LETTER
Metallo-β-lactamases (MBLs) threaten the clinical utility of β-lactam antibiotics by hydrolyzing penicillins, cephalosporins, and carbapenems. Moreover, they can also hydrolyze all clinically used inhibitors (e.g., clavulanic acid, sulbactam, and tazobactam) that protect β-lactam antibiotics from the activity of multidrug-resistant bacteria (1). Even the diazabicyclooctane (DBO)-based serine-β-lactamase (SBL) inhibitor avibactam, which was recently approved by the FDA, is hydrolyzed slowly by some MBLs (2).
The combination of avibactam and the monobactam antibiotic aztreonam has recently passed phase II clinical trials for the treatment of infections by multidrug-resistant Gram-negative bacteria producing MBLs (3). While SBL-mediated resistance to aztreonam has long been known via the evolution of SBLs (4), MBLs are not thought to hydrolyze aztreonam (5–7). Due to structural similarities between avibactam and aztreonam (Fig. 1A), particularly with respect to the sulfonate/sulfate substituent on the β-lactam/urea nitrogen, we were interested in examining the interaction between more recently discovered MBLs and aztreonam and the potential for new clinically relevant MBLs with monobactam hydrolyzing activity.
FIG 1.
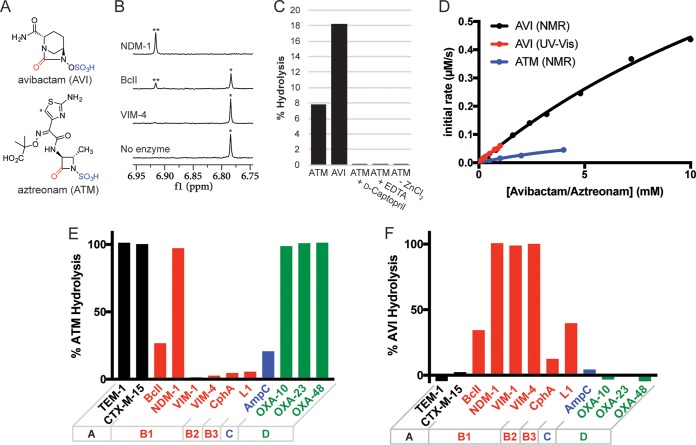
Hydrolysis of avibactam and aztreonam by NDM-1. (A) Structures of avibactam and aztreonam. (B) Extent of aztreonam (100 μM) hydrolysis by VIM-4, BcII, and NDM-1 (all 10 μM) after 16 h. Hydrolysis was monitored by NMR spectroscopy (700 MHz); the peaks labeled with an asterisk correspond to the aztreonam proton indicated with an asterisk in panel A, while the peaks labeled with two asterisks indicate the hydrolyzed product. (C) Extent of hydrolysis of a mixture of aztreonam (ATM; 1 mM) and avibactam (AVI; 1 mM) by NDM-1 (10 μM) after 50 min with 100 μM ZnCl2–50 mM Tris-d11 (pH 7.5)–10% D2O. The addition of d-captopril (500 μM) or EDTA (2 mM) or removal of ZnCl2 inhibited aztreonam hydrolysis. (D) Preliminary kinetic characterization of avibactam and aztreonam hydrolysis by NDM-1 as monitored by NMR and UV-Vis analysis. The NMR studies used 10 μM NDM-1, 100 μM ZnCl2, and the indicated concentration of avibactam or aztreonam in 50 mM Tris-d11 (pH 7.5)–10% D2O. The UV-Vis studies (monitored at 230 nm) used 10 μM NDM-1, 20 μM ZnCl2, and the indicated concentration of avibactam in 5 mM HEPES, pH 7.5 (2). Nonlinear regression analyses were performed using Prism 7 (GraphPad). (E and F) Hydrolysis of (E) aztreonam and (F) avibactam by a panel of β-lactamases covering classes A (black), B (red), C (blue), and D (green). Hydrolysis was measured by NMR after 24 h for samples consisting of enzyme (10 μM) and avibactam (400 μM) or aztreonam (1 mM) in 50 mM Tris-d11 (pH 7.5)–10% D2O.
We tested the hydrolysis of aztreonam by recombinant enzymes covering all three subclasses of MBLs (i.e., B1, B2, and B3). Following overnight incubation of a 1:10 ratio of MBL and aztreonam, the extent of hydrolysis was determined by nuclear magnetic resonance (NMR) spectroscopy. While Verona integron-encoded MBL-1 (VIM-1) (subclass B1), VIM-4 (B1), CphA (B2), and L1 (B3) did not hydrolyze aztreonam (within our limits of detection), the model MBL BcII (B1) showed partial hydrolysis, and New Delhi MBL-1 (NDM-1) (B1) fully hydrolyzed aztreonam under our assay conditions (Fig. 1B). The BcII data are in broad agreement with the previously observed “nonproductive” binding of aztreonam to BcII (8). Interestingly, no interaction between aztreonam and NDM-1 was observed by 19F-NMR analysis (9), suggesting that the binding interaction (e.g., Km) is quite weak. Therefore, more detailed kinetic analyses were performed.
The hydrolysis of aztreonam by NDM-1 was monitored over a shorter time scale (Fig. 1C), yielding a specific activity of 3.7 ± 0.4 nmol min−1 mg−1 using 10 μM NDM-1 and 1 mM aztreonam. The dependence of aztreonam hydrolysis on NDM-1 activity was confirmed by inhibition in the presence of EDTA and d-captopril, both inhibitors of MBLs (Fig. 1C). The hydrolysis of avibactam by NDM-1 was also shown by NMR analysis, which indicated that avibactam is hydrolyzed more quickly than aztreonam (Fig. 1C).
The kinetics of avibactam and aztreonam hydrolysis by NDM-1 were further investigated by UV-visible (UV-Vis) spectroscopy and NMR spectroscopy (Fig. 1D). Due to poor substrate turnover and the limitations associated with these detection methods, a full kinetic characterization was not possible; while the values obtained are expected to be imprecise, they may serve as estimates of substrate affinity and turnover. Although apparent Km and kcat values of ∼3 mM and ∼0.02 s−1 were obtained for the hydrolysis of avibactam by NDM-1 as monitored by UV-Vis, the limited substrate concentrations prevented accurate nonlinear regression analysis (Fig. 1D). Instead, based on the NMR studies which employed a wider range of substrate concentrations, avibactam had an apparent Km of ∼24 mM and an apparent kcat of ∼0.15 s−1 with NDM-1.
Aztreonam had an apparent Km of ∼9 mM and an apparent kcat of ∼0.014 s−1 with NDM-1 under the NMR assay conditions. By comparison, BcII had an approximate maximal kcat of ∼3 × 10−4 s−1 with aztreonam, while the other MBLs tested (for which we did not observe aztreonam hydrolysis by NMR) had calculated maximal kcat values of ∼6 × 10−5 s−1 based on the sensitivity limits of the NMR assay. To provide context with other β-lactamases, the kcat values for avibactam and aztreonam with NDM-1 are comparable to those determined previously for class D β-lactamases with carbapenems (10) and for class C β-lactamases with cephalosporins (11); these classes of enzymes are thought to contribute to resistance to these antibiotics in vivo.
The hydrolysis of avibactam and aztreonam was tested with a panel of 12 β-lactamases, as monitored by NMR (Fig. 1E and F). The β-lactamases tested belong to classes A (TEM-1, CTX-M-15), B (BcII, NDM-1, VIM-1, VIM-4, CphA, L1), C (AmpC), and D (OXA-10, OXA-23, OXA-48). While aztreonam was hydrolyzed efficiently by the class A and class D β-lactamases tested, the class C β-lactamase AmpC (from Pseudomonas aeruginosa) poorly catalyzed aztreonam hydrolysis. As indicated above, while most MBLs tested did not hydrolyze aztreonam, NDM-1 (and BcII to a lesser extent) displayed activity. Although no avibactam hydrolysis was observed for the class A, C, and D β-lactamases tested, MBLs belonging to subclasses B1, B2, and B3 all catalyzed avibactam hydrolysis (Fig. 1F).
These results challenge the widely held view that MBLs cannot hydrolyze aztreonam. Although the hydrolysis of avibactam and aztreonam by NDM-1 at the rate that we observed may well not be clinically relevant, the evolution of MBLs to more efficiently hydrolyze both substrates is likely. This proposal is analogous to what has been observed with the TEM SBLs; while TEM-1 does not efficiently hydrolyze aztreonam, TEM mutants with increased aztreonam hydrolyzing activity have been identified (12). Furthermore, MBL variants with greater activity may already exist in clinical isolates. Therefore, the potential for MBL-mediated resistance should be considered in evaluating the clinical use of avibactam and aztreonam, individually or in combination, as well as of other DBOs and monobactams.
ACKNOWLEDGMENTS
We thank the Medical Research Council and the Wellcome Trust for funding our work on MBLs. C.T.L. is grateful to the Canadian Institutes of Health Research.
REFERENCES
- 1.Prosperi-Meys C, Llabres G, de Seny D, Soto RP, Valladares MH, Laraki N, Frere JM, Galleni M. 1999. Interaction between class B β-lactamases and suicide substrates of active-site serine β-lactamases. FEBS Lett 443:109–111. doi: 10.1016/S0014-5793(98)01689-5. [DOI] [PubMed] [Google Scholar]
- 2.Abboud MI, Damblon C, Brem J, Smargiasso N, Mercuri P, Gilbert B, Rydzik AM, Claridge TD, Schofield CJ, Frère JM. 2016. Interaction of avibactam with class B metallo-β-lactamases. Antimicrob Agents Chemother 60:5655–5662. doi: 10.1128/AAC.00897-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandes P, Martens E. 2017. Antibiotics in late clinical development. Biochem Pharmacol 133:152–163. doi: 10.1016/j.bcp.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 4.Quinn JP, Miyashiro D, Sahm D, Flamm R, Bush K. 1989. Novel plasmid-mediated beta-lactamase (TEM-10) conferring selective resistance to ceftazidime and aztreonam in clinical isolates of Klebsiella pneumoniae. Antimicrob Agents Chemother 33:1451–1456. doi: 10.1128/AAC.33.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh TR. 2005. The emergence and implications of metallo-beta-lactamases in Gram-negative bacteria. Clin Microbiol Infect 11(Suppl 6):2–9. doi: 10.1111/j.1469-0691.2005.01264.x. [DOI] [PubMed] [Google Scholar]
- 6.Crandon JL, Nicolau DP. 2013. Human simulated studies of aztreonam and aztreonam-avibactam to evaluate activity against challenging gram-negative organisms, including metallo-β-lactamase producers. Antimicrob Agents Chemother 57:3299–3306. doi: 10.1128/AAC.01989-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshall S, Hujer AM, Rojas LJ, Papp-Wallace KM, Humphries RM, Spellberg B, Hujer KM, Marshall EK, Rudin SD, Perez F, Wilson BM, Wasserman RB, Chikowski L, Paterson DL, Vila AJ, van Duin D, Kreiswirth BN, Chambers HF, Fowler VG Jr, Jacobs MR, Pulse ME, Weiss WJ, Bonomo RA. 2017. Can ceftazidime-avibactam and aztreonam overcome β-lactam resistance conferred by metallo-β-lactamases in Enterobacteriaceae. Antimicrob Agents Chemother 61:e02243-16. doi: 10.1128/AAC.02243-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poeylaut-Palena AA, Tomatis PE, Karsisiotis AI, Damblon C, Mata EG, Vila AJ. 2007. A minimalistic approach to identify substrate binding features in B1 metallo-β-lactamases. Bioorg Med Chem Lett 17:5171–5174. doi: 10.1016/j.bmcl.2007.06.089. [DOI] [PubMed] [Google Scholar]
- 9.Rydzik AM, Brem J, van Berkel SS, Pfeffer I, Makena A, Claridge TD, Schofield CJ. 2014. Monitoring conformational changes in the NDM-1 metallo-β-lactamase by 19F NMR spectroscopy. Angew Chem Int Ed Engl 53:3129–3133. doi: 10.1002/anie.201310866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potron A, Rondinaud E, Poirel L, Belmonte O, Boyer S, Camiade S, Nordmann P. 2013. Genetic and biochemical characterisation of OXA-232, a carbapenem-hydrolysing class D β-lactamase from Enterobacteriaceae. Int J Antimicrob Agents 41:325–329. doi: 10.1016/j.ijantimicag.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez-Martínez JM, Poirel L, Nordmann P. 2009. Extended-spectrum cephalosporinases in Pseudomonas aeruginosa. Antimicrob Agents Chemother 53:1766–1771. doi: 10.1128/AAC.01410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantu C III, Huang W, Palzkill T. 1996. Selection and characterization of amino acid substitutions at residues 237–240 of TEM-1 β-lactamase with altered substrate specificity for aztreonam and ceftazidime. J Biol Chem 271:22538–22545. doi: 10.1074/jbc.271.37.22538. [DOI] [PubMed] [Google Scholar]