Abstract
Smoking cessation reduces the risk of cardiovascular disease (CVD), but also elevates fasting serum glucose (FSG) levels. The effect of post-cessation hyperglycemia on cardiovascular disease is unknown. The study population consisted of 127,066 men without type 2 diabetes from the Korean National Health Insurance System – Health Screening Cohort database. Change in smoking habits and FSG was determined by the difference in smoking status and FSG levels from the first (2002 and 2003) and second (2004 and 2005) health examinations. Continual smokers, quitters, ex-smokers, and never smokers were stratified according to FSG elevation. The study participants were followed-up for CVD and CVD-related death from 2006 to 2013. Compared to continual smokers, quitters had decreased risk of CVD among those without FSG elevation (hazard ratio, HR, 0.76, 95% confidence interval, CI, 0.66–0.86) and with FSG elevation (HR 0.83, 95% CI 0.72–0.96). Similarly, quitters had a tendency towards reduced risk of CVD-related death among those without FSG elevation (HR 0.74, 95% CI 0.51–1.09) and with FSG elevation (HR 0.68, 95% CI 0.46–1.03). Post-cessation hyperglycemia did not attenuate the beneficiary risk-reducing effects of quitting on CVD and CVD-related death.
Introduction
Smoking is considered one of the most important causes of preventable death worldwide1. In the Unites States alone, smoking is estimated to account for approximately 480,000 deaths, or 20 percent of deaths annually2. This is due to the fact that smoking causes a number of serious illnesses, including cardiovascular disease (CVD)3. While smoking cessation reduces the risk of CVD, quitting also has temporary side effects that may attenuate this beneficiary risk-reducing effect on CVD. Multiple studies have shown that smoking cessation temporarily increases the risk of type 2 diabetes, with quitters having a 14 to 54% increased risk of diabetes within the first two to three years4–9. This increase in type 2 diabetes risk may be due to the deterioration of glycemic control following smoking cessation, reflected by increased fasting serum glucose (FSG) or HbA1c levels after quitting depicted in previous studies9–12.
Elevation of FSG is positively associated with the risk of cardiovascular disease and CVD-related death13–15. Tanne and colleagues revealed that there was a positive relationship between FSG and cardiovascular disease15. Similarly, Wei and colleagues have shown that the risk of CVD-related mortality increased in rising FSG levels in a dose-responsive manner14. Finally, Barr and colleagues revealed that for every incremental 12.6 mg/dL increase in FSG levels among those above FSG of 90 mg/dL, there was a 30% increase in the risk of CVD-related mortality (hazard ratio, HR, 1.3, 95% confidence interval, CI, 1.1–1.4)13.
While smoking cessation reduces the risk of CVD and CVD-related death, whether or not post-cessation hyperglycemia attenuates the risk-reducing effect of quitting on CVD has not yet been explored. Therefore, we aimed to determine the effect smoking cessation with and without FSG elevation on the risk of CVD and CVD-related death using the Korean National Health Insurance System – National Health Screening Cohort (NHIS-HealS) database.
Results
Table 1 shows the descriptive characteristics of the study population. The mean values of initial FSG for continual smokers, quitters, ex-smokers, and never smokers are 91.4 mg/dL, 91.2 mg/dL, 92.3 mg/dL, and 91.6 mg/dL, respectively. The mean values of change in FSG between the second and first health examinations were 3.4 mg/dL, 3.9 mg/dL, 2.6 mg/dL, and 2.6 mg/dL for continual smokers, quitters, ex-smokers, and never smokers, respectively. The change in FSG for quitters was significantly greater than that for continual smokers, which was determined by the analysis of variance (ANOVA) method (p-value < 0.01).
Table 1.
Descriptive characteristics of the study population.
| Continual smokers | Quitters | Ex-smokers | Never smokers | |
|---|---|---|---|---|
| Number of people | 43,627 | 13,513 | 24,945 | 44,981 |
| Age, years, mean (SD) | 51.7 (7.8) | 52.5 (8.2) | 53.6 (8.8) | 54.6 (9.0) |
| Fasting serum glucose, mg/dL, mean (SD) | ||||
| First examination | 91.4 (13.1) | 91.2 (12.9) | 92.3 (12.7) | 91.6 (12.8) |
| Second examination | 94.8 (18.7) | 95.1 (18.3) | 95.0 (16.8) | 94.3 (16.8) |
| Change | 3.4 (19.9) | 3.9 (19.3) | 2.6 (17.6) | 2.6 (17.9) |
| Socioeconomic status, N (%) | ||||
| First quartile (highest) | 16,065 (36.8) | 5,730 (42.4) | 12,303 (49.3) | 19,718 (43.8) |
| Second quartile | 13,932 (31.9) | 4,000 (29.6) | 6,914 (27.7) | 13,035 (29.0) |
| Third quartile | 8,280 (19.0) | 2,314 (17.1) | 3,633 (14.6) | 7,636 (17.0) |
| Fourth quartile (lowest) | 5,350 (12.3) | 1,469 (10.9) | 2,095 (8.4) | 4,592 (10.2) |
| Physical activity, times per week, N (%) | ||||
| None | 19,930 (45.7) | 6,988 (51.7) | 9,025 (36.2) | 19,295 (42.9) |
| 1–2 | 15,407 (35.3) | 3,785 (28.0) | 8,659 (34.7) | 14,224 (31.6) |
| 3–4 | 4,984 (11.4) | 1,689 (12.5) | 4,265 (17.1) | 6,305 (14.0) |
| 5–7 | 3,306 (7.6) | 1,051 (7.8) | 2,996 (12.0) | 5,157 (11.5) |
| Alcohol consumption, drinks per week, N (%) | ||||
| None | 9,513 (21.8) | 6,630 (49.1) | 7,865 (31.5) | 22,207 (49.4) |
| 1–2 | 23,260 (53.3) | 5,251 (38.9) | 12,829 (51.4) | 17,932 (39.9) |
| ≥3 | 10,854 (24.9) | 1,632 (12.1) | 4,251 (17.0) | 4,842 (10.8) |
| Body mass index, kg/m2, mean (SD) | 23.6 (2.8) | 24.0 (2.8) | 24.2 (2.7) | 24.0 (2.7) |
| Systolic blood pressure, mmHg, mean (SD) | 126.6 (16.3) | 127.1 (16.4) | 127.6 (15.9) | 127.8 (16.4) |
| Diastolic blood pressure, mmHg, mean (SD) | 80.2 (10.9) | 80.4 (10.9) | 80.5 (10.7) | 80.6 (10.8) |
| Total cholesterol, mg/dL, mean (SD) | 196.6 (36.3) | 198.5 (36.6) | 197.5 (35.3) | 194.5 (34.9) |
| Charlson comorbidity index, % | ||||
| 0 | 20,217 (46.3) | 5,779 (42.8) | 9,756 (39.1) | 17,754 (39.5) |
| 1 | 13,349 (30.6) | 4,109 (30.4) | 8,053 (32.3) | 14,094 (31.3) |
| ≥2 | 10,061 (23.1) | 3,625 (26.8) | 7,136 (28.6) | 13,133 (29.2) |
Acronyms: SD, standard deviation; N, number of people.
Figure 1 depicts the effect of smoking habit change on change in FSG levels. The adjusted mean values (95% CI) for FSG change for continual smokers, quitters, ex-smokers, and never smokers were 3.35 (2.17–3.53) mg/dL, 3.85 (3.54–4.17) mg/dL, 2.64 (2.41–2.87) mg/dL, and 2.70 (2.52–2.87) mg/dL, respectively. Compared to continual smokers, the change in FSG levels was significantly greater among quitters (p value 0.008). Compared to never smokers, ex-smokers and never smokers had lower changes in FSG levels (p values < 0.001).
Figure 1.
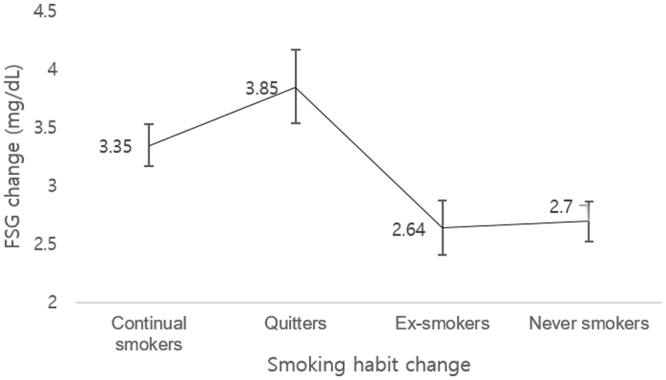
Effect of smoking habit change on change in fasting serum glucose. The adjusted mean values of fasting serum glucose was determined for continual smokers, quitters, ex-smokers, and never smokers. Adjusted means of change in fasting serum glucose calculated by linear regression analysis with adjustments for age, socioeconomic status, physical activity, alcohol consumption, body mass index, blood pressure, total cholesterol, and Charlson comorbidity index Acronyms: FSG, fasting serum glucose.
The association between smoking habit change with and without FSG elevation on CVD is shown in Table 2. Compared to continual smokers, quitters had reduced risk of CVD among those without FSG elevation (HR 0.76, 95% CI 0.66–0.86) and with FSG elevation (HR 0.83, 95% CI 0.72–0.96). Both quitters without FSG elevation and without FSG elevation had reduced risk myocardial infarction (HR 0.43, 95% CI 0.31–0.60 and HR 0.60, 95% CI 0.43–0.83, respectively). Quitters tended to have decreased risk of total stroke among those without FSG elevation (HR 0.87, 95% CI 0.74–1.01) and with FSG elevation (HR 0.91, 95% CI 0.77–1.06).
Table 2.
Effect of smoking habit change with and without fasting serum glucose elevation on cardiovascular disease.
| Continual smokers | Quitters | Ex-smokers | Never smokers | ||
|---|---|---|---|---|---|
| Cardiovascular disease | Without FSG elevation* | ||||
| Events | 1,057 | 278 | 483 | 927 | |
| Person-years | 190,574 | 57,650 | 112,965 | 203,865 | |
| aHR (95% CI) | 1.00 (reference) | 0.76 (0.66–0.86) | 0.62 (0.56–0.69) | 0.59 (0.54–0.65) | |
| With FSG elevation | |||||
| Events | 817 | 253 | 369 | 678 | |
| Person-years | 143,586 | 46,187 | 79,728 | 144,051 | |
| aHR (95% CI) | 1.00 (reference) | 0.83 (0.72–0.96) | 0.62 (0.55–0.71) | 0.58 (0.52–0.64) | |
| Myocardial infarction | Without FSG elevation | ||||
| Events | 251 | 44 | 77 | 130 | |
| Person-years | 193,142 | 58,388 | 114,212 | 206,254 | |
| aHR (95% CI) | 1.00 (reference) | 0.43 (0.31–0.60) | 0.40 (0.31–0.52) | 0.32 (0.25–0.40) | |
| With FSG elevation | |||||
| Events | 193 | 47 | 57 | 88 | |
| Person-years | 145,645 | 46,817 | 80,692 | 145,969 | |
| aHR (95% CI) | 1.00 (reference) | 0.60 (0.43–0.83) | 0.41 (0.30–0.55) | 0.31 (0.24–0.41) | |
| Total stroke | Without FSG elevation | ||||
| Events | 814 | 239 | 415 | 806 | |
| Person-years | 191,437 | 57,795 | 113,177 | 204,259 | |
| aHR (95% CI) | 1.00 (reference) | 0.87 (0.75–1.01) | 0.71 (0.64–0.80) | 0.69 (0.62–0.76) | |
| With FSG elevation | |||||
| Events | 638 | 211 | 313 | 602 | |
| Person-years | 144,182 | 46,308 | 79,949 | 144,340 | |
| aHR (95% CI) | 1.00 (reference) | 0.91 (0.77–1.06) | 0.68 (0.59–0.78) | 0.66 (0.59–0.74) | |
| Ischemic stroke | Without FSG elevation | ||||
| Events | 487 | 144 | 245 | 456 | |
| Person-years | 192,442 | 58,103 | 113,660 | 205,235 | |
| aHR (95% CI) | 1.00 (reference) | 0.86 (0.71–1.04) | 0.67 (0.57–0.79) | 0.62 (0.54–0.71) | |
| With FSG elevation | |||||
| Events | 390 | 125 | 170 | 332 | |
| Person-years | 144,912 | 46,567 | 80,326 | 145,212 | |
| aHR (95% CI) | 1.00 (reference) | 0.85 (0.69–1.04) | 0.57 (0.48–0.69) | 0.56 (0.48–0.65) | |
| Hemorrhagic stroke | Without FSG elevation | ||||
| Events | 170 | 48 | 65 | 160 | |
| Person-years | 193,445 | 58,364 | 114,236 | 206,189 | |
| aHR (95% CI) | 1.00 (reference) | 0.91 (0.65–1.26) | 0.59 (0.44–0.80) | 0.76 (0.60–0.96) | |
| With FSG elevation | |||||
| Events | 120 | 40 | 56 | 134 | |
| Person-years | 145,916 | 46,825 | 80,764 | 145,812 | |
| aHR (95% CI) | 1.00 (reference) | 1.02 (0.71–1.47) | 0.76 (0.55–1.05) | 0.96 (0.74–1.24) | |
*FSG elevation defined by an elevation of fasting serum glucose of more than the upper limit of 95% confidence interval for FSG change among quitters (>4.17mg/dL)
Hazard ratio calculated by Cox proportional hazards regression analysis with adjustments for age, socioeconomic status, physical activity, alcohol consumption, body mass index, baseline fasting serum glucose, blood pressure, total cholesterol, and Charlson comorbidity index
Acronyms: FSG, fasting serum glucose; aHR, adjusted hazard ratio; CI, confidence interval.
The effect of smoking habit change with and without FSG elevation on CVD-related death is shown in Table 3. Compared to continual smokers, quitters tended to have reduced risk of CVD-related death among those without FSG elevation (HR 0.74, 95% CI 0.51–1.09) and with FSG elevation (HR 0.68, 95% CI 0.46–1.03). Quitters among those with FSG elevation had decreased risk of myocardial infarction-related death (HR 0.26, 95% CI 0.11–0.62), compared to continual smokers. Both quitters with and without FSG elevation did not have reduced risk of total stroke-related death (HR 0.80, 95% CI 0.47–1.35 and HR 1.11, 95% CI 0.69–1.80, respectively). Sensitivity analyses on the effect of smoking habit change with and without FSG elevation on CVD and CVD-related death after excluding participants with events occurring within the first one to four years of follow-up are shown in Table 4. The effect of post-cessation hyperglycemia on CVD and CVD-related death was not altered after excluding events that occurred within the first one to four years of follow-up. Compared to continual smokers, quitters had reduced risk of CVD among those with and without FSG elevation after excluding participants diagnosed with CVD within the first four years of follow-up.
Table 3.
Effect of smoking habit change with and without fasting serum glucose elevation on cardiovascular disease-related death.
| Continual smokers | Quitters | Ex-smokers | Never smokers | ||
|---|---|---|---|---|---|
| Cardiovascular disease-related death | Without FSG elevation* | ||||
| Events | 133 | 34 | 72 | 127 | |
| Person-years | 194,009 | 58,546 | 114,444 | 206,670 | |
| aHR (95% CI) | 1.00 (reference) | 0.74 (0.51–1.09) | 0.68 (0.50–0.91) | 0.59 (0.46–0.76) | |
| With FSG elevation | |||||
| Events | 120 | 30 | 42 | 100 | |
| Person-years | 146,281 | 46,952 | 80,915 | 146,282 | |
| aHR (95% CI) | 1.00 (reference) | 0.68 (0.46–1.03) | 0.49 (0.34–0.70) | 0.58 (0.44–0.77) | |
| Myocardial infarction-related death | Without FSG elevation | ||||
| Events | 68 | 16 | 33 | 51 | |
| Person-years | 194,009 | 58,546 | 114,444 | 206,670 | |
| aHR (95% CI) | 1.00 (reference) | 0.68 (0.39–1.18) | 0.67 (0.44–1.03) | 0.50 (0.34–0.73) | |
| With FSG elevation | |||||
| Events | 59 | 6 | 20 | 29 | |
| Person-years | 146,281 | 46,952 | 80,915 | 146,282 | |
| aHR (95% CI) | 1.00 (reference) | 0.26 (0.11–0.62) | 0.50 (0.30–0.84) | 0.36 (0.22–0.57) | |
| Total stroke-related death | Without FSG elevation | ||||
| Events | 65 | 18 | 39 | 76 | |
| Person-years | 194,009 | 58,546 | 114,444 | 206,670 | |
| aHR (95% CI) | 1.00 (reference) | 0.80 (0.47–1.35) | 0.69 (0.46–1.03) | 0.68 (0.48–0.96) | |
| With FSG elevation | |||||
| Events | 61 | 24 | 22 | 71 | |
| Person-years | 146,281 | 46,952 | 80,915 | 146,282 | |
| aHR (95% CI) | 1.00 (reference) | 1.11 (0.69–1.80) | 0.48 (0.29–0.79) | 0.79 (0.55–1.13) | |
| Ischemic stroke-related death | Without FSG elevation | ||||
| Events | 19 | 5 | 15 | 32 | |
| Person-years | 194,009 | 58,546 | 114,444 | 206,670 | |
| aHR (95% CI) | 1.00 (reference) | 0.67 (0.25–1.82) | 0.76 (0.38–1.53) | 0.82 (0.45–1.50) | |
| With FSG elevation | |||||
| Events | 14 | 10 | 5 | 524 | |
| Person-years | 146,281 | 46,952 | 80,915 | 146,282 | |
| aHR (95% CI) | 1.00 (reference) | 2.02 (0.88–2.64) | 0.48 (0.17–1.35) | 1.16 (0.58–2.33) | |
| Hemorrhagic stroke-related death | Without FSG elevation | ||||
| Events | 25 | 2 | 15 | 22 | |
| Person-years | 194,009 | 58,546 | 114,444 | 206,670 | |
| aHR (95% CI) | 1.00 (reference) | — | 0.81 (0.42–1.58) | 0.59 (0.32–1.10) | |
| With FSG elevation | |||||
| Events | 25 | 13 | 10 | 20 | |
| Person-years | 146,281 | 46,952 | 80,915 | 146,282 | |
| aHR (95% CI) | 1.00 (reference) | 1.28 (0.79–3.15) | 0.61 (0.29–1.30) | 0.63 (0.34–1.18) | |
*FSG elevation defined by an elevation of fasting serum glucose of more than the upper limit of 95% confidence interval for FSG change among quitters (>4.17 mg/dL)
Hazard ratio calculated by Cox proportional hazards regression analysis with adjustments for age, socioeconomic status, physical activity, alcohol consumption, body mass index, baseline fasting serum glucose, blood pressure, total cholesterol, and Charlson comorbidity index
Acronyms: FSG, fasting serum glucose; aHR, adjusted hazard ratio; CI, confidence interval
Blank cells indicate no reliable values of hazard ratios and 95% confidence intervals due to the small number of events.
Table 4.
Sensitivity analysis of the effect of smoking habit change with and without fasting serum glucose elevation on cardiovascular disease and cardiovascular disease-related death after excluding participants with events occurring within the first one to four years of follow-up.
| Exclusion period | Continual smokers | Quitters | Ex-smokers | Never smokers | ||
|---|---|---|---|---|---|---|
| aHR (95% CI) | ||||||
| Cardiovascular disease | One year | Without FSG elevation* | 1.00 (reference) | 0.77 (0.67–0.89) | 0.64 (0.57–0.72) | 0.61 (0.55–0.67) |
| With FSG elevation | 1.00 (reference) | 0.85 (0.73–0.98) | 0.62 (0.54–0.70) | 0.56 (0.50–0.63) | ||
| Two years | Without FSG elevation | 1.00 (reference) | 0.82 (0.70–0.96) | 0.60 (0.52–0.69) | 0.56 (0.50–0.63) | |
| With FSG elevation | 1.00 (reference) | 0.78 (0.67–0.91) | 0.65 (0.57–0.73) | 0.61 (0.55–0.68) | ||
| Three years | Without FSG elevation | 1.00 (reference) | 0.82 (0.70–0.96) | 0.60 (0.52–0.69) | 0.56 (0.50–0.63) | |
| With FSG elevation | 1.00 (reference) | 0.79 (0.67–0.92) | 0.65 (0.57–0.75) | 0.64 (0.57–0.71) | ||
| Four years | Without FSG elevation | 1.00 (reference) | 0.83 (0.70–0.99) | 0.61 (0.52–0.71) | 0.55 (0.48–0.63) | |
| With FSG elevation | 1.00 (reference) | 0.83 (0.69–0.99) | 0.68 (0.58–0.78) | 0.63 (0.56–0.72) | ||
| Cardiovascular disease-related death | One year | Without FSG elevation | 1.00 (reference) | 0.81 (0.54–1.21) | 0.74 (0.54–1.00) | 0.64 (0.49–0.85) |
| With FSG elevation | 1.00 (reference) | 0.72 (0.47–1.10) | 0.49 (0.34–0.72) | 0.62 (0.46–0.83) | ||
| Two years | Without FSG elevation | 1.00 (reference) | 0.85 (0.55–1.29) | 0.77 (0.56–1.08) | 0.69 (0.52–0.92) | |
| With FSG elevation | 1.00 (reference) | 0.74 (0.47–1.16) | 0.52 (0.34–0.77) | 0.64 (0.47–0.88) | ||
| Three years | Without FSG elevation | 1.00 (reference) | 0.85 (0.54–1.35) | 0.83 (0.58–1.17) | 0.67 (0.49–0.92) | |
| With FSG elevation | 1.00 (reference) | 0.77 (0.47–1.26) | 0.54 (0.35–0.84) | 0.72 (0.51–1.01) | ||
| Four years | Without FSG elevation | 1.00 (reference) | 0.80 (0.48–1.34) | 0.79 (0.54–1.16) | 0.66 (0.47–0.93) | |
| With FSG elevation | 1.00 (reference) | 0.72 (0.42–1.23) | 0.48 (0.29–0.78) | 0.72 (0.50–1.03) |
*FSG elevation defined by an elevation of fasting serum glucose of more than the upper limit of 95% confidence interval for FSG change among quitters (>4.17 mg/dL)
Hazard ratio calculated by Cox proportional hazards regression analysis with adjustments for age, socioeconomic status, physical activity, alcohol consumption, body mass index, baseline fasting serum glucose, blood pressure, total cholesterol, and Charlson comorbidity index
Acronyms: FSG, fasting serum glucose; aHR, adjusted hazard ratio; CI, confidence interval.
The effect of smoking habit change on CVD without stratification by FSG elevation is shown in Supplemental Table S1. Compared to continual smokers, quitters (HR 0.79, 95% CI 0.72–0.87), ex-smokers (HR 0.62, 95% CI 0.57–0.68), and never smokers (HR 0.59, 95% CI 0.55–0.63) all had reduced risk CVD. Quitters, ex-smokers, and never smokers all had decreased risk of CVD-related death (HR 0.71, 95% CI 0.54–0.94, HR 0.59, 95% CI 0.47–0.74, and HR 0.58, 95% CI 0.48–0.70, respectively), compared to continual smokers.
Discussion
In this large-scale cohort study of over 120,000 men without type 2 diabetes, we have shown that post-cessation hyperglycemia does not attenuate the beneficiary risk-reducing effects of smoking cessation on CVD and CVD-related death. To our knowledge, this is the first study to investigate the effect of smoking habit change with and without FSG elevation on CVD and CVD-related death.
Similar to our results, several previous studies have shown that smoking cessation is associated with elevated FSG levels. Two separate studies investigating the changes in FSG levels after quitting revealed net gains of 8.1 mg/dL12 and 5.4 mg/dL11 of FSG after smoking cessation. Although the magnitude of FSG elevation upon smoking cessation in our study was smaller compared to those in previous studies, this may be explained by the fact that our study population was limited to non-diabetic male patients. While it has been suggested that post-cessation hyperglycemia was due to post-cessation weight gain, two recent studies have shown that this hyperglycemic state persisted even after adjusting for weight gain following smoking cessation9,10. The exact mechanisms causing post-cessation hyperglycemia is yet unclear and merit further investigation.
Multiple studies support the positive relationship between FSG levels and the risk of CVD13–16. Experimental studies have revealed that hyperglycemia disrupts the function of normal endothelial cells, contributes to the formation of atherosclerotic plaques, and accelerate the rupture of plaques, leading to thrombosis of the vessels16. Furthermore, previous epidemiological studies have shown that hyperglycemia is associated with arterial stiffness17, intima-media thickening of the endothelial wall, and endothelial dysfunction of the arterial wall18. While FSG elevation may increase the risk of CVD through these mechanisms, the results from our study suggest that the benefit of smoking cessation outweighs the potential deleterious effect of post-cessation hyperglycemia on the risk of CVD.
A large body of evidence indicates that smoking increases the risk of CVD. Recently, a meta-analysis of 25 cohorts and 503,905 participants revealed that smokers had elevated risk of CVD (HR 2.07, 95% CI 1.82–2.36) compared to never smokers3. Carbon monoxide from cigarette smoke is known to bind to hemoglobin, resulting in the inhibition of oxygen binding. The consequent chronic hypoxic state leads to increased blood viscosity, ultimately resulting in a hypercoagulable state due to smoking19. Furthermore, it has been shown that smokers have elevated levels of circulating fibrinogen20, which may promote thrombus formation synergistically with increased blood viscosity. Smoking also increases the formation of hydrogen peroxide and superoxide21, which could lead to increased thrombogenesis by inducing oxidative stress and cellular damage22. The decrease in these pathophysiological processes upon smoking cessation may have contributed to the reduction of CVD risk among quitters.
Quitting was more beneficial in reducing the risk of myocardial infarction compared total stroke, regardless of FSG elevation. This may explained by the fact that myocardial infarction and stroke, particularly hemorrhagic stroke, have distinct pathophysiological mechanisms. While myocardial infarction is the result of vessel occlusion, rupture of the vessel wall is the overriding mechanism for hemorrhagic stroke. Particularly, as one of the most important consequences of smoking is the development of a hypercoagulable state, it is reasonable to assume that smoking cessation may have a greater beneficiary effect on preventing myocardial infarction where hypercoagulability would elevate the risk of thrombogenesis. This is supported in a previous study showing that smoking cessation led to decreased risk of myocardial infarction (HR 0.43, 95% CI 0.34–0.53) but not hemorrhagic stroke (HR 0.82, 95% CI 0.64–1.06) compared to continual smokers23.
Several limitations need to be considered when interpreting the results of our study. First, as smoking status was determined by a questionnaire, it may not exactly reflect the actual smoking status of the study participant. Second, since smoking status was not followed-up after the second health examination, we do not know how the smoking status was altered afterwards. Third, as smoking habit change and FSG elevation were both measured during the same period, we cannot determine that smoking cessation preceded FSG elevation with certainty. However, multiple previous longitudinal studies have shown that quitting is associated with hyperglycemia11,12. Fourth, we used FSG levels in order to assess glycemic control as HbA1c values were not available. Further studies using HbA1c levels to determine the effect of post-cessation hyperglycemia on cardiovascular disease and CVD-related mortality are needed. However, in large-scale population studies, FSG has been used as a marker for glycemic control in multiple studies11,12,16. Fifth, the study population was limited to men aged 40 years or more. Further investigations studying the effect of post-cessation hyperglycemia on cardiovascular disease among younger aged adults and women are needed. Finally, myocardial infarction and total stroke were included in CVD, leaving out other cardiovascular events such as peripheral arterial disease that are known to be affected by smoking and hyperglycemia. While the focus of this study on major cardiovascular events, future studies investigating the association between post-cessation hyperglycemia and other cardiovascular events are needed.
Despite these limitations, the study has a number of strengths. To our knowledge, this is the first study to examine the effects of elevated FSG after smoking cessation on the risk of CVD and CVD-related death. Furthermore, the large study population, relatively long follow-up period, extensive number of covariates considered and accurate health records from the claims database add to the reliability of our results. Finally, extensive sensitivity analyses revealed that the risk reduction in CVD and CVD-related death upon smoking cessation regardless of FSG elevation was preserved after excluding events that occurred within the first four years of follow-up.
In conclusion, smoking cessation was associated with reduced risk of CVD and CVD-related death regardless of FSG elevation. Particularly, smoking cessation may be beneficial in reducing the risk of myocardial infarction regardless of FSG elevation. Smokers should be encouraged to quit in order to benefit from reduced risk of CVD and CVD-related death despite post-cessation hyperglycemia.
Methods
Participants
The study population was derived from the NHIS-HealS research database. The enrollment rate for the NHIS is 97% due to the fact that health insurance enrollment, provided by NHIS, became mandatory for all Korean citizens since the National Health Insurance Act in 1989. All enrollees who turn 40 years old are required to undergo biannual health examinations. Based on the data from these health examinations, the NHIS constructs datasets by a simple random sampling method and provides data on sociodemographics, hospital visits, and laboratory and other clinical data for research purposes. The data used in this study is directly available via the NHIS database registration system. Various fields of research have used this NHIS database for multiple epidemiological studies, and its validity has been described in detail elsewhere24,25.
A total of 176,422 men who participated in health examinations between the first (2002 to 2003) and second (2002 to 2003) periods with available smoking status and FSG values were recruited. Among them, we excluded 30,979 men who were diagnosed with diabetes during the first health examination period. Furthermore, 8,939 men who were diagnosed with cardiovascular disease and 341 men who passed away before the index date of 1 January 2006 were excluded. Then, 8,609 men with unwanted smoking status (new smokers and relapsers), 215 men with extreme outliers (lower and upper 0.05% of distribution)26 for FSG, and 1,481 men with missing values on covariates. Ultimately, the final study population consisted of 127,066 participants.
The Seoul National University Hospital Institutional Review Board (IRB) approved this study (IRB number: X-1701/378–902) and waived the requirement for informed consent from study participants as the NHIS database is anonymized in adherence to strict confidentiality guidelines. All experiments were performed in accordance with the relevant guidelines and regulations.
Key variables
Study participants were grouped according to the change in smoking habit, determined by a self-reported questionnaire, between the first and second health examinations. Participants were divided into continual smokers, quitters, ex-smokers, and never smokers. Fasting serum glucose, which was measured by a blood exam during each health examination, was used to determine FSG elevation. FSG elevation was defined as an increase of more than the upper limit of the 95% confidence interval for the adjusted mean value of FSG change among quitters (4.17 mg/dL). The participants were divided into those without FSG elevation and those with FSG elevation.
Within the NHIS database, hospital admission records were used to identify cases of CVD and CVD-related death. Cardiovascular disease was defined using the codes from the Tenth Revision of International Classification of Diseases (ICD-10) from the World Health Organization. Myocardial infarction (ICD-10 codes: I21, I22, I23, I24) and total stroke (ICD-10 codes: I60, I61, I62, I63, I64, I65, I66, I67, I68, I69) were included in CVD. Total stroke was further divided into ischemic stroke (ICD-10 codes: I63) and hemorrhagic stroke (ICD-10 codes: I60, I61, I62). We defined an event of CVD as cases with two or more days of hospital admission with at least one of the ICD-10 codes pertaining to CVD. Mortality was determined by participants with a death date between 1 January 2006 and 31 December 2013. Among those with a death date, cause-specific mortality was identified by the cause of death using the ICD-10 code.
Statistical analysis
All participants were followed-up starting 1 January 2006 and ended at the date of diagnosis of CVD, date of death, or 31 December 2013, whichever came first. Age (continuous, years), socioeconomic status (SES, categorical, first, second, third, and fourth quartiles), physical activity (categorical, none, 1–2, 3–4, and 5–7 times per week), alcohol consumption (categorical, none, 1–2, and 3 or more times), body mass index (BMI, continuous, kg/m2), systolic blood pressure (continuous, mmHg), diastolic blood pressure (continuous, mmHg), total cholesterol (continuous, mg/dL), baseline fasting serum glucose (continuous, mmHg), and Charlson comorbidity index (CCI, categorical, 0, 1, or 2 or more) were considered potential confounding covariates and extracted between 2002 and 2005. SES was categorized according to each patient’s insurance premium status. CCI was calculated using the ICD-10 code diagnoses for major comorbidities between 2002 and 2005 based on the claims database. The algorithm for the calculation of CCI by ICD-10 codes was adapted from another study27.
The adjusted mean values for FSG change for continual smokers, quitters, ex-smokers, and never smokers were calculated by linear regression analysis. We conducted Cox proportional hazards regression analyses to obtain the hazard ratios (HRs) and 95% confidence intervals (CI) of the risk of CVD and CVD-related mortality according to smoking habit change with and without FSG elevation. In all analyses, continual smokers were used as references. The multivariate-adjusted analysis was adjusted for age, BMI, blood pressure, baseline FSG, total cholesterol, physical activity, smoking status, drinking habit, SES, and CCI. To minimize the possibility of reverse-causality, in which smokers quit due to worsening health conditions that may elevate the risk of CVD, sensitivity analysis of the effect of smoking habit change with and without FSG elevation on CVD and CVD-related death were conducted by excluding participants with events occurring within the first one to four years of follow-up.
Statistical significance was defined as a p-value of less than 0.05 in a two-sided manner. All data collection and statistical analyses conducted in this study were done with SAS 9.3 (SAS Institute, Cary, NC, USA) and STATA 13.0 (StataCorp LP, College Station, TX, USA), respectively.
Electronic supplementary material
Acknowledgements
We would like to thank Hyeyoung Yoo for providing administrative support. This study used NHIS-HealS data (NHIS-2017-2-473) from the Korean NHIS.
Author Contributions
S.C., K.K., J.C., K.L., and S.M.P. contributed to the conception and design of the study. S.C., S.M.K., H.-Y.K., J.-H.J., M.H.C., K.L., and S.M.P. contributed to the analysis and interpretation of the data. S.C., K.L., and S.M.P. contributed to the drafting of the article. S.C., K.K., J.C., H.-Y.K., J.-H.J., M.H.C., K.L., and S.M.P. contributed to the critical revision for important intellectual content of the article. All authors approved the final copy of the article. S.C. conducted collection and assembly of the data. K.L. and S.M.P. are the co-corresponding authors and guarantors of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-16378-2.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kiheon Lee, Email: keyhoney@gmail.com.
Sang Min Park, Email: smpark.snuh@gmail.com.
References
- 1.Jha, P. et al. In Disease Control Priorities in Developing Countries (eds Jamison D. T., et al.) (2006). [PubMed]
- 2.Alberg AJ, Shopland DR, Cummings KM. The 2014 Surgeon General’s report: commemorating the 50th Anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking. Am J Epidemiol. 2014;179:403–412. doi: 10.1093/aje/kwt335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mons U, et al. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ. 2015;350:h1551. doi: 10.1136/bmj.h1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeh HC, Duncan BB, Schmidt MI, Wang NY, Brancati FL. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Ann Intern Med. 2010;152:10–17. doi: 10.7326/0003-4819-152-1-201001050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hur NW, et al. Smoking cessation and risk of type 2 diabetes mellitus: Korea Medical Insurance Corporation Study. Eur J Cardiovasc Prev Rehabil. 2007;14:244–249. doi: 10.1097/01.hjr.0000239474.41379.79. [DOI] [PubMed] [Google Scholar]
- 6.Will JC, Galuska DA, Ford ES, Mokdad A, Calle EE. Cigarette smoking and diabetes mellitus: evidence of a positive association from a large prospective cohort study. Int J Epidemiol. 2001;30:540–546. doi: 10.1093/ije/30.3.540. [DOI] [PubMed] [Google Scholar]
- 7.Davey Smith G, et al. Incidence of type 2 diabetes in the randomized multiple risk factor intervention trial. Ann Intern Med. 2005;142:313–322. doi: 10.7326/0003-4819-142-5-200503010-00006. [DOI] [PubMed] [Google Scholar]
- 8.Luo J, et al. Smoking cessation, weight gain, and risk of type 2 diabetes mellitus among postmenopausal women. Arch Intern Med. 2012;172:438–440. doi: 10.1001/archinternmed.2012.24. [DOI] [PubMed] [Google Scholar]
- 9.Oba S, et al. Smoking cessation increases short-term risk of type 2 diabetes irrespective of weight gain: the Japan Public Health Center-Based Prospective Study. PLoS One. 2012;7:e17061. doi: 10.1371/journal.pone.0017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lycett D, et al. The association between smoking cessation and glycaemic control in patients with type 2 diabetes: a THIN database cohort study. Lancet Diabetes Endocrinol. 2015;3:423–430. doi: 10.1016/S2213-8587(15)00082-0. [DOI] [PubMed] [Google Scholar]
- 11.Stein JH, et al. Smoking cessation and the risk of diabetes mellitus and impaired fasting glucose: three-year outcomes after a quit attempt. PLoS One. 2014;9:e98278. doi: 10.1371/journal.pone.0098278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SS, et al. The changes of blood glucose control and lipid profiles after short-term smoking cessation in healthy males. Psychiatry Investig. 2011;8:149–154. doi: 10.4306/pi.2011.8.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barr EL, et al. Continuous relationships between non-diabetic hyperglycaemia and both cardiovascular disease and all-cause mortality: the Australian Diabetes, Obesity, and Lifestyle (AusDiab) study. Diabetologia. 2009;52:415–424. doi: 10.1007/s00125-008-1246-y. [DOI] [PubMed] [Google Scholar]
- 14.Wei M, et al. Low fasting plasma glucose level as a predictor of cardiovascular disease and all-cause mortality. Circulation. 2000;101:2047–2052. doi: 10.1161/01.CIR.101.17.2047. [DOI] [PubMed] [Google Scholar]
- 15.Tanne D, Koren-Morag N, Goldbourt U. Fasting plasma glucose and risk of incident ischemic stroke or transient ischemic attacks: a prospective cohort study. Stroke. 2004;35:2351–2355. doi: 10.1161/01.STR.0000140738.94047.55. [DOI] [PubMed] [Google Scholar]
- 16.Park C, et al. Fasting glucose level and the risk of incident atherosclerotic cardiovascular diseases. Diabetes Care. 2013;36:1988–1993. doi: 10.2337/dc12-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Popele NM, et al. Impaired fasting glucose is associated with increased arterial stiffness in elderly people without diabetes mellitus: the Rotterdam Study. J Am Geriatr Soc. 2006;54:397–404. doi: 10.1111/j.1532-5415.2005.00614.x. [DOI] [PubMed] [Google Scholar]
- 18.Thomas GN, et al. Deleterious impact of “high normal” glucose levels and other metabolic syndrome components on arterial endothelial function and intima-media thickness in apparently healthy Chinese subjects: the CATHAY study. Arterioscler Thromb Vasc Biol. 2004;24:739–743. doi: 10.1161/01.ATV.0000118015.26978.07. [DOI] [PubMed] [Google Scholar]
- 19.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (1) N Engl J Med. 1992;326:242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- 20.Kannel WB, Wolf PA, Castelli WP, D’Agostino RB. Fibrinogen and risk of cardiovascular disease. The Framingham Study. JAMA. 1987;258:1183–1186. doi: 10.1001/jama.1987.03400090067035. [DOI] [PubMed] [Google Scholar]
- 21.Burke A, Fitzgerald GA. Oxidative stress and smoking-induced vascular injury. Prog Cardiovasc Dis. 2003;46:79–90. doi: 10.1016/S0033-0620(03)00076-8. [DOI] [PubMed] [Google Scholar]
- 22.Benowitz NL. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Prog Cardiovasc Dis. 2003;46:91–111. doi: 10.1016/S0033-0620(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 23.Song YM, Cho HJ. Risk of stroke and myocardial infarction after reduction or cessation of cigarette smoking: a cohort study in korean men. Stroke. 2008;39:2432–2438. doi: 10.1161/STROKEAHA.107.512632. [DOI] [PubMed] [Google Scholar]
- 24.Jee SH, et al. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355:779–787. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- 25.Park SM, et al. Prediagnosis Body Mass Index and Risk of Secondary Primary Cancer in Male Cancer Survivors: A Large Cohort Study. J Clin Oncol. 2016;34:4116–4124. doi: 10.1200/JCO.2016.66.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sung J, Song YM, Ebrahim S, Lawlor DA. Fasting blood glucose and the risk of stroke and myocardial infarction. Circulation. 2009;119:812–819. doi: 10.1161/CIRCULATIONAHA.108.776989. [DOI] [PubMed] [Google Scholar]
- 27.Sundararajan V, et al. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57:1288–1294. doi: 10.1016/j.jclinepi.2004.03.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


