Abstract
Purpose
The purpose of this study is to examine the spectrum of infertility diagnoses and assisted reproductive technology (ART) treatments in relation to risk of preterm birth (PTB) in singletons.
Methods
Population-based assisted reproductive technology surveillance data for 2000–2010 were linked with birth certificates from three states: Florida, Massachusetts, and Michigan, resulting in a sample of 4,370,361 non-ART and 28,430 ART-related singletons. Logistic regression models with robust variance estimators were used to compare PTB risk among singletons conceived with and without ART, the former grouped by parental infertility diagnoses and treatment modalities. Demographic and pregnancy factors were included in adjusted analyses.
Results
ART was associated with increased PTB risk across all infertility diagnosis groups and treatment types: for conventional ART, adjusted relative risks ranged from 1.4 (95% CI 1.0, 1.9) for male infertility to 2.4 (95% CI 1.8, 3.3) for tubal ligation. Adding intra-cytoplasmic sperm injection and/or assisted hatching to conventional ART treatment did not alter associated PTB risks. Singletons conceived by mothers without infertility diagnosis and with donor semen had an increased PTB risk relative to non-ART singletons.
Conclusions
PTB risk among ART singletons is increased within each treatment type and all underlying infertility diagnosis, including male infertility. Preterm birth in ART singletons may be attributed to parental infertility, ART treatments, or their combination.
Keywords: Assisted reproductive technology, Infertility, Preterm birth, Intra-cytoplasmic sperm injection, Assisted hatching, Donor oocytes or embryos
Introduction
Assisted reproductive technology (ART), a group of infertility treatments, involves the retrieval of gametes and their handling in the laboratory to achieve fertilization outside or inside the body. To maximize pregnancy rate, conventional ART treatment is often integrated with additional techniques, e.g., the use of donor gametes/embryos, transfer of frozen embryos, intra-cytoplasmic sperm injection (ICSI, insertion of a single sperm into an oocyte), and assisted hatching (AH, creation of a hole in the embryo’s zona pellucida to promote embryonic implantation). The modality of ART regimen utilized depends on the couples’ characteristics, infertility diagnosis, treatment history, and financial factors.
Studies have consistently detected an increased risk of shorter gestation and smaller newborn size among ART singletons compared with non-ART singletons [1–3]. Explanations for these findings are subject to debate. It is unclear if the increased risk is present irrespective of the ART techniques employed. Additionally, there is the challenge of disentangling excess ART-associated risk and excess risk due to underlying causes of infertility. Studies attempting to separate the ART treatment effect from the infertility effect have found modest increases in risks of preterm birth (PTB) and small newborn size among singletons born to infertile couples who eventually conceived without ART [4, 5]. While there are reports of PTB among singletons in association with either ART modalities or parental infertility diagnosis [6–10], this study considers simultaneously infertility diagnosis, treatment modalities, and gametes/embryos source. Further, we sought to estimate the risk of PTB attributed to ART treatments, in the absence of parental infertility. Therefore, we performed a three-state population-based retrospective cohort study to examine associations among infertility diagnoses, ART treatment modality, gametes/embryos source, and PTB in singletons.
Methods
Study population
The States Monitoring Assisted Reproductive Technology (SMART) collaborative monitors and studies ART-associated health outcomes within participating states [11]. The SMART collaborative includes the Centers for Disease Control and Prevention (CDC) and the Connecticut, Florida, Massachusetts, and Michigan Public Health agencies. CDC constructed a population-based dataset that linked ART data from the National ART Surveillance System (NASS) with records of all live births occurring in Massachusetts, Florida, and Michigan in 2000–2010 [12]. The probabilistic method successfully linked NASS and birth records with high linkage rate (87.8%) and good sensitivity (96.4) [11, 12]. We restricted this dataset to women aged 15–60 with singleton births between 22 and 44 weeks’ gestation; this represents a viable live birth range, and excludes the plurality effect on adverse birth outcomes. To control for differential birth outcomes among singletons from cryopreserved vs. fresh ART cycles, we excluded ART singletons born from cryopreserved embryos transfer.
ART and infertility
Three “exposure” variables were evaluated in relation to preterm birth: (1) infertility diagnosis, including one single infertility diagnosis (male infertility, unexplained infertility, endometriosis, diminished ovarian reserve, tubal disease, tubal ligation, ovulation disorders, uterine factor, or other infertility factor [immunologic, chromosomal, cancer chemotherapy, or other systemic disease]), or multiple diagnoses, i.e., any combination of those listed above; (2) treatment type, including ART/conventional, ART/ICSI, ART/AH, and ART/ICSI/AH);(3) gamete source, i.e., donor oocyte/embryo and donor semen.
Preterm birth
Data on gestational age at delivery are from birth certificates. Preterm birth (PTB) was defined as delivery before 37 completed weeks’ gestation, based on clinical estimate. This study obtained approval from the Institutional Review Boards of the Public Health agencies of participating states and the CDC.
Statistical analysis
Descriptive statistics, chi square, and linear regression for complex data were used to compare the maternal and infant characteristics between ART and non-ART groups. We applied statistical methodology for clustered data, e.g., generalized estimating equations (GEE), to account for more than one singleton birth to the same woman.
Associations among infertility diagnoses, ART treatment types, and PTB were examined using regression models with GEE. Risk ratios for PTB were estimated from average marginal predictions in the fitted logistic regression models [13]. We obtained crude and adjusted estimates from models with parity, maternal age, race/ethnicity, education level, state of residence, and delivery year as covariates. PTB was modeled as a binary outcome (yes/no).
The initial set of analyses compared PTB risk between non-ART and all ART singletons conceived with fresh embryos and grouped by couples’ infertility diagnoses and treatment modalities (conventional, ICSI, AH, and combined ICSI/AH). We repeated this analysis separating donor and non-donor embryos/oocytes. Our reference group for these analyses was non-ART singletons. Finally, in an effort to separate ART treatment effects from infertility effects, we focused on a specific ART group, i.e., women without an infertility diagnosis who conceived by use of partner or donor semen. In this last set of analyses, the referent group was ART singletons conceived by couples with male infertility using autologous semen; this allowed us to assess whether semen source, donor versus partner, influences PTB risk in ART singletons.
SUDAAN 11 (RTI) statistical software was used throughout the analyses to generate logistic and linear regression models.
Sensitivity analyses
To examine whether the extremes of maternal age influenced our results [14], we restricted the analyses to singletons born to women aged 21–44. ART therapy often involves the transfer of multiple embryos and first trimester loss of a co-twin has been linked to adverse birth outcomes among ART singletons [15, 16]. Therefore, in a second sensitivity analysis, we investigated the impact of early fetal loss on the surviving singleton by excluding all singleton births with a co-twin observed by an ultrasound at 6 weeks’ gestation.
Results
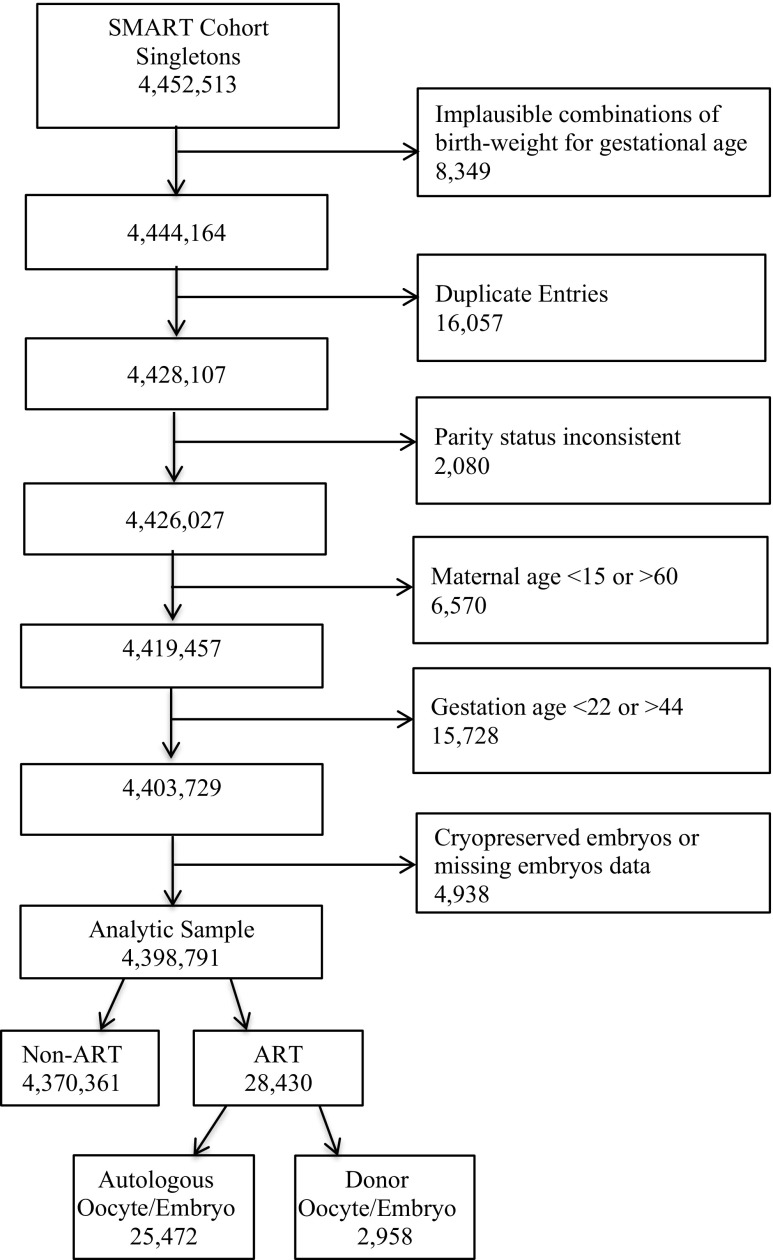
Our sample included 4,370,361 non-ART and 28,430 ART-associated singletons (Fig. 1). ART mothers of singletons were more likely to be non-Hispanic white, older, primipara, and to have attained a higher education level relative to non-ART mothers. Compared with non-ART singletons, a significantly higher percentage of ART singletons were born preterm (Table 1).
Fig. 1.
Flow chart of participants in the States Monitoring Assisted Reproductive Technology (SMART) dataset: Florida, Massachusetts, and Michigan, 2000–2010
Table 1.
Maternal and infant characteristics of ART and non-ART singleton live births in three states: Massachusetts, Michigan, and Florida, 2000–2010
| Maternal, infant characteristics | Non-ART | ART | P valuea |
|---|---|---|---|
| Sample size N (%) | 4,370,361 (99.3) | 28,430 (0.7) | <0.01 |
| Mean maternal age (SE) | 27.7 (0.003) | 35.5 (0.03) | <0.01 |
| Maternal race/ethnicityb | |||
| Non-Hispanic White | 2,520,523 (58) | 22,786 (81) | |
| Non-Hispanic Black | 779,578 (18) | 1061 (4) | |
| Hispanic | 811,492(19) | 2391 (9) | <0.01 |
| Asian/other | 226, 770 (5) | 1769 (6) | |
| Maternal educationb | |||
| High school or lower | 2,087,123 (48) | 3667 (13) | |
| Some college | 1,078,329 (25) | 5853 (21) | <0.01 |
| Bachelor’s or higher | 1,166,001 (27) | 18,759 (66) | |
| Parity | |||
| 0 | 1,822,354 (42) | 19,236 (68) | |
| 1 | 1,426,203 (33) | 6831 (24) | <0.01 |
| 2 | 686,514 (16) | 1563 (6) | |
| ≥ 3 | 419,992(10) | 711 (3) | |
| Newborn sex | |||
| Male | 2,238,639 (51) | 14,607 (51) | 0.6 |
| Female | 2,131,722 (49) | 13,823 (49) | |
| PTB <37 weeks | 25,172 (8) | 3258 (11) | |
| Term birth ≥37 weeks | 4,019,669 (92) | 350,692 (89) | <0.01 |
| State | |||
| Massachusetts | 812,962 (19) | 14,240 (50) | |
| Michigan | 1,306,547 (30) | 5179 (18) | <0.01 |
| Florida | 2,250,852 (52) | 9011 (32) | |
ART assisted reproductive technology, PTB preterm birth, SE standard error
a P value computed for complex data
b<1% missing
In this sample of ART singletons conceived from fresh embryos, the adjusted relative risk (aRR) of PTB was significantly increased for ART infants in most infertility subgroups and treatment types, compared with non-ART infants (Table 2). In the conventional ART group, an increased aRR for PTB was observed for all infertility diagnoses, with a range of 1.4 for male infertility to 2.4 for tubal ligation infertility. Within ART singletons, a significantly increased PTB risk was observed among those born to couples with tubal ligation compared with singletons of couples with endometriosis or male infertility. ART/ICSI, ART/AH, or ART with combined ICSI/AH were associated with more than twofold increase in PTB risk among singletons born to mothers with uterine factor infertility compared with non-ART singletons. Nonetheless, almost all treatment-diagnosis combinations were associated with increased risk of PTB in ART vs. non-ART singletons.
Table 2.
Relative risk of preterm birth among singleton live births in three states, Massachusetts, Michigan, and Florida, 2000–2010: includes non-ART referent and fresh embryo ART cycles stratified by infertility diagnosis and ART treatment type
| ART/infertility diagnosis | N (PTB %) | ART conventional (n = 9166) | ART ICSI (n = 11,180) | ART ICSI/AH (n = 5024) | |||
|---|---|---|---|---|---|---|---|
| Relative risk crude (CI) | Relative risk adjusteda (CI) | Relative risk crude (CI) | Relative risk adjusteda (CI) | Relative risk crude (CI) | Relative risk adjusteda (CI) | ||
| Non-ART | 4,370,361 (8) | Referent | Referent | Referent | Referent | Referent | Referent |
| Female infertility | |||||||
| Endometriosis | 1637 (10) | 1.3 (1.1, 1.7) | 1.6 (1.3, 2.0) | 1.2 (0.9, 1.6) | 1.4 (1.0, 1.8) | 1.5 (1.0, 2.1) | 1.7 (1.2, 2.5) |
| Diminished ovarian reserve | 2028 (14) | 1.6 (1.3, 1.9) | 1.7 (1.4, 2.1) | 2.1 (1.8, 2.5) | 2.1 (1.8, 2.5) | 1.4 (1.0, 2.0) | 1.5 (1.1, 2.1) |
| Tubal disease | 2697 (13) | 1.6 (1.4, 1.9) | 1.8 (1.6, 2.1) | 1.6 (1.3, 2.0) | 1.7 (1.3, 2.1) | 1.6 (1.2, 2.2) | 1.7 (1.3, 2.4) |
| Tubal ligation | 400 (15) | 2.5 (1.8, 3.3) | 2.4 (1.8, 3.3) | 1.1 (0.6, 1.9) | 0.8 (0.4, 1.6) | 1.1 (0.5, 2.9) | 1.1 (0.4, 2.7) |
| Ovulation disorder | 1681 (14) | 1.7 (1.4, 2.0) | 2.1 (1.7, 2.4) | 1.8 (1.5, 2.2) | 2.0 (1.6, 2.4) | 2.0 (1.4, 2.8) | 2.3 (1.6, 3.2) |
| Uterine factor | 252 (15) | 1.4 (0.8, 2.4) | 1.7 (1.0, 2.8) | 2.5 (1.5, 4.1) | 2.7 (1.7, 4.5) | 2.1 (0.9, 5.0) | 2.5 (1.0, 5.8) |
| Other | 2289 (12) | 1.4 (1.2, 1.7) | 1.8 (1.5, 2.1) | 1.7 (1.4, 2.1) | 1.9 (1.5, 2.3) | 1.5 (1.1, 2.1) | 1.8 (1.3, 2.5) |
| Male infertility | 6111 (9) | 1.1 (0.8, 1.5) | 1.4 (1.0, 1.9) | 1.2 (1.1, 1.4) | 1.4 (1.3, 1.6) | 1.0 (0.9, 1.2) | 1.2 (1.1, 1.5) |
| Unexplained infertility | 3964 (10) | 1.1 (1.0, 1.3) | 1.4 (1.2, 1.6) | 1.4 (1.1, 1.7) | 1.6 (1.3, 2.0) | 1.4 (1.0, 1.9) | 1.7 (1.2, 2.2) |
| > 1 infertility diagnosis | 7371 (12) | 1.5 (1.3, 1.7) | 1.7 (1.5, 2.0) | 1.6 (1.4, 1.7) | 1.7 (1.5, 1.9) | 1.5 (1.3, 1.7) | 1.6 (1.4, 1.8) |
aAdjusted for parity, age, race/ethnicity, education level, state of residence, and delivery year
ART assisted reproductive technology, PTB preterm birth, ICSI intra-cytoplasmic sperm injection, AH assisted hatching, CI confidence interval
Of the 28,430 ART singletons, 25,472 were conceived from autologous embryos/oocytes and 2958 from donor oocytes/embryos. After removing donor oocytes/embryos singletons, we observed that the PTB risk pattern in the remaining autologous embryos/oocytes singletons grouped by infertility diagnoses and ART treatments groups was similar to that of all ART singletons (data not shown). Due to small numbers, we could not adequately evaluate the donor oocytes/embryos singletons separately. The one exception was the group with diminished ovarian reserve; the risk of PTB among ART singletons conceived with donor oocyte/embryo by mothers with diminished ovarian diagnosis was significantly increased compared with non-ART singletons [OR = 1.9, 95% CI (1.5, 2.3)].
A separate analysis assessed ART singletons conceived by subfertile couples with male infertility but without female infertility diagnosis. This group had a 40% increased PTB risk, regardless of semen source (partner, donor), compared with non-ART singletons (Table 3).
Table 3.
Relative risk of preterm birth among singleton live births in three states, Massachusetts, Michigan, and Florida, 2000–2010: includes non-ART referent and ART mothers without female infertility diagnosis stratified by semen source
| ART/male infertility by semen source | N (% PTB) | Relative risk crude (CI) | Relative risk adjusted (CI)a |
|---|---|---|---|
| Non-ART | 4,370,361 (8) | Reference | Reference |
| ART/partner | 5857 (9) | 1.2 (1.1, 1.3) | 1.4 (1.3, 1.5) |
| ART/donor | 217 (10) | 1.3 (0.9, 1.9) | 1.4 (1.0, 2.1) |
aAdjusted for parity, age, race/ethnicity, education level, state of residence and delivery year
ART assisted reproductive technology, PTB preterm birth, CI confidence interval, singletons of subfertile male with missing semen source data were excluded (n = 1013)
Sensitivity analyses
The above results were essentially unchanged when maternal age was restricted to 21–44, or when singleton births that started as multi-fetal pregnancies were excluded.
Discussion
In this large, population-based dataset of live births, ART singletons grouped by both parental infertility diagnoses and ART treatment modalities had an increased risk of PTB compared with non-ART singletons. Increased PTB risk persisted after removing singletons with donor oocyte/embryo. Singletons born to couples with male infertility, but without female infertility diagnosis, also had an increased PTB risk, regardless of semen source (autologous or donor). The latter finding suggests the contribution of ART treatments to PTB risk for singletons conceived by couples absent of parental infertility.
A limited number of studies have investigated whether the heterogeneity of infertility diagnoses and treatments among ART populations is associated with the risk of poorer birth outcomes. One study observed a significantly increased number of PTB deliveries, above the expected, among ART singletons born to women with tubal factor infertility [17]. ART singletons born to couples with female infertility in general [18], or tubal factor infertility in particular [7], had an increased PTB risk compared with those born to couples with male infertility. Among all ART births, singletons and multiples, an increased PTB risk was associated with male factor, ovulation disorders, tubal inflammation, but not with endometriosis [9]. Only one study investigated PTB risk among singletons conceived with different ART treatment types (fresh, frozen, ICSI), but parental infertility diagnosis was not accounted for [18].
This study considered birth outcomes across infertility groups exposed to different ART techniques. Conventional ART cycles are often combined with ICSI, AH techniques, or both to improve the likelihood of success. In previous studies, ART/ICSI treatments were found to be associated with higher PTB rates and smaller newborn size compared with non-ART group [19–22]. However, these studies were not able to control for confounders such as cycle type (autologous/donor or fresh/frozen) and infertility diagnosis. One study investigated the added PTB risk of ICSI relative to the conventional ART among singletons and reported a protective effect for ICSI, but since ART therapies with fresh or frozen embryos were not assessed separately, the protective effect may have been driven by frozen cycles [22]. Our study is unique in its modeling of conventional ART therapy, ICSI, AH, and their combination among fresh/autologous cycles, to examine PTB risk across parental infertility diagnoses.
ART with donor gametes is the recommended treatment for patients with poor ovarian reserve, poor oocyte quality, and/or severe male infertility [23]. Previous reports hypothesized that oocyte donation induces immunological responses that may play a role in higher rates of hypertensive disorders and subsequent PTB in ART pregnancies with donated compared with autologous oocytes [24–26]. One small study observed an increased PTB risk among ART singletons conceived with donor oocytes compared with ART autologous oocytes [aOR 1.8] or compared with non-ART singletons [aOR 3.4] [27]. Perhaps due to its small sample size, this study did not further classify treatments as fresh or frozen embryo ART cycles. In our population-based study, we restricted the sample to fresh embryos and grouped ART cycles by technique type and oocytes/embryos source. The multivariate models controlled for several maternal and pregnancy characteristics. Our results suggest that ART singletons conceived with autologous or donor embryos/oocytes had increased PTB risk compared with non-ART singletons. However, in the donor stratum, small cell sizes in some treatment-diagnosis combinations resulted in insufficient power to estimate PTB risk among ART relative to non-ART singletons.
There is limited evidence of donor male gametes’ effects on PTB risk. A recent study did not detect significantly increased risks for PTB or small newborn size among singletons born following an intrauterine insemination (IUI) using donor compared with partner semen [28]. However, compared with ART singletons, IUI-conceived singletons had a lower PTB risk [28]. Within ART populations, similar crude risks of LBW or PTB were found among infants conceived with donor compared with partner sperm [29].
We were interested in sperm source for two reasons: (1) to assess associations with PTB and (2) to potentially separate an infertility effect from an ART effect. Our comparison included the non-ART group and the ART group diagnosed with male but not female infertility. By specifically examining ART cycles that use donor sperm in women with no reported infertility diagnoses, we reasoned that we have removed any excess PTB risk associated with female infertility and what remains is the PTB risk associated with this particular ART technology. Our results suggested that among ART users, semen source did not change the risk of PTB. Most important, we found that in the absence of parental infertility ART singletons conceived with gametes of fertile individuals had an increased risk for PTB compared with non-ART singletons; this suggests that when not confounded by underlying infertility, the ART treatment still confers excess PTB risk.
Our results remained robust to sensitivity analyses that investigated the influence of younger and older maternal age, or co-twin early loss on the risk of PTB. By restricting the analytic sample to mothers’ age 21–44, we obtained comparable maternal age distributions in the ART and non-ART groups. Further, we controlled for the impact of extremes of maternal age on the risk of preterm birth [30]. Similarly, exclusion of singleton births that originated from multi-fetal pregnancies removed morbidity associated with early loss of a co-twin that might impact comparisons between ART and non-ART subgroups [16].
The use of a large population-level ART surveillance data is a major strength of this study. This comprehensive dataset represents more than 97% of US-based ART cycles. Only few small clinics did not submit their data to NASS [31]. The large sample size and detailed ART treatment information provided sufficient power to examine PTB risk among subgroups of infertility diagnosis and treatment types, and to control for confounding related to ART treatment type by restricting our sample to fresh ART cycles.
This study also had limitations worth noting. We evaluated overall risk of PTB and did not have information to distinguish or separately assess spontaneous and medically indicated PTB. Data on previous PTB were also unavailable for the study period. We could not ascertain subfertility in the non-ART population, non-ART treatments, such as intra-uterine insemination, oral or injectable medications, or ART treatment pursued by this group out of state. Thus our effect sizes may be slightly biased, most likely attenuated. Finally, although the probabilistic linkage method for matching ART recipients to birth certificates achieved a high linkage rate (87.8%) and good validity, it is not error free [12].
Conclusions
These data indicate a significantly increased risk of PTB among ART singletons across most parental infertility diagnoses and treatment modalities. Our findings support the hypothesis that infertility may explain some of the ART association with PTB. We also found that, among ART singletons, even autologous oocytes/embryos had an increased PTB risk compared with non-ART singletons. Finally, the increased PTB risk among ART singletons from women with no infertility diagnosis and cycles using donor sperm suggests an ART PTB risk that cannot be explained by parental infertility alone.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Financial disclosures
M.P.D is a stockholder in and on the Board of Directors of Advanced Reproductive Care, and has received a grant from Serono.
Funding
This research was supported in part by a T32 Grant from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (T32-HD046377).
Ethical approval
The study received approval from the Institutional Review Boards of Florida, Massachusetts, Michigan, and the CDC.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol. 2004;103(3):551–563. doi: 10.1097/01.AOG.0000114989.84822.51. [DOI] [PubMed] [Google Scholar]
- 2.McDonald SD, Han Z, Mulla S, Murphy KE, Beyene J, Ohlsson A. Preterm birth and low birth weight among in vitro fertilization singletons: a systematic review and meta-analyses. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):138–148. doi: 10.1016/j.ejogrb.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 3.Xu XK, Wang YA, Li Z, Lui K, Sullivan EA. Risk factors associated with preterm birth among singletons following assisted reproductive technology in Australia 2007–2009–a population-based retrospective study. BMC Pregnan Childb. 2014;14(1):406. doi: 10.1186/s12884-014-0406-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messerlian C, Maclagan L, Basso O. Infertility and the risk of adverse pregnancy outcomes: a systematic review and meta-analysis. Hum Reprod. 2013;28(1):125–137. doi: 10.1093/humrep/des347. [DOI] [PubMed] [Google Scholar]
- 5.Basso O, Baird DD. Infertility and preterm delivery, birthweight, and caesarean section: a study within the Danish National Birth Cohort. Hum Reprod. 2003;18(11):2478–2484. doi: 10.1093/humrep/deg444. [DOI] [PubMed] [Google Scholar]
- 6.Dunietz GL, Holzman C, McKane P, Li C, Boulet SL, Todem D, et al. Assisted reproductive technology and the risk of preterm birth among primiparas. Fertil Steril. 2015; doi:10.1016/j.fertnstert.2015.01.015. [DOI] [PMC free article] [PubMed]
- 7.Kawwass JF, Crawford S, Kissin DM, Session DR, Boulet S, Jamieson DJ. Tubal factor infertility and perinatal risk after assisted reproductive technology. Obstet Gynecol. 2013;121(6):1263–1271. doi: 10.1097/AOG.0b013e31829006d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med. 2002;346(10):731–737. doi: 10.1056/NEJMoa010806. [DOI] [PubMed] [Google Scholar]
- 9.Stern JE, Luke B, Tobias M, Gopal D, Hornstein MD, Diop H. Adverse pregnancy and birth outcomes associated with underlying diagnosis with and without assisted reproductive technology treatment. Fertil Steril. 2015;103(6):1438–1445. doi: 10.1016/j.fertnstert.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levi Dunietz G, Holzman C, Zhang Y, Talge NM, Li C, Todem D, et al. Assisted reproductive technology and newborn size in singletons resulting from fresh and cryopreserved embryos transfer. PLoS One. 2017;12(1):e0169869. doi: 10.1371/journal.pone.0169869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mneimneh AS, Boulet SL, Sunderam S, Zhang Y, Jamieson DJ, Crawford S, et al. States monitoring assisted reproductive technology (SMART) collaborative: data collection, linkage, dissemination, and use. J Women’s Health (Larchmt) 2013;22(7):571–577. doi: 10.1089/jwh.2013.4452. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Cohen B, Macaluso M, Zhang Z, Durant T, Nannini A. Probabilistic linkage of assisted reproductive technology information with vital records, Massachusetts 1997–2000. Matern Child Health J. 2012;16(8):1703–1708. doi: 10.1007/s10995-011-0877-7. [DOI] [PubMed] [Google Scholar]
- 13.Bieler GS, Brown GG, Williams RL, Brogan DJ. Estimating model-adjusted risks, risk differences, and risk ratios from complex survey data. Am J Epidemiol. 2010;171(5):618–623. doi: 10.1093/aje/kwp440. [DOI] [PubMed] [Google Scholar]
- 14.Yogev Y, Melamed N, Bardin R, Tenenbaum-Gavish K, Ben-Shitrit G, Ben-Haroush A. Pregnancy outcome at extremely advanced maternal age. Am J Obstet Gynecol. 2010;203(6):558. doi: 10.1016/j.ajog.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 15.Luke B, Brown MB, Grainger DA, Stern JE, Klein N, Cedars MI, et al. The effect of early fetal losses on singleton assisted-conception pregnancy outcomes. Fertil Steril. 2009;91(6):2578–2585. doi: 10.1016/j.fertnstert.2008.03.068. [DOI] [PubMed] [Google Scholar]
- 16.Pinborg A, Lidegaard Ø, la Cour FN, Andersen AN. Consequences of vanishing twins in IVF/ICSI pregnancies. Hum Reprod. 2005;20(10):2821–2829. doi: 10.1093/humrep/dei142. [DOI] [PubMed] [Google Scholar]
- 17.Schieve LA, Ferre C, Peterson HB, Macaluso M, Reynolds MA, Wright VC. Perinatal outcome among singleton infants conceived through assisted reproductive technology in the United States. Obstet Gynecol. 2004;103(6):1144–1153. doi: 10.1097/01.AOG.0000127037.12652.76. [DOI] [PubMed] [Google Scholar]
- 18.Wang YA, Sullivan EA, Black D, Dean J, Bryant J, Chapman M. Preterm birth and low birth weight after assisted reproductive technology-related pregnancy in Australia between 1996 and 2000. Fertil Steril. 2005;83(6):1650–1658. doi: 10.1016/j.fertnstert.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 19.Henningsen AK, Pinborg A, Lidegaard O, Vestergaard C, Forman JL, Andersen AN. Perinatal outcome of singleton siblings born after assisted reproductive technology and spontaneous conception: Danish national sibling-cohort study. Fertil Steril. 2011;95(3):959–963. doi: 10.1016/j.fertnstert.2010.07.1075. [DOI] [PubMed] [Google Scholar]
- 20.Marino JL, Moore VM, Willson KJ, Rumbold A, Whitrow MJ, Giles LC, et al. Perinatal outcomes by mode of assisted conception and sub-fertility in an Australian data linkage cohort. PLoS One. 2014;9(1):e80398. doi: 10.1371/journal.pone.0080398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandey S, Shetty A, Hamilton M, Bhattacharya S, Maheshwari A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis. Hum Reprod Update. 2012;18(5):485–503. doi: 10.1093/humupd/dms018. [DOI] [PubMed] [Google Scholar]
- 22.Pinborg A, Wennerholm UB, Romundstad LB, Loft A, Aittomaki K, Soderstrom-Anttila V, et al. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update. 2013;19(2):87–104. doi: 10.1093/humupd/dms044. [DOI] [PubMed] [Google Scholar]
- 23.ASRM Recommendations for gamete and embryo donation: a committee opinion. Fertil Steril. 2013;99(1):47–62. doi: 10.1016/j.fertnstert.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 24.Levron Y, Dviri M, Segol I, Yerushalmi GM, Hourvitz A, Orvieto R, et al. The ‘immunologic theory’ of preeclampsia revisited: a lesson from donor oocyte gestations. Am J Obstet Gynecol. 2014;(4):211, 383.e1-5. doi:10.1016/j.ajog.2014.03.044. [DOI] [PubMed]
- 25.Soderstrom-Anttila V. Pregnancy and child outcome after oocyte donation. Hum Reprod Update. 2001;7(1):28–32. doi: 10.1093/humupd/7.1.28. [DOI] [PubMed] [Google Scholar]
- 26.Wiggins DA, Main E. Outcomes of pregnancies achieved by donor egg in vitro fertilization—a comparison with standard in vitro fertilization pregnancies. Am J Obstet Gynecol. 2005;192(6):2002–2006. doi: 10.1016/j.ajog.2005.02.059. [DOI] [PubMed] [Google Scholar]
- 27.Malchau SS, Loft A, Larsen EC, Aaris Henningsen AK, Rasmussen S, Andersen AN, et al. Perinatal outcomes in 375 children born after oocyte donation: a Danish national cohort study. Fertil Steril. 2013;99(6):1637–1643. doi: 10.1016/j.fertnstert.2013.01.128. [DOI] [PubMed] [Google Scholar]
- 28.Malchau SS, Loft A, Henningsen AK, Nyboe Andersen A, Pinborg A. Perinatal outcomes in 6,338 singletons born after intrauterine insemination in Denmark, 2007 to 2012: the influence of ovarian stimulation. Fertil Steril. 2014;102(4):1110–1116. doi: 10.1016/j.fertnstert.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 29.Gibbons WE, Cedars M, Ness RB. Toward understanding obstetrical outcome in advanced assisted reproduction: varying sperm, oocyte, and uterine source and diagnosis. Fertil Steril. 2011;95(5):1645–1649. doi: 10.1016/j.fertnstert.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 30.Vaughan DA, Cleary BJ, Murphy DJ. Delivery outcomes for nulliparous women at the extremes of maternal age–—a cohort study. BJOG Int J Obstet Gynaecol. 2014;121(3):261–268. doi: 10.1111/1471-0528.12311. [DOI] [PubMed] [Google Scholar]
- 31.Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Barfield WD. Assisted reproductive technology surveillance—United States, 2011. Morb Mort Weekly Rep Surveil Sum (Washington, DC : 2002) 2014;63(10):1–28. [PubMed] [Google Scholar]