Abstract
The use of immature oocytes subjected to in vitro maturation (IVM) opens interesting perspectives for fertility preservation where ovarian reserves are damaged by pathologies or therapies, as in PCO/PCOS and cancer patients. Human oocyte cryopreservation may offer some advantages compared to embryo freezing, such as fertility preservation in women at risk of losing fertility due to oncological treatment or chronic disease, egg donation and postponing childbirth. It also eliminates religious and/or other ethical, legal, and moral concerns of embryo freezing. In addition, a successful oocyte cryopreservation program could eliminate the need for donor and recipient menstrual cycle synchronization. Recent advances in vitrification technology have markedly improved the oocyte survival rate after warming, with fertilization and implantation rates comparable with those of fresh oocytes. Healthy live births can be achieved from the combination of IVM and vitrification, even if vitrification of in vivo matured oocytes is still more effective. Recently, attention is given to highlight whether vitrification procedures are more successful when performed before or after IVM, on immature GV-stage oocytes, or on in vitro matured MII-stage oocytes. In this review, we emphasize that, even if there are no differences in survival rates between oocytes vitrified prior to or post-IVM, reduced maturation rates of immature oocytes vitrified prior to IVM can be, at least in part, explained by underlying ultrastructural and biomolecular alterations.
Keywords: Oocyte, Vitrification, In vitro maturation, Ultrastructure, Transmission electron microscopy
Clinical IVM
Since its introduction in the 1990s, in vitro maturation (IVM) has emerged as an attractive infertility treatment option. Early experience with IVM yielded limited success in humans and satisfactory pregnancy rates were obtained when advances in IVM protocols were especially associated to stricter patient selection and alternative strategies, such transfer frozen replacement cycles of embryos derived from IVM oocytes [1, 2]. IVM has the potential to substitute for, or be an adjuvant to, standard in vitro fertilization (IVF) protocols for several reasons. It requires no or very little gonadotropin supplementation in vivo and it has been proposed as an alternative assisted reproduction approach to reduce drawbacks of controlled ovarian induction (COI) [2]. The most appropriate candidates for IVM are polycystic ovaries (PCO) and polycystic ovarian syndrome (PCOS) patients. By not using COI, the risk of ovarian hyperstimulation syndrome (OHSS) is diminished [3]. Along with the decreased risk of OHSS, reduction of cost per cycle, drug-related side effects and psychological stress are advantages of IVM protocol.
Antral follicle count (AFC) is important in selection for IVM in normo-ovulatory patients. If the number of follicles available for recovery is too low, the cycle outcome would be unsatisfactory. The parameters for selection of IVM candidates are: age ≤ 36 years, body mass index < 30 kg/m2, FSH < 10 mIU/mL, estradiol < 250 pmol/mL, AFC > 5, and antimüllerian hormone (AMH) > 1.3 ng/mL [4]. Initial work with clinical IVM was in the 1960s by Edwards [5]; others [6] reported the first baby using immature oocytes collected from unstimulated cycles in a donor oocyte program. While, Trounson et al. [1] reported the first birth from PCOS patients using the mother’s own oocytes in 1994. The first IVM attempts using oocytes collected from unstimulated ovaries date to the 1990s, but a systematic development of IVM occurred in the second half of same decade [2]. In IVM cycles, pregnancy rates are unsatisfactory when compared with IVF cycles, but patient selection can improve IVM outcomes. Several studies have reported the obstetric and perinatal outcomes and incidence of congenital malformation after IVM [2, 3]. Even if indications for IVM have expanded—including both normal and poor responders—limitations of IVM as an approach for fertility preservation include the low number of patients eligible (PCO/PCOS are only the 5–7% of the entire IVF patient population and few are the cancer patients who cannot undergo hormonal stimulation with high peak estradiol concentrations) and low maturation rates of GV oocytes (50–55% at best).
Oocyte vitrification technology
Although early successes were reported using human cryopreserved oocytes, this technology was widely perceived to be inefficient and subjected to concerns on safety until many of these worries were alleviated by fundamental validation studies in the 1990s. This led to the adoption of oocyte cryopreservation as a clinical tool, particularly in Italy, where pending legislative changes sought to outlaw embryo cryopreservation. Oocytes can also be cryopreserved by non-equilibrium procedures, such as vitrification, rapid cooling, and ultrarapid cooling [7]. Vitrification is an alternative approach to embryo/oocyte cryopreservation, which was described as a revolutionary technique. It is different from slow freezing in that it avoids the formation of ice crystals in the intracellular and extracellular spaces [8]. It is the solidification of a solution by an extreme elevation in viscosity at low temperatures without ice crystal formation, a process achieved by a combination of a high concentration of cryoprotective additives (CPAs) (4–8 mol/L) and an extremely high cooling rate [9, 10]. The common freezing solutions used for vitrification are composed of permeating (e.g., EG, G, DMSO, PG, acetamide; > 4 M) and non-permeating (e.g., sucrose, trehalose; > 0.5 M) agents [9]. The major drawback of the vitrification procedure is the cytoxicity connected to the high concentration of CPAs, making important the temperature control and a consciousness in the use of CPA cocktails [11]. Evidences on CPA cytotoxicity were strengthened by a report using Raman microspectroscopy, demonstrating an altered protein distribution of the oocyte cortex and a severe disruption of the cortical F-actin network after exposure of ovine oocytes to DMSO and EG [12]. Recently, the application of strategies to reduce CPA concentrations ranged from the increase in the vitrification medium viscosity, by macromolecular supplementation [7, 13], to the use of microdevices allowing to reduce the time available for ice nucleation and growth [14]. To further increase the cooling rate (> 10.000 °C/min) necessary for successful vitrification, the volume of the solution in which the oocytes are vitrified has been dramatically decreased (0.1–2 µL). To achieve this, special carrier systems (open and closed devices) were developed, such as open pulled straws, Flexipet-denuding pipettes, Cryotop, electron microscopy grids, cryoloops, or “Hemi-Straw” system. Up to now, the debate on open vs closed device is still opened and connected to the risk of cross-contamination as well as to clinical outcomes. The risk of contamination in open and closed vitrification devices was studied by evaluating the sterility conditions of the liquid nitrogen (LN), where contaminants of bacterial origin were found in the cryostorage containers both before and after their filling with LN [15]. However, procedures for decontamination, especially for open carriers, have been successfully produced [16]. Recent data from prospective studies are evidencing comparable maturation rates, fertilization rates, embryo developmental competence, clinical pregnancy, and live birth rates between the open and closed systems [17–20]. Comparing the open and closed systems, acceptable survival rates were reported for both [21].
Vitrification may be difficult to perform, because of the very concentrated and viscous volume of vitrification solutions in which the oocytes must be handled for a very limited amount of time (< 1 min) prior to and during vitrification. Similar to slow freezing, rapid warming is required for the optimal survival of vitrified oocytes.
Based on the current state of evidences, modern procedures to cryopreserve oocytes should no longer be considered experimental as declared in 2013 by the American Society for Reproductive Medicine (ASRM) and the Society for Assisted Reproductive Technology (SART). In fact, Cobo et al. [22] stated their findings from a randomized controlled trial of over 3000 fresh and 3000 vitrified oocytes (92.5% survival) in an oocyte donation program, confirming no detrimental effects of vitrification on subsequent development or implantation. Others, using the same vitrification protocols, also reported similar outcomes in oocyte donation programs [23]. Results obtained with the same technique in infertility program showed a trend towards lower clinical outcomes from vitrified oocytes, especially over the age of 34 [24]. With improving the results, application of vitrification for human oocyte cryopreservation is increasing but the number of oocytes necessary to achieve reasonable success should also take into account the patient’s age [25].
Vitrification and oocyte quality
Vitrification was developed as the promising technology to capitalize the reproductive potential of surplus oocytes and preserve fertility, even if slow freezing remains a suitable choice [26]. Starting from the assumption that clinical data tell that oocyte cryopreservation at the mature stage is a safe and efficient procedure, criteria to assess oocyte quality after warming are not yet standardized [27].
Recent advances in live cell imaging are becoming valuable tools to assess alterations in ooplasmic compartments. Intracellular calcium concentrations, indicators of cellular damage, have been determined on fresh, thawed, and warmed human oocytes, evidencing a better preservation of calcium dynamics in oocytes subjected to a close vitrification system than slow freezing [28]. However, following calcium transients for 16 h after fertilization by ICSI, warmed human MII-stage oocytes showed calcium transients occurring over a longer period of time, with a higher amplitude and a lower frequency, respect to the in vivo counterpart [29]. Outcomes of this study were not reported because calcium-sensitive fluorescent dyes and the high-intensity excitation light may impair embryo development [29]. Therefore, this method does not allow use of the same oocyte to follow the further embryonic development but can provide important data on the metabolic status of warmed oocytes. Interestingly, kinetics of embryos obtained from ICSI of vitrified/warmed human oocytes evidenced that oocyte vitrification has no consequence on the completion of oocyte meiosis, but affects the fertilization process and embryo kinetics at the zygote stage, thus evidencing that the detrimental effects of vitrification are probably connected to a pronuclei instability [30]. Variation in clustering and distribution of mitochondria can reflect developmental competence [31]. Mitochondrion selective probes can, indeed, be used to analyze mitochondrial patterns or variations in the mitochondrial membrane potential in vitrified/warmed oocytes. Decreased inner membrane potential was found in human oocytes vitrified after IVM, indicating damage of the mitochondrial functions [32]. However, the reduction in the ΔΨm seemed to spontaneously recover after 4 h in culture to levels of fresh oocytes [33]. Microfilaments can also be indicators of cryodamage, as reported by [34], where microfilament structure was altered in vitrified human oocytes and did not restore to the physiological configuration before than 3 h after thawing. Precocious cell cycle entry, abnormal chromatin configurations, and disruption of the actin cytoskeleton were observed in vitrified-warmed cumulus-enclosed GV-stage rat oocytes [35].
Oocyte in vitro maturation prior to and post-vitrification
In ART, 15–20% of the oocytes retrieved from patients is meiotically immature, even after controlled hormonal hyperstimulation (COI) [36, 37]. Since there is the possibility of an abnormal embryonic development and defective cytoplasmic maturation, these so called “leftover” oocytes are usually discarded because of their poor-quality. However, due to the not enough number of mature oocytes retrieved from the patients who are low responders or having an unsynchronized cohort of follicles, immature oocytes can increase the yield of total available oocytes. At the same time, cryopreservation of in vitro matured oocytes could meet the demand of patients who want to preserve fertility in case of pathologies or therapies damaging ovarian reserves [38]. Oocyte storage may also circumvent the ethical and legal problems encountered in embryo cryopreservation [39]. Apart from the debate about the definition and clinical outcome of IVM [40–42], the recent issue on performing IVM before or after vitrification is producing interesting data.
Although nowadays comparable high survival rates (80–85%) have been reported for oocytes vitrified prior to or post-IVM [43, 44], differences found in terms of cellular alterations and clinical outcomes claim for an impairment in the ability of human IVM oocytes to mature, fertilize, and cleave, that seems to be dependent on the stage in which vitrification occurs.
In early studies [45], the survival of GV oocytes was equivalent to that of mature oocytes (30%) following cryopreservation. However, only 20% of the surviving GV oocytes underwent subsequent maturation. The first baby from a vitrified IVM oocyte was reported in 2009 [46]. In this case, 89% of the oocytes extruded a polar body, 25% survived, 75% fertilized, and a singleton pregnancy was achieved. Several studies have undertaken comparison of freezing at either the immature stage or MII following IVM [47–50]. Cao et al. (2009) reported that maturation rates of GV oocytes after vitrification were significantly lower with respect to those of GV oocytes subjected to IVM and then vitrified at the MII stage (50.8 vs 70.4%, respectively) [47], thus suggesting a reduced oocyte maturation potential by vitrification at immature stage [48]. Our previous studies showed that almost 90% of immature oocytes survived after vitrification and IVM. However, even if oocyte morphology and zona pellucida birefringence were not altered [51, 52], the maturation potential was reduced by Cryotop vitrification of human immature GV-stage oocytes, as seen by reduced maturation rate and oocyte viability respect to the fresh counterpart [36, 53]. Superior maturation rates of GV and MI oocytes vitrified post-IVM (≈ 64%), have been demonstrated in terms of mitochondria, chromatin, and spindle formation, respect to those vitrified prior to IVM (≈ 33%) [44]. Moreover, no detrimental effects were seen on the meiotic spindle configuration between oocytes matured in vitro and those vitrified after IVM [32]. Differently, others stated that cryopreserving MII oocytes post-IVM is more efficient than at the immature stage pre-IVM [38]. In a recent report, several outcomes—ranging from survival to development to blastocyst rates—were higher in GV-stage oocytes vitrified at GV and then matured in vitro up to the MII-stage, with respect to those vitrified post-IVM [54]. However, oocytes matured in vitro from unstimulated ovaries and vitrified post-IVM, displayed reduced developmental potential, as indicated by only the 5.6% of the resulting embryos able to reach the blastocyst rate [55]. Data available in literature about vitrification prior or post-IVM are summarized in Table 1.
Table 1.
An overview of the main outcomes reported for human oocytes vitrified prior to or post-IVM
| Oocyte developmental stage | Vitrification post-IVM | Vitrification prior to IVM | Outcomes | References |
|---|---|---|---|---|
| GV (n. 103) (fIVM) | – | Yes | Maturation rate: 61.2% (fIVM), 33.3% (vIVM) | (Mohsenzadeh et al., 2012, [36]) |
| GV (n. 102) (vIVM) | ||||
| GV (n. 68) and MI (n. 32) (G1) | Yes (G1) | Yes (G2) | Fertilization rate/oocytes injected: 52.5% (G1), 45% (G2) | (Fasano et al., 2012) [43] |
| GV (n. 52) and MI (n. 32) (G2) from ICSI patients | ||||
| Cleaved embryos/oocytes fertilized: 52.4% (G1), 55.5% (G2) | ||||
| Developed blastocysts: 0 (G1), 0 (G2) | ||||
| GV (n. 72), MI (n. 29) (fIVM) | – | Yes (vIVM) | MII oocyte (matured): 59.4% (fIVM), 40.4% (vIVM) P < 0.001 | (Nazari et al., 2011) [51] |
| Oocyte arrest: 35.6% (fIVM), 15.7% (vIVM) Degeneration: 3% (fIVM), 42.7% (vIVM) <0.001 | ||||
| GV (n. 66), MI (n. 36) (vIVM) | ||||
| Parthenogenesis: 2% (fIVM), 1.2% (vIVM) | ||||
| GV (n. 150) (fIVM) | – | Yes (vIVM) | MII oocyte (matured): 75.33% (fIVM), 45.92% (vIVM) P < 0.001 | (Shahedi et al., 2013) [52] |
| GV (n. 151) (vIVM) | ||||
| Oocyte arrest: 15.33% (fIVM), 8.14% (vIVM) | ||||
| Degeneration: 6% (fIVM), 44.4% (vIVM) P < 0.001 | ||||
| Parthenogenesis: 3.33% (fIVM), 1.48 (vIVM) | ||||
| GV (n. 33), MI (n. 20) (fIVM) | – | Yes (vIVM) | Maturation: 87.9% (GV, fIVM), 90% (MI, fIVM), 31.6% (GV, vIVM), 71%(MI, vIVM) | Yazdanpanah et al., 2013 [53] |
| GV (n. 19), MI (n. 31) (vIVM) | ||||
| Oocyte arrest: 3% (GV, fIVM), 0% (MI, fIVM), 42.1%(GV, vIVM), 12.9%(MI, vIVM) | ||||
| Degeneration: 3% (GV, fIVM), 0% (MI, fIVM), 26.3% (GV, vIVM), 6.5% (MI, vIVM) | ||||
| Parthenogenesis: 3% (GV, fIVM), 5% (MI, fIVM), − (GV, vIVM), − (MI, vIVM) | ||||
| Maturation and degeneration: 3% (GV, fIVM), 0% (MI, fIVM), 0% (GV, vIVM), 3.2% (MI, vIVM) | ||||
| Maturation and parthenogenesis: – (GV, fIVM), − (MI, fIVM), 0% (GV, vIVM), 6.5% (MI, vIVM) | ||||
| GV (G2: n. 43, G3: n. 53) unsuitable for IVF-ICSI cycles | Yes (G2) | Yes (G3) | Survival rate: 95.6% (G2), 96.7% (G3) | (Molina et al., 2016) [54] |
| Maturation rate: 83.7% (G2), 56.6% (G3) | ||||
| Developmental rate to 2-cell embryos: 82.8% (G2) 69% (G3) | ||||
| Developmental rate to morula: 20.7% (G2) 0.0% (G3) | ||||
| Developmental rate to blastocyst: 3.5% (G2) 0.0% (G3) | ||||
| MII oocytes matured in vitro (n. 122) (vitro-MII) | – | Yes | Maturation rate: 72.4% (vitro-MII), 78.3% (cryo-MII) P > 0.05 | (Liu et al., 2016 [56]) |
| Abnormal spindle rate: 45% (vitro-MII), 78.9% (cryo-MII) P > 0.05 | ||||
| GV (n. 122) (cryo-MII) from infertile woman | ||||
| Abnormal chromosome configuration rate: 50% (vitro-MII), 84.2% (cryo-MII) P < 0.05 |
It is of note that the birth of a healthy child has been successfully obtained after the implantation of vitrified/warmed embryos obtained by IVM in a PCOS patient [57].
The pool of immature oocytes can also represent another option for cancer patients, reinforcing the need of clarification on the right timing for vitrification and IVM. In a retrospective analysis about immature oocytes retrieved from excised ovarian tissues, matured in vitro and then vitrified, the mean maturation rate following IVM was of the 79% [58]. In another study, immature oocytes from ovariectomy specimens and vitrified post-IVM, produced a 65% fertilization rate and one clinical pregnancy [59]. Lower overall maturation rates (30%) were obtained by immature human oocytes retrieved from discarded ovarian medulla tissues and subjected first to IVM and then to vitrification, but the post-warming survival rate was of the 64% [60]. If IVM was performed after IVM on MI-stage oocytes from cancer patients, maturation rates were significantly lower than those obtained after COI (39.1 vs 85.2%) [61]. The combination of IVM and vitrification on immature oocytes might, indeed, represent a complementary strategy for fertility preservation for those cancer patients where hyperstimulation cannot be applied.
Ultrastructure of oocytes vitrified prior to or post-IVM
Transmission electron microscopy (TEM) evaluation, alone or integrated with immunocytochemical approach, is especially effective in estimating how cooling rates and cryoprotectants affect the oocyte structural integrity [62]. However, even if TEM enables the best exploration of the cell ultrastructure, important limitations—such as relying on expensive technology, highly trained personnel, lengthy specimen preparation, and observation—make it hardly usable for the analysis of large numbers of samples.
The impact of vitrification on the oocyte fine morphology was investigated in different mammals and at different stages. However, results are yet inconclusive, with species-specific differences and, among the same species, confounding results can be obtained by the availability of different protocols and/or devices for vitrification.
Up to now, ultrastructural data mainly showed alterations to microvilli, mitochondria, lipid droplets, and cortical granules (CG) after vitrification [63–66].
However, available literature did not yet unravel which is, between immature and mature oocytes, the optimal maturation stage for vitrification. In porcine cumulus-oocyte-complexes (COCs), ultrastructural alterations were found after vitrification, but more severe at GV-stage, as seen by the separation of cumulus cell from the oocytes, fractured zona pellucida, interrupted gap junctions, reduced microvilli, and mitochondrial matrix density [63]. If vitrification occurred at the MII-stage, the morphology of mitochondria appeared normal, with cortical granules still lined under the oolemma and many vacuoles in the cytoplasm. Given the temperature-sensitive nature of the MII spindles, cryopreservation of immature stages may offer a theoretical advantage, due to the absence of the meiotic spindle and the presence of the nucleus. However, the percentage of oocytes with normal spindle organization decreased significantly in both GV- and MII-stage oocytes [63]. In bovine, data are controversial with a study reporting that oocytes vitrified at the GV-stage were more susceptible to ultrastructural modifications, including a significant premature CG exocytosis [64] and another in which the degree of cytoplasmic degenerations increased with the timing of vitrification, being more evident at MII-stage oocytes [65]. Specifically, from a marked reduction in the number of cortical granules, changes in the cytoplasmic density at the periphery, the beginning of the vacuolization process and mitochondrial clamping observed at GV-stage ultrastructural damages increased at the MII-stage with severe vacuolization, an irregular shape throughout the ooplasm, an almost complete lack of cortical granules and drastic mitochondrial degenerations indicating cell death [65]. In canine, even if no significant differences were found in the rates of GV oocytes reaching the MII-stage, vitrified/warmed immature oocytes showed important ultrastructural alterations [66].
Also in humans, comprehensive ultrastructural studies on vitrified/warmed oocytes, especially highlighting differences between the pre- and post-IVM vitrification, are scarce and conclusive evidences to identify top-quality cryopreserved oocytes were only recently generated.
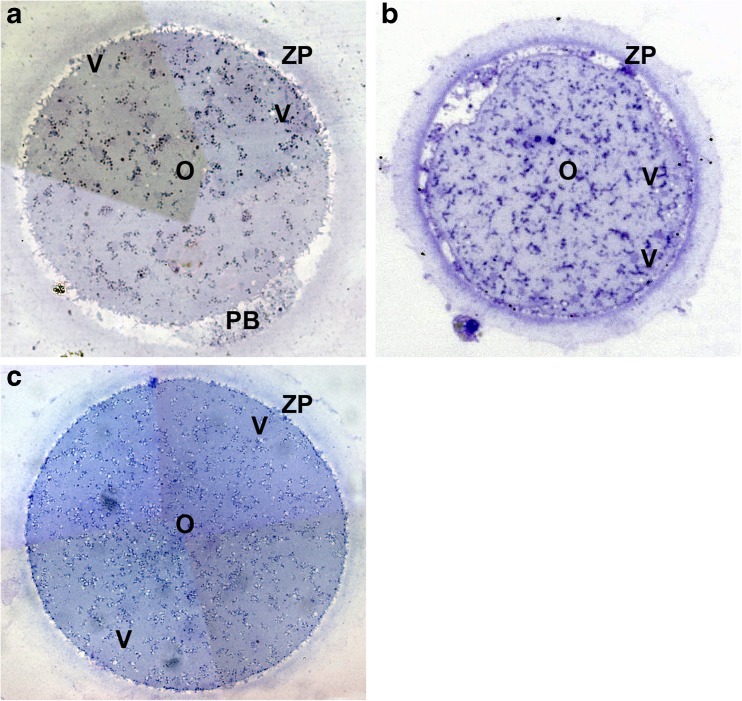
Vitrification/warming seems to not significantly impair human oocyte general microarchitecture. In fact, dimensions and density of the ooplasm by light microscopy (LM) examination of semithin sections was comparable to the fresh counterpart [21, 52, 67–69] (Fig. 1). However, human oocytes subjected to vitrification showed peculiar and stage-specific signs of ultrastructural alterations.
Fig. 1.
Fresh control (a) and vitrified-warmed (b, c) human oocytes. LM shows the general morphology and organelle microtopography. No explicit differences in shape, dimensions, and organelle distribution were found between fresh and vitrified oocytes. Apparent variations of ZP thickness is an effect of the section plane (not equatorial). Vacuoles were detected only sporadically in both fresh (a) and vitrified-warmed (b, c) oocytes. O oocyte, Va vacuoles, ZP zona pellucida, M-SER mitochondria-smooth endoplasmic reticulum aggregates, PB polar body (modified from: [69] Bianchi et al., 2014 (a, c) and [67] Nottola et al., 2009 (b))
Vitrified/warmed immature GV-stage human oocytes
A percentage of vitrified/warmed immature GV-stage oocytes showed, in fact, short and small microvilli irregularly distributed on the ooplasm and small vacuoles, occasionally large and isolated, abnormally located in the oocyte periphery. Noteworthy, important subcellular compartments such as cortical granules, SER, mitochondria, and nucleus did not show evident damages after warming [68].
In vitro matured metaphase II-stage oocytes from vitrified/warmed GV
When oocytes were vitrified at the GV-stage and then subjected to IVM, a series of alterations occurred in their cytoplasm. Part of these alterations, such as the presence of numerous, uniformly distributed, very large MV complexes (Fig. 2a, b) and of occasional, still migrating CGs in the deep cytoplasm (Fig. 2b), were also found in fresh IVM oocytes [70] and are presumably related to the prolonged culture (the former) or to an incomplete maturation (the latter).
Fig. 2.
Ultrastructure of human MII stage oocytes obtained from IVM of vitrified-warmed GV-stage oocytes. Mitochondria are generally rounded and provided with few peripheral or transverse cristae. Note the presence of complexes between mitochondria and vesicles of smooth endoplasmic reticulum (a, b). Dumbbell-shaped, possibly dividing mitochondria can be occasionally found in the ooplasm (asterisk in d). Extensive vacuolization is showed in c. Cortical granules are sparse in the ooplam (b) and form a discontinuous layer beneath the oolemma in vitrified-warmed oocytes (a, d). Microvilli are seen on the oolemma of vitrified-warmed oocytes (b, c). ZP zona pellucida, m mitochondria, mv microvilli, MV mitochondria-vesicle complexes, Ly lysosome, V vacuoles, CG cortical granules (modified from: [52] Shahedi et al., 2013)
Other alterations, such as various degrees of vacuolization (Fig. 2c) and the presence of a reduced amount of CGs (Fig. 2d), including the presence of isolated elements in the inner cytoplasm [71], may be due to the superimposition of a possible cryodamage [52]. The reduced number of CG, irrespective of the device used for vitrification (open or closed) (Fig. 3), is sometimes due to a premature exocytosis of the CG content into the perivitelline space (PVS), resulting in ZP hardening [26]. A compaction of the inner aspect of the ZP, with the loss of its typical filamentous texture, due to the presence of large areas of filaments packed together, was sometimes found in the same samples (Fig. 2a) [26]. Spindles and chromosomes showed a significantly higher abnormal configuration, with respect to MII-stage oocytes matured in vivo or in vitro [56].
Fig. 3.
Fresh (a) and vitrified-warmed MII oocytes (b, c). TEM micrograph showing the presence of a continuous layer of cortical granules under the oolemmal membrane (a), differently to vitrified-warmed oocytes, irrespective of the open (b) or closed (c) cryodevices used. Note the increased compaction of the inner aspect of the ZP in b in comparison with the looser texture in a, c. CG cortical granules, ZP zona pellucida, PVS perivitelline space, mv microvilli, V vacuole, SER smooth endoplasmic reticulum (modified from: [67] Nottola et al., 2009 (a, b); [69] Bianchi et al., 2014 (c))
Vitrified/warmed mature MII-stage human oocytes
Ultrastructural changes detected after vitrification/warming on MII-stage human oocytes mainly involved microvilli, vacuoles, smooth endoplasmic reticulum, mitochondria, and cortical granules. Normally, after warming, numerous long and thin microvilli projected into a PVS with regular shape, width, and content (Fig. 3) [52, 67, 68], but in about the 30% of warmed oocytes, focal surface areas with rare and/or short microvilli were found [67, 68].
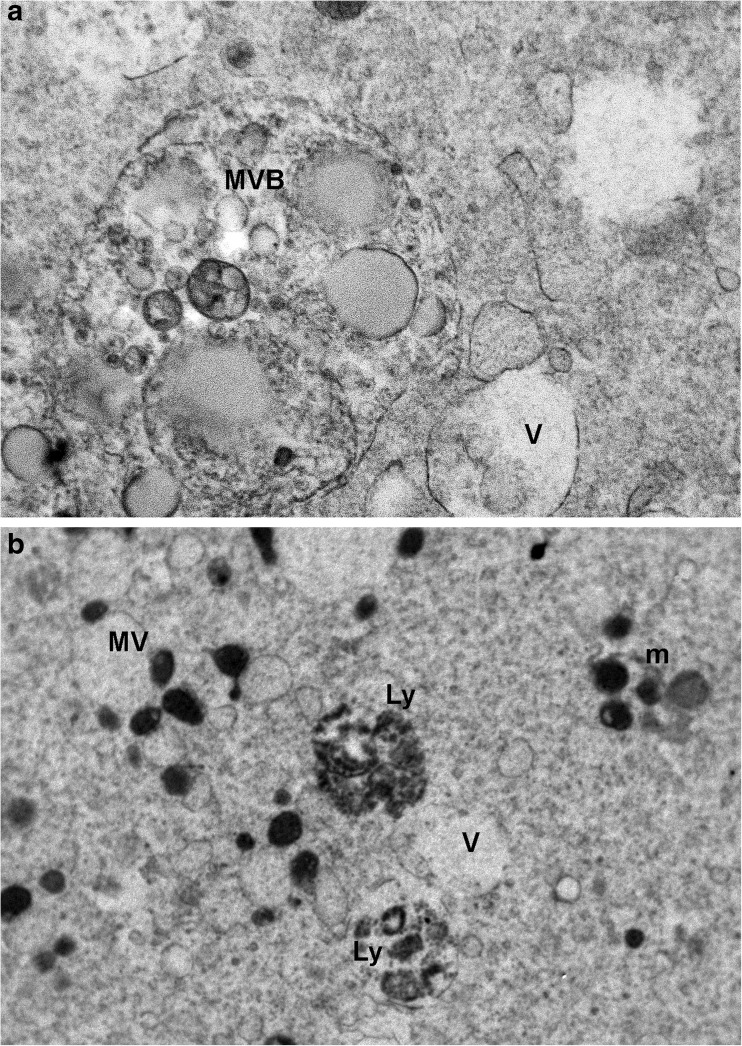
The occurrence of vacuolization seemed to be cryodevice-related, since a slighter degree was detected in human MII-stage oocytes vitrified by open carrier devices [21, 68] (Figs. 1b and 3b), with respect to closed ones [28, 69] (Fig. 1c). Regardless of their numbers, ultrastructural characteristics of vacuoles were the same: they appeared as empty structures, irregularly rounded and bordered by a membrane, often interrupted and in association with multivesicular bodies (MVBs) and lysosomes (Fig. 4) [26].
Fig. 4.
Vitrified-warmed MII stage oocytes. Ultrastructure of multivesicular bodies (a) and lysosomes, sometimes associated with vacuoles (b). MVB multivesicular body, Ly lysosome, m mitochondria, MV mithocondria-vacuoles complexes, V vacuoles (modified from: [67] Nottola et al., 2009 (a) and [69] Bianchi et al., 2014 (b))
Human oocytes after vitrification frequently showed typical M-SER aggregates, similar for dimensions, shape, and location to those observed in fresh controls (Fig. 5a, b). However, in a percentage of vitrified-warmed oocytes, small and slender, immature M-SER aggregates could be found (Fig. 5c) [67].
Fig. 5.
Voluminous aggregates between mitochondria and elements of smooth endoplasmic reticulum are seen in fresh (a) and vitrified-warmed MII stage oocytes (b). Immature M-SER aggregates can be also found after vitrification (c). M-SER mitochondria-smooth endoplasmic reticulum aggregates, V vacuole (modified from: [72] Coticchio et al., 2010 (a); [67] Nottola et al., 2009 (b, c))
Ultrastructural data available up to now seems, therefore, to underline that vitrification prior to IVM could be beneficial for the absence of the cryosensitive meiotic spindle and the presence of a compact nucleus protecting the genetic material. However, the reduction in the exchange surface between vitrified/warmed GV-stage human oocyte and corona radiata cells, deducible from the shortness and reduction of microvilli [69], could affect the subsequent IVM.
Mature MII-stage human oocytes vitrified/warmed post to IVM
In oocytes subjected to vitrification post-IVM, general features were physiologically preserved, with an abundant presence of mitochondria often associated to SER, to form M-SER aggregates, and less frequently to vesicles, to form MV complexes. The number of mitochondria was slightly, but not statistically, higher, respect to oocytes vitrified prior to IVM [71]. From the same study, it seems that oocytes cryopreserved after IVM show slight improvements in term of ultrastructural characteristics of cytoplasm maturation in respect to those vitrified before IVM [71].
Somatic compartments in IVM and vitrification
Cumulus and granulosa cells attracted the attention of researchers and clinical practitioners to unravel their role as protectors against vitrification-induced damage or as vulnerable to cryoinjuries. MII-stage human oocytes vitrified with or without corona radiata did not show differences in zona pellucida hardening and number of cortical granules but, after conventional IVF insemination, oocytes with an intact corona radiata had higher fertilization rates [73]. Human MII-stage COCs subjected to in vitro aging, evidenced a re-compaction of the CC cells combined with the loss of the elongated shape of the corona cells after in vitro culture, irrespective of the age of the patients [74]. Differently, meiotic spindle seemed to better recover after vitrification without cumulus cells, as seen by a significantly higher percentage of detectable spindle in human denuded MII oocytes [75]. The protective effects of corona radiata/cumulus cells during vitrification/warming was studied on equine COCs. Detrimental effects of CPAs on denuded GV-stage oocytes to reach MII-stage with a normal spindle were overcome in COCs by the presence of corona radiata/cumulus cells, probably protecting the oocyte against disruption of cytoplasmic factors important to meiotic spindle reassembly [76]. However, vitrified/warmed cumulus-enclosed GV-stage oocytes showed damages to tranzonal projections and microtubules in rhesus monkey [77], and the loss of transzonal projections in vitrified/warmed mouse oocytes from pre-antral follicles [78], showing how oocyte-cumulus cell contact and interactions, particularly strict at the GV-stage, are sensitive to cryopreservation.
Deleterious effects of vitrification, such as retraction of cumulus cell projections, were observed in vitrified sheep COCs [79], similar to immature vitrified COCs from primed rats, were a reduction of F-actin in ooplasm and oocyte cortex was associated to irreversible but irregular retraction of transzonal projections [35]. The mechanical removal of cumulus cells from immature bovine oocytes did not affect their maturation competence but reduced blastocyst rates, when compared with intact COCs [80]. Bovine oocytes subjected to IVM only, showed a different distribution of membrane-bound vesicles and lipid droplets between MII-stage denuded oocytes (DOs) and cumulus-enclosed oocytes (CEOs), associated to a significant reduction in total lipid level in DOs and to a differential expression of genes regulating energy metabolism, transcription and translation between CEO and DO [81]. Vitrification of GV-stage bovine COCs prior to IVM allowed more than 30% of blastocyst yields if cumulus cell layers were downsized, whereas only 13.4 and 23.7% of the matured oocytes deriving from denuded oocytes and full-size COCs reached the blastocyst stage, respectively [82].
Molecular integrity of IVM oocytes and vitrification
Among the genes expressed in germ cells, ZAR1, BMP15, GDF9, and NLRP5 transcripts were detected at high levels in the oocytes, as oocyte-specific markers [83]. ZAR1 was shown to be critical for early-phase embryo development. Importantly, knockout of this gene makes embryos incapable of developing beyond the first cleavage stage [84]. Also, NLRP5 is a maternal effect gene required for the development prior to the zygotic genome activation [85]. The NLRP5 transcript was detected during oocyte growth from primary follicles, and its level decreases post fertilization [86]. In addition, both BMP15 and GDF9 as germ cell-specific genes affect growth and function of oocytes [87].
Human IVM oocytes retrieved from unstimulated or hormonally stimulated cycles have a higher incidence of spindle defects and chromosomal misalignments compared with the in vivo matured oocytes [88, 89], as also demonstrated by ultrastructural study in animal models [90]. It was reported that IVM oocytes have a high incidence of aneuploidy [91] and the generated embryos are with high rate of fragmentation, deformity in blastomeres nucleation, and aneuploidy [92]. These phenomena indicate a failure in the cell cycle regulation, and many of the genes participating in this process were expressed in oocytes undergoing IVM [93].
Oocyte degeneration can be alleviated using vitrification technology. However, different factors of high cryoprotectant concentrations, cooling, and osmotic stress may initiate the apoptosis within MII oocytes, probably related to the activation of receptor- and mitochondria-mediated apoptotic pathways [94, 95]. However, inclusion of apoptosis inhibitors in the incubation medium after warming, improved the in vitro developmental ability of vitrified oocytes by increasing mitochondrial function, reducing apoptotic level and changing apoptosis-elated gene expression [96, 97]. Therefore, assessment of apoptosis genes expression is an efficient tool for assessing oocytes viability after vitrification. Bcl-2 family is apoptosis regulator, which control the permeabilization of the mitochondrial outer membrane; and are either pro-apoptotic such as Bax and BAD, or anti-apoptotic including Bcl-2 and Bcl-xL. In 2007, Dhali and associates showed that there is a correlation between the compromised development competency and alteration in expression level of Bax, Bcl2, and p53 genes in the vitrified embryos [97]. Cumulus cells also have a critical role in protecting oocyte against oxidative stress [98]. In this regard, Turathum et al. [66] reported that the expression levels of HSP70, Dnmt1 and SOD1 genes in control and vitrified-warmed canine oocytes with cumulus cells was comparable. Vitrification can also influence the epigenetic makeup of oocytes. It induced changes in histone H4 acetylation and histone H3 lysine 9 methylation in oocytes of pigs [99]. Additionally, vitrification significantly decreased the expression of Dnmt1 messenger RNA (mRNA) in mouse MII oocytes [100, 101]. However, in the absence of cryopreservation, the process of IVM may induce degeneration via apoptosis [102, 103]. This pathway may be activated by stimuli of temperature, toxicants, and oxidative stress. Four stages occur in the process of apoptosis. The first one is cell reception of a lethal stimulus, which is followed by activation of early signaling molecules of caspase 8, P53, or C-myc in the second stage. These signals are relayed into central regulators (e.g., Bax and Bcl-2) at stage three, which results in life or death of the cell. The final stage initiates effectors molecules of caspase 3 [94, 104]. Anchamparuthy et al. [105] demonstrated that transcript levels of Fas, FasL, Bax, and Bcl-2 increased post-vitrification and GV maturation. Our recent study evaluated the effects of vitrification on expression patterns of apoptosis-related (Bax, Bcl-2), DNMT1, and stress-related genes (Sod1, HSP70) of GV human oocytes retrieved from ICSI and matured by IVM technology [106]. Our results showed that the expression level of DNMT1 was reduced in the vIVM group, which may cause alteration in the patterns of DNA methylation, resulting in disruption of gene expression, genomic imprinting, and genome stabilization, leading to cell death. In this study, although the expression of apoptosis-related genes significantly upregulated in vitrified and fresh oocytes, the pro-apoptotic (Bax) mRNA level increased more than 14 times in vitrified oocytes. The ratio of Bcl-2 and Bax may be useful for measuring the oocytes tendency towards either survival or apoptosis. The results also showed that the ratio of pro-apoptotic to anti-apoptotic mRNA, was upregulated 4.3 times in vitrified oocytes [106]. This can, probably, justify the partial recovery capacity of oocytes after vitrification and IVM.
Conclusions
Immature oocyte IVM is a promising treatment of several types of infertility, without recourse to ovarian stimulation, especially for young women [107]. Cryopreservation of oocytes has gained importance with increased demand to preserve oocytes for future use. Vitrification is now routinely used in ARTs, also in association to IVM programs [47, 48]. However, alterations found on the oocyte ultrastructure by means of TEM, as reduction of the microvillar layer, vacuolization, increase of MV complexes and alteration of M-SER aggregates, could affect the oocyte mRNA content, as seen by an increase of stress and apoptosis related gene expression after vitrification/warming in human immature oocytes. Therefore, low maturation rates of immature oocytes vitrified prior to IVM could be explained by the observed alterations in their ultrastructure and gene expression profile. The use of vitrified immature human oocytes should be reconsidered for fertility preservation in cases of low or poor ovarian reserve, PCO/PCOS and cancer patients, although more studies have to be done to confirm these findings, especially to clarify the best maturation stage for vitrification.
Finally, it is worth noting that, despite the presence of numerous and different organelle alterations—from a slight to a moderate extent—in a high number of oocytes subjected to IVM and/or vitrification, these oocytes quite often survive these procedures and even undergo maturation and fertilization, with a fairly good success. At this regard, we speculate that some of the above described ultrastructural alterations may be transient, and a partial or total recovery may occur at fertilization or during early embryo development, due to organelle remodeling and membrane recycling [108]. We cannot exclude, however, that these alterations may persist as latent in these early stages, being responsible of defects that become visible only later, during fetal and post-natal life.
Acknowledgements
We are very grateful to Dr. Giovanni Coticchio for his valuable comments and for critically reviewing this manuscript.
Author contributions
MAK, AS and SA designed the study. MGP and MAK wrote the manuscript. SAN and GM critically revised the manuscript. All authors have read and approved the final version and submission of this manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Trounson A, Wood C, Kausche A. In vitro maturation and the fertilization and developmental competence of oocytes recovered from untreated polycystic ovarian patients. Fertil Steril. 1994;62:35362. doi: 10.1016/S0015-0282(16)56891-5. [DOI] [PubMed] [Google Scholar]
- 2.Lim KS, Chae SJ, Choo CW, Ku YH, Lee HJ, Hur CY, Lim JH, Lee WD. In vitro maturation: Clinical applications. Clin Exp Reprod Med. 2013;40(4):143–147. doi: 10.5653/cerm.2013.40.4.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gremeau AS, Andreadis N, Fatum M, Craig J, Turner K, McVeigh E, Child T. In vitro maturation or in vitro fertilization for women with polycystic ovaries? A case-control study of 194 treatment cycles. Fertil Steril. 2012;98(2):355–360. doi: 10.1016/j.fertnstert.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 4.Fadini R, Mignini Renzini M, Dal Canto M, Epis A, Crippa M, Caliari I, et al. Oocyte in vitro maturation in normo-ovulatory women. Fertil Steril. 2013;99:1162–1169. doi: 10.1016/j.fertnstert.2013.01.138. [DOI] [PubMed] [Google Scholar]
- 5.Edwards RG. Maturation in vitro of human ovarian oocytes. Lancet. 1965;2:926–929. doi: 10.1016/S0140-6736(65)92903-X. [DOI] [PubMed] [Google Scholar]
- 6.Cha KY, Koo JJ, Ko JJ, Choi DH, Han SY, Yoon TK. Pregnancy after in vitro fertilization of human follicular oocytes collected from nonstimulated cycles, their culture in vitro and their transfer in a donor oocyte program. Fertil Steril. 1991;55:109–113. doi: 10.1016/S0015-0282(16)54068-0. [DOI] [PubMed] [Google Scholar]
- 7.Konc J, Kanyó K, Kriston R, Somoskői B, Cseh S. Cryopreservation of embryos and oocytes in human assisted reproduction. Biomed Res Int. 2014;2014:307268. doi: 10.1155/2014/307268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at −196°C by vitrification. Nature. 1985;313:573–575. doi: 10.1038/313573a0. [DOI] [PubMed] [Google Scholar]
- 9.Rall WF, Fahy GM. Vitrification: an overview. In: Tucker MJ, Liebermann J, editors. Vitrification in assisted reproduction. London: Informa Healthcare; 2007. [Google Scholar]
- 10.Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod BioMed Online. 2005;11(3):300–308. doi: 10.1016/S1472-6483(10)60837-1. [DOI] [PubMed] [Google Scholar]
- 11.Lawson A, Ahmad H, Sambanis A. Cytotoxicity effects of cryoprotectants as single-component and cocktail vitrification solutions. Cryobiology. 2011;62(2):115–122. doi: 10.1016/j.cryobiol.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogliolo L, Murrone O, Piccinini M, Ariu F, Ledda S, Tilocca S, Albertini DF. Evaluation of the impact of vitrification on the actin cytoskeleton of in vitro matured ovine oocytes by means of Raman microspectroscopy. J Assist Reprod Genet. 2015;32(2):185–193. doi: 10.1007/s10815-014-0389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coello A, Campos P, Remohí J, Meseguer M, Cobo A. A combination of hydroxypropyl cellulose and trehalose as supplementation for vitrification of human oocytes: a retrospective cohort study. J Assist Reprod Genet. 2016;33(3):413–421. doi: 10.1007/s10815-015-0633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi JK, Huang H, He X. Improved low-CPA vitrification of mouse oocytes using quartz microcapillary. Cryobiology. 2015;70(3):269–272. doi: 10.1016/j.cryobiol.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molina I, Mari M, Martínez JV, Novella-Maestre E, Pellicer N, Pemán J. Bacterial and fungalcontamination risks in human oocyte and embryo cryopreservation: open versus closed vitrification systems. Fertil Steril. 2016;106(1):127–32. [DOI] [PubMed]
- 16.Parmegiani L, Accorsi A, Bernardi S, Arnone A, Cognigni GE, Filicori M. A reliable procedure for decontamination before thawing of human specimens cryostored in liquid nitrogen: three washes with sterile liquid nitrogen (SLN2) Fertil Steril. 2012;98(4):870–875. doi: 10.1016/j.fertnstert.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 17.Sarandi S, Herbemont C, Sermondade N, Benoit A, Sonigo C, Poncelet C, et al. A prospective study to compare the efficiency of oocyte vitrification using closed or open devices. Gynecol Obstet Fertil. 2016;44(5):280–4. [DOI] [PubMed]
- 18.Papatheodorou A, Vanderzwalmen P, Panagiotidis Y, Prapas N, Zikopoulos K, Georgiou I, Prapas Y. Open versus closed oocyte vitrification system: a prospective randomized sibling-oocyte study. Reprod BioMed Online. 2013;26(6):595–602. doi: 10.1016/j.rbmo.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 19.De Munck N, Santos-Ribeiro S, Stoop D, Van de Velde H, Verheyen G. Open versus closed oocyte vitrification in an oocyte donation programme: a prospective randomized sibling oocyte study. Hum Reprod. 2016;31(2):377–384. doi: 10.1093/humrep/dev321. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Zheng X, Yan J, Qiao J, Liu P. Neonatal outcomes after the transfer of vitrified blastocysts: closed versus open vitrification system. Reprod Biol Endocrinol. 2013;11:107. doi: 10.1186/1477-7827-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonetti A, Cervi M, Tomei F, Marchini M, Ortoliani F, Manno M. Ultrastructural evaluation of human metaphase II oocytes after vitrification: closed versus open devices. Fertil Steril. 2011;95:928–935. doi: 10.1016/j.fertnstert.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 22.Cobo A, Meseguer M, Remohi J, Pellicer A. Use of cryo-banked oocytes in an ovum donation programme: a prospective, randomized, controlled, clinical trial. Hum Reprod. 2010;25:2239–2246. doi: 10.1093/humrep/deq146. [DOI] [PubMed] [Google Scholar]
- 23.Herrero L, Martinez M, Garcia-Velasco JA. Current status of human oocyte and embryo cryopreservation. Curr Opin Obstet Gynecol. 2011;23:245–250. doi: 10.1097/GCO.0b013e32834874e2. [DOI] [PubMed] [Google Scholar]
- 24.Rienzi L, Romano S, Albricci L, Maggiulli R, Capalbo A, Baroni E, Colamaria S, Sapienza F, Ubaldi F. Embryo development of fresh “versus” vitrified metaphase II oocytes after ICSI: a prospective randomized sibling-oocyte study. Hum Reprod. 2010;25:66–73. doi: 10.1093/humrep/dep346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cobo A, García-Velasco JA, Coello A, Domingo J, Pellicer A, Remohí J. Oocyte vitrification as an efficient option for elective fertility preservation. Fertil Steril. 2016;105(3):755–764. doi: 10.1016/j.fertnstert.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 26.Khalili MA, Maione M, Palmerini MG, Bianchi S, Macchiarelli G, Nottola SA. Ultrastructure of human mature oocytes after vitrification. Eur J Histochem. 2012;56(3):e38. doi: 10.4081/ejh.2012.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khalili MA, Sultan AM, Mojibian M. Role of oocyte morphology on fertilization and embryo formation in assisted reproductive techniques. Mid East Fertil Soc J. 2005;10:72–77. [Google Scholar]
- 28.Gualtieri R, Mollo V, Barbato V, Fiorentino I, Iaccarino M, Talevi R. Ultrastructure and intracellular calcium response during activation in vitrified and slow-frozen human oocytes. Hum Reprod. 2011;26:2452–2460. doi: 10.1093/humrep/der210. [DOI] [PubMed] [Google Scholar]
- 29.Nikiforaki D, Vanden Meerschaut F, Qian C, De Croo I, Lu Y, Deroo T, Van den Abbeel E, Heindryckx B, De Sutter P. Oocyte cryopreservation and in vitro culture affect calcium signalling during human fertilization. Hum Reprod. 2014;29(1):29–40. doi: 10.1093/humrep/det404. [DOI] [PubMed] [Google Scholar]
- 30.Chamayou S, Romano S, Alecci C, Storaci G, Ragolia C, Palagiano A, Guglielmino A. Oocyte vitrification modifies nucleolar remodeling and zygote kinetics—a sibling study. J Assist Reprod Genet. 2015;32(4):581–586. doi: 10.1007/s10815-015-0446-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leoni GG, Palmerini MG, Satta V, Succu S, Pasciu V, Zinellu A, Carru C, Macchiarelli G, Nottola SA, Naitana S, Berlinguer F. Differences in the kinetic of the first meiotic division and in active mitochondrial distribution between prepubertal and adult oocytes mirror differences in their developmental competence in a sheep model. PLoS One. 2015;10(4):e0124911. doi: 10.1371/journal.pone.0124911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lei T, Guo N, Liu JQ, Tan MH, Li YF. Vitrification of in vitro matured oocytes: effects on meiotic spindle configuration and mitochondrial function. Int J Clin Exp Pathol. 2014;15(7(3)):1159–1165. [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C, Han S, Liu W, Wang Y, Huang G. Effect of vitrification on mitochondrial membrane potential in human metaphase II oocytes. J Assist Reprod Genet. 2012;29(10):1045–1050. doi: 10.1007/s10815-012-9848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ci Q, Li M, Zhang Y, Ma S, Gao Q, Shi Y. Confocal microscopic analysis of the microfilament configurations from human vitrification-thawed oocytes matured in vitro. Cryo-Letters. 2014;35(6):544–548. [PubMed] [Google Scholar]
- 35.Kim SS, Olsen R, Kim DD, Albertini DF. The impact of vitrification on immature oocyte cell cycle and cytoskeletal integrity in a rat model. J Assist Reprod Genet. 2014;31(6):739–747. doi: 10.1007/s10815-014-0216-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohsenzadeh M, Khalili MA, Nazari S, Jahromi VH, Agharahimi A, Halvaei I. Effect of vitrification on morphology and in vitro maturation outcome of human immature oocytes. Ital J Anat Embryol. 2012;117:190–198. [PubMed] [Google Scholar]
- 37.Brambillasca F, Guglielmo MC, Coticchio G, Mignini Renzini M, Dal Canto M, Fadini R. The current challenges to efficient immature oocyte cryopreservation. J Assist Reprod Genet. 2013;30(12):1531–1539. doi: 10.1007/s10815-013-0112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Combelles CM, Chateau G. The use of immature oocytes in the fertility preservation of cancer patients: current promises and challenges. Int J Dev Biol. 2012;56:919–929. doi: 10.1387/ijdb.120132cc. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Racowsky C, Combelles CM. Is it best to cryopreserve human cumulus-free immature oocytes before or after in vitro maturation? Cryobiology. 2012;65:79–87. doi: 10.1016/j.cryobiol.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Coticchio G. IVM in need of clear definitions. Hum Reprod. 2016;31(7):1387–1389. doi: 10.1093/humrep/dew110. [DOI] [PubMed] [Google Scholar]
- 41.Dahan MH, Tan SL, Chung J, Son WY. Clinical definition paper on in vitro maturation of human oocytes. Hum Reprod. 2016;31(7):1383–1386. doi: 10.1093/humrep/dew109. [DOI] [PubMed] [Google Scholar]
- 42.De Vos M, Smitz J, Thompson JG, Gilchrist RB. The definition of IVM is clear-variations need defining. Hum Reprod. 2016;31(11):2411–2415. doi: 10.1093/humrep/dew208. [DOI] [PubMed] [Google Scholar]
- 43.Fasano G, Demeestere I, Englert Y. In-vitro maturation of human oocytes: before or after vitrification? J Assist Reprod Genet. 2012;29(6):507–512. doi: 10.1007/s10815-012-9751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JA, Sekhon L, Grunfeld L, Copperman AB. In-vitro maturation of germinal vesicle and metaphase I eggs prior to cryopreservation optimizes reproductive potential in patients undergoing fertility preservation. Curr Opin Obstet Gynecol. 2014;26(3):168–173. doi: 10.1097/GCO.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 45.Mandelbaum J, Junca AM, Tibi C, Plachot M, Alnot MO, Rim H, et al. Cryopreservation of immature and mature hamster and human oocytes. Ann N Y Acad Sci. 1988;541:550–561. doi: 10.1111/j.1749-6632.1988.tb22291.x. [DOI] [PubMed] [Google Scholar]
- 46.Chian RC, Gilbert L, Huang JY, Demirtas E, Holzer H, Benjamin A, et al. Live birth after vitrification of in vitro matured human oocytes. Fertil Steril. 2009;91:372–376. doi: 10.1016/j.fertnstert.2007.11.088. [DOI] [PubMed] [Google Scholar]
- 47.Cao Y, Xing Q, Zhang ZG, Wei ZL, Zhou P, Cong L. Cryopreservation of immature and in vitro matured human oocytes by vitrification. Reprod BioMed Online. 2009;19:369–373. doi: 10.1016/S1472-6483(10)60170-8. [DOI] [PubMed] [Google Scholar]
- 48.Cao YX, Chian RC. Fertility preservation with immature and in vitro matured oocytes. Semin Reprod Med. 2009;27(6):456–464. doi: 10.1055/s-0029-1241055. [DOI] [PubMed] [Google Scholar]
- 49.Baka SG, Toth TL, Veeck LL, Jones HW, Jr, Muasher SJ, Lanzendorf SE. Evaluation of the spindle apparatus of in vitro matured human oocytes following cryopreservation. Hum Reprod. 1995;10:1816–1820. doi: 10.1093/oxfordjournals.humrep.a136182. [DOI] [PubMed] [Google Scholar]
- 50.Goud A, Goud P, Qian C, Van der Elst J, Van Maele G, Dhont M. Cryopreservation of human germinal vesicle stage and in vitro matured MII oocytes: influence of cryopreservation media on the survival, fertilization, and early cleavage divisions. Fertil Steril. 2000;74:487–494. doi: 10.1016/S0015-0282(00)00672-5. [DOI] [PubMed] [Google Scholar]
- 51.Nazari S, Khalili M, Esmaielzadeh F, Mohsenzadeh M. Maturation capacity, morphology and morphometric assessments of human immature oocytes after vitrification and in vitro maturation. Iran J Reprod Med. 2011;9:33–37. [PMC free article] [PubMed] [Google Scholar]
- 52.Shahedi A, Hoseini A, Khalili MA, Norouzian M, Salehi M, Piraei, et al. The effect of vitrificationon ultrastructure of human in vitro matured germinal vesicle oocytes. Eur J Obstet Gynecol Reprod Biol. 2013;167:69–75. doi: 10.1016/j.ejogrb.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 53.Yazdanpanah F, Khalili MA, Eftekhar M, Karimi H. The effect of vitrification on maturation and viability capacities of immature human oocytes. Arch Gynecol Obstet. 2013;288(2):439–444. doi: 10.1007/s00404-013-2777-0. [DOI] [PubMed] [Google Scholar]
- 54.Molina I, Gómez J, Balasch S, Pellicer N, Novella-Maestre E. Osmotic-shock produced by vitrification solutions improves immature human oocytes in vitro maturation. Reprod Biol Endocrinol. 2016;14(1):27. doi: 10.1186/s12958-016-0161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Imesch P, Scheiner D, Xie M, Fink D, Macas E, Dubey R, Imthurn B. Developmental potential of human oocytes matured in vitro followed by vitrification and activation. J Ovarian Res. 2013;6:30. doi: 10.1186/1757-2215-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu MH, Zhou WH, Chu DP, Fu L, Sha W, Li Y. Ultrastructural changes and methylation of human oocytes vitrified at the germinal vesicle stage and matured in vitro after thawing. Gynecol Obstet Investig. 2016;82(3):252–261. doi: 10.1159/000448143. [DOI] [PubMed] [Google Scholar]
- 57.Oliveira NP, Dutra CG, Frantz GN, Basso CG, Fortis MF, Frantz N. Embryos from in vitro maturation (IVM) technique can be successfully vitrified resulting in the birth of a healthy child. JBRA Assist Reprod. 2015;19(4):263–265. doi: 10.5935/1518-0557.20150050. [DOI] [PubMed] [Google Scholar]
- 58.Huang JY, Tulandi T, Holzer H, Tan SL, Chian RC. Combining ovarian tissue cryobanking with retrieval of immature oocytes followed by in vitro maturation and vitrification: an additional strategy of fertility preservation. Fertil Steril. 2008;89(3):567–572. doi: 10.1016/j.fertnstert.2007.03.090. [DOI] [PubMed] [Google Scholar]
- 59.Segers I, Mateizel I, Van Moer E, Smitz J, Tournaye H, Verheyen G, De Vos M. In vitro maturation (IVM) of oocytes recovered from ovariectomy specimens in the laboratory: a promising “ex vivo” method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. J Assist Reprod Genet. 2015;32(8):1221–1231. doi: 10.1007/s10815-015-0528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yin H, Jiang H, Kristensen SG, Andersen CY. Vitrification of in vitro matured oocytes collected from surplus ovarian medulla tissue resulting from fertility preservation of ovarian cortex tissue. J Assist Reprod Genet. 2016;33(6):741–746. doi: 10.1007/s10815-016-0691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sifer C, Sellam-Chokron O, Sermondade N, Cedrin-Durnerin I, Sonigo C, Herbemont C, Grynberg M. Should metaphase 1 and 2 stages oocytes be vitrified in the same time for fertility preservation? Future Oncol. 2016;12(20):2297–2305. doi: 10.2217/fon-2016-0096. [DOI] [PubMed] [Google Scholar]
- 62.Sathananthan AH, Trounson AO. Effects of culture and cryopreservation on human oocyte and embryo ultrastructure and function. In: Van Blerkom J, Motta PM, editors. Ultrastructure of human gametogenesis and early embryogenesis. Boston: Kluwer Academic Publ; 1989. pp. 181–199. [Google Scholar]
- 63.Wu C, Rui R, Dai J, Zhang C, Ju S, Xie B, et al. Effects of cryopreservation on the developmental competence, ultrastructure and cytoskeletal structure of porcine oocytes. Mol Reprod Dev. 2006;73:1454–1462. doi: 10.1002/mrd.20579. [DOI] [PubMed] [Google Scholar]
- 64.Fuku EJ, Liu J, Downey BR. In vitro viability and ultrastructural changes in bovine oocytes treated with a vitrification solution. Mol Reprod Dev. 1995;40(2):177–185. doi: 10.1002/mrd.1080400206. [DOI] [PubMed] [Google Scholar]
- 65.Sprícigo JF, Morais K, Ferreira AR, Machado GM, Gomes AC, Rumpf R, Franco MM, Dode MA. Vitrification of bovine oocytes at different meiotic stages using the Cryotop method: assessment of morphological, molecular and functional patterns. Cryobiology. 2014;69(2):256–265. doi: 10.1016/j.cryobiol.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 66.Turathum B, Saikhun K, Sangsuwan P, Kitiyanant Y. Effects of vitrification on nuclear maturation, ultrastructural changes and gene expression of canine oocytes. Reprod Biol Endocrinol. 2010;8:70. doi: 10.1186/1477-7827-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nottola SA, Coticchio G, Sciajno R, Gambardella A, Maione M, Scaravelli G, Bianchi S, Macchiarelli G, Borini A. Ultrastructural markers of quality in human mature oocytes vitrified using cryoleaf and cryoloop. Reprod BioMed Online. 2009;19(Suppl 3):17–27. doi: 10.1016/S1472-6483(10)60280-5. [DOI] [PubMed] [Google Scholar]
- 68.Palmerini MG, Antinori M, Maione M, Cerusico F, Versaci C, Nottola SA, Khalili MA, Antinori S. Ultrastructure of immature and mature human oocytes after Cryotop vitrification. J Reprod Dev. 2014;60(6):411–420. doi: 10.1262/jrd.2014-027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bianchi V, Macchiarelli G, Borini A, Lappi M, Cecconi S, Miglietta S, Familiari G, Nottola SA. Fine morphological assessment of quality of human mature oocytes after slow freezing or vitrification with a closed device: a comparative analysis. Reprod Biol Endocrinol. 2014;12:110. doi: 10.1186/1477-7827-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Coticchio G, Dal Canto M, Fadini R, Mignini Renzini M, Guglielmo MC, Miglietta S, Palmerini MG, Macchiarelli G, Nottola SA. Ultrastructure of human oocytes after in vitro maturation. Mol Hum Reprod. 2016;22(2):110–118. doi: 10.1093/molehr/gav071. [DOI] [PubMed] [Google Scholar]
- 71.Segovia Y, Victory N, Peinado I, García-Valverde LM, García M, Aizpurua J, et al. Ultrastructural characteristics of human oocytes vitrified before and after in vitro maturation. J Reprod Dev. 2017. doi:10.1262/jrd.2017-009. [DOI] [PMC free article] [PubMed]
- 72.Coticchio G, Borini A, Distratis V, Maione M, Scaravelli G, Bianchi V, Macchiarelli G, Nottola SA. Qualitative and morphometric analysis of the ultrastructure of human oocytes cryopreserved by two alternative slow cooling protocols. J Assist Reprod Genet. 2010;27(4):131–40. [DOI] [PMC free article] [PubMed]
- 73.Tong XH, Wu LM, Jin RT, Luo LH, Luan HB, Liu YS. Fertilization rates are improved after IVF if the corona radiata is left intact in vitrified-warmed human oocytes. Hum Reprod. 2012;27(11):3208–3214. doi: 10.1093/humrep/des295. [DOI] [PubMed] [Google Scholar]
- 74.Bianchi S, Macchiarelli G, Micara G, Linari A, Boninsegna C, Aragona C, Rossi G, Cecconi S, Nottola SA. Ultrastructural markers of quality are impaired in human metaphase II aged oocytes: a comparison between reproductive and in vitro aging. J Assist Reprod Genet. 2015;32(9):1343–1358. doi: 10.1007/s10815-015-0552-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Minasi MG, Fabozzi G, Casciani V, Ferrero S, Litwicka K, Greco E. Efficiency of slush nitrogen vitrification of human oocytes vitrified with or without cumulus cells in relation to survival rate and meiotic spindle competence. Fertil Steril. 2012;97(5):1220–1225. doi: 10.1016/j.fertnstert.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 76.Tharasanit T, Colleoni S, Galli C, Colenbrander B, Stout TA. Protective effects of the cumulus-corona radiata complex during vitrification of horse oocytes. Reproduction. 2009;137(3):391–401. doi: 10.1530/REP-08-0333. [DOI] [PubMed] [Google Scholar]
- 77.Vandevoort CA, Shirley CR, Hill DL, Leibo SP. Effects of cryoprotectants and cryopreservation on germinal vesicle-stage cumulus-oocyte complexes of rhesus monkeys. Fertil Steril. 2008;90(3):805–816. doi: 10.1016/j.fertnstert.2007.06.105. [DOI] [PubMed] [Google Scholar]
- 78.Trapphoff T, El Hajj N, Zechner U, Haaf T, Eichenlaub-Ritter U. DNA integrity, growth pattern, spindle formation, chromosomal constitution and imprinting patterns of mouse oocytes from vitrified pre-antral follicles. Hum Reprod. 2010;25(12):3025–3042. doi: 10.1093/humrep/deq278. [DOI] [PubMed] [Google Scholar]
- 79.Ebrahimi B, Valojerdi MR, Eftekhari-Yazdi P, Baharvand H. Ultrastructural changes of sheep cumulus-oocyte complexes following different methods of vitrification. Zygote. 2012;20(2):103–115. doi: 10.1017/S0967199410000638. [DOI] [PubMed] [Google Scholar]
- 80.Modina S, Beretta M, Lodde V, Lauria A, Luciano AM. Cytoplasmic changes and developmental competence of bovine oocytes cryopreserved without cumulus cells. Eur J Histochem. 2004;48(4):337–346. [PubMed] [Google Scholar]
- 81.Auclair S, Uzbekov R, Elis S, Sanchez L, Kireev I, Lardic L, Dalbies-Tran R, Uzbekova S. Absence of cumulus cells during in vitro maturation affects lipid metabolism in bovine oocytes. Am J Physiol Endocrinol Metab. 2013;304(6):E599–E613. doi: 10.1152/ajpendo.00469.2012. [DOI] [PubMed] [Google Scholar]
- 82.Tashima K, Kubo Y, Hirabayashi M, Hochi S. Downsizing cumulus cell layers to improve cryotolerance of germinal vesicle-stage bovine oocytes. Theriogenology. 2017;95:1–7. doi: 10.1016/j.theriogenology.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 83.Pennetier S, Uzbekova S, Perreau C, Papillier P, Mermillod P, Dalbie’s-Tran R. Spatio-temporal expression of the germ cell marker genes MATER, ZAR1, GDF9, BMP15, and VASA in adult bovine tissues, oocytes, and preimplantation embryos. Biol Reprod. 2004;71:1359–1366. doi: 10.1095/biolreprod.104.030288. [DOI] [PubMed] [Google Scholar]
- 84.Wu X, Viveiros MM, Eppig JJ, Bai Y, Fitzpatrick SL, Matzuk MM. Zygote arrest 1 (Zar1) is a novel maternal-effect gene critical for the oocyte-to-embryo transition. Nat Genet. 2003;33:187–191. doi: 10.1038/ng1079. [DOI] [PubMed] [Google Scholar]
- 85.Tong ZB, Gold L, Pfeifer KE, Dorward H, Lee E, Bondy CA, Dean J, Nelson LM. Mater, a maternal effect gene required for early embryonic development in mice. Nat Genet. 2000;26:267–268. doi: 10.1038/81547. [DOI] [PubMed] [Google Scholar]
- 86.Hamatani T, Ko MS, Yamada M, Kuji N, Mizusawa Y, Shoji M, Hada T, Asada H, Maruyama T, Yoshimura Y. Global gene expression profiling of preimplantation embryos. Hum Cell. 2006;19:98–117. doi: 10.1111/j.1749-0774.2006.00018.x. [DOI] [PubMed] [Google Scholar]
- 87.Dube JL, Wang P, Elvin J, Lyons KM, Celeste AJ, Matzuk MM. The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol Endocrinol. 1998;12:1809–1817. doi: 10.1210/mend.12.12.0206. [DOI] [PubMed] [Google Scholar]
- 88.Racowsky C, Kaufman ML. Nuclear degeneration and meiotic aberrations observed in human oocytes matured in vitro: analysis by light microscopy. Fertil Steril. 1992;58:750–755. doi: 10.1016/S0015-0282(16)55323-0. [DOI] [PubMed] [Google Scholar]
- 89.Cekleniak NA, Combelles CMH, Ganz DA, Fung J, Albertini DF, Racowsky C. A novel system for in vitro maturation of human oocytes. Fertil Steril. 2001;75:1185–1193. doi: 10.1016/S0015-0282(01)01789-7. [DOI] [PubMed] [Google Scholar]
- 90.Palmerini MG, Nottola SA, Leoni GG, Succu S, Borshi X, Berlinguer F, Naitana S, Bekmukhambetov Y, Macchiarelli G. In vitro maturation is slowed in prepubertal lamb oocytes: ultrastructural evidences. Reprod Biol Endocrinol. 2014;12:115. doi: 10.1186/1477-7827-12-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Magli MC, Ferraretti AP, Crippa A, Lappi M, Feliciani E, Gianaroli L. First meiosis errors in immature oocytes generated by stimulated cycles. Fertil Steril. 2006;86:629–635. doi: 10.1016/j.fertnstert.2006.02.083. [DOI] [PubMed] [Google Scholar]
- 92.Nogueira D, Staessen C, Van de Velde H, Van Steirteghem A. Nuclear status and cytogenetics of embryos derived from in vitro-matured oocytes. Fertil Steril. 2000;74:295–298. doi: 10.1016/S0015-0282(00)00642-7. [DOI] [PubMed] [Google Scholar]
- 93.Jones GM, Cram DS, Song B, Magli MC, Gianaroli L, Lacham-Kaplan O, Findlay JK, Jenkin G, Trounson AO. Gene expression profiling of human oocytes following in vivo or in vitro maturation. Hum Reprod. 2008;23(5):1138–1144. doi: 10.1093/humrep/den085. [DOI] [PubMed] [Google Scholar]
- 94.Ebrahimi B, Valojerdi MR, Eftekhari-Yazdi P, Baharvand H. In vitro maturation, apoptotic gene expression and incidence of numerical chromosomal abnormalities following cryotop vitrification of sheep cumulus-oocyte complexes. J Assist Reprod Genet. 2010;27(5):239–246. doi: 10.1007/s10815-010-9401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dai JJ, Niu YF, Wu CF, Zhang SH, Zhang DF. Both death receptor and mitochondria mediated apoptotic pathways participated the occurrence of apoptosis in porcine vitrified MII stage oocytes. Cryo-Letters. 2016;37(2):129–136. [PubMed] [Google Scholar]
- 96.Niu Y, Dai J, Chen Y, Wu C, Zhang S, Zhang D. Positive effect of apoptotic inhibitor z-vad-fmk on vitrified-thawed porcine mii stage oocytes. Cryo-Letters. 2016;37(3):188–195. [PubMed] [Google Scholar]
- 97.Dhali A, Anchamparuthy V, Butler S, Pearson R, Mullarky I, Gwazdauskas F. Gene expression and development of mouse zygotes following droplet vitrification. Theriogenology. 2007;68:1292–1298. doi: 10.1016/j.theriogenology.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 98.Tatemoto H, Sakurai N, Muto N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during in vitro maturation: role of cumulus cells. Biol Reprod. 2000;63:805–810. doi: 10.1095/biolreprod63.3.805. [DOI] [PubMed] [Google Scholar]
- 99.Spinaci M, Vallorani C, Bucci D, Tamanini C, Porcu E, Galeati G. Vitrification of pig oocytes induces changes in histone H4 acetylation and histone H3 lysine 9 methylation (H3K9) Vet Res Commun. 2012;36:165–171. doi: 10.1007/s11259-012-9527-9. [DOI] [PubMed] [Google Scholar]
- 100.Zhao XM, Ren JJ, Du WH, Hao HS, Wang D, Qin T, Liu Y, Zhu HB. Effect of vitrification on promoter CpG island methylation patterns and expression levels of DNA methyltransferase 1o, histone acetyltransferase 1, and deacetylase 1 in metaphase II mouse oocytes. Fertil Steril. 2013;100:256–261. doi: 10.1016/j.fertnstert.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 101.Cheng KR, Fu XW, Zhang RN, Jia GX, Hou YP, Zhu SE. Effect of oocyte vitrification on deoxyribonucleic acid methylation of H19, Peg3, and Snrpn differentially methylated regions in mouse blastocysts. Fertil Steril. 2014;102:1183–1190. doi: 10.1016/j.fertnstert.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 102.Hinck H, Smissen PVD, Heusterpreute M, Donnay I, Hertogh RD, Pampfer S. Identification of caspase 3 and caspase-activated deoxyribonuclease in rat blastocysts and their implication in the induction of chromatin degradation (but not nuclear fragmentation) by high glucose. Biol Reprod. 2001;64:555–562. doi: 10.1095/biolreprod64.2.555. [DOI] [PubMed] [Google Scholar]
- 103.Palmerini MG, Nottola SA, Tunjung WA, Kadowaki A, Bianchi S, Cecconi S, Sato E, Macchiarelli G. EGF-FSH supplementation reduces apoptosis of pig granulosa cells in co-culture with cumulus-oocyte complexes. Biochem Biophys Res Commun. 2016;481:159–164. doi: 10.1016/j.bbrc.2016.10.151. [DOI] [PubMed] [Google Scholar]
- 104.Morita Y, Perez GI, Paris F, Miranda S, Ehleiter D, Haimovitz-Friedman A, et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-1phosphatetherapy. Nat Med. 2000;6:1109–1114. doi: 10.1038/80442. [DOI] [PubMed] [Google Scholar]
- 105.Anchamparuthy V, Pearson R, Gwazdauskas F. Expression pattern of apoptotic genes in vitrified-thawed bovine oocytes. Reprod Domest Anim. 2010;45:e83–e90. doi: 10.1111/j.1439-0531.2009.01527.x. [DOI] [PubMed] [Google Scholar]
- 106.Shahedi A, Yeganeh F, Hosseini A, Khalili MA. Comparision of stress and apoptosis-related genes expression between vitrified and fresh human GV oocytes after in-vitro maturation. Iran J Reprod Med. 2014;12(1):29–30. [Google Scholar]
- 107.Chian RC, Cao YX. In vitro maturation of immature human oocytes for clinical application. Methods Mol Biol. 2014;1154:271–288. doi: 10.1007/978-1-4939-0659-8_12. [DOI] [PubMed] [Google Scholar]
- 108.Nottola SA, Albani E, Coticchio G, Palmerini MG, Lorenzo C, Scaravelli G, Borini A, Levi-Setti PE, Macchiarelli G. Freeze/thaw stress induces organelle remodeling and membrane recycling in cryopreserved human mature oocytes. J Assist Reprod Genet. 2016;33(12):1559–1570. doi: 10.1007/s10815-016-0798-x. [DOI] [PMC free article] [PubMed] [Google Scholar]