Abstract
Purpose
Cumulus cells (CC) play important roles in oocyte development and cumulus expressed genes can be used as markers for oocyte quality. This study aimed to investigate temporal changes in the expression of cumulus marker genes during oocyte maturation as possible biomarkers of embryo developmental competence in ovine.
Methods
Gene expression was assessed in the CC of the BCB+ (developmentally competent) and BCB- (developmentally poor) oocytes at 0, 12, and 24 h of in vitro maturation (IVM). Further, the association between the temporal cumulus gene expression and in vitro oocyte and embryo development was assessed.
Results
The maturation and blastocyst formation rates were found significantly greater for the BCB+ than the BCB- oocytes. At the 0 h of IVM, a significant upregulation in the expression of PTGS2, STAR, SDC2, LHR, FGF2, BCL2, IL7RA, HSPA1A, and IFNT was observed in the CC of the poor (BCB-) as compared to the competent (BCB+) oocytes. In contrast, it was observed that as maturation progressed, the cumulus expression of most of the favorable genes was reduced and was found significantly downregulated at the completion of IVM in the poor as compared to the competent oocytes.
Conclusions
The study revealed noticeable differences in the cumulus gene expression profile at different stages of IVM between ovine oocytes of differential developmental ability. The results indicated that the loss of cumulus gene expression along the maturation period in the poor oocytes was related to their intrinsic poor quality in the ovarian follicle.
Keywords: Gene expression, Cumulus cells, Oocyte, Embryo, In vitro development, Ovine
Introduction
The outcome of in vitro fertilization (IVF) is largely influenced by the developmental competence of oocytes. Competence is the ability of an oocyte to complete the process of maturation, to be fertilized and to support the early embryonic development. It is suggested that although culture conditions throughout in vitro embryo production may have a modest influence on the developmental potential of the early embryo, the quality of the oocyte at the start of the process is the key factor determining the proportion of oocytes developing to the blastocyst stage [1]. During follicular development and ovulation, oocytes are surrounded by cumulus cells (CC). These cells play important roles in acquiring competence of an oocyte during the process of maturation [2] and oocyte developmental competence is adversely affected if CC are removed before maturation [3]. One of the major functions of CC is the channeling of metabolites and nutrients to the oocyte, which is required for germinal vesicle breakdown and subsequent development to metaphase-II stage [1].
The assessment of the expression of CC marker genes as predictors of oocyte/embryo developmental competence has gained importance in recent years. Several cumulus expressed genes are crucial for oocyte maturation and development and assessment of their expression can provide a reliable method for choosing oocytes with greater potential for blastocyst formation and live birth [4]. The expression of gremlin 1 (GREM1), hyaluronan synthase 2 (HAS2), prostaglandin-endoperoxide synthase 2 (PTGS2), and steroidogenic acute regulatory protein (STAR) genes in CC correlates with the developmental ability of oocytes and morphology of embryos [5–7]. The receptors of FSH and LH are expressed in CC [8, 9], and it is proposed that LH contributes towards oocyte maturation exclusively through the cumulus expressed receptors [10, 11]. The cumulus expression of B-cell CLL/lymphoma 2 (BCL2) and BCL2-associated X protein (BAX) genes has been found to be associated with the development of oocytes [12, 13]. The interleukin-7 (IL-7) receptor (IL7RA) is expressed in CC and its expression increases with oocyte maturation [14]. Heat shock protein 70 (HSP70) gene (HSPA1A) is also expressed in CC under non-heat stress condition and critical roles of this protein in fertilization and embryo development have been suggested previously [15–17]. Although interferon tau (IFNT) expression is detectable in CC, its functional role in oocyte development is not established currently [18].
Brilliant cresyl blue (BCB) staining is an established non-invasive method for discriminating oocytes of differential developmental ability from a heterogeneous pool [5]. The principle of the method is that the intracellular enzyme glucose-6-phosphate dehydrogenase (G6PDH) metabolizes BCB stain absorbed by the oocytes following their incubation in the staining solution. The concentration of G6PDH is greater in the growing as compared to that of the grown oocytes. Therefore, the growing oocytes quickly metabolize the stain following incubation and the oocytes appear colorless (BCB-), but the grown oocytes with lesser G6PDH activity remain blue (BCB+).
In vitro embryo production and gene expression in oocytes, cumulus-oocyte complexes (COCs), and embryos have been studied extensively in ovine. Nevertheless, negligible information is currently available on the cumulus expressed genes and their association with the development of oocyte and or embryo in this species. Furthermore, meager information is available at present on the chronological changes in the profile of the cumulus expressed genes during oocyte maturation in ovine or any other mammalian species.
The study aimed to assess the temporal expression pattern of selected oocyte development-related genes at different stages of in vitro maturation (IVM) in the CC of the BCB-screened oocytes and to relate the expression profile with the oocyte developmental ability in ovine. It was hypothesized that the temporal expression pattern of the cumulus expressed genes would differ between the poor quality and the developmentally competent ovine oocytes.
Materials and methods
The chemicals used in the experiments were procured from Sigma-Aldrich Co., MO, USA unless otherwise mentioned.
Experimental design
Experiment 1
Maturation rate of the BCB-screened ovine oocytes following IVM was assessed. The BCB-screened COCs (BCB+ and BCB-) were matured in vitro for 24 h and denuded and nuclear maturation rate was determined by Hoechst staining. The experiment was conducted in 10 replicates (N = 347 in the BCB+ and N = 465 in the BCB- groups).
Experiment 2
Development of the BCB-screened ovine oocytes following IVM, IVF, and in vitro embryo culture (IVC) was assessed. The BCB-screened COCs (BCB+ and BCB-) were subjected to IVM, IVF, and IVC and the post-fertilization embryo development rate was recorded. The experiment was performed in four replicates (N = 144 in the BCB+ and N = 229 in the BCB- groups).
Experiment 3
Temporal expression pattern of the selected oocyte development-related genes was assessed in the CC of the BCB-screened ovine oocytes at different stages of IVM. The CC were collected from the individual BCB-screened COCs (BCB+ and BCB-) at the 0, 12, and 24 h of IVM and the cumulus gene expression was assessed by the real-time quantitative PCR (qPCR). The experiment was conducted in five replicates.
Oocyte collection, IVM, IVF, and IVC
Sheep ovaries were collected from a local abattoir and transported to the laboratory in a thermos containing warm (~37 °C) normal saline supplemented with strepto-penicillin (1.6 g/l; Cadila Healthcare Ltd., Vadodara, India) within 3–4 h of slaughter. The ovaries were washed thoroughly with strepto-penicillin-supplemented warm normal saline and excess tissues were removed. Oocytes were aspirated from the 2–6 mm follicles using a 20-G needle attached with a 2-ml syringe containing 500 μl of aspiration medium that was composed of HEPES-buffered M199 (Life Technologies Corporation, NY, USA) supplemented with heparin (50 IU/ml), gentamicin (50 μg/ml; Life Technologies Corporation, NY, USA), and fatty acid free BSA fraction V (4 mg/ml). The COCs with more than three layers of compact CC and homogeneous cytoplasm were collected for the experiments. The COCs were washed in aspiration medium and then subjected to the BCB staining (26 μM in DPBS) for 45 min at 38.5 °C and the BCB+ (blue-colored cytoplasm) and the BCB- (colorless cytoplasm) COCs were collected separately. The BCB-screened COCs were washed in DPBS and B199 medium that was composed of bicarbonate-buffered M199 (Life Technologies Corporation, NY, USA) supplemented sodium pyruvate (0.2 mM), gentamicin (50 μg/ml), cysteamine (0.1 mM), and FBS (10%; Life Technologies Corporation, NY, USA). Ten COCs in 10 μl of B199 medium were transferred into 40 μl drops of IVM medium (B199 supplemented with ovine-FSH, human-LH, and 17b-estradiol) that were overlaid with mineral oil and pre-incubated for at least 2 h at 38.5 °C in a CO2 incubator (5% CO2 in a humidified environment). The final concentrations of FSH, LH, estradiol, and FBS in the maturation drops were 0.01 U/ml, 0.02 U/ml, 1 μg/ml, and 10%, respectively. The COCs were matured for 24 h at 38.5 °C in a CO2 incubator.
IVF and IVC were performed according to the previously described methods [19] after suitable modifications. On the day of IVF, semen sample was collected from a healthy ram (~3 years of age) using a handheld electro ejaculator for sheep/goat. The neat semen was diluted with warm (37 °C) milk-egg yolk extender (1 g of nonfat dry milk powder, 90 mg of glucose, 500 μg of gentamicin, and 1 ml of egg yolk in a total volume of 10 ml) for a final spermatozoa concentration of 50 × 106/ml. The diluted semen sample was stored at 4 °C for approximately 3.5 h and then subjected to the swim-up separation in SOFH-IVF (without heparin) medium to prepare spermatozoa for IVF. Following the 24 h of IVM, the COCs were washed in SOFH-IVF and SOF-IVF (supplemented with 2% estrous ewe serum) media and 10 to 12 COCs in 10 μl of SOF-IVF medium were transferred into 30 μl drops of SOF-IVF medium that were overlaid with mineral oil and pre-incubated for at least 2 h at 38.5 °C in a CO2 incubator. Subsequently, 10 μl of prepared spermatozoa was added into each drop for a final volume of 50 μl and final spermatozoa concentration of 1 × 106/ml.
After 24 h of sperm-oocyte incubation in a CO2 incubator, CC were removed from the COCs by vortexing in 200 μl of SOFH-IVF medium for 5 min. Presumptive zygotes were washed in SOFH-IVF and SOF-IVC (supplemented with 10% FBS) media and transferred into 20 μl drops (8 to 10 zygotes/drop) of SOF-IVC medium that were overlaid with mineral oil and pre-incubated for at least 2 h at 38.5 °C in a CO2 incubator. IVC was performed for 192 h in a CO2 incubator and the embryos were transferred into fresh pre-incubated SOF-IVC drops at 96 h post culture. The embryos were evaluated at 24, 120, and 192 h post culture and different development stages were recorded.
Assessment of oocyte maturation
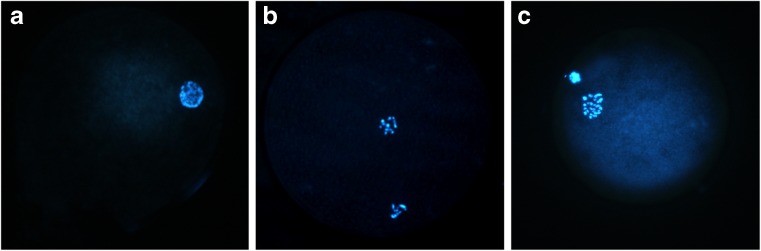
Oocyte maturation rate was assessed following the 24 h of IVM. The COCs were collected from the culture drops, placed in a 1.5-ml microcentrifuge tube containing 150 μl of aspiration medium supplemented with hyaluronidase (6000 U/ml), incubated for 5 min at 37 °C, vortexed for 5 min, and centrifuged at low speed briefly. The entire content of the tube was emptied in a 35 mm culture dish containing 2 ml of aspiration medium. The denuded oocytes were collected, washed in aspiration medium, incubated in Hoechst 33342 solution (5 μg/ml DPBS) for 30 min at 37 °C, and washed in aspiration medium. The stained oocytes were mounted on glass slides with DPX Mountant and screened under a fluorescent microscope with DAPI filter (wavelength 330–380 nm). Different nuclear maturation stages such as germinal vesicle (GV), telophase-I (T-I) and M-II were recorded and the oocytes at T-I and M-II stages were considered as matured. The proportion of oocytes that underwent meiosis resumption was calculated as the proportion of all oocytes other than those at GV stage (Fig. 1).
Fig. 1.
Nuclear maturation stages of the ovine oocytes following in vitro maturation (IVM) for 24 h. Oocytes were stained with Hoechst 33342. a Germinal vesicle. b Telophase-I. c Metaphase-II
Collection of CC, RNA isolation, and cDNA synthesis
The CC were collected from the BCB-screened COCs at the 0, 12, and 24 h of IVM. The COCs were evaluated under a stereo zoom microscope and the COCs with nearly similar cumulus mass were collected for RNA isolation in both groups (BCB+ and BCB-) at each time point. Denudation of the COCs was performed by subjecting them to hyaluronidase treatment and vortexing as described previously. Following the treatment, entire content of the microcentrifuge tube was placed in a 35-mm culture dish in the form of a drop and oocyte was removed from the drop. The leftover medium along with the CC was aspirated with the help of a micropipette and placed into a fresh 1.5-ml microcentrifuge tube and centrifuged (5000×g for 5 min at 4 °C). The supernatant was discarded and the cell pellet was washed with aspiration medium by centrifugation (5000×g for 5 min at 4 °C). The washed pellet was subjected to RNA extraction using TRI reagent following the manufacturer’s instructions and linear acrylamide (Life Technologies Corporation, NY, USA) was used for effective precipitation of RNA. The extracted total RNA was dissolved in nuclease-free water and subjected to RNase-free DNase I (Life Technologies Corporation, NY, USA) treatment following the manufacturer’s instructions. Following the DNase treatment, the RNA pellet was washed, air dried, dissolved in 10 μl of nuclease-free water, and reverse transcribed into first-strand complementary DNA (cDNA) immediately using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, CA, USA) following the manufacturer’s instructions. The synthesized cDNA was stored at −20 °C until used for gene expression analysis by qPCR.
Gene expression analysis
The qPCR assays of selected genes were performed in a Bio-Rad MyiQ™ qPCR detection System using SsoFast™ EvaGreen® Supermix (Bio-Rad Laboratories Inc., CA, USA). Briefly, 1 μl of cDNA, 0.25 μM of each primer and 1× EvaGreen mix were used in a total volume of 10 μl. The qPCR conditions were as follows: initial denaturation at 95 °C for 3 min and 40 cycles of 95 °C for 15 s and 60 °C for 30 s. The identity of amplified product was confirmed by agarose gel electrophoresis and melting curve analysis (initiated at 55 °C with 0.5 °C increments at each step of 10 s up to 95 °C). The details of the selected genes and the primer pairs used in the study are provided in Table 1. The relative changes in gene expression were determined using the 2−ΔΔCT method [28] and 18S was used as endogenous reference gene [2].
Table 1.
Details of the selected genes and primer pairs used for gene expression analysis
| Gene | Primer sequences (5′–3′) | Amplicon size | Reference/Genbank accession no. | Amplification efficiency, % (R 2) |
|---|---|---|---|---|
| GREM1 | F: TGCTGGAGTCCAGCCAAGA; R: GCACCAGTCTCGCTTCAGGTA | 65 | [20] | 97.04 (0.99) |
| HAS2 | F: CCTCATCATCCAAAGCCTG; R: ACATTTCCGCAAATAGTCTG | 138 | [21] | 97.47 (0.99) |
| PTGS2 | F: AGGAGGTCTTTGGTCTGGTG; R: TCTGGAACAACTGCTCATCG | 126 | [21] | 95.02 (0.99) |
| STAR | F: GCCAGGTGTTGAAGGCCA; R: TCTTTAACAGACTTGGAGGCTTCC | 104 | This study (NM_001009243.1) | 93.33 (0.99) |
| SDC2 | F: ATGCGATACACCAACAGCAGGA; R: GAAACCACAACGCTGAGCAAGA | 257 | [22] | 90.35 (0.99) |
| LHR | F: TGACTATGGTTTCTGCTTACCCAA; R: CCATAATGTCTTCACAGGGATTGA | 80 | [23] | 91.41 (0.99) |
| FSHR | F: GCCTGCCCATGGATATTGAC; R: TAGCAACCACAGATGACCACAAA | 97 | [23] | 92.78 (0.99) |
| FGF2 | F: AGTGTGTGCAAACCGTTACCTTGC; R: ATACTGCCCAGTTCGTTTCAGTGC | 172 | [24] | 90.18 (0.99) |
| FGF10 | F: TCCCCACCTGTCCGGCTGCT; R: CGCACATGCCTCCCCGCACT | 183 | This study (NM_001009230.1) | 94.88 (0.99) |
| BCL2 | F: GCCGAGATGTCCAGTCAGC; R: GACGCTCTCCACACACATGAC | 150 | [25] | 94.50 (0.99) |
| BAX | F: CATGGAGCTGCAGAGGATGA; R: GTTGAAGTTGCCGTCGGAAA | 100 | [25] | 98.19 (0.99) |
| IL7RA | F: ACCAAGCTGACACTCCTAC; R: CCATTCACTCCAGAAGCC | 107 | This study (XM_004017053.1) | 96.67 (0.99) |
| HSPA1A | F: CTGATCAAGCGCAACTCCA; R: AGCAGGTTGTTGTCCCGAGT | 131 | [26] | 98.84 (0.99) |
| IFNT | F: CCTGGACCGAATGAACAGA; R: AGGTTGAAGCTCTGCTGGAG | 150 | [26] | 94.55 (0.99) |
| 18S | F: AGAAACGGCTACCACATCCAA; R: CCTGTATTGTTATTTTTCGT | 90 | [27] | 96.09 (0.99) |
Statistical analysis
Statistical analyses were performed using the PASW 18.0.0 software package (SPSS/IBM, IL, USA). The values expressed in percentage for oocyte and embryo development rates were subjected to arcsine transformation before analyses [29]. The values expressed in fold change for gene expression were subjected to log2 transformation before analyses [30]. Student’s t test was used to analyze the variations in the oocyte and embryo development rates between the BCB-screened groups. The variations in the cumulus gene expression between the BCB-screened groups at each time point was analyzed by Student’s t test. A probability value of less than 0.05 was considered significant.
Results
In vitro development of the BCB-screened oocytes
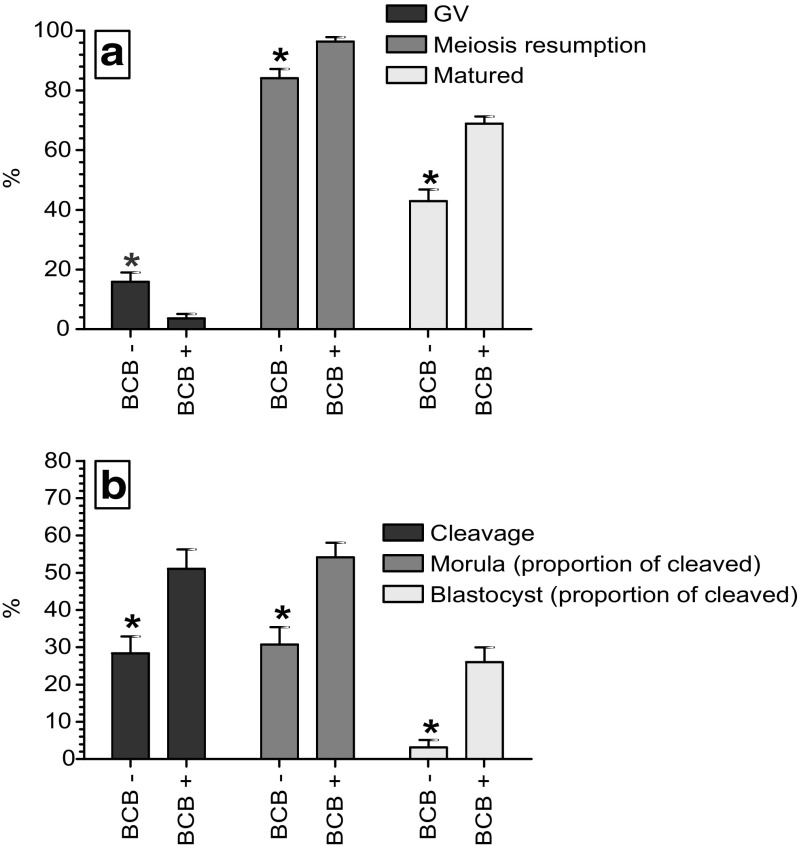
The variations in the oocyte development rates between the BCB-screened groups are depicted in Fig. 2. At the end of the 24 h of IVM, the proportion of oocytes at GV stage was found significantly (p < 0.05) lesser in the BCB+ (3.61%) as compared to that of the BCB- (15.9%) group. In contrast, the meiosis resumption and maturation rates following the 24 h of IVM were found significantly (p < 0.05) greater in the BCB+ (96.4 and 68.8%, respectively) as compared to that of the BCB- (84.1 and 42.9%, respectively) group. Similarly, following IVM, IVF, and IVC, the rates (%) of cleavage and morula (of cleaved embryos) and blastocyst (of cleaved embryos) formation were found significantly (p < 0.05) greater in the BCB+ (51.1, 54.2, and 26.0, respectively) than that of the BCB- (28.4, 30.7, and 3.11, respectively) group.
Fig. 2.
In vitro development (mean ± SE) of the BCB-screened ovine oocytes. a Maturation rate following in vitro maturation (IVM). b Embryo development following IVM, in vitro fertilization (IVF), and in vitro embryo culture (IVC). GV germinal vesicle stage, meiosis resumption all oocytes other than those at GV stage, matured oocytes at telophase-I and metaphase-II stages. The asterisk indicates a significant difference at p < 0.05
Gene expression in the CC obtained from the BCB-screened COCs
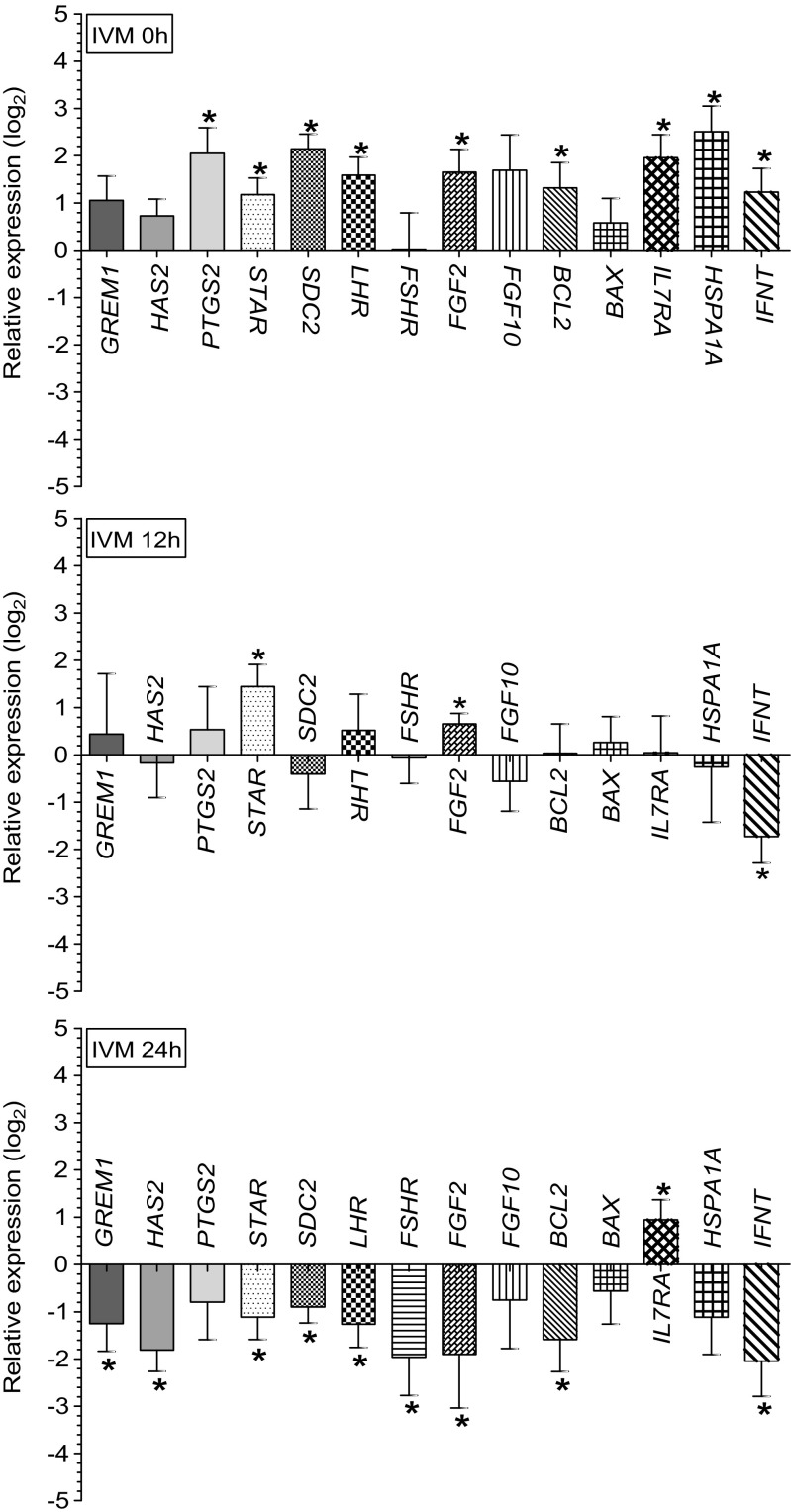
The variations in relative gene expression in the CC obtained from the BCB- as compared to the BCB+ oocytes at different stages of IVM (0, 12, and 24 h) are depicted in Fig. 3.
Fig. 3.
Variations (mean ± SE) in the relative expression of targeted genes in the cumulus cells (CC) of the BCB- (developmentally poor) as compared to the BCB+ (developmentally competent) ovine oocytes at different stages (0, 12, and 24 h) of in vitro maturation (IVM). The asterisk indicates a significant difference at p < 0.05
At the beginning of IVM (0 h), the cumulus expression of GREM1, HAS2, FSH receptor (FSHR), fibroblast growth factor 10 (FGF10), and BAX did not differ significantly between the groups. In contrast, the expression of PTGS2, STAR, syndecan 2 (SDC2), LH receptor (LHR), fibroblast growth factor 2 (FGF2), BCL2, IL7RA, HSPA1A, and IFNT was found significantly (p < 0.05) upregulated (4.15-, 2.26-, 4.42-, 3.00-, 3.14-, 2.48-, 3.88-, 5.69-, and 2.34-fold, respectively) in the CC of the BCB- as compared to the BCB+ oocytes.
At the mid stage of IVM (12 h), the cumulus expression of GREM1, HAS2, PTGS2, SDC2, LHR, FSHR, FGF10, BCL2, BAX, IL7RA, and HSPA1A was not significantly different between the groups. In contrast, the expression of STAR and FGF2 was found significantly (p < 0.05) upregulated (2.72- and 1.57-fold, respectively) and the expression of IFNT was found significantly (p < 0.05) downregulated (0.30-fold) in the CC of the BCB- as compared to the BCB+ oocytes.
At the completion of IVM (24 h), no significant difference was observed in the cumulus expression of PTGS2, FGF10, BAX, and HSPA1A between the groups. In contrast, a significant (p < 0.05) downregulation in the expression of GREM1, HAS2, STAR, SDC2, LHR, FSHR, FGF2, BCL2, and IFNT (0.42-, 0.28-, 0.46-, 0.53-, 0.41-, 0.25-, 0.27-, 0.33-, and 0.24-fold, respectively) and a significant (p < 0.05) upregulation in the expression of IL7RA (1.92-fold) was observed in the CC of the BCB- as compared to the BCB+ oocytes.
Discussion
The CC play important roles in the oocyte development process. The cells are involved in the maintenance of meiotic arrest and contribute to the cytoplasmic and nuclear maturation of oocytes [2, 31]. The present study aimed to investigate the temporal changes in the expression of selected genes at different stages of IVM in the CC derived from the ovine COCs of different developmental potential. The results indicated that the cumulus gene expression profile at the beginning and completion of IVM was distinctly different between the developmentally poor (BCB-) and the developmentally competent (BCB+) ovine oocytes.
The CC were derived from the BCB-screened COCs for assessing the association of oocyte developmental competence with CC gene expression. The BCB staining test is a proven non-invasive and non-perturbing method for selecting more developmentally competent oocytes [5]. In ovine, significantly greater maturation, cleavage, and blastocyst rates of the BCB+ as compared to the BCB- oocytes have been observed previously [32]. In the current study, significantly greater meiosis resumption and maturation rate following IVM and significantly greater cleavage and morulae and blastocyst formation rates following IVM, IVF, and IVC in the BCB+ as compared to the BCB- group indicated that the screening was effective in defining the oocyte pools of variable quality.
The expression profile of 14 selected genes was investigated at different stages of IVM in the CC derived from the BCB-screened oocytes. The selected genes were representative of previously identified CC markers of oocyte quality and development (GREM1, HAS2, PTGS2, STAR, SDC2, and HSPA1A), gonadotropin responsiveness (LHR and FSHR), FGF signaling (FGF2 and FGF10), apoptosis (BAX and BCL2), and interleukin (IL7RA) and interferon (IFNT) signaling.
GREM1 and HAS2 are involved in the regulation of cumulus function and final oocyte maturation, respectively [20, 33]. The PTGS2 expression in COCs may determine oocyte quality and timing of maturation and promotes the expansion and survival of CC [34–36]. The expression of STAR is promoted primarily by FSH and LH [37] and the STAR-knockout mice display incomplete follicular maturation and anovulation [38]. The SDC2 gene is involved in cell proliferation and cytoskeletal organization [22]. Enhanced expression of GREM1, HAS2, PTGS2, and STAR in CC has been reported to be associated with better oocyte and embryo development in human, bovine, and sheep [6, 7, 20, 31, 39]. An association between the cumulus expression of SDC2 and oocyte quality is suggested previously [22]. Our study revealed that the cumulus expression of GREM1, HAS2, PTGS2, STAR, and SDC2 was variable between the oocytes of different quality and at different stages of IVM. It was evident that the expression of these favorable genes except PTGS2 was significantly downregulated in the CC of the poor as compared to the competent oocytes at the completion of IVM. The results indicate the roles of these genes in the ovine oocyte maturation process, especially at the late stage.
FSH and LH play important roles in the regulatory mechanisms of oocyte maturation through their binding to specific receptors (LHR and FSHR), which are expressed on the surface of CC [8, 9, 40]. A significant downregulation in the expression of LHR and FSHR in the CC of the poor oocytes at the completion of IVM was evident in the current study. The results indicate that at the late maturation stage, the cumulus expression of these two genes is important for the ovine oocytes to acquire developmental competence.
FGF2 and FGF10 have been established as oocyte competent factors as they play important roles in the oocyte maturation process [41–43]. In the present study, a significant variation in the cumulus FGF2 expression between the experimental groups at different stages of IVM suggests its involvement in the oocyte maturation process in ovine. In contrast, similar cumulus FGF10 expression along the maturation period in both the experimental groups suggests that it is probably not a key factor contributing to the maturation process of ovine oocytes.
An inverse relationship exists between the proliferation and apoptosis of CC [44]. BCL2 and BAX are anti- and pro-apoptotic proteins respectively that participate in the mitochondria-dependent apoptosis pathway [12]. In human and buffalo, greater BCL2 and lesser BAX expressions in CC have been shown to contribute to the developmental competence of oocytes [12, 13]. In the current study, similar cumulus BAX expression in both the experimental groups along the maturation period indicates that ovine oocyte development during maturation was not influenced by the cumulus BAX expression. In contrast, a significant downregulation in the cumulus BCL2 expression at the completion of IVM and associated poor oocyte development indicate its decisive role in imparting oocyte developmental competence.
The role of IL-7 in oocyte maturation is not well defined currently. Nevertheless, the recent evidences suggest that IL-7 regulates the replication of CC and the cumulus expression of its receptor mRNA increases with oocyte maturation in mouse [14]. The current study revealed a significantly different cumulus IL7RA expression at the beginning and completion of IVM in the poor oocytes suggesting its likely involvement in the ovine oocyte maturation process.
The HSPA1A gene encodes HSP70 protein that protects cells against heat damage [45]. HSPA1A is also expressed in CC, oocytes, and embryos under non-heat stress condition and evidences suggest that it plays critical roles in fertilization and embryo development, most likely through its protective action against apoptosis [15–17]. In the current study, a significantly greater cumulus expression of HSPA1A and anti-apoptotic BCL2 was found in the CC of the poor as compared to the competent oocyte at the beginning of IVM. Further, it was observed that in the CC of the poor oocytes, the expression of BCL2 was reduced at the mid and late maturation stages that was coincided with the reduced cumulus HSPA1A expression in the same group at both the time points. The results suggest the likely involvement of HSP70 in regulating apoptosis of the ovine CC.
IFNT is primarily involved in the maternal recognition of pregnancy. Although the expression of IFNT has been detected previously in bovine CC [18], its involvement in the oocyte developmental process is not known currently. In the current study, a significant downregulation in the cumulus expression of IFNT at the mid and late maturation stages and associated poor oocyte development suggest the possible involvement of cumulus expressed IFNT in the oocyte maturation process.
In conclusion, the current study revealed noticeable differences in the cumulus gene expression profile at different stages of IVM between the ovine oocytes of differential developmental ability. It was evident that as maturation progressed, the cumulus expression of most of the favorable genes (GREM1, HAS2, STAR, SDC2, LHR, FSHR, FGF2, BCL2, and IFNT) was gradually reduced and was found significantly downregulated in the poor oocytes at the completion of IVM. The results indicated that the loss of cumulus gene expression along the maturation period in the poor oocytes was related to their intrinsic poor quality in the ovarian follicle. Further, under unoptimized in vitro condition, they were unable to follow a normal gene expression pattern as compared to the good oocytes. Future studies on the influence of the cumulus expression of these favorable genes on the post-fertilization developmental ability of individual oocytes are required if these genes are to be used as definitive markers for predicting embryo development in ovine.
Acknowledgements
This work was supported by the ICAR-National Agricultural Science Fund (grant number NFBSFARA/BSA-4005/2013-14).
References
- 1.Lonergan P, Fair T. Maturation of oocytes in vitro. Annu Rev Anim Biosci. 2016;4:10.1–10.14. doi: 10.1146/annurev-animal-022114-110822. [DOI] [PubMed] [Google Scholar]
- 2.Tesfaye D, Ghanem N, Carter F, Fair T, Sirard MA, Hoelker M, et al. Gene expression profile of cumulus cells derived from cumulus–oocyte complexes matured either in vivo or in vitro. Reprod Fertil Dev. 2009;21:451–461. doi: 10.1071/RD08190. [DOI] [PubMed] [Google Scholar]
- 3.Maedomari N, Kikuchi K, Ozawa M, Noguchi J, Kaneko H, Ohnuma K, et al. Cytoplasmic glutathione regulated by cumulus cells during porcine oocyte maturation affects fertilization and embryonic development in vitro. Theriogenology. 2007;67:983–993. doi: 10.1016/j.theriogenology.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update. 2007;13:289–312. doi: 10.1093/humupd/dml062. [DOI] [PubMed] [Google Scholar]
- 5.Bhardwaj R, Ansari MM, Pandey S, Parmar MS, Chandra V, Kumar GS, et al. GREM1, EGFR, and HAS2; the oocyte competence markers for improved buffalo embryo production in vitro. Theriogenology. 2016;86:2004–2011. doi: 10.1016/j.theriogenology.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 6.McKenzie LJ, Pangas SA, Carson SA, Kovanci E, Cisneros P, Buster JE, et al. Human cumulus granulosa cell gene expression: a predictor of fertilization and embryo selection in women undergoing IVF. Hum Reprod. 2004;19:2869–2874. doi: 10.1093/humrep/deh535. [DOI] [PubMed] [Google Scholar]
- 7.Valiollahpoor Amiri M, Deldar H, Ansari PZ. Impact of supplementary royal jelly on in vitro maturation of sheep oocytes: genes involved in apoptosis and embryonic development. Syst Biol Reprod Med. 2016;62:31–38. doi: 10.3109/19396368.2015.1088102. [DOI] [PubMed] [Google Scholar]
- 8.Méduri G, Charnaux N, Driancourt MA, Combettes L, Granet P, Vannier B, et al. Follicle-stimulating hormone receptors in oocytes? J Clin Endocrinol Metab. 2002;87:2266–2276. doi: 10.1210/jcem.87.5.8502. [DOI] [PubMed] [Google Scholar]
- 9.Vigone G, Merico V, Redi CA, Mazzini G, Garagna S, Zuccotti M. FSH and LH receptors are differentially expressed in cumulus cells surrounding developmentally competent and incompetent mouse fully grown antral oocytes. Reprod Fertil Dev. 2015;27:497–503. doi: 10.1071/RD13251. [DOI] [PubMed] [Google Scholar]
- 10.Bornslaeger EA, Schultz RM. Regulation of mouse oocyte maturation: effect of elevating cumulus cell cAMP on oocyte cAMP levels. Biol Reprod. 1985;33:698–704. doi: 10.1095/biolreprod33.3.698. [DOI] [PubMed] [Google Scholar]
- 11.Van Tol HTA, Van Eijk MJT, Mummery CL, Van den Hurk R, Bevers MM. Influence of FSH and hCG on the resumption of meiosis of bovine oocytes surrounded by cumulus cells connected to membrana granulosa. Mol Reprod Dev. 1996;45:218–224. doi: 10.1002/(SICI)1098-2795(199610)45:2<218::AID-MRD15>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 12.Filali M, Frydman N, Belot MP, Hesters L, Gaudin F, Tachdjian G, et al. Oocyte in-vitro maturation: BCL2 mRNA content in cumulus cells reflects oocyte competency. Reprod BioMed Online. 2009;19:71–84. doi: 10.1016/S1472-6483(10)61071-1. [DOI] [PubMed] [Google Scholar]
- 13.Jain A, Jain T, Kumar P, Kumar M, De S, Gohain M, et al. Follicle-stimulating hormone–induced rescue of cumulus cell apoptosis and enhanced development ability of buffalo oocytes. Domest Anim Endocrinol. 2016;55:74–82. doi: 10.1016/j.domaniend.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Cakmak H, Franciosi F, Zamah AM, Cedars MI, Conti M. Dynamic secretion during meiotic reentry integrates the function of the oocyte and cumulus cells. Proc Natl Acad Sci U S A. 2016;113:2424–2429. doi: 10.1073/pnas.1519990113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matwee C, Kamaruddin M, Betts DH, Basrur PK, King WA. The effects of antibodies to heat shock protein 70 in fertilization and embryo development. Mol Hum Reprod. 2001;7:829–837. doi: 10.1093/molehr/7.9.829. [DOI] [PubMed] [Google Scholar]
- 16.Camargo LS, Viana JH, Ramos AA, Serapião RV, de Sa WF, Ferreira AM, et al. Developmental competence and expression of the Hsp 70.1 gene in oocytes obtained from Bos indicus and Bos taurus dairy cows in a tropical environment. Theriogenology. 2007;68:626–632. doi: 10.1016/j.theriogenology.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 17.Payton RR, Rispoli LA, Saxton AM, Edwards JL. Impact of heat stress exposure during meiotic maturation on oocyte, surrounding cumulus cell, and embryo RNA populations. J Reprod Dev. 2011;57:481–491. doi: 10.1262/jrd.10-163M. [DOI] [PubMed] [Google Scholar]
- 18.Johnson KM, Alvarez X, Kubisch HM. Localization of interferon-tau in bovine embryos and cumulus cells by confocal microscopy. Reprod Fertil Dev 2004;16:239.
- 19.Dhali A, Anchamparuthy VM, Butler SP, Pearson RE, Gwazdauskas FC. In vitro development of bovine embryos cultured with stem cell factor or insulin-like growth factor-I following IVF with semen of two bulls having different field fertility. Anim Reprod Sci. 2009;116:188–195. doi: 10.1016/j.anireprosci.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Anderson RA, Sciorio R, Kinnell H, Bayne RAL, Thong KJ, Sousa PA, et al. Cumulus gene expression as a predictor of human oocyte fertilisation, embryo development and competence to establish a pregnancy. Reproduction. 2009;138:629–637. doi: 10.1530/REP-09-0144. [DOI] [PubMed] [Google Scholar]
- 21.Kyasari OR, Valojerdi MR, Farrokhi A, Ebrahimia B. Expression of maturation genes and their receptors during in vitro maturation of sheep COCs in the presence and absence of somatic cells of cumulus origin. Theriogenology. 2012;77:12–20. doi: 10.1016/j.theriogenology.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Salhab M, Pollet SD, Auclair S, Joly CG, Brisard D, Tran RD, et al. In vitro maturation of oocytes alters gene expression and signaling pathways in bovine cumulus cells. Mol Reprod Dev. 2013;80:166–182. doi: 10.1002/mrd.22148. [DOI] [PubMed] [Google Scholar]
- 23.Silva LM, Lazzarotto CR, Tavares KCS, Oliveira CHA, Silva AM, Fernandes CCL, et al. Comparative expression profiles of genes related to oocyte development in goats after long-term feeding with biodiesel castor industry residues. Anim Reprod Sci. 2014;148:32–41. doi: 10.1016/j.anireprosci.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Almeida AP, Saraiva MV, Alves Filho JG, Silva GM, Gonçalves RF, Brito IR, et al. Gene expression and immunolocalization of fibroblast growth factor 2 in the ovary and its effect on the in vitro culture of caprine preantral ovarian follicles. Reprod Domest Anim. 2012;47:20–25. doi: 10.1111/j.1439-0531.2011.01793.x. [DOI] [PubMed] [Google Scholar]
- 25.Ebrahimi B, Valojerdi MR, Yazdi PE, Baharvand H. In vitro maturation, apoptotic gene expression and incidence of numerical chromosomal abnormalities following cryotop vitrification of sheep cumulus-oocyte complexes. J Assist Reprod Genet. 2010;27:239–246. doi: 10.1007/s10815-010-9401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi I, Lee JH, Fisher P, Campbell KHS. Caffeine treatment of ovine cytoplasts regulates gene expression and foetal development of embryos produced by somatic cell nuclear transfer. Mol Reprod Dev. 2010;77:876–887. doi: 10.1002/mrd.21230. [DOI] [PubMed] [Google Scholar]
- 27.Catala MG, Izquierdo D, Uzbekova S, Morato R, Roura M, Romaguera R, et al. Brilliant cresyl blue stain selects largest oocytes with highest mitochondrial activity, maturation-promoting factor activity and embryo developmental competence in prepubertal sheep. Reproduction. 2011;142:517–527. doi: 10.1530/REP-10-0528. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Hao R, Zhang C, Lv L, Shi L, Yue W. Effects of AY9944 A-7 on gonadotropin-induced meiotic resumption of oocytes and development of parthenogenetic embryos in sheep. Theriogenology. 2015;83:30–37. doi: 10.1016/j.theriogenology.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 30.Rieu I, Powers SJ. Real-time quantitative RT-PCR: design, calculations, and statistics. Plant Cell. 2009;21:1031–1033. doi: 10.1105/tpc.109.066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feuerstein P, Cadoret V, Dalbies-Tran R, Guerif F, Bidault R, Royere D. Gene expression in human cumulus cells: one approach to oocyte competence. Hum Reprod. 2007;22:3069–3077. doi: 10.1093/humrep/dem336. [DOI] [PubMed] [Google Scholar]
- 32.Mohammadi-Sangcheshmeh A, Veshkini A, Hajarizadeh A, Jamshidi-Adegani F, Zhandi M, Abazari-Kia AH, et al. Association of glucose-6-phosphate dehydrogenase activity with oocyte cytoplasmic lipid content, developmental competence, and expression of candidate genes in a sheep model. J Assist Reprod Genet. 2014;31:1089–1098. doi: 10.1007/s10815-014-0264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoenfelder M, Einspanier R. Expression of hyaluronan synthases and corresponding hyaluronan receptors is differentially regulated during oocyte maturation in cattle. Biol Reprod. 2003;69:269–277. doi: 10.1095/biolreprod.102.011577. [DOI] [PubMed] [Google Scholar]
- 34.Calder MD, Caveney AN, Westhusin ME, Watson AJ. Cyclooxygenase-2 and prostaglandin E(2)(PGE(2)) receptor messenger RNAs are affected by bovine oocyte maturation time and cumulus-oocyte complex quality, and PGE(2) induces moderate expansion of the bovine cumulus in vitro. Biol Reprod. 2001;65:135–140. doi: 10.1095/biolreprod65.1.135. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi T, Morrow JD, Wang H, Dey SK. Cyclooxygenase-2-derived prostaglandin E(2) directs oocyte maturation by differentially influencing multiple signaling pathways. J Biol Chem. 2006;281:37117–37129. doi: 10.1074/jbc.M608202200. [DOI] [PubMed] [Google Scholar]
- 36.Gebhardt KM, Feil DK, Dunning KR, Lane M, Russell DL. Human cumulus cell gene expression as a biomarker of pregnancy outcome after single embryo transfer. Fertil Steril. 2011;96:47–52.e2. doi: 10.1016/j.fertnstert.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 37.Miller WL, Strauss JF., 3rd Molecular pathology and mechanism of action of the steroidogenic acute regulatory protein, StAR. J Steroid Biochem Mol Biol. 1999;69:131–141. doi: 10.1016/S0960-0760(98)00153-8. [DOI] [PubMed] [Google Scholar]
- 38.Hasegawa T, Zhao L, Caron KM, Majdic G, Suzuki T, Shizawa S, et al. Developmental roles of the steroidogenic acute regulatory protein (StAR) as revealed by StAR knockout mice. Mol Endocrinol. 2000;14:1462–1471. doi: 10.1210/mend.14.9.0515. [DOI] [PubMed] [Google Scholar]
- 39.Salhab M, Papillier P, Perreau C, Guyader-Joly C, Dupont J, Mermillod P, et al. Thymosins beta-4 and beta-10 are expressed in bovine ovarian follicles and upregulated in cumulus cells during meiotic maturation. Reprod Fertil Dev. 2010;22:1206–1221. doi: 10.1071/RD10015. [DOI] [PubMed] [Google Scholar]
- 40.Shimada M, Nishibori M, Isobe N, Kawano N, Terada T. Luteinizing hormone receptor formation in cumulus cells surrounding porcine oocytes and its role during meiotic maturation of porcine oocytes. Biol Reprod. 2003;68:1142–1149. doi: 10.1095/biolreprod.102.010082. [DOI] [PubMed] [Google Scholar]
- 41.Zhang K, Hansen PJ, Ealy AD. Fibroblast growth factor 10 enhances bovine oocyte maturation and developmental competence in vitro. Reproduction. 2010;140:815–826. doi: 10.1530/REP-10-0190. [DOI] [PubMed] [Google Scholar]
- 42.Rajput SK, Lee K, Zhenhua G, Di L, Folger JK, Smith GW. Embryotropic actions of follistatin: paracrine and autocrine mediators of oocyte competence and embryo developmental progression. Reprod Fertil Dev. 2013;26:37–47. doi: 10.1071/RD13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang K, Hansen PJ, Ealy AD. Fibroblast growth factor 2 promotes bovine oocyte meiotic maturation and developmental competence. Reprod Fertil Dev. 2010;23:236. doi: 10.1071/RDv23n1Ab276. [DOI] [PubMed] [Google Scholar]
- 44.Zhai B, Liu H, Li X, Dai L, Gao Y, Li C, et al. BMP15 prevents cumulus cell apoptosis through CCL2 and FBN1 in porcine ovaries. Cell Physiol Biochem. 2009;32:264–278. doi: 10.1159/000354435. [DOI] [PubMed] [Google Scholar]
- 45.Kregel KC. Heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]