Abstract
Leukemia is the most commonly diagnosed childhood cancer, although its etiology is still largely unknown. Growing evidence supports a role for infection in the etiology of acute lymphocytic leukemia (ALL), and the involvement of the immune system suggests that vaccination may also play a role. However, the findings presented in the published literature are inconsistent. Therefore, we conducted a PRISMA systematic review and meta-analysis. 14 studies were identified and meta-analyzed. Vaccinations studied comprised Bacillus Calmette-Guérin (BCG) vaccine, Triple vaccine, Hepatitis B vaccine (HBV), Polio, Measles, Rubella, Mumps, trivalent MMR vaccine and Haemophilus influenza type B (HiB) vaccine. We observed a protective association between any vaccination in the first year of life and risk of childhood leukemia (summary odds ratio (OR) 0.58 [95% confidence interval (CI) 0.36–0.91]). When individual vaccines were analysed, some evidence of an association was seen only for BCG (summary OR 0.73 [95% CI 0.50–1.08]). In conclusion, early vaccination appears to be associated with a reduced risk of childhood leukemia. This finding may be underpinned by the association observed for BCG. Given the relatively imprecise nature of the results of this meta-analysis, our findings should be interpreted cautiously and replicated in future studies.
Introduction
Leukemia is the most commonly diagnosed cancer in childhood, representing 31% of all cancer diagnoses1. Acute lymphocytic leukemia (ALL) and acute myelogenous leukemia (AML) are the most common subtypes. ALL alone accounts for 26% of cancers diagnosed from birth until four years of age. On the other hand, chronic leukemia is considered to be very rare in this age group2. Despite the great success in the treatment of ALL where the survival of children has steadily improved reaching 85% in both the United States and Europe, its etiology is still largely unknown3. A recent study by Ward et al. showed that the incidence of childhood acute leukemia in the United States had an annual increase rate of 0.7 percent for ALL and 1.1 percent for AML between 1975 and 20102. However, the cause of this rising incidence has not been identified. Improved diagnostic techniques and improved notifications may partly explain these observed increases.
Various risk factors for childhood leukemia have been identified, such as ionizing radiation during prenatal and postnatal life4,5, paternal smoking6–8, exposure to household pesticides9 and benzene10. On the other hand, early exposure to infections may be protective for childhood ALL. This was originally postulated by Greaves in 1997 when he stated that both the pattern and timing of infections in early life is critical to the developmental programming of the immune system11. These immunomodulatory changes start with the passage of the mother’s immunoglobulins through the placenta in early pregnancy and in breast milk which is considered to be the first exposure to bacteria and viruses. He argued that current lifestyles in developed countries characterized by less exposure to infections or early weaning prevent mothers from sufficiently providing immune modulation to their offspring. Moreover, the improvement of hygiene in developed countries decreased the exposure of children themselves to infections. Therefore, when the inappropriately programmed immune system in a genetically predisposed child is exposed to common microbes in an abnormal time frame, it causes an abnormal immunological response thus increasing the risk of leukemia11. A recent meta-analysis of 14 case-control studies using day-care attendance as an indicator of the increased likelihood of early exposure to infections has shown a strong protective association between day care attendance and risk of ALL12. The growing evidence in support of the role of infection in the etiology of childhood leukemia has led to an interest in a possible role of childhood vaccination.
Published studies have reported contradictory results; studies from USA13–18, Canada19,20 and Finland21,22 have reported a reduced risk of leukemia with Bacille Calmette–Guerin (BCG) vaccination. However, a study in New Zealand23 showed no effect. After a thorough review of the literature, we aimed to systematically review and meta-analyze the current literature to provide evidence regarding the association between a range of vaccines and the risk of childhood leukemia.
Material and Methods
Search Strategy
Our study was conducted according to the recommendations of the PRISMA statement24 (Supplementary Table 1). We registered the protocol at PROSPERO with the registration number CRD42015024816. In June 14th, 2015, an electronic search was conducted in nine medical databases/search engines: PubMed, Scopus, Google Scholar, ISI Web of science, WHO Global health library, POPLINE, VHL, NYAM (New York academy of medicine) and SIGLE (System for information on grey literature in Europe). The descriptor used for searching in eight databases, except Google Scholar was: “(immunization or vaccines or vaccine or vaccination or vaccinated or immunized) and (risk or risks or association or correlation or odds) and (leukemia or leukaemia or leukemic or leukaemic) and (child or children or childhood or baby or infant or infants or newborn or neonates or pediatrics or pediatric)”. In Google Scholar, we used the keywords as the exact phrases “childhood leukemia”/ “childhood leukaemia” and “immunization, vaccines, vaccine, vaccination, vaccinated, immunized” with the option with at least one of the words and selected “where my words occur in the title of the article”. For every database, all variants spelled with “s” instead of “z” were covered in the search strategy. In addition, we manually searched the reference lists of relevant articles.
Selection criteria
Studies that reported the association of early vaccination with leukemia in children under 20 years old and vaccines were included in this review with no restriction to language. Exclusion criteria were: (i) unreliably extracted data; (ii) leukemia cases reported in adults; (iii) overlapped data sets; (iv) abstract-only articles, review articles, theses, conference proceedings, books, and case reports. All studies identified were reviewed independently for eligibility by three authors and conflicts were resolved by seeking a discussion and consensus with senior researchers (NTH, EM, KH).
Data extraction
Three independent reviewers extracted the data from the included studies. Any discrepancies were resolved by discussion to reach a consensus or through consulting senior researchers. A form for data extraction was designed for the authors to use. Sample: (author, title, year of publication, journal name, name of the study, study design, country, duration of follow up, sample size, prevalence, age, gender, type of leukemia (eg. ALL, AML, lymphoblastic, myeloblastic), exposure assessment, statistical analysis and risk estimation through the reported odds ratios (OR) and relative risks (RR). Where these had not been estimated and only raw numbers of participants were provided in tables, we estimated crude ORs from the information available.
Quality assessment
The risk of bias of clinical trial25,26 was independently assessed by two reviewers using “The Cochrane Collaboration’s tool”. It consisted of six specific domains, including: sequence generation, allocation concealment, blinding of participants and personnel, incomplete outcome data, selective outcome reporting, and other sources of bias. The assessment of each domain was categorized as ‘low risk,’ ‘high risk,’ or ‘unclear risk’ of bias by two independent reviewers. Any differences between reviewers were resolved by discussion and by consultation from supervisor to reach a consensus if needed. Methodological quality assessment of observational studies14,15,17,19,22,23,27–32 was performed by using the risk of bias assessment tool of the “Cancer Council Australia”33. The metrics of this tool were as follows: subject selection (which included sub-metrics for case selection, control selection, and adequacy of case definition), comparability of groups on demographic characteristics and important potential confounders, ascertainment of exposure/treatment, and follow-up.
Statistical methods
Quantitative synthesis with meta-analysis was performed using Comprehensive Meta-analysis software version 2.0 (Biostat, New Jersey, USA). Summary odds ratios (ORs) and 95% confidence intervals [CIs] were computed for individual vaccines when there were two or more studies providing results for the association between that specific vaccine and risk of childhood leukemia. Heterogeneity between studies was assessed using the Q statistic and I-squared tests. Heterogeneity was considered statistically significant if the P-value was < 0.1 or I-squared value was > 50%34,35. Random and fixed effects models were used depending on the heterogeneity among the studies.
When there were ten or more studies of a particular vaccine, we performed meta regression for moderators to examine the potential effects of study characteristics on the summary effect measure. Subgrouping of early/late and type of vaccination was performed to examine the effect of age at vaccination and type of infection on risk of leukemia. The effect of study characteristics on the summary OR was considered significant when the p-value was less than 0.0536. Publication bias was evaluated by funnel plot and Egger’s regression where there were five or more studies assessing the effect of a particular variable. Publication bias was considered significant when the P-value was < 0.1. The Duvall and Tweedie’s trim-and-fill method was used to increase symmetry by adjusting for studies that appeared to be missing in the presence of publication bias37–39.
Results
Literature search and study characteristics
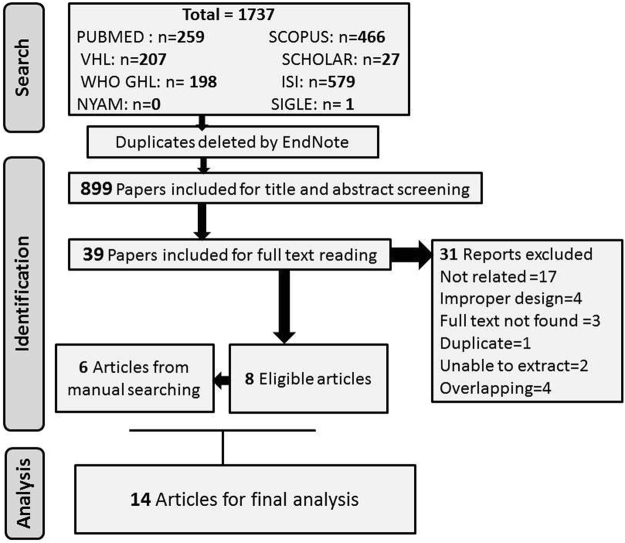
A total of 899 reports were included for title and abstract screening after automatic removal of duplicates in author, title, and date with Endnote software, and 39 articles were considered for full text reading. Only eight articles matched our selection criteria. The manual search of references also provided additional six reports. Among studies which were excluded from the manual search, there were two studies that did not provide a non-leukemic group40,41, two studies enrolled patients at more than 20 of age42,43, one study had overlapping participants44 and one study was a conference paper45. Hence, the total number of articles for final analysis was 14. Detailed characteristics of included studies are shown in Table 1. Twelve of the included studies were observational14,15,17,19,22,23,27–32, and two were clinical trials25,26 (Fig. 1). The results of meta-analysis of childhood leukemia and different types of vaccines are shown in Table 2.
Table 1.
Basic characteristics of included studies.
| Author/Year/Ref | Country | Leukemia | Vaccination | |||
|---|---|---|---|---|---|---|
| Type | Number | Method of Diagnosis | Type | Time | ||
| Crispen/1976 | USA | ND | 85356 | ND | BCG | ND |
| Dockerty/1999 | New Zealand | ALL | 121 | ND | BCG, MMR, Measles, Rubella, Oral Polio, DPT, Double vaccine, Hepatitis B | BCG: 3 months, 6 years, Measles: within 1 year |
| Nishi/1989 | Japan | ALL | 63 | ND | BCG and Measles | 1st and 2nd year |
| Groves/1999 | USA | ALL | 439 | ND | MMR,Oral Polio, DPT, Tetanus, Diphteria, Hib polysaccharide and conjugate, Hepatitis B | ND |
| Von Kries/2000 | Germany | AL | 129 | ND | BCG, MMR | BCG: at birth, MMR: after 1 year |
| MacArthur/2007 | Canada | ND | 399 | Hematology, Pathology | Mumps, Measles, Rubella, Diphtheria, Pertussis,Tetanus, Polio, Hepatitis, BCG, and others | 1st and 2nd year |
| Mallol.Mesnard/2007 | France | AML, ALL, AL | 726 | Bone marrow analysis | BCG, Diphtheria, Tetanus, Poliomyelitis, Pertussis, Hepatitis B, Hib, Pneumococcus, Meningococcus, Measles, Mumps, Rubella | 1st and 2nd year |
| Ma/2005 | USA | ND | 323 | ND | DTP, MMR, Polio, Hepatitis B, and Hib | ND |
| Petridou/1997 | Greece | ND | 153 | ND | DTP, BCG, Virus vaccines | At birth |
| Salonen/1976 | Finland | ND | 368 | Pathology, Clinical | BCG | 1–18 year |
| Comstock/1975 | USA | ND | 98 | ND | BCG | ND |
| Davignon/1971 | Canada | ND | 96 | ND | BCG | ND |
| Máthé/1974 | France | ND | 130 | ND | BCG | ND |
| Sutherland/1982 | England | ND | 28 | ND | BCG, vole-bacillus | ND |
Figure 1.
PRISMA flow diagram of studies’ screening and selection.
Table 2.
Meta-analysis of the association of childhood leukemia with different types of vaccines from fourteen included studies.
| Type of vaccines | No. of study | Total sample size (Leukemia/Control) | Heterogeneity | Model | Association with leukemia | Egger’s 2-tailed bias p-value | ||
|---|---|---|---|---|---|---|---|---|
| p-value | I 2 | p-value | Odds ratio (95% CI) | |||||
| Early vaccination | 7 | 3000/3719581 | 0.0001 | 77.9 | Random | 0.019 | 0.58 (0.36–0.91) | 0.60 |
| BCG vaccines | 12 | 3778/3760815 | 0.0001 | 77.7 | Random | 0.117 | 0.73 (0.50–1.08) | 0.47 |
| Mumps vaccines | 4 | 3431/4999 | 0.98 | 0 | Fixed | 0.95 | 1.01 (0.82–1.23) | 0.54 |
| Hib vaccines | 3 | 2528/4004 | 0.001 | 84.6 | Random | 0.83 | 1.06 (0.64–1.76) | 0.89 |
| HBV vaccines | 5 | 2266/3906 | 0.67 | 0 | Fixed | 0.95 | 1.00 (0.85–1.18) | 0.69 |
| Triple vaccines | 4 | 1986/2714 | 0.2 | 35.1 | Fixed | 0.56 | 1.10 (0.79–1.53) | 0.99 |
| Poliosip vaccines | 4 | 2390/2769 | 0.99 | 0 | Fixed | 0.93 | 0.98 (0.70–1.39) | 0.29 |
| MMR vaccines | 4 | 2302/2667 | 0.98 | 0 | Fixed | 0.78 | 0.96 (0.75–1.24) | 0.85 |
| Measles vaccines | 6 | 3715/5627 | 0.02 | 59.1 | Random | 0.81 | 1.02 (0.85–1.23) | 0.23 |
| Rubella vaccines | 5 | 3548/5267 | 0.96 | 0 | Fixed | 0.98 | 1.00 (0.82–1.23) | 0.10 |
IPooled odds ratios (OR) with corresponding 95% confidence intervals (95% CI) of the pooled results were calculated where more than one study had investigated the factor.
Quality assessment
Using the Cochrane collaboration’s assessment tool, the two clinical trials25,26 were of unclear risk of bias regarding allocation concealment and sequence generation while the domain of incomplete outcome data were at low risk. According to Cancer Council Australia’s quality assessment tool of case control studies14,15,17,19,22,23,27–32, at least two out of six domains were at low risk of bias for all case control studies. Every article has more than three domains were at low risk of bias except for the study of Nishi et al.30. However, the overall quality of evidence in this systematic review is low and the results should be interpreted cautiously (Supplementary Table 2A and Supplementary Table 2B).
Early vaccination
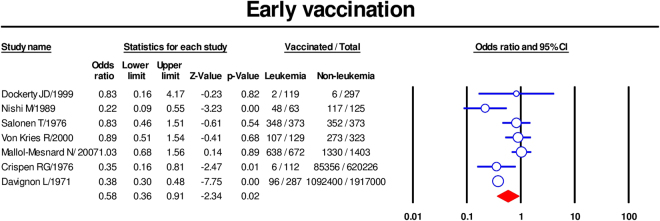
The association between childhood leukemia and early vaccination, which included BCG vaccine, DPT vaccine, double vaccine and HBV vaccine given within the first year of life, was meta-analyzed. The summary ORs for receiving any type of vaccine in the first year of life (based on seven studies)14,22,23,27,28,30,32 was 0.58 [95% CI 0.36–0.91] (Fig. 2). There was significant heterogeneity among included studies (p < 0.1, I2 = 78%,), but no publication bias was seen (Supplementary Fig. 1); Egger’s test P value was 0.3.
Figure 2.
Forest plot showing results of meta-analysis of association of early vaccination with childhood leukemia. Cl: confidence interval.
BCG vaccine
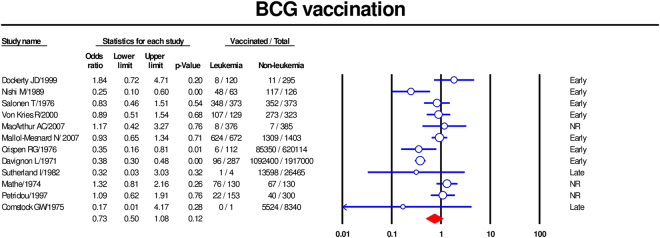
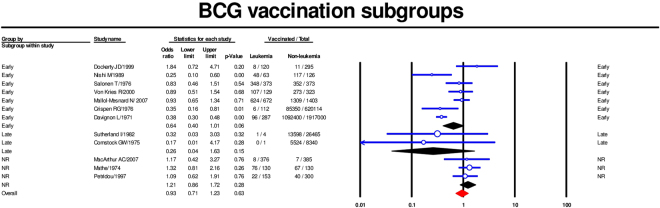
Twelve studies were included in the meta-analysis of the association between BCG vaccine and childhood leukemia14,19,22,23,25–32. Using a random-effects model, the summary OR was 0.73 [95% CI 0.50–1.08] (Fig. 3). Our subgroups analysis by age at BCG vaccination revealed that both early vaccination (less than one year old) (summary OR 0,64; 95% CI [0.4–1.01])14,22,23,27,28,30,32 and later vaccination (summary OR 0.26; 95% CI 0.04–1.63)25,26 were associated with a lower risk of childhood leukemia, although the latter estimate lacked precision as there were only two studies. There was no evidence of association when the timing of vaccination was not reported19,29,31 (Fig. 4). A meta-regression revealed little effect of publication year on the association of BCG vaccination and childhood leukemia (coefficient 0.02, 95% CI [−0.005, 0.045], p value = 0.06) (Supplementary Fig. 2).
Figure 3.
Forest plot showing results of meta-analysis of association of BCG vaccination with childhood leukemia. Cl: confidence interval.
Figure 4.
Forest plot showing subgroup analysis of association between early BCG vaccination and childhood leukemia. Cl: confidence interval.
Mumps vaccine
Meta-analysis of five studies showed no evidence of any association between mumps vaccination and childhood leukemia (summary OR 1.01 [95% CI 0.82–1.23])15,17,19,23,28. However, mumps vaccine was given alone in only one study28 and in a combined vaccination in four others15,17,19,23 so we could not perform a subgroup analysis (Supplementary Fig. 3).
HiB vaccine
Meta-analysis of three studies15,17,28 revealed no association between HiB vaccination and childhood leukemia (summary OR 1.06 [95% CI 0.64–1.76]) (Supplementary Fig. 4).
HBV vaccine
Four data sets were included in the meta-analysis of the association between HBV vaccination and childhood leukemia17,19,23,28. The result indicated that there was no association (summary OR 1.00 [95% CI 0.85–1.16]) (Supplementary Fig. 5).
Triple vaccine
Triple vaccine is a combination of diphtheria, tetanus and pertussis (DPT) vaccines. Meta-analysis of four studies15,17,23,31 revealed no association between Triple vaccine and childhood leukemia risk (summary OR 1.1 [95% CI 0.79–1.53]) (Supplementary Fig. 6).
Polio sip vaccine
Four datasets were included in the meta-analysis of the association between Polio vaccination and childhood leukemia risk19,24,28,29. The summary OR was 0.98 [95% CI 0.70–1.39] (Supplementary Fig. 7).
MMR vaccine
Four studies were included in the meta-analysis of the association between trivalent MMR vaccine and childhood leukemia19,24,28,29. No association was seen (summary OR 0.96 [95% CI 0.75–1.24]) (Supplementary Fig. 8).
Measles vaccine
There were six studies of the association between measles vaccination and childhood leukemia15,17,19,23,28,30. They provided seven datasets, as one study23 reported measles vaccine given both alone and in trivalent vaccine of MMR. No significant association between measles vaccine and leukemia was found (summary OR 0.96 [95% CI 0.71–1.30]). A subgroup meta-analysis was performed to investigate whether the association was affected by type of injection (alone or combined). No evidence of an association was seen in either subgroup (summary ORs were 0.84 [95% CI 0.39–1.83] and 1.02 [95% CI 0.66–1.59] respectively) (Supplementary Fig. 9).
Rubella vaccine
Meta-analysis of six datasets available from five studies15,17,19,23,28 revealed no evidence of an association between rubella vaccine and leukemia risk (summary OR 1.00 [95% CI 0.82–1.23]). Subgroup analysis indicated that rubella vaccination was not associated with leukemia risk whether given alone or in combined trivalent MMR vaccine (Supplementary Fig. 10).
Discussion
Our meta-analysis indicated that receiving any type of vaccine in the first year of life may be protective against childhood leukemia. When specific types of vaccine were separately analyzed, however, only the analysis of BCG revealed at least some evidence of a reduced risk. Because BCG is given early, it was not possible in this study to separate any potential protective effect of early vaccination from BCG vaccination itself. However, a protective association with BCG cannot be ruled out, given that no other individual vaccine was associated with a reduced risk of leukemia in this meta-analysis.
The protective association we observed between early vaccination (and/or BCG) and risk of childhood leukemia overall may involve similar biological pathways to those underlying the apparent reduction in leukemia risk associated with day-care attendance12. Similar to the potential for common infections during day-care attendance, exposure of children to microbial pathogen in vaccines early in life may mimic the role of infections in triggering the response of the naïve immune system. Schmiegelow et at proposed the hypothalamus-pituitary-adrenal axis hypothesis in which infections at early stage of life may alter plasma cortisol levels46. This can result in elimination of leukemic cells and reduce the risk of ALL46,47. Moreover, there is evidence that vaccines, both viral and bacterial, also have the ability to raise the plasma cortisol48–51. Thus, the adrenal hypothesis may explain in immunological terms the association between early vaccination and risk of leukemia. Such a mechanism would also be consistent with Greaves’ delayed-infection hypothesis52. However, this does not explain why BCG – but none of the other specific (mostly viral) vaccines – appeared to be associated (albeit weakly) with a reduction in leukemia risk.
Heterologous (or “off-target”) effects of BCG vaccination in children have been consistently observed53,54; outcomes for which there is some evidence of a protective include all-cause infant mortality32,53 and childhood cancer32. The biological pathways involved are unclear, but are thought to involve lymphocyte activation and/or innate immune memory that could promote protection beyond the intended target pathogen. Potential mechanisms are discussed in detail by Goodridge et al.53. In addition, many animal studies have reported beneficial effects of BCG in enhancing the immune response to unrelated pathogens55 and tumours. Theorically and practically, BCG vaccine has been utilized as an immunotherapy for a certain cancer56–58. The mechanism in which BCG vaccine ameliorates effectively cancer was not well-explored. Several studies investigated the alteration of different types of cytokines by BCG vaccine59–62, such as Th1’s cytokines (IFN-γ, TNF-α, IL-12) and Th2’s cytokines (IL-10, IL-4). These substances play roles in regulation of natural killer cells, macrophages or cytotoxic T lymphocytes cells61, which have antitumor effects. In addition, the antitumor activity of BCG vaccine through activating human dendritic cells, an antigen-present cells, has been studied63–67. This process was mediated by modulation of several cytokines such as IL2, IL10 and IFN gamma67. Taking specific antileukemia effect into consideration, studies were performed and their findings were promising. Jang et al.65 investigated the activity of dendritic cells of pediatric leukemia patients exposed by BCG, the result revealed an enhanced proliferation and maturation status. Recently, a BCG-derived heat shock protein, named HSP70, was investigated. Findings from animal and in vitro studies demonstrated an elevated immunogenicity effects against leukemia cells68,69.
Strengths
To the best of our knowledge, this is the first comprehensive systematic review and meta-analysis investigating the association between vaccination and childhood leukemia. All steps of this systematic review and meta-analysis were conducted in strict accordance with the Cochrane handbook of systematic reviews70 and reported according to the recommendations of the PRISMA statement24. Moreover, stratification according to time of vaccination (for BCG vaccination) and type of vaccination (with early vaccination, Measles vaccine and Rubella vaccines) was further examined.
Limitations
Although our systematic literature search was conducted on nine electronic libraries, the number of studies included in the final analysis was limited and it was not possible to conduct our meta-analysis by leukemia subtype. Furthermore, most of the included studies were observational. We were unable to evaluate the effects of several confounders such as prenatal and postnatal care, the nutrient supplies, and the microorganism exposure on the pooled results due to the lack of such information in the included studies. Further, some of the observational studies provided raw data only, from which we estimated crude study-specific ORs. Observational studies are subject to biases such as selection and recall bias. Except for Salonen 197622 which was at moderate risk of bias, the six remaining studies were at high risk of bias. However, these biases (if present) would most likely have similarly affected the results for all types of vaccinations, not just early vaccination and/or BCG itself. We acknowledge that the association seen with BCG vaccination was not statistically significant; however, this was likely due to a lack of statistical power and a relevant protective association remains a possibility. While seven studies reported early vaccination, four studies were before 1990 and only two were in 21st century; this led to some heterogeneity. Most studies reporting BCG vaccines were conducted before the 1980s. The design of those studies was not appropriate for evaluating the effect of BCG vaccination in risk of leukemia, because clinical trials were predominantly designed for protecting against tuberculosis. Additionally, three of them, which contained a large number of participants, reported leukemia deaths rather than leukemia cases14,26,27.
The change in vaccine schedule is worthy of note. According to Center for Disease Control and Prevention (CDC), many vaccines have currently been used for children at birth, such as HBV, or within the first year, such as DTA, Rotavirus, or Haemophilus influenza type b. These changes may alter the potential effect of early vaccine on childhood leukemia risk. Further studies should investigate the impact of these shifts in vaccine policy.
Conclusion
Our comprehensive systematic review and meta-analysis indicated that the early vaccination may reduce the risk of childhood leukemia, and this finding may be attributable to BCG vaccination. Replication of this finding is important, and its impact in the context of changing vaccination policy should be carefully evaluated.
Electronic supplementary material
Acknowledgements
This work was supported in part by a “Grant-in-Aid for Scientific Research (B)” (16H05844, 2016–2019 for Nguyen Tien Huy) from Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan and by the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID) for Kenji Hirayama. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author Contributions
N.T.H., M.E.M., K.N.D. contributed to study design, data analysis and writing manuscript. A.E., O.A.M., A.L.E., O.H., A.M.M., T.L.H.V., C.H. contributed to searching databases, screening articles and extracting data. A.S.F., A.P.P., W.M.A., M.M. contributed to preparing tables, figures and writing manuscript, E.M., K.H., N.T.H. contributed to data analysis and approved the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Mostafa Ebraheem Morra, Nguyen Dang Kien and Ahmed Elmaraezy contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-16067-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Elizabeth Milne, Email: Liz.Milne@telethonkids.org.au.
Nguyen Tien Huy, Email: nguyentienhuy@tdt.edu.vn.
References
- 1.Society, A. C. Childhood Leukemia, http://www.cancer.org/acs/groups/cid/documents/webcontent/003095-pdf.pdf (2015).
- 2.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 3.Greaves M. Infection, immune responses and the aetiology of childhood leukaemia. Nature Reviews Cancer. 2006;6:193–203. doi: 10.1038/nrc1816. [DOI] [PubMed] [Google Scholar]
- 4.Ron E, Preston DL, Mabuchi K, Thompson DE, Soda M. Radiation research. 1994;137:S98–112. doi: 10.2307/3578894. [DOI] [PubMed] [Google Scholar]
- 5.Preston DL, et al. Effect of recent changes in atomic bomb survivor dosimetry on cancer mortality risk estimates. Radiation research. 2004;162:377–389. doi: 10.1667/RR3232. [DOI] [PubMed] [Google Scholar]
- 6.Chang JS. Parental smoking and childhood leukemia. Methods Mol Biol. 2009;472:103–137. doi: 10.1007/978-1-60327-492-0_5. [DOI] [PubMed] [Google Scholar]
- 7.Milne E, et al. Parental prenatal smoking and risk of childhood acute lymphoblastic leukemia. Am J Epidemiol. 2012;175:43–53. doi: 10.1093/aje/kwr275. [DOI] [PubMed] [Google Scholar]
- 8.Farioli A, et al. Tobacco smoke and risk of childhood acute lymphoblastic leukemia: findings from the SETIL case-control study. Cancer causes & control: CCC. 2014;25:683–692. doi: 10.1007/s10552-014-0371-9. [DOI] [PubMed] [Google Scholar]
- 9.Ma X, et al. Critical windows of exposure to household pesticides and risk of childhood leukemia. Environmental Health Perspectives. 2002;110:955–960. doi: 10.1289/ehp.02110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cogliano VJ, Baan R, Straif K. Updating IARC’s carcinogenicity assessment of benzene. Am J Ind Med. 2011;54:165–167. doi: 10.1002/ajim.20916. [DOI] [PubMed] [Google Scholar]
- 11.Greaves M. Aetiology of acute leukaemia. The Lancet. 1997;349:344–349. doi: 10.1016/S0140-6736(96)09412-3. [DOI] [PubMed] [Google Scholar]
- 12.Urayama KY, Buffler PA, Gallagher ER, Ayoob JM, Ma X. A meta-analysis of the association between day-care attendance and childhood acute lymphoblastic leukaemia. International journal of epidemiology. 2010;39:718–732. doi: 10.1093/ije/dyp378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comstock G, Livesay V, Webster R. Leukaemia and BCG: A controlled trial. The Lancet. 1971;298:1062–1063. doi: 10.1016/S0140-6736(71)90381-3. [DOI] [PubMed] [Google Scholar]
- 14.Crispen R, Rosenthal S. BCG vaccination and cancer mortality. Cancer Immunology, Immunotherapy. 1976;1:139–142. doi: 10.1007/BF00205456. [DOI] [Google Scholar]
- 15.Groves FD, et al. Infant vaccinations and risk of childhood acute lymphoblastic leukaemia in the USA. Br J Cancer. 1999;81:175–178. doi: 10.1038/sj.bjc.6690668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckley JD, et al. Epidemiological characteristics of childhood acute lymphocytic leukemia. Analysis by immunophenotype. The Childrens Cancer Group. Leukemia. 1994;8:856–864. [PubMed] [Google Scholar]
- 17.Ma X, et al. Vaccination history and risk of childhood leukaemia. International Journal of Epidemiology. 2005;34:1100–1109. doi: 10.1093/ije/dyi113. [DOI] [PubMed] [Google Scholar]
- 18.Rosenthal R, et al. BCG vaccination and leukemia mortality. JAMA. 1972;222:1543–1544. doi: 10.1001/jama.1972.03210120041010. [DOI] [PubMed] [Google Scholar]
- 19.MacArthur AC, et al. Risk of childhood leukemia associated with vaccination, infection, and medication use in childhood: the Cross-Canada Childhood Leukemia Study. Am J Epidemiol. 2008;167:598–606. doi: 10.1093/aje/kwm339. [DOI] [PubMed] [Google Scholar]
- 20.Davignon L, Robillard P, Lemonde P, Frappier A. BCG vaccination and leukaemia mortality. The Lancet. 1970;296:638. doi: 10.1016/S0140-6736(70)91402-9. [DOI] [PubMed] [Google Scholar]
- 21.Salonen T, Saxén L. Risk indicators in childhood malignancies. International journal of cancer. 1975;15:941–946. doi: 10.1002/ijc.2910150609. [DOI] [PubMed] [Google Scholar]
- 22.Salonen T. Prenatal and perinatal factors in childhood cancer. Ann Clin Res. 1976;8:27–42. [PubMed] [Google Scholar]
- 23.Dockerty JD, et al. Infections, vaccinations, and the risk of childhood leukaemia. Br J Cancer. 1999;80:1483–1489. doi: 10.1038/sj.bjc.6690548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 25.Comstock GW, Martinez I, Livesay VT. Efficacy of BCG vaccination in prevention of cancer. Journal of the National Cancer Institute. 1975;54:835–839. [PubMed] [Google Scholar]
- 26.Sutherland I. BCG and vole bacillus vaccination in adolescence and mortality from leukaemia. Statistics in medicine. 1982;1:329–335. doi: 10.1002/sim.4780010410. [DOI] [PubMed] [Google Scholar]
- 27.Davignon L, Lemonde P, St-Pierre J, Frappier ABCG. vaccination and leukaemia mortality. Lancet (London, England) 1971;1:80–81. doi: 10.1016/S0140-6736(71)90804-X. [DOI] [PubMed] [Google Scholar]
- 28.Mallol-Mesnard N, et al. Vaccination and the risk of childhood acute leukaemia: the ESCALE study (SFCE) International journal of epidemiology. 2007;36:110–116. doi: 10.1093/ije/dyl270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathe G, Facy F, Hatton F, Halle-Pannenko O. BCG vaccination and acute leukaemia. Biomedicine / [publiee pour l’A.A.I.C.I.G.] 1974;21:132–134. [PubMed] [Google Scholar]
- 30.Nishi M, Miyake H. A case-control study of non-T cell acute lymphoblastic leukaemia of children in Hokkaido, Japan. J Epidemiol Community Health. 1989;43:352–355. doi: 10.1136/jech.43.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petridou E, et al. The risk profile of childhood leukaemia in Greece: a nationwide case-control study. Br J Cancer. 1997;76:1241–1247. doi: 10.1038/bjc.1997.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Von Kries R, Peter Grunert V, Kaletsch U, Michaelis J, Göbel U. Prevention of childhood leukemia by BCG vaccination in newborns? A population-based case-control study in Lower Saxony, Germany. Pediatric hematology and oncology. 2000;17:541–550. doi: 10.1080/08880010050122807. [DOI] [PubMed] [Google Scholar]
- 33.Olver, I. et al. Development of Clinical Practice Guidelines Using Cancer Council Australia’s Cancer Guidelines Wiki. (2014).
- 34.Zintzaras E, Lau J. Synthesis of genetic association studies for pertinent gene–disease associations requires appropriate methodological and statistical approaches. Journal of clinical epidemiology. 2008;61:634–645. doi: 10.1016/j.jclinepi.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ: British Medical Journal. 2003;327:557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munafo MR, Flint J. Meta-analysis of genetic association studies. TRENDS in Genetics. 2004;20:439–444. doi: 10.1016/j.tig.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 37.Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics, 1088–1101 (1994). [PubMed]
- 38.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. Jama. 2006;295:676–680. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 39.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Härö A. The effect of BCG-vaccination and tuberculosis on the risk of leukaemia. Developments in biological standardization. 1985;58:433–449. [PubMed] [Google Scholar]
- 41.Ambrosch F, Wiedermann G, Krepler P. Studies on the influence of BCG vaccination on infantile leukemia. Developments in biological standardization. 1986;58(Pt A):419–424. [PubMed] [Google Scholar]
- 42.Kendrick MA, Comstock GW. BCG vaccination and the subsequent development of cancer in humans. Journal of the National Cancer Institute. 1981;66:431–437. [PubMed] [Google Scholar]
- 43.Villumsen M, et al. Risk of lymphoma and leukaemia after bacille Calmette-Guerin and smallpox vaccination: a Danish case-cohort study. Vaccine. 2009;27:6950–6958. doi: 10.1016/j.vaccine.2009.08.103. [DOI] [PubMed] [Google Scholar]
- 44.Snider DE, Comstock GW, Martinez I, Caras GJ. Efficacy of BCG Vaccination in Prevention of Cancer: An Update: Brief Communication2. JNCI: Journal of the National Cancer Institute. 1978;60:785–788. doi: 10.1093/jnci/60.4.785. [DOI] [PubMed] [Google Scholar]
- 45.Rosenthal SR. Cancer precursors and their control by BCG. Developments in biological standardization. 1986;58(Pt A):401–416. [PubMed] [Google Scholar]
- 46.Schmiegelow K, Vestergaard T, Nielsen SM, Hjalgrim H. Etiology of common childhood acute lymphoblastic leukemia: the adrenal hypothesis. Leukemia. 2008;22:2137–2141. doi: 10.1038/leu.2008.212. [DOI] [PubMed] [Google Scholar]
- 47.Azevedo-Silva F, Camargo B, Pombo-de-Oliveira MS. Implications of infectious diseases and the adrenal hypothesis for the etiology of childhood acute lymphoblastic leukemia. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 2010;43:226–229. doi: 10.1590/s0100-879x2010007500011. [DOI] [PubMed] [Google Scholar]
- 48.El Yousfi M, et al. The inflammatory response to vaccination is altered in the elderly. Mechanisms of ageing and development. 2005;126:874–881. doi: 10.1016/j.mad.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 49.Oken E, Kasper DL, Gleason RE, Adler GK. Tetanus toxoid stimulation of the hypothalamic-pituitary-adrenal axis correlates inversely with the increase in tetanus toxoid antibody titers. The Journal of clinical endocrinology and metabolism. 1998;83:1691–1696. doi: 10.1210/jcem.83.5.4829. [DOI] [PubMed] [Google Scholar]
- 50.Pourcyrous M, Korones SB, Crouse D, Bada HS. Interleukin-6, C-reactive protein, and abnormal cardiorespiratory responses to immunization in premature infants. Pediatrics. 1998;101:E3. doi: 10.1542/peds.101.3.e3. [DOI] [PubMed] [Google Scholar]
- 51.Rowe J, et al. Th2-Associated Local Reactions to the Acellular Diphtheria-Tetanus-Pertussis Vaccine in 4- to 6-Year-Old Children. Infection and Immunity. 2005;73:8130–8135. doi: 10.1128/IAI.73.12.8130-8135.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greaves M. Speculations on the cause of childhood acute lymphoblastic leukemia. Leukemia. 1988;2:120–125. [PubMed] [Google Scholar]
- 53.Goodridge HS, et al. Harnessing the beneficial heterologous effects of vaccination. Nature reviews. Immunology. 2016;16:392–400. doi: 10.1038/nri.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pollard AJ, Finn A, Curtis N. Non-specific effects of vaccines: plausible and potentially important, but implications uncertain. Archives of Disease in Childhood. 2017 doi: 10.1136/archdischild-2015-310282. [DOI] [PubMed] [Google Scholar]
- 55.Freyne B, Marchant A, Curtis N. BCG-associated heterologous immunity, a historical perspective: experimental models and immunological mechanisms. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2015;109:46–51. doi: 10.1093/trstmh/tru196. [DOI] [PubMed] [Google Scholar]
- 56.Boehm BE, et al. Efficacy of bacillus Calmette-Guerin Strains for Treatment of Nonmuscle Invasive Bladder Cancer: A Systematic Review and Network Meta-Analysis. The Journal of urology. 2017;198:503–510. doi: 10.1016/j.juro.2017.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamat AM, et al. Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of bladder carcinoma. Journal for immunotherapy of cancer. 2017;5:68. doi: 10.1186/s40425-017-0271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer–a current perspective. Nature reviews. Urology. 2014;11:153–162. doi: 10.1038/nrurol.2014.15. [DOI] [PubMed] [Google Scholar]
- 59.Janaszek-Seydlitz W, et al. Effect of different Bacillus Calmette-Guerin substrains on growth inhibition of T24 bladder cancer cells and cytokines secretion by BCG activated peripheral blood mononuclear cells of PBMCs. Advances in clinical and experimental medicine: official organ Wroclaw Medical University. 2014;23:877–884. doi: 10.17219/acem/37330. [DOI] [PubMed] [Google Scholar]
- 60.Kleinnijenhuis J, et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. Journal of innate immunity. 2014;6:152–158. doi: 10.1159/000355628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu X, et al. Cytokines as effectors and predictors of responses in the treatment of bladder cancer by bacillus Calmette-Guerin. Future oncology (London, England) 2014;10:1443–1456. doi: 10.2217/fon.14.79. [DOI] [PubMed] [Google Scholar]
- 62.Portevin D, Young D. Natural killer cell cytokine response to M. bovis BCG Is associated with inhibited proliferation, increased apoptosis and ultimate depletion of NKp44(+)CD56(bright) cells. PloS one. 2013;8:e68864. doi: 10.1371/journal.pone.0068864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanarico N, et al. Different transcriptional profiles of human monocyte-derived dendritic cells infected with distinct strains of Mycobacterium tuberculosis and Mycobacterium bovis bacillus Calmette-Guerin. Clinical & developmental immunology. 2011;2011:741051. doi: 10.1155/2011/741051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thurnher M, et al. Bacillus Calmette-Guerin mycobacteria stimulate human blood dendritic cells. International journal of cancer. 1997;70:128–134. doi: 10.1002/(SICI)1097-0215(19970106)70:1<128::AID-IJC19>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 65.Yang J, et al. Effect of Bacillus Calmette-Guerin on the expansion of dendritic cells from peripheral blood of pediatric patients with leukemia in vitro. Zhongguo shi yan xue ye xue za zhi. 2010;18:1240–1243. [PubMed] [Google Scholar]
- 66.Kim KD, et al. Enhanced antigen-presenting activity and tumour necrosis factor-alpha-independent activation of dendritic cells following treatment with Mycobacterium bovis bacillus Calmette-Guerin. Immunology. 1999;97:626–633. doi: 10.1046/j.1365-2567.1999.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheadle EJ, Selby PJ, Jackson AM. Mycobacterium bovis bacillus Calmette-Guerin-infected dendritic cells potently activate autologous T cells via a B7 and interleukin-12-dependent mechanism. Immunology. 2003;108:79–88. doi: 10.1046/j.1365-2567.2003.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jimbo J, et al. Induction of leukemia-specific antibodies by immunotherapy with leukemia-cell-derived heat shock protein 70. Cancer science. 2008;99:1427–1434. doi: 10.1111/j.1349-7006.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ye Q, Wang ZH, Qin SK. Preparation of heat shock protein 70 (Hsp70) and idiotypic determinant single-chain antibody (Id-ScFv) in a patient with B-cell chronic lymphatic leukemia (B-CLL) and antitumor effect of peptide complex Hsp70-Id. Ai zheng = Aizheng = Chinese journal of cancer. 2008;27:133–138. [PubMed] [Google Scholar]
- 70.Collaboration, T. C. Cochrane Handbook for Systematic Review of Interventions. (2011).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.