumulation mediates various disorders. Diabetes, inflammation and acute ischemia/perfusion (I/R) in heart are previously reported but hypoxia-driven AGE accumulation in cancer cells is not yet fully discussed [11]. Here, we review the extensive role of AGE and the Receptor for Advanced Glycation End Products (RAGE) in cancer progression.
2. AGE and RAGE: Brief overview
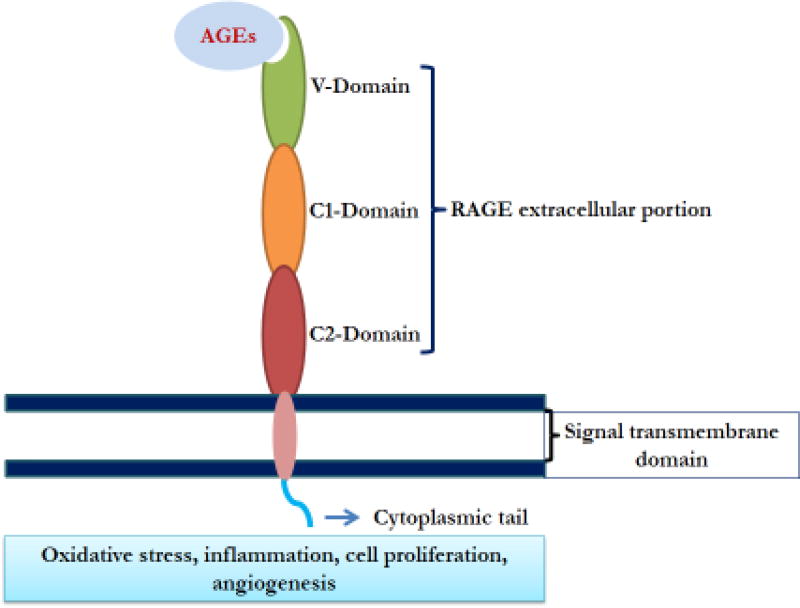
AGE are generated through non-enzymatic glycation and oxidation of proteins that play a significant role in the pathogenic progression of several metabolic disorders. These compounds are also known as glycotoxins [4, 12, 13]. Formation of Schiff base and Amadori products are considered as the first step in glycation where aldoses are coupled with free amino groups of proteins, lipids, nucleic acids. These intermediate forms are the dicarbonyls such as glyoxal (GO), methylglyoxal (MG), or 3-deoxyglucosone (3-DG) which is highly reactive [14, 15]. Finally, Amadori products react non-enzymatically with lysine or arginine residues to produce AGE. These dicarbonyls are also derived from lipid peroxidation, glycolysis as well as protein degradation [16]. AGE are either extracellular, derived from food items or intracellular that are derived from sugars [17]. AGE are broadly categorized into three types summarized in table 1. Apart from protein glycation, DNA glycation also produces AGE, which act as potential carcinogens [18]. The glycated DNA accumulates several structural changes including depurination, strand breaks, and mutations such as insertions, deletions, and transpositions [19]. RAGE is expressed on the cell surface and when bound to AGE initiates further signaling events. RAGE is a 35 kDa pattern recognition receptor and transmembrane protein belonging to the immunoglobulin (Ig) superfamily [20]. RAGE is also a receptor for Damage-Associated Molecular Pattern (DAMP) molecule that originates from damaged cells and alerts the immune system to tissue trauma. RAGE acts as a receptor for high mobility group box 1 (HMGB1), the prototypical DAMP, and S100 proteins along with AGE [21]. The detailed structure of RAGE is illustrated in figure 2. RAGE is composed of one extracellular domain, one transmembrane domain, and one cytoplasmic tail. The extracellular domain of RAGE consists of three immunoglobulin-like regions: one “V”-type followed by two “C”-type regions [22]. The transmembrane-spanning domain connects the intracellular region with the 43-amino acid cytosolic tail. The V-domain is critical for ligand-binding, whereas the cytosolic tail is essential for RAGE-mediated intracellular signaling. The V-domain of RAGE facilitates AGE binding which further activates an array of signaling pathways [23, 24] involved in tumorigenesis. The ligand and receptor bond in between RAGE-AGE is mediated by charge-charge interaction [25]. Binding of AGE to RAGE initiates several downstream events that lead to cell proliferation, autophagy, and carcinogenesis [26–28].
Table 1.
Types of advanced glycation end products (AGE)
| Type of AGE | Examples |
|---|---|
| Fluorescent cross-linking | Pentosidine and Crossline |
| Non-fluorescent cross-linking | Imadazolium dilysine cross-links, Alkyl formyl glycosyl pyrroles and Arginine–lysine imidazole crosslinks |
| Non-cross-linking | Pyrraline and N carboxymethyllysine |
Figure 2. Structure of receptor for advanced glycation end product (RAGE).
The RAGE is composed of three extracellular immunoglobulin (Ig)-like domains, a single transmembrane helix (light pink) and a short cytoplasmic (dark sky blue) domain. The Ig domains are V (shown in dark green), C1 (orange) and C2 domain (dark brown). The V-domain is responsible for binding to the AGE (faint blue) and cytoplasmic tail responses to the signaling pathways.
3. Role of AGE-RAGE complex in cancer progression
AGE-RAGE axis plays a critical role in tumor growth and metastasis when RAGE is activated upon binding with AGE [29]. There is a growing body of evidence that supports a correlation between AGE-RAGE expression and cancer malignancy. Few of them are melanoma [30], oral cancer [31], breast cancer [32, 33], gastric cancer [34], colorectal cancer [35, 36], pancreatic cancer [8, 37, 38], intestinal cancer [39], prostate cancer [40, 41], renal cancer [42] and leukaemia [43]. One contradictory finding [44] that needs to be studied in detail proposes tumor suppressive function of RAGE in lung cancer.
AGE-RAGE axis plays a pivotal role in cancer cell proliferation. One recent report explains AGE-RAGE-mediated cell proliferation in rat vascular smooth muscle cells [45]. Another study on breast cancer cell lines revealed RAGE-mediated cell proliferation and cancer progression [46]. RAGE also plays a key role in various metabolic activities of the cells. The proliferation of myeloid cells is regulated by AGE-RAGE interaction followed by MAPK, PI3K, JAK/STAT-mediated pathways [43]. Mammalian target of rapamycin (mTOR) which is a serine/threonine protein kinase is a potential RAGE target and regulates transcription, cell growth, cell proliferation, cell motility, cell survival and protein synthesis [47]. AGE-RAGE interaction also induces cancer promoting vascular endothelial growth factors (VEGF) in breast cancer cells [33]. Another study suggests that RAGE activation by AGE induces VEGF production and inflammatory response in human synoviocytes by modulating RAGE-NF-κB pathway [48]. Autophagy is the programmed cell survival process by which organelles and proteins are degraded after being engulfed by the autophagic vesicles followed by fusion with lysosomes [49]. This is recognized as a mechanism for tumor cell survival, which provides resistance to apoptosis [50]. AGE-RAGE-mediated autophagy induction is also reported in the literature. AGE-RAGE-mediated autophagy induction is associated with tumor cell survival. This pathway is operated through reactive oxygen species (ROS) generation. RAGE is considered as a positive regulator of autophagy and negative regulator of apoptosis during oxidative stress conditions. ROS generation is enhanced during AGE-RAGE interaction and hence induces the autophagy process [51]. The same paper also reported apoptosis-mediated enhanced cell death and diminished cell survival by autophagy during the H2O2 induced oxidative injury by suppressing RAGE expression. Studies have also established the fact that RAGE is a positive feedback regulator for NF-κB. This is so because knockdown of RAGE decreases H2O2-induced activity of NF-κB. Collectively, these findings suggest that RAGE is an important regulator of oxidative injury. These findings also provide the mechanistic insight of apoptosis-autophagy crosstalk mediated via ROS signaling during a process involving RAGE [52].
Apart from autophagy cellular senescence which limits the cell proliferation process can also play a significant role during cancer progression [53]. Stress induced premature cellular senescence is a biological process which is different from replicative senescence and induced during oxidative stress conditions [54–58]. It is believed that senescent cells show bimodal mode of action during cancer progression which includes suppressed tumour growth at early stage [54, 59–62] and tumor promoting effect during later stage [53]. These cells enhance the proliferative property of cells by secreting metalloproteases, growth factors and cytokines which are known as Senescence-Associated Secretory Phenotype (SASP) or Senescence Messaging Secretome (SMS) [54–56, 58, 63]. Involvement of senescence-mediated disease progression is also observed in other conditions such as diabetes [64], cardiovascular disease [65, 66], renal failure [67], arthritis [68] and neurodegenerative disorders [69]. Cancer cells acquire senescence bypass mechanism which overrides the replicative senescence process and escape from senescence mechanism [70, 71]. Recently RAGE-mediated premature senescence activation [72] has explained in the literature. Liu et al. [72] describe RAGE-mediated ER stress which further activates premature senescence via p21 signaling in diabetic nephropathy. However, detailed role of bimodal action of RAGE-mediated cancer progression as well as suppression needs to be studied under senescence activated conditions. Cancer cells possessing invasive property are metastatic in nature. Metastatic property of cancer cells is also influenced by AGE-RAGE interaction. Growth and invasion of melanoma cells was enhanced after AGE-RAGE interaction [73]. Kang et al. [74] provided evidence for upregulation of RAGE in cells forming a primary melanoma tumor that could contribute to the metastatic switch. The experimental analysis also suggested that the metastatic human melanoma cells, G361 exhibit higher levels of cellular proliferation and migration in the presence of RAGE activating AGE ligands. AGE-RAGE interaction also induces migratory potential of oral cancer cells by up-regulating MMP2 and 9 in an ERK-mediated manner [31]. Angiogenesis is the hallmark of cancer metastasis. The role of AGE-RAGE complex in angiogenesis is well documented in the literature [75, 76]. A very recent finding explains AGE-mediated angiogenesis process by moesin phosphorylation which is a ezrin-radixin-moesin (ERM) protein family protein through a RhoA/ROCK pathway in human umbilical vein endothelial cells [77]. Similarly, AGE-RAGE interaction enhances angiogenic potential in hepatocellular carcinoma cells by upregulating VEGF expression [78]. In addition, RAGE activation also increases endothelial permeability to macromolecules which is very common condition in tumor microvasculature. In a study by Tsuji et al. [79] promotion of angiogenesis in lymph node metastasis by latent membrane protein 1 is associated with an increased expression of RAGE. Autocrine VEGF is found to be a major mediator of AGEs driven angiogenesis. AGE–RAGE interaction reduces the pericyte number which in turn relieves the restriction on endothelial cell replication and facilitates angiogenesis [80].
RAGE plays diverse roles in the proliferation of cancer. For example in osteosarcoma cells, RAGE is over expressed and it further induces cell migration by ROS mediated mechanism [81]. AGE bind to and activate RAGE, which is a predominant modulator of inflammation-associated cancer and induces ROS that are an important regulator of cancer [16]. The mechanism of AGE-RAGE complex mediated cancer progression is different in different cancers and summarized in table 2.
Table 2.
Summary of the role of AGE-RAGE complex in different cancers
| Types of Cancer | Role of AGE-RAGE complex | References |
|---|---|---|
| Osteosarcoma | Promotes proliferation | (81) |
| Breast cancer | Induces proliferation, migration and invasion | (6, 32, 33, 44, 46) |
| Colorectal cancer | Biomarkers | (26) |
| Colorectal cancer | Induces tumorigenesis, cell proliferation | (35, 36) |
| Pancreatic cancer | Promotes tumorigenesis | (38, 74, 111) |
| Hepatocellular carcinoma | Enhances angiogenic potential Promotes proliferation | (27, 78) |
| Prostate cancer | Cancer cell proliferation | (41, 104, 126) |
| Oral cancer | Cancer migration | (31) |
| Gastric cancer | Cancer invasion | (34) |
4. Hypoxia: an inducer for AGE-RAGE mediated cancer progression
Hypoxia is a physiological condition which arises inside a solid tumor mass due to insufficient oxygen supply and links to multiple oncogenic pathways [82]. Hypoxia contributes to chemoresistance, radioresistance, angiogenesis, vasculogenesis, invasiveness and metastasis in solid tumors [83, 84]. Metabolic parameters like low glucose concentration, low oxygen concentration as well as low pH lead to the development of hypoxic regions within the tumor mass [85, 86]. These hypoxic cells also induce angiogenesis and evade the apoptotic mechanism of the cell [87].
Hypoxia-driven AGE accumulation and RAGE activation is well documented [88]. Gopal et al. [88] observed hypoxia-induced accumulation of fluorescence AGE such as LW-1 and s-RAGE. Hypoxic tumor cells shows metabolic shift from mitochondrial aerobic respiration to anaerobic glycolysis process [89]. These hypoxic cells induce accumulation of di-carbonyls which act as a precursor for AGE synthesis [16]. MG which is a major intermediate compound formed from glycolysis can also act as a precursor molecule for AGE [90]. MG readily reacts with proteins, lipids and nucleic acids to form AGE. Shinohara et al. [91] observed significant accumulation of MG at high glucose concentrations. Chang et al. [92] reported rapid generation of AGE after hypoxia exposure in endothelial cells which further activated RAGE-mediated signaling. These oxygen deficient cells actively participate in tumor growth and metastasis through activating several signaling events [93]. Another report demonstrated hypoxia-induced AGE formation and RAGE activation in macrophages [94]. RAGE-AGE interaction also mediates myocardial injury after ischemia attack [95]. Previously, hyperglycemia induced AGE activation followed by retinal neovascularization was studied by Shin et al. [96].
Cancer cells are hyperglycemic in nature and the enhanced glucose uptake induces aerobic glycolysis or Warburg’s effect [97]. Hyperglycemic condition is also linked with cancer migration, invasion as well as proliferation [98] and induces AGE accumulation. Detailed understanding of the AGE mediated cancer onset could open avenues in cancer therapeutics. The relation between hyperglycemia and epigenetic modification of oncogenic pathways has been recently studied. A finding by Dong et al. [99] demonstrated epigenetic silencing of fructose-1, 6-biphosphatase which is one of the critical gluconeogenic enzymes, increases glycolysis and NADPH production via the pentose phosphate pathway and a reduction in oxidative phosphorylation. This pathway is regulated by the transcription factor snail which also plays a major role in EMT progression. These metabolic alterations induce survival pathways that lead to cancer stem cell phenotype through lowering ROS generation and inducing β-catenin/TCF4 activation [99].
Further, AGE-RAGE interaction activates several signaling pathways that are involved in cancer progression during oxygen deficient condition. For example, AGE-RAGE interaction activates JNK as well as stat1 signaling during insufficient blood supply [100]. Hypoxia-induced RAGE upregulation was discussed in some recent findings, which further explain the role of AGE-RAGE interaction in hypoxic cancer cells. A recent report described hypoxia-induced RAGE expression in oxygen deficient hepatocellular carcinoma (HCC) [101]. This study showed that RAGE positive cell lines are more resistant to hypoxia as compared to RAGE negative cell lines in HCC. Suppression of RAGE expression by siRNA enhanced susceptibility towards hypoxia. This finding clarifies the significance of RAGE in adapting to the hypoxic resistant phenotype which is very critical for the survival of hypoxic cancer cells. Another study explained that hypoxia-mediated RAGE induces phosphorylation of Erk1/2, Akt and nuclear translocation of NF-κB. Once in the nucleus, NF-κB would contribute to cell survival and invasion under hypoxia, by maintaining RAGE and P2X7R expression levels and matrix metalloproteinases 2 and 9 synthesis [102]. Hypoxia induced AGE-RAGE mediated activation and their downstream signaling pathways are illusttrated in figure 3. These observations highlight the significance of hypoxia in AGE-RAGE signaling and cancer progression. Treatment of hypoxic cancer cells by targeting AGE-RAGE interaction will gain more attention in near future. One recent addition to the hypoxia-mediated AGE-RAGE activation is involvement of senescence-mediated malignancy in hypoxic cells. Several pro-senescent pathways are regulated in hypoxic cells [103]. Senescent cells showed SASP which induces secretion of immunomodulatory cytokines as well as several cell survival factors. Reports are there that explain role of hypoxic senescent fibroblasts in cancer progression [104]. Work done by Taddei et al. [104] showed correlation between hypoxia-induced senescent stroma and malignant property of prostate cancer cells. Their work further showed that hypoxia-induced miR-210 mediates tumor vessel formation as well as enhances aggressiveness of cancer [104].
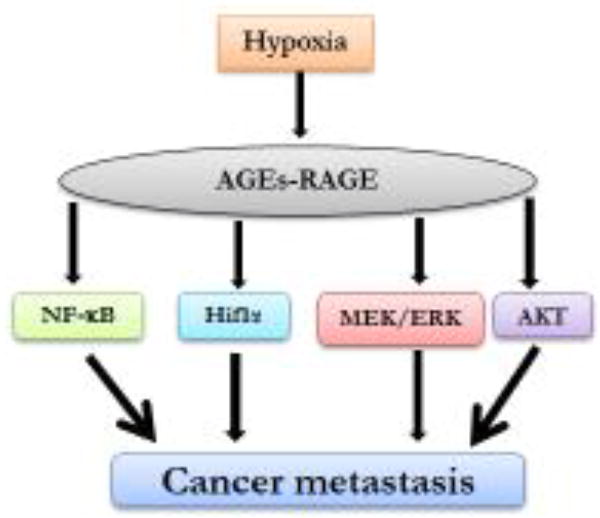
Figure 3. Hypoxia-induced AGE-RAGE-mediated signaling pathways.
Hypoxia induces AGE accumulation which further formed complex with RAGE and activates several downstream pathways including NF-Kb, Hif1α, ERK and AKT signaling. These pathways further contribute to cancer metastasis progression.
Hypoxia leads to development of aggressive phenotype and various hypoxia-driven factors actively participate in tumor cell survival and hamper clinical response to therapy [87]. Hypoxia inducible factor 1 (Hif1) is one of the major transcription factors upregulated in hypoxic microenvironment and it induces several downstream genes involved in cancer progression and metastasis [105]. The Hif1 expression is also regulated by AGE-RAGE mediated signaling which is explained in subsequent sections.
5. Hif1: a key player in AGE-RAGE complex mediated carcinogenesis
Hif1 is one of the major factors induced during hypoxia exposure and maintains oxygen homeostasis in the body. It helps to adapt in the oxygen deprived condition by regulating glucose uptake and anaerobic respiration of cells. Erythropoiesis, angiogenesis, cell survival, growth, metabolic adaption are influenced by Hif1-mediated pathways [106, 107]. Hif1 is a heterodimeric transcription factor composed of oxygen dependent alpha (α) subunit and constitutively expressed beta (β) subunit. The α subunit is stabilized in hypoxic condition and translocated to the nucleus where it interacts with the β subunit to form the complex which further activates transcription of various proteins involved in survival of cancer cells [108]. So the alpha subunit plays a critical role in the regulation of cell survival machinery in hypoxic cells. Both α and β subunits have similar structure [109] and the whole gene is subdivided into several domains. The amino-terminal domain consists of basic helix-loop-helix (bHLH) and PAS (Per-ARNT-Sim homology) domain. The carboxy-terminal domain (CTD) has two transactivation domains separated by one inhibitory domain (ID) [109, 110]. Hif1α has one oxygen-dependent degradation domain (ODD) towards its CTD which senses oxygen concentration inside the cells. As both Hif1α as well as AGE are upregulated in hypoxic cells, correlation between these two might have immense importance in cancer therapeutics.
Recently RAGE-mediated Hif1α activation was extensively studied. Kang et al. [111] showed that the interaction between RAGE and oncogenic KRAS induces Hif1α activation in pancreatic cancer cells. They found a novel mechanism of Hif1α activation involving NF-κB-RAGE-KRAS-Hif1α–mediated pathway in hypoxic pancreatic cancer cells. Inhibition of RAGE expression downregulates KRAS activation followed by Hif1α expression. Hence, RAGE-mediated signaling is necessary for downstream Hif1α activation. This upregulated Hif1α further induces aggressiveness of the pancreatic cancer mass. Further, knock down of RAGE as well as Hif1α decreased cell viability and induced apoptosis. These results suggest a role of RAGE in maintaining the viability of cancer cells [111]. It was further demonstrated that both mRNA and protein level of RAGE were increased in a hypoxic rich environment. This study further showed upregulation of RAGE expression in primary neurons subjected to hypoxia or oxygen-glucose deprivation, an in vitro model of ischemia [112]. AGE-mediated regulation of Hif1α transcriptional activity has also been reported [113]. The relation between RAGE-Hif1α was explained in another study that demonstrated RAGE mediated-Hif1α activation by suppressing p53 protein expression [114]. Correlation between AGE induced oxidative stress and Hif1α stabilization in Leidig’s cells was recently studied [115]. Bala et al. [116] demonstrated AGE-RAGE expression mediated Hif1α expression in HUVEC cells. In this study HUVEC cells were exposed to glycation modified human serum albumin (AGE-HSA) and expression of RAGE was measured. This study revealed upregulation of RAGE in HUVEC cells which is further accompanied by induced Hif1α expression. Knockdown of RAGE by using siRNA downregulated Hif1α expression. Furthermore, Hif1α targeted VEGF expression was significantly induced upon AGE-HSA treatment. These findings support the role of hypoxia in AGE-mediated angiogenesis and cancer progression. Another study also investigated AGE mediated VEGF accumulation [117] in hypoxic cells. Hif1a also plays a major role in bypassing oncogene induced senescence and induces premalignant property of the cells [118]. However, direct link between RAGE activation, hypoxia and Hif1α-mediated cancer progression by senescence bypassing is not yet established. Hence, further research in this field will open a new arena for cancer therapeutics. Further studies are needed to find out the detailed mechanism of AGE-mediated and Hif1α induced carcinogenesis in hypoxic cells.
6. Therapeutic strategies to target AGE-RAGE complex in cancer
Targeting AGE-RAGE interaction to control AGE mediated disorders has been extensively studied. Several therapeutic approaches have been adapted that include inhibition of AGE formation, accumulation, blocking of AGE-RAGE interaction and their signaling mediated pathways, use of RAGE blockers and anti-RAGE antibodies. Enzymatic degradation of AGE precursor like α-oxalaldehyde is another potential alternative method to inhibit AGE accumulation [119]. Targeting AGE mediated pathways in diabetes treatment has been elucidated in many reports. One such report demonstrated that inhibition of AGE-RAGE interaction protects against hyperglycemia induced fibrosis of peritoneal membrane [120]. It is interesting to note that benfotiamine-mediated AGE inhibition acts as a potential therapeutic approach to control type 2 diabetes [121]. Clinical trials of several anti-AGE molecules are reported and among them TM2002 showed better AGE inhibitory effect. TM2002 protects against AGEs mediated renal injury and cardiovascular disorders [122]. Aminoguanidine and Alagebrium are two chemicals that are widely used to inhibit AGE accumulation in diabetic kidney [123]. Another compound is pigment epithelium-derived factor that partly suppresses AGE-RAGE axis and helps to cure vascular complications of diabetic patients [124]. A pioneer work by Maeda et al. [124] showed the significant role played by aptamers to regulate AGE function. These are short single standard DNA or RNA molecules. This work demonstrated that binding of these aptamers to AGE further inhibits their binding to RAGE and reduces RAGE-mediated oxidative stress in diabetic nephropathy. These findings suggest that regulation of AGEs-RAGE axis plays a major role in the therapeutic aspects of glycation derived diabetes. In spite of significant advancement in the AGE-RAGE mediated carcinogenesis, only few therapeutic approaches are reported till date. Mizumoto et al. showed that inhibition of RAGE by using anti-RAGE antibody protects against pulmonary metastasis [125]. In another study, apoptosis induction in prostate cancer cell lines was achieved by targeted blocking of AGE receptors [126]. Food-mediated AGE accumulations are also reduced by lowering intake of food cooked at higher temperature [127]. Detection of AGE-RAGE complex has tremendous importance for the above mentioned therapeutic approaches. Different methods are used to quantify AGE accumulation, few of which are diode array detector (DAD), fluorescence detector, tandem mass spectrometer (MS/MS), gas chromatography coupled with MS and enzyme-linked immunosorbent assay (ELISA) [128]. Detection of AGE-RAGE complex is also performed by immunohistochemistry [129]. Autoantibodies are generated against glycated protein and DNA which detects AGE-RAGE complex [17, 130]. As RAGE-mediated senescence plays a pivotal role in cancer progression, detection of these senescencent cells might help to link with AGE-RAGE-mediated cancer. A pioneer work by Evangelou et al 2017 [131] identified a novel method which detects senescent cells in biological materials by using lipophilic, biotin-linked Sudan Black B (SBB) analogue. This method for detection of RAGE-mediated senescent cells could be further extended to detect AGE-RAGE-mediated complex. However, considering the significant increment in the incidence of AGE-RAGE mediated cancer, further therapeutic interventions studies are needed.
7. Conclusions and emerging questions
In this review, we summarized recent findings and advancements in AGE-RAGE mediated carcinogenesis. Hypoxia-induced regulation of AGE-RAGE-mediated carcinogenic pathway is gaining importance these days. The detailed mechanism of these signaling pathways are not yet fully explored, which will be very helpful to decipher new methods to cure cancer. There are so many open questions that remain unexplored. The most unanswered question is whether there is any direct modification in hypoxia-induced Hif1α molecule due to glycation or accumulation of AGE. As Hif1α is a major molecule involved in several carcinogenic pathways, this type of finding will create a new insight into AGE-mediated carcinogenesis. As most of the findings discussed here are based on in vitro studies, there is a need to conduct some relevant in vivo studies for accurate interpretation. It is interesting to note that diabetic patients are more prone to cancer [132]; hence correlation between these two diseases is needed to be studied in detail. As AGE are involved in both diabetes as well as cancer, another important question is whether existing therapies for AGE-mediated diabetes will be helpful for controlling AGEs-mediated cancer. Designing of selective inhibitors for AGE-RAGE mediated Hif1a signaling pathway will also prove to be an advanced strategy for cancer treatment.
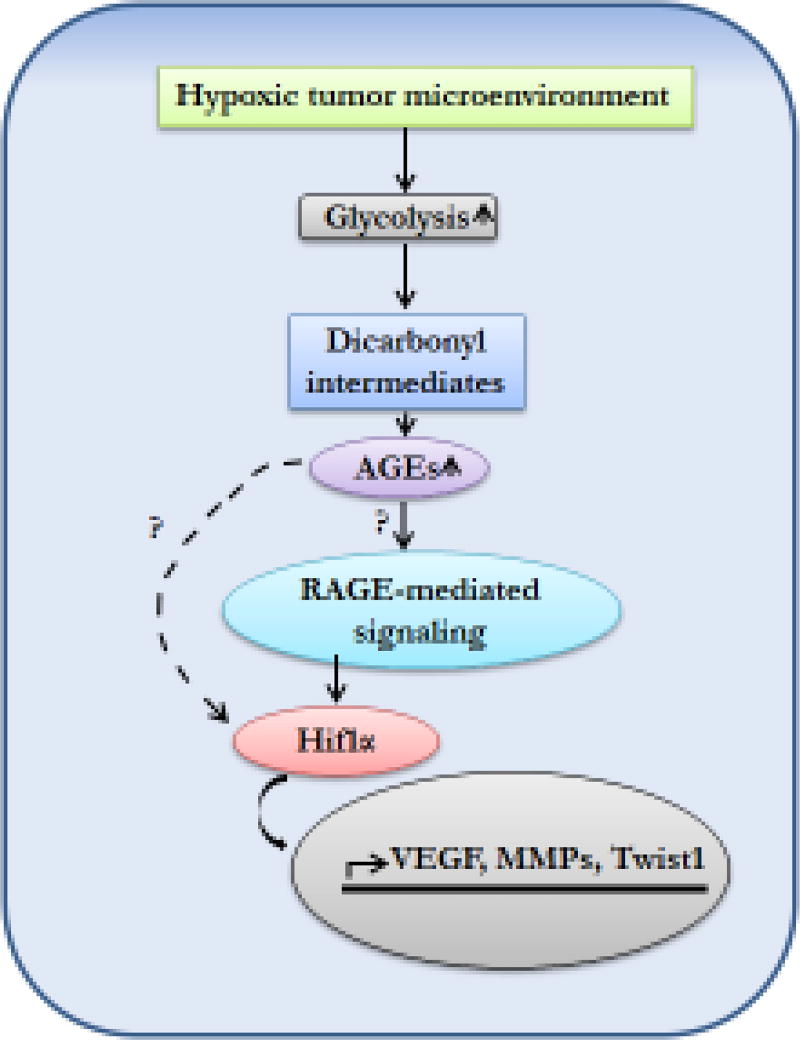
Figure 4. Hypoxia-driven AGE-RAGE-mediated carcinogenesis is induced by Hif1α.
Hypoxic microenvironment induces glycolysis and dicarbonyls accumulation. These dicarbonyls further trigger AGE generation. Once AGE interact with their receptors, RAGE, they activate Hif1α signaling pathways which further act as a transcription factor for synthesis of cell survival and metastasis related genes.
Acknowledgments
The corresponding author is supported by grants from the United States Public Health Service (R01CA160867; R01AR059742; P30AR066524) and the Department of Defense (W81XWH-15-1-0513).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Malik P, Chaudhry N, Mittal R, Mukherjee TK. Role of receptor for advanced glycation end products in the complication and progression of various types of cancers. Biochimica et biophysica acta. 2015;1850(9):1898–904. doi: 10.1016/j.bbagen.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 2.Gkogkolou P, Bohm M. Advanced glycation end products: Key players in skin aging? Dermato-endocrinology. 2012;4(3):259–70. doi: 10.4161/derm.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younus H, Anwar S. Prevention of non-enzymatic glycosylation (glycation): Implication in the treatment of diabetic complication. International journal of health sciences. 2016;10(2):261–77. [PMC free article] [PubMed] [Google Scholar]
- 4.Turner DP. Advanced glycation end-products: a biological consequence of lifestyle contributing to cancer disparity. Cancer research. 2015;75(10):1925–9. doi: 10.1158/0008-5472.CAN-15-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad S, Shahab U, Baig MH, Khan MS, Khan MS, Srivastava AK, Saeed M. Moinuddin Inhibitory effect of metformin and pyridoxamine in the formation of early intermediate and advanced glycation end-products. PloS one. 2013;8(9):e72128. doi: 10.1371/journal.pone.0072128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharaf H, Matou-Nasri S, Wang Q, Rabhan Z, Al-Eidi H, Al Abdulrahman A, Ahmed N. Advanced glycation endproducts increase proliferation, migration and invasion of the breast cancer cell line MDA-MB-231. Biochimica et biophysica acta. 2015;1852(3):429–41. doi: 10.1016/j.bbadis.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Teja M, Gronau JH, Breit C, Zhang YZ, Minamidate A, Caley MP, McCarthy A, Cox TR, Erler JT, Gaughan L, Darby S, Robson C, Mauri F, Waxman J, Sturge J. AGE-modified basement membrane cooperates with Endo180 to promote epithelial cell invasiveness and decrease prostate cancer survival. The Journal of pathology. 2015;235(4):581–92. doi: 10.1002/path.4485. [DOI] [PubMed] [Google Scholar]
- 8.Jiao L, Stolzenberg-Solomon R, Zimmerman TP, Duan Z, Chen L, Kahle L, Risch A, Subar AF, Cross AJ, Hollenbeck A, Vlassara H, Striker G, Sinha R. Dietary consumption of advanced glycation end products and pancreatic cancer in the prospective NIH-AARP Diet and Health Study. The American journal of clinical nutrition. 2015;101(1):126–34. doi: 10.3945/ajcn.114.098061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad S, Khan MS, Akhter F, Khan MS, Khan A, Ashraf JM, Pandey RP, Shahab U. Glycoxidation of biological macromolecules: a critical approach to halt the menace of glycation. Glycobiology. 2014;24(11):979–90. doi: 10.1093/glycob/cwu057. [DOI] [PubMed] [Google Scholar]
- 10.Chang J, Erler J. Hypoxia-mediated metastasis. Advances in experimental medicine and biology. 2014;772:55–81. doi: 10.1007/978-1-4614-5915-6_3. [DOI] [PubMed] [Google Scholar]
- 11.Shang L, Ananthakrishnan R, Li Q, Quadri N, Abdillahi M, Zhu Z, Qu W, Rosario R, Toure F, Yan SF, Schmidt AM, Ramasamy R. RAGE modulates hypoxia/reoxygenation injury in adult murine cardiomyocytes via JNK and GSK-3beta signaling pathways. PloS one. 2010;5(4):e10092. doi: 10.1371/journal.pone.0010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, Yong A, Striker GE, Vlassara H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. Journal of the American Dietetic Association. 2010;110(6):911–16. e12. doi: 10.1016/j.jada.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uribarri J, del Castillo MD, de la Maza MP, Filip R, Gugliucci A, Luevano-Contreras C, Macias-Cervantes MH, Markowicz Bastos DH, Medrano A, Menini T, Portero-Otin M, Rojas A, Sampaio GR, Wrobel K, Wrobel K, Garay-Sevilla ME. Dietary advanced glycation end products and their role in health and disease. Advances in nutrition. 2015;6(4):461–73. doi: 10.3945/an.115.008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad S, Moinuddin, Shahab U, Habib S, Salman Khan M, Alam K, Ali A. Glycoxidative damage to human DNA: Neo-antigenic epitopes on DNA molecule could be a possible reason for autoimmune response in type 1 diabetes. Glycobiology. 2014;24(3):281–91. doi: 10.1093/glycob/cwt109. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad MI, Ahmad S, Moinuddin Preferential recognition of methylglyoxal-modified calf thymus DNA by circulating antibodies in cancer patients. Indian journal of biochemistry & biophysics. 2011;48(4):290–6. [PubMed] [Google Scholar]
- 16.Lin JA, Wu CH, Lu CC, Hsia SM, Yen GC. Glycative stress from advanced glycation end products (AGEs) and dicarbonyls: An emerging biological factor in cancer onset and progression. Molecular nutrition & food research. 2016 doi: 10.1002/mnfr.201500759. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed N. Advanced glycation endproducts--role in pathology of diabetic complications. Diabetes research and clinical practice. 2005;67(1):3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Ashraf JM, Shahab U, Tabrez S, Lee EJ, Choi I, Aslam Yusuf M, Ahmad S. DNA Glycation from 3-Deoxyglucosone Leads to the Formation of AGEs: Potential Role in Cancer Auto-antibodies. Cell biochemistry and biophysics. 2016;74(1):67–77. doi: 10.1007/s12013-015-0713-6. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad S, Moinuddin, Dixit K, Shahab U, Alam K, Ali A. Genotoxicity and immunogenicity of DNA-advanced glycation end products formed by methylglyoxal and lysine in presence of Cu2+ Biochemical and biophysical research communications. 2011;407(3):568–74. doi: 10.1016/j.bbrc.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 20.Koch M, Chitayat S, Dattilo BM, Schiefner A, Diez J, Chazin WJ, Fritz G. Structural basis for ligand recognition and activation of RAGE. Structure. 2010;18(10):1342–52. doi: 10.1016/j.str.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sessa L, Gatti E, Zeni F, Antonelli A, Catucci A, Koch M, Pompilio G, Fritz G, Raucci A, Bianchi ME. The receptor for advanced glycation end-products (RAGE) is only present in mammals, and belongs to a family of cell adhesion molecules (CAMs) PloS one. 2014;9(1):e86903. doi: 10.1371/journal.pone.0086903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chuah YK, Basir R, Talib H, Tie TH, Nordin N. Receptor for advanced glycation end products and its involvement in inflammatory diseases. International journal of inflammation. 2013;2013:403460. doi: 10.1155/2013/403460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue J, Rai V, Singer D, Chabierski S, Xie J, Reverdatto S, Burz DS, Schmidt AM, Hoffmann R, Shekhtman A. Advanced glycation end product recognition by the receptor for AGEs. Structure. 2011;19(5):722–32. doi: 10.1016/j.str.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee EJ, Park JH. Receptor for Advanced Glycation Endproducts (RAGE), Its Ligands and Soluble RAGE: Potential Biomarkers for Diagnosis and Therapeutic Targets for Human Renal Diseases. Genomics & informatics. 2013;11(4):224–9. doi: 10.5808/GI.2013.11.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fritz G. RAGE: a single receptor fits multiple ligands. Trends in biochemical sciences. 2011;36(12):625–32. doi: 10.1016/j.tibs.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Jiao L, Chen L, Alsarraj A, Ramsey D, Duan Z, El-Serag HB. Plasma soluble receptor for advanced glycation end-products and risk of colorectal adenoma. International journal of molecular epidemiology and genetics. 2012;3(4):294–304. [PMC free article] [PubMed] [Google Scholar]
- 27.Yaser AM, Huang Y, Zhou RR, Hu GS, Xiao MF, Huang ZB, Duan CJ, Tian W, Tang DL, Fan XG. The Role of receptor for Advanced Glycation End Products (RAGE) in the proliferation of hepatocellular carcinoma. International journal of molecular sciences. 2012;13(5):5982–97. doi: 10.3390/ijms13055982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie J, Mendez JD, Mendez-Valenzuela V, Aguilar-Hernandez MM. Cellular signalling of the receptor for advanced glycation end products (RAGE) Cellular signalling. 2013;25(11):2185–97. doi: 10.1016/j.cellsig.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Yamagishi S, Matsui T, Fukami K. Role of receptor for advanced glycation end products (RAGE) and its ligands in cancer risk. Rejuvenation research. 2015;18(1):48–56. doi: 10.1089/rej.2014.1625. [DOI] [PubMed] [Google Scholar]
- 30.Lee EJ, Kim JY, Oh SH. Advanced glycation end products (AGEs) promote melanogenesis through receptor for AGEs. Scientific reports. 2016;6:27848. doi: 10.1038/srep27848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko SY, Ko HA, Shieh TM, Chang WC, Chen HI, Chang SS, Lin IH. Cell migration is regulated by AGE-RAGE interaction in human oral cancer cells in vitro. PloS one. 2014;9(10):e110542. doi: 10.1371/journal.pone.0110542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lata K, Mukherjee TK. Knockdown of receptor for advanced glycation end products attenuate 17alpha-ethinyl-estradiol dependent proliferation and survival of MCF-7 breast cancer cells. Biochimica et biophysica acta. 2014;1840(3):1083–91. doi: 10.1016/j.bbagen.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S. Metformin inhibits advanced glycation end products (AGEs)-induced growth and VEGF expression in MCF-7 breast cancer cells by suppressing AGEs receptor expression via AMP-activated protein kinase. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2013;45(5):387–90. doi: 10.1055/s-0032-1331204. [DOI] [PubMed] [Google Scholar]
- 34.Wang D, Li T, Ye G, Shen Z, Hu Y, Mou T, Yu J, Li S, Liu H, Li G. Overexpression of the Receptor for Advanced Glycation Endproducts (RAGE) is associated with poor prognosis in gastric cancer. PloS one. 2015;10(4):e0122697. doi: 10.1371/journal.pone.0122697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong SY, Takeuchi M, Hyogo H, McKeown-Eyssen G, Yamagishi S, Chayama K, O’Brien PJ, Ferrari P, Overvad K, Olsen A, Tjonneland A, Boutron-Ruault MC, Bastide N, Carbonnel F, Kuhn T, Kaaks R, Boeing H, Aleksandrova K, Trichopoulou A, Lagiou P, Vasilopoulou E, Masala G, Pala V, Santucci De Magistris M, Tumino R, Naccarati A, Bueno-de-Mesquita HB, Peeters PH, Weiderpass E, Quiros JR, Jakszyn P, Sanchez MJ, Dorronsoro M, Gavrila D, Ardanaz E, Rutegard M, Nystrom H, Wareham NJ, Khaw KT, Bradbury KE, Romieu I, Freisling H, Stavropoulou F, Gunter MJ, Cross AJ, Riboli E, Jenab M, Bruce WR. The Association between Glyceraldehyde-Derived Advanced Glycation End-Products Colorectal Cancer Risk, Cancer epidemiology biomarkers & prevention : a publication of the American Association for Cancer Research. cosponsored by the American Society of Preventive Oncology. 2015;24(12):1855–63. doi: 10.1158/1055-9965.EPI-15-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang H, Zhong Y, Zhou S, Peng L. Knockdown of RAGE expression inhibits colorectal cancer cell invasion and suppresses angiogenesis in vitro and in vivo. Cancer letters. 2011;313(1):91–8. doi: 10.1016/j.canlet.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 37.Grote VA, Nieters A, Kaaks R, Tjonneland A, Roswall N, Overvad K, Nielsen MR, Clavel-Chapelon F, Boutron-Ruault MC, Racine A, Teucher B, Lukanova A, Boeing H, Drogan D, Trichopoulou A, Trichopoulos D, Lagiou P, Palli D, Sieri S, Tumino R, Vineis P, Mattiello A, Arguelles Suarez MV, Duell EJ, Sanchez MJ, Dorronsoro M, Huerta Castano JM, Barricarte A, Jeurnink SM, Peeters PH, Sund M, Ye W, Regner S, Lindkvist B, Khaw KT, Wareham N, Allen NE, Crowe FL, Fedirko V, Jenab M, Romaguera D, Siddiq A, Bueno-de-Mesquita HB, Rohrmann S. The associations of advanced glycation end products its soluble receptor with pancreatic cancer risk: a case-control study within the prospective EPIC Cohort Cancer epidemiology biomarkers & prevention : a publication of the American Association for Cancer Research. cosponsored by the American Society of Preventive Oncology. 2012;21(4):619–28. doi: 10.1158/1055-9965.EPI-11-1139. [DOI] [PubMed] [Google Scholar]
- 38.Leclerc E, Vetter SW. The role of S100 proteins and their receptor RAGE in pancreatic cancer. Biochimica et biophysica acta. 2015;1852(12):2706–11. doi: 10.1016/j.bbadis.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heijmans J, Buller NV, Hoff E, Dihal AA, van der Poll T, van Zoelen MA, Bierhaus A, Biemond I, Hardwick JC, Hommes DW, Muncan V, van den Brink GR. Rage signalling promotes intestinal tumourigenesis. Oncogene. 2013;32(9):1202–6. doi: 10.1038/onc.2012.119. [DOI] [PubMed] [Google Scholar]
- 40.Yang S, Pinney SM, Mallick P, Ho SM, Bracken B, Wu T. Impact of Oxidative Stress Biomarkers and Carboxymethyllysine (an Advanced Glycation End Product) on Prostate Cancer: A Prospective Study. Clinical genitourinary cancer. 2015;13(5):e347–51. doi: 10.1016/j.clgc.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bao JM, He MY, Liu YW, Lu YJ, Hong YQ, Luo HH, Ren ZL, Zhao SC, Jiang Y. AGE/RAGE/Akt pathway contributes to prostate cancer cell proliferation by promoting Rb phosphorylation and degradation. American journal of cancer research. 2015;5(5):1741–50. [PMC free article] [PubMed] [Google Scholar]
- 42.Lin L, Zhong K, Sun Z, Wu G, Ding G. Receptor for advanced glycation end products (RAGE) partially mediates HMGB1-ERKs activation in clear cell renal cell carcinoma. Journal of cancer research and clinical oncology. 2012;138(1):11–22. doi: 10.1007/s00432-011-1067-0. [DOI] [PubMed] [Google Scholar]
- 43.Kim JY, Park HK, Yoon JS, Kim SJ, Kim ES, Ahn KS, Kim DS, Yoon SS, Kim BK, Lee YY. Advanced glycation end product (AGE)-induced proliferation of HEL cells via receptor for AGE-related signal pathways. International journal of oncology. 2008;33(3):493–501. [PubMed] [Google Scholar]
- 44.Elgamal OA, Park JK, Gusev Y, Azevedo-Pouly AC, Jiang J, Roopra A, Schmittgen TD. Tumor suppressive function of mir-205 in breast cancer is linked to HMGB3 regulation. PloS one. 2013;8(10):e76402. doi: 10.1371/journal.pone.0076402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma M, Guo X, Chang Y, Li C, Meng X, Li S, Du ZX, Wang HQ, Sun Y. Advanced glycation end products promote proliferation and suppress autophagy via reduction of Cathepsin D in rat vascular smooth muscle cells. Molecular and cellular biochemistry. 2015;403(1–2):73–83. doi: 10.1007/s11010-015-2338-x. [DOI] [PubMed] [Google Scholar]
- 46.Nasser MW, Wani NA, Ahirwar DK, Powell CA, Ravi J, Elbaz M, Zhao H, Padilla L, Zhang X, Shilo K, Ostrowski M, Shapiro C, Carson WE, 3rd, Ganju RK. RAGE mediates S100A7-induced breast cancer growth and metastasis by modulating the tumor microenvironment. Cancer research. 2015;75(6):974–85. doi: 10.1158/0008-5472.CAN-14-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hou X, Hu Z, Xu H, Xu J, Zhang S, Zhong Y, He X, Wang N. Advanced glycation endproducts trigger autophagy in cadiomyocyte via RAGE/PI3K/AKT/mTOR pathway. Cardiovascular diabetology. 2014;13:78. doi: 10.1186/1475-2840-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen YJ, Chan DC, Chiang CK, Wang CC, Yang TH, Lan KC, Chao SC, Tsai KS, Yang RS, Liu SH. Advanced glycation end-products induced VEGF production and inflammatory responses in human synoviocytes via RAGE-NF-kappaB pathway activation. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2016;34(5):791–800. doi: 10.1002/jor.23083. [DOI] [PubMed] [Google Scholar]
- 49.White E. The role for autophagy in cancer. The Journal of clinical investigation. 2015;125(1):42–6. doi: 10.1172/JCI73941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marino G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nature reviews. Molecular cell biology. 2014;15(2):81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. American journal of physiology, Endocrinology and metabolism. 2001;280(5):E685–94. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- 52.Kang R, Tang D, Livesey KM, Schapiro NE, Lotze MT, Zeh HJ., 3rd The Receptor for Advanced Glycation End-products (RAGE) protects pancreatic tumor cells against oxidative injury. Antioxidants & redox signaling. 2011;15(8):2175–84. doi: 10.1089/ars.2010.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davalos AR, Coppe JP, Campisi J, Desprez PY. Senescent cells as a source of inflammatory factors for tumor progression. Cancer metastasis reviews. 2010;29(2):273–83. doi: 10.1007/s10555-010-9220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gorgoulis VG, Halazonetis TD. Oncogene-induced senescence: the bright and dark side of the response. Current opinion in cell biology. 2010;22(6):816–27. doi: 10.1016/j.ceb.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 55.Munoz-Espin D, Serrano M. Cellular senescence: from physiology to pathology. Nature reviews. Molecular cell biology. 2014;15(7):482–96. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 56.Burton DG, Krizhanovsky V. Physiological and pathological consequences of cellular senescence. Cellular and molecular life sciences : CMLS. 2014;71(22):4373–86. doi: 10.1007/s00018-014-1691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Georgakopoulou EA, Tsimaratou K, Evangelou K, Fernandez Marcos PJ, Zoumpourlis V, Trougakos IP, Kletsas D, Bartek J, Serrano M, Gorgoulis VG. Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence. A method applicable in cryo-preserved and archival tissues. Aging. 2013;5(1):37–50. doi: 10.18632/aging.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liakou E, Mavrogonatou E, Pratsinis H, Rizou S, Evangelou K, Panagiotou PN, Karamanos NK, Gorgoulis VG, Kletsas D. Ionizing radiation-mediated premature senescence and paracrine interactions with cancer cells enhance the expression of syndecan 1 in human breast stromal fibroblasts: the role of TGF-beta. Aging. 2016;8(8):1650–69. doi: 10.18632/aging.100989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, Takaoka M, Nakagawa H, Tort F, Fugger K, Johansson F, Sehested M, Andersen CL, Dyrskjot L, Orntoft T, Lukas J, Kittas C, Helleday T, Halazonetis TD, Bartek J, Gorgoulis VG. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444(7119):633–7. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 60.Liontos M, Koutsami M, Sideridou M, Evangelou K, Kletsas D, Levy B, Kotsinas A, Nahum O, Zoumpourlis V, Kouloukoussa M, Lygerou Z, Taraviras S, Kittas C, Bartkova J, Papavassiliou AG, Bartek J, Halazonetis TD, Gorgoulis VG. Deregulated overexpression of hCdt1 and hCdc6 promotes malignant behavior. Cancer research. 2007;67(22):10899–909. doi: 10.1158/0008-5472.CAN-07-2837. [DOI] [PubMed] [Google Scholar]
- 61.Halazonetis TD, Gorgoulis VG, Bartek J. An oncogene-induced DNA damage model for cancer development. Science. 2008;319(5868):1352–5. doi: 10.1126/science.1140735. [DOI] [PubMed] [Google Scholar]
- 62.Liontos M, Niforou K, Velimezi G, Vougas K, Evangelou K, Apostolopoulou K, Vrtel R, Damalas A, Kontovazenitis P, Kotsinas A, Zoumpourlis V, Tsangaris GT, Kittas C, Ginsberg D, Halazonetis TD, Bartek J, Gorgoulis VG. Modulation of the E2F1-driven cancer cell fate by the DNA damage response machinery and potential novel E2F1 targets in osteosarcomas. The American journal of pathology. 2009;175(1):376–91. doi: 10.2353/ajpath.2009.081160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodier F, Campisi J. Four faces of cellular senescence. The Journal of cell biology. 2011;192(4):547–56. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Helman A, Klochendler A, Azazmeh N, Gabai Y, Horwitz E, Anzi S, Swisa A, Condiotti R, Granit RZ, Nevo Y, Fixler Y, Shreibman D, Zamir A, Tornovsky-Babeay S, Dai C, Glaser B, Powers AC, Shapiro AM, Magnuson MA, Dor Y, Ben-Porath I. p16(Ink4a)-induced senescence of pancreatic beta cells enhances insulin secretion. Nature medicine. 2016;22(4):412–20. doi: 10.1038/nm.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vasile E, Tomita Y, Brown LF, Kocher O, Dvorak HF. Differential expression of thymosin beta-10 by early passage and senescent vascular endothelium is modulated by VPF/VEGF: evidence for senescent endothelial cells in vivo at sites of atherosclerosis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15(2):458–66. doi: 10.1096/fj.00-0051com. [DOI] [PubMed] [Google Scholar]
- 66.Gorgoulis VG, Pratsinis H, Zacharatos P, Demoliou C, Sigala F, Asimacopoulos PJ, Papavassiliou AG, Kletsas D. p53-dependent ICAM-1 overexpression in senescent human cells identified in atherosclerotic lesions. Laboratory investigation; a journal of technical methods and pathology. 2005;85(4):502–11. doi: 10.1038/labinvest.3700241. [DOI] [PubMed] [Google Scholar]
- 67.Sturmlechner I, Durik M, Sieben CJ, Baker DJ, van Deursen JM. Cellular senescence in renal ageing and disease. Nature reviews. Nephrology. 2017;13(2):77–89. doi: 10.1038/nrneph.2016.183. [DOI] [PubMed] [Google Scholar]
- 68.Price JS, Waters JG, Darrah C, Pennington C, Edwards DR, Donell ST, Clark IM. The role of chondrocyte senescence in osteoarthritis. Aging cell. 2002;1(1):57–65. doi: 10.1046/j.1474-9728.2002.00008.x. [DOI] [PubMed] [Google Scholar]
- 69.McShea A, Harris PL, Webster KR, Wahl AF, Smith MA. Abnormal expression of the cell cycle regulators P16 and CDK4 in Alzheimer’s disease. The American journal of pathology. 1997;150(6):1933–9. [PMC free article] [PubMed] [Google Scholar]
- 70.Dimri GP. What has senescence got to do with cancer? Cancer cell. 2005;7(6):505–12. doi: 10.1016/j.ccr.2005.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leal JF, Fominaya J, Cascon A, Guijarro MV, Blanco-Aparicio C, Lleonart M, Castro ME, Ramon YCS, Robledo M, Beach DH, Carnero A. Cellular senescence bypass screen identifies new putative tumor suppressor genes. Oncogene. 2008;27(14):1961–70. doi: 10.1038/sj.onc.1210846. [DOI] [PubMed] [Google Scholar]
- 72.Liu J, Huang K, Cai GY, Chen XM, Yang JR, Lin LR, Yang J, Huo BG, Zhan J, He YN. Receptor for advanced glycation end-products promotes premature senescence of proximal tubular epithelial cells via activation of endoplasmic reticulum stress-dependent p21 signaling. Cellular signalling. 2014;26(1):110–21. doi: 10.1016/j.cellsig.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 73.Abe R, Shimizu T, Sugawara H, Watanabe H, Nakamura H, Choei H, Sasaki N, Yamagishi S, Takeuchi M, Shimizu H. Regulation of human melanoma growth and metastasis by AGE-AGE receptor interactions. The Journal of investigative dermatology. 2004;122(2):461–7. doi: 10.1046/j.0022-202X.2004.22218.x. [DOI] [PubMed] [Google Scholar]
- 74.Kang R, Loux T, Tang D, Schapiro NE, Vernon P, Livesey KM, Krasinskas A, Lotze MT, Zeh HJ., 3rd The expression of the receptor for advanced glycation endproducts (RAGE) is permissive for early pancreatic neoplasia. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(18):7031–6. doi: 10.1073/pnas.1113865109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoffmann S, Friedrichs U, Eichler W, Rosenthal A, Wiedemann P. Advanced glycation end products induce choroidal endothelial cell proliferation matrix metalloproteinase-2 and VEGF upregulation in vitro. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2002;240(12):996–1002. doi: 10.1007/s00417-002-0568-6. [DOI] [PubMed] [Google Scholar]
- 76.Yamagishi S, Yonekura H, Yamamoto Y, Katsuno K, Sato F, Mita I, Ooka H, Satozawa N, Kawakami T, Nomura M, Yamamoto H. Advanced glycation end products-driven angiogenesis in vitro. Induction of the growth and tube formation of human microvascular endothelial cells through autocrine vascular endothelial growth factor. The Journal of biological chemistry. 1997;272(13):8723–30. doi: 10.1074/jbc.272.13.8723. [DOI] [PubMed] [Google Scholar]
- 77.Wang Q, Fan A, Yuan Y, Chen L, Guo X, Huang X, Huang Q. Role of Moesin in Advanced Glycation End Products-Induced Angiogenesis of Human Umbilical Vein Endothelial Cells. Scientific reports. 2016;6:22749. doi: 10.1038/srep22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takino J, Yamagishi S, Takeuchi M. Glycer-AGEs-RAGE signaling enhances the angiogenic potential of hepatocellular carcinoma by upregulating VEGF expression. World journal of gastroenterology. 2012;18(15):1781–8. doi: 10.3748/wjg.v18.i15.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsuji A, Wakisaka N, Kondo S, Murono S, Furukawa M, Yoshizaki T. Induction of receptor for advanced glycation end products by EBV latent membrane protein 1 and its correlation with angiogenesis and cervical lymph node metastasis in nasopharyngeal carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(17):5368–75. doi: 10.1158/1078-0432.CCR-08-0198. [DOI] [PubMed] [Google Scholar]
- 80.Rojas A, Morales MA. Advanced glycation and endothelial functions: a link towards vascular complications in diabetes. Life sciences. 2004;76(7):715–30. doi: 10.1016/j.lfs.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Q, Jin Y, Zhao CF, Wang WJ, Liu GY. Receptor for advanced glycation end-products (RAGE) is overexpressed in human osteosarcoma and promotes the proliferation of osteosarcoma U-2OS cells in vitro. Genetics and molecular research : GMR. 2016;15(2) doi: 10.4238/gmr.15027817. [DOI] [PubMed] [Google Scholar]
- 82.Masson N, Ratcliffe PJ. Hypoxia signaling pathways in cancer metabolism: the importance of co-selecting interconnected physiological pathways. Cancer & metabolism. 2014;2(1):3. doi: 10.1186/2049-3002-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nature reviews. Cancer. 2011;11(6):393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 84.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nature reviews. Cancer. 2004;4(6):437–47. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 85.Kizaka-Kondoh S, Inoue M, Harada H, Hiraoka M. Tumor hypoxia: a target for selective cancer therapy. Cancer science. 2003;94(12):1021–8. doi: 10.1111/j.1349-7006.2003.tb01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Semenza GL. Hypoxia and cancer. Cancer metastasis reviews. 2007;26(2):223–4. doi: 10.1007/s10555-007-9058-y. [DOI] [PubMed] [Google Scholar]
- 87.Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression angiogenesis, metastasisand resistance to therapy. Hypoxia. 2015;3:83–92. doi: 10.2147/HP.S93413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gopal P, Gosker HR, Theije CC, Eurlings IM, Sell DR, Monnier VM, Reynaert NL. Effect of chronic hypoxia on RAGE and its soluble forms in lungs and plasma of mice. Biochimica et biophysica acta. 2015;1852(5):992–1000. doi: 10.1016/j.bbadis.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Molecular cancer. 2013;12:152. doi: 10.1186/1476-4598-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Allaman I, Belanger M, Magistretti PJ. Methylglyoxal, the dark side of glycolysis. Frontiers in neuroscience. 2015;9:23. doi: 10.3389/fnins.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shinohara M, Thornalley PJ, Giardino I, Beisswenger P, Thorpe SR, Onorato J, Brownlee M. Overexpression of glyoxalase-I in bovine endothelial cells inhibits intracellular advanced glycation endproduct formation and prevents hyperglycemia-induced increases in macromolecular endocytosis. The Journal of clinical investigation. 1998;101(5):1142–7. doi: 10.1172/JCI119885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chang JS, Wendt T, Qu W, Kong L, Zou YS, Schmidt AM, Yan SF. Oxygen deprivation triggers upregulation of early growth response-1 by the receptor for advanced glycation end products. Circulation research. 2008;102(8):905–13. doi: 10.1161/CIRCRESAHA.107.165308. [DOI] [PubMed] [Google Scholar]
- 93.Rojas A, Gonzalez I, Morales E, Perez-Castro R, Romero J, Figueroa H. Diabetes and cancer: Looking at the multiligand/RAGE axis. World journal of diabetes. 2011;2(7):108–13. doi: 10.4239/wjd.v2.i7.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu Y, Toure F, Qu W, Lin L, Song F, Shen X, Rosario R, Garcia J, Schmidt AM, Yan SF. Advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling and up-regulation of Egr-1 in hypoxic macrophages. The Journal of biological chemistry. 2010;285(30):23233–40. doi: 10.1074/jbc.M110.117457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bucciarelli LG, Kaneko M, Ananthakrishnan R, Harja E, Lee LK, Hwang YC, Lerner S, Bakr S, Li Q, Lu Y, Song F, Qu W, Gomez T, Zou YS, Yan SF, Schmidt AM, Ramasamy R. Receptor for advanced-glycation end products: key modulator of myocardial ischemic injury. Circulation. 2006;113(9):1226–34. doi: 10.1161/CIRCULATIONAHA.105.575993. [DOI] [PubMed] [Google Scholar]
- 96.Shin ES, Sorenson CM, Sheibani N. Diabetes and retinal vascular dysfunction. Journal of ophthalmic & vision research. 2014;9(3):362–73. doi: 10.4103/2008-322X.143378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 98.Ryu TY, Park J, Scherer PE. Hyperglycemia as a risk factor for cancer progression. Diabetes & metabolism journal. 2014;38(5):330–6. doi: 10.4093/dmj.2014.38.5.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S, Lin Y, Yao J, Shi J, Kang T, Lorkiewicz P, St Clair D, Hung MC, Evers BM, Zhou BP. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer cell. 2013;23(3):316–31. doi: 10.1016/j.ccr.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aleshin A, Ananthakrishnan R, Li Q, Rosario R, Lu Y, Qu W, Song F, Bakr S, Szabolcs M, D’Agati V, Liu R, Homma S, Schmidt AM, Yan SF, Ramasamy R. RAGE modulates myocardial injury consequent to LAD infarction via impact on JNK and STAT signaling in a murine model. American journal of physiology. Heart and circulatory physiology. 2008;294(4):H1823–32. doi: 10.1152/ajpheart.01210.2007. [DOI] [PubMed] [Google Scholar]
- 101.Hiwatashi K, Ueno S, Abeyama K, Kubo F, Sakoda M, Maruyama I, Hamanoue M, Natsugoe S, Aikou T. A novel function of the receptor for advanced glycation end-products (RAGE) in association with tumorigenesis and tumor differentiation of HCC. Annals of surgical oncology. 2008;15(3):923–33. doi: 10.1245/s10434-007-9698-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tafani M, Schito L, Pellegrini L, Villanova L, Marfe G, Anwar T, Rosa R, Indelicato M, Fini M, Pucci B, Russo MA. Hypoxia-increased RAGE and P2XR expression regulates tumor cell invasion through phosphorylation of Erk1/2 and Akt and nuclear translocation of NF-{kappa}B. Carcinogenesis. 2011;32(8):1167–75. doi: 10.1093/carcin/bgr101. [DOI] [PubMed] [Google Scholar]
- 103.Welford SM, Giaccia AJ. Hypoxia and senescence: the impact of oxygenation on tumor suppression. Molecular cancer research : MCR. 2011;9(5):538–44. doi: 10.1158/1541-7786.MCR-11-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taddei ML, Cavallini L, Comito G, Giannoni E, Folini M, Marini A, Gandellini P, Morandi A, Pintus G, Raspollini MR, Zaffaroni N, Chiarugi P. Senescent stroma promotes prostate cancer progression: the role of miR-210. Molecular oncology. 2014;8(8):1729–46. doi: 10.1016/j.molonc.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Masoud GN, Li W. HIF-1alpha pathway: role, regulation and intervention for cancer therapy. Acta pharmaceutica Sinica. B. 2015;5(5):378–89. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood reviews. 2013;27(1):41–53. doi: 10.1016/j.blre.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hubbi ME, Semenza GL. Regulation of cell proliferation by hypoxia-inducible factors. American journal of physiology. Cell physiology. 2015;309(12):C775–82. doi: 10.1152/ajpcell.00279.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tafani M, De Santis E, Coppola L, Perrone GA, Carnevale I, Russo A, Pucci B, Carpi A, Bizzarri M, Russo MA. Bridging hypoxia inflammation and estrogen receptors in thyroid cancer progression. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2014;68(1):1–5. doi: 10.1016/j.biopha.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 109.Wu D, Potluri N, Lu J, Kim Y, Rastinejad F. Structural integration in hypoxia-inducible factors. Nature. 2015;524(7565):303–8. doi: 10.1038/nature14883. [DOI] [PubMed] [Google Scholar]
- 110.Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiological reviews. 2012;92(3):967–1003. doi: 10.1152/physrev.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kang R, Hou W, Zhang Q, Chen R, Lee YJ, Bartlett DL, Lotze MT, Tang D, Zeh HJ. RAGE is essential for oncogenic KRAS-mediated hypoxic signaling in pancreatic cancer. Cell death & disease. 2014;5:e1480. doi: 10.1038/cddis.2014.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pichiule P, Chavez JC, Schmidt AM, Vannucci SJ. Hypoxia-inducible factor-1 mediates neuronal expression of the receptor for advanced glycation end products following hypoxia/ischemia. The Journal of biological chemistry. 2007;282(50):36330–40. doi: 10.1074/jbc.M706407200. [DOI] [PubMed] [Google Scholar]
- 113.Bondeva T, Heinzig J, Ruhe C, Wolf G. Advanced glycated end-products affect HIF-transcriptional activity in renal cells. Molecular endocrinology. 2013;27(11):1918–33. doi: 10.1210/me.2013-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hiwatashi K, Ueno S, Kubo F, Sakoda M, Tateno T, Hayashi T, Abeyama K, Natsugoe S. Relevance of apoptosis and tolerance to hypoxic stress in cells transfected with receptor for advanced glycation end products (RAGE) Anticancer research. 2009;29(4):1287–94. [PubMed] [Google Scholar]
- 115.Chen Y, Zhang Y, Ji H, Ji Y, Yang J, Huang J, Sun D. Involvement of hypoxia-inducible factor-1alpha in the oxidative stress induced by advanced glycation end products in murine Leydig cells. Toxicology in vitro : an international journal published in association with BIBRA. 2016;32:146–53. doi: 10.1016/j.tiv.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 116.Bala K, Gohil NK. Interaction of glycated protein and DFO mimicked hypoxia in cellular responses of HUVECs. Molecular bioSystems. 2012;8(10):2657–63. doi: 10.1039/c2mb25156f. [DOI] [PubMed] [Google Scholar]
- 117.Treins C, Giorgetti-Peraldi S, Murdaca J, Van Obberghen E. Regulation of vascular endothelial growth factor expression by advanced glycation end products. The Journal of biological chemistry. 2001;276(47):43836–41. doi: 10.1074/jbc.M106534200. [DOI] [PubMed] [Google Scholar]
- 118.Kilic Eren M, Tabor V. The role of hypoxia inducible factor-1 alpha in bypassing oncogene-induced senescence. PloS one. 2014;9(7):e101064. doi: 10.1371/journal.pone.0101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vlassara H, Uribarri J, Cai W, Striker G. Advanced glycation end product homeostasis: exogenous oxidants and innate defenses. Annals of the New York Academy of Sciences. 2008;1126:46–52. doi: 10.1196/annals.1433.055. [DOI] [PubMed] [Google Scholar]
- 120.De Vriese AS, Flyvbjerg A, Mortier S, Tilton RG, Lameire NH. Inhibition of the interaction of AGE-RAGE prevents hyperglycemia-induced fibrosis of the peritoneal membrane. Journal of the American Society of Nephrology : JASN. 2003;14(8):2109–18. doi: 10.1681/ASN.V1482109. [DOI] [PubMed] [Google Scholar]
- 121.Stirban A, Negrean M, Stratmann B, Gawlowski T, Horstmann T, Gotting C, Kleesiek K, Mueller-Roesel M, Koschinsky T, Uribarri J, Vlassara H, Tschoepe D. Benfotiamine prevents macro- and microvascular endothelial dysfunction and oxidative stress following a meal rich in advanced glycation end products in individuals with type 2 diabetes. Diabetes care. 2006;29(9):2064–71. doi: 10.2337/dc06-0531. [DOI] [PubMed] [Google Scholar]
- 122.Miyata T, Dan T. Inhibition of advanced glycation end products (AGEs): an implicit goal in clinical medicine for the treatment of diabetic nephropathy? Diabetes research and clinical practice. 2008;(82 Suppl 1):S25–9. doi: 10.1016/j.diabres.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 123.Forbes JM, Yee LT, Thallas V, Lassila M, Candido R, Jandeleit-Dahm KA, Thomas MC, Burns WC, Deemer EK, Thorpe SR, Cooper ME, Allen TJ. Advanced glycation end product interventions reduce diabetes-accelerated atherosclerosis. Diabetes. 2004;53(7):1813–23. doi: 10.2337/diabetes.53.7.1813. [DOI] [PubMed] [Google Scholar]
- 124.Maeda S, Matsui T, Takeuchi M, Yoshida Y, Yamakawa R, Fukami K, Yamagishi S. Pigment epithelium-derived factor (PEDF) inhibits proximal tubular cell injury in early diabetic nephropathy by suppressing advanced glycation end products (AGEs)-receptor (RAGE) axis. Pharmacological research. 2011;63(3):241–8. doi: 10.1016/j.phrs.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 125.Mizumoto S, Takahashi J, Sugahara K. Receptor for advanced glycation end products (RAGE) functions as receptor for specific sulfated glycosaminoglycans and anti-RAGE antibody or sulfated glycosaminoglycans delivered in vivo inhibit pulmonary metastasis of tumor cells. The Journal of biological chemistry. 2012;287(23):18985–94. doi: 10.1074/jbc.M111.313437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Elangovan I, Thirugnanam S, Chen A, Zheng G, Bosland MC, Kajdacsy-Balla A, Gnanasekar M. Targeting receptor for advanced glycation end products (RAGE) expression induces apoptosis and inhibits prostate tumor growth. Biochemical and biophysical research communications. 2012;417(4):1133–8. doi: 10.1016/j.bbrc.2011.12.060. [DOI] [PubMed] [Google Scholar]
- 127.Goldberg T, Cai W, Peppa M, Dardaine V, Baliga BS, Uribarri J, Vlassara H. Advanced glycoxidation end products in commonly consumed foods. Journal of the American Dietetic Association. 2004;104(8):1287–91. doi: 10.1016/j.jada.2004.05.214. [DOI] [PubMed] [Google Scholar]
- 128.Sharma C, Kaur A, Thind SS, Singh B, Raina S. Advanced glycation End-products (AGEs): an emerging concern for processed food industries. Journal of food science and technology. 2015;52(12):7561–76. doi: 10.1007/s13197-015-1851-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ashraf JM, Ahmad S, Choi I, Ahmad N, Farhan M, Tatyana G, Shahab U. Recent advances in detection of AGEs: Immunochemical bioanalytical and biochemical approaches. IUBMB life. 2015;67(12):897–913. doi: 10.1002/iub.1450. [DOI] [PubMed] [Google Scholar]
- 130.Shahab U, Tabrez S, Khan MS, Akhter F, Khan MS, Saeed M, Ahmad K, Srivastava AK, Ahmad S. Immunogenicity of DNA-advanced glycation end product fashioned through glyoxal and arginine in the presence of Fe(3)(+): its potential role in prompt recognition of diabetes mellitus auto-antibodies. Chemico-biological interactions. 2014;219:229–40. doi: 10.1016/j.cbi.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 131.Evangelou K, Lougiakis N, Rizou SV, Kotsinas A, Kletsas D, Munoz-Espin D, Kastrinakis NG, Pouli N, Marakos P, Townsend P, Serrano M, Bartek J, Gorgoulis VG. Robust, universal biomarker assay to detect senescent cells in biological specimens. Aging cell. 2017;16(1):192–197. doi: 10.1111/acel.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lan CC, Ko YC, Yu HS, Li WC, Wu CS, Lu YW, Yang YH, Chen GS. Psoriatic patients with diabetes are prone to develop digestive organ cancers: a population-based study in Taiwan. Journal of dermatological science. 2012;68(2):82–8. doi: 10.1016/j.jdermsci.2012.08.004. [DOI] [PubMed] [Google Scholar]