Abstract
Dendritic spines are mushroom-shaped postsynaptic compartments that host biochemical signal cascades important for synaptic plasticity and, ultimately, learning and memory. Signaling events in spines involve a signaling network composed of hundreds of signaling proteins interacting with each other extensively. Synaptic plasticity is typically induced by Ca2+ elevation in spines, which activates a variety of signaling pathways. This leads to changes in the actin cytoskeleton and membrane dynamics, which in turn causes structural and functional changes of the spine. Recent studies have demonstrated that the activities of these proteins have a variety of spatiotemporal patterns, which orchestrate signaling activity in different subcellular compartments at different timescales. The diffusion and the decay kinetics of signaling molecules play important roles in determining the degree of their spatial spreading, and thereby the degree of the spine specificity of the signaling pathway.
Main Text
Dendrites of a neuron typically receive information from 103 to 104 synapses and process the electrical information locally and globally in the millisecond regime. At slower timescales, biochemical signal transduction plays important roles in modifying the synaptic strength and dendritic excitability for administering the processing of electrical information. This signaling network, mediated by hundreds of species of signaling proteins that are connected extensively with each other (1, 2), is perhaps as complex as neuronal circuits in terms of the number of the species of the nodes and connections. Changes in signaling states control the synaptic strength and electrical properties of dendrites through the regulation of ion channels and transmitter receptors, tuning the way dendrites integrate electrical signals. This malleability of the neuron, or neuronal plasticity, is considered to be the basis of brain plasticity, learning, and memory.
Among hundreds of molecules in the signaling network, Ca2+ is perhaps the most important one. In the resting state, its concentration is typically in the 50–100 nM range, but increases 10–100-fold when neurons are activated (3). This triggers the activation of Ca2+-binding proteins, leading to the activation of cascades of biochemical events. Different spatiotemporal patterns of Ca2+ pulses are decoded in different ways by the network, regulating the structure and function of synapses and dendritic excitability and contributing to information storage by the neuronal circuit.
During the processing of biochemical information, the complicated structures of dendrites have profound effects on shaping the spatiotemporal pattern of signaling activity (4). For example, pyramidal neurons have apical and basal dendrites that branch extensively into secondary, tertiary, and fourth-degree dendrites. The branching patterns physically restrict biochemical signal spreading, serving as one of the factors that define the signaling pattern.
Dendrites of pyramidal neurons are decorated with dendritic spines, tiny protrusions emanating from the surface of dendrites. A mature dendritic spine is shaped like a mushroom with a small spherical “head” (0.01–1 μm3) that is connected to the dendrite through a narrow “neck” (length 0.1–1 μm and diameter ∼0.1 μm) (5). The narrow neck acts as a diffusion barrier and compartmentalizes biochemical signaling. Each spine typically houses one postsynaptic density (PSD), the receiving site of synaptic transmissions packed with signal transduction machinery.
Our understanding of the spatiotemporal regulation of biochemical signaling in dendrites has been dramatically improved by recent advances in signal imaging techniques based on fluorescence resonance energy transfer (FRET) in combination with photostimulation of single synapses (6). These studies revealed the temporal sequence of biochemical signaling cascades and their spatial spreading from the stimulated spines.
Given this context, we now focus on the crucial roles and mechanisms of biochemical signal transduction in dendrites for neuronal plasticity, with specific emphasis on long-term potentiation (LTP) of synaptic transmission and associated structural change of dendritic spines. It should be noted that this perspective focuses mainly on NMDA receptor-dependent plasticity in hippocampal pyramidal neurons; different mechanisms may apply to different systems and paradigms.
Structural and functional plasticity of dendritic spines
Spine head volume is linearly related with the size of the PSD and the sensitivity of spines to glutamate (5, 7). Thus, it is widely accepted that change in the volume of dendritic spines is the structural correlate of change in synaptic efficacy. For example, long-term enlargement of spine size is associated with LTP of synaptic transmission. Inducing LTP and long-term spine enlargement requires strong synaptic stimulation that causes large Ca2+ influx into the spine through NMDA receptors (8, 9, 10). During this process, both spine head volume and postsynaptic sensitivity to glutamate increases rapidly within a few minutes (9, 10). However, the size of PSD grows much slower, at a timescale of around 1 h (11, 12). Since synaptic strength increases immediately during LTP (9, 10), synaptic potentiation at the early stage may be caused by an increase in transmission by glutamate receptors at the postsynaptic surface near, but outside of, the PSD or an increase in the density of glutamate receptors in the PSD.
In contrast to LTP, long-term depression (LTD) of synaptic transmission is associated with spine shrinkage, induced by weak and prolonged stimulation (13, 14, 15). LTD can come in many varied forms; some depend on Ca2+ influx and others not, and some show wide-spreading spine shrinkage in multiple spines whereas others show only in stimulated spines (13, 14, 16).
It is unknown to what degree long-term changes in spine size are necessary for changing synaptic strength and vice versa. For both LTP and LTD, pharmacological properties for structural and electrophysiological plasticity are similar and thus these two are in general considered to be very well correlated (10, 15).
Overview of Ca2+-triggered signaling events
During the induction of long-term synaptic plasticity, pulses of brief Ca2+ elevations, which last only for 10–100 ms (17, 18), are translated into signaling that last for hours. Initial signaling is transduced by Ca2+-binding proteins in spines. Among these Ca2+-binding proteins, Calmodulin (CaM) is one of the most important, due to its ability to signal to many other signaling proteins including Ca2+/CaM-dependent kinases (CaMK) (19). In particular, CaMKII activation is known to be important for LTP induction—but not for maintenance—and learning (20). Their activity profile was measured with FRET sensors that measure the conformation change of CaMKII (8, 9, 21, 22). These studies revealed that CaMKII activation is restricted to stimulated spines and acts as a leaky integrator of calcium pulses with decay time constants of ∼6 s and ∼1 min. A number of proteins are phosphorylated by CaMKII during this time, which further relays the events to a longer timescale.
Ca2+-bound CaM also binds to Calcineurin, a phosphatase that is important for LTD and spine shrinkage (15, 23). Calcineurin activation has also been imaged in dissociated neurons. The activation threshold of Calcineurin is lower than that of CaMKII and thus LTD-inducing stimuli appear to be sufficient for activating Calcineurin (21). In response to LTP-inducing stimulation, Calcineurin activation spreads over several micrometers (21). The role of Calcineurin in LTP is not defined yet, but the spreading may be important for spine shrinkage that occurs in adjacent spines under some conditions (24).
Both CaMKII and Calcineurin activate/inactivate many downstream molecules. This includes a set of proteins that regulate the actin cytoskeleton. In particular, Rho GTPase proteins Rac1, Cdc42, and RhoA are three of the most studied small GTPase proteins that regulate cytoskeletal reorganization (25). Since spine structure is maintained by the actin cytoskeleton (26), regulation of these three molecules are the key for changing spine structure. During LTP, all of Rac1, Cdc42, and RhoA are activated by CaMKII, and all are required for inducing LTP and spine enlargement (27, 28, 29, 30, 31). Relaying the short CaMKII activation, Rac1, Cdc42, and RhoA sustain the activation over tens of minutes. Although the exact mechanisms linking CaMKII and small GTPase proteins are unknown, some of their activators (GTPase exchange factors), are known to be CaMKII-dependent and required for long-term synaptic plasticity (29, 32).
The activated Rho GTPase proteins in turn further activate a diverse collection of downstream molecules (25), a subset of which we present here. The Wiskott–Aldrich syndrome protein is activated only by Cdc42, whereas p21-activated kinase (PAK) is activated either by Cdc42 and Rac1 (25). RhoA activates a relatively distinct set of pathways such as Rho kinase (25). The Rac1/Cdc42-PAK pathway and the RhoA-Rho kinase pathway activate LIM kinase and its downstream protein cofilin (25). During LTP, cofilin decorates actin filaments to stabilize the filamentous structure, producing a stable complex that lasts for a long time (11).
Signaling events appear not to be restricted to intracellular processes. Recent studies found that extracellular signaling also plays important roles in synaptic plasticity. First, it has been shown that dendritic spines can release BDNF in a Ca2+-CaMKII-dependent manner, such that the released BDNF is bound to its receptor TrkB on the same spine (33, 34, 35). The activated TrkB signals to small GTPase proteins Rac1 and Cdc42 to regulate the actin cytoskeleton. It is not clear how CaMKII regulates BDNF release, but there may be release machinery that is phosphorylated by CaMKII (34). To give another example, it has been shown that lysosomes are fused to the plasma membrane and release Cathepsin B to activate a metalloproteinase MMP9 (36). MMP9 cleaves the fibers of the extracellular matrix to facilitate the growth of the spine. Release of BDNF and Cathepsin B both require the fusion of the internal membrane to the plasma membrane, providing a mechanism linking intracellular signaling to the extracellular domain.
Another important role of membrane trafficking during synaptic plasticity is the delivery of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors to the surface (37, 38). Since AMPA receptors are the major mediator of synaptic transmission, the trafficking of these receptors is critical for synaptic plasticity (39). Exocytosis of vesicles containing the receptors occurs rapidly during the induction of LTP both in the stimulated spine and adjacent area of the shaft, simultaneously increasing the receptor concentration and the membrane area in the spine (40, 41). Thus, this increases the postsynaptic sensitivity to glutamate and spine volume.
In addition to the activation of signaling proteins for rapid spine shape modifications, activity-dependent protein synthesis in dendrites is critical for maintaining synaptic plasticity for more than several hours (11, 35, 42, 43, 44). Many signaling pathways, including the TrkB, Rho GTPase, extracellular signal-regulated kinase (ERK), and protein kinase A (PKA) pathways, are known to regulate protein synthesis in dendrites (45). Protein synthesis machinery exists near the base of the spine neck, and is recruited to activated spines (46, 47). Thus, local protein synthesis near the spine may provide synthesis products to the stimulated spines to maintain long-term synaptic plasticity (44).
Mechanism of signal spreading
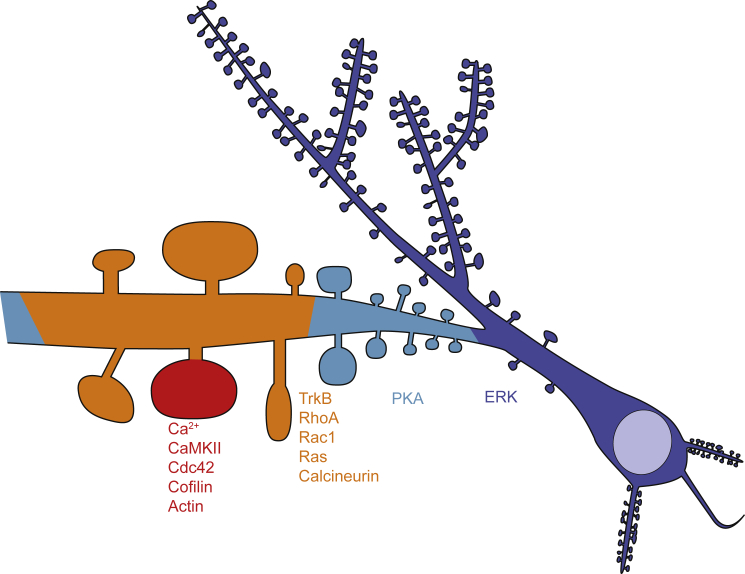
Diffusion is efficient at the micrometer scale, and hence the diffusion of signaling molecules is one of the most critical factors for determining the degree of spatial spreading of signaling activity. Since the distance between the nearest spines is typically less than a few micrometers, diffusion also defines how spine-specific the signaling is. The spreading of signaling activity has been measured using signal imaging in combination with two-photon glutamate uncaging, which can trigger signaling at a single spine. This allowed us to image the spatial profiles of protein kinases CaMKII, PKA, and ERK, and small GTPase proteins RhoA, Rac1, and Cdc42 (Fig. 1) (8, 9, 11, 27, 28, 48, 49).
Figure 1.
Illustration of signal spreading in response to stimulation of a single dendritic spine. Red: signaling restricted to the stimulated spine. Orange: signaling that spreads over a short stretch of dendritic segments (5–15 μm). Cyan: signaling that spreads over a longer distance. Dark blue: signaling that spreads into the nucleus. The area near the stimulated spines is magnified.
Spatial spreading of specific signaling molecules is determined by the balance between diffusion and inactivation rate: how far active molecules can diffuse before they are inactivated. In the simplest model assuming one dimensional diffusion of the molecules along the dendrite, the spatial profile of activity C is given as a function of the distance from the stimulated spine (x) and time (t) as
where Deff is the effective diffusion coefficient of the signaling molecule and τ is the time constant of the decay of molecular activity (50). At the steady state (t → ∞), C can be described as
where L is the length constant
Thus, temporal and spatial scales are tightly coupled. In addition to the gradient along the dendritic shaft above, the gradient at the spine neck can be also calculated as the flux of molecules through a path connecting the two compartments (the spine and the dendrite), and is known to be well-modeled by a function of the neck diameter, dendritic diameter, and the head size as well as Deff and τ (50).
It should be noted that the functions above only model the simplest conceivable scenario, and many other factors can affect the spreading of molecular activity. For example, when the upstream molecule also diffuses, the spreading will be the convolution of the spreading of the upstream molecule and the signal spreading described above. Furthermore, in dendrites with high spine density, the diffusion becomes anomalous, and Deff (not constant in this case) becomes smaller (51). Finally, the regeneration mechanisms by positive feedback may play an important role for signaling that spreads over a long distance (52, 53).
Despite the simplification, the above model fits well to the measured spreading of signaling activity. For instance, Ca2+ elevation and CaMKII activation are restricted to the stimulated spines. For Ca2+, since extrusion of Ca2+ is extremely fast (20–100 ms), although the effective diffusion of Ca2+ is also fast (1–65 μm2/s), the length constant predicted by the model is 0.1–2.5 μm, consistent with the measured length constant 1.2–1.9 μm (17, 18) (Table 1). For CaMKII activation, the decay time constant is much longer: ∼6 s (fast component) (8). However, CaMKII binds to many proteins and diffuses with much slower rate: ∼0.1 μm2/s, which gives the length constant L ∼0.8 μm (54) (Table 1). These predicted L values for CaMKII and Ca2+ are consistent with spine-specific activation of these molecules (Fig. 1).
Table 1.
The Diffusion Properties of Signaling Molecules and Their Spreading
| Molecule | Properties | Effective Diffusion Coefficient of Active Proteins Deff (μm2/s) | Inactivation Time Constant τ (s) | Calculated Length Constant L = (DeffL)1/2 (μm) | Measured Length Constant L (μm) | Restricted to Spines |
|---|---|---|---|---|---|---|
| Ca2+ | ion, binds to buffer | 1–65a | 0.02–0.1b | 0.1–2.5 | 1.2–1.9b | yesa |
| CaMKII | binds to PSD/actin | 0.1c | 6d | 0.8 | ∼1e | yese |
| Cdc42 | membrane | 0.5f | ∼200g,h | 10 | ∼1–2g,h | yYesg,h |
| Ras | membrane | 0.5f | ∼300i | 12 | ∼10i | noi |
| RhoA | membrane | 0.5f | ∼200g,h | 10 | ∼5h | noh |
| Rac1 | membrane | 0.5f | ∼1200g | ∼24 | ∼15j | noj |
| PKA | binds to AKAP/cytosolic/membrane | 0.5–10j | ∼200k | 10–45 | ∼15k | nok |
| ERK | cytosolic | 10l | ∼1200m | ∼110 | >50–200m | nom |
Another class of major signaling proteins, small GTPase proteins, are typically membrane tethered and diffuse at the rate of ∼0.5 μm2/s (50, 55, 56). Their decay rate is about a few minutes for Ras, RhoA, and Cdc42, which gives the predicted length constant of ∼10 μm (Table 1). This value is consistent with previous measurements of small GTPase activity using FRET sensors, with one exception: Cdc42. Although Cdc42 is as diffusible as other molecules, its activity was found to be restricted to stimulated spines (27) (Fig. 1). Rac1 activation lasts a little longer (∼20 min) and its activity spreads more than ∼15 μm, as predicted (Fig. 1; Table 1). The mechanisms underlying the compartmentalization of Cdc42 are not clear. One possible explanation is that there is a protein that continues to activate Cdc42 in spines, whereas Cdc42 is inactivated immediately after diffusing out of the spine (50). Although Cdc42 is critical for spine specificity of spine structural plasticity, the spreading of Ras, RhoA, and Rac1 are important for facilitating spine plasticity in surrounding spines, causing the cooperativity of synaptic plasticity among adjacent spines (28, 48). This may be the basis of clustered synaptic plasticity in vivo (57, 58, 59).
Cytosolic proteins typically diffuse ∼10 times faster than membrane-targeted proteins (∼10 μm2/s) (60, 61). The spreading of cytosolic protein kinases such as ERK has been measured to be more than several tens of micrometers (49). Since the activations of ERK decays over ∼20 min (49), their spreading can be calculated to be ∼100 μm, consistent with a recent study showing extremely wide spreading of ERK activation (Fig. 1; Table 1). This high degree of spreading is perhaps important for ERK in playing a role in synapse-to-nuclear signaling (53).
The activation profile of PKA has also been measured, and was found to be more than tens of micrometers (Fig. 1) (49). Considering the decay time of PKA activation (∼3 min), this value is consistent with that of cytosolic protein (Table 1) (49). However, recent studies suggest that the regulation of PKA diffusion is complicated: PKA catalytic domain (PKA-C) is bound to microtubules through its regulatory domain (PKA-R) and AKAP. When PKA is activated, PKA-C is released from PKA-R, and some fraction of PKA-C binds to the membrane via N-terminus myristoylation (62). The spreading of this fraction is calculated to be ∼5 μm. However, it should be noted that spatial spreading of PKA is also affected by the spreading of its upstream messenger cyclic AMP, which has not yet been measured.
Similar to the description of the spread of signaling activity, the spread of newly synthesized proteins would be determined by the balance between molecular diffusion and degradation. Recent studies showed insights into the spatial aspect of new protein synthesis. For example, Hayashi-Takagi et al. (63) produced an active synapse probe (AS-paRac), which consists of mRNA dendrite targeting elements of Arc, a PSD targeting domain of PSD-95, and photoactivatable Rac1 (paRac) for manipulating the actin cytoskeleton for 2–3 days. They found that this construct is accumulated in active spines in a spine-specific manner. More surprisingly, when they illuminated neurons expressing this construct, they could selectively shrink recently enlarged spines. This reversed the animal’s learning, suggesting that spine structural plasticity is important for learning and memory. Although the exact mechanism that causes the spine-specific accumulation remains elusive, one possible scenario is that the construct is synthesized in the stimulated spine and then the expressed protein is stationed there for a long time. Perhaps the slow diffusion of PSD95 causes this spine-specific localization of the synthesis product (64). In any case, the data using AS-paRac appear to support the hypothesis that protein synthesis can occur specific to stimulated spines.
On the other hand, there also exists evidence suggesting that the synthesized proteins spread over a long distance. For example, a recent study suggested that newly synthesized actin spreads over ∼10–20 μm from the stimulated spines, perhaps due to higher mobility of actin compared to PSD95 (47). Furthermore, it has been demonstrated that the induction of protein synthesis-dependent LTP (late-phase LTP) in one spine facilitates late-phase LTP in spines in the same dendrite over several tens of micrometers in a protein synthesis-dependent manner (42). Thus, these studies suggest that dendritic spines can share the synthesis product over a long distance. The differences in the degree of spreading perhaps reflect the differences in the mobility of the synthesized protein and their degradation rate (44).
Next steps
Our understanding of biochemical signal transduction underlying synaptic plasticity were improved dramatically by new techniques that allowed us to monitor signaling activity at single dendritic spines. However, revealing the nature of biochemical networks would require a larger coverage of the network elements. We currently have the activity profiles of ∼10 proteins. By extending this to ∼100 proteins, we expect to develop a much better understanding about the operational principles of such networks.
One problem with the current approaches is that most studies have used overexpressed sensors. Since a signaling reporter often includes the target signaling protein or its substrate, it likely causes profound effects on the cellular process and may change the cellular properties. Furthermore, it may affect the measured spatiotemporal dynamics. For example, our typical small GTPase sensor is made of the target small GTPase protein fused to a donor and its effector fused to an acceptor (65, 66). The overexpression of a small GTPase protein can saturate its activator (Guanosine nucleotide exchange factor) and inactivator (GTPase accelerating factor), and the overexpression of effectors would compete with GTPase accelerating factor (65, 66). Both of these would slow down the kinetics of the small GTPase activity. Recent advances in techniques for labeling endogenous proteins in specific cells including in vivo genome editing (67, 68, 69, 70), conditional knock-in (71), and intrabody (72) will open the possibility of imaging the activity of endogenous proteins. Thus, we expect to be able to image interactions and conformation changes of endogenous proteins using this approach.
Another obvious next step would be to measure signaling activity in neurons in vivo. Most signaling studies have been performed with well-defined stimulation in vitro. Extending the study to a more natural stimulation in vivo would be necessary to reveal biochemical states of neurons during learning and memory. However, under these situations, we no longer have control over which spines are stimulated and when. Thus, such study would require simultaneous imaging of several signaling proteins. This will allow us to correlate activities of different signaling elements both in space and time and thus analyze the connection in the network. Currently, it is possible to image two to three proteins at once (21, 73, 74, 75, 76), but it is still challenging to achieve sufficient sensitivity in all channels for single spine imaging in vivo. Development of new bright fluorophores with different spectral properties would be important for this application.
Finally, it is necessary to produce a mathematical framework to model biochemical processes with hundreds of nodes in dendrites. Although there have been attempts to produce graph theoretic models (1, 2), these models cannot simulate the dynamics of signaling and, in general, are simplified such that all the molecular reactions occur within a single compartment. However, we have already learned that spatiotemporal dynamics are important for controlling signaling at different subcellular components. Partial differential equation-based models have been used to simulate the precise spatiotemporal dynamics of signaling proteins in spines and dendrites (77). However, this approach appears to be computationally intensive even for simulating a few molecules, and implementing interaction networks with many components may be complicated. A better solution might be to build models with intermediate complexity for both aspects of signaling networks, for example a dynamic model with several spatial compartments (78).
Acknowledgments
I thank T. Yasuda for critical reading of the manuscript and the members of the Yasuda lab for discussion.
Editor: Brian Salzberg.
References
- 1.Azeloglu E.U., Iyengar R. Signaling networks: information flow, computation, and decision making. Cold Spring Harb. Perspect. Biol. 2015;7:a005934. doi: 10.1101/cshperspect.a005934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bromberg K.D., Ma’ayan A., Iyengar R. Design logic of a cannabinoid receptor signaling network that triggers neurite outgrowth. Science. 2008;320:903–909. doi: 10.1126/science.1152662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng K., Bard L., Rusakov D.A. Time-resolved imaging reveals heterogeneous landscapes of nanomolar Ca(2+) in neurons and astroglia. Neuron. 2015;88:277–288. doi: 10.1016/j.neuron.2015.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neves S.R., Tsokas P., Iyengar R. Cell shape and negative links in regulatory motifs together control spatial information flow in signaling networks. Cell. 2008;133:666–680. doi: 10.1016/j.cell.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris K.M., Stevens J.K. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J. Neurosci. 1989;9:2982–2997. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishiyama J., Yasuda R. Biochemical computation for spine structural plasticity. Neuron. 2015;87:63–75. doi: 10.1016/j.neuron.2015.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuzaki M., Ellis-Davies G.C., Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat. Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang J.Y., Parra-Bueno P., Yasuda R. CaMKII autophosphorylation is necessary for optimal integration of Ca2+ signals during LTP induction, but not maintenance. Neuron. 2017;94:800–808.e804. doi: 10.1016/j.neuron.2017.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S.J., Escobedo-Lozoya Y., Yasuda R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458:299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuzaki M., Honkura N., Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosch M., Castro J., Hayashi Y. Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron. 2014;82:444–459. doi: 10.1016/j.neuron.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer D., Bonhoeffer T., Scheuss V. Balance and stability of synaptic structures during synaptic plasticity. Neuron. 2014;82:430–443. doi: 10.1016/j.neuron.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 13.Hayama T., Noguchi J., Kasai H. GABA promotes the competitive selection of dendritic spines by controlling local Ca2+ signaling. Nat. Neurosci. 2013;16:1409–1416. doi: 10.1038/nn.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh W.C., Hill T.C., Zito K. Synapse-specific and size-dependent mechanisms of spine structural plasticity accompanying synaptic weakening. Proc. Natl. Acad. Sci. USA. 2013;110:E305–E312. doi: 10.1073/pnas.1214705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Q., Homma K.J., Poo M.M. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Wiegert J.S., Oertner T.G. Long-term depression triggers the selective elimination of weakly integrated synapses. Proc. Natl. Acad. Sci. USA. 2013;110:E4510–E4519. doi: 10.1073/pnas.1315926110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noguchi J., Matsuzaki M., Kasai H. Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron. 2005;46:609–622. doi: 10.1016/j.neuron.2005.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabatini B.L., Oertner T.G., Svoboda K. The life cycle of Ca(2+) ions in dendritic spines. Neuron. 2002;33:439–452. doi: 10.1016/s0896-6273(02)00573-1. [DOI] [PubMed] [Google Scholar]
- 19.Wayman G.A., Lee Y.S., Soderling T.R. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron. 2008;59:914–931. doi: 10.1016/j.neuron.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lisman J., Yasuda R., Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 2012;13:169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujii H., Inoue M., Bito H. Nonlinear decoding and asymmetric representation of neuronal input information by CaMKIIα and calcineurin. Cell Reports. 2013;3:978–987. doi: 10.1016/j.celrep.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 22.Takao K., Okamoto K., Hayashi Y. Visualization of synaptic Ca2+ /calmodulin-dependent protein kinase II activity in living neurons. J. Neurosci. 2005;25:3107–3112. doi: 10.1523/JNEUROSCI.0085-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulkey R.M., Endo S., Malenka R.C. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- 24.Oh W.C., Parajuli L.K., Zito K. Heterosynaptic structural plasticity on local dendritic segments of hippocampal CA1 neurons. Cell Reports. 2015;10:162–169. doi: 10.1016/j.celrep.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo L. Rho GTPases in neuronal morphogenesis. Nat. Rev. Neurosci. 2000;1:173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- 26.Hotulainen P., Hoogenraad C.C. Actin in dendritic spines: connecting dynamics to function. J. Cell Biol. 2010;189:619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murakoshi H., Wang H., Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedrick N.G., Harward S.C., Yasuda R. Rho GTPase complementation underlies BDNF-dependent homo- and heterosynaptic plasticity. Nature. 2016;538:104–108. doi: 10.1038/nature19784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herring B.E., Nicoll R.A. Kalirin and Trio proteins serve critical roles in excitatory synaptic transmission and LTP. Proc. Natl. Acad. Sci. USA. 2016;113:2264–2269. doi: 10.1073/pnas.1600179113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim I.H., Wang H., Yasuda R. Loss of Cdc42 leads to defects in synaptic plasticity and remote memory recall. eLife. 2014;3:e02839. doi: 10.7554/eLife.02839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haditsch U., Leone D.P., Palmer T.D. A central role for the small GTPase Rac1 in hippocampal plasticity and spatial learning and memory. Mol. Cell. Neurosci. 2009;41:409–419. doi: 10.1016/j.mcn.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller M.B., Yan Y., Mains R.E. Neuronal Rho GEFs in synaptic physiology and behavior. Neuroscientist. 2013;19:255–273. doi: 10.1177/1073858413475486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edelmann E., Cepeda-Prado E., Leßmann V. Theta burst firing recruits BDNF release and signaling in postsynaptic CA1 neurons in spike-timing-dependent LTP. Neuron. 2015;86:1041–1054. doi: 10.1016/j.neuron.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Harward S.C., Hedrick N.G., McNamara J.O. Autocrine BDNF-TrkB signalling within a single dendritic spine. Nature. 2016;538:99–103. doi: 10.1038/nature19766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka J., Horiike Y., Kasai H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319:1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Padamsey Z., McGuinness L., Emptage N.J. Activity-dependent exocytosis of lysosomes regulates the structural plasticity of dendritic spines. Neuron. 2017;93:132–146. doi: 10.1016/j.neuron.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jurado S., Goswami D., Malenka R.C. LTP requires a unique postsynaptic SNARE fusion machinery. Neuron. 2013;77:542–558. doi: 10.1016/j.neuron.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu D., Bacaj T., Südhof T.C. Postsynaptic synaptotagmins mediate AMPA receptor exocytosis during LTP. Nature. 2017;544:316–321. doi: 10.1038/nature21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malinow R., Malenka R.C. AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 40.Makino H., Malinow R. AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron. 2009;64:381–390. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patterson M.A., Szatmari E.M., Yasuda R. AMPA receptors are exocytosed in stimulated spines and adjacent dendrites in a Ras-ERK-dependent manner during long-term potentiation. Proc. Natl. Acad. Sci. USA. 2010;107:15951–15956. doi: 10.1073/pnas.0913875107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Govindarajan A., Israely I., Tonegawa S. The dendritic branch is the preferred integrative unit for protein synthesis-dependent LTP. Neuron. 2011;69:132–146. doi: 10.1016/j.neuron.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leal G., Comprido D., Duarte C.B. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology. 2014;76:639–656. doi: 10.1016/j.neuropharm.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Rangaraju V., Tom Dieck S., Schuman E.M. Local translation in neuronal compartments: how local is local? EMBO Rep. 2017;18:693–711. doi: 10.15252/embr.201744045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panja D., Bramham C.R. BDNF mechanisms in late LTP formation: a synthesis and breakdown. Neuropharmacology. 2014;76:664–676. doi: 10.1016/j.neuropharm.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 46.Ostroff L.E., Fiala J.C., Harris K.M. Polyribosomes redistribute from dendritic shafts into spines with enlarged synapses during LTP in developing rat hippocampal slices. Neuron. 2002;35:535–545. doi: 10.1016/s0896-6273(02)00785-7. [DOI] [PubMed] [Google Scholar]
- 47.Yoon Y.J., Wu B., Singer R.H. Glutamate-induced RNA localization and translation in neurons. Proc. Natl. Acad. Sci. USA. 2016;113:E6877–E6886. doi: 10.1073/pnas.1614267113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harvey C.D., Yasuda R., Svoboda K. The spread of Ras activity triggered by activation of a single dendritic spine. Science. 2008;321:136–140. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang S., Yasuda R. Imaging ERK and PKA activation in single dendritic spines during structural plasticity. Neuron. 2017;93:1315–1324.e1313. doi: 10.1016/j.neuron.2017.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yasuda R., Murakoshi H. The mechanisms underlying the spatial spreading of signaling activity. Curr. Opin. Neurobiol. 2011;21:313–321. doi: 10.1016/j.conb.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santamaria F., Wils S., Augustine G.J. The diffusional properties of dendrites depend on the density of dendritic spines. Eur. J. Neurosci. 2011;34:561–568. doi: 10.1111/j.1460-9568.2011.07785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka K., Augustine G.J. A positive feedback signal transduction loop determines timing of cerebellar long-term depression. Neuron. 2008;59:608–620. doi: 10.1016/j.neuron.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhai S., Ark E.D., Yasuda R. Long-distance integration of nuclear ERK signaling triggered by activation of a few dendritic spines. Science. 2013;342:1107–1111. doi: 10.1126/science.1245622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee S.J., Yasuda R. Spatiotemporal regulation of signaling in and out of dendritic spines: CaMKII and Ras. Open Neurosci. J. 2009;3:117–127. doi: 10.2174/1874082000903020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lommerse P.H., Blab G.A., Schmidt T. Single-molecule imaging of the H-ras membrane-anchor reveals domains in the cytoplasmic leaflet of the cell membrane. Biophys. J. 2004;86:609–616. doi: 10.1016/S0006-3495(04)74139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murakoshi H., Iino R., Kusumi A. Single-molecule imaging analysis of Ras activation in living cells. Proc. Natl. Acad. Sci. USA. 2004;101:7317–7322. doi: 10.1073/pnas.0401354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen J.L., Villa K.L., Nedivi E. Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron. 2012;74:361–373. doi: 10.1016/j.neuron.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fu M., Yu X., Zuo Y. Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature. 2012;483:92–95. doi: 10.1038/nature10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lai C.S., Franke T.F., Gan W.B. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2012;483:87–91. doi: 10.1038/nature10792. [DOI] [PubMed] [Google Scholar]
- 60.Bloodgood B.L., Sabatini B.L. Neuronal activity regulates diffusion across the neck of dendritic spines. Science. 2005;310:866–869. doi: 10.1126/science.1114816. [DOI] [PubMed] [Google Scholar]
- 61.Lidke D.S., Huang F., Lenormand P. ERK nuclear translocation is dimerization-independent but controlled by the rate of phosphorylation. J. Biol. Chem. 2010;285:3092–3102. doi: 10.1074/jbc.M109.064972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tillo S.E., Xiong W.H., Zhong H. Liberated PKA catalytic subunits associate with the membrane via myristoylation to preferentially phosphorylate membrane substrates. Cell Reports. 2017;19:617–629. doi: 10.1016/j.celrep.2017.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hayashi-Takagi A., Yagishita S., Kasai H. Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature. 2015;525:333–338. doi: 10.1038/nature15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gray, N. W., R. M. Weimer, …, K. Syoboda. Rapid distribution of synaptic PSD-95 in the neocortex in vivo. PLoS Biol. 4:e370. [DOI] [PMC free article] [PubMed]
- 65.Oliveira A.F., Yasuda R. Imaging the activity of Ras superfamily GTPase proteins in small subcellular compartments in neurons. Methods Mol. Biol. 2014;1071:109–128. doi: 10.1007/978-1-62703-622-1_9. [DOI] [PubMed] [Google Scholar]
- 66.Yasuda R., Harvey C.D., Svoboda K. Supersensitive Ras activation in dendrites and spines revealed by two-photon fluorescence lifetime imaging. Nat. Neurosci. 2006;9:283–291. doi: 10.1038/nn1635. [DOI] [PubMed] [Google Scholar]
- 67.Mikuni T., Nishiyama J., Yasuda R. High-throughput, high-resolution mapping of protein localization in mammalian brain by in vivo genome editing. Cell. 2016;165:1803–1817. doi: 10.1016/j.cell.2016.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suzuki K., Tsunekawa Y., Belmonte J.C. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature. 2016;540:144–149. doi: 10.1038/nature20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsunekawa Y., Terhune R.K., Matsuzaki F. Developing a de novo targeted knock-in method based on in utero electroporation into the mammalian brain. Development. 2016;143:3216–3222. doi: 10.1242/dev.136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Uemura T., Mori T., Tabuchi K. Fluorescent protein tagging of endogenous protein in brain neurons using CRISPR/Cas9-mediated knock-in and in utero electroporation techniques. Sci. Rep. 2016;6:35861. doi: 10.1038/srep35861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fortin D.A., Tillo S.E., Zhong H. Live imaging of endogenous PSD-95 using ENABLED: a conditional strategy to fluorescently label endogenous proteins. J. Neurosci. 2014;34:16698–16712. doi: 10.1523/JNEUROSCI.3888-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gross G.G., Junge J.A., Arnold D.B. Recombinant probes for visualizing endogenous synaptic proteins in living neurons. Neuron. 2013;78:971–985. doi: 10.1016/j.neuron.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Demeautis C., Sipieter F., Tramier M. Multiplexing PKA and ERK1&2 kinases FRET biosensors in living cells using single excitation wavelength dual colour FLIM. Sci. Rep. 2017;7:41026. doi: 10.1038/srep41026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim S.Y., Arai Y., Nagai T. Simultaneous imaging of multiple cellular events using high-accuracy fluorescence polarization microscopy. Microscopy (Oxf.) 2017;66:110–119. doi: 10.1093/jmicro/dfw110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laviv T., Kim B.B., Yasuda R. Simultaneous dual-color fluorescence lifetime imaging with novel red-shifted fluorescent proteins. Nat. Methods. 2016;13:989–992. doi: 10.1038/nmeth.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Woehler A. Simultaneous quantitative live cell imaging of multiple FRET-based biosensors. PLoS One. 2013;8:e61096. doi: 10.1371/journal.pone.0061096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramirez S.A., Raghavachari S., Lew D.J. Dendritic spine geometry can localize GTPase signaling in neurons. Mol. Biol. Cell. 2015;26:4171–4181. doi: 10.1091/mbc.E15-06-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown S.A., Moraru I.I., Loew L.M. Virtual NEURON: a strategy for merged biochemical and electrophysiological modeling. J. Comput. Neurosci. 2011;31:385–400. doi: 10.1007/s10827-011-0317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oliveira A.F., Yasuda R. Neurofibromin is the major ras inactivator in dendritic spines. J. Neurosci. 2014;34:776–783. doi: 10.1523/JNEUROSCI.3096-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]