Abstract
Purpose
The main purposes of the study were to investigate the endocrine function of ovarian tissue transplanted to heterotopic subcutaneous sites and the reproductive competence and telomere length of a nonhuman primate originating from transplanted tissue.
Methods
Ovarian cortex pieces were transplanted into the original rhesus macaques in the arm subcutaneously, in the abdomen next to muscles, or in the kidney. Serum estradiol (E2) and progesterone (P4) concentrations were measured weekly for up to 8 years following tissue transplantation. A monkey derived from an oocyte in transplanted ovarian tissue entered time-mated breeding and underwent controlled ovarian stimulation. Pregnancy and offspring were evaluated. Telomere lengths and oocytes obtained following controlled ovarian stimulation were assessed.
Results
Monkeys with transplants in the arm and abdomen had cyclic E2 of 100 pg/ml, while an animal with arm transplants had E2 of 50 pg/ml. One monkey with transplants in the abdomen and kidney had ovulatory cycles for 3 years. A monkey derived from an oocyte in transplanted tissue conceived and had a normal gestation until intrapartum fetal demise. She conceived again and delivered a healthy offspring at term. Controlled ovarian stimulations of this monkey yielded mature oocytes comparable to controls. Her telomere length was long relative to controls.
Conclusions
Heterotopic ovarian tissue transplants yielded long-term endocrine function in macaques. A monkey derived from an oocyte in transplanted tissue was reproductively competent. Her telomere length did not show epigenetically induced premature cellular aging. Ovarian tissue transplantation to heterotopic sites for fertility preservation should move forward cautiously, yet optimistically.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-017-1019-y) contains supplementary material, which is available to authorized users.
Keywords: Ovarian tissue transplantation, Steroid hormone, Fertility, Telomere, Primate
Introduction
About 852,000 new cancer cases are expected to be diagnosed in women of the USA in 2017, with 5.4% of the patients less than 49 years old (American Cancer Society). The prognosis for cancer patients has greatly improved with an approximately 70% 5-year survival rate, and therefore, attention is drawn to patients’ quality of life following survival. In women prior to or at reproductive age, the chemo- or radiation therapy used to cure their cancer is gametotoxic and may render them sub-fertile or infertile. The incidence of ovarian failure may approach 90% in patients undergoing high-dose chemotherapy [1].
Current approaches to preserve fertility in women before cancer treatment include ovarian transposition, as well as oocyte and embryo cryopreservation (American Cancer Society). However, ovarian transposition does not protect the ovary from total body irradiation or chemotherapy. Also, oocyte or embryo cryopreservation requires time to perform the assisted reproductive technology (ART) procedures, which delays cancer treatment, and is not suitable for prepubertal girls. One potential solution is to remove and cryopreserve ovarian tissue before cancer treatment for future utilization after completing therapy via autografting, xenografting, or culture. With autografting, ovarian tissue can be transplanted into the original patient orthotopically to the ovarian hilum or heterotopically to a distant site. To date, there have been 62 births worldwide from the transplantation of cryopreserved human ovarian tissue [2–4], with an additional 53 deliveries reported more recently [5].
Despite these successes, data are limited on the long-term endocrine function of heterotopic ovarian tissue transplants [6, 7] or on the long-term normalcy of offspring born after ovarian tissue transplantation in any primate species. Given that the first human pregnancies from ovarian tissue transplantation were achieved in 2004–2005 [8, 9], results regarding the offspring’s reproductive competence and lifespan are absent. Efforts are continuing to monitor children born following ovarian tissue transplantation to obtain information on their safety and health [10]. We previously reported fertility preservation via autografting in rhesus macaques and the first primate live birth derived from an oocyte after ovarian tissue transplantation subcutaneously to the abdomen and ARTs, i.e., in vitro fertilization (IVF) and embryo transfer [11]. Because ovarian structure, function, and regulation appear very similar in macaques and women, long-term studies in macaques may provide valuable information on offspring born following ovarian tissue transplantation.
Studies in rodents indicated that offspring born from ART procedures could have various health problems and short lifespan [12, 13], and it has been suggested that laboratory manipulations may induce epigenetic changes in oocytes [14], including telomere shortening, limiting the number of cell divisions that, in turn, could be responsible for aging at a cellular level [15].
Here, we report a follow-up investigation on the endocrine function over 8 years in macaques with ovarian tissue transplants in heterotopic subcutaneous sites. Studies were also performed to assess the reproductive competence, via spontaneous mating or ART, and the telomere length of a macaque derived from transplanted ovarian tissue.
Material and methods
Ovarian tissue transplantation and subsequent pregnancy
The general care and housing of rhesus macaques (Macaca mulatta) was provided by the Division of Comparative Medicine at the Oregon National Primate Research Center (ONPRC), Oregon Health & Science University (OHSU). Animals were pair-caged in a temperature-controlled (22 °C), light-regulated (12L:12D) room. The diet consisting of Purina monkey chow (Ralston-Purina, Richmond, IN, USA) was provided twice a day and supplemented with fresh fruit or vegetables once a day. Water was provided ad libitum. Animals were treated according to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals [16]. Protocols were approved by the ONPRC Institutional Animal Care and Use Committee.
This was a follow-up study including animals reported previously [11]. Seven oophorectomized macaques received fresh ovarian cortical tissue transplants autologously to the arm and abdomen (n = 4), kidney and abdomen (n = 2), and arm only (n = 1). Metaphase II (MII) oocytes were retrieved from transplants following recombinant human chorionic gonadotropin treatment and fertilized via intracytoplasmic sperm injection (ICSI). Following embryo transfer to the oviduct, an infant monkey was born in 2003 and named BRENDA.
Ovarian steroid assay
Peripheral blood samples were collected from monkeys following ovarian tissue transplantation on every Monday, Wednesday, and Friday for a year. Serum estradiol (E2) and progesterone (P4) concentrations were assayed using radioimmunoassay by the Endocrine Technology Support Core at ONPRC [17]. After year 1, blood samples were collected three times per week when serum E2 was greater than 20 pg/ml or P4 was greater than 2 ng/ml. Otherwise, phlebotomy was reduced to weekly draws. For monkeys with the arm and abdomen transplants, three were followed, i.e., blood sampling, for 2 years, and one was followed for 3 years. For monkeys with the kidney and abdomen transplants, one was followed for 8 years while the other was not followed due to significant scarring in transplant sites resulting from the surgery. The monkey with arm transplants was followed for 2 years.
Spontaneous mating and pregnancy monitoring
After birth, the general health of BRENDA was monitored by the veterinary staff in the Division of Comparative Medicine (DCM) at ONPRC. When BRENDA reached puberty and was of reproductive age (5 years old), she was transferred to the Time-mated Breeding Unit, DCM, ONPRC, where she was monitored for menses. Five days after menses, serum E2 and P4 levels were measured every other day. When serum E2 reached 200 pg/ml, BRENDA was paired with a reproductively competent male, whose prior fecundity was five pregnancies in 13 time-mated breedings. Pairing lasted for 5 days until ovulation was confirmed by mid-cycle E2 surge and rising serum P4 levels [18]. Pregnancy was confirmed at 7 weeks after the successful mating by serum P4 levels > 1.0 ng/ml and fetal cardiac activity using a GE Medical Systems Voluson© 730 Expert Doppler ultrasound instrument (General Electric Company, Waukesha, WI, USA). Fetal cardiac activity and biparietal diameter were also measured by ultrasonography at week 20, the third trimester of the typical 165-day gestation period. The third trimester ultrasound was used to estimate delivery date. A vaginal delivery of offspring occurred at the expected due date [18]. After being sufficiently postpartum, BRENDA was paired again at age 6 with the same male and conceived. This time, a Cesarean section was performed at the expected due date by the veterinary staff as described previously [18].
Controlled ovarian stimulation and oocyte retrieval
At ages 7–8, BRENDA underwent three controlled ovarian stimulations, followed by oocyte aspiration, performed by the ART Support Core at ONPRC as previously described [19]. First, 30 IU recombinant human follicle-stimulating hormone (FSH; IM, bid; NV Organon, Oss, Netherlands) was administered on menstrual cycle days 1–8, followed by 30 IU recombinant human FSH plus 30 IU recombinant human luteinizing hormone (IM, bid; EMD Serono, Inc., Rockland, MA, USA) on cycle days 7–8, and then a single injection of 1500 IU recombinant human chorionic gonadotropin (hCG; IM; Ovidrel; EMD Serono, Inc.) on cycle day 8. Laparoscopic oocyte retrieval was performed 35 h later. Serum steroid levels were assayed, antral follicle growth was evaluated by ultrasonography, and oocyte meiotic status was assessed via microscopy by the ART Support Core. During the same time period, the ART Support Core also performed controlled ovarian stimulations in additional nine age-matched monkeys using the same protocol with one stimulation per monkey.
Telomere length assay
A 3 ml blood sample was collected from BRENDA at age 7 and from 12 healthy control monkeys, both males and females of various ages (Supplemental Table 1). DNA was extracted using a DNeasy Blood and Tissue Kit (Qiagen, Inc., Valencia, CA, USA). Telomere length was assayed as previously described [20, 21] with modifications. Briefly, 10 ng DNA was used per reaction. DNA signal for telomere specific sequences were amplified by real-time PCR using a QuantiFast SYBR Green PCR kit (Qiagen, Inc.). Sequences of primers (900 nM/l) were (5′-3′) as follows: Telg, ACACTAAGGTTTGGGTTTGGGTTTGGGTTTGGGTTAGTGT; Telc, TGTTAGGTATCCCTATCCCTATCCCTATCCCTATCCCTAACA; Albugcr2, CGGCGGCGGGCGGCGCGGGCTGGGCGGCCATGCTTTTCAGCTCTGCAAGTC; and Albdgcr2, GCCCGGCCCGCCGCGCCCGTCCCGCCGAGCATTAAGCTCTTTGGCAACGTAGGTTTC. Thermal cycling consisted of 1 cycle of 15 min at 95 °C; 2 cycles of 15 s at 94 °C and 15 s at 49 °C; and 32 cycles of 15 s at 94 °C, 10 s at 62 °C, 15 s at 74 °C with signal acquisition, 10 s at 84 °C, and 15 s at 88 °C with signal acquisition. The 74 °C signal provided the cycle thresholds (Cts) for telomeres, and the 88 °C signal provided the Cts for the single copy gene (albumin). Telomere values were quantified using an Applied Biosystems 7900HT Fast Real-Time PCR System (Thermo Fisher Scientific Inc., Grand Island, NY, USA). The standard curve method was used to determine the T (telomere) and S (standard single copy gene) values for each experimental sample. Masked samples were run in triplicate, and the average was reported. For a given experimental sample, the T value is the number of nanograms of the reference DNA that matches the experimental sample for copy number of the telomere template. The S value is the number of nanograms of the reference DNA that matches the experimental sample for copy number of the standard single copy gene template. T/S, therefore, is a relative and dimensionless value. A sample with a T/S ratio greater than 1.0 has an average telomere length greater than that of the standard single copy gene. A sample with a T/S ratio less than 1.0 has an average telomere length shorter than that of the standard single copy gene [21].
Statistical analysis
Statistical analysis was performed using SigmaPlot 11 software (SPSS, Inc., Chicago, IL, USA). Oocyte numbers and relative telomere lengths were analyzed for BRENDA and control animals. Values are presented as mean ± SEM for control animals. A standard normal distribution table was used to identify the area under the standard normal curve in order to determine the probability of a specified range of distribution.
Results
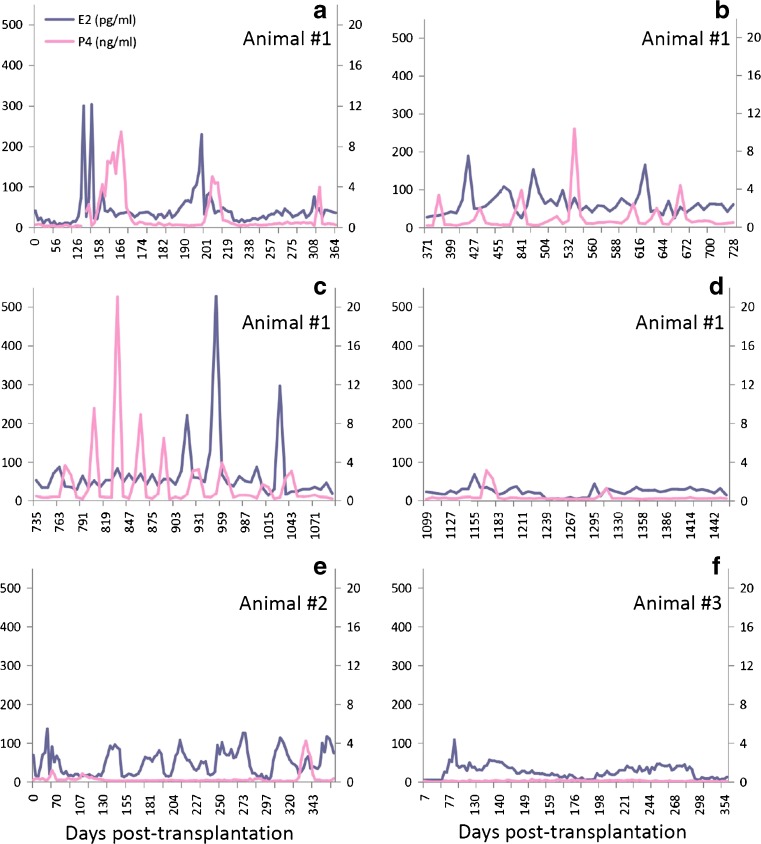
Serum concentrations of E2 were greater than 50 pg/ml in all monkeys within 150 days of ovarian tissue transplantation regardless of transplantation sites as previously reported [11]. A monkey with heterotopic ovarian tissue transplants in the abdomen and kidney ovulated sporadically for 3 years with E2 levels of 150–300 pg/ml and P4 levels greater than 3 ng/ml (Fig. 1a–c). The animal had two E2 surges in year 4 (Fig. 1d) and intermittent E2 production of 50 pg/ml up to year 8 post-transplantation (data not shown). The four monkeys with ovarian tissue transplants in the abdomen and arm had serum E2 levels that regularly increased to 100 pg/ml, with rare rises in P4 > 3 ng/ml, during the first year (representative data in Fig. 1e). Production of E2 and P4 became sporadic in year 2 post-transplantation, and only one animal had transient increases in E2 in year 3 (data not shown). The monkey with transplants in the arm only had 1 year of limited E2 levels up to 50 pg/ml, with no P4 rises (Fig. 1f), and then hormone levels became non-detectable in serum (data not shown). The menstrual cycle records for each monkey post-transplantation are summarized in Supplemental Table 2.
Fig. 1.
Serum estradiol (E2; blue line) and progesterone (P4; pink line) concentrations in representative monkeys with ovarian tissue transplants in the abdomen and kidney during years 1–4 (Animal #1; a–d), in the abdomen and arm during year 1 (Animal #2; e), and in the arm only during year 1 (Animal #3; f)
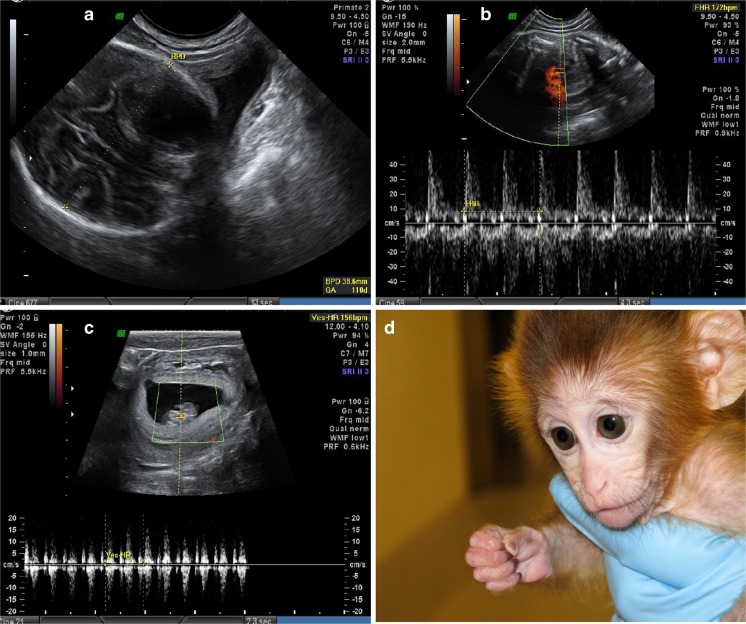
BRENDA was born following IVF and embryo transfer of an MII oocyte derived from ovarian tissue transplanted subcutaneously as previously reported [11]. BRENDA reached menarche at age 2.0 and had four menses during the first year, though her age at the time of her first luteinizing hormone surge is unknown (puberty in female macaques occurs between 2 and 5 years). She then had six menstrual cycles at ages 3–4 and seven menstrual cycles at ages 4–5. She conceived at age 5 after her first pairing with a male. Ultrasound evaluation at approximately 20 weeks of pregnancy showed a biparietal diameter of 38.6 mm (Fig. 2a) and a fetal heart rate of 172 bpm (Fig. 2b), which are normal in macaque fetuses. At term, BRENDA went into spontaneous labor, but arrested during the second stage resulting in intrapartum demise. An autopsy documented a normal term female infant with facial edema and intracranial hemorrhage consistent with a second-stage arrest. BRENDA resumed regular menstrual cycles after being sufficiently postpartum. She was paired again at age 6 and conceived on her first pairing. Ultrasound evaluation at approximately 7 weeks of pregnancy identified a normal fetal heart rate of 156 bpm (Fig. 2c). An elective Cesarean section at 157 days gestation delivered a healthy term female infant named Elizabeth OCTAVIA for “ovarian cortical transplant and viable infant afterwards” (Fig. 2d). OCTAVIA was nursed by a foster dam and reached menarche at age 2.5. She had three menstrual cycles at ages 2–3 and five menstrual cycles at ages 3–4, though her age at the time of her first luteinizing hormone surge is unknown.
Fig. 2.
Ultrasound images of biparietal diameter measurement (a) and fetal heart rate (b) of a 20-week fetus during BRENDA’s first spontaneous pregnancy, and fetal heart rate (c) of a 7-week fetus during BRENDA’s second spontaneous pregnancy. A healthy female infant monkey was born from BRENDA’s second pregnancy and named Elizabeth OCTAVIA (d)
BRENDA resumed regular menstrual cycles after the second pregnancy. During her seventh year, she underwent three controlled ovarian stimulations. She responded to each gonadotropin treatment as indicated by elevated serum E2 levels (peak level > 2000 pg/ml) prior to hCG treatment and P4 levels (> 6 ng/ml) post-hCG treatment. The number of oocytes retrieved per protocol from BRENDA and age-matched controls is summarized in Table 1. During the first stimulation, 18 MII oocytes were retrieved from BRENDA, with 23 ± 6 collected from control animals. Under the assumption that data from the control animals followed normal distribution, there was a 40.5% chance to observe an MII oocyte number as large as or less than 18 in the control population. At age 8, BRENDA also served as a surrogate dam on one occasion in the ART Support Core. She became pregnant following a single embryo transfer with three fetuses collected by a Cesarean section at 62 days gestation for another research study.
Table 1.
Numbers of oocytes retrieved from BRENDA and nine age-matched control monkeys following controlled ovarian stimulation
| Oocytes | BRENDA | Controls | ||
|---|---|---|---|---|
| 1st | 2nd | 3rd | 1 stimulation/monkey | |
| Total | 102 | 88 | 56 | 70 ± 14 |
| Metaphase II | 18 | 15 | 5 | 23 ± 6 |
| Metaphase I | 21 | 14 | 7 | 23 ± 10 |
| Germinal vesicle | 54 | 54 | 40 | 20 ± 6 |
| Degenerated | 9 | 5 | 4 | 4 ± 1 |
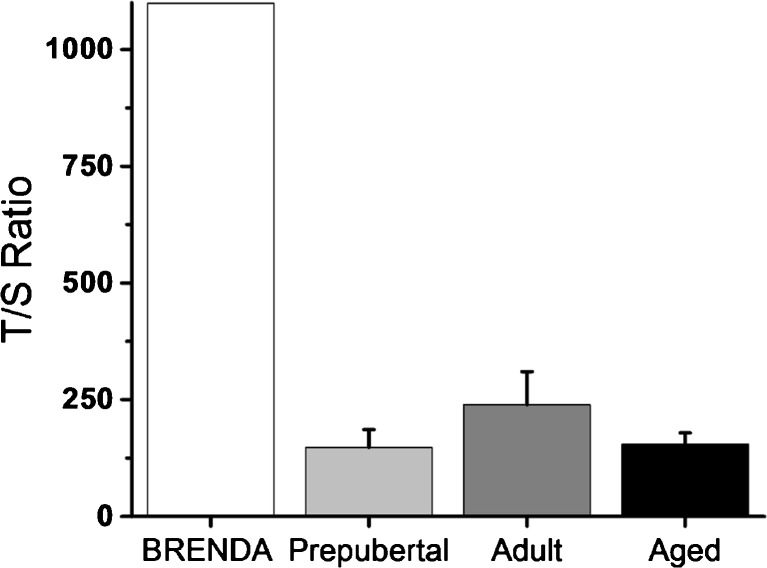
The ratio of copies of telomeres to copies of the standard single copy gene (T/S) was 1098.7 for BRENDA and 173.2 ± 24.3 for 12 control animals (147.7 ± 38.2 for prepubertal animals; 239.0 ± 70.9 for adult animals; 154.0 ± 25.1 for aged animals; Fig. 3). Under the assumption that results from the control animals followed a normal distribution, the probability of observing a T/S value as large as or greater than 1098.7 in the control population was 0.0%. BRENDA is currently 14 years old (similar to a 35-year-old woman).
Fig. 3.
Relative numbers of telomere copies to standard single copy gene copies (T/S) for BRENDA and control monkeys (four prepubertal animals, three adult animals, and five aged animals). Values are presented as mean ± SEM for control animals
Discussion
Ovarian tissue transplantation provides fertility preservation options to women facing gonadotoxic medical treatment, including cancer therapy. Compared with orthotopic transplantation, a heterotopic transplantation site can offer advantages including (a) greater numbers of ovarian tissue fragments transplanted, (b) ease of the transplantation procedure, and (c) better access for follicular monitoring and oocyte collection [7, 22]. To date, the optimal site for heterotopic ovarian tissue transplantation has not been clearly defined [7], though a few studies investigated subcutaneous sites. Ovarian function resumed after transplantation subcutaneously to the forearm [23] and the rectus muscle of the abdomen [6], but offspring have not been reported. In the current study, the animal with heterotopic transplants to the kidney and abdominal muscle exhibited the longest endocrine function, demonstrating the success of transplanting ovarian tissue to well-vascularized tissue. Animals with transplants to the arm and abdominal muscle had relatively less steroid hormone function, and the animal with transplants to the arm only had the least E2 production for the shortest period of time. The results are consistent with the duration of endocrine function described in heterotopic ovarian transplantation to subcutaneous sites in women. Fresh ovarian tissue resumed function after being transplanted subcutaneously to the forearm of women as indicated by periodic menstruation and follicular development for about 2 years post-transplantation [23]. Patients with frozen-thawed ovarian tissue transplanted to the rectus muscle of the abdomen were followed for 10 years in a previous study [6], wherein endocrine function in one patient continued for 7 years post-transplantation. However, for the current investigation, the study design is limited in that it did not compare single sites of transplantation.
While there have been concerns about the reproductive competence of mouse offspring born via ART [24] in 1999, Louise Brown became the first IVF-born baby to have a child of her own, indicating that women born from IVF can have healthy children. However, ovarian tissue transplantation adds complications to IVF treatment due to significant hypoxia during transplantation that may affect subsequent follicular development and oocyte maturation [6, 7]. Therefore, it is encouraging to demonstrate that BRENDA, born after ART procedures with an oocyte derived from ovarian tissue transplantation to a subcutaneous site, was able to achieve timely puberty and experienced three pregnancies via time-mated breeding or IVF resulting in a normal offspring. BRENDA also yielded comparable numbers of MII oocytes to those of age-matched control monkeys during controlled ovarian stimulation cycles, further illustrating her normal reproductive potential. In addition, Elizabeth OCTAVIA, daughter of BRENDA, reached puberty at the expected age for female macaques, portending normal reproductive development in the second generation.
There is growing evidence that an adverse in vitro environment for oocytes and subsequently derived embryos may negatively impact health of the offspring, e.g., mice demonstrate long-term detrimental health effects with epigenetic origins in IVF offspring following growth of immature oocytes and preimplantation embryos in suboptimal conditions in vitro [13, 25]. Here, BRENDA was examined for telomere length in peripheral white blood cells to evaluate potential epigenetic issues caused by non-native conditions during ovarian tissue transplantation, oocyte retrieval, and IVF. Surprisingly, BRENDA did not show premature cellular aging, as suggested by shortened telomeres [15], or compromised reproductive function, as predicted by short telomeres in human oocytes [26]. In contrast, BRENDA’s telomeres were longer than those of control monkeys. Telomerase activity in oocytes and early embryos is minimal and increases only at the blastocyst stage [27]. A DNA double-strand break mechanism called alternative lengthening of telomeres robustly elongates telomeres [27]; this mechanism is evident during early embryo development and may explain the long telomeres in BRENDA. Alternatively, there is evidence that hypoxia conditions can alter telomere length. Mild hypoxia increased telomerase activity and telomere length in human umbilical vein endothelial cells [28, 29], vascular smooth muscle cells [30], and stem cells [31]. Given the existence of a genetic component to telomere length, the long telomeres in BRENDA may also have been inherited from her parents. With preliminary data from only one animal, no definitive conclusions can be drawn based on the current finding and further studies are clearly warranted on oocytes, embryos, and offspring derived from heterotopic and orthotopic ovarian tissue transplantation.
In summary, fresh ovarian tissue transplanted to heterotopic subcutaneous sites can maintain ovarian steroid hormone production for years. BRENDA, the first primate derived from an oocyte in subcutaneously transplanted ovarian tissue, is fertile and also produced normal metaphase II oocytes during IVF cycles. Her telomeres do not appear to be shortened. While clinical information is collected continuously in order to gain general acceptance of ovarian tissue transplantation as an established procedure [10], results from the current nonhuman study are informative for the future of ovarian tissue banking with subsequent transplantation to improve fertility in women, including cancer patients.
Electronic supplementary material
(DOCX 14.8 kb)
(DOCX 15.8 kb)
Acknowledgements
We are grateful for the assistance provided by members of the Division of Comparative Medicine, the Endocrine Technology Support Core, the ART Support Core, and the Biostatistics and Bioinformatics Unit at ONPRC. We appreciate Drs. Theodore R. Hobbs, Dave L. Hess, and Byung S. Park for their valuable expertise.
Funding
This work was supported by the National Institutes of Health (NIH) Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) UL1 RR024926 (the Oncofertility Consortium: RL1 HD058293, HD058295, PL1 EB008542), NIH NICHD through cooperative agreement as part of the Specialized Cooperative Center Program in Reproduction and Infertility Research U54 HD18185, NIH Office of the Director P51OD011092 (Oregon National Primate Research Center), and the Bidwell Foundation.
Compliance with ethical standards
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-017-1019-y) contains supplementary material, which is available to authorized users.
References
- 1.Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001;7:535–543. doi: 10.1093/humupd/7.6.535. [DOI] [PubMed] [Google Scholar]
- 2.Donnez J, Dolmans MM. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet. 2015;32:1167–1170. doi: 10.1007/s10815-015-0544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Müller A, Keller K, Wacker J, Dittrich R, Keck G, Montag M, et al. Retransplantation of cryopreserved ovarian tissue: the first live birth in Germany. Dtsch Arztebl Int. 2012;109:8–13. doi: 10.3238/arztebl.2012.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunlop CE, Brady BM, McLaughlin M, Telfer EE, White J, Cowie F, et al. Re-implantation of cryopreserved ovarian cortex resulting in restoration of ovarian function, natural conception and successful pregnancy after haematopoietic stem cell transplantation for Wilms tumour. J Assist Reprod Genet. 2016;33:1615–1620. doi: 10.1007/s10815-016-0805-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van der Ven H, Liebenthron J, Beckmann M, Toth B, Korell M, Krüssel J, on behalf of the FertiPROTEKT network et al. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Hum Reprod. 2016;9:2031–2041. doi: 10.1093/humrep/dew165. [DOI] [PubMed] [Google Scholar]
- 6.Kim SS. Assessment of long term endocrine function after transplantation of frozen-thawed human ovarian tissue to the heterotopic site: 10 year longitudinal follow-up study. J Assist Reprod Genet. 2012;29:489–493. doi: 10.1007/s10815-012-9757-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SS. Revisiting the role of heterotopic ovarian transplantation: futility or fertility. J Asst Reprod Genet. 2014;28:141–145. doi: 10.1016/j.rbmo.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 8.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 9.Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. New Eng J Med. 2005;355:318–321. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 10.Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P, Andersen CY. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet. 2017;34:325–336. doi: 10.1007/s10815-016-0843-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DM, Yeoman R, Battaglia DE, Stouffer RL, Fanton JW, Wolf DP. Birth of a monkey after heterotopic transplantation of fresh ovarian tissue and assisted reproduction. Nature. 2004;428:137–138. doi: 10.1038/428137a. [DOI] [PubMed] [Google Scholar]
- 12.Eppig JJ, O’Brien M. Development in vitro of mouse oocytes from primordial follicles. J Biol Reprod. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- 13.Eppig JJ, O'Brien MJ, Wigglesworth K, Nicholson A, Zhang W, King BA. Effect of in vitro maturation of mouse oocytes on the health and lifespan of adult offspring. Hum Reprod. 2009;24:922–928. doi: 10.1093/humrep/den466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dennis C. Synthetic sex cells. Nature. 2003;424:364–366. doi: 10.1038/424364a. [DOI] [PubMed] [Google Scholar]
- 15.Blasco MA. The epigenetic regulation of mammalian telomeres. Nat Rev Genet. 2007;8:299–309. doi: 10.1038/nrg2047. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, et al. Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three-dimensional culture: effects of gonadotropins and insulin. Reproduction. 2010;140:685–697. doi: 10.1530/REP-10-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duffy DM, Stouffer RL. Follicular administration of a cyclooxygenase inhibitor can prevent oocyte release without alteration of normal luteal function in rhesus monkeys. Hum Reprod. 2002;17:2825–2831. doi: 10.1093/humrep/17.11.2825. [DOI] [PubMed] [Google Scholar]
- 18.Zelinski MB, Murphy MK, Lawson MS, Juirisicova A, Pau KYF, Toscano NP, et al. In vivo delivery of FTY720 prevents radiation-induced ovarian failure and infertility in adult female nonhuman primates. Fertil Steril. 2011;95:1440–1445. doi: 10.1016/j.fertnstert.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouhibi N, Zelinski-Wooten MB, Thomson JA, Wolf DP. Assisted fertilization and nuclear transfer in nonhuman primates. In: Wolf DP, Zelinski-Wooten MB, editors. Contemporary endocrinology™ assisted fertilization and nuclear transfer in mammals. Totowa: Humana Press, Inc.; 2001. pp. 253–284. [Google Scholar]
- 20.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37:e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lan Q, Cawthon R, Gao Y, Hu W, Hosgood HD, III, Barone-Adesi F, et al. Longer telomere length in peripheral white blood cells is associated with risk of lung cancer and the rs2736100 (CLPTM1L-TERT) polymorphism in a prospective cohort study among women in China. PLoS One. 2013;8:e59230. doi: 10.1371/journal.pone.0059230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod Update. 2009;15:649–665. doi: 10.1093/humupd/dmp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oktay K, Buyuk E, Rosenwaks Z, Rucinski J. A technique for transplantation of ovarian cortical strips to the forearm. Fertil Steril. 2003;80:193–198. doi: 10.1016/S0015-0282(03)00568-5. [DOI] [PubMed] [Google Scholar]
- 24.Li M, Zhao Y, Zhao CH, Yan J, Yan YL, Rong L, et al. High FSH decreases the developmental potential of mouse oocytes and resulting fertilized embryos, but does not influence offspring physiology and behavior in vitro or in vivo. Hum Reprod. 2013;28:1309–1323. doi: 10.1093/humrep/det014. [DOI] [PubMed] [Google Scholar]
- 25.Fernández-Gonzalez R, Moreira P, Bilbao A, Jiménez A, Pérez-Crespo M, Ramírez MA, et al. Long-term effect of in vitro culture of mouse embryos with serum on mRNA expression of imprinting genes, development, and behavior. Proc Natl Acad Sci U S A. 2004;101:5880–5885. doi: 10.1073/pnas.0308560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keefe DL, Liu L, Marquard K. Telomeres and aging-related meiotic dysfunction in women. Cell Molec Life Sci. 2007;64:139–143. doi: 10.1007/s00018-006-6466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keefe DL. Telomeres, reproductive aging, and genomic instability during early development. Reprod Sci. 2016;23:1612–1615. doi: 10.1177/1933719116676397. [DOI] [PubMed] [Google Scholar]
- 28.Guan J-Z, Guan W-P, Maeda T, Makino N. Different levels of hypoxia regulate telomere length and telomerase activity. Aging Clin Exp Res. 2012;24:213–217. doi: 10.1007/BF03325250. [DOI] [PubMed] [Google Scholar]
- 29.Guan J-Z, Guan W-P, Maeda T, Makino N. Alteration of telomere length and subtelomeric methylation in human endothelial cell under different levels of hypoxia. Arch Med Res. 2012;43:15–20. doi: 10.1016/j.arcmed.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Minamino T, Mitsialis SA, Kourembanas S. Hypoxia extends the life span of vascular smooth muscle cells through telomerase activation. Mol Cell Biol. 2001;21:3336–3342. doi: 10.1128/MCB.21.10.3336-3342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davy P, Allsopp R. Hypoxia: are stem cells in it for the long run? Cell Cycle. 2011;10:206–211. doi: 10.4161/cc.10.2.14535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 14.8 kb)
(DOCX 15.8 kb)