ABSTRACT
Antifungal susceptibility testing is an essential tool for guiding therapy, although EUCAST and CLSI reference methods are often available only in specialized centers. We studied the performance of an agar-based screening method for the detection of azole resistance in Aspergillus fumigatus cultures. The VIPcheck consists of four wells containing voriconazole, itraconazole, posaconazole, or a growth control. Ninety-six A. fumigatus isolates were used. Thirty-three isolates harbored a known resistance mechanism: TR34/L98H (11 isolates), TR46/Y121F/T289A (6 isolates), TR53 (2 isolates), and 14 isolates with other cyp51A gene point mutations. Eighteen resistant isolates had no cyp51A-mediated azole resistance. Forty-five isolates had a wild-type (WT) azole phenotype. Four technicians and two inexperienced interns, blinded to the genotype/phenotype, read the plates visually after 24 h and 48 h and documented minimal growth, uninhibited growth, and no growth. The performance was compared to the EUCAST method. After 24 h of incubation, the mean sensitivity and specificity were 0.54 and 1.00, respectively, with uninhibited growth as the threshold. After 48 h of incubation, the performance mean sensitivity and specificity were 0.98 and 0.93, respectively, with minimal growth. The performance was not affected by observer experience in mycology. The interclass correlation coefficient was 0.87 after 24 h and 0.85 after 48 h. VIPcheck enabled the selection of azole-resistant A. fumigatus colonies, with a mean sensitivity and specificity of 0.98 and 0.93, respectively. Uninhibited growth on any azole-containing well after 24 h and minimal growth after 48 h were indicative of resistance. These results indicate that the VIPcheck is an easy-to-use tool for azole resistance screening and the selection of colonies that require MIC testing.
KEYWORDS: VIPcheck, broth microdilution, antifungal resistance, susceptibility, azole resistance, antifungal susceptibility testing
INTRODUCTION
Azole resistance is an emerging problem in Aspergillus fumigatus (1), with increasing evidence that patients with azole-resistant aspergillosis fail azole therapy (2–5). Two routes of resistance selection have been signified, through patient therapy and through the exposure of A. fumigatus to azole fungicides in the environment. There are important differences between these routes of resistance selection, including patient risk factors and fungal resistance mechanisms. Resistance mechanisms that are associated with the environmental route include TR34/L98H and TR46/Y121F/T289A (6–8). Surveillance studies in The Netherlands show that of the clinical isolates that are azole resistant, between 83% and 95% harbor mutations associated with the environmental route, while approximately 15% exhibit an azole-resistant phenotype without known resistance mutations (9). As patients inhale these airborne azole-resistant spores, a resistant infection may occur in any Aspergillus disease and in patients who have never been treated with medical triazoles (3, 7, 10, 11). In one study, two-thirds of patients with azole-resistant A. fumigatus had not previously received azole therapy (4). Furthermore, azole-susceptible and azole-resistant A. fumigatus colonies may be concomitantly present in clinical cultures (12), and patients with invasive aspergillosis have been reported due to both azole-susceptible and azole-resistant colonies (13).
These observations pose a challenge for the diagnosis of azole resistance, as multiple colonies will need to be tested in culture-positive patients. This was also recommended by a group of experts who advocated testing of up to five colonies in patients who are to receive antifungal therapy in geographic regions with azole resistance (14). Two reference methods of antifungal susceptibility testing are available, broth microdilution based on EUCAST and CLSI standards (15, 16), but these assays are not widely available, and referral to a mycology reference center would cause a significant delay before results are available. We developed an agar-based method aimed to help identify A. fumigatus colonies that are resistant to itraconazole, voriconazole, and posaconazole. Any growth on the azole-containing agar is suggestive of azole resistance, and the isolate could then be selected for in vitro susceptibility testing. This would provide an easy-to-use screening method that can be used in any clinical microbiology laboratory.
The principle of agar-based detection of azole resistance in A. fumigatus has been extensively used in surveillance studies (4, 17). After further improvements to the format regarding the antifungal concentration and inoculation procedure (using a drop of suspension instead of a nose or sterile swab), the assay is now being commercialized (VIPcheck, Nijmegen, the Netherlands) and is currently available for research use only.
We investigated the performance of the VIPcheck as a diagnostic tool in the clinical microbiology laboratory. The performance of the VIPcheck was determined using a collection of well-characterized A. fumigatus isolates, with various known and with unknown resistance mechanisms, and the plates were read by both experienced and inexperienced observers.
(These data have previously been published at the 26th European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, The Netherlands, 9 to 12 April 2016, abstract no. P1618 [18].)
RESULTS
Isolates.
Thirty-three isolates harbored a known resistance mechanism: TR34/L98H (11 isolates); TR46/Y121F/T289A (6 isolates); TR53 (2 isolates); a substitution at codon G54 (G54W [3 isolates], G54E [2 isolates], and G54R [1 isolate]), M220 (M220V [1 isolate], M220R [1 isolate], M220I [1 isolate], and M220K [1 isolate]), G448 (G448S [2 isolates]), P216 (P216L [1 isolate]), and G138 (G138C [1 isolate]) (4, 19–26). For 18 isolates, no cyp51A-mediated azole resistance mechanism was found, indicating a yet-unknown mechanism causing resistance. The susceptibility data of the resistant isolates without cyp51A mutations can be found at Table S1 in the supplemental material. The phenotypically wild-type (WT) isolates did not harbor cyp51A resistance mechanisms.
Performance of the VIPcheck.
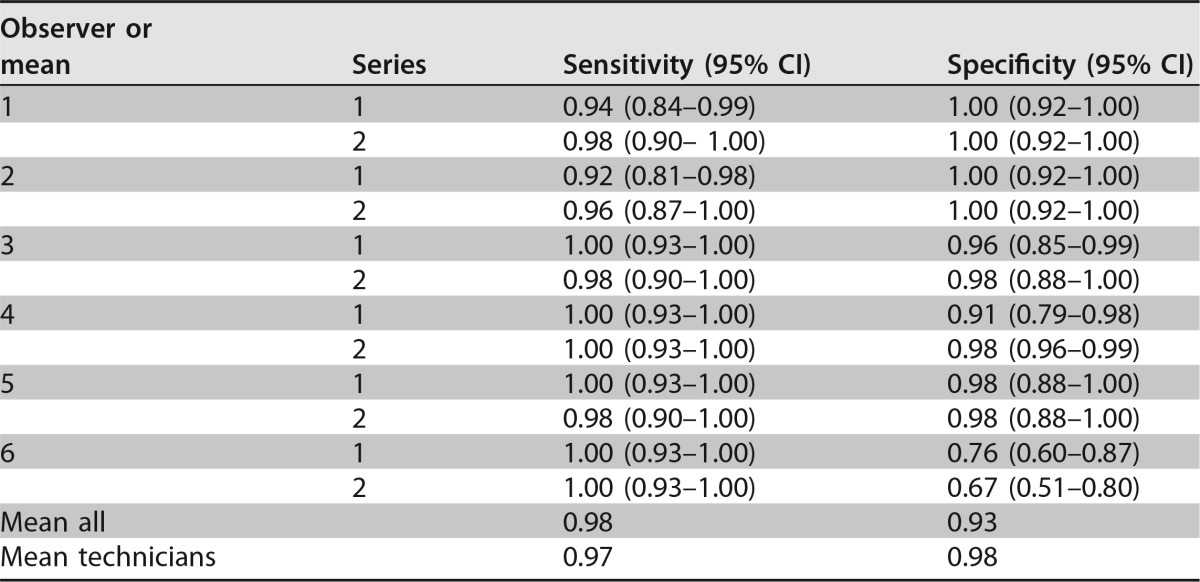
All isolated showed uninhibited (++) growth after 24 h in the control well without azoles (Fig. 1). Growth on any of the wells supplemented with azoles indicates azole resistance against the specific azole indicated. After 24 h of incubation, the mean sensitivity for all observers with minimal (+) growth as the threshold was 0.81, while the specificity was 0.91. With uninhibited growth as the threshold, the sensitivity decreased to 0.54, but no false positives were observed. At the 48-h/minimal growth endpoint, the mean sensitivity was 0.98 for all observers, while the mean specificity was 0.93 (Table 1).
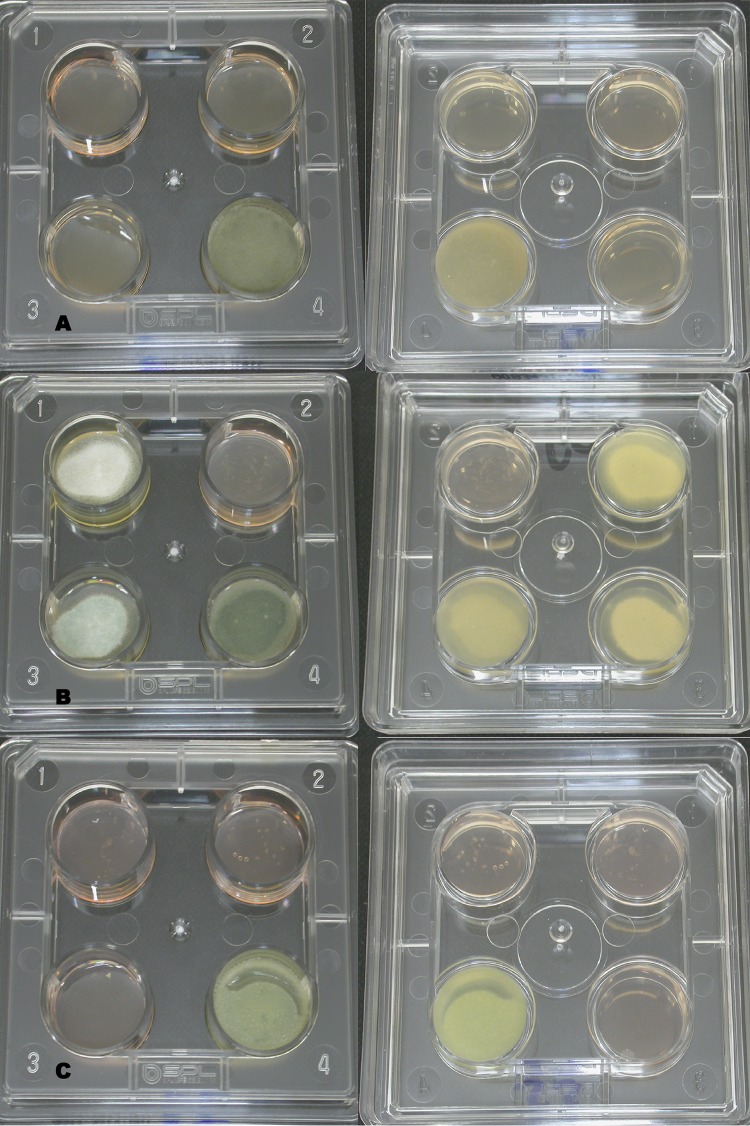
FIG 1.
Pictures of VIPcheck plates. Well 1, 4 mg/liter ITC; well 2, 2 mg/liter VRC; well 3, 0.5 mg/liter POS; well 4, growth control. (A) Front and back of a susceptible isolate after 48 h of incubation. (B) Front and back of a resistant isolate with uninhibited (++) growth after 48 h of incubation. (C) Front and back of a resistant isolate with minimal (+) growth after 48 h of incubation.
TABLE 1.
Performance of the VIPcheck after 24 h and 48 h of incubationa
| Growth condition | Sensitivity | Specificity |
|---|---|---|
| Minimal growth | ||
| 24 h | 0.81 | 0.91 |
| 48 h | 0.98 | 0.93 |
| Uninhibited growth | ||
| 24 h | 0.54 | 1.00 |
| 48 h | 0.92 | 0.99 |
The mean of 6 observers is displayed for 2 endpoints: minimal (+) growth and uninhibited (++) growth. The performance of the VIPcheck was compared to the EUCAST reference method.
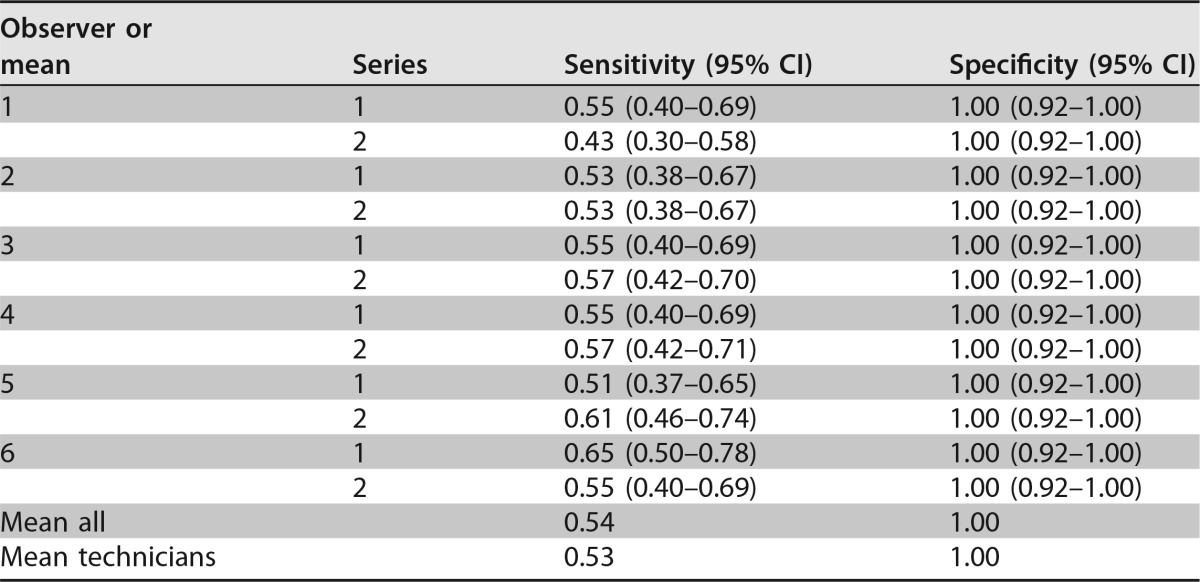
The sensitivity and specificity for the individual observers for reading after 24 h with uninhibited growth as the threshold are shown in Table 2 and for minimal growth at 24 h in Table 3. Overall, the sensitivity was higher among observers without experience in mycology, due to a lower threshold of minimal growth. As a consequence, a lower specificity was observed in observers without experience in mycology. In contrast, the specificity was 1.00 for the experienced observers. With uninhibited growth as the threshold for resistance, a lower sensitivity was seen, but specificity was near 100% (mean sensitivity/specificity for all observers, 0.92/0.99, respectively).
TABLE 2.
Performance of VIPcheck after 24 h of incubation with uninhibited (++) growth as the thresholda
Performance of the VIPcheck after 24-h incubations for 96 Aspergillus fumigatus isolates: 45 azole susceptible and 51 azole resistant in two replicates. Performance is reported for each observer and series individually. “Mean all” is the mean of all sensitivities and specificities. “Mean technicians” is the mean performance for observers 1 to 4. Observers 1 and 2 were highly experienced mycology technicians, observers 3 and 4 were bacterial technicians without experience in mycology, and observers 5 and 6 were inexperienced interns.
TABLE 3.
Performance of VIPcheck after 48 h of incubation with minimal (+) growth as the thresholda
Performance of the VIPcheck after 48-h incubations for 96 A. fumigatus isolates: 45 azole susceptible and 51 azole resistant in two replicates. Performance is reported for each observer and series individually. “Mean all” is the mean of all sensitivities and specificities. “Mean technicians” is the mean performance for observers 1 to 4. Observers 1 and 2 were highly experienced mycology technicians, observers 3 and 4 were bacterial technicians without experience in mycology, and observers 5 and 6 were inexperienced interns.
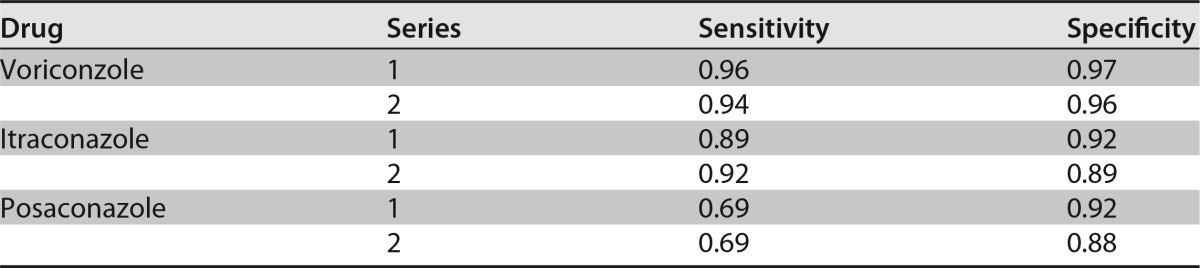
For individual drugs, the mean sensitivity/specificity for all observers and the duplicates together were 0.90/0.96 for itraconazole, 0.95/0.90 for voriconazole, and 0.69/0.90 for posaconazole with minimal growth as the threshold. The performances for the individual drugs for the two duplicates separately can be found in Table 4. Subgroup analysis revealed a mean sensitivity of 0.96 with uninhibited growth as the threshold and 0.99 with minimal growth as the threshold for the 33 isolates with known resistance mechanisms. The mean sensitivity of the isolates with environmental mutations was 1.00 with uninhibited growth as the threshold.
TABLE 4.
Performance of VIPcheck after 48 h of incubation for the individual drugsa
Performance of the VIPcheck after 48-h incubations for 96 A. fumigatus isolates: 45 azole susceptible and 51 azole resistant in two replicates. Performance is reported for the mean of all 6 observers for each drug individually.
The agreement between observers was assessed with the interclass correlation coefficient (ICC). The ICC estimated for all observers was 0.87 (P < 0.001) with uninhibited growth as the threshold after 24 h. For 48 h with minimal growth as the threshold, the ICC was 0.85 (P < 0.001). The ICC for the microbiology technicians (observers 1 to 4) was higher, with 0.90 and 0.93 for readings at 24 h and 48 h, respectively. The reproducibility between the series incubated by the inexperienced observer and the experienced observer was assessed. Kappa's agreement values between series 1 and 2 were 0.85 and 0.87 (P > 0.001) for all observers and 0.86 and 0.86 (P > 0.001) for the technicians with uninhibited growth at 24 h and with minimal growth as the threshold at 48 h, respectively.
DISCUSSION
We evaluated a new strategy that could enable the early detection of azole resistance in A. fumigatus. As azole-susceptible and azole-resistant A. fumigatus colonies may be concomitantly present in clinical cultures, MIC testing of multiple colonies would be required to detect or rule out azole resistance. This is, however, laborious, costly, and not broadly applicable, as many clinical microbiology laboratories have limited experience with MIC testing of molds. Sending isolates to reference mycology centers causes a delay, which might compromise patient outcome. An approach that uses agar-based screening of A. fumigatus colonies might enable the selection of A. fumigatus isolates that have a high probability of azole resistance and require MIC testing. An important condition for such an approach is the reliability and reproducibility of agar-based screening.
Our study showed a sensitivity and specificity of agar-based screening using VIPcheck ranging between 0.92 and 1.00 and 0.67 and 1.00, respectively, and the test was easy to read by both experienced and inexperienced technicians. Furthermore, the high agreement between series 1 and 2 indicated that a lack of experience in inoculation of the plates does not influence the performance of the VIPcheck.
At 24 h, uninhibited growth was easy to recognize, and using this endpoint resulted in a specificity of 1.00 for all observers. However, only half of the resistant isolates showed uninhibited growth after 24 h of incubation (mean sensitivity for all observers, 0.55). However, most observers could not discriminate minimal growth and no growth, which resulted in many false positive-results when minimal growth was used as the endpoint. Therefore, after 24 h of incubation, the VIPcheck plate should be checked for uninhibited growth as an indication for resistance.
At 48 h, the difference between minimal and no growth was easier to recognize (Fig. 1b), regardless of the observers' background. This was demonstrated by an ICC of above 0.80, which indicates good reproducibility between observers, and a sensitivity of 0.92 with a threshold of uninhibited growth. However, four resistant isolates did not show uninhibited growth in the antifungal wells after 48 h for one of the two duplicates. Small white dots that could be distinguished from the medium gave clues to its resistance to azoles, as is seen in Fig. 1c. Observers 3 to 6 documented these as minimal growth, but observers 1 and 2 did not. One of the four isolates that was missed by some observers had a known resistance mechanism (M220K, isolate ID V059-27); for the other three isolates, no known cyp51A mutations were found (Table 5). Isolates with non-cyp51A-mediated resistance might develop through patient therapy and consequently may exhibit a fitness cost, such as reduced growth rate. These isolates might be more difficult to detect with the VIPcheck than those that harbor environmental resistance mutations. The group with environmental resistance mutations appears not to have a fitness cost, as they otherwise would not survive in the environment in competition with WT A. fumigatus isolates. Therefore, it is important to further analyze any growth on the azole-containing agar, as this might indicate resistance. The proportion of patient-derived resistance and environmental resistance will differ between hospitals depending on the patient population, although in many regions, environmental resistance mutations appear to dominate.
TABLE 5.
Characteristics of resistant A. fumigatus strains missed with VIPchecka
| Strain | cyp51 | MIC 1 (mg/liter) |
MIC 2 (mg/liter) |
No. missed by VIPcheck/total no. |
Strain characteristics | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ITC | VOR | POS | ITC | VOR | POS | Series 1 | Series 2 | |||
| V059-27 | M220K | >16 | 2 | 0.25 | >16 | >16 | 2 | 2/6 | 0/6 | Only minimal growth in EUCAST 96-well plate; discrepancy in MIC between measurements 1 and 2 |
| V130-18 | >16 | 4 | 0.5 | 4 | 4 | 0.5 | 2/6 | 2/6 | Only very minimal growth after 48 h on VIPcheck plates and growth plate; isolated from a patient with long-term azole treatment; discrepant MIC results | |
| V156-11 | >16 | 4 | 2 | >16 | 4 | 0.5 | 0/6 | 3/6 | No specific growth characteristics | |
| V159-73 | >16 | 2 | 2 | >16 | 2 | 0.5 | 1/6 | 0/6 | No specific growth characteristics | |
Strain and patient characteristics were added when available. There were discrepancies in the MIC results between measurements 1 and 2 for isolates V059-27 and V130-18. Strain characteristics were added for the 4 missed strains.
The experienced observers 1 and 2 had worked with the VIPcheck before, and their previous experience using the agar-based resistance detection for azole resistance surveillance was that all isolates showed uninhibited growth at 48 h. Therefore, they were less aware that some resistant isolates with rare mutations may show only minimal growth after 48 h. The high specificity of 1.00 and lower sensitivity for these observers indicate that their personal threshold for minimal growth was higher than for the inexperienced observers.
The performance of individual wells as an indicator for specific drug resistance is lower, especially for posaconazole. The plates are developed to distinguish between azole-susceptible and (multi)azole-resistant phenotypes. Based on these results, theoretically, isolates that are monoresistant to posaconazole have higher chance to be missed by the VIPcheck. However, such a phenotype has not been described in the literature, and virtually all isolates resistant to posaconazole or voriconazole are cross-resistant to itraconazole (27). Furthermore, isavuconazole was not added to the plates. Isavuconazole resistance is highly correlated with voriconazole resistance, and monoresistance to isavuconazole alone has not been documented; thus, resistance to isavuconazole is unlikely to be missed (28).
There are some limitations of this study. The performance of the VIPcheck was evaluated in a single center using stored isolates from our database. This gave us the chance to include a substantial number of resistant isolates with diverse resistance genotypes, and it made it possible to perform reliability and reproducibility tests. The percentage of uncommon resistance mechanisms in this collection was higher than that observed in clinical practice; thus, the isolates used in this study do not resemble a real-life situation. The results should thus be confirmed in a prospective setting and in a multicenter setting.
The VIPcheck provides an easy-to-use, sensitive, and specific screening method for discrimination between azole-susceptible and -resistant isolates of A. fumigatus. Uninhibited growth after 24 h can be used as an indicator for azole resistance. At 48 h, minimal growth should be used as the threshold for azole resistance. The assay can be used in nonexpert laboratories to screen A. fumigatus cultures for triazole resistance and select isolates that subsequently need MIC testing. The assay provides a reliable indication of resistance and thus may guide the choice of antifungal therapy while awaiting the results of MIC testing. Such an approach would reduce the delay of appropriate antifungal therapy, although further studies are needed to explore this strategy. As the time to administration of effective antifungal therapy is an important factor for a successful clinical response, this might help improve patient survival.
MATERIALS AND METHODS
Isolates.
Ninety-six A. fumigatus isolates collected between 1994 and 2014 were used for the validation of the VIPcheck plates. Isolates were selected from the fungal culture collection of the Radboud University Medical Center, Nijmegen, The Netherlands. All but three isolates were cultured from patient specimens. Forty-five isolates were azole susceptible based on EUCAST broth microdilution reference method and clinical breakpoints. Fifty-one isolates showed an azole-resistant phenotype to one or more of the mold-active triazoles itraconazole, posaconazole, and/or voriconazole (29). For all isolates, the full cyp51A gene and promoter region had been sequenced (4). Isolates were stored in glycerol broth at −80°C and subcultured twice on Sabouraud dextrose agar to produce fresh conidia. The in vitro activities of voriconazole, itraconazole, and posaconazole were tested in accordance with the EUCAST broth microdilution reference method (15). The same culture was then used to inoculate the VIPcheck.
Quality control.
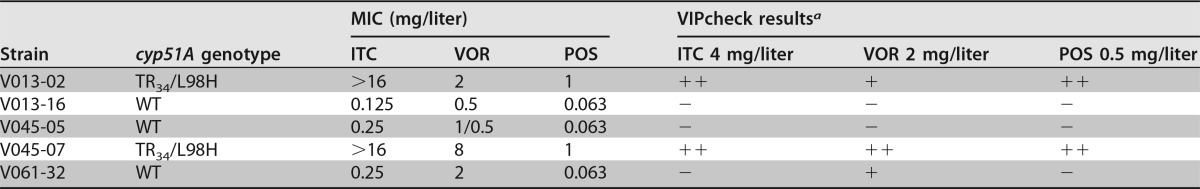
Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were used as quality controls for EUCAST susceptibility testing. The quality control routine for a batch of VIPcheck plates consists of the inoculation of five A. fumigatus isolates with known phenotype: two azole-susceptible isolates; one isolate resistant for itraconazole and posaconazole and intermediate for voriconazole resulting in minimal growth in the well supplemented with voriconazole; one isolate resistant for both itraconazole, posaconazole, and voriconazole; and one isolate with intermediate susceptibility for voriconazole resulting in minimal growth. The MICs and genotype of the quality control (QC) strains can be found at Table 6. All EUCAST susceptibility results and VIPcheck results were conform to the quality control requirements.
TABLE 6.
Characteristics of quality control strains
+, minimal growth; ++ uninhibited growth; −, no growth.
Inoculation of VIPcheck.
Briefly, a wet sterile swab was used to collect conidia from an A. fumigatus colony to make a 0.5 to 2 McFarland suspension visually in 1 ml of sterile water. A disposable pipette was used to add one drop of the suspension (25 μl) to each of the four wells. The wells contain either 4 mg/liter itraconazole (ITC), 2 mg/liter voriconazole (VRC), or 0.5 mg/liter posaconazole (POS). The fourth well is the growth control. The lid was put on the VIPcheck, and the plates were incubated for 48 h at 37°C. The presence of growth was determined after 1 day and 2 days of incubation.
Performance of the VIPcheck.
In this study, 96 isolates were tested in duplicate (series 1 and 2) to assess the reproducibility and reliability. Series 1 was inoculated by an inexperienced researcher (observer 5) who had not worked with the VIPcheck before. The second series was inoculated by an experienced mycology technician (observer 1). All plates from both series were read by six observers who were blinded for the genotype and phenotype of the different isolates to assess the interobserver correlation. Observers 1 and 2 were experienced mycology technicians. Observers 3 and 4 were experienced bacteriological technicians without experience in mycology. Observers 5 and 6 were not trained in microbiology: observer 5 was a medical doctor, and observer 6 was a first year intern. Observers were instructed to read the plates and document (i) (close to) uninhibited (++) growth, (ii) minimal (+) growth, or (iii) no growth for each individual well at both 24 h and 48 h.
Statistics.
Pilot testing indicated sensitivity close to 1.00 after 48 h for the VIPcheck. To achieve a 0.99 sensitivity with a 95% confidence interval (CI) of above 0.88, the sample size of the resistant isolates was calculated to be 51 (30). All calculations were performed using SPSS (version 22) and GraphPad Prism (version 5). Using the EUCAST MICs as the golden standard, the performance of the VIPcheck was evaluated using a 2 by 2 cross table. The outcomes reported are sensitivity and specificity, with concomitant 95% CI calculated using GraphPad Prism with the two-tailed Fisher's exact test. Means were calculated using SPSS, and no CI were reported.
The interclass correlation coefficient (ICC) was calculated with SPSS using a mixed model, and the single measurement value is reported as the result. To assess the reproducibility among the duplicates, agreement between series 1 and series 2 was calculated as the Cohen's kappa with SPSS, which corrects for chance.
Supplementary Material
ACKNOWLEDGMENTS
We thank T. van Oorsouw, C. van der Rijt-Van den Biggelaar, and M. Vermeeren for assisting with the reading of the VIPcheck results.
We received no external funding for the study.
VIPcheck is a trademark registered by Radboud University Medical Center, Department of Medical Microbiology, Mycology Section, Nijmegen, The Netherlands. P.E.V. is the founder of VIPcheck. P.E.V. has received research grants from Gilead Sciences, Astellas, Merck Sharp & Dohme (MSD), F2G, and Bio-Rad, is a speaker for Gilead Sciences and MSD, and is on the advisory boards for Pfizer, MSD, and F2G. All other authors declare no competing interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01250-17.
REFERENCES
- 1.Chowdhary A, Kathuria S, Xu J, Meis JF. 2013. Emergence of azole-resistant Aspergillus fumigatus strains due to agricultural azole use creates an increasing threat to human health. PLoS Pathog 9:e1003633. doi: 10.1371/journal.ppat.1003633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Linden JW, Snelders E, Kampinga GA, Rijnders BJ, Mattsson E, Debets-Ossenkopp YJ, Kuijper EJ, Van Tiel FH, Melchers WJ, Verweij PE. 2011. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007–2009. Emerg Infect Dis 17:1846–1854. doi: 10.3201/eid1710.110226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snelders E, van der Lee HA, Kuijpers J, Rijs AJ, Varga J, Samson RA, Mellado E, Donders AR, Melchers WJ, Verweij PE. 2008. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med 5:e219. doi: 10.1371/journal.pmed.0050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Linden JW, Camps SM, Kampinga GA, Arends JP, Debets-Ossenkopp YJ, Haas PJ, Rijnders BJ, Kuijper EJ, van Tiel FH, Varga J, Karawajczyk A, Zoll J, Melchers WJ, Verweij PE. 2013. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin Infect Dis 57:513–520. doi: 10.1093/cid/cit320. [DOI] [PubMed] [Google Scholar]
- 5.Steinmann J, Hamprecht A, Vehreschild MJ, Cornely OA, Buchheidt D, Spiess B, Koldehoff M, Buer J, Meis JF, Rath PM. 2015. Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J Antimicrob Chemother 70:1522–1526. doi: 10.1093/jac/dku566. [DOI] [PubMed] [Google Scholar]
- 6.Verweij PE, Snelders E, Kema GH, Mellado E, Melchers WJ. 2009. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect Dis 9:789–795. doi: 10.1016/S1473-3099(09)70265-8. [DOI] [PubMed] [Google Scholar]
- 7.Verweij PE, Chowdhary A, Melchers WJ, Meis JF. 2016. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis 62:362–368. doi: 10.1093/cid/civ885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meis JF, Chowdhary A, Rhodes JL, Fisher MC, Verweij PE. 2016. Clinical implications of globally emerging azole resistance in Aspergillus fumigatus. Philos Trans R Soc Lond B Biol Sci. 371:20150460. doi: 10.1098/rstb.2015.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Greeff SC, Mouton JW. 2017. NethMap 2017: consumption of antimicrobial agents and antimicrobial resistance among medically important bacteria in the Netherlands/MARAN 2017: monitoring of antimicrobial resistance and antibiotic usage in animals in the Netherlands in 2016. RIVM report 2017-0056, 149–154. [Google Scholar]
- 10.Pelaez T, Monteiro MC, Garcia-Rubio R, Bouza E, Gomez-Lopez A, Mellado E. 2015. First detection of Aspergillus fumigatus azole-resistant strain due to Cyp51A TR46/Y121F/T289A in an azole-naive patient in Spain. New Microbes New Infect 6:33–34. doi: 10.1016/j.nmni.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Astvad KM, Jensen RH, Hassan TM, Mathiasen EG, Thomsen GM, Pedersen UG, Christensen M, Hilberg O, Arendrup MC. 2014. First detection of TR46/Y121F/T289A and TR34/L98H alterations in Aspergillus fumigatus isolates from azole-naive patients in Denmark despite negative findings in the environment. Antimicrob Agents Chemother 58:5096–5101. doi: 10.1128/AAC.02855-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard SJ, Pasqualotto AC, Anderson MJ, Leatherbarrow H, Albarrag AM, Harrison E, Gregson L, Bowyer P, Denning DW. 2013. Major variations in Aspergillus fumigatus arising within aspergillomas in chronic pulmonary aspergillosis. Mycoses 56:434–441. doi: 10.1111/myc.12047. [DOI] [PubMed] [Google Scholar]
- 13.Kolwijck E, van der Hoeven H, de Sevaux RG, ten Oever J, Rijstenberg LL, van der Lee HA, Zoll J, Melchers WJ, Verweij PE. 2016. Voriconazole-susceptible and voriconazole-resistant Aspergillus fumigatus coinfection. Am J Respir Crit Care Med 193:927–929. doi: 10.1164/rccm.201510-2104LE. [DOI] [PubMed] [Google Scholar]
- 14.Verweij PE, Ananda-Rajah M, Andes D, Arendrup MC, Bruggemann RJ, Chowdhary A, Cornely OA, Denning DW, Groll AH, Izumikawa K, Kullberg BJ, Lagrou K, Maertens J, Meis JF, Newton P, Page I, Seyedmousavi S, Sheppard DC, Viscoli C, Warris A, Donnelly JP. 2015. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updat 21–22:30–40. doi: 10.1016/j.drup.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Arendrup MC, Guinea J, Cuenca-Estrella M, Meletiadis J, Mouton JW, Lagrou K, Howard SJ, Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST) . 2015. EUCAST definitive document E.Def 9.3. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds, version 9.3. European Committee for Antimicrobial Susceptibility Testing, Växjö, Sweden: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_9_3_Mould_testing_definitive.pdf. [Google Scholar]
- 16.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.Van der Linden JW, Arendrup MC, Van der Lee HAL, Melchers WJ, Verweij PE. 2009. Azole containing agar plates as a screening tool for azole resistance of Aspergillus fumigatus. Mycoses 52:19. doi: 10.1111/j.1439-0507.2009.01781.x. [DOI] [Google Scholar]
- 18.Buil JB, van der Lee HA, Rijs AJMM, Zoll J, Hovestadt JAMF, Melchers WJG, Verweij PE. 2016. Agar based screening of azole resistance in Aspergillus fumigatus, abstr P1618. 26th Eur Cong Clin Microbiol Infect Dis, Amsterdam, The Netherlands, 9 to 12 April 2016. [Google Scholar]
- 19.Howard SJ, Webster I, Moore CB, Gardiner RE, Park S, Perlin DS, Denning DW. 2006. Multi-azole resistance in Aspergillus fumigatus. Int J Antimicrob Agents 28:450–453. doi: 10.1016/j.ijantimicag.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Mann PA, Parmegiani RM, Wei SQ, Mendrick CA, Li X, Loebenberg D, DiDomenico B, Hare RS, Walker SS, McNicholas PM. 2003. Mutations in Aspergillus fumigatus resulting in reduced susceptibility to posaconazole appear to be restricted to a single amino acid in the cytochrome P450 14alpha-demethylase. Antimicrob Agents Chemother 47:577–581. doi: 10.1128/AAC.47.2.577-581.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diaz-Guerra TM, Mellado E, Cuenca-Estrella M, Rodriguez-Tudela JL. 2003. A point mutation in the 14alpha-sterol demethylase gene cyp51A contributes to itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother 47:1120–1124. doi: 10.1128/AAC.47.3.1120-1124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nascimento AM, Goldman GH, Park S, Marras SA, Delmas G, Oza U, Lolans K, Dudley MN, Mann PA, Perlin DS. 2003. Multiple resistance mechanisms among Aspergillus fumigatus mutants with high-level resistance to itraconazole. Antimicrob Agents Chemother 47:1719–1726. doi: 10.1128/AAC.47.5.1719-1726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellete B, Raberin H, Morel J, Flori P, Hafid J, Manhsung RT. 2010. Acquired resistance to voriconazole and itraconazole in a patient with pulmonary aspergilloma. Med Mycol 48:197–200. doi: 10.3109/13693780902717018. [DOI] [PubMed] [Google Scholar]
- 24.Mellado E, Garcia-Effron G, Alcazar-Fuoli L, Cuenca-Estrella M, Rodriguez-Tudela JL. 2004. Substitutions at methionine 220 in the 14alpha-sterol demethylase (Cyp51A) of Aspergillus fumigatus are responsible for resistance in vitro to azole antifungal drugs. Antimicrob Agents Chemother 48:2747–2750. doi: 10.1128/AAC.48.7.2747-2750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mellado E, Garcia-Effron G, Alcazar-Fuoli L, Melchers WJ, Verweij PE, Cuenca-Estrella M, Rodriguez-Tudela JL. 2007. A new Aspergillus fumigatus resistance mechanism conferring in vitro cross-resistance to azole antifungals involves a combination of cyp51A alterations. Antimicrob Agents Chemother 51:1897–1904. doi: 10.1128/AAC.01092-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camps SM, van der Linden JW, Li Y, Kuijper EJ, van Dissel JT, Verweij PE, Melchers WJ. 2012. Rapid induction of multiple resistance mechanisms in Aspergillus fumigatus during azole therapy: a case study and review of the literature. Antimicrob Agents Chemother 56:10–16. doi: 10.1128/AAC.05088-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Tudela JL, Alcazar-Fuoli L, Mellado E, Alastruey-Izquierdo A, Monzon A, Cuenca-Estrella M. 2008. Epidemiological cutoffs and cross-resistance to azole drugs in Aspergillus fumigatus. Antimicrob Agents Chemother 52:2468–2472. doi: 10.1128/AAC.00156-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gregson L, Goodwin J, Johnson A, McEntee L, Moore CB, Richardson M, Hope WW, Howard SJ. 2013. In vitro susceptibility of Aspergillus fumigatus to isavuconazole: correlation with itraconazole, voriconazole, and posaconazole. Antimicrob Agents Chemother 57:5778–5780. doi: 10.1128/AAC.01141-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verweij PE, Howard SJ, Melchers WJ, Denning DW. 2009. Azole-resistance in Aspergillus: proposed nomenclature and breakpoints. Drug Resist Updat 12:141–147. doi: 10.1016/j.drup.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Flahault A, Cadilhac M, Thomas G. 2005. Sample size calculation should be performed for design accuracy in diagnostic test studies. J Clin Epidemiol 58:859–862. doi: 10.1016/j.jclinepi.2004.12.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.