Abstract
Purposes
At the moment of sex reassignment surgery (SRS), the ovarian tissue is sometimes cryopreserved as fertility preservation option for female-to-male trans men, also called trans men. During this preparation, cumulus-oocyte-complexes (COCs) can be found and in vitro matured. It is not known if these oocytes are developmentally competent. In order to use these oocytes for fertility preservation and subsequent fertilization, a normal spindle structure before and after vitrification is necessary.
Methods
A total of 680 COCs were collected from trans men (n = 16) at the time of SRS and after testosterone treatment. The COCs were subjected to in vitro maturation and those that reached the metaphase II stage (MII) were collected and split into two groups; group 1 was immediately fixed for spindle staining and group 2 was first vitrified and warmed followed by spindle staining. Statistical analysis was performed by Fisher’s exact test.
Results
After 48 h in vitro maturation, 38.1% of COCs were at MII stage. Those oocytes were split in two groups: (1) 126 MII oocytes in the noncryopreservation group and (2) 133 MII oocytes underwent cryopreservation through vitrification. The oocyte survival rate, after 2 h warming, was 67.7%. Both the noncryopreserved and the vitrified group showed comparable results concerning normal spindle structure and chromosomes alignment, 85.7% vs. 92.2% (P = 0.27).
Conclusions
Spindle structure analysis and chromosomal alignment after vitrification seem normal in in vitro matured COCs collected during the tissue processing of ovaries in trans men at the time of SRS. The MII oocytes do not seem to be morphologically affected by prolonged testosterone treatment.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-017-0976-5) contains supplementary material, which is available to authorized users.
Keywords: Human ovarian medulla tissue, Metaphase II oocytes, Vitrification, Spindle structure, In vitro maturation
Introduction
Gender dysphoria is a condition in which a person experiences discrepancy between the sex assigned at birth and the gender they identify themselves with. In our centre, female-to-male, also called trans men, are treated in a multi-disciplinary approach, including cross-sex hormone therapy and if desired by the patient, sex reassignment surgery (SRS). Hormone therapy with anti-oestrogens and androgens is used in the majority of trans men. After at least 1 year of masculinisation therapy, SRS can be offered. This SRS results in an irreversible loss of natural reproductive capabilities in trans men. During the last decade, trans men present themselves at a much younger age, when they do not yet have children nor have a defined fertility wish [1]. The need to discuss reproductive choices with transgender persons before starting hormonal treatment is therefore necessary. For preservation of their fertility options, in theory, there are three possibilities available for trans men: oocyte banking, embryo banking and banking of the ovarian tissue (OT) [2]. The study of Wierckx et al. [3] showed that in a questionnaire half of the corresponding trans men would like to have children and 37.5% would have cryopreserved oocytes if it had been possible; however, cryopreservation of oocytes requires hormonal stimulation, oocyte retrieval and oocyte vitrification. Hormonal stimulation has to be performed before cross hormone treatment and includes multiple endovaginal ultrasound monitoring, which might be perceived as emotionally difficult in this patient cohort. Additionally, the majority of the patients are in favour of a fast transition. For these reasons, oocyte cryopreservation is most of the time not the preferred fertility preservation technique chosen by trans men. If taking steps towards preservation of fertility, most trans men will prefer to cryopreserve the OT at the time of hysterectomy and oophorectomy. This choice will not require an additional surgical procedure, nor frequent vaginal ultrasound monitoring and does not defer the patient from a fast transition process.
It has been shown that the androgen treatment does not deplete the primordial follicles present in the cortical ovarian strips from trans men, nor does it affects the developmental capacity of the follicles upon xenotransplantation [4]. However, the results of in vitro maturation (IVM) of human primordial follicles starting from the cryopreserved OT without the use of xenotransplantation are still far for implementation in a clinical setting [5–7]. The studies involving optimisation of this technique are mostly situated in the field of oncology where patients would have the chance to fulfil their child wish using their own gametes after being declared cancer free. This technique could also be potentially interesting for trans men. The future use of the cryopreserved OT is transplantation and although technically possible, it would restore female endocrine function, which might be an unwanted side effect for trans men. IVM of primordial follicles towards mature oocytes is not a realistic option at this moment; however, there might be other possibilities aside from ovarian stimulation to obtain mature oocytes. The cryopreservation of OT implies that the cortical region comprised of the primordial follicles is cryopreserved, and the medulla, the inner part of the ovary, is discarded. Literature [8–10] has shown that this medulla tissue comprises of growing follicles, mostly antral follicles. The immature oocytes obtained from these antral follicles are collected from nonstimulated ovaries as cumulus-oocyte-complexes (COCs). These COCs can subsequently be matured in vitro up to the metaphase II (MII) stage [11–13]. This option maximises the amount of functional oocytes preserved for the patient at the moment of processing and cryopreservation of the tissue. The matured MII oocytes can be stored by vitrification for future use in assisted reproduction [11–13].
Recently, our group [14] described a normal distribution of cortical follicles in the ovaries of trans men after more than a year of testosterone treatment. The work confirmed the presence and IVM potential of COCs obtained during the ovarian tissue processing. Moreover, normal spindle structures and chromosome alignments were detected in the resulting MII oocytes after 48 h IVM. In order to use these oocytes for fertility preservation purposes, cryopreservation by vitrification is necessary as most trans men do not yet have a defined child wish, nor a partner at the time of their transition and SRS. Before assessing fertilisation and embryo development studies in these oocytes, realistic oocyte survival warming rates and normal spindle structures and chromosome alignment after warming are necessary.
Methods
Collection of COCs
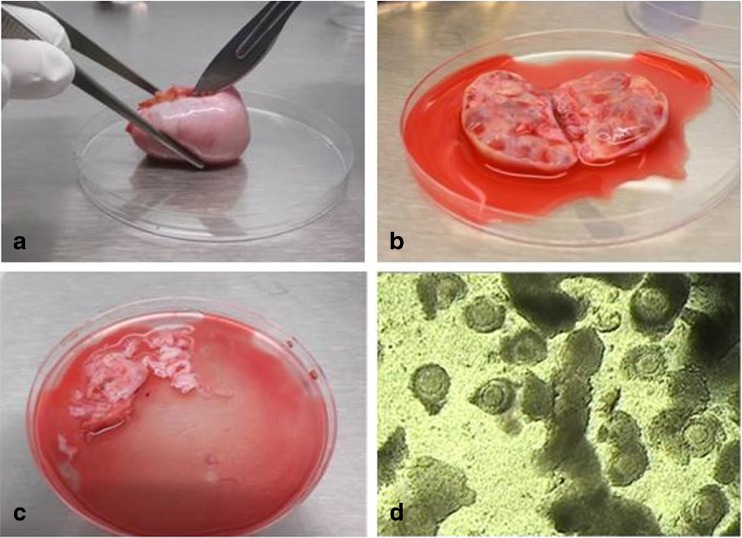
COCs were obtained from consenting trans men (n = 16) with a mean age of 24.1 ± 6.1 years during the OT cryopreservation. All trans men were on testosterone treatment prior to the OT preservation (on average 53.6 ± 21.0 weeks) (supplementary Table 1). During the process of isolating the ovarian cortex (Fig. 1a), the medulla was removed carefully by scraping with very sharp scalpels. During this process, the medulla was collected in the petri dish containing the manipulation medium (Fig. 1b), Leibovitz L-15* medium (Life Technologies, Merelbeke, Belgium), supplemented with 0.45% human serum albumin (Red Cross, Belgium). The dishes with the discarded material, the medulla and the manipulation medium in which the preparation of the cortical tissue took place were examined under the stereomicroscope (Olympus SZX 7) for the presence of COCs (Fig. 1c). The entire petri dish is carefully evaluated field by field. All COCs that were found were transferred to a prewarmed gamete buffer medium (Cook Ireland Ltd., Limerick, Ireland) at 37 °C that served as a collection medium during the entire procedure. All collected COCs were assumed to contain immature oocytes and were considered for IVM. All COCs were cultured in vitro for 44–48 h starting from the moment of COC collection. The COCs that reached the MII stage were collected and per patient randomly divided into two groups: group 1 was immediately fixed for spindle staining and analysis; and group 2 was first vitrified and warmed, those oocytes that survived after 2 h warming procedure were fixed for spindle staining and analysis. (Detailed numbers per study group and per patient are given in the supplementary Table 1.)
Fig. 1.
Ovaries obtained through laparoscopy (a); Bisected ovary with late antral follicles in which immature oocytes are located, clearly visible in the interior of the ovary (b), Remaining tissue (=medulla) with suspension of possible immature COCs (c) and COCs during manipulation of ovarian cortex tissue and before maturation (d)
The use of the tissues for the current study was approved by the Ghent University Hospital Ethical Committee (UZ Ghent reference: 2015/0124–Belgian registration number: B670201523543)
IVM medium and maturation assessment
All COCs were cultured individually in 25 μl drops of IVM medium overlaid with preincubated embryo-tested light mineral oil in a humidified atmosphere at 5% CO2, 6% O2 in air at 37 °C. The IVM preparation as described elsewhere [15] consisted of tissue culture medium 199 (TCM 199,Sigma, Bornem, Belgium) supplemented with 10 ng/ml epidermal growth factor (Sigma), 1 μg/ml estradiol (Sigma), 10 mIU/ml recombinant FSH (Puregon), 0.5 mIU/ml HCG (Pregnyl), 1 mM l-glutamine (Sigma), 0.3 mM sodium pyruvate (Sigma), 0.8% HSA, 100 IU/ml penicillin G (Sigma) and 100 μg/ml streptomycin sulphate (Sigma).
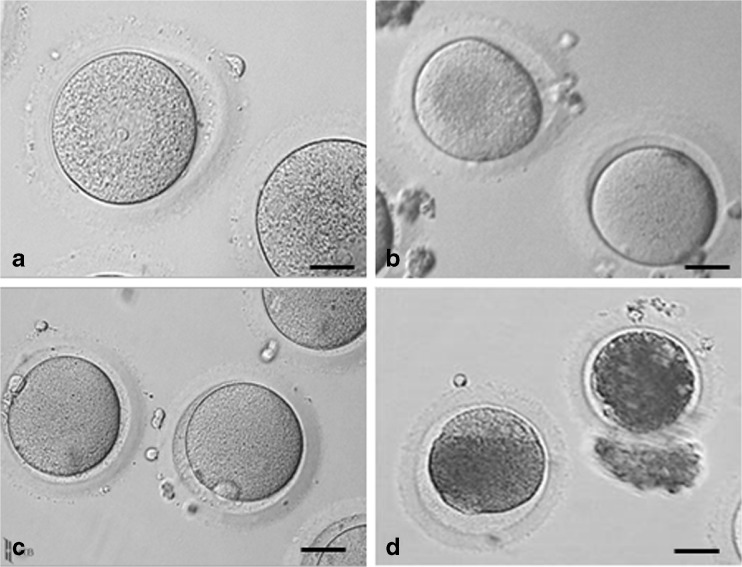
After 48 h IVM, cumulus cells were removed by mechanical denudation. The denuded oocytes were then evaluated based on their nuclear stage (Fig. 2), oocytes were classified as: germinal vesicle (GV) stage (Fig. 2a), GV breakdown (GVBD) or metaphase I (MI) (Fig. 2b), metaphase II (MII) (Fig. 2c) and degenerated oocytes (Fig. 2d). Degenerated oocytes were not further included in this study.
Fig. 2.
Oocyte maturation assessment after 44–48 h IVM. a Germinal vesicle oocytes (GV) (scale bar 40.0 μm). b Germinal vesicle breakdown (GVBD) oocyte or metaphase I oocytes (MI) (scale bar 42.1 μm). c metaphase II oocytes (MII) (scale bar 47.0 μm). d degenerated oocytes (scale bar 45.1 μm)
Vitrification and warming
Vitrification was carried out using the Irvine Scientific Vitrification Freeze Kit containing (i) an equilibration solution (ES) (7.5% (v/v) ethylene glycol +7.5% (v/v) dimethylsulphoxide (DMSO) in an M-199 HEPES-buffered medium supplemented with 20% dextran serum supplement and 35 μg/ml Gentamycin) and (ii) a vitrification solution (VS) (15% (v/v) ethylene glycol +15% (v/v) DMSO +0.5 M sucrose in a M-199 HEPES-buffered medium supplemented with 20% dextran serum supplement and 35 μg/ml Gentamycin). Briefly, the MII oocyte was placed in a 25 μl droplet for 1 min at 37 °C containing gamete buffer (Cook Ireland Ltd., Limerick, Ireland). The gamete buffer droplet containing the oocyte was merged with an adjacent droplet of 25 μl ES and kept at room temperature for 3 min. This droplet was merged with a second, new adjacent droplet of 25 μl ES and kept at room temperature for 3 min. Then the oocyte was placed in 25 μl ES for 6 min at room temperature. Finally the oocytes were incubated in four drops of 25 μl vitrification solution droplet for less than 1 min at room temperature, followed by loading the oocyte into a cryotop straw (Kitazato) using minimal volume. The top was plunged into liquid nitrogen. Moving the cryotop in the plastic straw was done in the liquid nitrogen.
For warming, the Irvine Scientific Vitrification Thaw Kit was used with some modifications. The kit contained (i) a thawing solution (TS) (1 mol/l sucrose in an M-199 HEPES-buffered medium supplemented with 20% DSS), (ii) a dilution solution (DS) (0.5 mol/l sucrose in an M-199 HEPES-buffered medium supplemented with 20% DSS) and (iii) a washing solution (WS) (M-199 HEPES-buffered medium supplemented with 20% DSS). The cryotop containing the oocyte was taken out of the liquid nitrogen and was immediately placed in 1 ml prewarmed TS for 1 min at 37 °C, followed by 3 min in DS (500 μl) and 5 min in WS (500 μl), both at room temperature. Finally, the oocytes were washed in the culture medium and placed in the incubator.
The survival rate of the warmed oocytes was assessed (a) immediately, (b) 1 h and (c) 2 h after warming. Oocytes were considered “morphologically survived” if there was no dark/ degenerated or contracted ooplasm present.
Spindle staining by immuno-fluorescent labelling
Fresh and vitrified MII oocytes were fixed and extracted in a microtubule-stabilizing buffer, as described elsewhere [16]. Briefly, to visualize microtubules, MII oocytes were incubated in the presence of a mixture of mouse monoclonal anti-α, β-tubulin (1:200; Sigma) overnight at 4 °C, followed by Alexa-Fluor-488 conjugated goat-anti-mouse immunoglobulin G (1:200; Molecular Probes, Life Technologies, Merelbeke, Belgium) for 2 h at 37 °C. Chromatin was stained with ethidium homodimer-2 (1:500; Molecular Probes, Life Technologies) for 1 h at 37 °C. Labelled MII oocytes were washed and mounted in Mowiol 4–88 (Sigma) containing 0.01% phenylenediamine (Sigma) as an anti-fading reagent.
Assessment of meiotic spindle and chromosome configuration by confocal microscopy
Images of labelled MII oocytes were observed using a Nikon scanning laser confocal microscope with a ×60 oil immersion objective (Nikon, NA = 1.4).
A three-dimensional image of the micro-tubular structure and chromosomes was rendered from Z-axis stacks (0.75 μm/step) by using ImageJ software (rsbweb.nih.gov/ij/), which was projected into a single image as shown in Fig. 3.
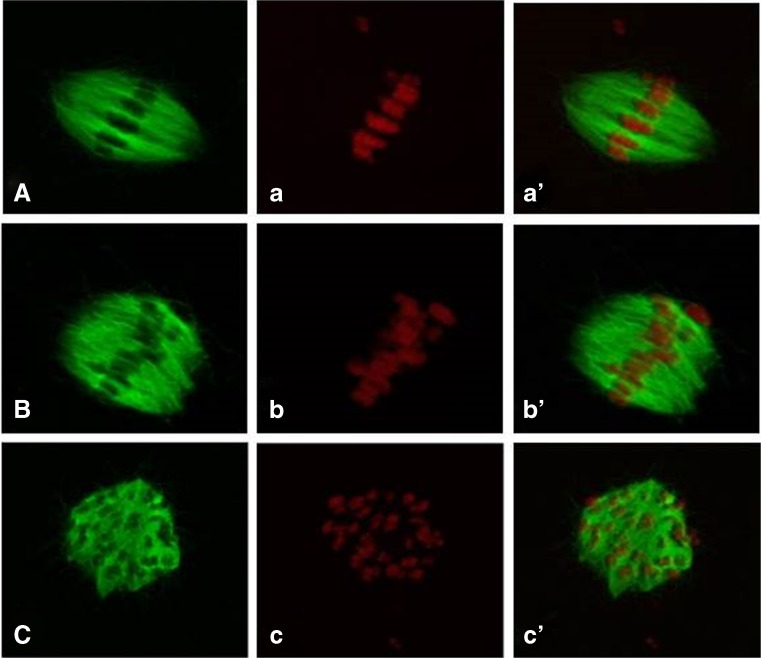
Fig. 3.
Confocal three-dimensional reconstructions of microtubules (green) and chromosomes (red) are showing representative patterns used in the classification of spindles as bipolar (A, a′), bipolar with irregularities at either the spindle poles (B, b′) or equator (C, c′). Similarly, chromosomes were classified as aligned (a, a′), mostly aligned with ≤6 chromosomes away from the spindle equatorial region (b, b′) and dispersed with >6 chromosomes away or all chromosomes located throughout the spindle (c, c′). In summary, normal spindle complexes are shown in a′ and b′ and an abnormal spindle complex is shown in c′
Meiotic spindle configuration of the oocytes was analysed based on the classification described by Combelles et al. [17]; normal, if microtubules were barrel shaped and formed two opposite pointed poles (Fig. 3A); bipolar, slightly aberrant, if shape was bipolar with no pointed poles (Fig. 3B); abnormal, if shape was bipolar with irregularities at the equator or at the poles; abnormal, if the microtubules were non bipolar shaped, including mono- or multi-polar instances (Fig. 3C). Based on chromosomal distribution at the metaphase plate, oocytes were classified as followed; normal, if chromosomes were aligned at equatorial plate (Fig. 3a); normal, if ≤6 chromosomes separated from the equatorial region (according to Combelles et al. [17]) (Fig. 3b); abnormal, if dispersed, or >6 chromosomes away from equatorial region or all chromosomes located throughout the spindle (according to Combelles et al. [17]) (Fig. 3c).
In this study, we distinguished two groups for the overall classification of the spindle formation, taken into account both the shape of the microtubules in combination with the chromosomal distribution: (i) normal spindle formation being an oocyte displaying a normal shaped spindle with aligned chromosomes (Fig. 3a′, b′) and (ii) abnormal spindle formation being an oocytes having an abnormal spindle shape (Fig. 3C) and/or having abnormal chromosomes position (Fig. 3c) were abnormal (Fig. 3c′).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 7 and SPSS 20. Data were analysed by Fisher’s exact and chi-square test as appropriate. Value of P < 0.05 indicated statistical significance.
Results
Maturation of COCs
In total, 680 COCs were collected from the medulla suspension of 16 trans men.
The average weight of both ovarian medullae per person was 7.28 ± 3.51 g. The average number of COCs collected per trans men was 42.5, ranging from minimum 5 to maximum 174 COCs. After 48 h IVM, the overall MII oocyte rate was 38.1% (259/680), of which 27.1% (184/680) reached the MI-stage, 18.1% (123/680) were at the GV-stage and 16.8% (114/680) were degenerated oocytes.
The IVM rate for trans men below 20 years of age (31.7%) was comparable with patients aged 20–30 years (39.0%) and above 30 years (30.2%) (P = 0.38) (Table 1).
Table 1.
IVM rate of COCs collected during the processing of two whole ovaries, according to the age of the trans men included in the study
| Age | Patients (n) |
Age mean ± SD |
AMH mean (range) |
Oocytes collected (n) (range) |
Oocytes collected/patient mean ± SD |
IVM rate (%) (range) |
|---|---|---|---|---|---|---|
| <20 years | 4 | 18.3 ± 0.5 | 8.4 (1.3–17.0) | 245 (7–174) | 61.3 ± 76.2 | 31.7 (14.3–46.2) |
| 20–30 years | 9 | 23.0 ± 2.9 | 5.5 (0.8–17.0) | 369 (5–118) | 41.0 ± 35.7 | 39.0 (20.5–63.6) |
| >30 years | 3 | 35.0 ± 1.0 | 2.2 (2.0–2.4) | 66 (17–27) | 22.0 ± 5.0 | 30.2 (22.7–44.4) |
No statistical significant differences were observed in the IVM rate after 44–48 h IVM culture, among the three groups.
Vitrification–Warming
Those 259 MII oocytes were split randomly into two groups: (group 1) 126 MII oocytes in the nonvitrification group, and (group 2) 133 MII oocytes underwent the vitrification–warming procedure. Immediately after warming, the survival rate was 73.7% (98/133); after 1 h postwarming 68.4% (91/133) and after 2 h postwarming 67.7% (90/133). These 90 survived MII oocytes were fixed and stained for spindle and chromosome analysis. The postwarming survival of oocytes from trans men below 20 years of age (71.3%) was comparable with patients aged 20–30 years (69.7%) and above 30 years (66.7%) (P = 0.38) (Table 2).
Table 2.
Survival rate of vitrified-warmed oocytes according to the age of the trans men
| Age | Patients (n) |
Age mean ± SD |
AMH mean (range) |
IVM rate (%) |
Vitrified MII oocytes (n) (range) |
Survived oocytes after 2 h warming (%) (range) |
|---|---|---|---|---|---|---|
| <20 years | 4 | 18.3 ± 0.5 | 8.4 (1.3–17.0) | 31.7 | 50 (1–39) | 71.3 (25.0–100.0) |
| 20–30 years | 9 | 23.0 ± 2.9 | 5.5 (0.8–17.0) | 39.0 | 72 (1–24) | 69.7 (33.3–100.0) |
| >30 years | 3 | 35.0 ± 1.0 | 2.2 (2.0–2.4) | 30.2 | 11 (2–6) | 66.7 (50.0–83.3) |
No statistical significant differences were observed in the survival rate after 2 h warming between the three age groups
Chromosome arrangement and spindle organization
Normal spindle complexes, displaying a normal spindle and a normal chromosome alignment were found in both the nonvitrified and the vitrified group (87.1 and 92.2%, respectively) (P = 0.27) (Table 3). Looking more in detail separately to the spindle on the one hand and the chromosome alignment on the other hand, the following observations were made: Bipolar spindles with pointed poles were found in over 50% of the IVM oocytes both in the nonvitrified compared to the vitrified group (57.3% (71/124) vs. 53.3% (48/90), P = 0.58) and around 40% of the oocytes showed bipolar spindle structures in the absence of a pointed pole in both groups (nonvitrified 37.1% (46/124) vs. vitrified–warmed 41.1% (37/90), P = 0.57). The proportion of completely aligned chromosomes in the MII oocytes of the nonvitrified group was comparable to those of the vitrified group (66.1% (82/124) vs. 62.2% (56/90), P = 0.57).
Table 3.
Spindle characteristics and chromosome alignment of nonvitrified and vitrified-warmed oocytes
| Nonvitrified | Vitrified | P value | |
|---|---|---|---|
| No. of analysed MII-oocytes | 124 | 90 | |
| Normal spindle structureb | 108 (87.1) | 83 (92.2) | P = 0.27 |
| Abnormal spindle structurec | 16 (12.9) | 7 (0.78) | P = 0.27 |
| Detailed spindle pattern | |||
| Normal shape | 117 (94.4) | 85 (94.4) | P = 1.00 |
| Bipolar, pointed poles (%a) | 71 (57.3) | 48 (53.3) | P = 0.58 |
| Bipolar, flattened poles (%a) | 46 (37.1) | 37 (41.1) | P = 0.57 |
| Abnormal shape | 7 (5.6) | 5 (5.6) | P = 1.00 |
| Bipolar, irregularities at poles or equatorial plate (%a) | 3 (2.4) | 1 (1.1) | P = 0.64 |
| Non bipolar, mono- or or multi-polar (%a) | 4 (3.2) | 4 (4.5) | P = 0.73 |
| Detailed chromosome pattern | |||
| Normal | 108 (87.1) | 83 (92.2) | P = 0.27 |
| Aligned (%a) | 82 (66.1) | 56 (62.2) | P = 0.57 |
| Mostly aligned with ≤6 chromosomes away of equatorial plate (%a) | 26 (21.0) | 27 (30.0) | P = 0.15 |
| Abnormal | |||
| Dispersed (%a) | 16 (12.9) | 7 (7.8) | P = 0.27 |
aPercentage on the total number analysed metaphase II oocytes (MII)
bNormal spindle structure showed a normal spindle pattern combined with normal chromosome pattern
cAbnormal spindle structure showed an abnormal spindle pattern combination abnormal chromosome pattern
Oocytes where the chromosome alignment has ≤6 chromosomes slightly away of the equatorial plate are also considered normal (according to Combelles et al. [17]). The appearance of this specific subset of normal alignment was also comparable between the groups (21.0% (26/124) vs. 30.0% (27/90), P = 0.15) (Table 3).
Discussion
In the present study, the spindle structure before and after vitrification of IVM MII oocytes in trans men at the moment of ovarian tissue cryopreservation was investigated. IVM is used in literature for both the transvaginal ultrasound-guided aspiration of immature oocytes from visible antral follicles and the collection and maturation of COC while preparing the ovarian tissue. Even the latter technique is sometimes a combination of isolating the COCs from the visible antral follicles on the ovarian cortex by using a syringe on the one hand and the collection of the COCs after the cortex had been dissected from the medulla [13]. These different approaches of obtaining COCs have resulted in an amount of publications with many different in vitro maturation timings and different maturation rates. For the ease of interpretation of our findings and the possible differences in results found in literature, we would like to indicate that this report is solely on the IVM of COCs collected after the preparation of the OVArian tissue which we would like to call IViMOVA. This technique has also been called ex vivo collection of oocytes by several groups [11, 18]. During the preparation of the ovarian tissue, we did not aspirate the visible antral follicles first, the tissue was immediately processed for cortex preservation as was the case in reports by some other groups [8, 9, 13, 18].
The so-called IViMOVA technique has already been introduced many years ago [8, 9] for oncological patients and has recently gained new attention as IVM techniques have become more and more routine in IVF laboratories [10, 11, 13, 18].
This IViMOVA approach could also be interesting for other patient groups suffering from gonadotoxic treatments. Our research group is currently investigating if this approach could also be used for fertility preservation programs for trans men [14]. Ghent University Hospital has a center of excellence in transgender health and therefore, maybe more than other fertility centres, we are being consulted for fertility preservation tailored specifically to the needs of these patients. Since most of the trans men choose to undergo bilateral oophorectomy at the time of SRS, there is a possibility to obtain MII by means of IViMOVA at that time.
For all patients included in this study (see supplementary Table 1), COCs could be collected at the time of OT manipulation and all patients, except one, had at least two MII oocytes after IViMOVA. These oocytes were distributed randomly in a nonvitrified and vitrified group. For one trans man (18-years old), we could retrieve seven COCs and only one oocyte matured to the MII stage. We chose to vitrify the oocyte, which also survived the warming process and showed a normal spindle complex. There are some important differences in our setup compared to the data in literature that have to be taken into account. First, the amount of COCs collected in trans persons is on average three to four times higher than reported in literature [11, 13], since both ovaries are procured and prepared in trans patients instead of one ovary. This is in contrast to oncological patients, where in general a complete unilateral or even partial unilateral oophorectomy is performed. When comparing the amount of COCs to the age group of the patients [13], it is clear that on average the most COCs were collected in the group <20 years. The amount of COCs declines with the patients’ age in contrast with the results of Yin et al. [13] were also prepubertal children were included in the age group <20 years. In our study, the minimum age limit of a patients included in the study is 18 years. This is due to the Belgian legislation were SRS is only possible at the age of 18 years. Not only age, but also AMH is correlated with the amount of COCs found in IViMOVA and this is in accordance with previous results published by our group [14].
The average maturation rate found in literature, 36% [11], 31% [12] and recently 28.1% [18] are comparable with the maturation rate found in this study (38.1%) (range 30.2–39.0%). IViMOVA oocytes were randomly distributed in two groups where spindle analysis was performed on the fresh oocytes or after vitrification and warming. The survival rate of the IViMOVA oocytes obtained from trans men (67.7%) is comparable to what is found in literature on survival rates of IVM-vitrified oocytes derived from unstimulated and primed ovaries (67.5%) [19]. Based on the data from the COC collection, maturation and survival after vitrification and warming, all trans men in our patient cohort could potentially benefit from this fertility preservation technique. Before these oocytes can be used in a clinical setting, the developmental capacity needs to be addressed. A first qualitative marker for oocyte functionality is the demonstration of a normal spindle structure after vitrification and warming [17, 20, 21]. During the spindle analysis we could notice that there were two types of bipolar spindles, those who were pointed also called focused and those that were flattened. This phenomenon was seen both in the nonvitrified MII oocyte and those that were vitrified. We analysed the spindles by confocal imaging and did, however, not perform any morphometric measurements. The latter could have given more detailed information of the spindle length, width and microtubule volume. The group of Coticchio et al. [21] did study morphometric and morphologic parameters of human MII oocytes of stimulated cycles matured in vivo or left over cumulus free germinal vesicle oocytes matured in vitro and showed that none of the morphometric parameters was actually associated with chromosome alignment on the metaphase plate. Overall, the majority of the spindle formation in the IViMOVA oocytes before and after vitrification was found to be normal; 87.1% compared to 92.2%, respectively (P = 0.27).
In this report, we show that vitrification and warming does not result in a loss of correct spindle alignment.
Conclusion
The results from this study show that COCs derived from trans men’s ovarian tissue can be in vitro matured, vitrified and warmed and show normal spindle complexes. Although small in number, these oocytes might serve as a potential fertility preservation method for trans men. Fertility preservation is best performed before any gonadotoxic treatment, as would also be the best practice for trans persons starting cross hormone therapy. However, if this difficult decision would have been postponed in certain patients, it might be a possibility to obtain and preserve IViMOVA oocytes at the time of SRS since, at least from a morphological point of view, the oocytes seem not affected by testosterone treatment. We do emphasize that this possibility for trans men is, at the moment, considered extremely experimental and not to be introduced in the clinic at all since the fertilisation and normal embryo development has not yet been determined.
Electronic supplementary material
(DOCX 19 kb)
Acknowledgments
This work is supported by the Fonds voor Wetenschappelijk Onderzoek (FWO) TBM T001816 N and the Gender Identity Research and Education Society (GIRES) foundation.
Compliance with ethical standards
Author disclosure statements
No competing financial interests exist.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-017-0976-5) contains supplementary material, which is available to authorized users.
References
- 1.De Sutter P, Kira K, Verschoor A, Hotimsky A. The desire to have children and the preservation of fertility in transsexual women: a survey. International Journal of Transgenderism. 2002;6:3. [Google Scholar]
- 2.De Roo C, Tilleman K, T'Sjoen G, De Sutter P. Fertility options in transgender people. Int Rev Psychiatry. 2016;28:112–119. doi: 10.3109/09540261.2015.1084275. [DOI] [PubMed] [Google Scholar]
- 3.Wierckx K, Van Caenegem E, Pennings G, Elaut E, Dedecker D, Van de Peer F, Weyers S, De Sutter P, T’Sjoen G. Reproductive wish in transsexual men. Hum Reprod. 2012;27:483–487. doi: 10.1093/humrep/der406. [DOI] [PubMed] [Google Scholar]
- 4.Van den Broecke R, Van der Elst J, Liu J, Hovatta O, Dhont M. The female-to-male transsexual patient: a source of human ovarian cortical tissue for experimental use. Hum Reprod. 2001;16:145–147. doi: 10.1093/humrep/16.1.145. [DOI] [PubMed] [Google Scholar]
- 5.Telfer EE, McLaughlin M. In vitro development of ovarian follicles. Semin Reprod Med. 2011;29:15–23. doi: 10.1055/s-0030-1268700. [DOI] [PubMed] [Google Scholar]
- 6.Telfer EE, McLaughlin M. Strategies to support human oocyte development in vitro. Int J Dev Biol. 2012;56:901–907. doi: 10.1387/ijdb.130001et. [DOI] [PubMed] [Google Scholar]
- 7.Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod. 2008;23:1151–1158. doi: 10.1093/humrep/den070. [DOI] [PubMed] [Google Scholar]
- 8.Huang JY, Tulandi T, Holzer H, Tan SL, Chian RC. Combining ovarian tissue cryo banking with retrieval of immature oocytes followed by in vitro maturation and vitrification: an additional strategy of fertility preservation. Fertil Steril. 2008;89:567–572. doi: 10.1016/j.fertnstert.2007.03.090. [DOI] [PubMed] [Google Scholar]
- 9.Fasano G, Moffa F, Dechène J, Englert Y, Demeestere I. Vitrification of in vitro matured oocytes collected from antral follicles at the time of ovarian tissue cryopreservation. Reprod Biol and endocrinology. 2011;9:150. doi: 10.1186/1477-7827-9-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilken-Jensen HN, Kristensen SG, Jeppesen JV, Andersen CY. Developmental competence of oocytes isolated from surplus medulla tissue in connection with cryopreservation of ovarian tissue for fertility preservation. ACTA Obstetricia et Gynecologica Scnadinavica. 2013;93:32–37. doi: 10.1111/aogs.12264. [DOI] [PubMed] [Google Scholar]
- 11.Segers I, Mateizel I, Van Moer E, Smitz J, Tournaye H, Verheyen G, De Vos M. In vitro maturation (IVM) of oocytes recovered from ovarectomy specimens in the laboratory: a promising “ex vivo” method of oocyte cryopreservation resulting in the first report of an ongoing pregnancy in Europe. J Assist Reprod Genet. 2015;32:1221–1231. doi: 10.1007/s10815-015-0528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abir R, Ben-Aharon I, Garor R, Yaniv I, Ash S, Stemmer SM, Ben-Haroush A, Freud E, Kravarusic D, Sapir O, Fisch B. Cryopreservation of in vitro matured oocytes in addition to ovarian tissue freezing for fertility preservation in paediatric female cancer patients before and after cancer therapy. Hum Reprod. 2016;31:750–762. doi: 10.1093/humrep/dew007. [DOI] [PubMed] [Google Scholar]
- 13.Yin H, Kristensen SG, Jiang H, Rasmussen A, Yding Andersen C. Survival and growth of isolated pre-antral follicles from human ovarian medulla tissue during long-term 3D culture. Hum Reprod. 2016;31:1531–1539. doi: 10.1093/humrep/dew049. [DOI] [PubMed] [Google Scholar]
- 14.De Roo C, Lierman S, Tilleman K, Peynshaert K, Braeckmans K, Caanen M, Lambalk CB, Weyers S, T’Sjoen G, Cornelissen R, De Sutter P. Ovarian tissue cryopreservation in female-to-male transgender people: insights into ovarian histology and physiology after prolonged androgen treatment. RBM Online. 2017. doi:10.1016/j.rbmo.2017.03.008. [DOI] [PubMed]
- 15.Nikiforaki D, Vanden Meerschaut F, Qian C, De Croo I, Lu Y, Deroo T, Van den Abbeel E, Heindryckx B, De Sutter P. Oocyte cryopreservation and in vitro culture affect calcium signalling during human fertilization. Hum Reprod. 2014;29(1):29–40. doi: 10.1093/humrep/det404. [DOI] [PubMed] [Google Scholar]
- 16.Mattson BA, Albertini DF. Oogenesis: chromatin and microtubule dynamics during meiotic prophase. Mol Reprod Dev. 1990;25:374–383. doi: 10.1002/mrd.1080250411. [DOI] [PubMed] [Google Scholar]
- 17.Combelles CMH, Ceyhan ST, WangH RC. Maturation outcome are improved following cryoleaf vitrification of immature human oocytes when compared to choline-based slow-freezing. J Assist Reprod Genet. 2011;28:1183–1192. doi: 10.1007/s10815-011-9674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fasano G, Dechène J, Antonacci R, Biramane J, Vannin AS, Van Langendonckt A, et al. Outcomes of immature oocytes collected from ovarian tissue for cryopreservation in adult and prepubertal patients. Reprod BioMed Online. 2017; doi:10.1016/j.rbmo.2017.03.007. [DOI] [PubMed]
- 19.Shalom-Paz E, Almong B, Shehata F, Huang J, Holzer H, Chian RC, Son WY, Tan SL. Fertility preservation for breast-cancer patients using IVM followed by oocyte or embryo vitrification. Reprod BioMed Online. 2010;21:566–571. doi: 10.1016/j.rbmo.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Mandelbaum J, Anastasiou O, Lévy R, Guérin JF, de Larouzière V, Antoine JM. Effects of cryopreservation on the meiotic spindle of human oocytes. Eur J Obstet Gynecol Reprod Biol. 2004;5(113 Suppl 1):S17–S23. doi: 10.1016/j.ejogrb.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Coticchio G, Bromfield JJ, Sciajno R, Gambardella A, Scaravelli G, Borini A, Albertini DF. Vitrification may increase the rate of chromosome misalignment in the metaphase II spindle of human mature oocytes. Reprod BioMed Online. 2009;19(3):29–34. doi: 10.1016/S1472-6483(10)60281-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 19 kb)