ABSTRACT
High treatment failure rates for Plasmodium falciparum malaria have been reported in Colombia for chloroquine, amodiaquine, and sulfadoxine-pyrimethamine. Artemisinin combination therapies were introduced in 2006 in Colombia, where artemether-lumefantrine (AL) is currently used to treat uncomplicated P. falciparum malaria. Artemisinin (ART) resistance was initially observed in Southeast Asia as an increased parasite clearance time, manifesting as a positive thick-blood smear on day 3 after treatment (D3 positivity). Recently, mutations in the propeller domain of the P. falciparum kelch13 gene (K13 propeller) have been associated with ART resistance. In this study, we surveyed AL effectiveness at D3 and molecular markers of drug resistance among 187 uncomplicated P. falciparum cases in 4 regions of Colombia from June 2014 to July 2015. We found that 3.2% (4/125) of patients showed D3 positivity, 100% (163/163) of isolates carried wild-type K13 propeller alleles, 12.9% (23/178) of isolates had multiple copies of the multidrug resistance 1 gene (mdr1), and 75.8% (113/149) of isolates harbored the double mutant NFSDD mdr1 haplotype (the underlining indicates mutant alleles). These data suggest that ART resistance is not currently suspected in Colombia but that monitoring for lumefantrine resistance and AL failures should continue.
KEYWORDS: Colombia, K13, Mdr1, antimalarial agents, artemether, artemisinin, drug resistance, lumefantrine, malaria
INTRODUCTION
Colombia is considered a geographical hot spot for the emergence of antimalarial resistance. Around 60,000 malaria cases, caused by Plasmodium falciparum and P. vivax in similar proportions, are reported annually in the country (1, 2). The treatment for uncomplicated P. falciparum malaria in Colombia was chloroquine (CQ) monotherapy until the early 1980s, when CQ resistance appeared and combinations of CQ or amodiaquine (AQ) with sulfadoxine-pyrimethamine (SP) were used. Based on 44% and 97% CQ failure rates (3, 4), the combination of AQ and SP (AQ+SP) officially became the frontline treatment in 1999 and was used throughout Colombia until 2006. At that time, artemether-lumefantrine (AL)—an artemisinin (ART) combination therapy (ACT)—was introduced throughout Colombia except for Antioquia state, where the combination of artesunate and mefloquine (AS-MQ), plus primaquine for P. falciparum gametocyte elimination, was used for 1 year (5).
Most antimalarial efficacy studies performed in Colombia have followed World Health Organization (WHO) recommendations, measuring 3 basic outcomes: (i) early treatment failure (danger signs or severe malaria during the 3 initial days after treatment in the presence of parasitemia or an increase in the parasite count of more than 25% by day 3 [D3] after treatment); (ii) late treatment failure (danger signs or severe malaria in the presence of parasitemia on any day between D4 and the last day of follow-up [D28 or D42] or the presence of parasitemia on any day between D4 and the last day of follow-up [D28 or D42] with axillary temperature of ≥37.5°C); and (iii) adequate response in which the patient remains without parasitemia and clinical signs during the entire follow-up period (6). The results of antimalarial efficacy studies carried out in Colombia from 1978 to 2008 are summarized in Table S1 in the supplemental material.
ART resistance was first suspected in 2008 in Cambodia, where it was described as corresponding to an increased parasite clearance time (7). This finding was later confirmed in a controlled efficacy study, which showed that Cambodian patients treated with artesunate (AS) followed by mefloquine (MQ) cleared their parasitemias up to 95 h after treatment, compared with Thai patients who cleared their parasitemias within 48 h (8). In recent years, multicenter studies have mapped the extent of ART resistance, showing that it is spreading in mainland Southeast Asia (SEA) but has not yet emerged in Africa (9).
Characterization of ART-resistant parasites was initially difficult because the conventional in vitro susceptibility assay, which determines the 50% inhibitory concentration (IC50) of antimalarial drugs, was unable to discriminate ART-resistant parasites and ART-sensitive parasites (8, 10). In 2013, ex vivo and in vitro versions of a novel ring-stage survival assay (RSA), which better mimicked parasite exposure to ART in treated patients, were able to clearly identify ART-resistant parasites associated with delayed parasite clearance times (11). More recently, mutations in the propeller domain of the P. falciparum kelch13 gene (K13, Pf3D7_1343700) were found to be highly prevalent in parasite populations of Cambodia and other Southeast Asian countries (9, 12). In SEA, K13 propeller polymorphism is considered a reliable molecular marker of ART-resistant P. falciparum parasites, which show increased survival rates in RSAs and delayed clearance (i.e., “D3 positivity”) in ACT-treated patients (13). Although D3 positivity is considered a useful tool to detect the emergence of ART resistance in a population (14), especially in resource-limited countries where standardized parasite clearance studies (15) are not feasible, the results can be confounded by host immunity and initial parasite density (14); therefore, follow-up studies to obtain parasite clearance half-life data are needed to confirm D3-positivity rates.
The use of ACTs was proposed as a strategy to delay the development of resistance to individual antimalarial drugs. Since the spread of P. falciparum resistance occurs in patients treated with ACTs, it is therefore important to understand parasite responses to both ART and its partner drugs. The parasite's food vacuole plays very important roles in the action of many antimalarial drugs. ABC-family transporters such as P. falciparum CQ resistance transporter (PfCRT) and P-glycoprotein homologue 1 (Pgh1) are involved in parasite resistance to CQ and amino-alcohol quinolones such as lumefantrine (LF), halofantrine (HF), and MQ. Resistant parasites show transporter modifications that enable them to increase the efflux of drugs from the food vacuole or from the cytoplasm (16). Previous studies have proposed that increased copy numbers and mutations in codons 86, 184, 1034, 1042, and 1246 of the P. falciparum mdr1 gene that encodes Pgh1 modulate parasite responses to ACT partner drugs (17).
Since 2006, only 3 studies (18–20) have used WHO-recommended protocols to measure the antimalarial efficacy of AL in Colombia; however, those studies did not use specific methods to study ART resistance. In addition, therapeutic efficacy studies are logistically complicated and expensive to perform in Colombia and their findings may not fully reflect the reality of field conditions (21). The objectives of this study were to determine the effectiveness of AL treatment among patients with uncomplicated P. falciparum malaria in 4 regions of Colombia; to survey parasite isolates for the presence of K13 propeller mutations associated with ART resistance in SEA; and to survey for increased mdr1 copy numbers and mdr1 single nucleotide polymorphisms (SNPs) associated with MQ and LF (22) resistance.
RESULTS
From June 2014 to July 2015, we enrolled 187 patients: 103 in Chocó, 61 in Nariño, 21 in Antioquia, and 2 in Córdoba (see Fig. S1 and Table S2 in the supplemental material). The median (interquartile range [IQR]) age of patients was 26 years (18 to 38.5), and 36.9% of them were women (Table S3). Of these 187 patients, 23.5% were students, 20.9% gold miners, 17.6% housewives, and 38.0% farmers, lumbermen, construction workers, fishermen, or ranchers. The median (IQR) parasite density was 3,300 parasites/μl (1,840 to 7,000).
Among the 187 enrolled patients, 66.8% (125/187) were successfully followed up on D3 (Table S2). The median (IQR) age of these patients was 26 years (17 to 37), and 40.0% of them were women (Table S3). The median (IQR) parasite density was 3,400 parasites/μl (1,810 to 7,940).
Among the 125 patients evaluated on D3, 3.2% (4/125) showed D3 positivity by microscopy, which was confirmed by nested PCR (nPCR) (Table S4). These 4 patients had D0 and D3 parasite densities of 900 to 8,000 and 40 to 2,120 parasites/μl, respectively. Five additional patients showed D3 gametocytemia by microscopy, which was confirmed by nPCR (Table S4). These 5 patients had D0 parasite densities of 2,200 to 17,000 parasites/μl and D3 gametocyte densities of 40 to 760 gametocytes/μl. All 9 patients with asexual or sexual parasitemia on D3 were enrolled in Chocó. D0 parasite densities were significantly different between D3-negative and D3-positive samples by nPCR (P < 0.05 [Kruskal-Wallis test]) but not microscopy (see Fig. S2).
We used a new PCR amplification and sequencing protocol (Fig. 1) to successfully sequence the entire K13 propeller domain in 87.2% (163/187) of D0 samples and found that all of them carried wild-type K13 propeller alleles. All 9 nPCR-positive samples on D3 also carried wild-type alleles (Table S5). We successfully estimated mdr1 copy numbers in 95.2% (178/187) of D0 samples and 100% (4/4) of D3-positive samples. We detected multicopy mdr1 genotypes in 12.9% (23/178) of D0 samples and 50% (2/4) of D3-positive samples. Among the 178 D0 samples, 8, 10, 3, 1, and 1 samples had 2, 3, 4, 5, and 6 copies of mdr1, respectively (Table 1). Two D3-positive samples had 2 copies of mdr1 (Table S5). Multicopy mdr1 genotypes were detected in Chocó, Nariño, and Antioquia—but not in Córdoba, where we genotyped only 2 samples (Table 1).
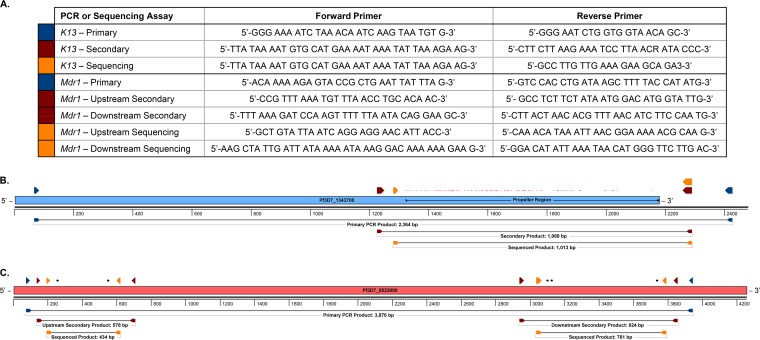
FIG 1.
Primers and schematic representations of K13 and mdr1 nested PCR and sequencing strategies. New nested PCR and sequencing primers (A) were developed to capture the full-length K13 propeller region (B) and the 5 haplotype-defining mdr1 SNPs (C). The primers in the table are color-coded to match their positions in the schematics. Positions of reported K13 propeller region SNPs (reviewed in reference 13) are marked in red. Positions of the 5 mdr1 haplotype SNPs (described in reference 48) are marked in black. Schematics were generated using SeqBuilder v.14 (DNASTAR).
TABLE 1.
Distribution of mdr1 copy numbers among 178 P. falciparum isolates from Colombian states
| Colombian state | No. (%) of isolates with indicated no. of mdr1 copies |
% of samples with increased mdr1 copy no. (no. of samples with increased mdr1 copy no./total no. of samples) | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Chocó | 84 | 5 | 6 | 2 | 1 | 0 | 14.3 (14/98) |
| Nariño | 53 | 3 | 2 | 1 | 0 | 0 | 10.2 (6/59) |
| Antioquia | 16 | 0 | 2 | 0 | 0 | 1 | 15.8 (3/19) |
| Córdoba | 2 | 0 | 0 | 0 | 0 | 0 | 0 (0/2) |
| Total | 155 (87.0) | 8 (4.5) | 10 (5.6) | 3 (1.7) | 1 (0.6) | 1 (0.6) | 12.9 (23/178) |
We used a new PCR amplification and sequencing protocol (Fig. 1) to genotype mdr1 codons 86, 184, 1034, 1042, and 1246 in 96.2%, 94.1%, 90.4%, 90.4%, and 82.3% of the 187 D0 samples. Of those samples successfully genotyped, 100% carried the wild-type N86 allele; 98.3% the mutant 184F allele; 99.4% the wild-type S1034 allele; 100% the mutant 1042D allele; and 24.0% the mutant 1246Y allele. Genotyping the codon 1246 SNP was likely less successful due to necessary trimming of poor-quality 5′ sequence from the downstream nPCR product. We successfully genotyped all 5 mdr1 SNPs in 79.7% (149/187) of D0 samples and found the NFSDD and NFSDY haplotypes in 75.8% (113/149) and 24.2% (36/149) of these samples, respectively (Table 2) (boldface underlined characters indicate mutant alleles). All nine D3 samples with asexual or sexual parasitemia by microscopy carried the NFSDD (n = 6) or NFSDY (n = 3) haplotypes (Table S5).
TABLE 2.
Distribution of mdr1 SNP haplotypes among P. falciparum isolates with 2 or 3 mdr1 SNPs from Colombian states
| Colombian state | No. (%) of NFSDD haplotypes | No. (%) of NFSDY haplotypes |
|---|---|---|
| Chocó | 59 (74.7) | 20 (25.3) |
| Nariño | 53 (94.6) | 3 (5.4) |
| Antioquia | 0 (0) | 13 (100) |
| Córdoba | 1 (100) | 0 (0) |
| Total | 113 (75.8) | 36 (24.2) |
DISCUSSION
To the best of our knowledge, this is the first report of a study investigating the effectiveness of routine AL treatment for malaria, K13 propeller alleles, and mdr1 copy number and SNP data from the same Colombian samples. Due to low sample numbers in Córdoba, we can only estimate the prevalence of these phenotypes and genotypes in Chocó, Nariño, and Antioquia. Using our new sequencing protocol, which captures the entire K13 propeller region, we found no evidence for the presence of K13 propeller mutations. This finding is similar to that recently reported for 66 samples from Nariño, Guaviare, and Córdoba in 2012 to 2013 (20), and for 162 samples from Brazil in 2010 to 2013 (23), and contrasts sharply with the deep reservoir of K13 propeller polymorphism in Africa (24, 25), where parasite diversity and transmission rates are much higher (26). However, we did identify 4/125 (3.2%) patients with D3 positivity, suggesting the possibility that a subset of parasites is becoming less susceptible to ART through mutation in other loci, that some individuals have relatively poor parasite-clearing immune responses, or that some patients were not self-administering their remaining doses of AL. Although we did not perform a classic therapeutic efficacy study, our findings reflect the AL response of patients under ordinary day-to-day conditions in Colombia. The low D3-positivity rate that we measured is consistent with similarly low estimates (0% to 8.6%) in a systematic review of 11 efficacy studies in South America (14), with a 0% estimate in a recent report of 90 patients in 3 Colombian localities (20), and with low (0% to 1.7%) early treatment failure rates following ACT in Colombia in 2000 to 2007 (3, 5, 18, 27). Overall, our data suggest that a small minority of Colombian parasites survives ART exposure at 72 h and thus could evolve genetic changes that confer decreased susceptibility to LF.
We found multicopy (up to 6 copies) mdr1 genotypes in 12.9% (23/178) of samples, which is lower than the 32% prevalence recently reported for P. falciparum isolates from Nariño, Guaviare, and Córdoba in 2011 to 2012 (20). Whether these mdr1 polymorphisms were selected previously by MQ exposure or more recently by LF exposure or both remains to be investigated. Elsewhere in South America, no mdr1 amplification was observed in 26 isolates from Brazil in the late 1990s (28), in 162 isolates from Brazil in 2010 to 2013 (23), or in 104 and 62 isolates from Peru in 1999 and 2006 to 2007, respectively (29), a short time after the introduction of ACT. On the other hand, 12.5% (5/40) and 8.7% (2/23) of isolates from Surinam in 2005 and 2009, respectively, carried 2 mdr1 copies (30). While the lack of recent published studies prevents us from determining whether these mdr1 polymorphisms are becoming more prevalent over time in Colombia or elsewhere in South America, recent mdr1 copy number data from Aponte et al. (20) show that, collectively, 32.0% (26/81) of isolates in Córdoba, Nariño, and Guaviare in 2012 to 2013 had multicopy mdr1 genotypes. Specifically, 67%, 17%, 10%, 2.5%, and 2.5% of 81 isolates had 1, 2, 3, 4, and 5 copies, respectively—a copy number distribution like that in our study (Table 1). Given that a >1.5-fold increase in mdr1 copy number has been associated with a >2-fold increase in LF IC50 (from 8.8 nM to 18.9 nM) (16), it is possible that AL usage is driving this genetic variation. Overall, these data suggest that multicopy mdr1 genotypes are prevalent in areas of malaria endemicity of Colombia and that future studies are needed to determine whether (i) the prevalence of increased mdr1 copy numbers is rising, (ii) parasites are evolving decreased susceptibility to LF in vitro, and (iii) AL therapeutic efficacy is dropping. Importantly, a recent study (31) found that mdr1 amplification—even in the absence of K13 propeller mutation—is a risk factor for AS-MQ failure along the Thailand-Myanmar border, further justifying continued monitoring for this genetic change in Colombia.
We also found the NFSDD and NFSDY mdr1 haplotypes in 75.8% and 24.2% of samples that were successfully genotyped for all 5 SNPs. These data differ moderately from those of a previous study (32), which found the NFSDD and NFSDY haplotypes in 40.0% and 53.3% of samples, respectively, from the Colombian Pacific Coast 16 years ago. Since there is a high probability of finding clonal P. falciparum infections in regions of malaria endemicity in Colombia (33), it is possible that we have repeatedly sampled the same clone in our study. No clear associations between these common mdr1 SNPs and treatment failures have been found in South America. Although the wild-type N86 allele can be selected by LF (34) and is commonly found in Colombian parasites (93.3% of isolates from the Pacific Coast in 1999 to 2001 [32], 97.2% of isolates from Antioquia in 2002 [35], 100% in our study), it has not been significantly associated with CQ, AQ, and MQ treatment failure (35) or CQ, AQ, and MQ susceptibility in vitro (36). Aponte et al. found similar allele frequencies in 13 samples from Guaviare, Nariño, and Córdoba, where they found that 100% of samples carried the wild-type 86N allele and mutant 184F and 1042D alleles and that 77% of them had the mutant 1246Y allele. In Benin, however, the wild-type N86 allele was selected for in recurrent infections after AL treatment and was associated with decreased LF and MQ susceptibility ex vivo (37). Remarkably, 100% of our Colombian isolates carried the wild-type N86 allele, as did 100% of Brazilian isolates in 2012 to 2013 (38). It is not known whether this is because LF is selecting the N86 allele or because CQ—which has limited use in the treatment of vivax malaria—is failing to select the 86Y allele, which is found at increased levels in some regions following CQ use and is associated with decreased CQ susceptibility in vitro in Asia (39). We also found that 98.3% of our samples carried the 184F mutation, similarly to the 92.8% and 100% prevalences reported for Colombian Pacific Coast samples (32) and Brazilian samples (38). The relationship between the 184F mutation and antimalarial drug responses is unclear, as it has been associated with decreased in vitro susceptibility to MQ, HF, and quinine (QN) in some studies (37, 40) but not in others (37, 41). In Senegal, however, the single mutant 86Y-Y184 haplotype—which was rare in our study—has been significantly associated with increased in vitro susceptibility to AQ, MQ, and LF (41). It is possible that the N86-184F haplotype that we commonly identified in our samples, which has been associated with decreased susceptibility to LF, MQ, QN, and piperaquine (37), has been selected over the drug-susceptible 86Y-184Y haplotype.
Regarding other mdr1 SNP positions, we found the wild-type S1034 allele in 99.4% of our isolates and the mutant 1042D allele in 100%. These findings are similar to the 100% S1034 and 93.3% 1042D prevalences reported for Colombian isolates (20, 32) and to the 84% 1034C and 100% 1042D prevalences reported for Brazilian isolates (38). Although SNPs at positions 1034 and 1042 have not been adequately evaluated, the 1042D mutation has been associated with increased in vitro susceptibility to LF in Thailand in 1999 to 2002, when AS-MQ was being used to treat falciparum malaria (42). Finally, we found that 24.0% of our samples carried the 1246Y mutation. Most of these samples were from Chocó and Antioquia, where AS-MQ was used to treat falciparum malaria for 1 year and where it is suspected that HF is illegally available; thus, it is possible that these drugs have selected for this mutant allele. In contrast, Aponte et al. found that 77% of samples in Guaviare and Córdoba, where AL therapy has been used since 2006, carried the 1246Y mutation (20). The 1246Y mutation is rare in Africa (34) but is common in South America. For example, this mutation was found in 100% of samples in Brazil's Amazonas state in 2012 to 2013 (38), 60% of samples in Colombia's Pacific Coast region in 1999 to 2001 (32), and 92% of samples in northwest Colombia in 2004 to 2005 (36). While some studies have associated the 1246Y mutation with decreased in vitro susceptibility to AQ (32) or MQ and LF (16), others have found no associations with decreased in vitro susceptibility to MQ, QN, or AQ (36).
Our study had several strengths and limitations. It provided estimates of D3 positivity after AL treatment under field conditions, as previous studies (21, 43, 44) have done, as well as the prevalence of K13 propeller mutations, mdr1 copy numbers, and 5 mdr1 SNPs in 4 Colombian provinces. Our methodology using PCR amplification and sequencing of the entire K13 propeller domain enabled us to screen for previously reported mutations in C-terminal codons G709, T703, and H719, which we were unable to do using previously published protocols. Our study was unable to account for the potentially confounding role of naturally acquired immunity in parasite clearance, mostly because age is not a good surrogate for immunity in semi-immune human populations. Also, we could not assess the efficacy of AL or parasite susceptibility to LF in vitro or relate these phenotypes to D3 positivity rates or genetic markers of drug resistance, as we could not confirm adherence to the 3-day ACT regimen or measure ex vivo drug susceptibility in our field settings. Moreover, we identified only 4 D3-positive patients (all of which had an initial parasite density of <10,000 parasites/μl) and therefore could not investigate parasite correlates of D3 positivity. Finally, we did not genotype our parasite isolates to investigate whether we have repeatedly sampled persistent, clonal parasite populations that may be circulating in Colombia (33). If they are, and if some mutations are genetically fixed within them, this could partly explain the very high prevalence of double and triple mdr1 mutants, for example, or the absence of K13 propeller polymorphism (unlike in Africa) in our study.
In summary, our report provides baseline prevalence data for D3-positivity rates under routine treatment conditions, K13 propeller mutations, increased mdr1 copy numbers, and 5 mdr1 SNPs, which can now be monitored prospectively in Chocó, Nariño, and Antioquia. Parasites from these states do not currently show evidence of ART resistance but do show evidence of increased mdr1 copy numbers and multiple mdr1 SNPs. Future studies are needed to investigate whether any of these mdr1 polymorphisms confer decreased susceptibility to LF in vitro or are associated with decreased AL efficacy in patients and to monitor for increasing prevalence of K13 propeller mutations. This report thus provides a view of genetic variation in two resistance-related genes (mdr1 and K13) in the country in South America with the third-greatest rate of malaria endemicity and raises the possibility of parasite resistance in Colombia spreading to neighboring countries.
MATERIALS AND METHODS
Study sites and patients.
Colombia is a tropical country located in northwest South America with an area of 1,141,748 km2 and 48 million inhabitants, 23% of whom are at risk of malaria parasite infection. This study was conducted in 4 regions of malaria endemicity: Nariño, Chocó, Córdoba, and Antioquia (see Fig. S1 in the supplemental material). These regions report >90% of P. falciparum malaria cases annually, and their principal economic activities are gold mining, fishing, and cocoa and banana cultivation. Patients diagnosed with P. falciparum malaria by expert microscopists at health care posts were invited to participate in the study. Inclusion criteria were modified from WHO recommendations and included parasite density of >500 parasites/μl and <50,000 parasites/μl, uncomplicated malaria, age >7 years and <65 years, and willingness to return to the clinic on D3. Patients with higher parasite densities were excluded because Colombia's national guide for malaria treatment (45) recommends that they be treated with a parenteral antimalarial. Patients aged ≤7 years were excluded because they are uncommon in our study populations and cannot ethically provide assent. Patients with danger signs like repetitive vomiting and diarrhea, which may lead to dehydration, were also excluded per the recommendations of the WHO and Colombian guidelines.
Enrollment and follow-up.
Patients who fulfilled the inclusion criteria were invited to participate in the study. After written informed consent was obtained, a finger-prick blood sample was taken to prepare thick-blood smears and dried Whatman 3MM filter-paper blood spots. Complete AL (Coartem) treatment consisted of 6 doses of 1.4 to 4 mg of artemether/kg of body weight and 10 to 16 mg of LF/kg dosed according to age over 3 days. After blood sampling, the first dose of AL was given and the patient observed for vomiting for 1 h, and the remaining 5 doses were given to the patient for self-administration (routine practice in Colombia). Patients were provided transportation back to the clinic about 72 h later, at which time a “D3” finger-prick blood sample was taken for a thick-blood smear and dried filter-paper blood spot. This study was approved by the Ethics Committee of the School of Medicine, Universidad de Antioquia, Medellín, Colombia.
P. falciparum microscopy.
Parasites were visualized in thick-blood smears stained with Field or Romanowsky modified stains. Parasites were counted against 200 leukocytes, assuming a leukocyte count of 8,000/μl. Smears were considered negative for parasitemia only after 500 leukocytes were counted. Field- and reference laboratory-based microscopists provided the first and second counts; discrepancies were reconciled by a third microscopist under blind conditions.
DNA extraction.
DNA was extracted from dried filter-paper blood spots using a QIAamp DNA minikit (Qiagen, Valencia, CA) and following the manufacturer's protocol.
Nested PCR to detect parasite infection.
Nested PCRs (nPCRs) to confirm P. falciparum or P. vivax infection on D0 and D3 were performed as described previously (46), with some modifications to primer annealing temperature and number of cycles, and used primers Rplu6 (5′-TTA AAA TTG TTG CAG TTA AAA CG-3′) and Rplu5 (5′-CCT GTT GTT GCC TTA AAC TTC-3′) to amplify DNA from both species in the primary PCR and rFAL1 (5′-TTA ACC TGG TTT GGG AAA ACC AA ATA TAT T-3′) and rFAL2 (5′-ACA CAA TGA ACT CAA TCA TGA CTA CCC GTC-3′) to detect P. falciparum or rVIV1 (5′-CGC TTC TAG CTT AAT CCA CAT AAC TGA TAC-3′) and rVIV2 (5′-ACT TCC AAG CCG AAG CAA AGA AAG TCC TTA-3′) to detect P. vivax in the nPCR. The master mix reaction mixture contained 2 mM MgCl2, 0.1 mM deoxynucleoside triphosphates (dNTPs), 0.125 μM primers, and 0.5 U Taq DNA polymerase (Thermo Fisher Scientific, Waltham, MA).
Nested PCR to amplify the entire K13 propeller domain.
We developed a new PCR method to amplify the entire K13 propeller domain (Fig. 1). The primary PCR primers (forward [FWD]) 5′-GGG AAA ATC TAA ACA ATC AAG TAA TGT G-3′ and (reverse [REV]) 5′-GGG AAT CTG GTG GTA ACA GC-3′ amplified the expected 2,364-bp product under the following conditions: 95°C for 5 min; 40 cycles of 95°C for 30 s, 52°C for 30 s, 62°C for 30 s, and 65°C for 2.5 min; and final extension at 65°C for 5 min. The nested PCR primers (FWD) 5′-TTA TAA AAT GTG CAT GAA AAT AAA TAT TAA AGA AG-3′ and (REV) 5′-CTT CTT AAG AAA TCC TTA ACR ATA CCC-3′ amplified the expected 1,068-bp product under the following conditions: 95°C for 5 min; 40 cycles of 95°C for 30 s, 50°C for 30 s, 62°C for 30 s, and 65°C for 1 min; and final extension at 65°C for 5 min. Platinum PCR SuperMix (Invitrogen, Carlsbad, CA) was used as the master mix and was supplemented with a 300 nM concentration of each primer. Final volumes for primary and nested PCRs were 16.5 μl (15 μl master mix plus 1.5 μl DNA template) and 33 μl (30 μl master mix plus 3 μl DNA template), respectively.
Sequencing the K13 propeller domain.
The PCR-amplified products were sent to Macrogen (Rockville, MD, USA) for double-stranded sequencing of the entire K13 propeller domain, using 5 μM primers (FWD) 5′-TTA TAA AAT GTG CAT GAA AAT AAA TAT TAA AGA AG-3′ and (REV) 5′-GCC TTG TTG AAA GAA GCA GA3-3′ (Fig. 1). For sequence analysis, ambiguous end sequences were trimmed and assembled against the P. falciparum 3D7 K13 propeller domain using Sequencher version 5.1 software (Gene Codes Corporation, Ann Arbor, MI). Using our new set of primers improved the possibility of covering the complete C-terminal end (containing 17 codons) of the K13 propeller domain.
Quantitative PCR to estimate mdr1 copy number.
Mdr1 copy number was estimated as described previously (47), using a 20-μl reaction volume containing 2 to 8 μl DNA template, 20 μl SensiFast SYBR green I (Bioline, Taunton, MA), and 300 nM primers (FWD) 5′-CAA GTG AGT TCA GGA ATT GGT AC-3′ and (REV) 5′-GCC TCT TCT ATA ATG GAC ATG G-3′ for mdr1 and primers (FWD) 5′-GCC TCT TCT ATA ATG GAC ATG G-3′ and (REV) 5′-TTT CAG CTA TGG CTT CAT CAA A-3′ for ldh in a 96-well plate (Bio-Rad, Hercules, CA) and a CFX real-time PCR machine (Bio-Rad). Conditions for quantitative PCR (qPCR) were 95°C for 15 min followed by 40 cycles of 95°C for 15 s, 58°C for 30 s, and 72°C for 20 s. At the end of reactions, the cycle threshold (CT) was manually set to the level reflecting the best kinetic PCR parameters, and melting curves were analyzed. Relative copy number was estimated based on the ΔΔCT method (2ΔΔCT), as follows: [(CTmdr1 − CTldh)sample − (CTmdr1 − CTldh)3D7]. Each reaction was done in triplicate, and the 3D7 and Dd2 P. falciparum reference strains were used to calibrate 1 and 2 mdr1 copies, respectively. Assays with a standard deviation of >25% were repeated. Samples with values of 1.69 to 2.49 were deemed to have 2 copies; 2.50 to 3.49, 3 copies; 3.50 to 4.49, 4 copies; 4.50 to 5.49, 5 copies; and 5.50 to 6.49, 6 copies. Samples showing multiple mdr1 copies were tested again in an independent assay, and the estimate with a lower standard deviation was chosen for analysis. In validated assays in which strain 3D7 values were normalized to 1 mdr1 copy, strain Dd2 values ranged from 1.80 to 2.25 copies.
PCR amplification and sequencing of mdr1.
A long PCR was set up to amplify a large 3,870-bp region of mdr1 (Fig. 1). The primary PCR primers (FWD) 5′-ACA AAA AGA GTA CCG CTG AAT TAT TTA G-3′ and (REV) 5′-GTC CAC CTG ATA AGC TTT TAC CAT ATG-3′ amplified the expected 3,876-bp product under the following conditions: 95°C for 5 min; 40 cycles of 95°C for 30 s, 52°C for 30 s, 62°C for 30 s, and 65°C for 4 min; and final extension at 65°C for 10 min. Two nPCRs were then performed to amplify 2 shorter fragments (Fig. 1). To capture SNPs at codons 86 and 184, the nested primers (FWD) 5′-CCG TTT AAA TGT TTA ACC TGC ACA AC-3′ and (REV) 5′-GCC TCT TCT ATA ATG GAC ATG GTA TTG-3′ amplified the expected 578-bp product under the following conditions: 95°C for 5 min; 40 cycles of 95°C for 30 s, 52°C for 30 s, 62°C for 30 s, and 65°C for 1 min; and final extension at 65°C for 5 min. To capture SNPs at codons 1032, 1042, and 1246, the nested primers (FWD) 5′-TTT AAA GAT CCA AGT TTT TTA ATA CAG GAA GC-3′ and (REV) 5′-CTT ACT AAC ACG TTT AAC ATC TTC CAA TG-3′ amplified the expected 924-bp product under the following conditions: 95°C for 5 min; 40 cycles of 95°C for 30 s, 54°C for 30 s, 62°C for 30 s, and 65°C for 1.5 min; and final extension at 65°C for 5 min. The primary and nested PCR volumes were 15 μl and 30 μl, respectively, and the reaction mixtures contained Platinum PCR SuperMix (Invitrogen), and 1.5 μl genomic DNA (primary PCR) or 3 μl primary PCR product (nPCR). For sequencing the upstream fragment, the primers (FWD) 5′-GCT GTA TTA ATC AGG AGG AAC ATT ACC-3′ and (REV) 5′-CAA ACA TAA ATT AAC GGA AAA ACG CAA G-3′ were used. To sequence the downstream fragment, the primers (FWD) 5′-AAG CTA TTG ATT ATA AAA ATA AAG GAC AAA AAA GAA G-3′ and (REV) 5′-GGA CAT ATT AAA TAA CAT GGG TTC TTG AC-3′ were used. Sequences were analyzed using Sequencher version 5.1 software. Samples with low quality were resequenced using an increased amount of template DNA.
Statistical analysis.
Raw data were organized using Microsoft Excel, and Kruskal-Wallis and Dunn's tests were performed using GraphPad Prism version 6.0 (GraphPad Software, La Jolla, CA).
Supplementary Material
ACKNOWLEDGMENTS
We thank our patients and their families who participated in this study, Pharath Lim for assistance with the qPCR assay, and the following health centers for their collaboration: Divino Niño Hospital and Department Institute of Health in Tumaco, Nariño; Ismael Roldan Hospital and Laboratory of Public Health in Qubdo, Chocó; Nuestra Señora del Carmen Hospital in El Bagre, Antioquia; and Divino Niño Health Care Center in Puerto Libertador, Córdoba.
This study was supported by the Proyecto Malaria Colombia-Global Fund, Pan American Health Organization (PAHO), Universidad de Antioquia, and the Intramural Research Program of the NIAID, NIH. M. Montenegro's fellowship was supported by the Colciencias Doctoral Program.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01036-17.
REFERENCES
- 1.Instituto Nacional de Salud. 2015. Boletin epidemiologico semana 52. Instituto Nacional de Salud, Bogotá, Colombia: http://www.ins.gov.co/boletin-epidemiologico/Boletn%20Epidemiolgico/2015%20Boletin%20epidemiologico%20Semana%2052.pdf. [Google Scholar]
- 2.Instituto Nacional de Salud. 2014. Boletin epidemiológico semana 51. Instituto Nacional de Salud, Bogotá, Colombia: http://www.ins.gov.co/boletin-epidemiologico/Boletn%20Epidemiolgico/2014%20Boletin%20epidemiologico%20Semana%2051.pdf. [Google Scholar]
- 3.Blair S, Carmona-Fonseca J, Piñeros JG, Ríos A, Alvarez T, Alvarez G, Tobón A. 2006. Therapeutic efficacy test in malaria falciparum in Antioquia, Colombia. Malar J 5:14. doi: 10.1186/1475-2875-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osorio LE, Giraldo LE, Grajales LF, Arriaga AL, Andrade AL, Ruebush TK, Barat LM. 1999. Assessment of therapeutic response of Plasmodium falciparum to chloroquine and sulfadoxine-pyrimethamine in an area of low malaria transmission in Colombia. Am J Trop Med Hyg 61:968–972. doi: 10.4269/ajtmh.1999.61.968. [DOI] [PubMed] [Google Scholar]
- 5.Vásquez AM, Sanín F, Alvarez LG, Tobón A, Ríos A, Blair S. 2009. Therapeutic efficacy of a regimen of artesunate-mefloquine-primaquine treatment for Plasmodium falciparum malaria and treatment effects on gametocytic development. Biomedica 29:307–319. (In Spanish.) doi: 10.7705/biomedica.v29i2.32. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. 2009. Methods for surveillance of antimalarial drug efficacy. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/atoz/9789241597531/en/. [Google Scholar]
- 7.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM; Artemisinin Resistance in Cambodia 1 (ARC1) Study Consortium. 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med 359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 8.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, et al. 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amaratunga C, Sreng S, Suon S, Phelps ES, Stepniewska K, Lim P, Zhou C, Mao S, Anderson JM, Lindegardh N, Jiang H, Song J, Su XZ, White NJ, Dondorp AM, Anderson TJ, Fay MP, Mu J, Duong S, Fairhurst RM. 2012. Artemisinin-resistant Plasmodium falciparum in Pursat province, western Cambodia: a parasite clearance rate study. Lancet Infect Dis 12:851–858. doi: 10.1016/S1473-3099(12)70181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witkowski B, Amaratunga C, Khim N, Sreng S, Chim P, Kim S, Lim P, Mao S, Sopha C, Sam B, Anderson JM, Duong S, Chuor CM, Taylor WR, Suon S, Mercereau-Puijalon O, Fairhurst RM, Menard D. 11 September 2013. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in-vitro and ex-vivo drug-response studies. Lancet Infect Dis doi: 10.1016/S1473-3099(13)70252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Ménard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Ménard D. 2014. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairhurst RM, Dondorp AM. 2016. Artemisinin-resistant Plasmodium falciparum malaria. Microbiol Spectr 4(3). doi: 10.1128/microbiolspec.EI10-0013-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das D, Price RN, Bethell D, Guerin PJ, Stepniewska K. 2013. Early parasitological response following artemisinin-containing regimens: a critical review of the literature. Malar J 12:125. doi: 10.1186/1475-2875-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flegg JA, Guerin PJ, White NJ, Stepniewska K. 2011. Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator. Malar J 10:339. doi: 10.1186/1475-2875-10-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veiga MI, Ferreira PE, Jörnhagen L, Malmberg M, Kone A, Schmidt BA, Petzold M, Björkman A, Nosten F, Gil JP. 2011. Novel polymorphisms in Plasmodium falciparum ABC transporter genes are associated with major ACT antimalarial drug resistance. PLoS One 6:e20212. doi: 10.1371/journal.pone.0020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffing S, Syphard L, Sridaran S, McCollum AM, Mixson-Hayden T, Vinayak S, Villegas L, Barnwell JW, Escalante AA, Udhayakumar V. 2010. pfmdr1 amplification and fixation of pfcrt chloroquine resistance alleles in Plasmodium falciparum in Venezuela. Antimicrob Agents Chemother 54:1572–1579. doi: 10.1128/AAC.01243-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatz C, Soto J, Nothdurft HD, Zoller T, Weitzel T, Loutan L, Bricaire F, Gay F, Burchard GD, Andriano K, Lefevre G, De Palacios PI, Genton B. 2008. Treatment of acute uncomplicated falciparum malaria with artemether-lumefantrine in nonimmune populations: a safety, efficacy, and pharmacokinetic study. Am J Trop Med Hyg 78:241–247. [PubMed] [Google Scholar]
- 19.Carrasquilla G, Baron C, Monsell EM, Cousin M, Walter V, Lefevre G, Sander O, Fisher LM. 2012. Randomized, prospective, three-arm study to confirm the auditory safety and efficacy of artemether-lumefantrine in Colombian patients with uncomplicated Plasmodium falciparum malaria. Am J Trop Med Hyg 86:75–83. doi: 10.4269/ajtmh.2012.11-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aponte S, Guerra AP, Alvarez-Larrotta C, Bernal SD, Restrepo C, Gonzalez C, Yasnot MF, Knudson-Ospina A. 2017. Baseline in vivo, ex vivo and molecular responses of Plasmodium falciparum to artemether and lumefantrine in three endemic zones for malaria in Colombia. Trans R Soc Trop Med Hyg 111:71–80. doi: 10.1093/trstmh/trx021. [DOI] [PubMed] [Google Scholar]
- 21.Sondo P, Derra K, Diallo-Nakanabo S, Tarnagda Z, Zampa O, Kazienga A, Valea I, Sorgho H, Owusu-Dabo E, Ouedraogo JB, Guiguemde TR, Tinto H. 2015. Effectiveness and safety of artemether-lumefantrine versus artesunate-amodiaquine for unsupervised treatment of uncomplicated falciparum malaria in patients of all age groups in Nanoro, Burkina Faso: a randomized open label trial. Malar J 14:325. doi: 10.1186/s12936-015-0843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkatesan M, Gadalla NB, Stepniewska K, Dahal P, Nsanzabana C, Moriera C, Price RN, Martensson A, Rosenthal PJ, Dorsey G, Sutherland CJ, Guerin P, Davis TM, Menard D, Adam I, Ademowo G, Arze C, Baliraine FN, Berens-Riha N, Bjorkman A, Borrmann S, Checchi F, Desai M, Dhorda M, Djimde AA, El-Sayed BB, Eshetu T, Eyase F, Falade C, Faucher JF, Froberg G, Grivoyannis A, Hamour S, Houze S, Johnson J, Kamugisha E, Kariuki S, Kiechel JR, Kironde F, Kofoed PE, LeBras J, Malmberg M, Mwai L, Ngasala B, Nosten F, Nsobya SL, Nzila A, Oguike M, Otienoburu SD, Ogutu B, et al. 2014. Polymorphisms in Plasmodium falciparum chloroquine resistance transporter and multidrug resistance 1 genes: parasite risk factors that affect treatment outcomes for P. falciparum malaria after artemether-lumefantrine and artesunate-amodiaquine. Am J Trop Med Hyg 91:833–843. doi: 10.4269/ajtmh.14-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ladeia-Andrade S, de Melo GN, de Souza-Lima RDC, Salla LC, Bastos MS, Rodrigues PT, Luz F, Ferreira MU. 2016. No clinical or molecular evidence of Plasmodium falciparum resistance to artesunate-mefloquine in northwestern Brazil. Am J Trop Med Hyg 95:148–154. doi: 10.4269/ajtmh.16-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor SM, Parobek CM, DeConti DK, Kayentao K, Coulibaly SO, Greenwood BM, Tagbor H, Williams J, Bojang K, Njie F, Desai M, Kariuki S, Gutman J, Mathanga DP, Martensson A, Ngasala B, Conrad MD, Rosenthal PJ, Tshefu AK, Moormann AM, Vulule JM, Doumbo OK, Ter Kuile FO, Meshnick SR, Bailey JA, Juliano JJ. 2015. Absence of putative artemisinin resistance mutations among Plasmodium falciparum in sub-Saharan Africa: a molecular epidemiologic study. J Infect Dis 211:680–688. doi: 10.1093/infdis/jiu467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MalariaGEN Plasmodium falciparum Community Project. 4 March 2016. Genomic epidemiology of artemisinin resistant malaria. Elife doi: 10.7554/eLife.08714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conway DJ. 2007. Molecular epidemiology of malaria. Clin Microbiol Rev 20:188–204. doi: 10.1128/CMR.00021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osorio L, Gonzalez I, Olliaro P, Taylor WR. 2007. Artemisinin-based combination therapy for uncomplicated Plasmodium falciparum malaria in Colombia. Malar J 6:25. doi: 10.1186/1475-2875-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zalis MG, Pang L, Silveira MS, Milhous WK, Wirth DF. 1998. Characterization of Plasmodium falciparum isolated from the Amazon region of Brazil: evidence for quinine resistance. Am J Trop Med Hyg 58:630–637. doi: 10.4269/ajtmh.1998.58.630. [DOI] [PubMed] [Google Scholar]
- 29.Bacon DJ, McCollum AM, Griffing SM, Salas C, Soberon V, Santolalla M, Haley R, Tsukayama P, Lucas C, Escalante AA, Udhayakumar V. 2009. Dynamics of malaria drug resistance patterns in the Amazon basin region following changes in Peruvian national treatment policy for uncomplicated malaria. Antimicrob Agents Chemother 53:2042–2051. doi: 10.1128/AAC.01677-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labadie-Bracho M, Adhin MR. 2013. Increased pfmdr1 copy number in Plasmodium falciparum isolates from Suriname. Trop Med Int Health 18:796–799. doi: 10.1111/tmi.12118. [DOI] [PubMed] [Google Scholar]
- 31.Phyo AP, Ashley EA, Anderson TJ, Bozdech Z, Carrara VI, Sriprawat K, Nair S, White MM, Dziekan J, Ling C, Proux S, Konghahong K, Jeeyapant A, Woodrow CJ, Imwong M, McGready R, Lwin KM, Day NP, White NJ, Nosten F. 2016. Declining efficacy of artemisinin combination therapy against P. falciparum malaria on the Thai-Myanmar border (2003-2013): the role of parasite genetic factors. Clin Infect Dis 63:784–791. doi: 10.1093/cid/ciw388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Echeverry DF, Holmgren G, Murillo C, Higuita JC, Björkman A, Gil JP, Osorio L. 2007. Short report: polymorphisms in the pfcrt and pfmdr1 genes of Plasmodium falciparum and in vitro susceptibility to amodiaquine and desethylamodiaquine. Am J Trop Med Hyg 77:1034–1038. [PubMed] [Google Scholar]
- 33.Echeverry DF, Nair S, Osorio L, Menon S, Murillo C, Anderson TJ. 2013. Long term persistence of clonal malaria parasite Plasmodium falciparum lineages in the Colombian Pacific region. BMC Genet 14:2. doi: 10.1186/1471-2156-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiaco K, Teixeira J, Machado M, do Rosário V, Lopes D. 2015. Evaluation of artemether-lumefantrine efficacy in the treatment of uncomplicated malaria and its association with pfmdr1, pfatpase6 and K13-propeller polymorphisms in Luanda, Angola. Malar J 14:504. doi: 10.1186/s12936-015-1018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montoya P, Tobón A, Blair S, Carmona J, Maestre A. 2007. Polymorphisms of the pfmdr1 gene in field samples of Plasmodium falciparum and their association with therapeutic response to antimalarial drugs and severe malaria in Colombia. Biomedica 27:204–215. (In Spanish.) doi: 10.7705/biomedica.v27i2.216. [DOI] [PubMed] [Google Scholar]
- 36.Restrepo-Pineda E, Arango E, Maestre A, Do Rosário VE, Cravo P. 2008. Studies on antimalarial drug susceptibility in Colombia, in relation to Pfmdr1 and Pfcrt. Parasitology 135:547–553. doi: 10.1017/S0031182008004307. [DOI] [PubMed] [Google Scholar]
- 37.Dahlström S, Aubouy A, Maïga-Ascofaré O, Faucher JF, Wakpo A, Ezinmègnon S, Massougbodji A, Houzé P, Kendjo E, Deloron P, Le Bras J, Houzé S. 2014. Plasmodium falciparum polymorphisms associated with ex vivo drug susceptibility and clinical effectiveness of artemisinin-based combination therapies in Benin. Antimicrob Agents Chemother 58:1–10. doi: 10.1128/AAC.01790-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aguiar AC, Pereira DB, Amaral NS, De Marco L, Krettli AU. 2014. Plasmodium vivax and Plasmodium falciparum ex vivo susceptibility to anti-malarials and gene characterization in Rondônia, West Amazon, Brazil. Malar J 13:73. doi: 10.1186/1475-2875-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pirahmadi S, Zakeri S, Afsharpad M, Djadid ND. 2013. Mutation analysis in pfmdr1 and pfmrp1 as potential candidate genes for artemisinin resistance in Plasmodium falciparum clinical isolates 4 years after implementation of artemisinin combination therapy in Iran. Infect Genet Evol 14:327–334. doi: 10.1016/j.meegid.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Lim P, Wongsrichanalai C, Chim P, Khim N, Kim S, Chy S, Sem R, Nhem S, Yi P, Duong S, Bouth DM, Genton B, Beck HP, Gobert JG, Rogers WO, Coppee JY, Fandeur T, Mercereau-Puijalon O, Ringwald P, Le Bras J, Ariey F. 2010. Decreased in vitro susceptibility of Plasmodium falciparum isolates to artesunate, mefloquine, chloroquine, and quinine in Cambodia from 2001 to 2007. Antimicrob Agents Chemother 54:2135–2142. doi: 10.1128/AAC.01304-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wurtz N, Fall B, Pascual A, Fall M, Baret E, Camara C, Nakoulima A, Diatta B, Fall KB, Mbaye PS, Diémé Y, Bercion R, Wade B, Pradines B. 2014. Role of Pfmdr1 in in vitro Plasmodium falciparum susceptibility to chloroquine, quinine, monodesethylamodiaquine, mefloquine, lumefantrine, and dihydroartemisinin. Antimicrob Agents Chemother 58:7032–7040. doi: 10.1128/AAC.03494-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price RN, Uhlemann AC, van Vugt M, Brockman A, Hutagalung R, Nair S, Nash D, Singhasivanon P, Anderson TJ, Krishna S, White NJ, Nosten F. 2006. Molecular and pharmacological determinants of the therapeutic response to artemether-lumefantrine in multidrug-resistant Plasmodium falciparum malaria. Clin Infect Dis 42:1570–1577. doi: 10.1086/503423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Oliveira AM, Chavez J, de Leon GP, Durand S, Arrospide N, Roberts J, Cabezas C, Marquino W. 2011. Efficacy and effectiveness of mefloquine and artesunate combination therapy for uncomplicated Plasmodium falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg 85:573–578. doi: 10.4269/ajtmh.2011.11-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Congpuong K, Bualombai P, Banmairuroi V, Na-Bangchang K. 2010. Compliance with a three-day course of artesunate-mefloquine combination and baseline anti-malarial treatment in an area of Thailand with highly multidrug resistant falciparum malaria. Malar J 9:43. doi: 10.1186/1475-2875-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan American Health Organization. 2010. Guía para la atención clínica integral del paciente con malaria. http://www.paho.org/col/index.php?option=com_docman&view=download&category_slug=publicaciones-ops-oms-colombia&alias=1220-guia-para-la-atencion-clinica-integral-del-paciente-con-malaria&Itemid=688.
- 46.Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN. 1993. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol 61:315–320. doi: 10.1016/0166-6851(93)90077-B. [DOI] [PubMed] [Google Scholar]
- 47.Lim P, Dek D, Try V, Eastman RT, Chy S, Sreng S, Suon S, Mao S, Sopha C, Sam B, Ashley EA, Miotto O, Dondorp AM, White NJ, Su XZ, Char MC, Anderson JM, Amaratunga C, Menard D, Fairhurst RM. 2013. Ex vivo susceptibility of Plasmodium falciparum to antimalarial drugs in western, northern, and eastern Cambodia, 2011–2012: association with molecular markers. Antimicrob Agents Chemother 57:5277–5283. doi: 10.1128/AAC.00687-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foote SJ, Kyle DE, Martin RK, Oduola AM, Forsyth K, Kemp DJ, Cowman AF. 1990. Several alleles of the multidrug-resistance gene are closely linked to chloroquine resistance in Plasmodium falciparum. Nature 345:255–258. doi: 10.1038/345255a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.