Abstract
Purpose
The aim of this study is to evaluate the feasibility of using a hand-made silver container for the cryopreservation of human ovarian cortex.
Methods
Human ovarian cortex tissues were vitrified using an open vitrification system (OVS) of needle immersed vitrification (NIV) and two closed vitrification systems (CVS) of a plastic vial (plastic CVS) and a silver container (silver CVS). Outcomes of vitrification were evaluated morphologically and histologically by in vitro culture and xenotransplantation. The apoptosis of primordial follicles was assessed by TUNEL staining. The production of E2 and P4 was examined by a chemiluminescent immunoassay. Blood vessels were visualized with CD31 staining.
Results
Compared with the fresh ovarian cortex tissue, ovarian cortex tissues that were vitrified using the three different carriers and then warmed showed significantly reduced percentages of normal primordial follicles, viability of primordial follicles, E2 and P4 levels during in vitro culture and decreased amounts of blood vessels. However, much better outcomes were obtained with NIV and silver CVS than with plastic CVS, based on the better morphology and viability of primordial follicles, higher E2 and P4 production during an in vitro culture, and greater numbers of blood vessels after xenografting. Importantly, the outcomes of ovarian cortex cryopreservation with silver CVS were similar and comparable to those with NIV.
Conclusions
The hand-made silver container as a CVS is a promising carrier for the cryopreservation of the human ovarian cortex.
Keywords: Closed vitrification system, Silver container, Ovarian cortex, Primordial follicles, Needle immersed vitrification
Introduction
The cryopreservation of human ovarian tissue was proven to be feasible in 19 women aged 19–44 years in 1996, when it offered a new method for fertility preservation [1]. Since the first infant was born after the transplantation of frozen/thawed ovarian tissue in 2004 [2], over 86 live births have occurred after the orthotopic auto-transplantation of cryopreserved tissue [3–5]. Because the ovaries are very sensitive to cytotoxic drugs, ovarian cortex cryopreservation has become an urgent and high-demand technology for young female cancer patients who must undergo advanced chemotherapy and radiotherapy [6], as well as for young female patients with non-oncological systemic diseases such as autoimmune and hematological conditions that sometimes require chemotherapy, radiotherapy, or marrow transplantation [7]. Compared to the traditional oocyte cryopreservation, the cryopreservation of ovarian tissues does not require ovarian stimulation and preserve gonadal function in prepubertal and adult patients [8]; moreover, this technology offers a promising option for women at high risk for premature ovarian failure and sterility [9].
Currently, the cryopreservation of human ovarian cortex is carried out in two ways: slow freezing (SF) or vitrification (VT). Although most successful infant births from the cryopreservation of human ovarian cortex were achieved by SF [8, 10], to the best of our knowledge, at least three live births have been reported using VT followed by in vitro activation [11, 12]. Nevertheless, the SF technique has some limitations: (1) ice crystals may form and cause cellular damage [13] and (2) the procedure requires several hours and expensive equipment, limiting its wide application. In the past decade, VT has been developed as a new technique for cryopreservation, which is usually characterized by the brief exposure to high concentrations of cryoprotectants before storage in liquid nitrogen [14]. Due to the high-viscosity environment, rapid cooling during VT can prevent ice crystal formation. However, the successful cryopreservation of ovarian cortex tissues using the VT technique requires a rapid exposure time, an appropriate type and concentration of cryoprotectants, and an optimal holding device.
The VT of ovarian tissues is generally carried out using an open vitrification system (OVS) or a closed vitrification system (CVS) [15]. Several carrier tools have been applied as OVSs for oocytes and embryos with successful results, including the cryoloop [16], copper electron microscope grids [17], a solid surface vitrification (SSV) device [18], and a direct cover vitrification (DCV) device [19]. Previous studies have developed a novel OVS, referred to as needle immersed vitrification (NIV), which has achieved good results with VT [10, 11, 20, 21]. In this method, mouse and human ovarian tissues are directly exposed to liquid nitrogen and vitrified in an acupuncture needle [20]. Nevertheless, its main disadvantage, the direct exposure of human ovarian tissues to liquid nitrogen, may introduce a high risk of bacterial or viral infection [22, 23]. To avoid bacterial or viral contamination, the CVS was developed. In this system, the ovarian tissue is usually enclosed in a cryovial/cryotube or container (plastic CVS) so it is not directly exposed to liquid nitrogen. The plastic cryovial/cryotube has been used for the VT of human oocytes, embryos, and ovarian tissues [24, 25], but the plastic wall of the cryovial/cryotube often leads to relatively slow cooling and warming speeds which may negatively affect the outcomes of rapid VT. In recent years, several researchers have focused on designing enclosed metal containers for the VT of ovarian tissues. Bos-Mikich et al. found that a simple hand-made aluminum foil caddy was suitable for the VT of the mouse ovary [26]; moreover, another group successfully vitrified bovine ovarian tissues using a metal container [27]. Nevertheless, there has been no attempt to implement metal containers for the VT of the human ovarian cortex. One of the promising metal containers is silver, as it conducts heat extremely well with a conductivity of 429 W/m Kelvin (W/m.K); this conductivity is much faster than that of plastic (0.2 W/m.K) and aluminum (237 W/m.K) [28]. Thus, in this study, it was hypothesized that silver should be an optimal carrier tool for human ovarian tissue CVS.
Hence, in this work, the efficacy of a hand-made silver container as a CVS (silver CVS) for the VT of the human ovarian cortex was investigated. The cryopreservation outcomes using the silver CVS were evaluated by ovarian histology, apoptosis of primordial follicles, secretion of estradiol (E2) and progesterone (P4) during an in vitro culture and re-vascularization after an in vivo transplantation in comparison to outcomes obtained with NIV and a plastic CVS.
Materials and methods
Collection of human ovarian tissues
This study was approved by the Institutional Ethics Committee of West China Medical Center at Sichuan University. Human ovarian samples were collected from eight patients, aged 27.2 ± 3.1 years, who underwent an oophorectomy (three patients) or ovarian cystectomy (five patients). Informed consents were obtained from all patients. Ovarian tissues were retrieved by laparoscopy and transported to the laboratory on ice within 20 min. Ovarian tissues from each patient were cut into 4 to 6 mm2 pieces at a thickness of about 2 mm using a No. 22 scalpel. Ovarian cortical slices from each patient were randomly assigned to four evenly distributed groups: non-treated fresh control, NIV, plastic CVS, and silver CVS, as shown in Table 1.
Table 1.
Assignment of ovarian slices from each patient to four groups
| Pt no. | Groups | HE/TUNEL | In vitro culture | Transplantation | Total number |
|---|---|---|---|---|---|
| 1 | Fresh | 2 | 0 | 6 | 32 |
| Plastic CVS | 2 | 0 | 6 | ||
| NIV | 2 | 0 | 6 | ||
| Sliver CVS | 2 | 0 | 6 | ||
| 2 | Fresh | 2 | 0 | 6 | 32 |
| Plastic CVS | 2 | 0 | 6 | ||
| NIV | 2 | 0 | 6 | ||
| Sliver CVS | 2 | 0 | 6 | ||
| 3 | Fresh | 2 | 2 | 6 | 40 |
| Plastic CVS | 2 | 2 | 6 | ||
| NIV | 2 | 2 | 6 | ||
| Sliver CVS | 2 | 2 | 6 | ||
| 4 | Fresh | 2 | 2 | 3 | 28 |
| Plastic CVS | 2 | 2 | 3 | ||
| NIV | 2 | 2 | 3 | ||
| Sliver CVS | 2 | 2 | 3 | ||
| 5 | Fresh | 2 | 2 | 3 | 28 |
| Plastic CVS | 2 | 2 | 3 | ||
| NIV | 2 | 2 | 3 | ||
| Sliver CVS | 2 | 2 | 3 | ||
| 6 | Fresh | 2 | 2 | 0 | 16 |
| Plastic CVS | 2 | 2 | 0 | ||
| NIV | 2 | 2 | 0 | ||
| Sliver CVS | 2 | 2 | 0 | ||
| 7 | Fresh | 2 | 2 | 0 | 16 |
| Plastic CVS | 2 | 2 | 0 | ||
| NIV | 2 | 2 | 0 | ||
| Sliver CVS | 2 | 2 | 0 | ||
| 8 | Fresh | 2 | 2 | 0 | 16 |
| Plastic CVS | 2 | 2 | 0 | ||
| NIV | 2 | 2 | 0 | ||
| Sliver CVS | 2 | 2 | 0 |
Design of silver container
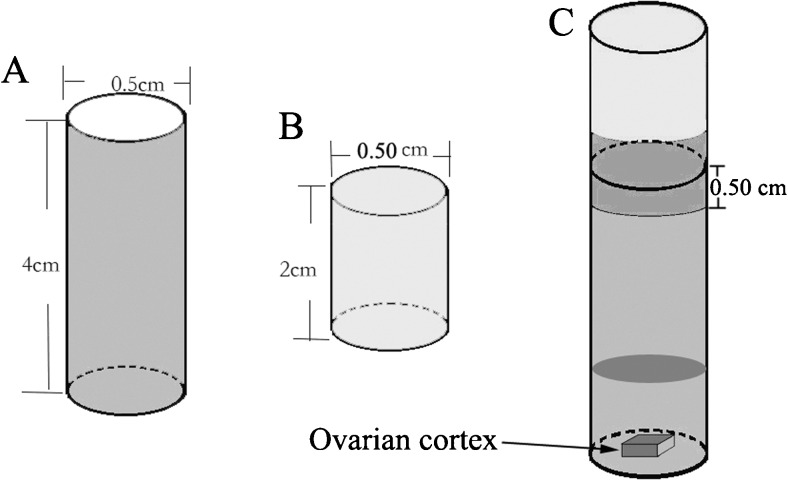
To avoid the weaknesses of plastic containers and utilize the advantage of fast heat transfer through silver, a 4-cm-tall silver cylindrical container was designed with a diameter of 0.5 cm and a 0.2-mm thick wall and closed bottom (Fig. 1a). Because the fabrication of a silver cap for the tube was difficult, a 2-cm-tall polyvinyl fluoride (PVF) cap with a 0.5-cm opening was designed (Fig. 1b). The silver tube and PVF cap can be tightly closed with a 0.5-cm overlap by clockwise screwing and then sealed with paraffin film (Fig. 1c). Both the silver tube and PVF cap were produced in our laboratory (Chinese Patent No. ZL201320633196.9).
Fig. 1.
Diagram illustrating the design of the silver CVS container. a A 4.0-cm-tall silver tube, with a 0.5-cm diameter and 0.2-mm thickness of the wall and bottom. b A 2.0-cm-tall PVF cap with a 0.5-cm opening and 0.2-mm thickness of the wall and bottom. c Illustration of the ovarian cortex and an appropriate amount of cryoprotectants in a silver container. The PVF cap was tightly closed on the silver tube with a 0.5-cm overlap by clockwise screwing. The cap was then sealed with paraffin film
VT procedures
For the three different VT procedures, ovarian tissues were dehydrated similarly using a two-step regimen with increasing concentrations of cryoprotectant as previously described [21]. Briefly, the cortical tissue pieces were pretreated with an equilibration solution for 10 min at room temperature (22–25 °C). The equilibration solution consisted of 1.35 mol/L (7.5%, v/v) ethylene glycol (EG, Sigma-Aldrich, St. Louis, MO), 1.06 mol/L (7.5%, v/v) dimethyl sulfoxide (DMSO, Sigma-Aldrich) and 20% fetal bovine serum (FBS, Gibco, Life Technologies, Grand Island, NY) in Dulbecco’s phosphate-buffered saline medium (DPBS, HyClone, Thermo Fisher Scientific Inc., Waltham, MA). Then the tissues were immersed in a VT solution consisting of 2.69 mol/L EG (15%), 2.11 mol/L DMSO (15%), and 0.5 mol/L sucrose for 2 min. The NIV procedure was performed with a special carrier (acupuncture needle), as previously described [20, 21]. After two dehydration steps, the tissues in the acupuncture needle were directly immersed in liquid nitrogen (−196 °C). Finally, the vitrified tissues were transferred to a liquid nitrogen-filled cryovial and stored in liquid nitrogen for at least 1 week.
The VT in the plastic CVS was carried out as previously described with minor modifications [29]. Cortical slices were dehydrated using the same regimens for NIV. After cryoprotectant loading, cortical slices were transferred to 1.8-ml NUNC cryotubes (Nunclon, Roskilde, Denmark) containing a minimum volume of VT medium. Then the cryotubes were tightly closed with an internal thread cap and immediately immersed in liquid nitrogen. The vitrified ovarian tissues were stored in liquid nitrogen for least 1 week. For VT in our hand-made silver containers, dehydrated cortical tissues were transferred to the silver container with about 0.2–0.3 ml of VT medium and sealed tightly with a PVF cap. Subsequently, the container was immediately immersed in liquid nitrogen and stored for at least 1 week.
Warming procedure
The same warming procedure was used for all cortical tissues vitrified in liquid nitrogen. Ovarian cortical slices were quickly removed from the cryovial or container and serially transferred to solutions of 1, 0.5, and 0.25 mol/L sucrose in DPBS supplemented with 20% FBS medium at 37 °C in a 5% CO2 humidified incubator for 5 min each.
Ovarian histology and evaluation of primordial follicles
Fresh and vitrified/warmed ovarian cortex slices were fixed in 4% paraformaldehyde, dehydrated, embedded in paraffin, and serially sectioned at 5 μm thickness. To evaluate the histological changes of primordial follicles, cortical sections from every ten sections of each slice were stained with hematoxylin and eosin (HE) and observed under light microscopy. All primordial follicles with a visible oocyte nucleus in each section were counted. The quality of primordial follicles was evaluated by the morphology of oocytes and granulosa cells. Primordial follicles were considered abnormal if any granulosa cell or oocyte showed a pyknotic nucleus, cytoplasm contraction, or clumping of the chromatin material, as described by Gougeon [30]. According to these criteria, primordial follicles were classified as normal or abnormal.
TUNEL assay
To examine cell apoptosis, every tenth slice from each cortical tissue was chosen to detect DNA fragments by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL kit; Enzo Life Sciences, Farmingdale, NY). The TUNEL assay was carried out following the instructions of the kit. The positive apoptotic signal was determined by apparent and intense brown staining of the nucleus. Only primordial follicles with a visible nucleolus were counted. When the oocyte and/or >50% of the granulosa cells exhibited positive TUNEL staining, the primordial follicle was considered to be apoptotic [31].
In vitro culture of ovarian tissues
Partial ovarian cortical tissues from six patients were pooled and cultured in vitro as previously described [21]. Fresh and vitrified/warmed cortical tissues were cut into approximately 1 mm3 pieces and placed in 24-well culture plates (Corning Incorporated, Corning, NY) pretreated with Matrigel extracellular matrix (Becton Dickinson, Stockholm, Sweden). Four pieces of tissue from each group were placed in one well (two wells per group) with 800 μl of the culture medium, as previously described [21]. The cortical tissues were cultured in 5% CO2 in air at 37 °C for 14 days. Half of the culture medium (400 μl) was replaced every other day (days 2, 4, 6, 8, 10, 12, and 14), and the removed samples were stored at −80 °C for the analysis of steroid concentrations. The morphology of cortex tissues was observed under light microscopy at the end of the experiment (day 14).
Measurement of ovarian steroid hormones
It has been demonstrated that the growth of ovarian tissue was associated with the elevated production of E2 and P4 after cryopreservation in vitro [32, 33]. Here, the concentrations of E2 and P4 in the culture medium were measured by chemiluminescent immunoassay kits (Abbott Laboratories, Chicago, IL) following the manufacturer’s instructions. The measurements were carried out on an automated AxSYM (Abbott Laboratories). The sensitivities for the E2 and P4 assays were 0.1 and 5.0 ng/mL, respectively.
Xenografting of ovarian cortical tissues
Partial ovarian cortical tissues from five patients in each group were pooled and prepared for xenografting. Forty-eight (12 per group, three per time point) 8-week-old severe combined immunodeficient (SCID) female mice (Charles River, Beijing, China) were used in this study. The mice were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). Surgery was carried out under strictly aseptic conditions. An approximately 5-mm dorsal incision was cut with surgical scissors. Two fresh or vitrified/warmed ovarian cortex tissues were grafted into the subcutaneous area of each mouse, as previously described [34, 35], and the skin was closed. The mice were sacrificed by cervical dislocation on days 3, 7, 14, and 21 after ovarian tissue transplantation. The transplanted ovarian cortical tissues were retrieved, fixed in 4% formaldehyde and embedded in paraffin for a histological analysis.
CD31 immunostaining
Located at endothelial cell junctions, CD31 is a transmembrane glycoprotein highly expressed in the endothelium which plays an essential role in transendothelial cellular migration [36, 37]. Hence, it was used to evaluate the re-vascularization of xenografted ovarian cortical tissues. Briefly, xenografted ovarian cortical tissues were sectioned into 5-μm thickness, dewaxed, and rehydrated. The immunohistochemistry was conducted using a commercial ABC kit (Vector Laboratories, Inc., Burlingame, CA). The sections were blocked in horse serum for 1 h at room temperature (RT) and then incubated with rabbit monoclonal anti-human CD31 antibody (1:300 dilution; Abcam, Cambridge, MA) at 4 °C overnight. After three washes, the sections were incubated with biotinylated anti-rabbit secondary antibody for 30 min at RT and then incubated with AB reagent for 30 min at RT. After three washes, the sections were stained with 3, 3′-diaminobenzidine (DAB) solution and counterstained with hematoxylin. To assess vascular density, CD31-positive vessels in three randomly selected fields of each section were counted at ×400 magnification. The mean value was used as the final vascular density for each section. Sections immunostained for CD31 were evaluated by two independent pathologists who were blinded to this study.
Statistical analysis
The data were expressed as mean ± standard error of mean (SEM). Statistical analyses were performed using SPSS® software Version 18.0 (IBM, Chicago, IL). One-way and two-way analyses of variance (ANOVA) were used to analyze the differences in the continuous variables among the different groups. A P value less than 0.05 was considered statistically significant.
Results
Effects of different VT methods on human ovarian histology
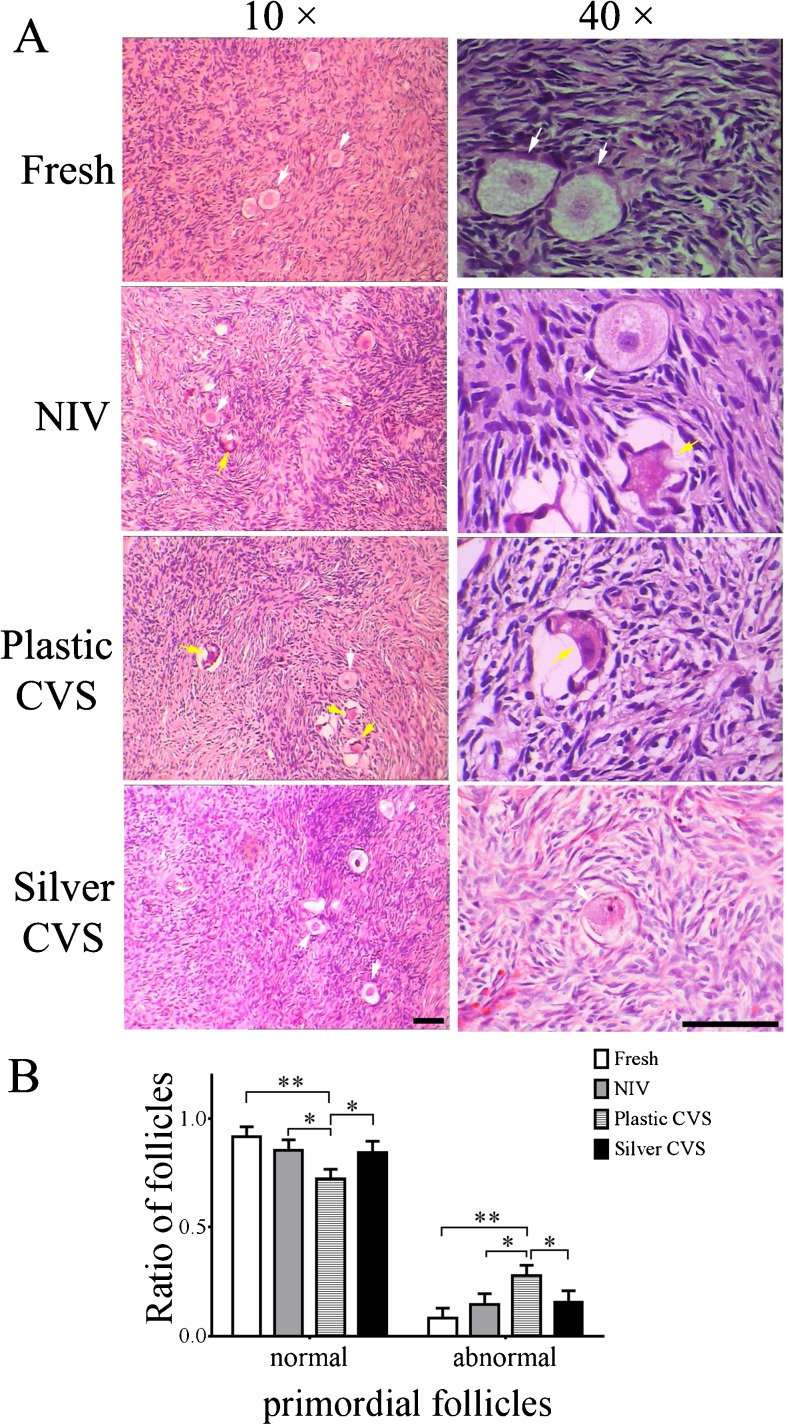
To examine the effects of different VT methods on the cryopreservation of the human ovarian cortex, the integrity of more than 150 primordial follicles in each group was assessed by HE staining. Representative images of normal and abnormal primordial follicles are shown in Fig. 2a. Although most of the primordial follicles in all groups were preserved with a good morphology after cryopreservation, different amounts of abnormal primordial follicles among the four groups were observed (Fig. 2a). The percentages of morphologically normal and abnormal primordial follicles in each group were calculated. The percentage of morphologically normal primordial follicles in the plastic CVS group was significantly lower than those in the fresh, NIV and silver CVS groups (P < 0.05 compared to NIV and silver CVS groups or P < 0.01 compared to the fresh group, Fig. 2b). No significant difference was noted in the percentages of morphologically normal and abnormal primordial follicles among the fresh, NIV and silver groups (P > 0.05, Fig. 2b).
Fig. 2.
Histology of primordial follicles after VT with different methods. a Representative images of normal (white arrow) and/or abnormal (yellow arrow) primordial follicles in the different groups. Scale bar =20.00 μm. b Statistical analysis of the percentages of normal and abnormal primordial follicles in different groups (n = 8) carried out by one-way ANOVA. *P < 0.05; **P < 0.01
Effects of different VT methods on the apoptosis of primordial follicles
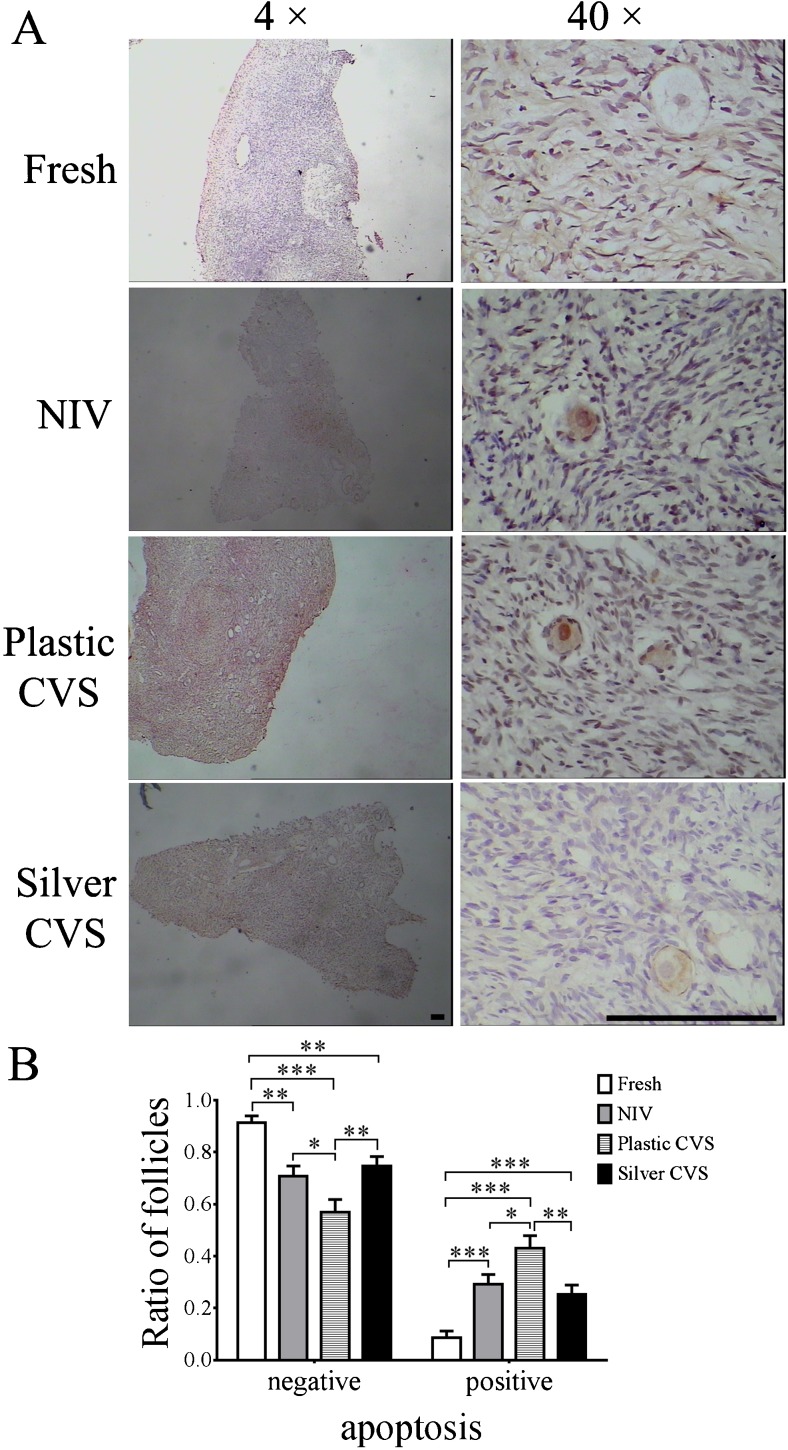
To further verify the presence of abnormal primordial follicles in each group, the apoptosis of primordial follicles was examined by TUNEL assay. The morphology of follicles was shown in Fig. 3a, and more than 150 primordial follicles in each group were assessed. The percentage of apoptotic primordial follicles in the plastic CVS group was significantly higher than those in the fresh, NIV, and silver CVS groups (P < 0.05 compared to NIV group, P < 0.01 compared to the silver CVS group or P < 0.001 compared to the fresh group, as shown in Fig. 3b). Interestingly, the percentages of apoptotic primordial follicles in the NIV and silver CVS groups were significantly higher than in the fresh ovarian cortex group (P < 0.001, Fig. 3b), although no significant difference in the percentages of morphologically normal and abnormal primordial follicles among the fresh, NIV and silver groups was found (P > 0.05, Fig. 2b). Moreover, there was no significant difference in the percentage of apoptotic primordial follicles between the NIV and silver CVS groups (P > 0.05, Fig. 3b). Accordingly, the percentages of normal (apoptosis-negative) primordial follicles in all groups exhibited an opposite trend to the percentage of apoptotic primordial follicles (P < 0.05, P < 0.01, or P < 0.001; please see Fig. 3b).
Fig. 3.
Viability of primordial follicles after VT with different methods. a Representative images of negative and/or positive staining for apoptosis in primordial follicles in the different groups. Scale bar =50.00 μm. b Statistical analysis for the percentages of negative and positive apoptotic primordial follicles in different groups (n = 8), which was carried out by one-way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001
Effects of different VT methods on the secretion of E2 and P4 from cortex tissues during in vitro culture
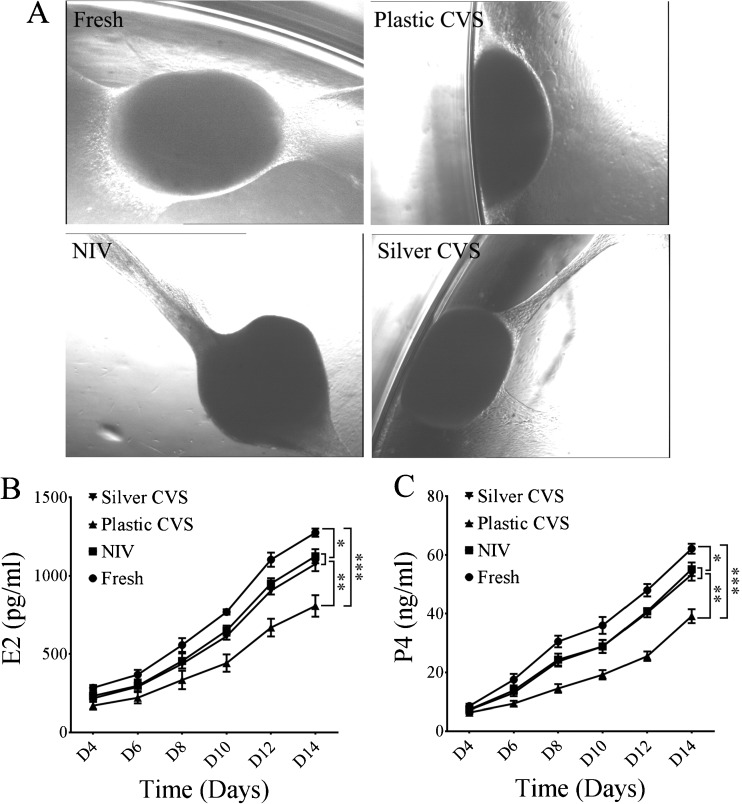
To evaluate the growth potential of vitrified/warmed ovarian cortex, the morphology of the ovarian cortex tissues was observed after 14 days of in vitro culture. It was found that the cortex tissue samples were round, oval or flat (Fig. 4a). In addition to the morphological change, the secretion of E2 and P4 in the culture medium was examined from fresh and vitrified/warmed cortex tissues. As shown in Fig. 4b, c, the levels of E2 and P4 were highest in the fresh ovarian cortex group and the lowest in the plastic CVS group at all time points. The levels of E2 and P4 in the plastic CVS group were obviously lower than those in the fresh group after day 6 and those in the NIV and silver CVS groups after day 10 (for E2) or day 8 (for P4) (P < 0.01 compared to NIV and silver CVS groups or P < 0.001 compared to the fresh group; kindly reference Fig. 4b, c). Moreover, the levels of E2 and P4 in the fresh group were notably higher than those in the NIV and silver CVS groups after day 10 (for E2) or day 8 (for P4) (P < 0.05; see Fig. 4b, c). Importantly, there was no significant difference in the levels of E2 and P4 between the NIV and silver CVS group at any time point (P > 0.05, as shown in Fig. 4a, b).
Fig. 4.
Effects of different VT methods on ovarian growth and the steroidogenesis of vitrified/warmed human ovarian tissues during an in vitro culture. a The morphology of ovarian cortex tissues after 14 days of in vitro culture. b E2 production from the ovarian cortex during an in vitro culture (n = 6). c P4 production from the ovarian cortex during an in vitro culture (n = 6). The statistical analysis was conducted by one-way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001
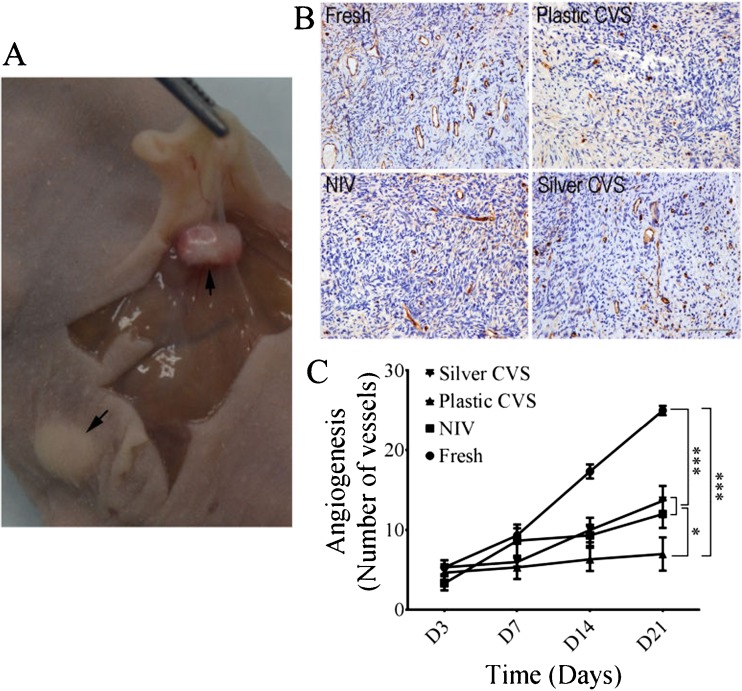
Effects of different VT methods on ovarian re-vascularization in the xenograft model
At 21 days after xenografting, the obvious growth of cortex tissues and the formation of new blood vessels were seen on the surface of all cortex tissues (Fig. 5a). The re-vascularization capacity of all groups was assessed according to the number of CD31-positive vessels (Fig. 5b). The number of ovarian vessels increased in a time-dependent manner in the fresh, NIV and silver CVS groups, but not in the plastic CVS group (Fig. 5c). There was no significant difference in the vessel numbers before day 7, but the numbers of ovarian vessels in the NIV and silver CVS groups on day 21 (P < 0.05) and in the fresh group on days 14 and 21 were significantly higher than those in the plastic CVS group (P < 0.001; Fig. 5c). Moreover, the numbers of ovarian vessels in the fresh group were significantly higher than those in the NIV and silver groups on days 14 and 21 (P < 0.001, Fig. 5b). No obvious difference in the number of ovarian vessels between the NIV and silver CVS groups was found at any time point (P > 0.05, Fig. 5C).
Fig. 5.
The re-vascularisation of ovarian cortex tissues after xenografting in nude mice. a Photograph of xenografted ovarian cortex tissues after 21 days. b Representative images of CD31 staining in ovarian cortex tissues 21 days after xenografting. Scale bar =50.00 mm. c Quantification of CD-31–positive blood vessels at different time points after xenografting. The statistical analysis was conducted by two-way ANOVA (n = 15). *P < 0.05; ***P < 0.001
Discussion
Many researchers have recently used the VT technique for the cryopreservation of the ovarian cortex with significant improvements [3]. To date, two major methods (OVS and CVS) and different vitrification carriers have been used to vitrify ovarian tissues, but each method and VT carrier have unique advantages and limitations [15–19, 38]. In this study, we innovatively designed a silver container and determined its encouraging effect on the VT of the human ovarian cortex.
Compared with oocytes and embryos, the structure of human ovarian tissue is more complex, consisting of different types of cells (oocytes, granulosa cells, blood) and fibrous stroma. Maintaining the cryostability of the tissue is challenging. Primordial follicles are an important biomarker for assessing ovarian reserve or the fertility potential of a woman. Moreover, primordial follicles have been found to be more resistant to cryoinjury, and thus, the morphology and integrity of primordial follicles are commonly used to assess ovarian tissues after cryostorage [39]. Therefore, in this study, the morphology and viability of primordial follicles were mainly used to evaluate the efficacy of the different VT methods. Based on morphology, a higher percentage of abnormal primordial follicles in vitrified/warmed ovarian cortex vitrified using the plastic CVS was found compared to these percentages among fresh ovarian cortex and vitrified/warmed ovarian cortex vitrified by NIV and with the silver CVS. However, the percentages of normal and abnormal primordial follicles after NIV and VT with the silver CVS were like those of fresh ovarian cortex. These data indicated that NIV and the silver CVS were better than the plastic CVS for the VT of the human ovarian cortex. In addition to the morphological difference, a high rate of apoptotic primordial follicles was seen after cryostorage using the plastic CVS, NIV and the silver CVS compared to that in the fresh ovarian cortex, suggesting that the VT procedures adversely affect the viability of primordial follicles. However, the primordial follicles vitrified by NIV and using the silver CVS showed better viability (significantly lower percentage of apoptotic primordial follicles) than those preserved using the plastic CVS, which is consistent with the good morphology of primordial follicles vitrified by NIV and with the silver CVS. In our previous study, TUNEL staining was used to evaluate the quality of stroma cells after NIV and slow freezing; better stroma cell preservation was determined after NIV (21). In our preliminary study, the apoptosis of stroma cells in these four systems was compared, and no significant difference was found. Hence, in this study the apoptosis of stroma cells was not investigated anymore. Interestingly, a recent study observed high rates of atretic follicles and apoptotic stromal cells after the use of NIV compared with after slow freezing plus the improvement protocol (host melatonin treatment and graft incubation with biological glue + vitamin E + vascular endothelial growth factor-A) [40]. However, no significant difference regarding apoptosis in stroma cells after VT was noted in this study. Moreover, the work finished by Abir et al. [40] mainly focused on the comparison of different protocols rather than VT devices. Additionally, the diameter of the acupuncture needle used in our NIV study was 0.18 mm, while the insulin needle with a diameter of 0.25 mm was used in Abir’s study [40]. It is possible that the needle with a larger diameter may do harm to the ovarian tissue during the NIV procedure which needs further investigation.
A previous study demonstrated that in vitro follicular growth after VT was associated with the increased production of E2 and P4 [38]. In the current study, the levels of E2 and P4 were gradually increased in all groups with time in culture. Moreover, these levels in the fresh, NIV and silver CVS groups were significantly higher than those in the plastic CVS group, which was consistent with the in vitro results for ovarian growth. Additionally, it was demonstrated that the level of AMH in the culture medium was increased during the growth of follicles from primordial to developing follicles [41, 42]; hence, it will be interesting to examine the level of AMH in the culture medium to test the viability of follicular in a future study. Blood vessels are highly sensitive to freezing, and re-vascularization is an important index for assessing the efficacy of vitrification [43]. Indeed, compared with fresh ovarian tissue, ovarian tissues vitrified by the three different VT methods and then warmed showed poorer re-vascularization after xenografting in nude mice. But the ovarian tissues vitrified by NIV and with the silver CVS showed much better re-vascularization than those processed with the plastic CVS. These results supported the notion that NIV and the silver CVS have an obvious beneficial effect on the preservation of ovarian functions when compared to the plastic CVS.
Ovarian tissue cryopreservation has been attempted using dozens of different VT devices, but all previously reported devices led to significant losses in the follicular pool compared with that of fresh ovarian tissue [44]. In this study, we did find that fresh ovarian tissues had better morphology, greater viability, less apoptosis, higher E2 and P4 levels, and better re-vascularization compared with vitrified/warmed ovarian tissues prepared using the different VT devices. It is well-accepted that, compared with a traditional CVS such as a cryovial/cryotube, OVSs such as the cryoloop and SSV, DCV, and NIV techniques exhibited a faster cooling rate and led to better vitrification results [3]. Nevertheless, most microorganisms can survive in liquid nitrogen; thus, the OVS has a major weakness regarding its high risk of contamination due to the direct exposure of ovarian tissue to liquid nitrogen. Cross-contamination between liquid nitrogen and embryos has been reported if cells or tissues are not protected well by a sealed container [45]. Recently, some researchers have attempted to develop an appropriate carrier for a CVS, which is the critical strategy for the CVS procedure [3]. Although the cryovial/cryotube is commonly used for the VT of human oocytes and embryos [9], due to rapid thermal conductivity, metal containers possessed better VT outcomes for ovarian tissues (in mice and cattle) compared with cryovials/cryotubes [25, 26]. Based on the physical properties of silver, its high conductivity seems to be a better compound for vitrification than other metals such as aluminum, iron, and stainless steel [28]. Moreover, the feasibility of NIV for the cryopreservation of mouse and human ovarian tissues has been investigated previously [20, 27].
Very importantly, the silver container was innovatively designed, and it was demonstrated that the silver CVS could achieve outcomes of ovarian cortex cryopreservation like those of NIV; these findings strongly support the success of silver CVS for ovarian cortex cryopreservation. Although in vitro growth, E2 and P4 production and the formation of blood vessels have been determined in this work, it will be more convincing to examine the developmental potential of secondary follicles and even the fertilization of oocytes from the vitrified/warmed human ovarian cortex preserved by the silver CVS in a future study. Additionally, the ophthalmic scissors and No. 22 scalpel were used to cut ovarian tissues at 2 mm thickness, and parafilm was used to secure the seal between the silver container and cap. Nevertheless, it would be better to use a special chopping tool to cut ovarian tissues with consistent thickness, and the long-term stability of parafilm in liquid nitrogen should be verified. Practically, ovarian tissue’s cryopreservation would be stored long term. Future investigation is thus needed to improve the technology to make a more appropriate cap for the silver container.
Conclusion
In this work, a silver container was designed, and it was then demonstrated that the use of the silver CVS resulted in primordial follicles with better morphology and viability, higher E2 and P4 production, and more blood vessels compared with plastic CVS. Furthermore, the outcomes of cryopreservation using the silver CVS were quite comparable to those achieved with the OVS and NIV. Use of this closed system for the vitrification of human tissue avoids the risk of bacterial or viral infection and offers a successful protective effect. Therefore, the silver CVS is a promising carrier for the cryopreservation of the human ovarian cortex.
Acknowledgments
This research was financially supported by Science and Technology Bureau of Chengdu City (2014-HM01-00045-SF). All the authors are very grateful for the technical support from Dr. Yan Wang from Stanford University and Dr. Yong-Can Huang from the University of Hong Kong for manuscript revision.
Author contributions
Zhun Xiao design of the study, revising it critically for important intellectual content; Yaoyao Zhang acquisition of data, drafting the article; Wei Fan analysis and interpretation of data, all authors approve the version to be submitted.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Footnotes
Zhun Xiao and Yaoyao Zhang contribute equally to this study.
References
- 1.Hovatta O, Silye R, Krausz T, Abir R, Margara R, Trew G, et al. Cryopreservation of human ovarian tissue using dimethylsulphoxide and propanediol-sucrose as cryoprotectants. Hum Reprod. 1996;116:1268–1272. doi: 10.1093/oxfordjournals.humrep.a019370. [DOI] [PubMed] [Google Scholar]
- 2.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;3649443:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 3.Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;3533:318–321. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 4.Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P, Anderson CY, et al. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue:focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet. 2017;34:325–336. doi: 10.1007/s10815-016-0843-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;2310:2266–2272. doi: 10.1093/humrep/den244. [DOI] [PubMed] [Google Scholar]
- 6.Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin’s disease. Oncologist. 2007;1212:1437–1442. doi: 10.1634/theoncologist.12-12-1437. [DOI] [PubMed] [Google Scholar]
- 7.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Restoration of ovarian function after orthotopic (intraovarian and periovarian) transplantation of cryopreserved ovarian tissue in a woman treated by bone marrow transplantation for sickle cell anaemia: case report. Hum Reprod. 2006;211:183–188. doi: 10.1093/humrep/dei268. [DOI] [PubMed] [Google Scholar]
- 8.Donnez J, Dolmans MM. Fertility preservation in women. Nat Rev Endocrinol. 2013;912:735–749. doi: 10.1038/nrendo.2013.205. [DOI] [PubMed] [Google Scholar]
- 9.Donnez J, Dolmans MM. Cryopreservation and transplantation of ovarian tissue. Clin Obstet Gynecol. 2010;534:787–796. doi: 10.1097/GRF.0b013e3181f97a55. [DOI] [PubMed] [Google Scholar]
- 10.Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 2013;110:17474–17479. doi: 10.1073/pnas.1312830110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30:608–615. doi: 10.1093/humrep/deu353. [DOI] [PubMed] [Google Scholar]
- 12.Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod. 2012;182:59–67. doi: 10.1093/molehr/gar082. [DOI] [PubMed] [Google Scholar]
- 13.Klocke S, Bundgen N, Koster F, Eichenlaub-Ritter U, Griesinger G. Slow-freezing versus vitrification for human ovarian tissue cryopreservation. Arch Gynecol Obstet. 2015;2912:419–426. doi: 10.1007/s00404-014-3390-6. [DOI] [PubMed] [Google Scholar]
- 14.Amorim CA, Curaba M, Van Langendonckt A, Dolmans MM, Donnez J. Vitrification as an alternative means of cryopreserving ovarian tissue. Reprod BioMed Online. 2011;232:160–186. doi: 10.1016/j.rbmo.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Posillico S, Kader A, Falcone T, Agarwal A. Ovarian tissue vitrification: modalities, challenges and potentials. Current Women’s Health Reviews. 2010;64:352–366. doi: 10.2174/157340410793362104. [DOI] [Google Scholar]
- 16.Liu L, Milroy C, Peterson CM, Carrell DT. Successful cryoloop vitrification and subsequent in vitro maturation of mouse preantral follicles. Syst Biol Reprod Med. 2011;573:149–153. doi: 10.3109/19396368.2010.536297. [DOI] [PubMed] [Google Scholar]
- 17.Rahimi G, Isachenko E, Isachenko V, Sauer H, Wartenberg M, Tawadros S, et al. Comparison of necrosis in human ovarian tissue after conventional slow freezing or vitrification and transplantation in ovariectomized SCID mice. Reprod BioMed Online. 2004;92:187–193. doi: 10.1016/S1472-6483(10)62128-1. [DOI] [PubMed] [Google Scholar]
- 18.Lin TC, Yen JM, Kuo TC, Gong KB, Hsu KH, Hsu TT. Comparison of the developmental potential of 2-week-old preantral follicles derived from vitrified ovarian tissue slices, vitrified whole ovaries and vitrified/transplanted newborn mouse ovaries using the metal surface method. BMC Biotechnol. 2008;8:38. doi: 10.1186/1472-6750-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou XH, Wu YJ, Shi J, Xia YX, Zheng SS. Cryopreservation of human ovarian tissue: comparison of novel direct cover vitrification and conventional vitrification. Cryobiology. 2010;602:101–105. doi: 10.1016/j.cryobiol.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Xiao Z, Li L, Fan W, Li SW. Novel needle immersed vitrification: a practical and convenient method with potential advantages in mouse and human ovarian tissue cryopreservation. Hum Reprod. 2008;2310:2256–2265. doi: 10.1093/humrep/den255. [DOI] [PubMed] [Google Scholar]
- 21.Xiao Z, Wang Y, Li L, Luo S, Li SW. Needle immersed vitrification can lower the concentration of cryoprotectant in human ovarian tissue cryopreservation. Fertil Steril. 2010;946:2323–2328. doi: 10.1016/j.fertnstert.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Bielanski A, Nadin-Davis S, Sapp T, Lutze-Wallace C. Viral contamination of embryos cryopreserved in liquid nitrogen. Cryobiology. 2000;402:110–116. doi: 10.1006/cryo.1999.2227. [DOI] [PubMed] [Google Scholar]
- 23.Bielanski A, Bergeron H, Lau PC, Devenish J. Microbial contamination of embryos and semen during long term banking in liquid nitrogen. Cryobiology. 2003;462:146–152. doi: 10.1016/S0011-2240(03)00020-8. [DOI] [PubMed] [Google Scholar]
- 24.Isachenko V, Isachenko E, Reinsberg J, Montag M, van der Ven K, Dorn C, et al. Cryopreservation of human ovarian tissue: comparison of rapid and conventional freezing. Cryobiology. 2007;553:261–268. doi: 10.1016/j.cryobiol.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Bagis H, Akkoc T, Tass A, Aktoprakligil D. Cryogenic effect of antifreeze protein on transgenic mouse ovaries and the production of live offspring by orthotopic transplantation of cryopreserved mouse ovaries. Mol Reprod Dev. 2008;754:608–613. doi: 10.1002/mrd.20799. [DOI] [PubMed] [Google Scholar]
- 26.Bos-Mikich A, Marques L, Rodrigues JL, Lothhammer N, Frantz N. The use of a metal container for vitrification of mouse ovaries, as a clinical grade model for human ovarian tissue cryopreservation, after different times and temperatures of transport. J Assist Reprod Genet. 2012;2911:1267–1271. doi: 10.1007/s10815-012-9867-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aquino D, Danielli L, Rigon P, Lothhammer N, Frantz N, Bos-Mikichi A. Ovarian tissue vitrification: the use of a novel metal closed system for clinical grade cryopreservation. JBRA assisted reproduction. 2014;18:12–15. doi: 10.5935/1518-0557.20140086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makinson R. The thermal conductivity of metals. In: mathematical proceedings of the Cambridge philosophical society. Cambridge: Univ Press; 1938. pp. 474–497. [Google Scholar]
- 29.Sheikhi M, Hultenby K, Niklasson B, Lundqvist M, Hovatta O. Clinical grade vitrification of human ovarian tissue: an ultrastructural analysis of follicles and stroma in vitrified tissue. Hum Reprod. 2011;263:594–603. doi: 10.1093/humrep/deq357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod. 1986;12:81–87. doi: 10.1093/oxfordjournals.humrep.a136365. [DOI] [PubMed] [Google Scholar]
- 31.Depalo R, Nappi L, Loverro G, Bettocchi S, Caruso ML, Valentini AM, et al. Evidence of apoptosis in human primordial and primary follicles. Hum Reprod. 2003;1812:2678–2682. doi: 10.1093/humrep/deg507. [DOI] [PubMed] [Google Scholar]
- 32.Li YB, Zhou CQ, Yang GF, Wang Q, Dong Y. Modified vitrification method for cryopreservation of human ovarian tissues. Chin Med J. 2007;120:110–114. [PubMed] [Google Scholar]
- 33.Morimoto Y, Oku Y, Sonoda M, Haruki A, Ito K, Hashimoto S, et al. High oxygen atmosphere improves human follicle development in organ cultures of ovarian cortical tissues in vitro. Hum Reprod. 2007;22:3170–3177. doi: 10.1093/humrep/dem314. [DOI] [PubMed] [Google Scholar]
- 34.Kim SS, Soules MR, Battaglia DE. Follicular development, ovulation, and corpus luteum formation in cryopreserved human ovarian tissue after xenotransplantation. Fertil Steril. 2002;78:77–82. doi: 10.1016/S0015-0282(02)03144-8. [DOI] [PubMed] [Google Scholar]
- 35.Rahimi G, Isachenko E, Isachenko V, Sauer H, Wartenberg M, Tawadros S, et al. Comparison of necrosis in human ovarian tissue after conventional slow freezing or vitrification and transplantation in ovariectomized SCID mice. Reprod BioMed Online. 2004;9:187–193. doi: 10.1016/S1472-6483(10)62128-1. [DOI] [PubMed] [Google Scholar]
- 36.Zocchi MR, Ferrero E, Leone BE, Rovere P, Bianchi E, Toninelli E, Pardi R, et al. CD31/PECAM-1-driven chemokine-independent transmigration of human T lymphocytes. Eur J Immunol. 1996;26:759–767. doi: 10.1002/eji.1830260406. [DOI] [PubMed] [Google Scholar]
- 37.Perez-Atayde AR, Sallan SE, Tedrow U, Connors S, Allred E, Folkman J, et al. Spectrum of tumor angiogenesis in the bone marrow of children with acute lymphoblastic leukemia. Am J Pathol. 1997;150:815–821. [PMC free article] [PubMed] [Google Scholar]
- 38.Ting AY, Yeoman RR, Campos JR, Lawson MS, Mullen SF, Fahy GM, et al. Morphological and functional preservation of pre-antral follicles after vitrification of macaque ovarian tissue in a closed system. Hum Reprod. 2013;285:1267–1279. doi: 10.1093/humrep/det032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hovatta O. Methods for cryopreservation of human ovarian tissue. Reprod BioMed Online. 2005;106:729–734. doi: 10.1016/S1472-6483(10)61116-9. [DOI] [PubMed] [Google Scholar]
- 40.Abir R, Fisch B, Fisher N, Samara N, Lerer-Serfaty G, Magen R, et al. Attempts to improve human ovarian transplantation outcomes of needle-immersed vitrification and slow-freezing by host and graft treatments. J Assist Reprod Genet. 2017;34:633–644. doi: 10.1007/s10815-017-0884-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Younis AJ, Lerer-Serfaty G, Stav D, Sabbah B, Shochat T, Kessler-Icekson G, et al. Extracellular-like matrices and leukaemia inhibitory factor for in vitro culture of human primordial follicles. Reprod Fertil Dev. 2017; doi:10.1071/RD16233. [DOI] [PubMed]
- 42.Lande Y, Fisch B, Tsur A, Farhi J, Prag-Rosenberg R, Ben-Haroush A, et al. Short-term exposure of human ovarian follicles to cyclophosphamide metabolites seems to promote follicular activation in vitro. Reprod BioMed Online. 2017;34:104–114. doi: 10.1016/j.rbmo.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Rahimi G, Isachenko V, Kreienberg R, Sauer H, Todorov P, Tawadros S, et al. Re-vascularisation in human ovarian tissue after conventional freezing or vitrification and xenotransplantation. Eur J Obstet Gynecol Reprod Biol. 2010;1491:63–67. doi: 10.1016/j.ejogrb.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 44.Herraiz S, Novella-Maestre E, Rodriguez B, Diaz C, Sanchez-Serrano M, Mirabet V, et al. Improving ovarian tissue cryopreservation for oncologic patients: slow freezing versus vitrification, effect of different procedures and devices. Fertil Steril. 2014;1013:775–784. doi: 10.1016/j.fertnstert.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 45.Simopoulou M, Asimakopoulos B, Bakas P, Boyadjiev N, Tzanakaki D, Creatsas G. Oocyte and embryo vitrification in the IVF laboratory: a comprehensive review. Folia Med (Plovdiv) 2014;563:161–169. doi: 10.2478/folmed-2014-0023. [DOI] [PubMed] [Google Scholar]