Abstract
Background
Microbiome-modulating interventions are promising for treatment and prevention of metabolic syndrome. The number of probiotic strains demonstrated ability to decrease cholesterol level in vivo, however, it was poorly confirmed in a clinical setting. The aim was to study the effects of L. acidophilus IMV B-7279, L. casei IMV B-7280, B. animalіs VKL and B. animalіs VKB separately and in various compositions on the level of serum cholesterol, gut microbiota contents and liver morphology on a high-calorie-induced obesity model in BALB/c mice.
Materials and methods
We used for the study female BALB/c mice 6–8 weeks old (18–24 g). Experimental animals were fed by a fat-enriched diet (FED), and 8 experimental groups were formed (12 mice in each group) to test strains of probiotic bacteria L. delbrueckii subsp. bulgaricus IMV B-7281, L. casei IMV B-7280, B. animalіs VKL and B. animalіs VKB and compositions. We used ultrasound for in vivo assessment of the liver and visceral (mesenteric) fat size. In the blood serum of the obese mice, the level of cholesterol was estimated. The liver morphology and gut microbiota of obese mice were studied.
Results
We revealed that after treatment with all of the studied probiotic bacteria and compositions of B. animalis VKL/B. animalis VKB/L. casei IMV B-7280, the weight of obese mice decreased, and cholesterol and its fraction levels in serum were reduced. The size of the liver slightly decreased after treatment with L. delbrueckii subsp. bulgaricus IMV B-7281, B. аnimalis VKB or probiotic compositions; we observed reduction of the mesenteric fat size after injection of all these probiotic bacteria (separately) and probiotic compositions. We defined the strain-dependent effects on serum lipid profiles, liver morphology and the gut microbiota. The B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 composition effectively recovered the liver morphological structure of obese mice. The number of Lactobacillus spp., Bifidobacterium spp. and coliform bacteria increased, the number of staphylococci and streptococci reduced, and the number of microscopic fungi significantly decreased in the gut of obese mice after treatment with L. casei IMV B-7280, L. delbrueckii subsp. bulgaricus IMV B-7281, B. animalis (separately) or their compositions.
Conclusion
L. casei IMV B-7280 (separately) and a composition of B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 are effective at decreasing the weight of obese mice, decreasing cholesterol level, restoring the liver morphology and beneficially modulating the gut microbiome in high-calorie-induced obesity.
Keywords: Predictive preventive personalized medicine, Metabolic syndrome, Obesity, Lactobacillus, Bifidobacterium, Cholesterol, Liver, Gut microbiota, Mouse model: mesenteric fat, Ultrasound
Overview
Microbiota and metabolic syndrome: Evidence of probiotic interventions efficacy for hypercholesterolemia, liver disease and the gut microbiota in obesity
Metabolic syndrome (MetS) is a violation of metabolism that leads to the development of obesity, liver disease, hypertension, dyslipidemia, hyperglycemia and insulin resistance and still is a large global challenge [1–4]. MetS is associated with altered metabolism of lipids, carbohydrates, insulin, violations of antioxidant systems, and development of inflammatory reactions [5–7]. Obesity in adults and children is a global epidemic in many countries worldwide, is often associated with hyperglycemia, hypertriglyceridemia, dyslipidemia and hypertension and is considered as the main risk factor for cardiovascular diseases [7, 8]. The World Health Organization (WHO) has predicted cardiovascular diseases (CVD) to remain the leader in causes of death, and by 2030, will affect up to 23.6 million people worldwide [9].
The development/updating of a panel of biomarkers of MetS [4, 10] is needed for diagnosis and prediction of metabolic diseases, prevention and personalized treatment. The importance of the prognostic and diagnostic significance of total cholesterol and its fractions was extensively proved by experimental and clinical studies [11] to be a major risk factor for coronary heart disease. Up to date cholesterol management requires statin therapy in increasing the target level for a low-density lipoprotein (LDL) cholesterol level of 4.9 mmol/L in patients with atherosclerotic CVD [12].
Microecological violations in various organs are triggers for development of metabolic diseases and related pathological processes, based on altered lipid metabolism. In particular, a number of experimental and clinical studies revealed the link between cholesterol metabolism disorder and other substances in such metabolic diseases as obesity and changes in gut microbiota [13–16], which is manifested primarily in increasing the number of Gram-positive Firmicutes and a decrease in the number of Gram-negative Bacteroidetes [13, 14].
The key role of gut microbiota in the development of metabolic diseases has been previously confirmed in the obesity models of mice. Thus, colonization of sterile mice by cecal microbiota of mice with obesity resulted in greater weight gain and fat accumulation compared to ordinary mice (Turnbaugh, 2008) [17]. The human gut microbiome can be modified by diet alteration, potentially facilitating adaptation to new dietary habits [18]. Probiotic strains demonstrated effectiveness in cholesterol lowering under high-cholesterol diet [19], and the role of cholesterol intake remain debatable vs. gut microbiota as a main trigger of obesity development and atherosclerosis. The mechanisms have been defined to explain the influence of gut microbiota on cholesterol metabolism [20–31]. The broad associations of liver function and development of nonalcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH) with gut microbiota was rigorously studied [32–34].
Microbiome-modulating interventions (mostly via probiotic strains) is a promising approach for treatment and prevention [35–42]. However, definitive evidence in many aspects is lacking for their use, and the legislative process is complex and not effective enough [43]. A number of probiotic strains have demonstrated the ability to decrease cholesterol levels in vivo in studies from the last decades [44–47]. Thus, recent in vivo studies showed that use of the probiotics and/or prebiotics can effectively reduce serum/plasma cholesterol levels, improving overall lipid profiles [47]; in particular Lactobacilli demonstrates a clear hypocholesterolemic effect in vivo [47–52]. The meta-analysis performed by Shimizu et al. showed that probiotic supplementation could be useful in the primary prevention of hypercholesterolemia and may lead to reductions in risk factors for cardiovascular disease. In addition, this meta-analysis showed that long-term (over 4 weeks) probiotic consumption resulted in a statistically significant decrease in cholesterol levels [53]. The meta-analysis of controlled short-term probiotic intervention studies (as many as 2000), excluding open-label studies, showed a decrease of total cholesterol and LDL cholesterol after fermented yoghurt product use. Long-term studies are still needed to confirm the sustained effects of probiotics on lipid profiles [54].
Different strains of Lactobacillus (LAB) affect not only the metabolism of cholesterol and triglycerides, but also normalize gut microbiota [23], beneficially modulate liver function [22], and decrease accumulation of fat [55, 56]. Clinical studies showed some decrease in the level of total cholesterol and LDL cholesterol after use of probiotics, including Lactobacillus, Bifidobacterium, Pediococcus, Leuconostoc and Enterococcus [57, 58]; however, very few confirmed its efficacy evidentiarily, likely due to bias imposed on the designs. On the other hand, other past studies and meta-analyses have also shown that probiotics and prebiotics had insignificant effects on lipid profiles, debating its hypocholesterolemic potential [53, 59–63]. Despite the ability for L. acidophilus to reduce cholesterol in vitro, no effect was seen in volunteers [63].
An important criterion for selection of probiotic strains for development of such drugs is their ability to normalize the metabolism of lipid spectrum components, mostly cholesterol. In various experimental models of metabolic diseases and in vitro, it was shown that some strains of Lactic acid bacteria (LAB) and Bifidobacteria had effective hypocholesterolemic activity associated with enzymatic degradation of bile acids, direct binding of cholesterol by cell walls of bacteria [20–25], and changes in expression of several genes involved in lipid metabolism [20].
There are two challenges to achieve an evidence-based and personalized approach in the use of probiotics for MetS:
the selection of the most effective relevant strain among existing strains; also challenging is the discovery of new strains for next-generation probiotics and development of live biotherapeutic products [64];
the appropriate stratification of the host responder according to age, gender and phenotype, using dynamic monitoring of a set of translational biomarkers for early detection and prevention of MetS via nutritional modulation [65]. Specific high-risk groups such as infants, the elderly and the immuno-compromised [59] require highly individualized prescriptions of probiotics and/or prebiotics.
We speculate that immune-centered theory [66, 67], claiming that gut microbiota can influence immune function beyond the gut, would be crucially helpful for choosing appropriate probiotic bacteria in the personalized clinical setting [42, 68]. Therefore, the selected probiotics should improve lipid metabolism by reducing the level of total cholesterol and LDL cholesterol. It is important to consider carbohydrate metabolism, insulin, the development of inflammatory reactions and maintaining a long-term effect after diet and weight loss. and considering long-term diet programming in cases of metabolic diseases [69].
We previously found that the probiotic strains L. casei IMV B-7280, L. delbrueckii subsp. bulgaricus IMV B-7281, B. animalіs VKL and B. animalіs VKB (separately) and in various compositions had effective hypocholesterolemic activity in vitro [52]. These probiotic strains have a high level of ability to balance the immune response via induction of various cytokines and modifying gut microbiota (Lazarenko et al., 2017) [68], and were effective for monosodium glutamate-induced obesity [70]. Our recent data demonstrated strong correlations between bacteria wall elasticity with immune-modulatory properties that facilitate selection of the most effective probiotic drugs [71].
Thus, based on the data discussed above, here we hypothesize that:
the strains demonstrating efficacy in vitro and having high immune modulatory activity, namely L. acidophilus IMV B-7279, L. casei IMV B-7280, B. animalіs VKL and B. animalіs VKB, separately and in compositions should demonstrate beneficial effects on serum level of cholesterol, on the gut microbiota and liver morphology in obese mice;
selecting strains for human applications is effective under appropriate assessment of reliable markers to evaluate obesity and MetS, including imaging data [42, 70, 72]; ultrasound of visceral (mesenteric) fat is representable, fast and most relevant for screening small animals like mice;
the efficacy of selected strains should receive rigorous confirmation in the clinical trials if properly designed.
The aim was to study the effects of L. acidophilus IMV B-7279, L. casei IMV B-7280, B. animalіs VKL and B. animalіs VKB separately and in various compositions on the level of cholesterol in the blood serum, on the gut microbiota contents and morphology of the liver in a high-calorie-induced ultrasound-assisted obesity model on BALB/c mice; and to overview the up-to-date evidence on using probiotics for MetS manifestions.
Materials and methods
The research was conducted in compliance with the standards of the Convention on Bioethics of the Council of Europe’s ‘Europe Convention for the Protection of Vertebrate Animals’ used for experimental and other scientific purposes’ (1997), the general ethical principles of animal experiments, approved by the First National Congress on Bioethics Ukraine (September 2001) in compliance with the Law of Ukraine of 21.02.2006 № 3447-IV «On protection of animals from abuse”, and with other international agreements and national legislation in this field. Animals were kept in a vivarium that was accredited in accordance with the ‘standard rules on ordering, equipment and maintenance of experimental biological clinics (vivarium)’. Instruments used in research were under metrological control. The number of animals used was as minimal as necessary; in vivo ultrasound allowed minimization of animal use with the highest informativeness. No human subjects were involved in the study.
Вacterial strain, media and growth conditions
L. delbrueckii subsp. bulgaricus IMV B-7281, L. casei IMV B-7280, B. animalіs VKL and B. animalіs VKB allocated from associated culture during laboratory studies of the fermented biological material from the intestines of humans were used in our study. L. delbrueckii subsp. bulgaricus IMV B-7281 and L. casei IMV B-7280 are deposited in the Ukrainian collection of microorganisms (D.K. Zabolotny Institute of Microbiology and Virology of NAS of Ukraine, Kyiv, Ukraine). The study was performed using bacteria lyophilized in a Cuddon freeze dryer FD1500 (New Zealand). Before each experiment, the viability of lyophilized strains of LAB and Bifidobacteria were tested by monitoring their growth on the Man–Rogosa–Sharpe (MRS) or bifidum-agar media, respectively, at 37 °C for 24–48 h.
Animals, diets and design of experiment
Experimental studies were conducted on female BALB/c mice at the age of 6–8 weeks (18–24 g). During the experiment, animals were kept in standard vivarium conditions, in plastic cages in a separate room at a steady temperature (20–22 °C), they were provided with the full mixed feed and had free access to automatic water bowls.
The mice were fed a fat-enriched diet (FED) composed of 30% fats, 40% proteins and 30% carbohydrates during 21 days to model obesity. From the 22nd day, these mice received standard full mixed feed and L. delbrueckii subsp. bulgaricus IMV B-7281, L. casei IMV B-7280, B. animalіs VKL, B. animalіs VKB (each strain separately) or B. animalis VKB/B. animalis VKL (ratio 1:1) or B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 (ratio 1:1:2) compositions. The probiotic bacteria were suspended in sterile phosphate-buffered saline (PBS). Mice received these probiotic bacteria and probiotic compositions in the dose of 500 μl and 1 x 106 cells per animal, orally daily during 7 days.
Eight experimental groups were formed from BALB/c mice (12 mice in each group): 1) intact mice that received standard diet (control); 2) mice that received FED; 3) mice that received FED and L. casei IMV B-7280; 4) mice that received FED and L. delbrueckii subsp. bulgaricus IMV B-7281; 5) mice that received FED and B. animalis VKB; 6) mice that received FED and B. animalis VKL; 7) mice that received FED and composition B. animalis VKB/B. animalis VKL; 8) mice that received FED and composition B. animalis VKL/B. animalis VKB/L. casei IMV B-7280.
Blood samples were taken from the tail vein on the 7th, 15th and 21st days from the beginning of FED and on the 4th, 9th, 15th, 21st and 30th days from the beginning of probiotic strains injection. All liver tissue depots were removed on the 30th day, rinsed with PBS and weighed.
Measuring body weight and ultrasonic studies of the liver and omentum
Body weight was measured once a week. Before sacrifice, mice were fasted for 12 h and anesthetized with diethyl ether.
Ultrasound examination of mice
We performed ultrasonography (US) of mice using linear 5–12-MHz frequency probes of an ultrasound scanner (Philips/ATL HDI 5000, Netherlands). For screening and examination of the mice, we evaluated the reliable ultrasound parameters, as follows (Fig. 1):
for screening all mice involved in the study on the longitudinal (sagittal) ultrasound probe position, we measured the thickness of mesenteric fat (omentum, largest part of visceral fat; the threshhold was considered as 1.5 mm) and collected records of longitudinal and transverse organo-complex scans (in sagittal and transverse probe positions) and measured the largest longitudinal liver size (via a subcostal approach);
the additional parameters, which were accessible to evaluate in small animals (in few mice) of all groups – liver echogenicity, kidneys structure, spleen length, visceral vessels, muscle thickness at the midfemoral level.
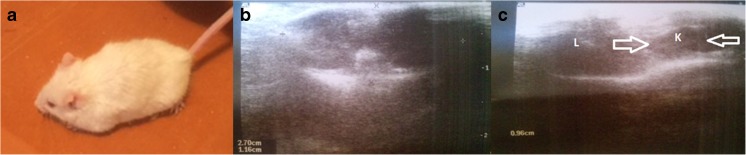
Fig. 1.
Ultrasound examination of mice along the experiment; (A) obese mouse (general view); (B) cross-section abdominal scan (transverse organo-complex record); (C) organs evaluation on US, L liver, K kidney
Cholesterol study
The concentration of free and ester cholesterol in the blood serum was determined according to the modified colorimetric method of Zlatkis-Zak with ferric chloride. The principle of this method is that free or ester cholesterol is oxidized by ferric chloride in the presence of acetic, sulfuric and phosphoric acids with the formation of unsaturated red-violet color products. Determination of the concentration of free and ester cholesterol was carried out according to the calibration chart on the spectrophotometer “MultiScan EX” with the optical wave length λ = 530 nm. The concentration of cholesterol id expressed in mg/ml.
Analysis of fecal microbiota
The number of LAB, Bifidobacteria, staphylococci, streptococci, coliform bacteria and microscopic fungi was determined in the intestinal contents. Different dilutions of aliquots of intestinal contents were plated on specific agar mediums to determine the quantity of different taxonomic groups of microorganisms. We counted the number of colony-forming units (CFU) of LAB, Bifidobacteria by seeding samples on MRS agar (MRSA) and bifidum agar (BA) medium, respectively, microscopic fungi on Sabouraud agar, coliform bacteria on ENDO, staphylococci on Baird–Parker agar (Merck, Germany), and streptococci on KF Streptococcus agar (Merck, Germany), given that one such colony corresponds to one bacterium. LAB, microscopic fungi, coliform bacteria, staphylococci and streptococci were cultured in aerobic conditions, and Bifidobacteria in anaerobic conditions, at 37 °C for 48 h.
Histological analysis
Liver samples from each mouse were rinsed with sterilized PBS, fixed in 10% formalin/PBS and then embedded in paraffin for staining with hematoxylin and eosin (H&E). Microscopic images (AxioObser Z1, Germany) were received at a magnifications of ×200, ×400 and ×1000.
Statistical analysis
Statistical analysis was performed by using one-way analysis of variance (ANOVA), using Epi Info software (USA, version 8.0) and Microsoft Office Excel. Numeric data were presented as mean arithmetic values and their standard deviations (M ± m). For single comparisons, values of P were determined using Student’s t test. Differences between groups were defined significant if P < 0.05.
The sample sizes were estimated based on previously obtained data and predicted differences between two means and standard deviations and considered expected death of animals.
Results
The changes of mice weight and ultrasound assessment of the liver and mesenteric fat
On the 7th day from the start of FED, we observed the changes in mice behavior: animals became lethargic, lost appetite and their body temperature increased. The results of weight studying of obese mice that received probiotic bacteria or probiotic compositions are presented in Table. 1.
Table 1.
Weight change of mice that received fat-enriched diet and probiotic bacteria or probiotic compositions
| Group of animals | Mice weight, g/term of observation | ||||
|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | Day 21 | Day 30 | |
| Mice that received FED | 20.3 ± 1.3 | 23.4 ± 0.5 | 25.7 ± 0.1 | 26.1 ± 0.2 | 26.3 ± 0.7 ** |
| Mice that received FED and L. casei IMV B-7280 | 23.6 ± 0.9 | 24.7 ± 1.1 | 27.4 ± 1.9** | 27.2 ± 1.1** | 23.5 ± 1.4# |
| Mice that received FED and L. delbrueckii subsp. bulgaricus ІМV В-7281 | 23.6 ± 1.0 | 29.5 ± 0.8** | 26.9 ± 0.7** | 26.8 ± 0.2** | 23.6 ± 1.5# |
| Mice that received FED and B. аnimalis VKB | 20.5 ± 4.6 | 22.4 ± 2.0 | 23.4 ± 1.0 | 23.9 ± 0.9** | 18.2 ± 1.9*# |
| Mice that received FED and B. аnimalis VKL | 21.2 ± 0.8 | 22.2 ± 0.4 | 23.3 ± 0.8 | 24.2 ± 0.9** | 20.4 ± 0.6*# |
| Mice that received FED and B. animalis VKB/B. animalis VKL | 21.0 ± 2.0 | 22.2 ± 1.2 | 23.4 ± 1.9 | 23.3 ± 1.7 | 23.2 ± 3.2 |
| Mice that received FED and B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 | 22.8 ± 1.4 | 24.6 ± 1.8 | 24.8 ± 1.6 | 26.9 ± 0.6** | 22.8 ± 1.6# |
| Mice that received FED and L. casei IMV B-7280/L. delbrueckii subsp. bulgaricus ІМV В-7281 | 18.5 ± 2.8 | 18.9 ± 2.7 | 19.45 ± 0.9 | 22.5 ± 1.0** | 17.0 ± 3.9*# |
Significant differences with the mice weight (all together) on the 21st day of FED are represented by * (P < 0.05); significant differences with the weight of mice that received FED on the 14th day after probiotic bacteria administration are presented by # (P < 0.05); significant differences with mice weight before FED (day 0) are presented by ** (P < 0.05)
The weight of mice that received FED increased by 6 g compared with mice that received a standard diet, confirming the development of obesity. But, the weight of obese mice that received L. casei IMV B-7280, L. delbrueckii subsp. bulgaricus IMV B-7281, B. аnimalis VKB, B. аnimalis VKL (separately) or B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 and L. casei IMV B-7280/L. delbrueckii subsp. bulgaricus IMV B-7281 probiotic compositions was decreased compared with obese mice that did not receive probiotic bacteria (control group). The weight of obese mice decreased more efficiently after injection of B. аnimalis VKB (separately) or L. casei IMV B-7280/L. delbrueckii subsp. bulgaricus ІМV В-7281 composition. However, the B. animalis VKB/B. animalis VKL composition did not alter the weight of obese mice.
Concurrently, we observed an increasing of the size of liver and mesenteric fat in obese mice compared with intact mice receiving a standard diet (Figs. 1, 2, and 3 and Table 2).
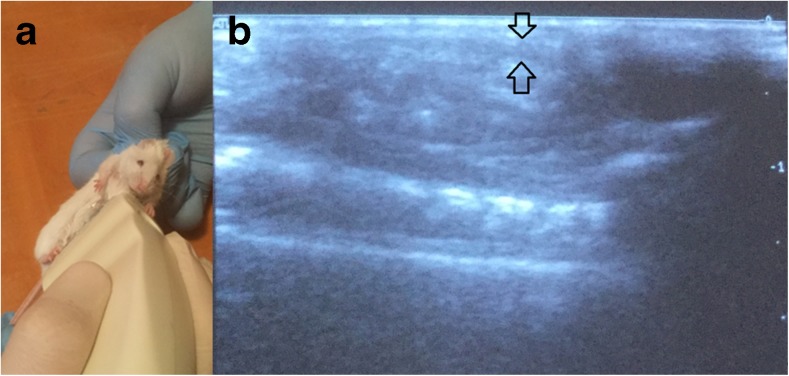
Fig. 2.
Ultrasound examination (A) to obtain longitudinal scans of the abdominal cavity of mice along the experiment (sagittal organo-complex records); B measurement of visceral (mesenteric) fat
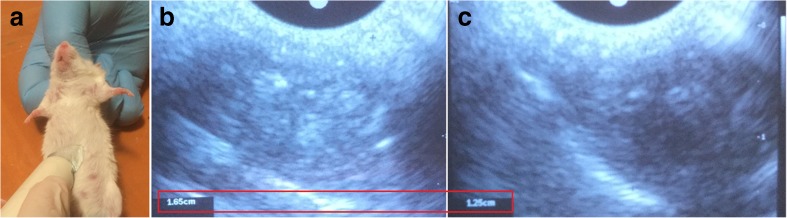
Fig. 3.
Ultrasound examination of the liver (A) during the experiment; (B) the enlarged liver of obese mouse; (C) liver size decreasing after treatment
Table 2.
Results of ultrasound studies of the liver and mesenteric fat of mice that received fat-enriched diet and probiotic strains of bacteria
| Group of mice | Liver size, mm | Mesenteric fat size, mm |
|---|---|---|
| Intact mice | 1.17 ± 0.02 | 0.13 ± 0.02 |
| Mice that received FED | 1.56 ± 0.02* | 0.18 ± 0.01* |
| Mice that received FED and L. casei IMV B-7280 | 1.50 ± 0.02 | 0.13 ± 0.02# |
| Mice that received FED and L. delbrueckii subsp. bulgaricus ІМV В-7281 | 1.40 ± 0.02# | 0.10 ± 0.02# |
| Mice that received FED and B. аnimalis VKB | 1.30 ± 0.02# | 0.08 ± 0.02# |
| Mice that received FED and B. аnimalis VKL | 1.70 ± 0.02 | 0.09 ± 0.02# |
| Mice that received FED and B. animalis VKB/B. animalis VKL | 1.45 ± 0.02# | 0.10 ± 0.02# |
| Mice that received FED and B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 | 1.45 ± 0.02# | 0.11 ± 0.02# |
| Mice that received FED and L. casei IMV B-7280/L. delbrueckii subsp. bulgaricus ІМV В-7281 | 1.45 ± 0.02# | 0.11 ± 0.02# |
Significant differences of intact mice are presented by * (P < 0.05); significant differences of obese mice are presented by # (P < 0.05)
Registration of longitudinal ultrasound scans of visceral organo-complex records of experimental mice allowed us to conduct fast screening measurements in a large number of animals (over 100) and precise post-processing analysis (Fig. 2). The size of the liver significantly increased on US after FED (p < 0.05), and slightly decreased after L. delbrueckii subsp. bulgaricus IMV B-7281, B. аnimalis VKB or probiotic composition injection into obese mice. Reduction of the mesenteric fat (omentum) size was observed in response to all these probiotic bacteria separately and the probiotic compositions. Femoral muscle thickness at the mid-femoral level was rather stable on US; therefore, fat/muscle ratio depended on visceral fat thickness. This allowed us to avoid bias, e.g. mass might be altered by gut hyperactivity induced by probiotics and/or changes of animals’ appetite, etc. We observed insignificant changes in kidney structure, but it is rather hard to make significant conclusions in the current study.
The cholesterol level in serum of obese mice that received probiotic bacteria
The level of total, free and ester (esterified) cholesterol was increased in the serum of obese mice (within 21 days); we also revealed a reduction of the esterification index on the 21st day compared with intact mice (Fig. 4a).
Fig. 4.
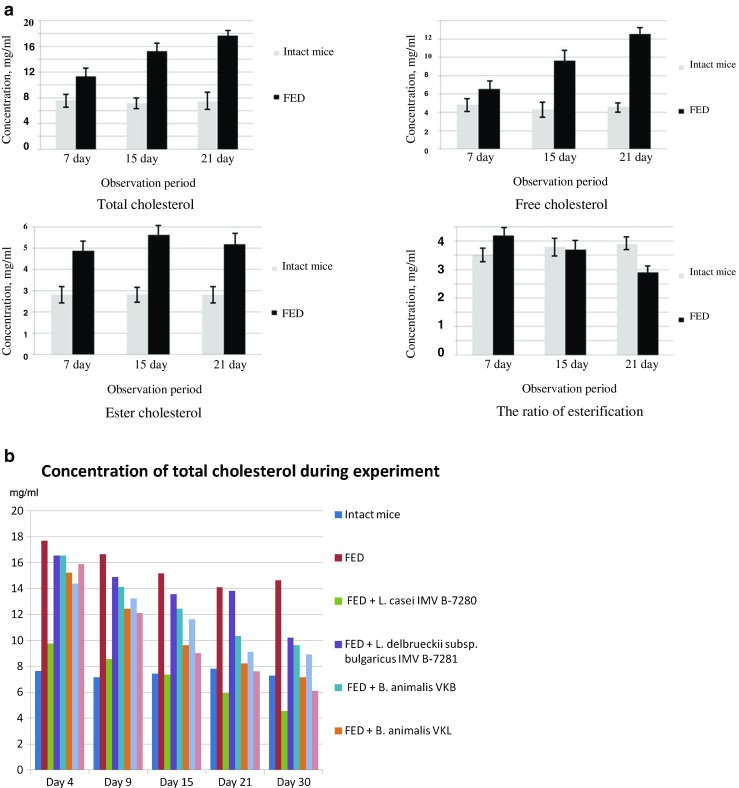
Cholesterol levels; (A) the concentration of total, free and ester cholesterol in the blood serum of BALB/c line mice that received FED; (B) changes of total cholesterol levels in the blood serum during the experiment in all groups
Note that the concentration of free, total and ester cholesterol in serum of obese mice remained at a high level over the next 30 days, when they already received the standard diet (Table 3, Fig. 4b).
Table 3.
Concentration of total, free and ester cholesterol in the blood serum of BALB/c mice treated with probiotic bacteria or probiotic compositions
| Group of mice | Concentration of cholesterol (mg/ml) term of observation after probiotic bacterial strains injection | ||||
|---|---|---|---|---|---|
| Day 4 | Day 9 | Day 15 | Day 21 | Day 30 | |
| Concentration of total cholesterol (sum of free and ester cholesterol), mg/ml | |||||
| Intact mice | 7.64 ± 0.35 | 7.16 ± 0.31 | 7.43 ± 0.17 | 7.83 ± 0.11 | 7.28 ± 0.14 |
| Mice that received FED | 17.71 ± 0.76* | 16.66 ± 0.28* | 15.18 ± 0.37* | 14.09 ± 0.70* | 14.63 ± 0.53* |
| Mice that received FED + L. casei IMV B-7280 | 9.77 ± 0.06* | 8.56 ± 0.15* | 7.37 ± 0.25* | 5.93 ± 0.48*• | 4.56 ± 0.23*• |
| Mice that received FED + L. delbrueckii subsp. bulgaricus ІМV В-7281 | 16.55 ± 0.15* | 14.89 ± 0.48•* | 13.56 ± 0.38•* | 13.81 ± 0.81* | 10.23 ± 0.63•* |
| Mice that received FED + B. animalis VKB | 16.55 ± 0.65* | 14.12 ± 0.36•* | 12.45 ± 0.61•* | 10.35 ± 0.45•* | 9.63 ± 0.63•* |
| Mice that received FED + B. animalis VKL | 15.22 ± 0.38•* | 12.45 ± 0.74•* | 9.64 ± 0.64•* | 8.22 ± 0.39• | 7.15 ± 0.47• |
| Mice that received FED + B. animalis VKB/B. animalis VKL | 14.38 ± 0.21* | 13.22 ± 0.08* | 11.63 ± 0.19* | 9.13 ± 0.59* | 8.91 ± 0.10* |
| Mice that received FED + B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 | 15.88 ± 0.26* | 12.11 ± 0.35•* | 9.03 ± 0.35•* | 7.62 ± 0.95• | 6.11 ± 0.69• |
| Concentration of free cholesterol | |||||
| Intact mice | 4.82 ± 0.36 | 4.34 ± 0.31 | 4.61 ± 0.18 | 5.01 ± 0.11 | 4.46 ± 0.14 |
| Mice that received FED | 12.33 ± 0.77* | 11.27 ± 0.29* | 9.77 ± 0.37* | 8.68 ± 0.71* | 9.21 ± 0.53* |
| Mice that received FED + L. casei IMV B-7280 | 8.41 ± 0.06* | 7.19 ± 0.15* | 5.99 ± 0.25* | 4.53 ± 0.49*• | 3.15 ± 0.23*• |
| Mice that received FED + L. delbrueckii subsp. bulgaricus ІМV В-7281 | 7.12 ± 0.55•* | 6.89 ± 0.62•* | 5.15 ± 0.37• | 5.59 ± 0.84• | 4.85 ± 0.17• |
| Mice that received FED + B. animalis VKB | 11.13 ± 0.82* | 10.67 ± 0.45* | 8.11 ± 0.81* | 8.22 ± 0.37* | 7.78 ± 0.55* |
| Mice that received FED + B. animalis VKL | 10.46 ± 0.71* | 9.79 ± 0.43•* | 8.56 ± 0.33* | 7.16 ± 0.62* | 6.02 ± 0.27•* |
| Mice that received FED + B. animalis VKB/B. animalis VKL | 9.72 ± 0.22* | 8.54 ± 0.08* | 6.93 ± 0.19* | 4.41 ± 0.60* | 4.19 ± 0.10*• |
| Mice that received FED + B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 | 8.65 ± 0.35•* | 8.12 ± 0.41•* | 6.52 ± 0.89•* | 6.03 ± 0.63• | 4.45 ± 0.40• |
| Concentration of ester cholesterol | |||||
| Intact mice | 2.82 ± 0.04 | 2.83 ± 0.03 | 2.82 ± 0.05 | 2.82 ± 0.03 | 2.84 ± 0.01 |
| Mice that received FED | 5.31 ± 0.09* | 5.45 ± 0.14* | 5.44 ± 0.04* | 5.43 ± 0.06* | 5.38 ± 0.08* |
| Mice that received FED + L. casei IMV B-7280 | 1.31 ± 0.02*• | 1.37 ± 0.04*• | 1.42 ± 0.02*• | 1.45 ± 0.02*• | 1.43 ± 0.03*• |
| Mice that received FED + L. delbrueckii subsp. bulgaricus ІМV В-7281 | 9.41 ± 0.05•* | 8.12 ± 0.21•* | 8.37 ± 0.18•* | 8.25 ± 0.16•* | 5.33 ± 0.09* |
| Mice that received FED + B. animalis VKB | 5.47 ± 0.11* | 3.38 ± 0.04•* | 4.24 ± 0.03•* | 2.17 ± 0.03•* | 1.83 ± 0.01•* |
| Mice that received FED + B. animalis VKL | 4.73 ± 0.13*• | 2.65 ± 0.08• | 1.11 ± 0.01•* | 1.05 ± 0.01•* | 1.19 ± 0.02•* |
| Mice that received FED + B. animalis VKB/B. animalis VKL | 4.67 ± 0.11*• | 4.62 ± 0.07*• | 4.75 ± 0.07*• | 4.71 ± 0.07*• | 4.79 ± 0.07*• |
| Mice that received FED + B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 | 7.33 ± 0.04•* | 4.12 ± 0.03•* | 2.55 ± 0.03• | 1.59 ± 0.02•* | 1.69 ± 0.01•* |
| The ratio of esterification (the ratio of ester cholesterol to total cholesterol) | |||||
| Intact mice (control) | 0.35 ± 0.02 | 0.38 ± 0.01 | 0.39 ± 0.02 | 0.37 ± 0.02 | 0.40 ± 0.03 |
| Mice that received FED | 0.28 ± 0.03 | 0.33 ± 0.01 | 0.37 ± 0.01 | 0.38 ± 0.03 | 0.36 ± 0.01 |
| Mice that received FED + L. casei IMV B-7280 | 0.13 ± 0.05 | 0.16 ± 0.02 | 0.19 ± 0.09 | 0.24 ± 0.04 | 0.31 ± 0.03 |
| Mice that received FED + L. delbrueckii subsp. bulgaricus ІМV В-7281 | 0.59 ± 0.04•* | 0.57 ± 0.03•* | 0.64 ± 0.03•* | 0.63 ± 0.04•* | 0.54 ± 0.04•* |
| Mice that received FED + B. animalis VKB | 0.34 ± 0.01 | 0.22 ± 0.02•* | 0.34 ± 0.02 | 0.22 ± 0.01•* | 0.17 ± 0.01•* |
| Mice that received FED + B. animalis VKL | 0.32 ± 0.03 | 0.23 ± 0.02•* | 0.12 ± 0.01•* | 0.14 ± 0.01•* | 0.16 ± 0.01•* |
| Mice that received FED + B. animalis VKB/B. animalis VKL | 0.32 ± 0.09 | 0.34 ± 0.01 | 0.40 ± 0.08 | 0.51 ± 0.02 | 0.54 ± 0.09 |
| Mice that received FED + B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 | 0.47 ± 0.02•* | 0.36 ± 0.01 | 0.27 ± 0.02• | 0.20 ± 0.02•* | 0.29 ± 0.02* |
Significant differences with intact mice are represented by * (P < 0.05); significant difference with obese mice that did not receive probiotic bacteria are presented by • (P < 0.05)
It was established that L. casei IMV B-7280, L. delbrueckii subsp. bulgaricus IMV B-7281, B. animalіs VKL and B. animalіs VKB (separately) or probiotic compositions significantly decreased the level of total and free cholesterol in the serum of obese mice compared with control group (Table 3).
The hypocholesterolemic effect of probiotic strains of LAB and Bifidobacteria, which we investigated, was definitely strain-dependent. Thus, L. casei IMV B-7280 was most efficient among probiotic bacteria and probiotic compositions which we investigated; the level of serum total cholesterol on the 15th, 21st and 30th days after L. casei IMV B-7280 administration in obese mice was even lower compared with intact mice.
As shown in Table 3, the level of free serum cholesterol in obese mice reduced more efficiently after administration of two LAB strains: L. casei IMV B-7280 and L. delbrueckii subsp. bulgaricus IMV B-7281(separately) compared with B. animalіs VKL and B. animalіs VKB (separately) throughout the observation period (P < 0.05) and B. animalis VKB/B. animalis VKL and B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 compositions on the 4, 9 and 15th days. The level of free serum cholesterol in obese mice that received L. casei IMV B-7280 or B. animalis VKB/B. animalis VKL composition on the 21st and 30th days was even lower than in intact mice. The impact of these strains of LAB and Bifidobacteria and their compositions on the level of ester serum cholesterol varied. L. сasei IMV B-7280, B. animalis VKL, B. animalis VKB/B. animalis VKL composition (throughout the observation period), B. animalis VKB or B. animalis VKB/B. animalis VKL/L. casei IMV B-7280 composition (on the 15–30th days) decreased the level of ester cholesterol in the serum compared with control group and even intact mice (Тable 3). L. delbrueckii subsp. bulgaricus IMV B-7281 differed from other strains by the fact that under its influence, the level of ester cholesterol in obese mice, on the contrary, significantly increased. The esterification ratio (the ratio of ester cholesterol to total cholesterol) rose throughout the observation period in mice receiving L. delbrueckii subsp. bulgaricus IMV B-7281, and on the 4th day after administration of B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 composition to obese mice compared with mice of all other groups (Table 1). In the serum of obese mice that received probiotic bacteria or probiotic compositions, the ratio of esterification was decreased compared with control group and even with intact mice.
Note that the level of serum cholesterol remained reduced on the 9–30th days, i.e. after obese mice received these probiotic bacteria or probiotic compositions.
Morphology of the liver
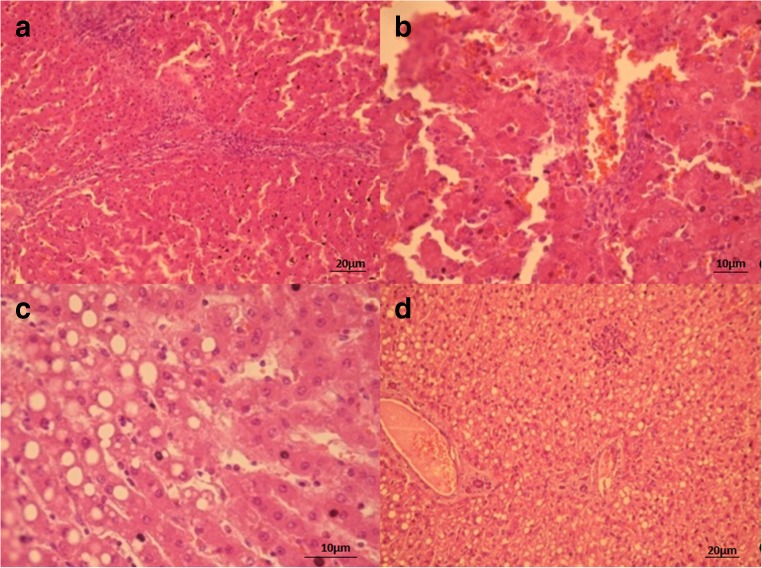
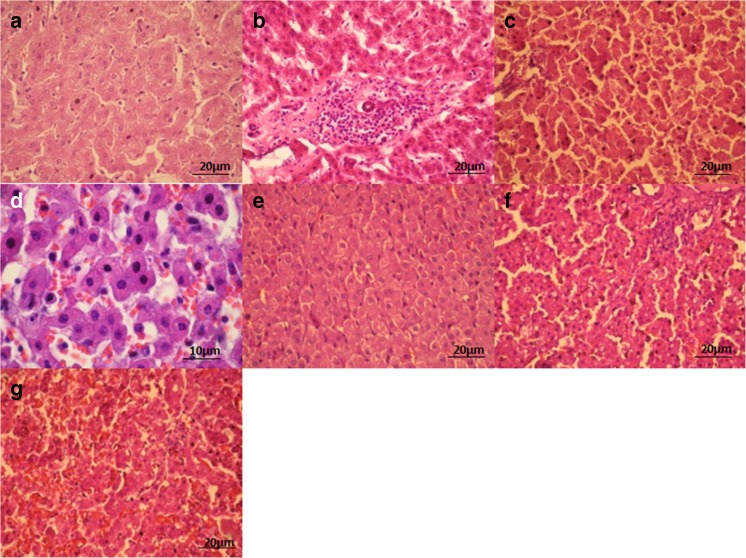
The liver tissue structure was unchanged in intact mice; pathomorphological changes were not revealed (Fig. 5). In the liver of obese mice, we found hemorrhages, hepatocytes fatty degeneration and necrosis (Fig. 5).
Fig. 5.
Liver tissue of intact (A, B) and obese (C, D) mice. (A, B) structure of liver tissue is preserved as normal, pathologic changes not detected. (C, D) fatty degeneration/necrosis of hepatocytes (A – x200 magnification, B – x400 magnification)
The morphological changes of liver tissue of obese mice that received L. casei IMV B-7280, L. delbrueckii subsp. bulgaricus IMV B-7281, B. animalіs VKL or B. animalіs VKB, as well as B. animalіs VKL/B. animalіs VKB or B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 compositions are presented in the Fig. 6.
Fig. 6.
Liver tissue of mice that received FED and L. casei IMV B-7280 (A), B. animalіs VKL (B), B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 (C), B. animalis VKB (D, E), L. delbrueckii subsp. bulgaricus IMV B-7281 (F), B. animalis VKB/B. animalis VKL composition (G). (A) restoring liver structure, lipid inclusions are detected in hepatocytes. Partial necrosis and fatty degeneration of hepatocytes (x400 magnification). (B) restoring structure, lipid inclusions in hepatocytes and existing isolated sites with lymphocytes and macrophages infiltrates are detected; partial necrosis and fatty degeneration of hepatocytes (×400 magnification). (C) restoring structure, degenerative changes not detected. Partial necrosis of hepatocytes (x400 magnifications). (D, E) partial necrosis and fatty degeneration of hepatocytes (D – ×1000 magnification, E – ×400 magnification). (F) necrosis and fatty degeneration of hepatocytes (×400 magnification). (G) necrosis and fatty degeneration of hepatocytes (×400 magnification)
We revealed that L. casei IMV B-7280, B. animalіs VKL or B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 composition recovered the liver structure of obese mice. After injection of this probiotic composition to obese mice, the dystrophic changes in the liver were not detected, while partial necrosis and fatty degeneration of hepatocytes remained in the liver of obese mice treated with L. casei IMV B-7280 or B. animalіs VKL. Fatty degeneration and necrosis of hepatocytes decreased after treatment with these probiotic bacteria or probiotic compositions. Yet, hemorrhages in the liver were not found in obese mice treated with L. casei IMV B-7280 or B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 composition. However, after injection of B. animalіs VKB, L. delbrueckii subsp. bulgaricus IMV B-7281 or B. animalіs VKL/B. animalіs VKB composition to obese mice, we found necrosis and fatty degeneration of hepatocytes.
The treatment with B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 composition effectively recovered the liver morphological structure in obese mice. L. casei IMV B-7280 and B. animalіs VKL (separately) restored the liver morphological structure of obese mice to a lesser degree. B. animalіs VKB or L. delbrueckii subsp. bulgaricus IMV B-7281 (separately) and B. animalіs VKL/B. animalіs VKB composition were ineffective.
The changes of the gut microbiota of obese mice that received probiotic strains or probiotic compositions
As demonstrated on the Table 4, obesity in mice was associated with increasing the total number of microorganisms (on the 4th–15th days) and changes in spectrum of gut microbiota. Thus, we observed decreasing the number of Lactobacillus spp. (throughout the observation period), Bifidobacterium spp. (on the 4th and 9th days) and coliform bacteria (on the 9th–30th days). However, the number of Gram-positive cocci (staphylococci, streptococci; throughout the observation period) and fungal species (on the 4th and 15th days) increased compared with intact mice.
Table 4.
Spectrum of gut microbiota of obese mice that received probiotic bacteria or probiotic compositions, M ± m
| Group of mice | Day | Number of microorganisms, that were sowed on the nutrient mediums, Lg CFU/mg | ||||||
|---|---|---|---|---|---|---|---|---|
| Baird–Parker agar | KF-Streptococcus agar | MRSA | BA | ENDO | Sabouraud agar | MPA | ||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Intact mice | – | 2.65 ± 0.09 | 3.25 ± 0.05 | 2.68 ± 0.02 | 2.15 ± 0.02 | 4.15 ± 0.09 | 2.77 ± 0.05 | 3.11 ± 0.09 |
| Mice that received FED | 4 | 4.11 ± 0.11* | 6.75 ± 0.12* | 0.52 ± 0.06* | 1.44 ± 0.06* | 3.75 ± 0.09 | 3.19 ± 0.07* | 4.47 ± 0.07* |
| 9 | 4.22 ± 0.09* | 6.58 ± 0.05* | 1.35 ± 0.02* | 1.25 ± 0.04* | 3.42 ± 0.08* | 3.22 ± 0.09* | 4.74 ± 0.06* | |
| 15 | 4.13 ± 0.03* | 5.12 ± 0.06* | 1.10 ± 0.01* | 2.06 ± 0.07 | 3.11 ± 0.04* | 3.07 ± 0.11 | 4.32 ± 0.03* | |
| 21 | 4.44 ± 0.05* | 5.67 ± 0.08* | 1.22 ± 0.05* | 2.12 ± 0.09 | 2.67 ± 0.02* | 3.11 ± 0.06 | 3.76 ± 0.04* | |
| 30 | 3.92 ± 0.12* | 6.47 ± 0.03* | 1.41 ± 0.03* | 2.11 ± 0.03 | 2.52 ± 0.03* | 3.16 ± 0.08 | 3.98 ± 0.03* | |
| Mice that received FED + L. casei IMV B-7280 | 4 | 3.89 ± 0.06* | 5.91 ± 0.05*• | 2.77 ± 0.06• | 2.92 ± 0.07*• | 3.90 ± 0.08 | 2.60 ± 0.11• | 4.26 ± 0.09* |
| 9 | 3.87 ± 0.12* | 5.11 ± 0.07*• | 3.49 ± 0.08*• | 3.32 ± 0.03*• | 4.22 ± 0.11• | 0*• | 4.17 ± 0.06*• | |
| 15 | 3.52 ± 0.09*• | 5.22 ± 0.02* | 4.78 ± 0.11*• | 3.11 ± 0.05* | 4.40 ± 0.15• | 0*• | 4.56 ± 0.07* | |
| 21 | 2.99 ± 0.03*• | 4.46 ± 0.10*• | 3.52 ± 0.05*• | 3.65 ± 0.04* | 4.13 ± 0.09• | 1.65 ± 0.06*• | 4.89 ± 0.10*• | |
| 30 | 2.45 ± 0.08• | 4.21 ± 0.07*• | 3.57 ± 0.09*• | 2.99 ± 0.08* | 4.86 ± 0.09*• | 1.98 ± 0.02*• | 4.23 ± 0.08* | |
| Mice that received FED + L. delbrueckii subsp. bulgaricus ІМV В-7281 | 4 | 4.45 ± 0.09* | 6.62 ± 0.09* | 1.16 ± 0.06*• | 1.65 ± 0.03* | 4.40 ± 0.09• | 3.65 ± 0.09* | 5.22 ± 0.09*• |
| 9 | 4.61 ± 0.11* | 5.89 ± 0.11*• | 2.11 ± 0.04*• | 2.16 ± 0.03• | 5.06 ± 0.08*• | 2.79 ± 0.07 | 5.37 ± 0.12*• | |
| 15 | 3.87 ± 0.07* | 6.03 ± 0.13*• | 2.25 ± 0.11*• | 2.27 ± 0.09 | 5.11 ± 0.11*• | 2.86 ± 0.11 | 5.62 ± 0.09*• | |
| 21 | 4.14 ± 0.09* | 5.63 ± 0.09* | 2.67 ± 0.09• | 2.08 ± 0.01 | 4.98 ± 0.09*• | 3.01 ± 0.09 | 5.64 ± 0.08*• | |
| 30 | 3.12 ± 0.03*• | 5.49 ± 0.07*• | 2.53 ± 0.12• | 2.19 ± 0.05 | 4.55 ± 0.12• | 2.92 ± 0.12 | 5.76 ± 0.09*• | |
| Mice that received FED + B. animalis VKB | 4 | 4.12 ± 0.11* | 5.92 ± 0.12*• | 1.52 ± 0.03*• | 2.22 ± 0.09• | 4.02 ± 0.12 | 2.17 ± 0.06*• | 4.78 ± 0.06* |
| 9 | 3.51 ± 0.04*• | 5.16 ± 0.09*• | 1.99 ± 0.05*• | 3.14 ± 0.06*• | 4.14 ± 0.04• | 1.63 ± 0.03*• | 5.21 ± 0.08*• | |
| 15 | 2.74 ± 0.03• | 4.22 ± 0.03*• | 2.11 ± 0.04*• | 3.92 ± 0.07* | 4.96 ± 0.07*• | 1.26 ± 0.02*• | 5.23 ± 0.09*• | |
| 21 | 2.98 ± 0.06• | 4.11 ± 0.05*• | 2.60 ± 0.02• | 3.62 ± 0.07* | 4.32 ± 0.09• | 2.54 ± 0.07• | 5.11 ± 0.12*• | |
| 30 | 3.12 ± 0.07*• | 4.07 ± 0.09*• | 2.65 ± 0.09• | 3.13 ± 0.03* | 4.07 ± 0.04• | 2.82 ± 0.04 | 4.98 ± 0.06*• | |
| Mice that received FED + B. animalis VKL | 4 | 3.61 ± 0.03*• | 6.17 ± 0.15* | 2.15 ± 0.05*• | 2.10 ± 0.05• | 3.80 ± 0.06* | 2.99 ± 0.10 | 4.87 ± 0.05* |
| 9 | 3.41 ± 0.07*• | 5.03 ± 0.06*• | 2.66 ± 0.09• | 3.42 ± 0.06*• | 3.96 ± 0.08• | 2.76 ± 0.04• | 4.61 ± 0.06* | |
| 15 | 4.06 ± 0.12* | 4.48 ± 0.02*• | 2.98 ± 0.12• | 3.65 ± 0.11* | 4.67 ± 0.11*• | 2.54 ± 0.07• | 4.17 ± 0.03* | |
| 21 | 3.21 ± 0.08*• | 3.62 ± 0.03• | 3.16 ± 0.06*• | 2.95 ± 0.06* | 3.72 ± 0.05*• | 2.80 ± 0.11 | 3.89 ± 0.04* | |
| 30 | 3.10 ± 0.05*• | 3.44 ± 0.05• | 3.25 ± 0.04*• | 2.63 ± 0.04* | 3.31 ± 0.09*• | 3.06 ± 0.09 | 3.51 ± 0.02• | |
| Mice that received FED + B. animalis VKB / B. animalis VKL | 4 | 4.10 ± 0.11* | 6.12 ± 0.18* | 1.77 ± 0.07*• | 3.79 ± 0.06*• | 3.89 ± 0.08 | 2.20 ± 0.07*• | 4.77 ± 0.07* |
| 9 | 3.78 ± 0.13* | 5.93 ± 0.11*• | 2.16 ± 0.11*• | 4.26 ± 0.07*• | 4.20 ± 0.09• | 2.11 ± 0.05*• | 5.12 ± 0.12* | |
| 15 | 3.77 ± 0.06*• | 5.11 ± 0.14* | 2.38 ± 0.10• | 4.61 ± 0.03* | 4.30 ± 0.04• | 1.96 ± 0.03*• | 5.34 ± 0.11*• | |
| 21 | 3.11 ± 0.06*• | 4.16 ± 0.09*• | 2.55 ± 0.09• | 5.14 ± 0.06* | 3.58 ± 0.03*• | 2.63 ± 0.08• | 4.98 ± 0.09*• | |
| 30 | 2.62 ± 0.03• | 3.41 ± 0.04• | 3.06 ± 0.03*• | 5.03 ± 0.07* | 3.97 ± 0.09*• | 2.79 ± 0.06 | 4.33 ± 0.05*• | |
| Mice that received FED + B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 | 4 | 3.61 ± 0.02*• | 5.11 ± 0.14*• | 2.17 ± 0.03*• | 2.86 ± 0.07*• | 4.12 ± 0.08• | 1.60 ± 0.03*• | 4.78 ± 0.08* |
| 9 | 2.87 ± 0.02• | 5.23 ± 0.12*• | 2.77 ± 0.07• | 4.14 ± 0.11*• | 4.55 ± 0.03*• | 0*• | 4.35 ± 0.07* | |
| 15 | 2.45 ± 0.04• | 4.46 ± 0.03*• | 3.58 ± 0.09*• | 5.10 ± 0.09* | 4.92 ± 0.09*• | 1.20 ± 0.04*• | 3.87 ± 0.05*• | |
| 21 | 2.97 ± 0.09• | 4.05 ± 0.08*• | 4.13 ± 0.05*• | 4.83 ± 0.13* | 5.16 ± 0.12*• | 1.11 ± 0.01*• | 3.65 ± 0.06 | |
| 30 | 3.14 ± 0.06*• | 3.86 ± 0.05*• | 4.19 ± 0.08*• | 4.22 ± 0.17* | 5.22 ± 0.16*• | 1.62 ± 0.03*• | 3.27 ± 0.07• | |
Significant differences with intact mice are represented by * (P < 0.05); significant differences with obese mice that did not receive probiotic bacteria are presented by • (P < 0.05)
The number of Lactobacillus spp., Bifidobacterium spp. and coliform bacteria increased in the gut of obese mice that received L. casei IMV B-7280, L. delbrueckii subsp. bulgaricus IMV B-7281, B. animalis VKB or B. animalis VKL (separately) or probiotic compositions, and the number of staphylococci and streptococci in most cases were reduced in different periods of observation compared with control or intact mice, although the total number of bacteria remained high.
We noted the higher number of Lactobacillus spp. in the gut of obese mice treated with L. casei IMV B-7280 (on the 9th–30th days), B. animalis VKL (on the 21st and 30th days), as well as treated with B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 (on the 15th–30th days) and B. animalis VKB/B. animalis VKL (on the 30th day) compositions compared with all other groups of mice (including intact mice; P < 0.05). The number of Bifidobacteria was higher (P < 0.05) even compared with all other groups of mice (including intact ones) after treatment with L. casei IMV B-7280 (on the 4th–30th days), B. animalis VKB or B. animalis VKL (on the 9th–30th days), and B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 or B. animalis VKB/B. animalis VKL (on the 4th–30th days) compositions. A significant increase in the number of both Lactobacillus spp. and Bifidobacterium spp. in the gut was observed after administration of L. casei IMV B-7280 and B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 composition to obese mice; the lesser increasing was observed after treatment with B. animalis VKL (only on the 21st–30th days) or B. animalis VKB/B. animalis VKL composition (on the 30th day).
The number of coliform bacteria in the gut of obese mice that received L. delbrueckii subsp. bulgaricus IMV B-7281 (on the 9th–21st days) or B. animalis VKB (on the 15th day) or B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 composition (on the 9th–30th days) was even higher than in intact mice.
The treatment with L. casei IMV B-7280, L. delbrueckii subsp. bulgaricus IMV B-7281, B. animalis VKB and B. animalis VKL or B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 composition significantly decreased the number of microscopic fungi in the gut of obese mice compared with the control and/or intact mice. The number of microscopic fungi in the gut was significantly lower after obese mice received L. casei IMV B-7280 or B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 composition (P < 0.05).
On the other hand, the treatment with L. casei IMV B-7280, L. delbrueckii subsp. bulgaricus IMV B-7281, B. animalis VKB or B. animalis VKL (separately) and B. animalis VKB/B. animalis VKL or B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 compositions did not normalize the total number of bacteria and the number of Gram-positive bacteria in the gut of obese mice; however, the number of Lactobacillus spp., Bifidobacterium spp. and Gram-negative bacteria (coliform bacteria) significantly increased.
Discussion
The normalization of lipid metabolism and treatment of gut microbiota violations using probiotics is a pivotal treatment and preventive strategy for patients with obesity and metabolic diseases. Our results are supposed to add evidence to the issue and are promising to facilitate clinical application.
Probiotics affect physiological functions and metabolic processes directly or through the normalization of microbiocenosis of mucous membranes of various organs and body systems; however, the range of their biological activity is a strain-dependent characteristic (Arora, 2012, 2013 Guo, 2011, Cho, 2015, Lumeng, 2013) [57, 58, 73–75].
Thus, bacterial strains have different probiotic effects on metabolic disease and obesity.
For example, in clinical and experimental studies, probiotic bacteria L. plantarum and L. gasseri reduced the body weight (Million, 2012; Wu, 2015) [31, 76] and cholesterol level [77] but, on the contrary, L. acidophilus, L. fermentum or L. ingluviei increase the body weight [76], and L. acidophilus NCDC 13 had no impact on obesity [73].
In the current study, we have defined that the probiotic bacteria L. casei IMV B-7280, L. delbrueckii subsp. bulgaricus IMV B-7281, B. animalis VKB and B. animalis VKL (separately) or B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 and B. animalis VKB/B. animalis VKL compositions decreased the weight of obese BALB/c mice, which was associated with decreased cholesterol level in serum and partial normalization of intestinal biocenosis. This was manifested in the increased number of Lactobacillus spp., Bifidobacterium spp. and coliform bacteria. Furthermore, a slight decreasing of the liver size and mesenteric fat thickness in obese mice was also observed under the effect of these probiotic bacteria and probiotic compositions. Other strains of LAB have also demonstrated hypocholesterolemic activity in vivo on mice models of metabolic diseases. The level of cholesterol decreased in obese mice after administration of L. plantarum strain K21 [31, 77], L. acidophilus NS1 [30], L. curvatus HY7601 and L. plantarum KY1032 [22]. L. rhamnosus CCFM1107 decreased the level of cholesterol in the liver and serum of mice with alcoholic impact of the liver [28] After administration of L. acidophilus to obese mice with damaged livers after a cholesterol-enriched diet, a reduction of cholesterol level both in serum and liver was observed [29]. L. acidophilus ATCC 4356 protected mice from atherosclerosis by reducing the level of cholesterol in blood plasma [23], and L. plantarum CAI6 and L. plantarum SC4 had a protective effect in models of cardiovascular disease in hyperlipidemic mice by reducing the level of total and low-density lipoprotein cholesterol [24].
Recently, the “bile salt hydrolase hypothesis” (BSH) has been proposed [48, 78–80]. Deconjugated bile acids are transformed into secondary bile acids by colonic microbes, which is most acceptable to explain cholesterol-lowering mechanics of probiotics together with direct binding to cholesterol, producing propionic and butyric acids and reducing the synthesis of cholesterol in the liver. Gut microbiota can regulate cholesterol metabolism via the following mechanisms: 1) direct effects on enzyme systems in liver cells and other organs that produce endogenous cholesterol; 2) increasing the regeneration rate of the intestinal villi cells, which produce endogenous cholesterol; 3) impact on absorption of cholesterol from the intestine, which depends on the transit of neutral sterols through the intestine, the concentration of ions (mainly calcium ions), the affinity of cell receptors to lipoproteins or microorganisms, that are involved in the transformation of cholesterol; 4) reduction of cholesterol level via the hydrolysis system of deconjugation of bile acids and direct binding of cholesterol by cell walls; 5) degradation and transformation of bile acids and steroid hormones, the concentration change of which can lead to strengthening or inhibition of cholesterol synthesis; 6) decreasing pH value in the gut, which ultimately leads to a decrease of bile acid synthesis in the liver and inhibition of cholesterol synthesis [20–31, 78–80]. Bosch et al. [78] concluded that the above characteristics suggest that the strain is an excellent candidate for reducing high blood cholesterol levels.
We have defined the clear strain-dependent hypocholesterolemic effect of the studied strains. Thus, L. casei IMV B-7280, L. delbrueckii subsp. bulgaricus IMV B-7281, B. animalis VKB and B. animalis VKL (separately) or B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 and B. animalis VKB/B. animalis VKL compositions also impact the level of various fractions of serum cholesterol (total, free and ester cholesterol). The decrease of the total cholesterol level in serum occurred as a result of the effects of L. casei IMV B-7280, and the level of free serum cholesterol was more effectively reduced under the effect of L. casei IMV B-7280 and L. delbrueckii subsp. bulgaricus IMV B-7281, compared with B. animalis VKB and B. animalis VKL. These two strains of LAB showed more effective in vitro hypocholesterolemic activity than B. animalis VKB and B. animalis VKL. Under the treatment with L. casei IMV B-7280 and L. delbrueckii subsp. bulgaricus IMV B-7281, the assimilation of cholesterol from the gastrointestinal tract or its binding was probably more intensive. A possible explanation might be the ability of different LAB strains to decrease the pH value in the gut, evoking deconjugation of the bile acids that bind cholesterol at low pH values. However, more research is needed to elucidate this mechanism.
The best probiotic composition with hypocholesteremic activity was B. animalis VKL/B. animalis VKB/L. casei IMV B-7280; their cholesterol-lowering activity on the serum-free cholesterol was better from these two Bifidobacteria strains in monoculture.
The level of ester cholesterol and the ratio of esterification, which is an important functional indicator of the liver function, significantly increased under the effect of L. delbrueckii subsp. bulgaricus IMV B-7281 throughout the observation period and only on the 4th day after the administration of B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 composition to obese mice. This probably occurred by reducing the level of free cholesterol under the effect of these probiotic strains and probiotic composition, and an increasing of the synthesis of ester cholesterol in the liver happens as a physiologically normal process. In the blood serum of obese mice that received L. casei IMV B-7280, B. animalis VKB and B. animalis VKL (separately) or B. animalis VKB/B. animalis VKL composition, the level of serum ester cholesterol and the ratio of esterification decreased. However, to obtain explanations for this, further research is required.
The rate of esterification is an indirect marker of the liver function, a site where cholesterol esters are synthesized. Furthermore, we observed that the liver morphological structure of obese mice was effectively recovered by B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 composition. The liver morphological structure of obese mice was restored to a lesser extent by L. casei IMV B-7280 and B. animalіs VKL (separately). Other probiotic bacteria and probiotic composition were ineffective. This proved that the restoration of the morphological structure of the liver of obese mice is associated with an improvement in lipid metabolism.
Probiotics are known to improve the liver function via the socalled ‘gut-liver axis’ [32–34, 56, 81], and may delay the progression of NAFLD, likely via both decreasing endotoxemia by downregulating serum lipopolysaccharides (LPS) and liver Toll-like receptor (TLR)-4, and improving gut flora alteration [81].
The gut microbiota is associated with obesity development and is changed by the hypocholesterolemic effect of probiotics. L. casei IMV B-7280, L. delbrueckii subsp. bulgaricus IMV B-7281, B. animalis VKB or B. animalis VKL (separately) and B. animalis VKB/B. animalis VKL or B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 compositions modified the gut microbiota in obese mice.
The gut microbiota in humans and mice is represented mainly by two phylotypes: Bacteroidetes and Firmicutes [82, 83]. The reduction in the number of Gram-negative Bacteroidetes bacteria and increase in the number of Gram-positive Firmicutes was revealed in the gut on different obesity models in mice and human studies with excess weight [13], although other studies have shown that the decrease in the ratio of Bacteroidetes:Firmicutes had no relationship to obesity in humans [84]. In another study on the obesity model, the reduction of Lactobacillus spp. and increase in C. perfringens was observed in the intestinal contents of mice [77]. We have found that in the intestinal contents of obese mice, the number of Lactobacillus spp., Bifidobacterium spp. and coliform bacteria decreased, and the number of Gram-positive cocci—staphylococci and streptococci—on the contrary, increased. This has proven that the intestinal microbiota can be an additional factor contributing to the development of obesity in mice.
L. casei IMV B-7280, L. delbrueckii subsp. bulgaricus IMV B-7281, B. animalis VKB and B. animalis VKL (separately) or B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 and B. animalis VKB/B. animalis VKL compositions changed the microflora of the intestinal contents of obese mice (increased the number of Lactobacillus spp., Bifidobacterium spp. and coliform bacteria) against the background of reducing the level of serum cholesterol that can provide a natural, safe alternative protection from obesity.
Treatment of obese mice with L. casei IMV B-7280 or B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 composition significantly increased the number of Lactobacillus spp. and Bifidobacterium spp., in comparison with mice of other groups. The levels of serum total, free and ester cholesterol were more effectively decreased under the treatment of L. casei IMV B-7280, and to a lesser extent under the influence of B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 composition.
Importantly, L. casei IMV B-7280 had the best hypocholesterolemic activity in vitro, as well as the best adhesiveness to epithelial cells compared with other strains that we investigated. L. delbrueckii subsp. bulgaricus IMV B-7281, B. animalis VKB and B. animalis VKL (separately) and B. animalis VKB/B. animalis VKL composition are probably less effective in cases of obesity.
Defining causality vs. correlation: is an inflammation in focus?
Identifying the causative associations of obesity and the human microbiota is still a challenge [75, 85–90]. Communication between the microbiota and immunity alters the metabolic responses during obesity and MetS. The beneficial bacteria can induce pro-inflammatory or regulatory immune responses, depending on the individual phenotype of the gut microbiome, and dietary habits [91]. Obesity coincides with low-level chronic inflammation in metabolic tissues. This obesity-related ‘metabolic inflammation’ involving adipose tissue, liver and muscle, which are key regulators of whole-body glucose homoeostasis, drives immunological underpinnings of insulin resistance and cardiovascular disease [65, 91]. Thus, the study by Fåk et al. demonstrated associations between immune modulatory and hypocholesterolemic properties of L. reuteri ATCC PTA 4659 probiotic strain which partly prevented diet-induced obesity in Apoe−/− mice, yet, induced no effects on blood cholesterol or atherosclerosis and likewise no effect on inflammatory markers (on macrophages or T-cell numbers in plaques) [92]. Lipopolysaccharide, the cell wall component of Gram-negative bacteria in the gut, are supposed as an important trigger of chronic inflammation associated with obesity (Cani et al.) [93].
However, the associations between immune modulatory vs. hypocholesterolemic activity has not yet been finally elucidated.
Obesity-induced endotoxemia [94] and liver dysfunction might be modulated by beneficial microbes via immune response, e.g., by TLR [81] to inhibit the cholesterol synthesis signaling pathway in the liver.
Thus, based on our preliminary data and series of in vitro and in vivo studies and data of trials that show efficacy in both settings [19], we speculate that the ability of the strain to decrease cholesterol may be associated with its immune-modulatory properties.
To tighten up the research power in order to predict outcomes for probiotic studies in a clinical setting and smart utilization of in vivo data is an important task.
Calls for new studies and translation: a personalized approach for microbiome-modulating interventions is needed
Many novel treatment techniques (including probiotics), despite showing their effectiveness, still lack rigorous scientific support [42]. Many of usual, every-day practice treatments found to be effective are still not supported by level I evidence. Probiotic research and the translation thereof is a cornerstone to solve this limitation, possibly successful only via changing health care and extensive public–private partnerships and regulatory bodies [95]. Successful translation of microbiome research will require: a research community; recognition of the effects of food microbiomes and its ingredients on health; appropriate regulations; and trusted products with a clear health benefit to consumers [95].
Selection of LAB as the most effective for lowering cholesterol [61, 78] requires an effective research agenda for translation and requires high validity for prediction results in a clinical setting based on studies in vivo.
Recently, we demonstrated the sequence of strains based on bacterial wall elasticity [71], which correlated with immune modulatory properties. The rigidity of the cell walls among LAB strains was distributed as follows: Lactobacillus acidophilus IMV B-7279 > Lactobacillus casei IMV B-7280 > Lactobacillus delbrueckii subsp. bulgaricus IMV B-7281; and among the Bifidobacteria strains: B. animalis VKB > B. animalis VKL. We suggested the bacterial wall elasticity evaluation as a fast and accurate method to assess parameters of probiotic strains to predict their immune-modulatory properties. According to our observations, strains with the most pronounced immune-modulatory properties also demonstrate a high efficacy in decreasing cholesterol levels; the correlation between in vitro/in vivo studies in decreasing cholesterol levels has been shown, e.g., for L. casei IMV B-7280. There are examples of successful clinical implementation [22].
The major strains demonstrating beneficial properties for health in vivo have to be clinically effective and chosen for further studies to be tested more precisely. This approach to choose an appropriate strain would be helpful considering strong biases in the clinical trials.
Evidence might be lacking when a personalized approach (or at least individualized or person-centered) should be initially supposed, but not applied.
The recent advances in predictive, preventive and personalized medicine (PPPM) and/or so-called precision medicine (see debates on term clarification in [96]) open a new era in utilization of the microbiome in human health for patient-tailored preventative or early treatment measures. The successful implementation of PPPM has been made in many domains [42, 96–98]. The recent advances are facilitating microbiome-wide association studies, which are analogous to genome-wide association studies [99]. Personalized modulation of the microbiome via nutritional and pre-, pro- and post-biotic intervention suppose dramatic increasing of their efficacy and level of evidence [42, 100, 101].
In order to achieve this ambitious goal, a diagnostic and predictive panel with a reliable model for stratification of MetS is needed to be created via host profiling using dynamic monitoring of a set of translational biomarkers [4, 10, 65, 101, 102]. A basic panel should include data of the host’s sex, age, phenotype and metabolic profile with estimation of levels of cholesterol, lipids, glucose, insulin resistance [10], uric acid, leptin, adiponectin, plasminogen activator inhibitor-1, interleukin-6, interleukin-10, tumor necrosis factor-α, oxidized LDL and paraoxonase-1; imaging data on liver, kidney structure/function, organs vascularity patterns, etc.
Microbiome biomarkers are those related to the etiological role of gut microbiota, like lipopolysaccharide binding protein (LBP), C-reactive protein (CRP), fasting insulin, and homeostasis model assessment of insulin resistance (HOMA-IR) [65], and other host-associated factors influencing the gut microbiota, like variation in vitamin D receptors [101].
Flammer syndrome markers (including NO, endothelin-1, questionnaire data) [102], physical activity patterns and a broad data set on dietary experience [91] should be considered.
Gender aspects for the use of probiotics are unclear; immune response was reported to have differences in both sexes [103], as gut microbiota differs in men and women and its impact on insulin sensitivity. Therefore, women are considered to be less sensitive to gut microbiota-associated metabolic diseases than men. A low-fat diet is efficacious in reducing the concentrations of TC, HDL-C, and LDL-C but not in reducing TG and TC/HDL-C ratio in women, yet is efficacious in premenopausal women [104].
Study of microbiome under stress, physical and psychical exercises should provide a source of potential biomarkers. The emerging evidence implicating microbiota in stress-related disorders and crosslinks between lifestyle and microbiome provide an intriguing hypothesis to stratify patients according to the response on stress, sterile environment, Flammer phenotype- and hypoxia-related patterns [105–107], and also consider the biomarkers, based on physical activity, movement patterns like gait analysis and feedback-based tests [108], etc. Our recent research in Antarctica provides novel insights in these domains [107]. This early detection and stratification of patients with MetS will support treatment and prevention via nutritional and lifestyle modulation [65].
Dose & periodicity of probiotic treatments during studies and antiobesity programs
The recent review of dose–responses of probiotics suggests that studying higher doses for this endpoint would be most worthwhile. The lack of a clear dose–response for the endpoints does not mean the effect does not exist. In particular, lower doses (less than 108 CFU/day) are lacking and may explain why a non-effective dose is not commonly identified [109]. Evidence-based recommendations for treatment indications for probiotics suggested a dose of 109 or higher [37]. Thus, in some cases, dose can be increased. In a volunteer study by Larsen et al. [110], the recovery of a group receiving 1011 CFU/day of probiotic strain was demonstrated. High doses of probiotics in humans are well-tolerated [110].
Recommendations on a probiotic treatment duration, breaks between sessions and dietary regime during and after treatment [90] have not been finalized. Heinsen et al. [111] showed that beneficial changes of both gut microbiome diversity and metabolism in obese humans under weight loss intervention were not sustained during weight maintenance.
The correct selection of an optimal time frame for intervention during an antiobesity program is a critical point effecting clinical success. In our study, the cholesterol levels remained increased long after receiving FED, even on the standard diet (see Table 3). On the other hand, the level of serum cholesterol in the current study remained reduced in mice after receiving probiotic bacteria or probiotic compositions.
The recent findings suggested that the microbiome should be targeted during antiobesity programs [111]; close interplay between nutritional modulation of gut microbiota and healthy aging was demonstrated [112]. Thus, calorie restriction can effectively lengthen the lifespan in various animal models, and has the health-promoting potential of balancing gut microbiota. This is possible due to the competition between the host and gut bacteria for nutrients, which may determine the composition of the feeding medium for homoeostatic control of microbiota in the lower gut. This ‘oligotrophic condition’ is recommended to preserve one’s lifespan [112].
Probiotic study design
Important to consider are appropriate designs for conducting, publishing, and communicating results of clinical studies involving probiotic applications in human participants [113, 114]. The experts of the International Scientific Association for Probiotics and Prebiotics (ISAPP) suggest [111] following four recommendations to conduct clinical studies of probiotic and/or prebiotic use: to define the end goal to reach a highest clinical effect and impact; design the study to maximize the chance of a positive response; choose which strain(s) and/or product(s) should be used and why; and carefully select the study cohort.
Nevertheless, we have to admit, that proper design of probiotic clinical trials is rather unfeasible in a large cohorts, especially when done unpersonalized.
Use of preclinical imaging can strongly extend results of an experiment. We suggested a simple and effective preclinical US imaging technique using equipment of general use, applicable on models of mice, rats and larger animals. The evaluated parameters as a cross-section area of the body, visceral fat and liver size are more reliable and much more informative that the mass of an animal, since it allows avoiding bias, induced, e.g., by congestion, gut hyperactivity induced by probiotics and an animals’appetite, etc. Registration records of longitudinal ultrasound scan of visceral organocomplexes of experimental mice is a method of screenings for study designs with a large number of animals. Mesenteric fat is the largest deposit in the abdominal cavity [115]. We can consider the threshold of mesenteric fat (greater omentum) thickness in mice as high as 1.5 mm for obesity. Since the muscle thickness was observed to be rather stable, the muscle/fat ratio increased during the obesity model. Visceral fat is largely represented by brown adipose tissue (BAT) and can be activated by the sympathetic nervous system or hormones to produce heat [72]. Contrast-enhanced ultrasound (CEUS) can be effective for visceral fat imaging identification, since BAT is a highly vascularized tissue [72]. Additional measuring of subcutaneous fat deposits (dorso-lumbal, gluteal and subcutaneous) and assessment of fat and organic blood flow should be also relevant preclinical imaging markers.
The information regarding colonic microbiota and the colonic mucosa; muscles and nerves in colon can be obtained using non-invasive hybrid techniques, including computed tomography (CT), magnetic resonance imaging (MRI), and US [116].
Conclusion
L. casei IMV B-7280 (separately) and a composition of B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 are effective at decreasing the weight of obese mice, decreasing cholesterol level, restoring the liver morphology and beneficially modulating the gut microbiome in high-calorie-induced obesity in a mouse model.
These strains are most promising for creation of probiotic preparations for application in humans.
Ultrasound was feasible and informative in a large amount of small animals and extended the possibilities of the experiment.
Outlooks and recommendations
We believe that a comprehensive approach for evaluating the efficacy of probiotic strains on an obesity model allows one to select the strains for creating effective probiotic preparations for prevention and treatment of metabolic diseases, which could be recommended for further preclinical and clinical studies.
However, further research of the impact of L. casei IMV B-7280, L. delbrueckii subsp. bulgaricus IMV B-7281, B. animalis VKB and B. animalis VKL (separately) or B. animalis VKL/B. animalis VKB/L. casei IMV B-7280 and B. animalis VKB/B. animalis VKL compositions is needed.
Microbiome-wide association studies would be the best option to follow up current research with multiparameter stratification patients with MetS, including data regarding lipids, carbohydrate metabolism, antioxidant system, inflammatory response, etc. on the largest cohorts possible.
The research focus should on potentially increasing the efficacy and the level of evidence via utilization of potentiated effects of probiotic compositions (mixtures) [117] and additional use of prebiotics. Thus, a new vision on prebiotics has been formulated by ISAPP experts in a new consensus [118], supposing that a prebiotic has no need to be involved in the broad metabolism of a beneficial microorganism but rather bias it towards the benefit of the host’s health. It has been proposed that the definition of a prebiotic is ‘a substrate that is selectively utilized by host microorganisms conferring a health benefit’. This opens an opportunity to test substances that were not previously considered as prebiotics and can be suggested for use with probiotic strains with synergized activity. Thus, potential candidates can be initially suggested as follows: fenugreek [94], nanomaterials, based on gold, nanoceria [119], selenium [29, 120], etc.
The use of probiotics is regulated by the guidelines of a number of organizations including WHO and Food and Agriculture Organization (FAO) [35], World Gastroenterology Organization (WGO) [36], ISAPP [37, 113, 118], European Food Safety Authority (EFSA) [121], United European Gastroenterology Organization (UEG), EPMA [96, 97, 122] and others.
The legislative process is complex and has been recently criticized; in particular, for the EU ‘adjudicate claims for probiotics is severely flawed, as has been stated by many outstanding scientists, companies and organizations’ [43].
Considering the anticipated rapid microbiome and probiotics research progress in the scope of PPPM, the unification of multidisciplinary approaches is needed, and should involve the EPMA to join leading regulatory bodies in this field in Europe and globally, firstly considering preparation of a ‘PPPM position paper on microbiome and probiotics’.
Acknowledgements
The study was conducted with the support of the State Agency on Science, Innovations and Informatisation of Ukraine.
Author’s information
RVB, M.D., Ph.D. is a researcher of the Inteferon Department of Zabolotny Institute of Microbiology and Virology, National Academy of Sciences of Ukraine and a medical doctor in the Clinical Hospital ‘Pheophania’ of the State Affairs Department, and National Representative of the European Association for Predictive, Preventive and Personalized Medicine (EPMA) in Ukraine.
Professor LML, D.Sc., LPB Ph.D., VVM Ph.D., and OVN Ph.D. are researchers in the Inteferon Department of Zabolotny Institute of Microbiology and Virology, National Academy of Sciences of Ukraine.
OMD Ph.D. is a medical doctor at JSC SPC ‘DiaprofMed’.
Professor MYS, Ph.D., D.Sci. is a corresponding member of the National Academy of Sciences of Ukraine and the director of the Interferon Department of Zabolotny Institute of Microbiology and Virology, NAS of Ukraine.
Authors’ contributions
RVB suggested the idea and design of the study, participated in experiments, did the ultrasound survey, study analysis, prepared the discussion, formulated future outlooks, and performed the second and final article drafting.
LML, LPB and VVM performed the experiments on animals and analysis of the study.
LML and LPB prepared the first draft of the manuscript, did the literature analysis, interpreted the results, and performed the statistical analysis.
OVN did the liver morphology study.
OMD participated in analysis of the study.
MYS did the organization, manuscript revision, data interpretation, and contributed to the overall development of the studied topic.
All authors read and approved the final manuscript.
Funding
Not applicable.
Compliance with ethical standards
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Competing interests
Authors declare that they have no competing interests.
Contributor Information
Rostyslav V. Bubnov, Email: rostbubnov@gmail.com
Lidiia P. Babenko, Email: babenkolidiia@gmail.com
Liudmyla M. Lazarenko, Email: lazarenkolm@gmail.com
Mykola Ya. Spivak, Email: n.spivak@ukr.net
References
- 1.WHO: Obesity and overweight: Fact sheet N. 311. http://www.who.int/mediacentre/factsheets/fs311/en/ Accessed 19 July 2017.
- 2.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2010;375(9710):181–3. 10.1016/S0140-6736(09)61794-3. [DOI] [PubMed]
- 3.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr Opin Cardiol. 2006;21(1):1–6. doi: 10.1097/01.hco.0000200416.65370.a0. [DOI] [PubMed] [Google Scholar]
- 4.Srikanthan K, Feyh A, Visweshwar H, Shapiro JI, Sodhi K. Systematic review of metabolic syndrome biomarkers: a panel for early detection, management, and risk stratification in the west Virginian population. Int J Med Sci. 2016;13(1):25–38. 10.7150/ijms.13800. [DOI] [PMC free article] [PubMed]
- 5.Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107(5):450–455. doi: 10.1016/S0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 6.Ndumele CE, Nasir K, Conceiçao RD, Carvalho JAM, Blumenthal RS, Santos RD. Hepatic steatosis, obesity, and the metabolic syndrome are independently and additively associated with increased systemic inflammation. Arterioscler Thromb Vasc Biol. 2011;31(8):1927–32. 10.1161/ATVBAHA.111.228262. [DOI] [PMC free article] [PubMed]
- 7.Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378(9793):804–14. 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed]
- 8.Gortmaker SL, Swinburn BA, Levy D, Carter R, Mabry PL, Finegood DT, et al. Changing the future of obesity: science, policy, and action. Lancet. 2011;378(9793):838–47. 10.1016/S0140-6736(11)60815-5. [DOI] [PMC free article] [PubMed]
- 9.WHO “Cardiovascular Disease,” Fact sheet no. 317, WHO, Geneva, Switzerland, 2009, http://www.who.int/mediacentre/factsheets/fs317/en/print.html.
- 10.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Crit Pathw Cardiol. 2005;4(4):198–203. doi: 10.1097/00132577-200512000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Adachi S. Factors associated with metabolic syndrome that affect prognosis in heart failure patients. Circ J. 2016;80(3):596–7. 10.1253/circj.CJ-16-0053. [DOI] [PubMed]
- 12.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3(4):213–23. 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed]
- 14.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 15.Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol. 2010;26(1):5–11. 10.1097/MOG.0b013e328333d751. [DOI] [PubMed]
- 16.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–9. 10.1038/nature11552. [DOI] [PubMed]
- 17.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31. [DOI] [PubMed]
- 18.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014 Jan 23;505(7484):559–63. 10.1038/nature12820. [DOI] [PMC free article] [PubMed]
- 19.Bendali F, Kerdouche K, Hamma-Faradji S, Drider D. In vitro and in vivo cholesterol lowering ability of lactobacillus pentosus KF923750. Benefic Microbes. 2017;8(2):271–80. 10.3920/BM2016.0121. [DOI] [PubMed]
- 20.Kondo S, Kamei A, Xiao JZ, Iwatsuki K, Abe K. Bifidobacterium breve B-3 exerts metabolic syndrome-suppressing effects in the liver of diet-induced obese mice: a DNA microarray analysis. Benefic Microbes. 2013;3:247–51. 10.3920/BM2012.0019. [DOI] [PubMed]
- 21.Kondo S, Xiao JZ, Satoh T, Odamaki T, Takahashi S, Sugahara H, et al. Antiobesity effects of Bifidobacterium breve strain B-3 supplementation in a mouse model with high-fat diet-induced obesity. Biosci Biotechnol Biochem. 2010;74(8):1656–1661. doi: 10.1271/bbb.100267. [DOI] [PubMed] [Google Scholar]
- 22.Park DY, Ahn YT, Park SH, Huh CS, Yoo SR, Yu R, et al. Supplementation of lactobacillus curvatus HY7601 and lactobacillus plantarum KY1032 in diet-induced obese mice is associated with gut microbial changes and reduction in obesity. PLoS One. 2013;8(3):e59470. 10.1371/journal.pone.0059470. [DOI] [PMC free article] [PubMed]
- 23.Huang Y, Wang J, Quan G, Wang X, Yang L, Zhong L. Lactobacillus acidophilus ATCC 4356 prevents atherosclerosis via inhibition of intestinalcholesterol absorption in apolipoprotein E-knockout mice. Appl Environ Microbiol. 2014;80(24):7496–504. 10.1128/AEM.02926-14. [DOI] [PMC free article] [PubMed]
- 24.Wang LX, Liu K, Gao DW, Hao JK. Protective effects of two lactobacillus plantarum strains in hyperlipidemic mice. World J Gastroenterol. 2013;19(20):3150–6. 10.3748/wjg.v19.i20.3150. [DOI] [PMC free article] [PubMed]
- 25.Wang BG, Xu HB, Wei H, Zeng ZL, Xu F. Oral administration of Bifidobacterim bifidum for modulating microflora, acid and bile resistance, and physiological indices in mice. Can J Microbiol. 2015;61(2):155–63. 10.1139/cjm-2014-0694. [DOI] [PubMed]
- 26.Kim SH, Huh CS, Choi ID, Jeong JW, Ku HK, Ra JH, et al. The anti-diabetic activity of Bifidobacterium lactis HY8101 in vitro and in vivo. J Appl Microbiol. 2014;117(3):834–45. 10.1111/jam.12573. [DOI] [PubMed]
- 27.Moya-Pérez A, Romo-Vaquero M, Tomás-Barberán F, Sanz Y, García-Conesa MT. Hepatic molecular responses to Bifidobacterium pseudocatenulatum CECT 7765 in a mouse model of diet-induced obesity. Nutr Metab Cardiovasc Dis. 2014;24(1):57–64. 10.1016/j.numecd.2013.04.011. [DOI] [PubMed]
- 28.Tian F, Chi F, Wang G, Liu X, Zhang Q, Chen Y, et al. Lactobacillus rhamnosus CCFM1107 treatment ameliorates alcohol-induced liver injury in amouse model of chronic alcohol feeding. J Microbiol. 2015;53(12):856–63. 10.1007/s12275-015-5239-5. [DOI] [PubMed]
- 29.Nido SA, Shituleni SA, Mengistu BM, Liu Y, Khan AZ, Gan F, et al. Effects of selenium-enriched Probiotics on lipid metabolism, Antioxidative status, Histopathological lesions, and related gene expression in mice fed a high-fat diet. Biol Trace Elem Res. 2016;171(2):399–409. 10.1007/s12011-015-0552-8. [DOI] [PubMed]
- 30.Song M, Park S, Lee H, Min B, Jung S, Park S, et al. Effect of lactobacillus acidophilus NS1 on plasma cholesterol levels in diet-induced obese mice. J Dairy Sci. 2015;98(3):1492–501. 10.3168/jds.2014-8586. [DOI] [PubMed]
- 31.Wu CC, Weng WL, Lai WL, Tsai HP, Liu WH, Lee MH, et al. Effect of lactobacillus plantarum strain K21 on high-fat diet-fed obese mice. Evid Based Complement Alternat Med. 2015;2015:391767. 10.1155/2015/391767. [DOI] [PMC free article] [PubMed]
- 32.Tilg H, Cani PD, Mayer EA. Gut microbiome and liver diseases. Gut. 2016;65:2035–2044. doi: 10.1136/gutjnl-2016-312729. [DOI] [PubMed] [Google Scholar]
- 33.Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014 Sep 4;513(7516):59–64. 10.1038/nature13568. [DOI] [PubMed]
- 34.Thomas H. NAFLD: a gut microbiome signature for advanced fibrosis diagnosis in NAFLD. Nat Rev Gastroenterol Hepatol. 2017;14(7):388. 10.1038/nrgastro.2017.67. [DOI] [PubMed]
- 35.WHO/FAO Scientific document. http://who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf. Accessed 19 July 2017.
- 36.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The international scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014(8):506–14. 10.1038/nrgastro.2014.66. [DOI] [PubMed]
- 37.WGO Updates guidelines on probiotics and prebiotics http://www.worldgastroenterology.org/UserFiles/file/guidelines/Probiotics-and-prebiotics-English2017.pdf Accessed 28 June 2017.
- 38.Sung V, Cabana MD, D’Amico F, Deshpande G, Dupont C, Indrio F, et al. Lactobacillus reuteri DSM17938 for managing infant colic: protocol for an individual participant data meta-analysis. BMJ Open. 2014;4(12):e006475. 10.1136/bmjopen-2014-006475. [DOI] [PMC free article] [PubMed]
- 39.Sanders ME, Merenstein DJ, Ouwehand AC, Reid G, Salminen S, Cabana MD, et al. Probiotic use in at-risk populations. J Amer Pharmacy Assoc. 2016;56:680–6. [DOI] [PubMed]
- 40.Reid G, Abrahamsson T, Bailey M, Bindels LB, Bubnov R, Ganguli K, et al. How do probiotics and prebiotics function at distant sites? Benef Microbes. 2017;8(4):521–533. 10.3920/BM2016.0222. [DOI] [PubMed]
- 41.The International Scientific Association for Probiotics and Prebiotics (ISAPP) Meeting Report. http://isappscience.org/annual-meeting-reports/ Accessed 19 July 2017.
- 42.Bubnov RV, Spivak MY, Lazarenko LM, Bomba A, Boyko NV. Probiotics and immunity: provisional role for personalized diets and disease prevention. EPMA J. 2015;6:14. doi: 10.1186/s13167-015-0036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reid G. Quo vadis – EFSA? Benefic Microbes. 2011;2(3):177–81. 10.3920/BM2011.0026. [DOI] [PubMed]
- 44.Mital BK, Garg SK. Anticarcinogenic, hypocholesterolemic, and antagonistic activities of lactobacillus acidophilus. Crit Rev Microbiol. 1995;21(3):175–214. doi: 10.3109/10408419509113540. [DOI] [PubMed] [Google Scholar]
- 45.Moruisi KG, Oosthuizen W, Opperman AM. Phytosterols/stanols lower cholesterol concentrations in familial hypercholesterolemic subjects: a systematic review with meta-analysis. J Am Coll Nutr. 2006;25(1):41–48. doi: 10.1080/07315724.2006.10719513. [DOI] [PubMed] [Google Scholar]
- 46.Agerbaek M, Gerdes LU, Richelsen B. Hypocholesterolaemic effect of a new fermented milk product in healthy middle-aged men. Eur J Clin Nutr. 1995;49(5):346–52. [PubMed]
- 47.Hunter PM, Hegele RA. Functional foods and dietary supplements for the management of dyslipidaemia. Nat Rev Endocrinol. 2017;13(5):278–88. 10.1038/nrendo.2016.210. [DOI] [PubMed]
- 48.Kumar M, Nagpal R, Kumar R, et al. Cholesterol-lowering Probiotics as potential biotherapeutics for metabolic diseases. Exp Diabetes Res. 2012;2012:902917. 10.1155/2012/902917. [DOI] [PMC free article] [PubMed]
- 49.Simons LA, Amansec SG, Conway P. Effect of lactobacillus fermentum on serum lipids in subjects with elevated serum cholesterol. Nutr Metab Cardiovasc Dis. 2006;16:531–535. doi: 10.1016/j.numecd.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Park YH, Kim JG, Shin YW, Kim HS, Kim YJ, Chun T, et al. Effects of lactobacillus acidophilus 43121 and a mixture of lactobacillus casei and Bifidobacterium longum on the serum cholesterol level and fecal sterol excretion in hypercholesterolemia-induced pigs. Biosci Biotechnol Biochem. 2008;72:595–600. doi: 10.1271/bbb.70581. [DOI] [PubMed] [Google Scholar]
- 51.Lye HS, Rahmat-Ali GR, Liong MT. Mechanisms of cholesterol removal by lactobacilli under conditions that mimic the human gastrointestinal tract. Int Dairy J. 2010;20:169–175. doi: 10.1016/j.idairyj.2009.10.003. [DOI] [Google Scholar]
- 52.Babenko LP, Sokolviak OY, Мokrozub VV, Nechypurenko OO, Demchenko OM, Bubnov RV, et al. Lactobacillus and Bifidobacterium probiotic strains reduce cholesterol levels and affect the gut microbiota in obese mice. United European Gastroenterol J. 2016;4(5S):A374–5. 10.1177/2050640616663689.
- 53.Shimizu M, Hashiguchi M, Shiga T, Tamura HO, Mochizuki M. Meta-analysis: effects of Probiotic supplementation on lipid profiles in normal to mildly Hypercholesterolemic individuals. PLoS One. 2015;10(10):e0139795. doi: 10.1371/journal.pone.0139795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agerholm-Larsen L, Bell ML, Grunwald GK, Astrup A. The effect of a probiotic milk product on plasma cholesterol: a meta-analysis of short-term intervention studies. Eur J Clin Nutr. 2000;54(11):856–860. doi: 10.1038/sj.ejcn.1601104. [DOI] [PubMed] [Google Scholar]
- 55.Aronsson L, Huang Y, Parini P, Korach-André M, Håkansson J, Gustafsson JÅ, et al. Decreased fat storage by lactobacillus paracasei is associated with increased levels of angiopoietin-like 4 protein (ANGPTL4). PLoS One. 2010;5(9):e13087. 10.1371/journal.pone.0013087. [DOI] [PMC free article] [PubMed]
- 56.Ma X, Hua J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol. 2008;49(5):821–30. 10.1016/j.jhep.2008.05.025. [DOI] [PMC free article] [PubMed]
- 57.Guo Z, Liu XM, Zhang QX, Shen Z, Tian FW, Zhang H, et al. Influence of consumption of probiotics on the plasma lipid profile: a meta-analysis of randomised controlled trials. Nutr Metab Cardiovasc Dis. 2011;21(11):844–50. 10.1016/j.numecd.2011.04.008. [DOI] [PubMed]
- 58.Cho YA, Kim J. Effect of Probiotics on blood lipid concentrations: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2015;94(43):e1714. 10.1097/MD.0000000000001714. [DOI] [PMC free article] [PubMed]
- 59.Ooi L-G, Liong M-T. Cholesterol-lowering effects of Probiotics and Prebiotics: a review of in vivo and in vitro findings. Int J Mol Sci. 2010;11(6):2499–522. 10.3390/ijms11062499. [DOI] [PMC free article] [PubMed]
- 60.Greany KA, Bonorden MJ, Hamilton-Reeves JM, McMullen MH, Wangen KE, Phipps WR, et al. Probiotic capsules do not lower plasma lipids in young women and men. Eur J Clin Nutr. 2008;62:232–237. doi: 10.1038/sj.ejcn.1602719. [DOI] [PubMed] [Google Scholar]
- 61.Wang J, Zhang H, Chen X, Chen Y. Menghebilige, Bao Q. Selection of potential probiotic lactobacilli for cholesterol-lowering properties and their effect on cholesterol metabolism in rats fed a high-lipid diet. J Dairy Sci. 2012;95(4):1645–1654. doi: 10.3168/jds.2011-4768. [DOI] [PubMed] [Google Scholar]
- 62.Sharma S, Puri S, Kurpad AV. Potential of Probiotics in Hypercholesterolemia: A Review of In Vitro and In Vivo Findings. Altern Ther Health Med. 2017;23:7. [PubMed]
- 63.Lewis SJ, Burmeister S. A double-blind placebo-controlled study of the effects of lactobacillus acidophilus on plasma lipids. Eur J Clin Nutr. 2005;59(6):776–780. doi: 10.1038/sj.ejcn.1602139. [DOI] [PubMed] [Google Scholar]
- 64.O’Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol. 2017;2:17057. 10.1038/nmicrobiol.2017.57. [DOI] [PubMed]
- 65.Xiao S, Zhao L. Gut microbiota-based translational biomarkers to prevent metabolic syndrome via nutritional modulation. FEMS Microbiol Ecol. 2014;87(2):303–14. 10.1111/1574-6941.12250. [DOI] [PMC free article] [PubMed]
- 66.Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediat Inflamm. 2010;2010. 10.1155/2010/289645. [DOI] [PMC free article] [PubMed]
- 67.Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12(1):5–9. doi: 10.1038/ni0111-5. [DOI] [PubMed] [Google Scholar]
- 68.Lazarenko LM, Babenko LP, Bubnov RV, Demchenko OM, Zotsenko VM, Boyko NV, et al. Imunobiotics are the novel biotech drugs with antibacterial and Immunomodulatory properties. Mikrobiol Z. 2017;79(1):66–75.
- 69.Liu R, Hong J, Xu X, Feng Q, Zhang D, Gu Y, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med. 2017;23(7):859–68. 10.1038/nm.4358. [DOI] [PubMed]
- 70.Savcheniuk OA, Virchenko OV, Falalyeyeva TM, Beregova Tetyana V, Babenko LP, Lazarenko LM, et al. The efficacy of probiotics for monosodium glutamate-induced obesity: dietology concerns and opportunities for prevention. EPMA J. 2014;5:2. [DOI] [PMC free article] [PubMed]
- 71.Mokrozub VV, Lazarenko LM, Sichel LM, Bubnov RV, Spivak MY. The role of beneficial bacteria wall elasticity in regulating innate immune response. EPMA J. 2015;6:13. doi: 10.1186/s13167-015-0035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baron DM, Clerte M, Brouckaert P, Raher MJ, Flynn AW, Zhang H, et al. In vivo noninvasive characterization of brown adipose tissue blood flow by contrast ultrasound in mice. Circ Cardiovasc Imaging. 2012 Sep 1;5(5):652–9. [DOI] [PMC free article] [PubMed]
- 73.Arora T, Anastasovska J, Gibson G, Tuohy K, Sharma RK, Bell J, et al. Effect of Lactobacillus acidophilus NCDC 13 supplementation on the progression of obesity in diet-induced obese mice. Br J Nutr. 2012;108(8):1382–9. 10.1017/S0007114511006957. [DOI] [PubMed]
- 74.Arora T, Singh S, Sharma RK. Probiotics: interaction with gut microbiome and antiobesity potential. Nutrition. 2013;29(4):591–6. 10.1016/j.nut.2012.07.017. [DOI] [PubMed]
- 75.Lumeng CN. Innate immune activation in obesity. Mol Asp Med. 2013;34(1):12–29. 10.1016/j.mam.2012.10.002. [DOI] [PMC free article] [PubMed]
- 76.Million M, Angelakis E, Paul M, Armougom F, Leibovici L, Raoult D. Comparative meta-analysis of the effect of lactobacillus species on weight gain in humans and animals. Microb Pathog. 2012;53(2):100–8. 10.1016/j.micpath.2012.05.007. [DOI] [PubMed]
- 77.Michael DR, Davies TS, Moss JWE, Calvente DL, Ramji DP, Marchesi JR, et al. The anti-cholesterolaemic effect of a consortium of probiotics: an acute study in C57BL/6J mice. Sci Rep. 2017 Jun 6;7(1):2883. 10.1038/s41598-017-02889-5. [DOI] [PMC free article] [PubMed]
- 78.Bosch M, Fuentes MC, Audivert S, Bonachera MA, Peiró S, Cuñé J. Lactobacillus plantarum CECT 7527, 7528 and 7529: probiotic candidates to reduce cholesterol levels. J Sci Food Agric. 2014;94(4):803–9. 10.1002/jsfa.6467. [DOI] [PubMed]
- 79.Kim G, Lee BH. Biochemical and molecular insights into bile salt hydrolase in the gastrointestinal microflora-a review. Asian Australas J Anim Sci. 2005;18:1505. doi: 10.5713/ajas.2005.1505. [DOI] [Google Scholar]
- 80.Jones ML, Martoni CJ, Parent M, Prakash S. Cholesterol-lowering efficacy of a microencapsulated bile salt hydrolase-active lactobacillus reuteri NCIMB 30242 yoghurt formulation in hypercholesterolaemic adults. Br J Nutr. 2012;107(10):1505–13. 10.1017/S0007114511004703. [DOI] [PubMed]
- 81.Xue L, He J, Gao N, Lu X, Li M, Wu X, et al. Probiotics may delay the progression of NAFLD by restoring the gut microbiota structure and improving intestinal endotoxemia. Sci Rep. 2017;7:45176. 10.1038/srep45176. [DOI] [PMC free article] [PubMed]
- 82.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Relman diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Frank DN, Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104(34):13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes. 2008;32(11):1720–4. 10.1038/ijo.2008.155. [DOI] [PubMed]
- 85.Zhao L. The gut microbiota and obesity: from correlation to causality. Nat Rev Microbiol. 2013;11(9):639–47. 10.1038/nrmicro3089. [DOI] [PubMed]
- 86.Wang J, Tang H, Zhang C, Zhao Y, Derrien M, Rocher E, et al. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015;9(1):1–15. 10.1038/ismej.2014.99. [DOI] [PMC free article] [PubMed]
- 87.Stenman LK, Burcelin R, Lahtinen S. Establishing a causal link between gut microbes, body weight gain and glucose metabolism in humans - towards treatment with probiotics. Benefic Microbes. 2016;7(1):11–22. 10.3920/BM2015.0069. [DOI] [PubMed]
- 88.Geurts L, Neyrinck AM, Delzenne NM, Knauf C, Cani PD. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: novel insights into molecular targets and interventions using prebiotics. Benefic Microbes. 2014;5(1):3–17. 10.3920/BM2012.0065. [DOI] [PubMed]
- 89.Cani PD, Knauf C. How gut microbes talk to organs: the role of endocrine and nervous routes. Mol Metab. 2016;5(9):743–52. 10.1016/j.molmet.2016.05.011. [DOI] [PMC free article] [PubMed]
- 90.Chen J, He X, Huang J. Diet effects in gut microbiome and obesity. J Food Sci. 2014;79(4):R442–51. 10.1111/1750-3841.12397. [DOI] [PubMed]
- 91.McPhee JB, Schertzer J. Immunometabolism of obesity and diabetes: microbiota link compartmentalized immunity in the gut to metabolic tissue inflammation. Clin Sci. 2015;129:1083–96. 10.1042/CS20150431. [DOI] [PubMed]
- 92.Fåk F, Bäckhed F. Lactobacillus reuteri prevents diet-induced obesity, but not atherosclerosis, in a strain dependent fashion in Apoe−/− mice. PLoS One. 2012;7(10):e46837. 10.1371/journal.pone.0046837. [DOI] [PMC free article] [PubMed]
- 93.Cani PD, Delzenne NM. Interplay between obesity and associated metabolic disorders: new insights into the gut microbiota. Curr Opin Pharmacol. 2009;9(6):737–743. doi: 10.1016/j.coph.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 94.Konopelniuk VV, Goloborodko II, Ishchuk TV, Synelnyk TB, Ostapchenko LI, Spivak MY, et al. Efficacy of fenugreek-based bionanocomposite on renal dysfunction and endogenous intoxication in high-calorie diet-induced obesity rat model—comparative study. EPMA J. 2017. 10.1007/s13167-017-0098-2. [DOI] [PMC free article] [PubMed]
- 95.Petschow B, Doré J, Hibberd P, Dinan T, Reid G, Blaser M, et al. Probiotics, prebiotics, and the host microbiome: the science of translation. Ann N Y Acad Sci. 2013;1306:1–17. [DOI] [PMC free article] [PubMed]
- 96.Golubnitschaja O, Baban B, Boniolo G, Wang W, Bubnov R, Kapalla M, et al. Medicine in the early twenty-first century: paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016;7:23. 10.1186/s13167-016-0072-4. [DOI] [PMC free article] [PubMed]
- 97.Golubnitschaja O, Costigliola V, EPMA General report & recommendations in predictive, preventive and personalised medicine 2012: white paper of the European Association for Predictive, preventive and personalised medicine. EPMA J. 2012;3(1):14. doi: 10.1186/1878-5085-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Akhmetov I, Bubnov RV. Innovative payer engagement strategies: will the convergence lead to better value creation in personalized medicine? EPMA J. 2017;8:1. 10.1007/s13167-017-0078-6. [DOI] [PMC free article] [PubMed]
- 99.Gilbert JA, Quinn RA, Debelius J, Xu ZZ, Morton J, Garg N, et al. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature. 2016;535(7610):94–103. 10.1038/nature18850. [DOI] [PubMed]
- 100.Zmora N, Zeevi D, Korem T, Segal E, Elinav E. Taking it personally: personalized utilization of the human microbiome in health and disease. Cell Host Microbe. 2016;19(1):12–20. 10.1016/j.chom.2015.12.016. [DOI] [PubMed]
- 101.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet. 2016;48(11):1396–406. 10.1038/ng.3695. [DOI] [PMC free article] [PubMed]
- 102.Bubnov R, Polivka J Jr, Zubor P, Koniczka K, Golubnitschaja O. Pre-metastatic niches in breast cancer: are they created by or prior to the tumour onset? “Flammer syndrome” relevance to address the question. EPMA J. 2017;8:141–57. 10.1007/s13167-017-0092-8. [DOI] [PMC free article] [PubMed]
- 103.Most J, Goossens GH, Reijnders D, Canfora EE, Penders J, Blaak EE. Gut microbiota composition strongly correlates to peripheral insulin sensitivity in obese men but not in women. Benefic Microbes. 2017;16:1–6. 10.3920/BM2016.0189. [DOI] [PubMed]
- 104.Wu L, Ma D, Walton-Moss B, He Z. Effects of low-fat diet on serum lipids in premenopausal and postmenopausal women: a meta-analysis of randomized controlled trials. Menopause. 2014;21(1):89–99. doi: 10.1097/GME.0b013e318291f5c2. [DOI] [PubMed] [Google Scholar]
- 105.Moloney RD, Desbonnet L, Clarke G, Dinan TG, Cryan JF. The microbiome: stress, health and disease. Mamm Genome. 2014;25(1–2):49–74. 10.1007/s00335-013-9488-5. [DOI] [PubMed]
- 106.Cerdá B, Pérez M, Pérez-Santiago JD, Tornero-Aguilera JF, González-Soltero R, Larrosa M. Gut microbiota modification: another piece in the puzzle of the benefits of physical exercise in health? Front Physiol. 2016;7:51. 10.3389/fphys.2016.00051. [DOI] [PMC free article] [PubMed]
- 107.Moiseyenko Y, Sukhorukov V, Pyshnov G, Mankovska I, Rozova K, Miroshnychenko O, et al. Antarctica challenges the new horizons in predictive, preventive, personalized medicine: preliminary results and attractive hypothesis for multi-disciplinary prospective studies in the Ukrainian ‘Akademik Vernadsky’ station. EPMA J. 2016;7(1):11. 10.1186/s13167-016-0060-8. [DOI] [PMC free article] [PubMed]
- 108.NY Dynamic Neuromuscular Rehabilitation & Physical Therapy Clinic. https://nydnrehab.com/ Accessed 19 July 2017.
- 109.Ouwehand AC. A review of dose-responses of probiotics in human studies. Benefic Microbes. 2017;8(2):143–51. 10.3920/BM2016.0140. [DOI] [PubMed]
- 110.Larsen CN, Nielsen S, Kaestel P, Brockmann E, Bennedsen M, Christensen HR, et al. Dose-response study of probiotic bacteria Bifidobacterium animalis subsp lactis BB-12 and lactobacillus paracasei subsp paracasei CRL-341 in healthy young adults. Eur J Clin Nutr. 2006 Nov;60(11):1284–93. [DOI] [PubMed]
- 111.Heinsen FA, Fangmann D, Müller N, Schulte DM, Rühlemann MC, Türk K, et al. Beneficial effects of a dietary weight loss intervention on human gut microbiome diversity and metabolism are not sustained during weight maintenance. Obes Facts. 2016;9(6):379–91. 10.1159/000449506. [DOI] [PMC free article] [PubMed]
- 112.Zhang C, Li S, Yang L, Huang P, Li W, Wang S, et al. Structural modulation of gut microbiota in life-long calorie-restricted mice. Nat Commun. 2013;4:2163. 10.1038/ncomms3163. [DOI] [PMC free article] [PubMed]
- 113.Shane AL, Cabana M, Vidry S, Merenstein D, Hummelen R, Ellis CL, et al. Guide to designing, conducting, publishing, and communicating results of clinical studies involving probiotic applications in human participants. Gut Microbes. 2010;1:243–53. [DOI] [PMC free article] [PubMed]
- 114.Reid G, Gaudier E, Guarner F, Huffnagle GB, Macklaim JM, Munoz AM, et al. Walter J; international scientific Association for Probiotics and Prebiotics. Responders and non-responders to probiotic interventions: how can we improve the odds? Gut Microbes. 2010;1(3):200–4. 10.4161/gmic.1.3.12013. [DOI] [PMC free article] [PubMed]
- 115.Cinti S. The Adipose Organ: Implications For Prevention And Treatment Of Obesity. In M.L. Frelut (Ed.), The ECOG’s eBook on Child and Adolescent Obesity: 2015. Retrieved from http://ebook.ecog-obesity.eu/chapter-biology/adipose-organ-implications-prevention-treatment-obesity/?utm_source=text&utm_medium=article-link&utm_campaign=ebook-en Accessed 28 June 2017.
- 116.Karunatilaka KS, Cameron EA, Martens EC, Koropatkin NM, Biteen JS. Superresolution imaging captures carbohydrate utilization dynamics in human gut symbionts. MBio. 2014;5(6):e02172. doi: 10.1128/mBio.02172-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim SJ, Park SH, Sin HS, Jang SH, Lee SW, Kim SY, Kwon B, Yu KY, Kim SY, Yang DK. Hypocholesterolemic effects of Probiotic mixture on diet-induced Hypercholesterolemic rats. Nutrients. 2017. 10.3390/nu9030293. [DOI] [PMC free article] [PubMed]
- 118.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: the international scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017. 10.1038/nrgastro.2017.75. [DOI] [PubMed]
- 119.Kobyliak N, Virchenko O, Falalyeyeva T, Kondro M, Beregova T, Bodnar P, et al. Cerium dioxide nanoparticles possess anti-inflammatory properties in the conditions of the obesity-associated NAFLD in rats. Biomed Pharmacother. 2017;90:608–14. 10.1016/j.biopha.2017.03.099. [DOI] [PubMed]
- 120.Yu I-T, Ju C-C, Lin J, Wu H-L, Yen H-T. Effects of probiotics and selenium combination on the immune and blood cholesterol concentration of pigs. J Anim Feed Sci. 2004;13(4):625–34. 10.22358/jafs/67630/2004.
- 121.EFSA NDA. Panel (EFSA panel on dietetic products, nutrition and allergies), 2016. General scientific guidance for stakeholders on health claim applications. EFSA J. 2016;14(1):4367. 10.2903/j.efsa.2016.4367. [DOI] [PMC free article] [PubMed]
- 122.Grech G, Zhan X, Yoo BC, Bubnov R, Hagan S, Danesi R, et al. EPMA position paper in cancer: current overview and future perspectives. EPMA J. 2015;6(1):9. 10.1186/s13167-015-0030-6. [DOI] [PMC free article] [PubMed]