ABSTRACT
Foodborne Vibrio vulnificus infections are associated with higher rates of sepsis and mortality than wound infections; however, antibiotic efficacy studies have not been performed in foodborne infection models. The efficacies of ceftriaxone, cefepime, doxycycline, ciprofloxacin, and combination therapy were assessed in V. vulnificus intestinal infection in mice in order to model foodborne infections. In accordance with prior studies of cefotaxime, cefepime was synergistic with doxycycline and ciprofloxacin in vitro; combination therapy significantly decreased bacterial growth, by ≥2 log10 units, from that with antibiotic monotherapy (P < 0.01). In vivo, survival rates in the ceftriaxone (50%), doxycycline (79%), and ciprofloxacin (80%) groups were significantly higher than those in the control group (0%) (P < 0.0001). Survival was significantly higher with ceftriaxone-doxycycline (91%) or ceftriaxone-ciprofloxacin (100%) therapy than with ceftriaxone (50%) (P ≤ 0.05). Survival with cefepime-doxycycline (96%) or cefepime-ciprofloxacin (90%) therapy was significantly higher than that with cefepime alone (20%) (P < 0.001). There was no difference in survival between the combination therapy groups. Thus, we conclude that combination therapy was the most effective treatment for foodborne V. vulnificus septicemia. In a septic patient with a recent ingestion of raw seafood, cefepime in combination with doxycycline or ciprofloxacin should be initiated for coverage of resistant Gram-negative organisms and V. vulnificus pending a microbiological diagnosis. Once a diagnosis of foodborne V. vulnificus septicemia is established, treatment can safely transition to ceftriaxone in combination with doxycycline or ciprofloxacin.
KEYWORDS: Vibrio vulnificus, cefepime, ceftriaxone, doxycycline, ciprofloxacin, foodborne infection, foodborne septicemia, mouse
INTRODUCTION
Vibrio vulnificus is a Gram-negative pathogen found in marine and estuarine environments worldwide (1). The two most common clinical syndromes caused by V. vulnificus are foodborne disease and wound infections; foodborne disease results from the ingestion of raw seafood, particularly oysters, and wound infections occur after skin lesions are exposed to contaminated seawater (2, 3). According to U.S. surveillance data, patients with foodborne illness have higher rates of septicemia (87% versus 55%) and death (61% versus 17%) than those with wound infections (2, 3). Since V. vulnificus disseminates from the gastrointestinal tract to the bloodstream, patients with foodborne infections can progress rapidly to septicemia, disseminated intravascular coagulation, and multiorgan failure within days of consuming seafood (4, 5). Of concern, recent epidemiological studies have reported an increase in the incidence of V. vulnificus infections worldwide. According to FoodNet, an active surveillance network of foodborne diseases in the United States, the annual incidence of V. vulnificus per 100,000 population increased from 0.01 to 0.05 between 1996 and 2010 (6). Furthermore, warming ocean patterns have coincided with the emergence of V. vulnificus infections along the northern U.S. Atlantic coast and Baltic Sea (7, 8).
Given the severity of foodborne V. vulnificus infections, effective empirical treatments and timely diagnosis are essential for halting the progression of disease and improving clinical outcomes (9). Based on in vitro and in vivo studies, the Centers for Disease Control and Prevention (CDC) recommend a fluoroquinolone or a third-generation cephalosporin in combination with a tetracycline for the treatment of V. vulnificus infections (9–12). A time-kill study found that cefotaxime and minocycline were synergistic in vitro, since subinhibitory concentrations of cefotaxime with minocycline reduced V. vulnificus growth by 6 orders of magnitude from that with either antibiotic alone (10). These results translated in vivo: cefotaxime-and-minocycline combination therapy resulted in survival rates significantly higher than those with minocycline monotherapy in a wound infection model in mice (11). Synergy was attributed to the fact that the antibiotics target different processes essential for V. vulnificus growth: cefotaxime targets cell wall growth, while minocycline inhibits protein synthesis (11). Fluoroquinolones were found to be equivalent in efficacy to combination therapy with cefotaxime and minocycline in wound infection models (12). The activity of fluoroquinolones against V. vulnificus may be related to their ability to decrease the production of the V. vulnificus multifunctional autoprocessing repeats-in-toxin (MARTXVv) toxin, the primary virulence factor associated with mortality in mice (13). Because all prior antibiotic efficacy studies were performed in wound infection models, there is a significant gap in our knowledge about the optimal antimicrobial therapy for foodborne V. vulnificus infections. Since foodborne infections are associated with worse clinical outcomes than wound infections, studies are needed to assess the efficacy of antibiotics in an intestinal V. vulnificus infection model.
While CDC treatment guidelines are applicable once a V. vulnificus diagnosis is confirmed, fluoroquinolones, third-generation cephalosporins, and tetracyclines are rarely administered in the initial management of sepsis pending a microbiological diagnosis. For initial treatment, the Surviving Sepsis Campaign recommends the initiation of extended-spectrum β-lactam antibiotics, including cefepime and piperacillin-tazobactam, for coverage of resistant Gram-negative organisms (14). Although sepsis is the most common initial presentation of patients with foodborne V. vulnificus infections, there are no studies on the efficacy of extended-spectrum β-lactam antibiotics for V. vulnificus foodborne septicemia. While one is awaiting a microbiological diagnosis for a septic patient with a recent history of raw seafood consumption, it is essential for the initial empirical antibiotic regimen to provide effective treatment for a possible foodborne V. vulnificus infection, since the illness can progress to death within days.
In this study, a mouse model of intestinal infection now routinely used in bacterial pathogenesis studies (15–18) was employed to assess antibiotic efficacy for the treatment of foodborne V. vulnificus infection. The antibiotics recommended by the CDC based on prior wound infection studies were tested, including ceftriaxone, doxycycline, ciprofloxacin, and combination therapy. We also assessed the efficacy of an extended-spectrum β-lactam antibiotic routinely administered in the empirical treatment of sepsis: cefepime. Cefepime was tested because prior antibiotic susceptibility studies of clinical and environmental V. vulnificus isolates have found lower levels of resistance to cefepime (3%) than to penicillins (10% to 100%) (19–22). In addition, it was hypothesized that cefepime, a fourth-generation cephalosporin, would perform as effectively as other cephalosporins (the first-generation cephalosporin cefazolin and the third-generation cephalosporin cefotaxime) in combination with tetracyclines and fluoroquinolones in vitro and in vivo against V. vulnificus (10, 11, 13, 23, 24). In an analysis of U.S. surveillance data, we found that 38% of patients with V. vulnificus bacteremia received an extended-spectrum β-lactam antibiotic, highlighting the need to assess the efficacy of these antimicrobials against foodborne V. vulnificus infections. In agreement with prior studies on cefotaxime, cefepime was synergistic with doxycycline and ciprofloxacin in vitro against V. vulnificus. The ceftriaxone-doxycycline, ceftriaxone-ciprofloxacin, cefepime-doxycycline, and cefepime-ciprofloxacin combination therapies were the most effective antibiotic regimens for intestinal V. vulnificus infections in mice.
RESULTS
Thirty-eight percent of patients with V. vulnificus bacteremia in the United States received an extended-spectrum β-lactam antibiotic.
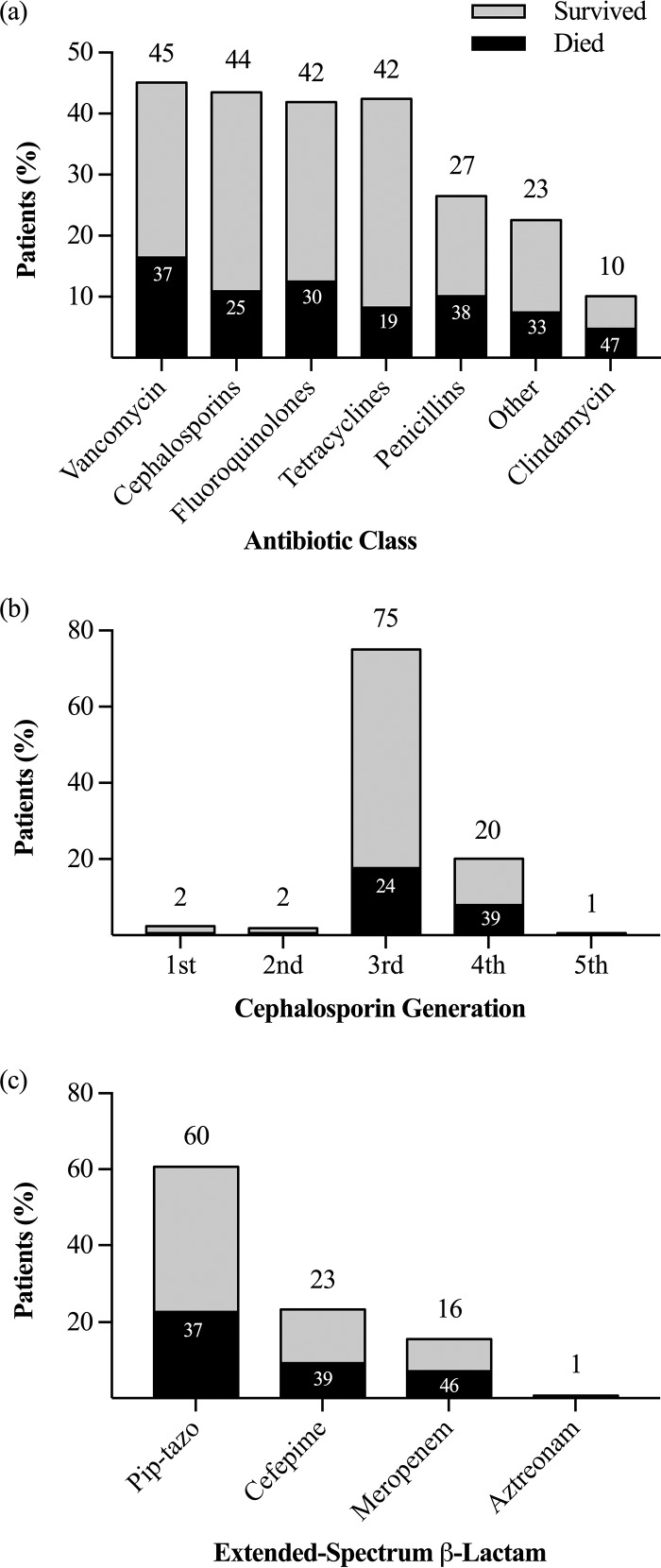
Between 2007 and 2013, 500 cases of V. vulnificus bacteremia were reported to the Cholera and Other Vibrios Illness Surveillance (COVIS), and information on antimicrobial therapy was available for 377 (75%) of these. The most commonly prescribed antibiotics were vancomycin (45%), cephalosporins (44%), fluoroquinolones (42%), tetracyclines (42%), and penicillins (27%) (Fig. 1a). For the 164 (44%) patients treated with a cephalosporin, those most frequently administered were third-generation cephalosporins (ceftriaxone, ceftazidime, cefotaxime) (75%) and cefepime (20%) (Fig. 1b). A total of 142 (38%) patients were treated with an extended-spectrum β-lactam with coverage of resistant Gram-negative organisms, including piperacillin-tazobactam (60%), cefepime (23%), and meropenem (16%) (Fig. 1c). Approximately one-third of patients with V. vulnificus bacteremia (118 patients) died despite receiving antibiotic therapy. The antibiotics with the highest associated mortality rates included clindamycin (47%), meropenem (46%), cefepime (39%), piperacillin-tazobactam (37%), and vancomycin (37%).
FIG 1.
Mortality rates by antibiotic class (a), cephalosporin generation (b), and extended-spectrum β-lactam (c). (a) Frequency of patients with V. vulnificus bacteremia who received antibiotics of each class (n = 377). (b) Frequency of each generation of cephalosporins among patients on cephalosporins (n = 164). (c) Frequency of each antibiotic among patients on an extended-spectrum β-lactam antibiotic (n = 142). Pip-tazo, piperacillin-tazobactam.
Cefepime was synergistic with doxycycline and ciprofloxacin in vitro against V. vulnificus.
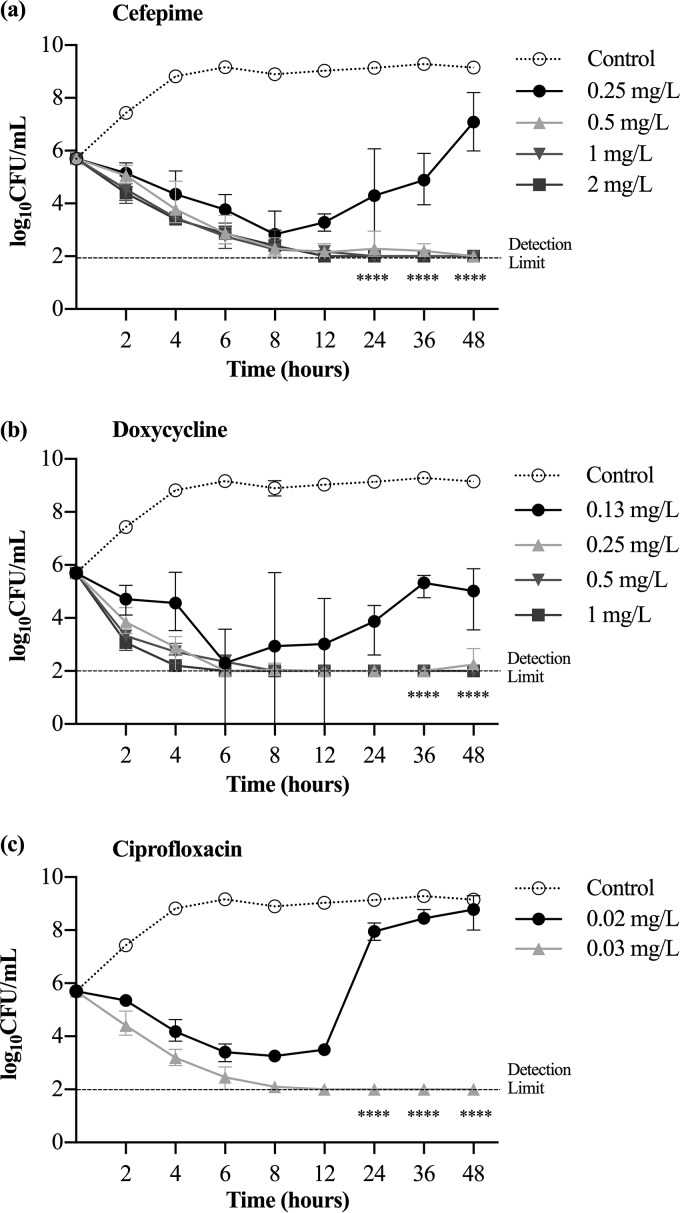
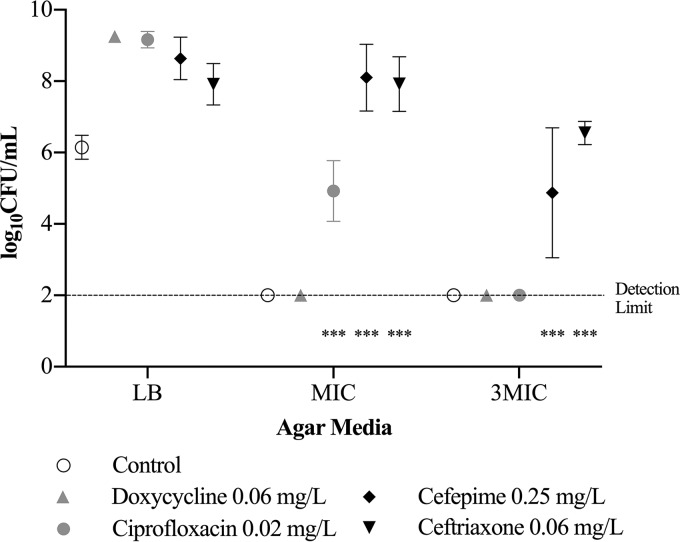
For V. vulnificus M06-24/O, the ceftriaxone MIC was 0.06 mg/liter; the cefepime MIC, 0.25 mg/liter; the doxycycline MIC, 0.13 mg/liter; and the ciprofloxacin MIC, 0.03 mg/liter. The antibiotic concentration with minimal bacterial inhibition was 0.06 mg/liter for ceftriaxone, 0.25 mg/liter for cefepime, 0.13 mg/liter for doxycycline, and 0.02 mg/liter for ciprofloxacin; bacterial growth at these antibiotic concentrations was >2 log10 CFU/ml higher than the detection limit at 36 and 48 h (P < 0.0001) (Fig. 2). V. vulnificus developed antibiotic resistance after suspension in 0.06 mg/liter ceftriaxone and 0.25 mg/liter cefepime (the concentration with minimal bacterial inhibition) for 48 h, as evidenced by the fact that bacterial growth was 2 log10 CFU/ml greater than that of the control on the respective antibiotic agar at the MIC and three times the MIC (P < 0.001) (Fig. 3).
FIG 2.
Inhibition of growth curves at various concentrations of cefepime (a), doxycycline (b), and ciprofloxacin (c). The concentration of antibiotic with minimal bacterial inhibition was 0.25 mg/liter for cefepime, 0.13 mg/liter for doxycycline, and 0.02 mg/liter for ciprofloxacin. Bacterial growth is represented as means ± standard deviations. The antibiotic concentration with minimal growth inhibition had a level of bacterial growth significantly higher, by ≥2 log10 units, than the detection limit (****, P < 0.0001).
FIG 3.
Antibiotic resistance in V. vulnificus after suspension in antibiotic concentrations with minimal bacterial inhibition for 48 h. Bacterial growth is represented as means ± standard deviations on LB agar and the respective antibiotic agar at the MIC and at three times the MIC (3MIC). Asterisks indicate antibiotic groups that had bacterial growth significantly higher, by 2 log10 units, than the control (***, P < 0.001).
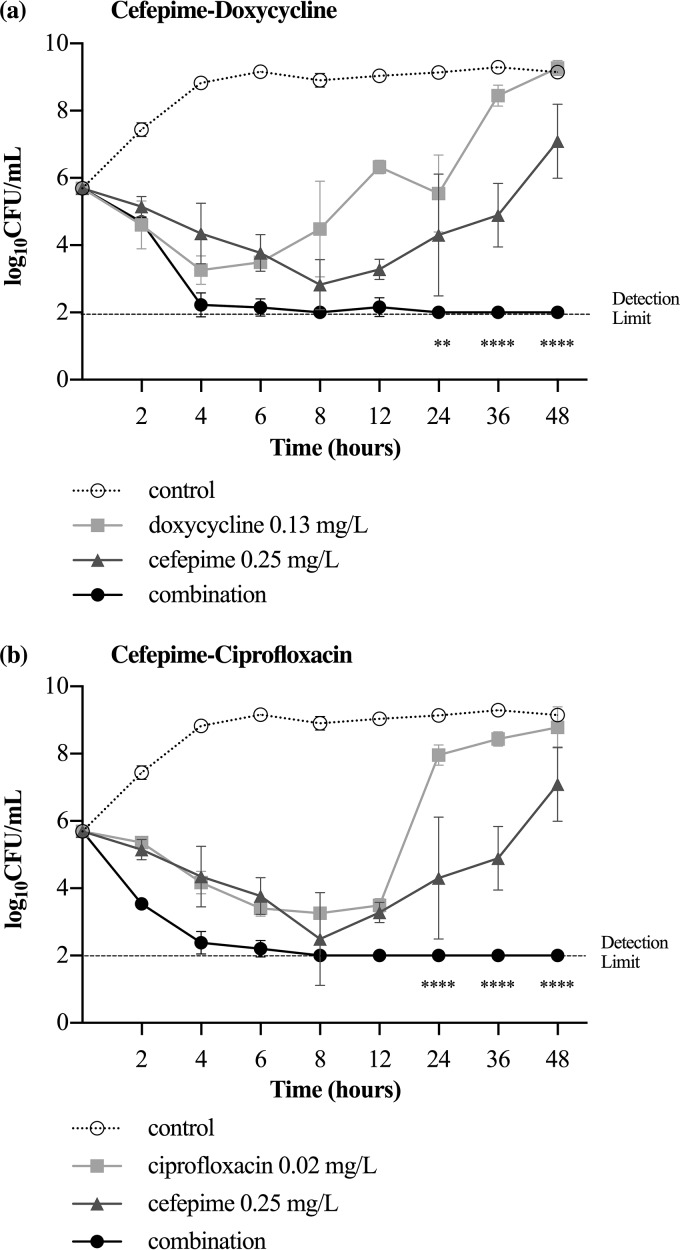
The combination of cefepime at 0.25 mg/liter and doxycycline at 0.13 mg/liter significantly decreased bacterial growth by >2 log10 CFU/ml from that with the antibiotics alone at 24 h (P < 0.01), 36 h, and 48 h (P < 0.0001) (Fig. 4a). The combination of cefepime at 0.25 mg/liter and ciprofloxacin at 0.03 mg/liter significantly decreased bacterial growth by >2 log10 CFU/ml from that with the antibiotics alone at 24, 36, and 48 h (P < 0.0001) (Fig. 4b).
FIG 4.
Inhibition of growth curves for V. vulnificus after incubation with cefepime, doxycycline, or a combination (a) and cefepime, ciprofloxacin, or a combination (b). Bacterial growth is represented as means ± standard deviations. The antibiotic combination significantly decreased bacterial growth, by ≥2 log10 CFU/ml, from that with the individual antibiotics (**, P < 0.01; ****, P < 0.0001).
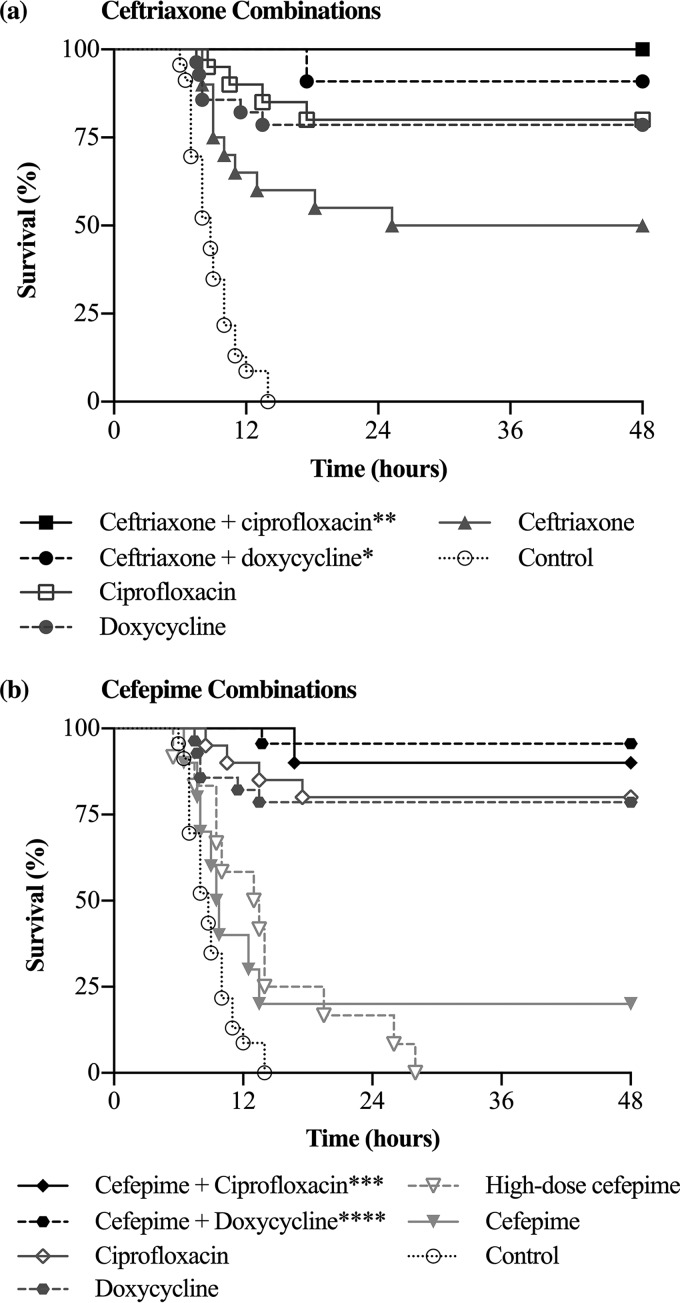
Survival rates in the ceftriaxone, doxycycline, and ciprofloxacin groups were significantly higher than those in the control group.
All mice in the intestinal infection control group (n = 23) died within 14 h. In the groups receiving antibiotics, the survival rate was 80% with ciprofloxacin (n = 20), 79% with doxycycline (n = 28), 50% with ceftriaxone (n = 20), 20% with cefepime (n = 10), and 0% with high-dose cefepime (n = 12) (Fig. 5). Survival was significantly higher in the ceftriaxone, doxycycline, and ciprofloxacin groups than in the control group (P < 0.0001). When antibiotic groups were compared, there was no significant difference in survival between the ciprofloxacin and doxycycline groups (P = 0.82) or between the cefepime and high-dose cefepime groups (P = 0.99). Survival was significantly higher in the ceftriaxone group than in the cefepime group (P ≤ 0.05).
FIG 5.
(a) Survival rates of mice treated with ceftriaxone alone or ceftriaxone combination therapy after V. vulnificus intestinal infection. Survival rates for the ceftriaxone-doxycycline (*, P ≤ 0.05) and ceftriaxone-ciprofloxacin (**, P < 0.01) groups were significantly higher than those for the ceftriaxone group. (b) Survival rates of mice treated with cefepime alone or cefepime combination therapy. Survival in the cefepime-doxycycline (****, P < 0.0001) and cefepime-ciprofloxacin (***, P < 0.001) groups was significantly higher than that in the cefepime group.
Ceftriaxone in combination with doxycycline or ciprofloxacin improved survival over that with ceftriaxone monotherapy for intestinal V. vulnificus infection.
The survival rate in the ceftriaxone-doxycycline group (n = 11) was 91%, and that in the ceftriaxone-ciprofloxacin group (n = 11) was 100% (Fig. 5a). Relative to that for the ceftriaxone group, survival was significantly improved in the ceftriaxone-doxycycline (P ≤ 0.05) and ceftriaxone-ciprofloxacin (P < 0.01) groups. The survival curves did not differ significantly between the doxycycline and ceftriaxone-doxycycline groups (P = 0.35), the ciprofloxacin and ceftriaxone-ciprofloxacin groups (P = 0.12), and the ceftriaxone-doxycycline and ceftriaxone-ciprofloxacin groups (P = 0.32).
Cefepime in combination with doxycycline or ciprofloxacin improved survival over that with cefepime monotherapy for intestinal V. vulnificus infection.
The survival rate in the cefepime-doxycycline group (n = 23) was 96%, and that in the cefepime-ciprofloxacin group (n = 10) was 90% (Fig. 5b). Survival was significantly improved in the cefepime-doxycycline (P < 0.0001) and cefepime-ciprofloxacin (P < 0.001) groups over that in the group receiving cefepime alone. Survival rates did not differ significantly between the ciprofloxacin and cefepime-ciprofloxacin groups (P = 0.48), the doxycycline and cefepime-doxycycline groups (P = 0.07), and the cefepime-doxycycline and cefepime-ciprofloxacin groups (P = 0.56).
There was no significant difference in survival rates between combination therapy groups.
The survival rate in the ceftriaxone-doxycycline group did not differ significantly from those in the cefepime-doxycycline (P = 0.61) and cefepime-ciprofloxacin (P = 0.92) groups. The survival rate in the ceftriaxone-ciprofloxacin group did not differ significantly from those in the cefepime-doxycycline (P = 0.49) and cefepime-ciprofloxacin (P = 0.29) groups.
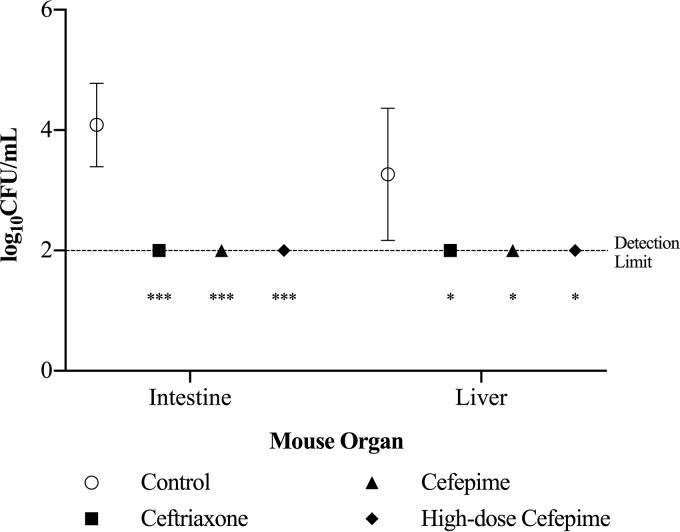
There was no emergence of antibiotic resistance to ceftriaxone, cefepime, or high-dose cefepime in vivo.
Because the ceftriaxone, cefepime, and high-dose cefepime groups had the highest mortality rates, the in vivo experiments for these treatment groups were repeated in order to assess the emergence of antibiotic resistance. At 8 h after infection (6 h after treatment), the liver and intestines were excised in order to be assessed for antibiotic-resistant V. vulnificus. There was no bacterial growth from the livers and intestines of the antibiotic-treated mice (Fig. 6). Each antibiotic-treated group had significantly less bacterial growth, by 2 log10 CFU/ml, in the intestines (P < 0.0001) and the liver (P < 0.05) than the control group. There was no bacterial growth from the excised organs of the antibiotic-treated mice on the respective antibiotic agar at the MIC and three times the MIC.
FIG 6.
V. vulnificus isolated from organs 8 h after mice were inoculated intragastrically (or 6 h after the initiation of antimicrobial therapy). Bacterial growth is represented as means ± standard deviations. No bacterial growth was detected in the intestines and livers of antibiotic-treated mice. Asterisks indicate antibiotic groups that had significantly less bacterial growth than the control (***, by 2 log10 CFU/ml [P < 0.0001]; *, by log10 CFU/ml [P < 0.05]).
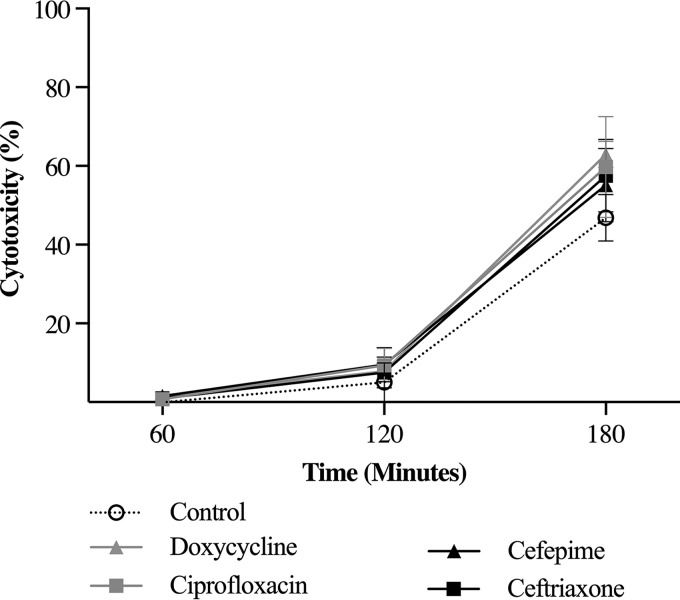
Despite differences in efficacy in vivo, cefepime, ceftriaxone, ciprofloxacin, and doxycycline had the same effect on V. vulnificus cytotoxicity.
There was no significant difference in V. vulnificus cytotoxicity between the control group and the ceftriaxone, cefepime, ciprofloxacin, and doxycycline groups at 60, 120, and 180 min (Fig. 7).
FIG 7.
Effects of subinhibitory levels of antibiotics on V. vulnificus cytotoxicity. HeLa cells were suspended in antibiotics at a concentration of ¼× MIC and were inoculated with V. vulnificus at a multiplicity of infection of 2. There was no significant difference in cytotoxicity between the antibiotic groups and the control at 60, 120, and 180 min.
DISCUSSION
Timely administration of effective antimicrobial therapy is required to halt the progression of foodborne V. vulnificus infection to septicemia and death. Indeed, studies have reported that human survival is strongly correlated with the immediate administration of appropriate antimicrobial therapy (9). Our study found that ciprofloxacin and doxycycline were the most effective antimicrobial agents for intestinal V. vulnificus infections in mice. The efficacy of fluoroquinolones against V. vulnificus wound infections was established previously (12), and this study confirms their utility for foodborne V. vulnificus infections. However, it was unexpected for doxycycline monotherapy to provide a survival benefit in a model of foodborne V. vulnificus infection, since it was found to be ineffective in a wound infection model. In a study by Chuang et al., the mortality rate was 100% among mice treated with minocycline after a subcutaneous injection of V. vulnificus (11). The enhanced efficacy of doxycycline for intestinal infections may be related to its ability to achieve high concentrations in the small intestine and the liver (25). Because doxycycline is excreted in the bile to the feces, drug concentrations are 10 to 25 times higher in the bile than in the serum (25).
In our analysis of COVIS surveillance data, about one-third of patients with V. vulnificus bacteremia received an extended-spectrum β-lactam with coverage of resistant Gram-negative organisms, highlighting the need to assess the efficacy of these antibiotics for foodborne infections. Meropenem, cefepime, and piperacillin-tazobactam were among the antibiotics associated with the highest mortality rates; disease severity was likely a confounding factor, since patients with the most severe infections are usually treated with broad-spectrum antibiotics. The extended-spectrum β-lactam cefepime was tested because the rates of cefepime resistance among clinical and environmental isolates are lower than the rates of resistance to penicillins (20, 21). Although cefepime had good activity against V. vulnificus in vitro, cefepime at standard and high doses did not provide a survival benefit in the intestinal infection model. Similarly, ceftriaxone effectively decreased V. vulnificus growth in vitro but provided a survival benefit to only 50% of mice in vivo. These findings were consistent with those of prior studies on the efficacy of other cephalosporins against V. vulnificus wound infections in mice (11, 13, 24, 26). After subcutaneous injection of V. vulnificus, the mortality rate was 100% for cefazolin- or cefotaxime-treated mice (11, 24, 26). Cefotaxime administered at five times the standard dose did not improve survival in the V. vulnificus wound infection model (mortality, 92%) (11). COVIS surveillance data also suggest that cephalosporins are not effective against V. vulnificus infections (9). In an analysis of 1,599 patients with V. vulnificus septicemia, gastroenteritis, and wound infection reported to the CDC, Wong et al. found that mortality was significantly higher among patients treated with cephalosporin monotherapy (all generations) (37%) than among those receiving fluoroquinolone monotherapy (17%) (9).
The emergence of antibiotic resistance in vivo did not explain the increased mortality among mice treated with cephalosporins. Although V. vulnificus developed resistance to ceftriaxone and cefepime in vitro when suspended in the concentration with minimal bacterial inhibition, both antibiotics effectively eliminated V. vulnificus from the intestines and the liver by 6 h after the initiation of treatment. Furthermore, development of V. vulnificus resistance to cefepime followed by outgrowth in vivo was unlikely, since serum cefepime concentrations were at least 40 times higher than the MIC (0.25 mg/liter). For the cefepime and high-dose cefepime groups, the antibiotic dosing was based on a pharmacokinetic study of cefepime in mice conducted by Maglio et al. (27). According to the study, the cefepime and high-dose cefepime groups received doses to achieve serum cefepime concentrations of 10 to 100 mg/liter and 50 to 500 mg/liter, respectively, over the 48-h treatment period (27). As confirmed in our cefepime time-kill study, a cefepime concentration of 0.5 mg/liter was sufficient to suppress V. vulnificus growth over the 48-h period during which the mice were observed (Fig. 2a).
To explain the differences in survival, it was hypothesized that ciprofloxacin and doxycycline inhibited rtxA1 transcription and MARTXVv toxin production to a greater degree than ceftriaxone and cefepime. Ciprofloxacin and doxycycline both inhibit processes essential for protein synthesis: ciprofloxacin inhibits DNA gyrase, an enzyme that prevents the supercoiling of DNA during replication and transcription (28), and doxycycline binds to the 30S ribosomal subunit and blocks the binding of aminoacyl-tRNA to mRNA (29). A prior study by Jang et al. found that ciprofloxacin significantly decreased rtxA1 transcription and V. vulnificus cytotoxicity from those with cefotaxime (13). In contrast, our study found that the antibiotics did not differ in V. vulnificus cytotoxicity, suggesting that the antibiotics had no influence on MARTXVv toxin production. Jang et al. may have overestimated the role of ciprofloxacin in V. vulnificus cytotoxicity, since there was a modest but significant difference of 20% in cytotoxicity between antibiotics. As seen in Fig. 7, cytotoxicity increased over time in our study, and despite variations at the midpoint, all antibiotics had 60% cytotoxicity by 180 min.
Differences in antibiotic efficacy may be related to how rapidly the antibiotics are able to halt the progression of a V. vulnificus infection. Because ciprofloxacin and doxycycline target processes essential for bacterial survival, these antibiotics can quickly eliminate the initial bacterial inoculum and any subsequent bacterial replicates (28, 29). Targeting the inoculum and subsequent replicates may halt the activation of a systemic inflammatory response. In contrast, because cephalosporins inhibit the final stage of cell wall synthesis, bacterial replicates are targeted, but the initial inoculum can activate an uncontrolled pathogenic systemic inflammatory response (30). In our in vivo experiments, although ceftriaxone and cefepime effectively eliminated V. vulnificus from the gastrointestinal organs by 6 h after treatment, we hypothesize that an uncontrolled systemic inflammatory response had already been activated, leading to high mortality rates. Indeed, a recent study has shown that it is the cytokine storm linked to uncontrolled proinflammatory responses that leads to the death of mice from V. vulnificus infections (31).
Despite differences in survival between the individual antibiotics, the efficacy of both cephalosporins for intestinal V. vulnificus infections increased with the addition of doxycycline or ciprofloxacin. The ceftriaxone-doxycycline, ceftriaxone-ciprofloxacin, cefepime-doxycycline, and cefepime-ciprofloxacin groups had the highest survival rates among the treatment groups. The improved efficacy of combination therapy in wound and foodborne V. vulnificus infections can be attributed to the synergistic interaction of the antibiotics, as demonstrated in vitro. Although survival did not differ statistically between the combination therapy, doxycycline, and ciprofloxacin groups, the additional survival benefit with combination therapy may be clinically meaningful in the setting of an infection with high rates of septicemia and death.
In contrast to prior antibiotic efficacy studies, there were no differences in survival between the combination therapy groups. In a wound infection model, Jang et al. found that survival in the cefotaxime-ciprofloxacin group (75%) was significantly higher than that in the cefotaxime-minocycline group (33%) (13). The differences in survival were attributed to the ability of ciprofloxacin to decrease MARTXVv transcription and the resulting V. vulnificus cytotoxicity (13). Our study found that there was no difference in V. vulnificus cytotoxicity between ciprofloxacin and the control over a 180-min period. For intestinal V. vulnificus infections, doxycycline may be as effective as ciprofloxacin in combination therapy, since both drugs penetrate gastrointestinal tissues at high concentrations (25, 32).
Overall, our study found that the most effective treatments for V. vulnificus foodborne septicemia include ceftriaxone-doxycycline, ceftriaxone-ciprofloxacin, cefepime-doxycycline, and cefepime-ciprofloxacin. For patients presenting in septic shock with a recent history of raw seafood consumption, we recommend initiating cefepime with doxycycline or ciprofloxacin for appropriate coverage of V. vulnificus and Gram-negative resistant organisms while awaiting microbiological cultures. Once a diagnosis of V. vulnificus is confirmed, the antibiotics can be safely deescalated to ceftriaxone with doxycycline or ciprofloxacin.
MATERIALS AND METHODS
Antibiotic use for V. vulnificus bacteremia in the United States.
COVIS is a national passive surveillance system established by the CDC and the Food and Drug Administration (FDA) to monitor the incidence of Vibrio infections in the United States (6, 33). Health care providers submit a report that includes patient demographic, clinical, and epidemiologic information (6, 33). Deidentified COVIS data from 2007 through 2013 were obtained in order to assess the use of antibiotics for V. vulnificus bacteremia in the United States. A maximum of three antibiotics were reported, but start and end dates were not released, so as to protect patient confidentiality. Without prescribing dates, it was not known whether antibiotics were administered concurrently or consecutively, so data were recorded as the patient simply having received at least one dose of the reported antibiotics over the term of treatment. The mortality rate was determined for each antibiotic class, cephalosporin generation, and extended-spectrum β-lactam.
In vitro antibiotic synergy testing.
V. vulnificus M06-24/O, a strain representative of U.S. clinical isolates, was used throughout the study. In vitro susceptibilities of V. vulnificus M06-24/O were determined for cefepime hydrochloride (U.S. Pharmacopeia Reference Standard, Rockville, MD), ceftriaxone sodium, doxycycline hyclate, and ciprofloxacin (Sigma-Aldrich, St. Louis, MO) using the broth microdilution method described in the Clinical and Laboratory Standards Institute (CLSI) guidelines (34). In a round-bottom 96-well plate, serial 2-fold antibiotic dilutions were performed in cation-adjusted Mueller-Hinton broth (CAMHB). Bacteria were inoculated into each well to achieve a final bacterial concentration of 5 × 105 CFU/ml. The inoculated plates were incubated at 30°C in an ambient air incubator. The MIC was the lowest antibiotic concentration that inhibited visible bacterial growth at 16 h. MIC results were confirmed using a SpectraMax M3 multimode microplate reader (Molecular Devices, Sunnyvale, CA); visible growth was defined as an optical density (OD) greater than 0.1 at 600 nm. Escherichia coli ATCC 25922 was used as the positive control.
In vitro synergism was established previously with time-kill assays for the third-generation cephalosporin cefotaxime in combination with minocycline and ciprofloxacin against V. vulnificus (10, 23). To maintain consistency with the prior V. vulnificus antibiotic efficacy studies, time-kill assays were performed as described previously in order to assess if cefepime was synergistic with doxycycline or ciprofloxacin in vitro (10, 23). For cefepime, doxycycline, and ciprofloxacin, volumetric dilutions were performed using CAMHB in 17- by 100-mm test tubes to make antibiotic concentrations of ¼× MIC, ½× MIC, MIC, 2× MIC, and 4× MIC. M06-24/O was inoculated in each test tube to achieve a bacterial concentration of 5 × 105 CFU/ml. The broth cultures were incubated at 30°C and 250 rpm. For each antibiotic concentration, bacterial counts were measured at 2, 4, 6, 8, 12, 24, 36, and 48 h. The antibiotic concentration with minimal growth inhibition—the highest concentration with bacterial growth >2 log10 CFU/ml higher than the detection limit at 48 h—was identified. The detection limit was 1 × 102 CFU/ml, based on the dilution method used to count bacterial colonies.
The emergence of antibiotic resistance was determined for V. vulnificus suspended in the antibiotic concentrations with minimal growth inhibition for 48 h. Broth cultures from the time-kill assays at 48 h were plated onto Luria-Bertani (LB) agar and the respective antibiotic agar at MIC and three times the MIC. Antibiotic resistance was defined as bacterial growth >2 log10 CFU/ml higher than that of the control on the 3× MIC antibiotic agar.
The cefepime-doxycycline and cefepime-ciprofloxacin combinations were tested using the antibiotic concentrations with minimal growth inhibition. Bacterial growth was measured over 48 h. The antibiotic combination was synergistic if bacterial growth was 2 log10 lower than the starting inoculum and the growth with each antibiotic alone (10, 23).
i.g. V. vulnificus infection of mice.
Mouse infections were conducted under protocols approved by the Northwestern University Institutional Animal Care and Use Committee. Each treatment group included 10 to 28 female CD-1 IGS mice (Charles River, Wilmington, MA) aged 32 to 38 days. The 10 treatment groups comprised control (0.9% sodium chloride), ceftriaxone, cefepime, high-dose cefepime, doxycycline, ciprofloxacin, ceftriaxone-doxycycline, ceftriaxone-ciprofloxacin, cefepime-doxycycline, and cefepime-ciprofloxacin groups. The cefepime and high-dose cefepime groups were tested to establish the optimal dose for V. vulnificus treatment. Mice were infected intragastrically (i.g.) as reported previously (17). Mice were anesthetized with an intraperitoneal injection of 10 μg/ml ketamine and 10 μg/ml xylazine suspended in 100 μl of phosphate buffer solution (PBS). By use of a feeding needle, mice were inoculated intragastrically with 50 μl of 8.5% sodium bicarbonate to neutralize the stomach, followed by 2 × 106 CFU of M06-24/O (10 times the 50% lethal dose [LD50]). To confirm the bacterial dose administered, the inoculum was plated onto LB agar and was incubated at 30°C for 16 h (17).
Two hours after the intragastric bacterial inoculation, intraperitoneal antibiotics were administered for 48 h. Antibiotic dosing was based on prior mouse studies using V. vulnificus wound infection models: ceftriaxone (Hikma Farmaceutica, Terrugem, Portugal) at 50 mg/kg of body weight every 12 h (11), doxycycline (Frenius Kabi USA, Lake Zurich, IL) at 6 mg/kg every 24 h (11), and ciprofloxacin (Claris Injectibles Ltd., Gujarat, India) at a 16-mg/kg loading dose followed by 8 mg/kg every 12 h (12). Based on a study of cefepime pharmacokinetics in mice (27), the cefepime (Hospira, Inc., Lake Forest, IL) group received 80 mg/kg every 6 h (peak concentration, 175.7 mg/liter; rate of elimination, 0.64 h−1) and the high-dose cefepime group received 300 mg/kg every 6 h (peak concentration, 510.5 mg/liter; rate of elimination, 0.46 h−1). In another study of cefepime pharmacokinetics in mice using an alternative assay for measuring drug plasma levels, cefepime administered subcutaneously at a notably lower dose of 50 mg/kg had a half-life of 1.07 h and a bioavailability of 77% (35). Mice were observed every 2 h for the first 24 h and then every 6 h for the next 24 h for the primary endpoint, death.
The emergence of antibiotic resistance in vivo was determined for the antibiotics with the highest mortality rates: ceftriaxone, cefepime, and high-dose cefepime. In each treatment group, three mice were anesthetized, inoculated with V. vulnificus intragastrically, and treated with the respective antibiotics 2 h after infection. For the recovery of V. vulnificus from the gastrointestinal organs, the mice were euthanized 8 h after infection (6 h after antibiotic treatment). This time point was chosen for two reasons: (i) death in the cefepime and high-dose cefepime treatment groups started around hour 8 of infection, and (ii) serum cefepime concentrations were at trough levels, since it is dosed every 6 h. The liver and intestines were excised and were homogenized in PBS as described previously (17). The CFU count per organ was calculated by plating serially diluted homogenates onto CHROMagar Vibrio (CHROMagar, Springfield, NJ), a medium that isolates and detects Vibrio species based on differential colony colors (green-blue to turquoise-blue for V. vulnificus). The homogenates were plated on antibiotic agar at the MIC and three times the MIC to be assessed for the emergence of antibiotic resistance in vivo.
Effect of antibiotics on V. vulnificus cytotoxicity.
Cytotoxicity assays were performed as described previously to assess the effect of the antibiotics on V. vulnificus MARTXVv production (13). HeLa cervical epithelial cells (ATCC CLL-2) were seeded in six-well culture plates and were incubated at 37°C under 5% CO2 for 16 h to reach 2 × 105 cells per well. The medium was changed to Dulbecco's modified Eagle's medium (DMEM) with an antibiotic concentration of ¼× MIC. Subinhibitory antibiotic concentrations were administered to assess the impact of the antibiotics on V. vulnificus MARTXVv production. The treatment groups included cefepime, ceftriaxone, ciprofloxacin, and doxycycline. The positive control was DMEM alone. M06-24/O was washed with PBS and was inoculated at a multiplicity of infection (MOI) of 2 (4 × 105 CFU per well). Lactate dehydrogenase (LDH) release, a marker of cytotoxicity, was measured from the culture supernatant at 60, 120, and 180 min using the CytoTox96 nonradioactive cytotoxicity assay (Promega, Madison, WI) according to the manufacturer's protocol. Results were read on a SpectraMax M3 multimode microplate reader (Molecular Devices, Sunnyvale, CA) at 490 nm. The culture supernatant at 180 min was plated on LB agar and was incubated at 30°C for 16 h to ensure that the antibiotics were administered at subinhibitory concentrations.
Statistical analysis.
Statistical analyses were performed using GraphPad Prism, version 7.00 for Mac OS X (GraphPad Software, La Jolla, CA). The time-kill and cytotoxicity assays were analyzed using two-way ANOVA (analysis of variance) and Tukey's multiple-comparison test. Survival analyses were conducted using the log rank test. Significance was set at a P value of ≤0.05.
ACKNOWLEDGMENTS
We thank Chao Qi for providing the E. coli ATCC 25922 strain and assisting with the broth microdilution methods. We thank Anna Newton and Erin Burdette at the CDC for the COVIS data. We thank Michael Angarone for advice on study design and review of the manuscript.
The study was supported by the Ruth L. Kirschstein Institutional National Research Service Award Training Grants in Translational Research Training in Infectious Diseases (grant T32A1095207, to S.A.T.) and Cellular and Molecular Basis of Disease (grant T32 GM08061, to H.E.G.), and National Institutes of Health grants R01AI092825-05 and R01AI098369-04 (to K.J.F.S.). The funder had no role in study design, data analysis, or publication submission.
REFERENCES
- 1.Huang KC, Weng HH, Yang TY, Chang TS, Huang TW, Lee MS. 2016. Distribution of fatal Vibrio vulnificus necrotizing skin and soft-tissue infections: a systematic review and meta-analysis. Medicine (Baltimore) 95:e2627. doi: 10.1097/MD.0000000000002627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menon MP, Yu PA, Iwamoto M, Painter J. 2014. Pre-existing medical conditions associated with Vibrio vulnificus septicaemia. Epidemiol Infect 142:878–881. doi: 10.1017/S0950268813001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro RL, Altekruse S, Hutwagner L, Bishop R, Hammond R, Wilson S, Ray B, Thompson S, Tauxe RV, Griffin PM. 1998. The role of Gulf Coast oysters harvested in warmer months in Vibrio vulnificus infections in the United States, 1988–1996. Vibrio Working Group. J Infect Dis 178:752–759. doi: 10.1086/515367. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto K, Ohshige K, Fujita N, Tomita Y, Mitsumizo S, Nakashima M, Oishi H. 2010. Clinical features of Vibrio vulnificus infections in the coastal areas of the Ariake Sea, Japan. J Infect Chemother 16:272–279. doi: 10.1007/s10156-010-0050-Z. [DOI] [PubMed] [Google Scholar]
- 5.Zhao H, Xu L, Dong H, Hu J, Gao H, Yang M, Zhang X, Chen X, Fan J, Ma W. 2015. Correlations between clinical features and mortality in patients with Vibrio vulnificus infection. PLoS One 10:e0136019. doi: 10.1371/journal.pone.0136019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newton A, Kendall M, Vugia DJ, Henao OL, Mahon BE. 2012. Increasing rates of vibriosis in the United States, 1996–2010: review of surveillance data from 2 systems. Clin Infect Dis 54(Suppl 5):S391–S395. doi: 10.1093/cid/cis243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Roux F, Wegner KM, Baker-Austin C, Vezzulli L, Osorio CR, Amaro C, Ritchie JM, Defoirdt T, Destoumieux-Garzon D, Blokesch M, Mazel D, Jacq A, Cava F, Gram L, Wendling CC, Strauch E, Kirschner A, Huehn S. 2015. The emergence of Vibrio pathogens in Europe: ecology, evolution, and pathogenesis (Paris, 11–12th March 2015). Front Microbiol 6:830. doi: 10.3389/fmicb.2015.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vezzulli L, Grande C, Reid PC, Helaouet P, Edwards M, Hofle MG, Brettar I, Colwell RR, Pruzzo C. 2016. Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proc Natl Acad Sci U S A 113:E5062–E5071. doi: 10.1073/pnas.1609157113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong KC, Brown AM, Luscombe GM, Wong SJ, Mendis K. 2015. Antibiotic use for Vibrio infections: important insights from surveillance data. BMC Infect Dis 15:226. doi: 10.1186/s12879-015-0959-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuang YC, Liu JW, Ko WC, Lin KY, Wu JJ, Huang KY. 1997. In vitro synergism between cefotaxime and minocycline against Vibrio vulnificus. Antimicrob Agents Chemother 41:2214–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chuang YC, Ko WC, Wang ST, Liu JW, Kuo CF, Wu JJ, Huang KY. 1998. Minocycline and cefotaxime in the treatment of experimental murine Vibrio vulnificus infection. Antimicrob Agents Chemother 42:1319–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang HJ, Chang MC, Ko WC, Huang KY, Lee CL, Chuang YC. 2002. In vitro and in vivo activities of newer fluoroquinolones against Vibrio vulnificus. Antimicrob Agents Chemother 46:3580–3584. doi: 10.1128/AAC.46.11.3580-3584.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang HC, Choi SM, Kim HK, Kim SE, Kang SJ, Park KH, Ryu PY, Lee TH, Kim YR, Rhee JH, Jung SI, Choy HE. 2014. In vivo efficacy of the combination of ciprofloxacin and cefotaxime against Vibrio vulnificus sepsis. PLoS One 9:e101118. doi: 10.1371/journal.pone.0101118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. 2013. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 15.Kwak JS, Jeong HG, Satchell KJ. 2011. Vibrio vulnificus rtxA1 gene recombination generates toxin variants with altered potency during intestinal infection. Proc Natl Acad Sci U S A 108:1645–1650. doi: 10.1073/pnas.1014339108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong HG, Satchell KJ. 2012. Additive function of Vibrio vulnificus MARTXVv and VvhA cytolysins promotes rapid growth and epithelial tissue necrosis during intestinal infection. PLoS Pathog 8:e1002581. doi: 10.1371/journal.ppat.1002581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gavin HE, Beubier NT, Satchell KJ. 2017. The effector domain region of the Vibrio vulnificus MARTX toxin confers biphasic epithelial barrier disruption and is essential for systemic spread from the intestine. PLoS Pathog 13:e1006119. doi: 10.1371/journal.ppat.1006119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim BS, Gavin HE, Satchell KJ. 2015. Distinct roles of the repeat-containing regions and effector domains of the Vibrio vulnificus multifunctional-autoprocessing repeats-in-toxin (MARTX) toxin. mBio 6:e00324–15. doi: 10.1128/mBio.00324-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elmahdi S, DaSilva LV, Parveen S. 2016. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: a review. Food Microbiol 57:128–134. doi: 10.1016/j.fm.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Baker-Austin C, McArthur JV, Lindell AH, Wright MS, Tuckfield RC, Gooch J, Warner L, Oliver J, Stepanauskas R. 2009. Multi-site analysis reveals widespread antibiotic resistance in the marine pathogen Vibrio vulnificus. Microb Ecol 57:151–159. doi: 10.1007/s00248-008-9413-8. [DOI] [PubMed] [Google Scholar]
- 21.Pan J, Zhang Y, Jin D, Ding G, Luo Y, Zhang J, Mei L, Zhu M. 2013. Molecular characterization and antibiotic susceptibility of Vibrio vulnificus in retail shrimps in Hangzhou, People's Republic of China. J Food Prot 76:2063–2068. doi: 10.4315/0362-028X.JFP-13-161. [DOI] [PubMed] [Google Scholar]
- 22.Radu S, Elhadi N, Hassan Z, Rusul G, Lihan S, Fifadara N, Yuherman, Purwati E. 1998. Characterization of Vibrio vulnificus isolated from cockles (Anadara granosa): antimicrobial resistance, plasmid profiles and random amplification of polymorphic DNA analysis. FEMS Microbiol Lett 165:139–143. doi: 10.1111/j.1574-6968.1998.tb13138.x. [DOI] [PubMed] [Google Scholar]
- 23.Kim DM, Lym Y, Jang SJ, Han H, Kim YG, Chung CH, Hong SP. 2005. In vitro efficacy of the combination of ciprofloxacin and cefotaxime against Vibrio vulnificus. Antimicrob Agents Chemother 49:3489–3491. doi: 10.1128/AAC.49.8.3489-3491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang HJ, Ko WC, Chuang YC, Chen CC, Chiang SR. 2010. Cefazolin plus minocycline against a clinical isolate of Vibrio vulnificus: in vitro and animal studies. Jpn J Infect Dis 63:16–18. [PubMed] [Google Scholar]
- 25.Vojtova V, Urbanek K. 2009. Pharmacokinetics of tetracyclines and glycylcyclines. Klin Mikrobiol Infekc Lek 15:17–21. (In Czech.) [PubMed] [Google Scholar]
- 26.Bowdre JH, Hull JH, Cocchetto DM. 1983. Antibiotic efficacy against Vibrio vulnificus in the mouse: superiority of tetracycline. J Pharmacol Exp Ther 225:595–598. [PubMed] [Google Scholar]
- 27.Maglio D, Ong C, Banevicius MA, Geng Q, Nightingale CH, Nicolau DP. 2004. Determination of the in vivo pharmacodynamic profile of cefepime against extended-spectrum-beta-lactamase-producing Escherichia coli at various inocula. Antimicrob Agents Chemother 48:1941–1947. doi: 10.1128/AAC.48.6.1941-1947.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bearden DT, Danziger LH. 2001. Mechanism of action of and resistance to quinolones. Pharmacotherapy 21:224S–232S. doi: 10.1592/phco.21.16.224S.33997. [DOI] [PubMed] [Google Scholar]
- 29.Chopra I, Roberts M. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev 65:232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barradell LB, Bryson HM. 1994. Cefepime. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 47:471–505. [DOI] [PubMed] [Google Scholar]
- 31.Murciano C, Lee CT, Fernandez-Bravo A, Hsieh TH, Fouz B, Hor LI, Amaro C. 2017. MARTX toxin in the zoonotic serovar of Vibrio vulnificus triggers an early cytokine storm in mice. Front Cell Infect Microbiol 7:332. doi: 10.3389/fcimb.2017.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vance-Bryan K, Guay DR, Rotschafer JC. 1990. Clinical pharmacokinetics of ciprofloxacin. Clin Pharmacokinet 19:434–461. doi: 10.2165/00003088-199019060-00003. [DOI] [PubMed] [Google Scholar]
- 33.Dechet AM, Yu PA, Koram N, Painter J. 2008. Nonfoodborne Vibrio infections: an important cause of morbidity and mortality in the United States, 1997–2006. Clin Infect Dis 46:970–976. doi: 10.1086/529148. [DOI] [PubMed] [Google Scholar]
- 34.Clinical and Laboratory Standards Institute. 2015. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—10th ed. CLSI document M07-A10, CLSI, Wayne, PA. [Google Scholar]
- 35.Bu W, Sexton H, Fan X, Torres P, Houston P, Heyman I, Liu L. 2010. The novel sensitive and high throughput determination of cefepime in mouse plasma by SCX-LC/MS/MS method following off-line microElution 96-well solid-phase extraction to support systemic antibiotic programs. J Chromatogr B Analyt Technol Biomed Life Sci 878:1623–1628. doi: 10.1016/j.jchromb.2010.03.045. [DOI] [PubMed] [Google Scholar]