Abstract
Purpose
Adding preimplantation genetic screening to in vitro fertilization has been shown to increase live birth rate in women older than 37. However, preimplantation genetic screening is an expensive procedure. Information on the cost-effectiveness of preimplantation genetic screening can help inform clinical decision making.
Methods
We constructed a decision analytic model for a hypothetical fresh, autologous in vitro fertilization cycle (with versus without preimplantation genetic screening) for women older than age 37 who had a successful oocyte retrieval and development of at least one blastocyst. The model incorporated probability and cost estimates of relevant clinical events based on data from published literature. Sensitivity analyses were performed to examine the impact of changes in model input parameters.
Results
In base-case analysis, IVF-PGS offered a 4.2 percentage point increase in live birth rate for an additional cost of $4509, yielding an incremental cost-effectiveness ratio (ICER) of $105,489 per additional live birth. This ICER was below the expected cost of $145,063 for achieving one live birth with IVF (assuming an average LBR of 13.4% and $19,415 per cycle for this patient population). Sensitivity analysis suggested that ICER improved substantially with decreases in PGS cost and increases in PGS effectiveness. Monte Carlo simulation showed PGS to be cost-effective in 93.9% of iterations at an acceptability cutoff of $145,063.
Conclusions
Considering the expected cost of achieving one live birth with IVF, PGS is a cost-effective strategy for women older than 37 undergoing IVF. Additional research on patients’ willingness-to-pay per live birth would further inform our understanding regarding the cost-effectiveness of PGS.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-017-1001-8) contains supplementary material, which is available to authorized users.
Keywords: Cost-effectiveness, Preimplantation genetic screening, In vitro fertilization, Willingness-to-pay
Introduction
In vitro fertilization (IVF) is an effective tool for the treatment of infertility, with live birth rates per started cycle as high as 37.1% for women younger than age 35 [1]. However, it is increasingly common for women to delay childbearing into their late 30’s and early 40’s [2]. These women face the inevitable biological clock which translates to decreased live birth rates (LBR). IVF is largely insufficient to overcome age-related infertility, only achieving live birth rates of 19.5% for women age 38–40, 9.7% for women age 41–42, and 3.1% for women over age 42 [1]. The decreasing live birth rate with advancing maternal age has predominantly been attributed to diminishing ovarian reserve [3] and, most significantly, increased rates of aneuploidy in the oocytes of aging women [4].
To maximize the success of IVF, a variety of techniques have been employed to aid in the selection of the embryo which will yield the best chance for a subsequent live birth [5]. One widely used approach, preimplantation genetic screening (PGS), attempts to avoid selection of embryos with aneuploidy for transfer. In the early years of PGS, when genetic analysis was restricted to only a few chromosomes, a large randomized controlled trial showed no improvements in clinical pregnancy or live birth rates [6]. However, several important methodologic improvements over the past decade now allow the full complement of chromosomes to be assayed with greater accuracy [5]. A recent analysis of US-wide IVF cycles shows an increased live birth rate for women older than age 37 who use PGS, with an adjusted odds ratio of 1.43 (95% confidence interval, 1.26–1.62) [7].
While beneficial in this age group for the desired outcome of infertility treatment (i.e., live birth), PGS is costly and, at the current time, rarely covered by medical insurance. Information on the cost-effectiveness of PGS for women older than age 37 undergoing IVF can help patients, clinicians, and insurers in their decision regarding whether to utilize and pay for this new technology. Therefore, the purpose of this study is to develop a decision analytic model to analyze the cost-effectiveness of an IVF cycle with versus without PGS in improving live birth rates in this population.
Material and methods
Decision model
We constructed a decision analytic model for a hypothetical fresh, autologous IVF cycle with PGS versus IVF alone for women older than age 37 who had a successful oocyte retrieval and development of at least one blastocyst. The model incorporated main clinical events including PGS, blastocyst transfer, clinical pregnancy, miscarriage, pregnancy termination, and live birth (Fig. 1). Effectiveness is measured by live birth rate. The analysis was performed from a health care sector perspective and included all costs associated with the medical care of the relevant events. Because data for this study exclusively came from the published literature, it was exempt from review by our institutional review board.
Fig. 1.
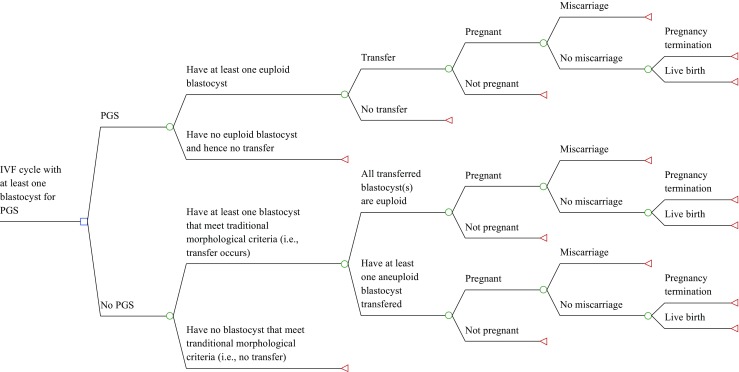
Decision tree model for a hypothetical fresh, autologous IVF cycle (with versus without genetic screening (PGS)) for women older than age 37 who had a successful oocyte retrieval and at least one blastocyst
The model began at the time point of having at least one blastocyst (i.e., after controlled ovarian hyperstimulation, oocyte retrieval, oocyte fertilization, and initial embryo culture). Because blastocyst biopsy is the standard of care for PGS in the USA [8], the availability of a blastocyst was considered a requirement for PGS to be utilized. Although the clinical decision of whether to use PGS is often made before beginning an IVF cycle, the clinical action of proceeding with PGS does not take place until this later time point. In the event that there are not enough embryos to culture to the blastocyst stage, an intention to treat with PGS would need to be abandoned; thus, for the purposes of a decision analytic model, the decision only happens after a blastocyst becomes available. Moreover, the probabilities and costs are equal between the treatment arms up until that time point, so the earlier components of IVF would not affect our assessment of the cost-effectiveness of PGS. Therefore, nothing is lost and model parsimony is gained by starting the model at this time point.
There are several key assumptions in the model. It was assumed that all IVF-PGS cycles with at least one euploid blastocyst would have an embryo transfer. We did not consider embryo cryopreservation and subsequent frozen embryo transfer after PGS, as data on the results of this strategy come from small, single-center trials [9] and may not be broadly applicable. We also assumed that an IVF alone cycle (without PGS) involved the transfer of at most two embryos; due to lack of detailed data, the probability of clinical pregnancy and miscarriage in such a cycle was assumed to be the same whether one or both of the transferred embryos were euploid. In addition, as intrauterine fetal demise is a rare event [7], we did not include it in our model. Finally, consistent with prior literature [10], it was assumed that all pregnant patients who did not miscarry would have an amniocentesis, and that all patients with evidence of aneuploidy would have genetic counseling and termination in the second trimester by dilation and evacuation. For simplicity, our model did not include chorionic villus sampling for prenatal diagnosis.
Parameter estimates
A thorough review of the literature was performed to identify base-case estimate and plausible range of the probabilities for the main clinical events in our model (Table 1) [7, 11–16]. For the majority of the base-case parameters, we used estimates from a recent study of all cycles in the US National ART Surveillance System performed in 2011 and 2012 [7]. This publication is the largest analysis of IVF-PGS cycles to date and includes data from nearly all IVF centers in the USA, making the findings more generalizable than the results of single-center analyses. Although the source publication does not contain detail on the PGS methodology employed for each cycle, the majority of PGS cycles in the studied time period employed array CGH. Our parameter estimates obtained from this study were based on women older than age 37 who had PGS for aneuploidy screening for an autologous IVF cycle, excluding those who had preimplantation genetic testing for other reasons (e.g., single-gene disorders or known parental chromosomal abnormality). Given the lack of multicenter data with more detailed age stratification, the base-case analysis was limited to all women older than age 37. However, single-center studies which included age stratifications were incorporated into the model when establishing minimum and maximum plausible values for probability parameters in sensitivity analyses [13, 14]. For probability parameters where no literature was available to guide the choice of minimum and maximum plausible values, we set these values at ±25% of the base-case estimate.
Table 1.
Estimates of probability-related parameters used to determine the cost-effectiveness of PGS for women older than 37 using IVF
| Parameter | Base-case estimate | Minimum value | Maximum value | Sources |
|---|---|---|---|---|
| Probability that a cycle has at least one euploid blastocyst (PGS arm) | 64.0% | 36.4% | 82.0% | [7, 11, 13] |
| Probability that a blastocyst called euploid by PGS is transferred | 100.0% | – | – | Authors’ Assumption |
| Probability that a blastocyst called aneuploid by PGS is transferred | 0.0% | – | – | Authors’ Assumption |
| Probability of live birth in a pregnancy that results from euploid blastocysts and is not miscarried | 99.8% | 99.0% | 100% | [7, 13] |
| Probability that at least one blastocyst meets traditional morphological criteria for transfer (non-PGS arm) | 66.0% | 49.5% | 94.5% | [7, 14] |
| Probability that only one blastocyst is transferred in a transferred cycle (non-PGS arm) | 11.4% | 8.6% | 14.3% | [7, 13] |
| Probability that a blastocyst selected for transfer by traditional morphological criteria is aneuploid (non-PGS arm) | 30.6% | 19.0% | 55.2% | [12, 15] |
| Adjusted odds ratio of clinical pregnancy in a PGS guided cycle relative to a non-PGS guided cycle | 1.18 | 1.05 | 3.86 | [7, 13] |
| Probability of clinical pregnancy in a non-PGS guided cycle | 42.5% | 31.9% | 49.5% | [7, 13] |
| Adjusted odds ratio of miscarriage (per pregnancy) in a PGS guided cycle relative to a non-PGS guided cycle | 0.55 | 0.32 | 0.70 | [7, 13] |
| Probability of miscarriage in a pregnancy resulting from a non-PGS guided cycle | 26.0% | 19.6% | 39.0% | [7, 13, 14, 16] |
| Probability of live birth in a pregnancy that results from a non-PGS guided cycle and is not miscarried | 97.8% | 97.3% | 99.0% | [7] |
Estimates of cost parameters were also determined from a review of the literature when possible (Table 2) [10, 17–20]. Of note, the cost estimate for management of miscarriage was based upon an expanded care model for early pregnancy loss, which incorporated expectant, medical, and surgical management options weighted by patient preferences [18]. Because our model begins after culture of embryos to blastocysts, our model did not use cost estimates for the entire IVF cycle [10, 17]. Rather, we estimated the cost of an embryo transfer separately based upon the price at our own institution charged to self-pay patients (Yale Fertility Center). All cost parameters were inflation adjusted to 2016 US dollars using the medical care component of the consumer price index [21]. Minimum and maximum plausible cost estimates were obtained from the literature when available or set at ±25% of the base-case cost.
Table 2.
Estimates of cost-related parameters (in 2016 US Dollars) used to determine the cost-effectiveness of PGS for women older than 37 using IVF
| Parameter | Base-case estimate | Minimum value | Maximum value | Sources |
|---|---|---|---|---|
| Cost of PGS | $4546 | $3410 | $13,449 | [17] |
| Cost of a fresh embryo transfer | $3025 | $2269 | $3782 | –a |
| Cost of miscarriage management | $1155 | $866 | $1443 | [18] |
| Cost of genetic counseling | $146 | $109 | $182 | [20] |
| Cost of amniocentesis | $1397 | $699 | $2794 | [10] |
| Cost of second trimester pregnancy termination | $1852 | $1358 | $2037 | [19] |
Minimum and maximum values were set at ±25% of the base-case value when no plausible range was available from the literature
aCost of fresh embryo transfer was based upon the cost at the authors’ institution (Yale Fertility Center)
Data analysis
We used the incremental cost-effectiveness ratio (ICER) to assess the additional cost associated with IVF-PGS (as compared to IVF alone) per additional live birth achieved. ICER was calculated by dividing the difference in expected cost by the difference in expected live birth rate between the two approaches, i.e., IVF-PGS versus IVF alone. In the absence of an established threshold for determining cost-effectiveness of interventions improving live birth rates, we calculated the expected cost for achieving one live birth with IVF alone for women older than age 37 to be $145,063 (assuming an average live birth rate of 13.4% per IVF cycle [1] and an average cost of $19,415 per IVF cycle [17]). We used this value as the threshold for evaluating the cost-effectiveness of IVF-PGS.
We performed both deterministic and probabilistic sensitivity analyses to assess the impact of uncertainty in parameter estimates on cost-effectiveness results. In deterministic sensitivity analysis, we varied each of the input parameters from their minimum to maximum values one at a time (one-way sensitivity analysis) and examined changes in the cost-effectiveness of IVF-PGS with decreasing PGS costs and increasing effectiveness for IVF-PGS at achieving clinical pregnancy (two-way sensitivity analysis). In addition, Monte Carlo simulation was performed, wherein all parameters were varied simultaneously with a triangular distribution bounded by the defined minimum and maximum values. One thousand iterations of the simulation were performed. Analysis was performed using TreeAge Pro 2013 (Williamstown, MA).
Results
In the base-case analysis, after the steps leading to the availability of at least one blastocyst, IVF-PGS had an expected cost of $6888, while IVF alone cost $2379, yielding a cost differential of $4509 (Table 3). The expected live birth rate associated with IVF-PGS was 4.2 percentage points higher than with IVF alone (24.9 vs. 20.7% per blastocyst-generating cycle), leading to an incremental cost-effectiveness ratio (ICER) of $105,489 for one additional live birth achieved with IVF-PGS (Table 3). Compared to the expected cost of $145,063 for women older than age 37 to achieve one live birth with IVF, IVF-PGS appears to be a cost-effective approach.
Table 3.
Base-case analysis of the cost-effectiveness of IVF-PGS versus IVF alone for women older than 37 who had a successful oocyte retrieval and at least one blastocyst
| Expected live birth rate | Expected costa | |
|---|---|---|
| IVF-PGS | 24.9% | $6887.83 |
| IVF alone | 20.7% | $2378.67 |
| Incremental | 4.2% | $4509.16 |
| Incremental cost-effectiveness ratio (ICER) | $105,488.59 |
aExpected cost reflects cost after generation of at least one blastocyst (i.e. not including costs associated with stimulation/monitoring, retrieval, fertilization, or culture)
One-way sensitivity analyses suggested that the estimated ICER was most influenced by the cost of PGS, the odds ratio of IVF-PGS (versus IVF alone) for achieving clinical pregnancy and avoiding miscarriage, the probability of having a euploid embryo in a PGS cycle, and the probability of having a transferrable embryo in a non-PGS cycle (Supplemental Fig. 1). For each of these parameters, variation in values within its expected range resulted in instances of ICER greater than $145,063, the threshold for cost-effectiveness in our model, suggesting that the cost-effectiveness of IVF-PGS is sensitive to each of these parameters.
Two-way sensitivity analysis was performed to simultaneously vary the value of two key parameters: PGS cost and the odds ratio of IVF-PGS (versus IVF alone) for achieving clinical pregnancy (Supplemental Fig. 2). Decreases in PGS cost and increases in PGS effectiveness both improved the estimated ICER. At the minimum modeled value for PGS cost, $3410, and the maximum modeled value for odds ratio of clinical pregnancy for IVF-PGS (versus IVF alone), 3.86, the ICER improved to $21,350 for an additional live birth.
To further assess the impact of uncertainty in the probability and cost parameters on our findings, we performed Monte Carlo simulation. Results from the 1000 iterations of the simulation were presented in a cost-effectiveness plane (Fig. 2a) and cost-effectiveness acceptability curve (Fig. 2b). When using $145,063 as the cutoff, IVF-PGS was cost-effective in 93.9% of the iterations. In comparison, when using a cost-effectiveness acceptability level of $52,074, IVF-PGS was cost-effective in 50% of the iterations.
Fig. 2.
Results of Monte Carlo simulation. a The cost-effectiveness plane, with each dot reflecting the incremental cost and incremental live birth rate between IVF-PGS and IVF alone from one iteration of the simulation (n = 1000 iterations).b The cost-effectiveness acceptability curve, reflecting the percentage of iterations where IVF-PGS was more cost-effective than IVF alone at different levels of willingness-to-pay thresholds
Discussion
Infertility treatments are costly and, in the USA, most couples are financially responsible for these costs. Therefore, newer treatments that afford an improvement in live birth rates have to be balanced with the cost and be subjected to an unbiased cost-effectiveness analysis. Our analysis is the first to evaluate the cost-effectiveness of modern PGS approaches, drawing on recent evidence of its effectiveness from a large nationwide sample [7]. For women older than 37 years of age who undergo IVF and produce enough quality embryos to have the option of PGS, our decision analytic model shows that, compared with IVF alone, the use of PGS is expected to have an extra cost of $105,489 for one additional live birth achieved. When evaluated at an acceptability threshold of $145,063, PGS is a cost-effective approach to improve live birth rate, and 93.9% of the iterations in Monte Carlo simulation were in favor of PGS as a cost-effective approach.
For treatment of infertility, there is no established threshold for determining the cost-effectiveness of an additional live birth achieved [17, 22, 23]. Although many CEAs continue to use a threshold of $50,000 per quality-adjusted life-year (QALY), there is scant data on QALY for health states related to treatment of infertility. Moreover, a cutoff of $50,000 per QALY has come under scrutiny as rigid and outdated, with many scholars decrying its near-universal application [24]. Many studies report willingness-to-pay much higher than $50,000 per QALY [25]. Prior fertility-related CEAs have selected arbitrary thresholds of $50,000 or $100,000 per live birth, citing parallelism to QALY-based cost-effectiveness research [17, 23]. In this study, we based our cost-effectiveness acceptability cutoff ($145,063) upon the average cost of achieving live birth with IVF alone in women older than 37. One earlier study, albeit a little outdated, showed an even higher willingness-to-pay of $167,906 per live birth (inflation adjusted 2016 US dollars) when surveying women [22], suggesting that it is reasonable to consider such a high cost-effectiveness threshold. It is imperative that further research be performed to better understand the amount various stakeholders, such as insurers and patients, are willing to pay for interventions to increase the probability of live birth.
Notably, our cost-effectiveness analysis was performed from a health care sector perspective. Although it was recently recommended that CEAs be performed from both the health care sector perspective and the societal perspective [26], the lack of high-quality data on patients’ and their spouses’ productivity losses associated with IVF treatments precluded us from accurately modeling costs from a societal perspective. As the PGS approach helps improve live birth rate and hence reduce productivity loss associated with additional IVF cycles, our analysis presents a conservative assessment for the overall cost-effectiveness of PGS. Further research collecting detailed information on productivity loss associated with IVF treatments will enable more comprehensive evaluation of the cost-effectiveness of interventions from societal perspective.
Given our finding that PGS is a cost-effective approach for achieving live birth among women older than age 37, incorporation of PGS in IVF cycles of these women may be considered in clinical care. In the USA in 2011 and 2012, only 7.8% of IVF cycles in this age group used PGS [7]. Previous literature suggests that cost is one of the most significant determinants of whether or not patients use PGS [27]. Although the absolute cost of the PGS procedure is still high, findings from our study regarding the expected incremental cost per additional live birth achieved may be useful when counseling patients about this option to help them make informed decisions. Additionally, insurers who provide IVF coverage may consider paying for PGS for this patient population, given that the expected cost per additional live birth achieved with PGS is below the expected cost of IVF alone for achieving one live birth.
The parameters identified as most influential in determining the cost-effectiveness of PGS in our model, such as the effectiveness of IVF-PGS (versus IVF alone) at both achieving clinical pregnancy and avoiding miscarriage, point to key areas for further research to help us better understand the cost-effectiveness of this evolving technology. Many questions have emerged in recent years about the robustness of PGS for aneuploidy detection, due to reports of euploid pregnancies after false positive results and the increased detection of mosaicism with modern PGS approaches [28–30]. Additionally, the false negative rate of PGS, although reportedly low [31], remains unclear. Resolving these questions remains an essential goal in future PGS research. The ideal study to refine these parameters would be a randomized controlled trial using modern PGS approaches in women of the age group of interest, such as the ongoing STAR trial (NCT02268786, clinicaltrials.gov).
Another area of future research which can further improve our understanding of the cost implications of PGS would be the potential benefit of PGS for improving success of elective single embryo transfer (eSET) cycles. While the use of IVF-PGS itself does not appear to impact the rates of multiple gestation [7], IVF-PGS with eSET has been proposed as a strategy to minimize the rates of multiple gestations without sacrificing live birth rates [32, 33]. If this hypothesis is supported with age-stratified data comparing IVF-PGS with eSET both to IVF alone with eSET and to IVF alone with double embryo transfer, there will be additional health benefit and cost savings attributable to a PGS-based strategy for avoiding undesired and costly outcomes of multiple pregnancies.
An additional aspect of fertility treatment which is in need of further exploration is the impact of PGS on cumulative live birth rates from multiple embryo transfers from a single IVF stimulation cycle. In an era of effective embryo cryopreservation, it has been argued that PGS cannot improve the cumulative outcomes of stepwise transfer of the entire embryonic cohort, for it does not improve (but rather identify) the chromosomal content of the embryos in a pool [34]. A recent randomized controlled trial by Rubio and colleagues substantiates this claim, showing no difference in cumulative live birth rate between IVF-PGS and IVF alone cycles, but it also shows that PGS can significantly reduce the miscarriage rate and decrease the time to live birth [16]. As this small study (approximately 100 couples per treatment arm) is the first to rigorously examine the questions of cumulative live birth rate and time-to-live-birth, the generalizability of its findings is yet to be established. Further research on these distinct PGS outcomes would be informative and can help facilitate rigorous modeling of these aspects of PGS in future cost-effectiveness analyses.
Our model has several limitations to consider. First, we obtained parameter estimates from the published literature which may not always include the exact patient population we intended. For example, many of our parameter values were based on the Chang et al. study which included recurrent pregnancy loss patients in the IVF-PGS arm [7]; these patients’ responses may not be applicable to patients using IVF for infertility alone. However, our findings were robust across a range of parameter values as shown in our thorough sensitivity analysis, strongly suggesting that the cost-effectiveness of the approach would be maintained even if the technique is less effective in specific subpopulations. Second, although our model used adjusted odds ratios to measure the effects of PGS versus no PGS approaches to reduce potential confounding from baseline patient characteristics, our parameter estimates did not account for potential differences in IVF response by race and BMI, which have been shown to influence IVF success rates [35, 36]. Third, due to a lack of conclusive data on its impact on live birth rate [9], our decision analytic model did not include the option of IVF-PGS cycles in which embryos are frozen after biopsy and then transferred in a subsequent cycle. This strategy is the preferred approach of some practitioners for all of their IVF patients [9], and it is often chosen out of necessity if blastocyst biopsy is performed [37]. As more conclusive data emerges on the relative effectiveness of frozen/thawed embryo transfers in IVF-PGS cycles, it will be clinically informative to model the cost-effectiveness of such an approach; although there is a known increase in cost for IVF with a frozen/thaw cycle [9], the impact on live birth rate must be first clarified to inform such a cost-effectiveness analysis.
The implementation of expensive technologies in healthcare, even when shown to offer an incremental improvement to outcomes, requires careful consideration of their relative cost-effectiveness. Our decision analytic model suggests that considering the expected cost of achieving one live birth with IVF, PGS is a cost-effective approach to improve live birth rate in women older than age 37 using IVF. Further research into the appropriate threshold values to use in evaluating cost-effectiveness of infertility treatments, as well as additional studies to refine the key probability parameters and to provide evidence for the impact of frozen embryo cycles would help improve future assessments of the cost-effectiveness of using PGS to guide embryo selection in IVF cycles.
Electronic supplementary material
The tornado diagram shows change in estimated incremental cost-effectiveness ratio (ICER) of IVF-PGS compared to IVF without PGS when each model input parameter was varied from its minimum value to maximum value (one-way sensitivity analysis). (PDF 199 kb)
Two-way Sensitivity Analysis. This figure shows change in estimated incremental cost-effectiveness ratio (ICER) of IVF-PGS compared to IVF without PGS the cost of PGS and the odds ratio of pregnancy with IVF-PGS (vs. PGS alone) were varied simultaneously. (PDF 209 kb)
Acknowledgements
We thank Ms. Jeani Chang (CDC) and Dr. Vanessa Dalton for kindly sharing unpublished data from their manuscripts for inclusion in our analysis.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
This study was presented in preliminary form at the 2017 American College of Obstetrics and Gynecology Annual Clinical and Scientific Meeting, San Diego, CA.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-017-1001-8) contains supplementary material, which is available to authorized users.
References
- 1.Centers for Disease Control and Prevention ASfRM, Society for Assisted Reproductive Technology, 2014 Assisted Reproductive Technology National Summary Report. In: US Dept of Health and Human Services, editor. Atlanta (GA)2016.
- 2.Mathews T, Hamilton BE. Mean age of mothers is on the rise: United States, 2000-2014. NCHS data brief. 2016(232):1–8. [PubMed]
- 3.American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and the Practice Committee of the American Society of Reproductive Medicine Female age-related fertility decline. Committee opinion no. 589. Fertil Steril. 2014;101(3):633–634. doi: 10.1016/j.fertnstert.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 4.Munne S, Alikani M, Tomkin G, Grifo J, Cohen J. Embryo morphology, developmental rates, and maternal age are correlated with chromosome abnormalities. Fertil Steril. 1995;64(2):382–391. doi: 10.1016/S0015-0282(16)57739-5. [DOI] [PubMed] [Google Scholar]
- 5.Gardner DK, Meseguer M, Rubio C, Treff NR. Diagnosis of human preimplantation embryo viability. Hum Reprod Update. 2015;21(6):727–747. doi: 10.1093/humupd/dmu064. [DOI] [PubMed] [Google Scholar]
- 6.Mastenbroek S, Twisk M, van Echten-Arends J, Sikkema-Raddatz B, Korevaar JC, Verhoeve HR, et al. In vitro fertilization with preimplantation genetic screening. N Engl J Med. 2007;357(1):9–17. doi: 10.1056/NEJMoa067744. [DOI] [PubMed] [Google Scholar]
- 7.Chang J, Boulet SL, Jeng G, Flowers L, Kissin DM. Outcomes of in vitro fertilization with preimplantation genetic diagnosis: an analysis of the United States Assisted Reproductive Technology Surveillance Data, 2011-2012. Fertil Steril. 2016;105(2):394–400. doi: 10.1016/j.fertnstert.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott KL, Hong KH, Scott RT., Jr Selecting the optimal time to perform biopsy for preimplantation genetic testing. Fertil Steril. 2013;100(3):608–614. doi: 10.1016/j.fertnstert.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Coates A, Kung A, Mounts E, Hesla J, Bankowski B, Barbieri E, et al. Optimal euploid embryo transfer strategy, fresh versus frozen, after preimplantation genetic screening with next generation sequencing: a randomized controlled trial. Fertil Steril 2017;107(3):723–730 e3. [DOI] [PubMed]
- 10.Mersereau JE, Plunkett BA, Cedars MI. Preimplantation genetic screening in older women: a cost-effectiveness analysis. Fertil Steril. 2008;90(3):592–598. doi: 10.1016/j.fertnstert.2007.07.1307. [DOI] [PubMed] [Google Scholar]
- 11.Demko ZP, Simon AL, McCoy RC, Petrov DA, Rabinowitz M. Effects of maternal age on euploidy rates in a large cohort of embryos analyzed with 24-chromosome single-nucleotide polymorphism–based preimplantation genetic screening. Fertil Steril. 2016;105(5):1307–1313. doi: 10.1016/j.fertnstert.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 12.Forman EJ, Upham KM, Cheng M, Zhao T, Hong KH, Treff NR, et al. Comprehensive chromosome screening alters traditional morphology-based embryo selection: a prospective study of 100 consecutive cycles of planned fresh euploid blastocyst transfer. Fertil Steril. 2013;100(3):718–724. doi: 10.1016/j.fertnstert.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 13.Kang H-J, Melnick AP, Stewart JD, Xu K, Rosenwaks Z. Preimplantation genetic screening: who benefits? Fertil Steril. 2016; [DOI] [PubMed]
- 14.Lee HL, McCulloh DH, Hodes-Wertz B, Adler A, McCaffrey C, Grifo JA. In vitro fertilization with preimplantation genetic screening improves implantation and live birth in women age 40 through 43. J Assist Reprod Genet. 2015;32(3):435–444. doi: 10.1007/s10815-014-0417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fragouli E, Alfarawati S, Spath K, Wells D. Morphological and cytogenetic assessment of cleavage and blastocyst stage embryos. Mol Hum Reprod. 2014;20(2):117–126. doi: 10.1093/molehr/gat073. [DOI] [PubMed] [Google Scholar]
- 16.Rubio C, Bellver J, Rodrigo L, Castillon G, Guillen A, Vidal C, et al. In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertil Steril. 2017;107(5):1122–1129. doi: 10.1016/j.fertnstert.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Murugappan G, Ohno MS, Lathi RB. Cost-effectiveness analysis of preimplantation genetic screening and in vitro fertilization versus expectant management in patients with unexplained recurrent pregnancy loss. Fertil Steril. 2015;103(5):1215–1220. doi: 10.1016/j.fertnstert.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Dalton VK, Liang A, Hutton DW, Zochowski MK, Fendrick AM. Beyond usual care: the economic consequences of expanding treatment options in early pregnancy loss. Am J Obstet Gynecol. 2015;212(2):177.e1–177.e6. doi: 10.1016/j.ajog.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 19.Jones RK, Kooistra K. Abortion incidence and access to services in the United States, 2008. Perspect Sex Reprod Health. 2011;43(1):41–50. doi: 10.1363/4304111. [DOI] [PubMed] [Google Scholar]
- 20.Evans M, Sonek J, Hallahan T, Krantz D. Cell-free fetal DNA screening in the USA: a cost analysis of screening strategies. Ultrasound Obstet Gynecol. 2015;45(1):74–83. doi: 10.1002/uog.14693. [DOI] [PubMed] [Google Scholar]
- 21.Statistics BoL. Consumer Price Index: Bureau of Labor Statistics; [March 24, 2017]. Available from: http://www.bls.gov/cpi/.
- 22.Neumann PJ, Johannesson M. The willingness to pay for in vitro fertilization: a pilot study using contingent valuation. Med Care. 1994;32(7):686–699. doi: 10.1097/00005650-199407000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Resetkova N, Humm K, Penzias A, Sakkas D, Lannon B. IVF proves cost effective compared to minimal stimulation embryo transfer, but minimal stimulation is a cost neutral alternative for women under age 35. Fertil Steril. 2015;104(3):e205. doi: 10.1016/j.fertnstert.2015.07.637. [DOI] [Google Scholar]
- 24.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 25.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Mak. 2000;20(3):332–342. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 26.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 27.Gebhart MB, Hines RS, Penman A, Holland AC. How do patient perceived determinants influence the decision-making process to accept or decline preimplantation genetic screening? Fertil Steril. 2016;105(1):188–193. doi: 10.1016/j.fertnstert.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 28.Gleicher N, Vidali A, Braverman J, Kushnir VA, Barad DH, Hudson C, et al. Accuracy of preimplantation genetic screening (PGS) is compromised by degree of mosaicism of human embryos. Reprod Biol Endocrinol. 2016;14 [DOI] [PMC free article] [PubMed]
- 29.Greco E, Minasi MG, Fiorentino F. Healthy babies after intrauterine transfer of mosaic aneuploid blastocysts. N Engl J Med. 2015;373(21):2089–2090. doi: 10.1056/NEJMc1500421. [DOI] [PubMed] [Google Scholar]
- 30.Munne S, Grifo J, Wells D. Mosaicism: “survival of the fittest” versus “no embryo left behind”. Fertil Steril. 2016;105(5):1146–1149. doi: 10.1016/j.fertnstert.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Kung A, Munne S, Bankowski B, Coates A, Wells D. Validation of next-generation sequencing for comprehensive chromosome screening of embryos. Reprod BioMed Online. 2015;31(6):760–769. doi: 10.1016/j.rbmo.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Ubaldi FM, Capalbo A, Colamaria S, Ferrero S, Maggiulli R, Vajta G, et al. Reduction of multiple pregnancies in the advanced maternal age population after implementation of an elective single embryo transfer policy coupled with enhanced embryo selection: pre- and post-intervention study. Hum Reprod. 2015;30(9):2097–2106. doi: 10.1093/humrep/dev159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forman EJ, Hong KH, Franasiak JM, Scott RT., Jr Obstetrical and neonatal outcomes from the BEST Trial: single embryo transfer with aneuploidy screening improves outcomes after in vitro fertilization without compromising delivery rates. Am J Obstet Gynecol. 2014;210(2):157e1–157e6. doi: 10.1016/j.ajog.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Cedars MI. Fresh versus frozen: initial transfer or cumulative cycle results: how do we interpret results and design studies? Fertil Steril. 2016;106(2):251–256. doi: 10.1016/j.fertnstert.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Provost MP, Acharya KS, Acharya CR, Yeh JS, Steward RG, Eaton JL, et al. Pregnancy outcomes decline with increasing body mass index: analysis of 239,127 fresh autologous in vitro fertilization cycles from the 2008-2010 Society for Assisted Reproductive Technology registry. Fertil Steril. 2016;105(3):663–669. doi: 10.1016/j.fertnstert.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Humphries LA, Chang O, Humm K, Sakkas D, Hacker MR. Influence of race and ethnicity on in vitro fertilization outcomes: systematic review. Am J Obstet Gynecol. 2016;214(2):212 e1–212e17. doi: 10.1016/j.ajog.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Sermon K, Capalbo A, Cohen J, Coonen E, De Rycke M, De Vos A, et al. The why, the how and the when of PGS 2.0: current practices and expert opinions of fertility specialists, molecular biologists, and embryologists. Mol Hum Reprod. 2016;22(8):845–857. doi: 10.1093/molehr/gaw034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The tornado diagram shows change in estimated incremental cost-effectiveness ratio (ICER) of IVF-PGS compared to IVF without PGS when each model input parameter was varied from its minimum value to maximum value (one-way sensitivity analysis). (PDF 199 kb)
Two-way Sensitivity Analysis. This figure shows change in estimated incremental cost-effectiveness ratio (ICER) of IVF-PGS compared to IVF without PGS the cost of PGS and the odds ratio of pregnancy with IVF-PGS (vs. PGS alone) were varied simultaneously. (PDF 209 kb)



