ABSTRACT
Acinetobacter baumannii is responsible for 10% of all nosocomial infections and has >50% mortality rates when causing ventilator-associated pneumonia. In this proof-of-concept study, we evaluated SPR741, an antibiotic adjuvant that permeabilizes the Gram-negative membrane, in combination with rifampin against AB5075, an extensively drug-resistant (XDR) A. baumannii strain. In standard in vitro assays and in a murine pulmonary model, we found that this drug combination can significantly reduce bacterial burden and promote animal survival despite an aggressive infection.
KEYWORDS: Acinetobacter, ESKAPE pathogens, animal models, antibacterial, antibiotic adjuvants, antibiotic resistance, antibiotics, mouse model, pulmonary model, virulent strain
TEXT
Acinetobacter baumannii gained notoriety as the bacterial species most frequently isolated from U.S. soldiers from 2004 to 2010, with >3,500 infections associated with war wounds (1–4). Presently, A. baumannii is a significant problem worldwide because of extensively drug-resistant (XDR) strains and the aggressive nature of some infections causing ventilator-associated pneumonia (5–7). Recently, the World Health Organization cited a critical need for A. baumannii research because of increased drug resistance and lack of treatments (8). To address the immediate need, research strategies such as antibiotic adjuvants, which involve the current antibiotic armamentarium, may provide a faster path to clinical application.
Antibiotic adjuvants are typically small molecules that sensitize a bacterium to clinically approved antibiotics (9). Specific examples with respect to A. baumannii include 2-aminoimidazole-based compounds, which disrupt two-component signaling (10), and anthracyclines, which potentiate activity of rifampin and linezolid (11). Here, we evaluated SPR741 (formerly NAB741), a polymyxin-B-derived molecule specifically designed to minimize the nephrotoxicity associated with this antibacterial class (12). SPR741 has reduced positive charge and lacks the highly lipophilic fatty-acid side chain present in polymyxins, which are the two structural features responsible for clinical nephrotoxicity (12, 13). As a proof of concept, we tested a combination of SPR741 and rifampin because colistin and rifampin have proven to be an effective combination in both mouse models and in patients (14–18). Rifampin was also previously tested against AB5075, a highly virulent XDR strain, in a murine pulmonary model of infection (19), which facilitated dosing for this study.
First, the SPR741/rifampin combination was tested in vitro against A. baumannii. The MICs for SPR741 and rifampin were determined for AB5075 to be 128 μg/ml and 4.0 μg/ml, respectively, using standard CLSI methods in cation-adjusted Mueller-Hinton broth (CAMHB) (20). This combination was assessed with the checkerboard method to determine fractional inhibitory concentration (FIC) (21), where synergistic activity was defined by an FIC of ≤0.5 (22). The MIC of rifampin dropped from 4.0 to 0.5 μg/ml in the presence of 2.0 μg/ml SPR741, an 8-fold reduction, thus producing an FIC of 0.14 and indicating synergy. An isobologram was generated from these results (Fig. 1A).
FIG 1.
(A) An isobologram generated from checkerboard assays where AB5075 was grown with increasing concentrations of SPR741 and rifampin. (B) Time-kill assay of SPR741, rifampin, and combinations against XDR-A. baumannii. AB5075 was grown overnight in CAMHB, then subcultured into CAMHB for 2 h. Cultures were inoculated 1:10 into CAMHB alone or with 2.0 μg/ml SPR741, 1.0 μg/ml rifampin, or both SPR741 and rifampin at their respective concentrations. Time points were taken at 0, 2, 4, 6, and 24 h, and samples were plated for CFU. Only the combination of rifampin and SPR741 was statistically significant (red line), as tested by two-way ANOVA (P = 0.0048).
Next, we examined whether this synergy was applicable across the whole species of A. baumannii. We analyzed a previously described 28-strain diversity set (19) and determined MICs for all strains. A combination of 4.0 μg/ml of SPR741 and 1.0 μg/ml rifampin inhibited the growth of 96% of the strains, with a minimum 4-fold reduction of most MICs. AB3027 was the exception, as it is significantly resistant to rifampin (MIC > 128 μg/ml; Table 1).
TABLE 1.
A. baumannii strains used in this study with individual MIC values
| A. baumannii strain | MIC fora: |
Growth in presence of SPR741 (4.0 μg/ml) + rifampin (1.0 μg/ml)b | |
|---|---|---|---|
| Rifampin | SPR741 | ||
| AB967 | 4 | <64 | − |
| AB2828 | 2 | 256 | − |
| AB3340 | 2 | 256 | − |
| AB3560 | 4 | 128 | − |
| AB3638 | 2 | 256 | − |
| AB3785 | 4 | 128 | − |
| AB3806 | 2 | 256 | − |
| AB3927 | >256 | 256 | + |
| AB4025 | 4 | 128 | − |
| AB4026 | 4 | >256 | − |
| AB4027 | 4 | >256 | − |
| AB4052 | 4 | 256 | − |
| AB4269 | 8 | >256 | − |
| AB4448 | 4 | <64 | − |
| AB4456 | 4 | 128 | − |
| AB4490 | 4 | 128 | − |
| AB4498 | 4 | 256 | − |
| AB4795 | 2 | 128 | − |
| AB4857 | 4 | 256 | − |
| AB4878 | 4 | 256 | − |
| AB4932 | 16 | <64 | − |
| AB4957 | 4 | 256 | − |
| AB4991 | 4 | 128 | − |
| AB5001 | 4 | 256 | − |
| AB5075 | 2 | 128 | − |
| AB5197 | 4 | 256 | − |
| AB5256 | 4 | 128 | − |
| AB5674 | 2 | 128 | − |
| AB5711 | 4 | 128 | − |
MICs were determined separately in the presence of the combination of SPR741 at 4.0 μg/ml and rifampin at 1.0 μg/ml.
−, no growth; +, growth.
To further evaluate activity, time-kill assays (3 biological replicates) were performed with 2.0 μg/ml SPR741 and 1.0 μg/ml rifampin against AB5075 grown in CAHMB as previously described (23). Each drug used alone had little effect on growth. In contrast, the combination resulted in fewer than 10 organisms (limit of detection) on LB plates at 2, 4, and 6 h (Fig. 1B), a significant result (two-way ANOVA, P = 0.0048). This result confirmed that the combination of SPR741/rifampin had a bactericidal, synergistic effect that should be further tested in vivo.
The methods for the murine pulmonary model of A. baumannii infection were previously detailed (19), and were conducted similarly for this study. In pilot experiments, we initially evaluated three doses of SPR741 (40, 60, or 80 mg/kg) once daily (QD) or twice daily (BID), with 5.0 mg/kg or 10.0 mg/kg doses of rifampin also provided QD or BID. Only the BID-treated mice survived over the course of 1 week, suggesting that QD treatment was not sufficient (data not shown). Next, two independent experiments were conducted using 10 mice per group. Four hours after A. baumannii inoculation, groups were treated with sterile saline (negative control), 5.0 mg/kg rifampin, 60 mg/kg SPR741 BID, or the combination of SPR741 40 mg/kg or 60 mg/kg SPR741 with 5.0 mg/kg rifampin BID for the next 3 days. The survival rate for 60 mg/kg SPR741 combined with 5.0 mg/kg rifampin BID was 90% (Fig. 2A), a significant success for this aggressive infection model (Mantel-Cox test, P < 0.0027). In contrast, untreated animals or mice receiving SPR741 alone succumbed to infection (Fig. 2A). Rifampin alone only provided 50% survival (Fig. 2A).
FIG 2.
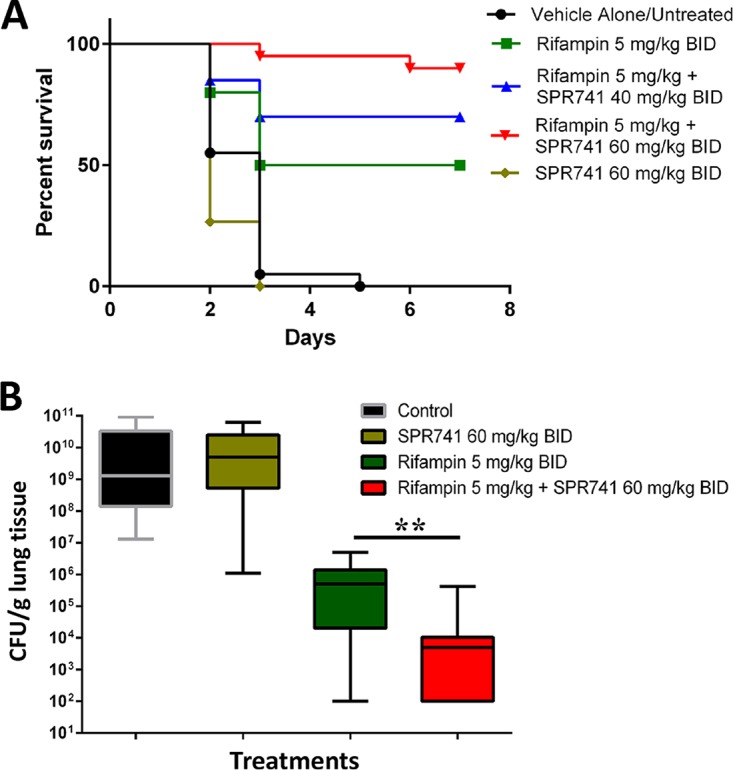
(A) Mice were intranasally inoculated with 5.0 × 106 CFU AB5075 and treated with rifampin 5 mg/kg BID (green line), SPR741 60 mg/kg BID (yellow line), the combination of these doses at 40 mg/kg SPR741 BID (blue line) or 60 mg/kg SPR741 BID (red line), or sterile saline (vehicle alone, untreated control; black line). The data presented is a combination of two biological replicates of 10 mice/group for a total of 20 mice (n = 20). Mice were monitored daily for signs of morbidity and mortality. Results for all groups were statistically significant (P < 0.05) compared to each other via the Mantel-Cox test (Graphpad Prism), except for the untreated control (black line) and SPR741-alone groups (yellow line). (B) Box-and-whisker plots of log10 CFU/g of lung tissue on day 2 postinoculum. Mice were treated with rifampin at 5 mg/kg BID (green box), SPR741 60 mg/kg BID (yellow box), the combination of these doses (red box), or sterile saline (vehicle alone, control; black box) for 2 days. Boxes show median and interquartile ranges, while whiskers represent 95% confidence interval (CI). Groups were compared each day via the Mann-Whitney U test. ** represents P values of <0.01 (P = 0.0029). These data are pooled from two biological replicates with at least 6 mice per group and 13 to 16 mice total per test condition.
Separate experiments were then conducted to evaluate bacterial burden via CFU (CFU/g of lung tissue). Mice were sacrificed on day 2 before the untreated control animals succumbed to infection, as previously described (19). These results mirrored the survival results, where the combination of SPR741/rifampin decreased bacterial burden by 6.0 log10 CFU/g compared to the vehicle-alone control (Mann-Whitney U test, P < 0.0001) (Fig. 2B). When comparing the combination of SPR741/rifampin to rifampin treatment alone, a 2.0-log10 reduction in burden was seen with the addition of SPR741, which was also statistically significant (Mann-Whitney U test, P = 0.0029) (Fig. 2B).
This investigation is a promising start with regard to in vivo safety and efficacy of SPR741 combinations against Gram-negative pathogens. In pilot experiments, more mice did succumb (80% survival) with 80 mg/kg BID doses of SPR741. The reason for this is unclear, but clearance and complete animal survival are difficult to achieve in this model. AB5075 is highly aggressive and bacteria reach high numbers in lung tissue, followed by dissemination into the bloodstream and colonization of other organs, including heart, spleen, and kidneys (4, 19). With regard to toxicity, previously presented results (P. Shastri and S. Coleman, ASM Microbe, Boston, MA, 16 to 20 June 2016) determined that the 60 mg/kg dose in mice scales to a human dose of approximately 200 to 400 mg. SPR741 demonstrated a no-observed-adverse-effect-level (NOAEL) of >60 mg/kg/day in cynomolgus monkeys (S. Coleman, M. Bleavins, T. Lister, M. Vaara, and T.R. Parr, ASM Microbe, Boston, MA, 16 to 20 June 2016), while nephrotoxicity was observed at 12 mg/kg/day with polymyxin B. Spero Therapeutics recently completed SPR741 dosing in healthy volunteers (https://clinicaltrials.gov/ct2/show/NCT03022175, ClinicalTrials registration no. NCT03022175). With these prior results and the data obtained from this proof-of-concept study, more preclinical investigations of SPR741-antibiotic combinations are warranted to evaluate efficacy against other bacterial species. Furthermore, animal models mimicking other clinical indications and evaluating pharmacokinetics/pharmacodynamics (PK/PD) are also currently being pursued.
ACKNOWLEDGMENTS
We thank the other members of Spero Therapeutics, Inc. and the Wound Infections Department at WRAIR for their support; these studies would not be possible without them.
The research presented here was partially supported by grant PR150337 awarded to M.J.P. (Spero Therapeutics, Inc.) and D.V.Z (WRAIR) from the Peer-Reviewed Medical Research Program.
M.J.P. and T.L. are employees of Spero Therapeutics, Inc.
Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting true views of the Department of the Army or the Department of Defense.
Research was conducted under an approved animal protocol (16-BRD-48S) in an AAALACi-accredited facility in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 2011 edition.
REFERENCES
- 1.Keen EF III, Murray CK, Robinson BJ, Hospenthal DR, Co EM, Aldous WK. 2010. Changes in the incidences of multidrug-resistant and extensively drug-resistant organisms isolated in a military medical center. Infect Control Hosp Epidemiol 31:728–732. doi: 10.1086/653617. [DOI] [PubMed] [Google Scholar]
- 2.Yun HC, Murray CK, Roop SA, Hospenthal DR, Gourdine E, Dooley DP. 2006. Bacteria recovered from patients admitted to a deployed U.S. military hospital in Baghdad, Iraq. Mil Med 171:821–825. [DOI] [PubMed] [Google Scholar]
- 3.Yun HC, Branstetter JG, Murray CK. 2008. Osteomyelitis in military personnel wounded in Iraq and Afghanistan. J Trauma 64:S163–S168. doi: 10.1097/TA.0b013e318160868c. [DOI] [PubMed] [Google Scholar]
- 4.Hobson DW, Schuh JC, Zurawski DV, Wang J, Arbabi S, McVean M, Funk KA. 2016. The first cut is the deepest: the history and development of safe treatments for wound healing and tissue repair. Int J Toxicol 35:491–498. doi: 10.1177/1091581816656804. [DOI] [PubMed] [Google Scholar]
- 5.Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. 2017. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 30:409–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spellberg B, Bonomo RA. 2014. The deadly impact of extreme drug resistance in Acinetobacter baumannii. Crit Care Med 42:1289–1291. doi: 10.1097/CCM.0000000000000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potron A, Poirel L, Nordmann P. 2015. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: mechanisms and epidemiology. Int J Antimicrob Agents 45:568–585. doi: 10.1016/j.ijantimicag.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 8.WHO. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization, Geneva, Switzerland: http://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/. [Google Scholar]
- 9.Wright GD. 2016. Antibiotic adjuvants: rescuing antibiotics from resistance. Trends Microbiol 24:862–871. doi: 10.1016/j.tim.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Thompson RJ, Bobay BG, Stowe SD, Olson AL, Peng L, Su Z, Actis LA, Melander C, Cavanagh J. 2012. Identification of BfmR, a response regulator involved in biofilm development, as a target for a 2-aminoimidazole-based antibiofilm agent. Biochemistry 51:9776–9778. doi: 10.1021/bi3015289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox G, Koteva K, Wright GD. 2014. An unusual class of anthracyclines potentiate Gram-positive antibiotics in intrinsically resistant Gram-negative bacteria. J Antimicrob Chemother 69:1844–1855. doi: 10.1093/jac/dku057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaara M, Siikanen O, Apajalahti J, Fox J, Frimodt-Moller N, He H, Poudyal A, Li J, Nation RL, Vaara T. 2010. A novel polymyxin derivative that lacks the fatty acid tail and carries only three positive charges has strong synergism with agents excluded by the intact outer membrane. Antimicrob Agents Chemother 54:3341–3346. doi: 10.1128/AAC.01439-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corbett D, Wise A, Langley T, Skinner K, Trimby E, Birchall S, Dorali A, Sandiford S, Williams J, Warn P, Vaara M, Lister T. 2017. Potentiation of antibiotic activity by a novel cationic peptide: potency and spectrum of activity of SPR741. Antimicrob Agents Chemother 61:e00200-17. doi: 10.1128/AAC.00200-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claeys KC, Fiorvento AD, Rybak MJ. 2014. A review of novel combinations of colistin and lipopeptide or glycopeptide antibiotics for the treatment of multidrug-resistant Acinetobacter baumannii. Infectious diseases and therapy 3:69–81. doi: 10.1007/s40121-014-0051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viehman JA, Nguyen MH, Doi Y. 2014. Treatment options for carbapenem-resistant and extensively drug-resistant Acinetobacter baumannii infections. Drugs 74:1315–1333. doi: 10.1007/s40265-014-0267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pachon-Ibanez ME, Docobo-Perez F, Lopez-Rojas R, Dominguez-Herrera J, Jimenez-Mejias ME, Garcia-Curiel A, Pichardo C, Jimenez L, Pachon J. 2010. Efficacy of rifampin and its combinations with imipenem, sulbactam, and colistin in experimental models of infection caused by imipenem-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 54:1165–1172. doi: 10.1128/AAC.00367-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song JY, Cheong HJ, Lee J, Sung AK, Kim WJ. 2009. Efficacy of monotherapy and combined antibiotic therapy for carbapenem-resistant Acinetobacter baumannii pneumonia in an immunosuppressed mouse model. Int J Antimicrob Agents 33:33–39. doi: 10.1016/j.ijantimicag.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Durante-Mangoni E, Signoriello G, Andini R, Mattei A, De Cristoforo M, Murino P, Bassetti M, Malacarne P, Petrosillo N, Galdieri N, Mocavero P, Corcione A, Viscoli C, Zarrilli R, Gallo C, Utili R. 2013. Colistin and rifampicin compared with colistin alone for the treatment of serious infections due to extensively drug-resistant Acinetobacter baumannii: a multicenter, randomized clinical trial. Clin Infect Dis 57:349–358. doi: 10.1093/cid/cit253. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs AC, Thompson MG, Black CC, Kessler JL, Clark LP, McQueary CN, Gancz HY, Corey BW, Moon JK, Si Y, Owen MT, Hallock JD, Kwak YI, Summers A, Li CZ, Rasko DA, Penwell WF, Honnold CL, Wise MC, Waterman PE, Lesho EP, Stewart RL, Actis LA, Palys TJ, Craft DW, Zurawski DV. 2014. AB5075, a highly virulent isolate of Acinetobacter baumannii, as a model strain for the evaluation of pathogenesis and Antimicrobial Treatments. mBio 5:e01076–01014. doi: 10.1128/mBio.01076-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing, 26th ed Approved standard M100-S26. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 21.Garcia LS. 2010. Synergism testing: broth microdilution checkerboard and broth macrodilution methods, p 140–162. In Garcia LS. (ed), Clinical microbiology procedures handbook, 3rd ed ASM Press, Washington, DC. [Google Scholar]
- 22.Hall MJ, Middleton RF, Westmacott D. 1983. The fractional inhibitory concentration (FIC) index as a measure of synergy. J Antimicrob Chemother 11:427–433. doi: 10.1093/jac/11.5.427. [DOI] [PubMed] [Google Scholar]
- 23.Joly-Guillou ML, Decré D, Herrman JL, Bourdelier E, Bergogne-Bérézin E. 1995. Bactericidal in-vitro activity of beta-lactams and beta-lactamase inhibitors, alone or associated, against clinical strains of Acinetobacter baumannii: effect of combination with aminoglycosides. J Antimicrob Chemother 36:619–629. doi: 10.1093/jac/36.4.619. [DOI] [PubMed] [Google Scholar]



