ABSTRACT
We have identified the first case of an fks1 hot spot 1 point mutation causing echinocandin resistance in a clinical Aspergillus fumigatus isolate recovered from a chronic pulmonary aspergillosis patient with an aspergilloma who first failed azole and polyene therapy and subsequently failed micafungin treatment.
KEYWORDS: Aspergillus fumigatus, chronic pulmonary aspergillosis, echinocandin resistance, fks1 mutation, micafungin
TEXT
Invasive aspergillosis (IA) and chronic pulmonary aspergillosis (CPA) are both life-threatening mycoses mainly caused by the fungal pathogen Aspergillus fumigatus. Triazole antifungal drugs represent first-line therapy for all forms of aspergillosis. Voriconazole is used as the primary azole for treatment of IA, while both itraconazole and voriconazole are used to treat CPA; posaconazole is more often used for prophylaxis (1, 2). Even though acquired resistance during therapy is rare, epidemiological studies have shown that Aspergillus azole resistance has increased over the past decade in patients with prolonged exposure to azoles, notably those with CPA (3). Mutations in the cyp51A gene leading to amino acid substitutions in the target enzyme lanosterol 14α-demethylase are the major mechanism of azole resistance (1, 4, 5), yet non-cyp51A-mediated resistance has been recently reported (6).
Micafungin (MCF) is a member of the echinocandin class of antifungal agents that targets the fungal cell wall by the inhibition of the β-(1,3)-glucan synthase (GS), an enzyme unique to fungi and responsible for the synthesis of the major cell wall component, β-(1,3)-d-glucan. MCF has demonstrated in vitro and in vivo activity against A. fumigatus, as well as synergistic activity when used in combination therapy with azoles (isavuconazole, itraconazole, or voriconazole) or with amphotericin B (7, 8). The first-in-class echinocandin, caspofungin, was initially approved by the FDA to treat patients with IA refractory to conventional therapy. Subsequently, micafungin has also been used to treat patients with IA as a second-line drug. Echinocandin resistance in Candida spp. has been linked to mutations in the FKS genes, which encode the β-(1,3)-glucan synthase enzymes (9–11). In A. fumigatus, an engineered point mutation in the hot spot 1 region of the fks1 gene was also sufficient to confer resistance to echinocandin drugs (12). Yet, to date, no echinocandin-resistant A. fumigatus isolates harboring a characteristic fks mutation have been recovered from patients after exposure to an echinocandin. In this study, we report the first case of an fks1 hot spot 1 point mutation causing echinocandin resistance in a clinical A. fumigatus isolate recovered from a chronic pulmonary aspergillosis patient who initially failed azole and polyene therapy and subsequently failed echinocandin therapy.
A 66-year-old lifelong-nonsmoking female complained of weight loss, fatigue, and severe breathlessness. The patient had severe kyphoscoliosis as a child, which was treated with the insertion of spinal rods in early adulthood. She had suffered recurrent chest infections for many years. She first presented in 2001 with an irritating cough, and several treatments with antibiotics failed to alleviate it. After 2 years, the cough worsened. The patient then coughed up large amounts of blood (hemoptysis), requiring intensive care admission; this was treated with embolization and oral tranexamic acid. She continued to cough and produced green sputum and lost weight. Her Aspergillus precipitin (IgG) titer was high, and a computed tomography (CT) scan demonstrated chronic cavitary pulmonary aspergillosis with a large fungal ball (aspergilloma). She started itraconazole therapy in 2005 (200 mg twice daily [BID]) but failed to respond despite satisfactory blood drug levels, and she was switched to voriconazole in 2006 (150 mg BID). Considerable improvement was seen initially, and the patient gained weight. Voriconazole therapy continued for 2 years. However, the Aspergillus precipitin titer remained high, and the cough continued. Further tests showed that trough plasma levels of voriconazole were above 0.5 mg/liter; however, the Aspergillus fumigatus isolates recovered were resistant to itraconazole, voriconazole, and posaconazole. She received intravenous (i.v.) amphotericin B (120 mg daily) for 3 weeks without any impairment of renal function. She had further intermittent courses of therapy with amphotericin B at the same daily dose without improvement over the following year. In June 2009, the patient started i.v. micafungin at 150 mg 6 times weekly with oral terbinafine (250 mg BID). She improved substantially and continued to take micafungin along with terbinafine to minimize the risk of resistance. She remained on this combination until she developed more hemoptysis, and therapy was discontinued in January 2012. She remained off therapy for over 2 years. Hemoptysis recurred, and she was trialed on isavuconazole in 2015/2016 (200 mg three times daily for 2 days and then 200 mg a day). A timeline of the antifungal therapy of the patient is shown in Fig. 1A.
FIG 1.
(A) Timeline for patient antifungal therapy. Collection of the Aspergillus species isolates for the current microbiological study is shown (triangles). (B) Isolates recovered from the patient grown for 2 days at 37°C on a PDA plate. (C) A. fumigatus clinical isolates 24053A and 24053B grown for 4 days at 37°C on PDA plates. Isolate 24053B grows at a lower rate and sporulates very poorly compared to the rest of isolates collected from the patient.
Once micafungin therapy was initiated, 12 consecutive Aspergillus species isolates (11 A. fumigatus and 1 A. flavus) were recovered from sputum cultures (Fig. 1B) and were submitted for antifungal susceptibility testing in accordance with the guidelines described in CLSI document M38-A2 (13). The drugs used were isavuconazole (ISA; Astellas Pharma USA, Inc., Northbrook, IL), itraconazole (ITR; Sigma-Aldrich, St. Louis, MO), voriconazole (VRC; Pfizer, Inc., New York, NY), posaconazole (POS; Merck Sharp & Dohme Corp., Rahway, NJ), amphotericin B (AMB; Sigma-Aldrich), caspofungin (CSF; Merck Sharp & Dohme Corp.), micafungin (MCF; Astellas Pharma USA, Inc.), and terbinafine (TERB) (Novartis Pharmaceuticals, East Hanover, NJ). Multilocus sequencing typing (MLST) of the 11 A. fumigatus strains collected from the patient was conducted (14). The promoter and open reading frame of the cyp51A (Afu6g12400) and the fks1 (Afu4g06890) genes were sequenced to identify mutations that account for resistance (Table 1).
TABLE 1.
Oligonucleotides used for fks1 and cyp51A PCR and sequencing in A. fumigatus
| No. | Oligonucleotide name | Sequence 5′→3′ | Purpose |
|---|---|---|---|
| 1 | AfFKS1 −751F | CCTGAGTTGGTGGTCAAT | Affks1 PCR amplification |
| 2 | AfFKS1 6378R | GACTGGCGAAACACGTTG | |
| 3 | AfFKS1 −211F | CTGCGACTCGAGATTCAG | |
| 4 | AfFKS1 697F | GCATGCGCAACATGTATG | |
| 5 | AfFKS1 1562F | CGCACAATCGCTTTACAC | |
| 6 | AfFKS1 1947F | CGTCAGTATGTGGCTAGC | Affks1 sequencing |
| 7 | AfFKS1 2405F | GATTTCTCAAGTTTGGAATGC | |
| 8 | AfFKS1 3043F | CGATCAAGCTCCTGTACC | |
| 9 | AfFKS1 3401F | GTCTGACAACCAGAATCAC | |
| 10 | AfFKS1 4269F | GTCCAGGAACTGACAGAG | |
| 11 | AfFKS1 5117F | CGAAGTCATGTTCTTCCTTG | |
| 12 | AfFKS1 5931R | CTTCGAGGCGCTGGATAC | |
| 13 | AfCyp51A −991F | CGTCGATCTGTGTGACAC | Afcyp51A PCR amplification |
| 14 | AfCyp51A 1993R | CTAGAAGGAGCAGGACTG | |
| 15 | AfCyp51A −819F | CATGCTGGGAGGAATCTC | |
| 16 | AfCyp51A −147F | GCTGGTCTCTCATTCGTC | Afcyp51A sequencing |
| 17 | AfCyp51A 502F | AGAGTCTCATGTGCCACT | |
| 18 | AfCyp51A 1151F | CACTCCTCTATTCACTCTATC |
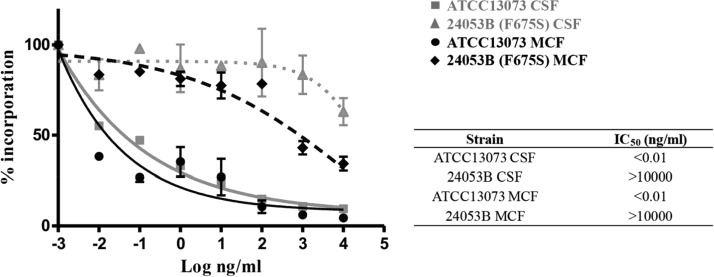
Twelve isolates were collected from the patient after MCF treatment; 11 of them were classified as A. fumigatus, and 1 isolate was classified as A. flavus after internal transcribed spacer PCR (ITS-PCR) identification. All A. fumigatus isolates but one (24053B) grew well on potato dextrose agar (PDA) plates, showing the typical dark-green colonies (Fig. 1B); in contrast, 24053B presented a diminished growth rate and a reduction in sporulation, hence, its white colony color (Fig. 1C). To assess the genetic diversity and the potential relatedness of the A. fumigatus isolates recovered from the patient, MLST was carried out, since a previous study in patients with aspergilloma reported the extreme genetic diversity in isolates recovered from fungal balls (15). MLST revealed only two different sequence types (STs), ST9 and ST12 (Table 2). According to the CLSI epidemiological cutoff values (ECVs) (16), all isolates except one (45755) were classified as wild type (WT) for all the triazole drugs tested. Sequencing of the cyp51A gene revealed five mutations unlinked to azole resistance in all A. fumigatus isolates (17) (Table 2). Since no ECVs have been established for terbinafine or micafungin, three WT strains (ATCC 13073, Af293, and R21 [18]) were included in the study for comparison reasons. The clinical isolates showed an MIC range of 0.5 to 1 μg/ml for terbinafine compared to 0.5 to 2 μg/ml for the control strains (Table 2); hence, we considered the clinical isolates to be WT for this antifungal drug. Regarding echinocandins, isolate 24053B showed a 16.6- to 33.3- and 66.6-fold increase in minimum effective concentrations (MECs) for CSF and MCF, respectively, compared to the control strains. The other 11 isolates recovered from the patient showed a WT phenotype to all echinocandins assayed (Table 2). The reduced in vitro susceptibilities to echinocandin drugs were confirmed in GS enzyme assays. Product-entrapped β-(1,3)-glucan synthase complexes were extracted from the prototype WT strain ATCC 13073 and the 24053B clinical isolate, as previously described (10, 11), and the echinocandin inhibition parameter 50% inhibitory concentration (IC50) was determined. The fks1-encoded enzyme extracted from the 24053B clinical strain showed a multilog increase in IC50s compared to the WT (>106-fold change for both CSF and MCF) (Fig. 2), indicating that it was nearly insensitive to drug. DNA sequence analysis of the published A. fumigatus Af293 fks1 gene revealed a point mutation at nucleotide position 2072 (T to C) in the 24053B isolate. This nucleotide change conferred a Phe-to-Ser amino acid substitution in codon position 675, the first codon of the highly conserved hot spot 1 region of fks1 (nucleotides [nt] 2071 to 3003→amino acids [aa] 675 to 684) (Table 3). The equivalent mutation in this hot spot 1 region is prominent and well known to confer echinocandin resistance in Candida albicans (F641S) and other Candida spp., and it has been linked with echinocandin clinical failure (9–11). It is noteworthy that the 24053B isolate was not recovered from any other samples collected from the patient, even though micafungin therapy failed. As has been described previously, multiple strains are present in aspergillomas, and the strain that is grown from sputum often does not reflect the full spectrum of strains present. It is likely that the patient still harbors MCF-resistant strains. The addition of terbinafine did not prevent the emergence of resistance to MCF, although it is possible it delayed its emergence.
TABLE 2.
MIC/MEC distributions of the antifungal drugs tested in the study for the Aspergillus species clinical isolates
| No. or WTa | Lab no. | Date received (day/mo/yr) | Species | MIC/MEC (mg/liter) |
Cyp51A changesb | Fks1 changesa | ST | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ISA | ITR | POS | VOR | CSF | MCF | AMB | TERB | |||||||
| 1 | 22432 | 2/9/2010 | A. fumigatus | 0.25 | 0.25 | 0.12 | 0.25 | 0.12 | 0.03 | 2 | 1 | Y46F, V172 M, T248N, E255D, K427E | ND | 9 |
| 2 | 23525 | 5/4/2010 | A. fumigatus | 0.25 | 0.25 | 0.12 | 0.12 | 0.12 | 0.03 | 2 | 0.5 | Y46F, V172 M, T248N, E255D, K427E | ND | 9 |
| 3 | 24053A | 6/15/2010 | A. fumigatus | 0.25 | 0.25 | 0.25 | 0.25 | 0.12 | 0.03 | 2 | 1 | Y46F, V172 M, T248N, E255D, K427E | S53G | 12 |
| 4 | 24053B | 6/15/2010 | A. fumigatus | 0.5 | 0.25 | 0.25 | 1 | 2 | 2 | 2 | 1 | Y46F, V172 M, T248N, E255D, K427E | S53G and F675S | 9 |
| 5 | 24555 | 7/28/2010 | A. fumigatus | 0.25 | 0.25 | 0.25 | 0.25 | 0.12 | 0.03 | 2 | 0.5 | Y46F, V172 M, T248N, E255D, K427E | ND | 9 |
| 6 | 29576A | 7/27/2011 | A. flavus | 0.25 | 0.5 | 0.25 | 0.25 | 0.12 | 0.03 | 2 | 0.06 | WTc | ND | ND |
| 7 | 29576B | 7/27/2011 | A. fumigatus | 0.25 | 0.25 | 0.25 | 0.25 | 0.12 | 0.03 | 2 | 0.5 | Y46F, V172 M, T248N, E255D, K427E | ND | 9 |
| 8 | 30906 | 10/25/2011 | A. fumigatus | 0.25 | 0.5 | 0.25 | 0.12 | 0.12 | 0.03 | 2 | 1 | Y46F, V172 M, T248N, E255D, K427E | ND | 12 |
| 9 | 33460 | 4/10/2012 | A. fumigatus | 0.25 | 0.5 | 0.25 | 0.12 | 0.12 | 0.03 | 2 | 1 | Y46F, V172 M, T248N, E255D, K427E | ND | 9 |
| 10 | 45755 | 12/30/2013 | A. fumigatus | 2 | 1 | 1 | 0.5 | 0.12 | 0.03 | 2 | 1 | Y46F, V172 M, T248N, E255D, K427E | ND | 12 |
| 11 | 53619 | 1/28/2015 | A. fumigatus | 0.25 | 0.5 | 0.25 | 0.25 | 0.12 | 0.03 | 2 | 0.5 | Y46F, V172 M, T248N, E255D, K427E | ND | 9 |
| 12 | 62194 | 12/24/2015 | A. fumigatus | 0.25 | 0.5 | 0.25 | 0.12 | 0.12 | 0.03 | 2 | 0.5 | Y46F, V172 M, T248N, E255D, K427E | ND | 9 |
| ATCC 13073 | A. fumigatus | 0.25 | 0.25 | 0.25 | 0.25 | 0.12 | 0.03 | 2 | 1 | ND | S53G | ND | ||
| R21 | A. fumigatus | 0.25 | 0.25 | 0.25 | 0.25 | 0.12 | 0.03 | 2 | 2 | ND | S53G | ND | ||
| Af293 | A. fumigatus | 0.12 | 0.12 | 0.12 | 0.12 | 0.06 | 0.03 | 1 | 0.5 | ND | WT | ND | ||
The three WT strains (ATCC 13073, R21, and Af293) were included for comparison purposes.
Reference strain used was Af293. ND, not determined.
Reference strain used was A. flavus NRRL3357.
FIG 2.
Echinocandin kinetic inhibition profiles for wild-type ATCC 13073 and clinical isolate 24053B. Product-entrapped β-(1,3)-glucan synthase complexes were assessed by incorporation of [3H]-UDP-glucose into radiolabeled product and evaluated using a sigmoidal-response (variable-slope) curve. Echinocandin inhibition kinetics yielding 50% inhibitory concentrations (IC50s) are expressed in nanograms per milliliter.
TABLE 3.
fks1 hot spot 1 sequencing of the A. fumigatus 24053B isolate
| Strain |
fks1 HS1a |
|
|---|---|---|
| nt | aa | |
| Af293 (reference) | TTCCTGACCCTGTCTTTCAAGGATCCGATCCG | FLTLSFKDPI |
| ATCC 13073 | TTCCTGACCCTGTCTTTCAAGGATCCGATCCG | FLTLSFKDPI |
| 24053B | TCCCTGACCCTGTCTTTCAAGGATCCGATCCG | SLTLSFKDPI |
HS1, hot spot 1. Nucleotide and amino acid changes have been highlighted in bold letters.
In conclusion, a resistance-associated point mutation in the well-conserved hot spot 1 region of fks1 conferring an F675S amino acid substitution was found in A. fumigatus isolate 24053B recovered from a patient on micafungin therapy for CPA. The mutant strain yielded a β-(1,3)-glucan synthase enzyme with highly reduced (>5 to 6 log orders) sensitivity to echinocandin drugs, resulting in elevated MECs and echinocandin clinical failure. To date, this is the first reported case of echinocandin resistance due to a characteristic point mutation in the fks1 gene in an A. fumigatus clinical isolate.
ACKNOWLEDGMENT
This work was supported by a grant from Astellas Pharma, Inc., to D.S.P.
REFERENCES
- 1.Verweij PE, Howard SJ, Melchers WJ, Denning DW. 2009. Azole-resistance in Aspergillus: proposed nomenclature and breakpoints. Drug Resist Updat 12:141–147. doi: 10.1016/j.drup.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Dekkers BG, Bakker M, van der Elst KC, Sturkenboom MG, Veringa A, Span LF, Alffenaar JC. 2016. Therapeutic drug monitoring of posaconazole: an update. Curr Fungal Infect Rep 10:51–61. doi: 10.1007/s12281-016-0255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Linden JW, Arendrup MC, Warris A, Lagrou K, Pelloux H, Hauser PM, Chryssanthou E, Mellado E, Kidd SE, Tortorano AM, Dannaoui E, Gaustad P, Baddley JW, Uekötter A, Lass-Flörl C, Klimko N, Moore CB, Denning DW, Pasqualotto AC, Kibbler C, Arikan-Akdagli S, Andes D, Meletiadis J, Naumiuk L, Nucci M, Melchers WJ, Verweij PE. 2015. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg Infect Dis 21:1041–1044. doi: 10.3201/eid2106.140717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Ingen J, van der Lee HA, Rijs AJ, Snelders E, Melchers WJ, Verweij PE. 2015. High-level pan-azole-resistant aspergillosis. J Clin Microbiol 53:2343–2345. doi: 10.1128/JCM.00502-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfaller MA. 2012. Antifungal drug resistance: mechanism, epidemiology and consequences for treatment. 125(1 Suppl):S3–S13. doi: 10.1016/j.amjmed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Chowdhary A, Sharma C, Hagen F, Meis JF. 2014. Exploring azole antifungal drug resistance in Aspergillus fumigatus with special reference to resistance mechanisms. Future Microbiol 9:697–711. doi: 10.2217/fmb.14.27. [DOI] [PubMed] [Google Scholar]
- 7.Enoch DA, Idris SF, Aliyu SH, Micallef C, Sule O, Karas JA. 2014. Micafungin for the treatment of invasive aspergillosis. J Infection 68:507–526. doi: 10.1016/j.jinf.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Katragkou A, McCarthy M, Meletiadis J, Petraitis V, Moradi PW, Strauss GE, Fouant MM, Kovanda LL, Petraitiene R, Roilides E, Walsh TJ. 2014. In vitro combination of isavuconazole with micafungin or amphotericin B deoxycholate against medically important molds. Antimicrob Agents Chemother 58:6934–6937. doi: 10.1128/AAC.03261-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Effron G, Katiyar SK, Park S, Edlind TD, Perlin DS. 2008. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob Agents Chemother 52:2305–2312. doi: 10.1128/AAC.00262-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Effron G, Lee S, Park S, Cleary JD, Perlin DS. 2009. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-beta-d-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob Agents Chemother 53:3690–3699. doi: 10.1128/AAC.00443-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Effron G, Park S, Perlin DS. 2009. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob Agents Chemother 53:112–122. doi: 10.1128/AAC.01162-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocha EM, Garcia-Effron G, Park S, Perlin DS. 2007. A Ser678Pro substitution in Fks1p confers resistance to echinocandin drugs in Aspergillus fumigatus. Antimicrob Agents Chemother 51:4174–4176. doi: 10.1128/AAC.00917-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, vol 28 CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 14.Bain JM, Tavanti A, Davidson AD, Jacobsen DD, Shaw D, Gow NA, Odds FC. 2007. Multilocus sequence typing of the pathogenic fungus Aspergillus fumigatus. J Clin Microbiol 45:1469–1477. doi: 10.1128/JCM.00064-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard SJ, Pasqualotto C, Anderson MJ, Leatherbarrow H, Albarrag AM, Harrison E, Gregson L, Bowyer P, Denning DW. 2013. Major variations in Aspergillus fumigatus arising within aspergillomas in chronic pulmonary aspergillosis. Mycoses 56:434–441. doi: 10.1111/myc.12047. [DOI] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2016. Epidemiological cut off values for antifungal susceptibility testing, 1st ed CLSI document M59. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.Alanio A, Cabaret O, Sitterle E, Costa JM, Brisse S, Cordonnier C, Bretagne S. 2012. Azole preexposure affects the Aspergillus fumigatus population in patients. Antimicrob Agents Chemother 56:4948–4950. doi: 10.1128/AAC.05990-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niki Y, Bernard EM, Edwards FF, Schmitt HJ, Yu B, Armstrong D. 1991. Model of recurrent pulmonary aspergillosis in rats. J Clin Microbiol 29:1317–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]