ABSTRACT
Antibiotic resistance and recurrence of bacterial vaginosis (BV), a polymicrobial infection, justify the need for novel antimicrobials to counteract microbial resistance to conventional antibiotics. Previously, two series of cationic amphiphiles (CAms) which self-assemble into supramolecular nanostructures with membrane-lytic properties were designed with hydrophilic head groups and nonpolar domains. The combination of CAms and commonly prescribed antibiotics is suggested as a promising strategy for targeting microorganisms that are resistant to conventional antibiotics. Activities of the CAms against Gardnerella vaginalis ATCC 14018, a representative BV pathogen, ranged from 1.1 to 24.4 μM. Interestingly, the tested healthy Lactobacillus species, especially Lactobacillus plantarum ATCC 39268, were significantly more tolerant of CAms than the selected pathogens. In addition, CAms prevented biofilm formation at concentrations which did not influence the normal growth ability of G. vaginalis ATCC 14018. Furthermore, the biofilm minimum bactericidal concentration (MBC-Bs) of CAms against G. vaginalis ATCC 14018 ranged from 58.8 to 425.6 μM, while much higher concentrations (≥850 μM) were required to produce ≥3-log reductions in the number of biofilm-associated lactobacilli. The conventional antibiotic metronidazole synergized strongly with all tested CAms against planktonic cells and biofilms of G. vaginalis ATCC 14018. The synergism between CAms and the tested conventional antibiotic may be considered a new, effective, and beneficial method of controlling biofilm-associated bacterial vaginosis.
KEYWORDS: AMP mimics, antimicrobials, Gardnerella vaginalis, biofilm, bacterial vaginosis
INTRODUCTION
Bacterial vaginosis (BV) is a noninflammatory, polymicrobial infection in women of reproductive age (1). Generally, the infection occurs due to a decrease in protective lactobacillus species, leading to the overgrowth of pathogenic anaerobic bacteria that are naturally present at low concentrations within the vaginal lumen (2). The mechanism by which the loss of lactobacilli occurs is not yet clear (3); however, the decrease in the commensal lactobacillus population causes an increase in pH due to reduced lactic acid production and increased fatty acid production by anaerobic bacteria, making the vaginal environment more suitable for opportunistic pathogen growth and unfavorable for lactobacillus growth (4). BV affects 10% to 30% of women in developed nations (5), making it three to four times more common than urinary tract infections and three times more prominent than Trichomonas vaginalis infection (6). While often asymptomatic, BV can lead to serious complications, including but not limited to abortion or premature birth in pregnant women, pelvic inflammatory disease, endometriosis, and infertility (2). During early pregnancy, the cost of screening and treating women for BV is quite high; total costs can amount to over $490,000 (7), not including recurrences. This issue calls for a safe, effective, and economically conscious treatment of the disease. Although no single bacterium can be considered the sole causative agent of BV, Gardnerella vaginalis, an opportunistic anaerobic pathogen, is the predominant bacterial species isolated from BV infections; G. vaginalis forms a thick biofilm and produces toxins as effective virulence factors, increasing bacterial resistance to conventional antibiotics (8). The bacterium has been detected in over 98% of BV cases and often exhibits a symbiotic relationship with other anaerobes (9).
Current treatment of bacterial vaginosis includes conventional antibiotics, most often 400 to 600 mg of metronidazole taken orally (10). Metronidazole is readily taken up through passive diffusion by anaerobic bacteria, in which it is activated within the cytoplasm and then reduced to its active form by electron transport, causing inhibition of DNA synthesis (11). Despite its initial effectiveness against the unwanted bacteria, the antibiotic often fails to fully eradicate the pathogenic biofilm (2). Pathogenic polymicrobial biofilms are especially difficult to eradicate; such biofilms occur in infections such as BV, with G. vaginalis being the predominant BV-associated pathogen (12).
Antibiotic resistance is an impending issue concerning the overuse of antibiotics; Gardnerella strains are increasingly becoming resistant to both clindamycin and, to a lesser extent, metronidazole (13–15). This leads to ineffective treatment of the infection, and often to recurring infection: approximately 80% of treated women will have another episode of BV within 1 year of treatment (16, 17). Therefore, resistance to antibiotics calls for alternative treatments, which leads to an interest in antimicrobial peptides (AMPs). AMPs are proteinaceous substances with low molecular weights and broad-spectrum antimicrobial activity against bacteria, fungi, and viruses (18). AMP-mimicking cationic amphiphilic compounds (CAms; either bola-like or gemini-like) effectively target the lipopolysaccharide (LPS) layer of the cell membrane in Gram-negative microorganisms; at the same time, eukaryotic cell membranes have a high cholesterol content and low anionic charge, which places them out of the target range of most AMPs (19). AMPs can form pores in the cell membrane or disable the proton motive force, as discussed by Wimley and Hristova (20). By targeting the LPS layer of the cell membrane, the cationic AMPs interact with the negatively charged membranes of the bacteria and the biofilm surface electrostatically, killing active and dormant cells or slowing their growth; the hydrophobic domain also interacts with and disrupts the hydrophobic membrane, resulting in cell death (21).
Previously, two series of CAms which self-assemble into supramolecular nanostructures with membrane-lytic properties were designed as AMP mimics with hydrophilic heads and nonpolar domains. In this study, the antimicrobial and antibiofilm activities of CAms (with nomenclatures of G8 and G10, based on the hydrophobic arm length) with ether or ester linkages (22) were determined against G. vaginalis ATCC 14018 and Lactobacillus species by establishing the MIC, the biofilm MIC (MIC-B), and the biofilm minimum bactericidal concentration (MBC-B) of each antimicrobial. The nature of antimicrobial interactions between the CAms and metronidazole against planktonic and biofilm cells was investigated as well.
RESULTS
CAms effectively inhibit planktonic cells of BV-associated pathogens but not lactobacilli.
The antimicrobial potential of CAms against planktonic G. vaginalis ATCC 14018 was determined and compared to their activity against healthy vaginal lactobacilli. The results illustrate that G10 compounds were more active than G8 compounds (Table 1; Fig. 1). The MICs of CAms against G. vaginalis ATCC 14018 were lower than their MICs against two lactobacilli, with significant differences (P < 0.05) for G10 compounds. However, no significant difference between the susceptibility of G. vaginalis ATCC 14018 and that of Lactobacillus gasseri ATCC 33323/Lactobacillus crispatus ATCC 33197 was detected for both types of compounds (Table 1).
TABLE 1.
MICs of CAms with different linker types and hydrophobic arm lengths against G. vaginalis and against healthy Lactobacillus species
| Microorganism | MIC (μM) |
P value for G. vaginalis vs lactobacilli |
MIC (μM) |
P value for G. vaginalis vs lactobacilli |
||
|---|---|---|---|---|---|---|
| G8 ether | G8 ester | G10 ether | G10 ester | |||
| G. vaginalis | 2.5 ± 0.98 | 18.5 ± 7.9 | 0.83 ± 0.0 | 2.3 ± 0.91 | ||
| L. rhamnosus | 38.5 ± 17.6 | 94.03 ± 32.1 | >0.05 | 17.6 ± 7.7 | 12.7 ± 0.0 | <0.05 |
| L. plantarum | 118 ± 34.1 | 185 ± 64.4 | ≤0.05 | 34.8 ± 15.9 | 33.9 ± 14.6 | <0.05 |
| L. gasseri | 9.7 ± 4.3 | 46.2 ± 16.6 | >0.05 | 4.4 ± 1.9 | 12.7 ± 0.0 | >0.05 |
| L. crispatus | 7 ± 0.0 | 55.8 ± 0.0 | >0.05 | 6.3 ± 0 | <3.1 ± 0 | >0.05 |
| Prevotella bivia | 28.4 | 111.6 | 6.6 | 6.3 | ||
| M. curtisii | 28.4 | 93.1 | 5.4 | 1.6 | ||
| Peptostreptococcus anaerobius | 3.6 | 13.9 | 1.6 | 3.1 | ||
FIG 1.
Structures of the tested CAms showing differences in linker type (ether or ester) and hydrophobic arm length (n = 8 or 10).
Since G. vaginalis ATCC 14018 is not the sole bacterial cause of BV, CAms were evaluated against the other most commonly isolated BV-associated pathogens: Mobiluncus curtisii ATCC 35241, Peptostreptococcus anaerobius ATCC 27337, and Prevotella bivia ATCC 29303. Peptostreptococcus anaerobius ATCC 27337 was more susceptible to CAms than M. curtisii ATCC 35241 and Prevotella bivia ATCC 29303 were, and again, G10 ether and G10 ester were more active than G8 compounds against selected pathogens (Table 1). The results showed that the MICs of G10 CAms against the three tested pathogens were significantly lower than their MICs against Lactobacillus plantarum ATCC 39268 and Lactobacillus rhamnosus 160 (P < 0.01), while no significant differences were identified when G8 compounds were used against the pathogens.
MICs and sub-MICs of CAms inhibit G. vaginalis ATCC 14018 biofilm formation.
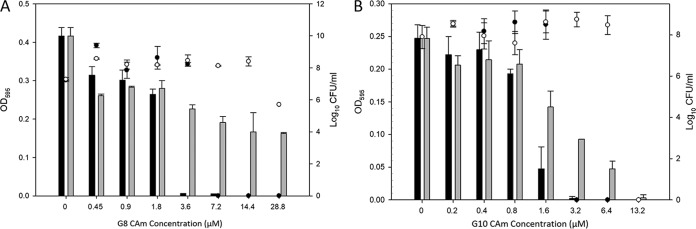
The antibiofilm activity of the CAms was evaluated in vitro against BV-associated G. vaginalis ATCC 14018 in 96-well tissue culture microplates (Fig. 2). The MIC-B values of G8 ether (Fig. 2A) and G10 ether (Fig. 2B) were within their MIC ranges: 3.6 μM G8 ether was required to inhibit >90% of biofilm formation, and 1.6 μM G10 ether and 3.2 μM G10 ester prevented >50% of biofilm formation compared to that of the untreated control. G8 ester showed 50% biofilm inhibition when the sub-MIC of 7.2 μM was used. Interestingly, the MIC-B50 and MIC-B90 of the CAms did not influence the growth of biofilm-associated bacterial cells.
FIG 2.
MIC-B values of G8 compounds (A) and G10 compounds (B) against G. vaginalis biofilm. The data show biofilm formation (black bars) and bacterial growth (●) after treatment with G8/G10 ether and biofilm formation (gray bars) and bacterial growth (○) after treatment with G8/G10 ester.
Lactobacillus biofilms are tolerant of CAm concentrations that are bactericidal for G. vaginalis ATCC 14018 biofilms.
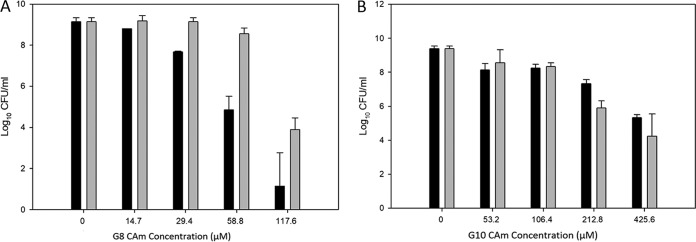
Despite being less active against planktonic G. vaginalis ATTC 14018 cells, G8 compounds were more active than G10 compounds against preformed biofilms (Fig. 3). Specifically, G10 ether and G10 ester had an MBC-B of 425.6 μM against G. vaginalis ATCC 14018 (Fig. 3B). At the same time, only 58.8 μM G8 ether and 117.6 μM G8 ester were sufficient to produce >3-log reductions in the number of biofilm-associated G. vaginalis ATCC 14018 organisms (Fig. 3A). The CAms' effectiveness against biofilms of the studied Lactobacillus species was evaluated and compared to the bactericidal activity against G. vaginalis ATCC 14018 biofilm. The data showed that the MBC-Bs of CAms against the G. vaginalis ATCC 14018 biofilm were lower than their MBC-Bs against all of the tested Lactobacillus species, with significant differences (P < 0.01) (Table 2).
FIG 3.
MBC-B values of G8 compounds (A) and G10 compounds (B) against a preformed biofilm of G. vaginalis. The data show the log10 CFU per milliliter of biofilm cells (black bars) after treatment with G8/G10 ether and the log10 CFU per milliliter of biofilm cells (gray bars) after treatment with G8/G10 ester.
TABLE 2.
MBC-B values of CAms against G. vaginalis and against healthy Lactobacillus speciesa
| Microorganism | MBC-B (μM) |
|||
|---|---|---|---|---|
| G8 ether | G8 ester | G10 ether | G10 ester | |
| G. vaginalis | 58.8 | 117.6 | 425.6 | 425.6 |
| L. rhamnosus | 940 | >890 | >850 | >810 |
| L. plantarum | 940 | >890 | >850 | >810 |
| L. gasseri | 940 | >890 | >850 | 810 |
| L. crispatus | 940 | >890 | >850 | 810 |
For all comparisons of G. vaginalis versus Lactobacillus species, the P value was <0.01.
CAms synergize with metronidazole against planktonic cells and biofilms of G. vaginalis ATCC 14018.
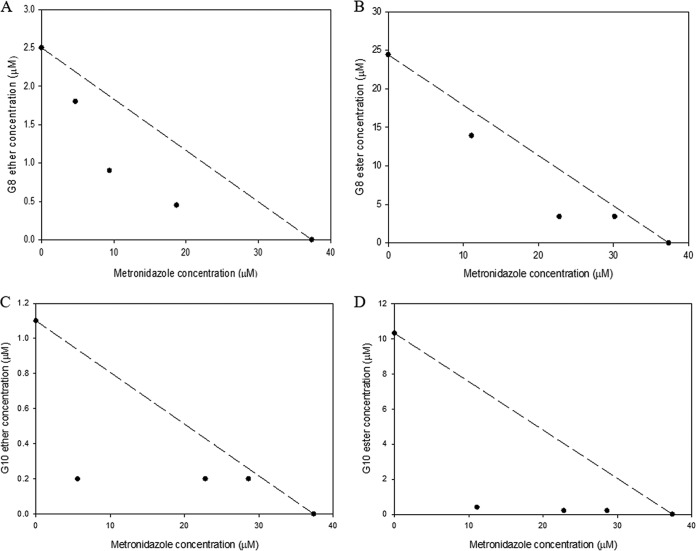
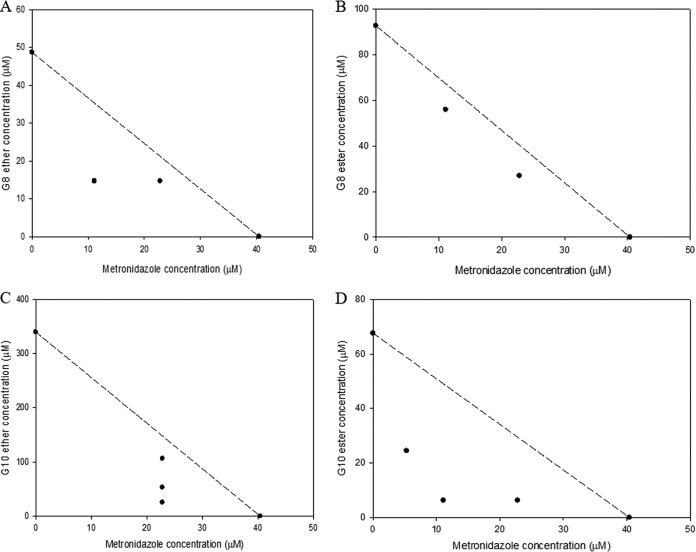
According to our results (Fig. 4 and 5), the CAms synergized with metronidazole against both planktonic cells and biofilms of G. vaginalis ATCC 14018. Metronidazole inhibited the growth of planktonic cells of G. vaginalis ATCC 14018 at an MIC of 37.4 μM, which was much lower than the concentration that inhibited the growth of vaginal lactobacilli (>1,168.5 μM). The MIC of G8 ether in combination with metronidazole decreased >5-fold compared to that of G8 ether alone (0.45 μM in combination versus 2.5 μM alone). Regarding biofilm inhibition, the MBC-B of G8 ether in combination with metronidazole decreased >3-fold compared to that of G8 ether used alone (11.1 μM in combination versus 40.4 μM alone). Overall, when metronidazole was used in combination with the tested CAms, the metronidazole MIC against planktonic cells was >7-fold lower, and its MIC-B against G. vaginalis ATCC 14018 about 3-fold lower, than the corresponding values for metronidazole alone (4.7 μM and 11.1 μM in combination versus 37.4 μM and 40.4 μM alone for planktonic cells and biofilms, respectively). The MIC of G8 ester in combination with metronidazole decreased >7-fold from that when it was used alone (3.4 μM in combination versus 24.4 μM alone). For G. vaginalis ATCC 14018 biofilms, the MBC-B of G8 ester in combination with metronidazole decreased >3-fold from that when it was used alone (26.9 μM in combination versus 92.7 μM alone). When G10 compounds were used in combination with metronidazole, the MIC of G10 decreased >5-fold from that when it was used alone (0.2 μM in combination versus 1.1 μM alone). In the same regard, the MBC-B of G10 ether in combination with metronidazole decreased >13-fold from that when it was used alone (25.7 μM and 7 μM in combination versus 340 μM alone). Furthermore, the MIC of G10 ester in combination with metronidazole decreased 50-fold from that when it was used alone (0.2 μM in combination versus 10 μM alone). Lastly, for G. vaginalis ATCC 14018 biofilms, the MBC-B of G10 ester in combination with metronidazole decreased >10-fold from that when it was used alone (6.3 μM in combination versus 67.7 μM alone).
FIG 4.
Isobolograms of metronidazole with CAms against planktonic cells of G. vaginalis. The graphs are for combinations of metronidazole with G8 ether (A), G8 ester (B), G10 ether (C), and G10 ester (D).
FIG 5.
Isobolograms of metronidazole with CAms against biofilm cells of G. vaginalis. The graphs are for combinations of metronidazole with G8 ether (A), G8 ester (B), G10 ether (C), and G10 ester (D).
Mechanism of CAm action against G. vaginalis ATCC 14018 biofilms.
To confirm that CAms act as membrane-targeting compounds, biofilm-associated G. vaginalis ATCC 14018 was treated with the G8 antimicrobial both alone at its MBC-B and in combination with metronidazole. Scanning electron microscopy (SEM) showed considerable morphological and ultrastructural changes in treated biofilm cells compared to untreated cells (Fig. 6). Prior to treatment with CAms, G. vaginalis ATCC 14018 cells within the biofilm showed a coccobacillus shape and smooth surfaces (Fig. 6A). In contrast, open holes, deep craters, severe membrane deformation, and cellular debris were noticed within the G8 ether-treated sample, providing strong evidence of membrane disruption and damage (Fig. 6B), with fragmentation also observed in the polar regions of the biofilm cells. In addition, degradation and deformation of biofilm exopolysaccharide (EPS) structures were observed. A combination of G8 ether and metronidazole was also used against a biofilm of G. vaginalis ATCC 14018 (Fig. 6C); in addition to EPS reduction, greater killing activity was observed, indicating the importance of antimicrobial combinations for the successful eradication of a biofilm and its biomass.
FIG 6.
SEM images. (A) CAm-untreated G. vaginalis biofilm; (B) G. vaginalis biofilm treated with 58.8 μM G8 ether; (C) G. vaginalis biofilm treated with a combination of 29.4 μM G8 ether and 40.4 μM metronidazole.
DISCUSSION
Traditional antibiotics are falling short when it comes to effectively treating bacterial infections. Biofilm-associated infections have become increasingly resistant to antibiotics, often resulting in recurrences that can lead to severe health consequences. Thus, there is an urgent need for alternative medications and treatment strategies. Bacterial vaginosis is one such infection that is often resistant to conventional antibiotics, primarily clindamycin and, more rarely, metronidazole (16). The infection arises from a decrease in commensal lactobacilli and an increase in anaerobic pathogenic species, predominantly G. vaginalis, which is often the main causative agent of BV (12). Recurrence of bacterial vaginosis is common within 3 to 12 months, often due to failure of treatment with conventional antibiotics and therefore a failed eradication and/or regrowth of pathogenic bacteria (16). In this study, we utilized cationic amphiphilic molecules (CAms) to control biofilm formation of BV-associated G. vaginalis ATCC 14018. As bacterial vaginosis is a polymicrobial infection, CAms were evaluated against the most frequently isolated BV anaerobes, including M. curtisii ATCC 35241, Prevotella bivia ATCC 29303, and Peptostreptococcus anaerobius ATCC 27337. The CAms were also evaluated against Lactobacillus species to observe the possible effect of CAms on the commensal bacteria, which are responsible for the stability and health of the vaginal microbiota (23).
Biofilm-associated BV is characterized by the overgrowth of opportunistic pathogenic bacteria, such as G. vaginalis, Prevotella bivia, Peptostreptococcus anaerobius, and M. curtisii; all four of these pathogens have shown increasing resistance to conventional antibiotics (24). In evaluations against planktonic cells of the pathogens, the most effective CAms were the ether compounds, particularly G10 ether. As shown in Table 1, G10 ether had the lowest MIC among those of the evaluated CAms against each pathogen. In evaluations of biofilm inhibition of G. vaginalis ATCC 14018, G10 ether at 1.6 μM was quite effective (Fig. 2B), as was G8 ether at 3.6 μM (Fig. 2A). Overall, we observed that Peptostreptococcus anaerobius ATCC 27337 was the most sensitive of the tested BV-associated pathogens, and Prevotella bivia ATCC 29303 and M. curtisii ATCC 35241 were more resistant to the highest concentration of each CAm (Table 1). The CAms used in this study (Fig. 1) consisted of hydrophilic head groups and nonpolar domains on opposite ends of the amphiphile's backbone, resulting in a facially amphiphilic conformation (22). The CAms' mode of action against the persistent pathogens, i.e., insertion and disruption of the cytoplasmic membrane, makes them efficient as antibacterial agents (25). CAms have been found to be more effective against Gram-positive bacteria than against Gram-negative organisms (22). Compared to those for G. vaginalis ATCC 14018 and Peptostreptococcus anaerobius ATCC 27337, the higher MIC values for Prevotella bivia ATCC 29303 and M. curtisii ATCC 35241 can be attributed to the existence of an additional lipopolysaccharide (LPS) layer in the Gram-negative organisms; the extra LPS layer forms a hydrophilic barrier, preventing the hydrophobic CAms from docking on the cytoplasmic membrane (22). Resistance of M. curtisii to antibiotics, such as metronidazole and clindamycin, has been reported in some studies (26, 27). It was suggested that 67.9% of BV recurrences are due to M. curtisii, which is frequently isolated from recurrent infections after initial treatment. M. curtisii, Prevotella bivia, and Peptostreptococcus anaerobius coexist with G. vaginalis to form a thick and adherent multispecies biofilm, as reported recently (28–31). G. vaginalis is often classified as a Gram-variable bacterium, meaning that it exhibits characteristics of both Gram-positive and Gram-negative bacteria. The thickness of its peptidoglycan layer varies depending on the cell phase. The G. vaginalis cell wall exhibits Gram-positive characteristics in the early exponential phase, but as the life cycle advances, the peptidoglycan layer decreases in thickness and the cell stains as Gram negative. Overall, cationic amphiphiles have been shown to be quite effective against anaerobes. Sovadinova et al. (32) evaluated AMP-mimetic amphiphilic polymethacrylate derivatives against the anaerobe Propionibacterium acnes and found them to be highly effective in eradicating the bacterium. Algburi et al. (28) evaluated the natural antimicrobials subtilosin and lauramide arginine ethyl ester (LAE) against G. vaginalis ATCC 14018. The cationic LAE compound altered the permeability of the cytoplasmic membrane and was shown to further enhance the antibiotic susceptibility of the bacterial cells, as did subtilosin A, without bactericidal effects on the healthy vaginal lactobacilli (28).
Lactobacilli are crucial to the stability and health of the vaginal microenvironment; they play a pivotal role in controlling the pH, which allows for inhibition of overgrowth of opportunistic pathogenic bacteria (30). Studies that evaluate an antimicrobial agent against pathogenic bacteria often neglect the effect of the agent on the protective microbiota. The CAms used in this study were evaluated against strains of four species of Lactobacillus: L. rhamnosus 160, L. plantarum ATCC 39268, L. gasseri ATCC 33323, and L. crispatus ATCC 33197. The most effective CAm on G. vaginalis ATCC 14018, G10 ether, had a much smaller effect on the Lactobacillus species, especially on L. plantarum ATCC 39268 and L. rhamnosus 160. Overall, we observed that the MIC values of CAms for each species of Lactobacillus were significantly greater than those for G. vaginalis ATCC 14018, leading us to propose that the CAms may selectively target pathogens while having little or no effect on the protective microbiota.
Most often, persistent infections arise from bacterial biofilms rather than solely from planktonic cells (33). Biofilms consist of a multitude of microbial cells that are associated with a surface and enclosed in a matrix consisting of extracellular polymeric substances (EPS), which often contributes to the antimicrobial resistance properties of biofilms (34). Quorum sensing has been considered to be the main line of communication between microbial cells in a community, allowing for the construction and/or dissolution of a biofilm (35). It has been demonstrated that prevention of biofilm formation, which includes the prevention of bacterial attachment and/or communication or changing the substrate surface properties, is more effective than treatment of a biofilm, as demonstrated by Shah et al. (36). Preventing biofilm formation can help to lower the required antimicrobial concentration due to the easier targeting of planktonic cells. In our study, we observed that biofilms of G. vaginalis ATCC 14018 were inhibited at the sub-MICs of CAms without influencing bacterial growth (Fig. 2A and B). One possible explanation for this occurrence is the interruption of quorum sensing by the CAms. de la Fuente-Núñez et al. reported that the small synthetic cationic peptide 1037 significantly affected the swarming motility (dependent on flagellin and quorum sensing) of bacterial cells and inhibited biofilm formation (37). We tested the four most promising CAms at sub-MICs for the ability to inhibit quorum sensing. For Gram-positive bacteria, the Fe(III) reduction assay was utilized to evaluate all four compounds as quorum sensing inhibitors. For Gram-negative bacteria, the CAms were evaluated as quorum sensing inhibitors by use of Chromobacterium violaceum as a microbial reporter (38). As a result of these experiments, no quorum sensing inhibition was observed in either Gram-positive or Gram-negative bacteria for any of the four compounds. Consequently, we speculate that there are different mechanisms involved in the interactions of these compounds with bacterial cells.
Another possible explanation is that CAms at sub-MICs coated the attachment surface, preventing the bacterial cells from gathering on the surface and thereby interfering with biofilm formation. Segev-Zarko et al. (39) demonstrated the ability of AMPs to reduce bacterial adhesion to surfaces and inhibit biofilm formation due to their capability of coating either the surface of a biomaterial or the bacterium itself. The findings of Beckloff et al. (40) seem to conflict with this observation. The AMP mimetic used in their study, meta-phenylene ethynylene (mPE), required a very high concentration, nearly 40 times the strength of the concentration needed to eradicate a biofilm of Staphylococcus aureus, to inhibit the biofilm.
Overall, the MIC-Bs of CAms against G. vaginalis ATCC 14018 were lower than the MIC values against the tested Lactobacillus species, reinforcing the observation that the CAms seem to selectively target pathogenic cells rather than the microorganisms of the healthy commensal microbiota.
The biofilm bactericidal activity of CAms was evaluated against preformed biofilms of G. vaginalis as well as Lactobacillus species. Unlike their activity against planktonic cells, G8 compounds were more effective than G10 compounds against preformed biofilms of G. vaginalis ATCC 14018 and Lactobacillus strains. In addition, the highly hydrophobic compounds may aggregate within the biofilm matrix, delaying the delivery of antimicrobial to the biofilm-associated cells.
All of the Lactobacillus biofilms were significantly more tolerant of CAms than the pathogenic biofilm of G. vaginalis ATCC 14018. It is important for the health of the vaginal microbiota that lactobacillus biofilms be kept intact and tolerant of antimicrobials. Lactobacillus species ferment glycogen secreted by vaginal epithelial cells into lactic acid, which controls the microenvironment of the vagina by maintaining a low pH and preventing the overgrowth of pathogens (41). Biofilm formation by protective bacteria, such as lactobacilli, enhances their beneficial properties and promotes their antimicrobial potential. Jones and Versalovic (42) reported a promotion of cytokine and antimicrobial production by Lactobacillus reuteri growing in a biofilm. Moreover, a biofilm of L. reuteri may possibly eradicate and reoccupy a biofilm of G. vaginalis (23), controlling pathogenic infection. Therefore, it is a crucial observation that the CAms had very little, if any, bactericidal effect on the Lactobacillus biofilms.
Metronidazole was used in this study because it is frequently prescribed by obstetrics/gynecology (OB/GYN) doctors for the treatment of BV (10, 43). The synergistic activity of metronidazole combined with natural antimicrobials enhanced its antimicrobial potential (28). In this study, the synergistic interactions between metronidazole and CAms against G. vaginalis ATTC 14018 planktonic and biofilm cells were investigated using isobolograms, and all CAms synergized with metronidazole against G. vaginalis ATCC 14018. Compared to the other combinations, G10 ester synergized with metronidazole more effectively than G8 compounds did, reducing the required concentration of each of the combined antimicrobials to kill planktonic and biofilm-associated cells. Synergism of two or more antimicrobials can improve the efficacy of each antimicrobial while lowering the risk of antimicrobial resistance, especially if the antimicrobials each have different modes of action (44). Using natural or novel antimicrobials either alone or in combination with conventional antibiotics does not carry the same consequences as those with the use of conventional antibiotics, such as metronidazole, alone (28). The National Institutes of Health has outlined a new approach to combating antibiotic resistance by combining conventional antibiotics with complementary methods, i.e., naturally derived antimicrobials (45). As shown in the resulting isobolograms, metronidazole synergized with each of the CAms against G. vaginalis ATCC 14018, with G10 compounds synergizing most strongly with metronidazole. Thus, the higher the CAm concentration, the lower the metronidazole concentration needed for effective inhibition of G. vaginalis ATCC 14018.
The SEM data in this study, together with the findings of Zhang et al. (22), suggest that the antimicrobial action of CAms is associated with the disruption of cellular membrane permeability, ultimately reducing the opportunity for bacterial resistance to develop. These promising results may potentially lead to a decrease in conventional antibiotic use for treatment of bacterial vaginosis, thereby decreasing the risk for antibiotic-resistant infections.
Conclusions.
Antibiotic resistance of persistent infections is an important challenge which requires significant attention toward the goal of finding alternative antimicrobial treatments. Here we reported on synthetic CAms and their antimicrobial and antibiofilm activities against BV-associated pathogens. The structure of CAms can be modified in several different ways and their biocidal potential improved to counteract resistance to antibiotics. CAms have effectively prevented and eradicated biofilms of G. vaginalis at low concentrations (in the micromolar range), without any harmful effects on the protective vaginal microbiota. In addition, CAms have shown synergistic activity with metronidazole against planktonic cells and biofilms of G. vaginalis ATTC 14018. Taken together, our results show that CAms are a promising new generation of antimicrobials with the potential to transform modern therapeutic strategies for treating bacterial vaginosis.
MATERIALS AND METHODS
Synthesis of Gn formulations.
Ether-linked and ester-linked cationic gemini-like amphiphiles (Gn, where “G” stands for gemini and n denotes the total carbon number of each hydrophobic arm) with various hydrophobic chain lengths (Fig. 1) were synthesized in high yields following procedures described by Zhang et al. (22).
Bacterial strains and growth conditions.
G. vaginalis ATCC 14018 was grown overnight in brain heart infusion (Difco, Sparks, MD) supplemented with 3% horse serum (JRH Biosciences, KS) (sBHI), while BHI supplemented with 1% glucose (BHIG) was used for biofilm formation assays. Each experiment using G. vaginalis ATCC 14018 was performed anaerobically (10% hydrogen, 5% carbon dioxide, and 85% nitrogen) within an anaerobic glove box (Coy Laboratory Products Inc., Grass Lake, MI, USA). The following strains of four species of vaginal Lactobacillus were used in this study to evaluate the possible harmful effects of CAms on beneficial bacteria: Lactobacillus plantarum ATCC 39268, Lactobacillus rhamnosus 160 (provided by A. A. Aroutcheva, Rush University Medical Center, Chicago, IL), Lactobacillus crispatus ATCC 33197, and Lactobacillus gasseri ATCC 33323. All four strains were grown for 18 to 24 h in DeMan, Rogosa, and Sharpe broth (MRS broth; Difco BD, Franklin Lakes, NJ, USA) under aerobic conditions at 37°C. For lactobacillus biofilm formation, MRS broth supplemented with 1% glucose and 2% sucrose (Fisher Scientific, Waltham, MA, USA) was utilized.
The antimicrobial activity of CAms was also tested against Peptostreptococcus anaerobius ATCC 27337, Mobiluncus curtisii ATCC 35241, and Prevotella bivia ATCC 29303, all of which, in addition to G. vaginalis ATCC 14018, are the most commonly isolated anaerobes from BV-infected women. These anaerobes were maintained within the anaerobic chamber and transferred daily into fresh sBHI.
Stock solution preparation of CAm compounds.
To prepare a stock solution, the CAm compounds were dissolved in double-distilled water (ddH2O) and incubated at 37°C with shaking at 250 rpm for 10 min. Each solution was then serially diluted 2-fold with sBHI broth to determine the MIC against planktonic cells and with BHIG broth to identify the MIC-B/MBC-B against biofilm cells.
MICs of CAms.
According to the Clinical and Laboratory Standards Institute (46), the MIC is defined as the lowest concentration of antimicrobial compound producing no visible growth or complete inhibition of bacterial growth for 24 h. To determine MIC values, a broth microdilution assay was performed as described by Algburi et al. (28), with minor modifications. Briefly, a 24-h culture of G. vaginalis ATCC 14018 was diluted with a suitable volume of culture medium to achieve ∼106 CFU/ml. The number of bacterial cells was identified using the spot plate method (29). The CAms were serially diluted 2-fold with sBHI in a non-tissue-culture 96-well microplate (Falcon, Corning Incorporated, Corning, NY, USA), resulting in a final volume of 100 μl for each well. Aliquots of 100 μl of the diluted cell suspension (∼106 CFU/ml) were added to the wells of a microplate that was treated with different concentrations of CAms. To avoid culture medium evaporation, 75 μl of mineral oil (Sigma-Aldrich, St. Louis, MO) was added to each treated well. The microplate was then transferred to a plate reader (model 550; Bio-Rad Laboratories, Hercules, CA, USA) and incubated anaerobically at 37°C for 24 to 36 h. The kinetic reading was analyzed statistically, and the MIC and sub-MICs of each CAm compound were determined.
MIC-B determination.
The MIC-B50 of an antimicrobial is defined as the lowest concentration of the antimicrobial that inhibits 50% of treated biofilm compared to an untreated one (control), while the MIC-B90 is defined as the minimum concentration that inhibits 90% of biofilm growth (47). G. vaginalis ATCC 14018 cells were grown overnight in sBHI broth anaerobically at 37°C. The overnight culture was diluted 1:100 (vol/vol) in BHIG broth to achieve ∼106 CFU/ml. The CAms were diluted 2-fold with BHIG in a 96-well tissue culture microplate (Falcon, Corning Incorporated, Corning, NY, USA), with a final volume of 100 μl in each well. Aliquots of 100 μl of diluted cell suspensions at 106 CFU/ml were added separately to different concentrations of CAms in a 96-well tissue culture microplate. The microplate was then incubated for 48 h at 37°C under anaerobic conditions. After incubation, the number of nonadherent bacterial cells was counted using a spot plate method and compared to the number of cells of the untreated control. The intact biofilm was then stained with 0.1% crystal violet (CV).
Biofilm staining using CV.
Biofilm staining using CV was performed as described by Borucki et al. (48), with minor modifications. Briefly, after counting of the nonadherent cells, the biofilm was fixed at 60°C for 60 min in an inverted position in an incubator (New Brunswick Scientific Co., Inc., NJ, USA). To each well, 125 μl of 0.1% CV was added and left at room temperature for 20 min. Each well was then rinsed three or four times with 200 μl of ddH2O and left for 15 min to dry at room temperature. To solubilize the CV-stained biofilm, 200 μl of 95% ethanol in water was added, and the microplate was incubated at 4°C for 30 min. One-hundred-microliter samples of solubilized CV were then transferred to a new flat-bottomed 96-well microplate. The absorbance of each sample was measured using a plate reader at 595 nm (model 550; Bio-Rad Laboratories, Hercules, CA, USA).
MBC-B determination.
The MBC-B is defined as the minimum concentration of an antibacterial agent that causes a ≥3-log reduction in the number of viable cells compared to the number of positive-control cells (49). The assay was performed according to the method of Algburi et al. (28), with minor modifications, namely, a 24-h culture of G. vaginalis ATCC 14018 was diluted to 107 CFU/ml. For the biofilm formation assay, aliquots of 200 μl were added to a 96-well tissue culture microplate, sealed with amplification tape (Nalge Nunc International, Rochester, NY, USA), and incubated for 24 to 36 h at 37°C. After incubation, the planktonic cells were removed by gently washing the biofilm twice with 200 μl of fresh broth. The biofilm was then treated with 200 μl of a predetermined concentration of the tested compound. The microplate was incubated for 24 h at 37°C under anaerobic conditions. After incubation, the antimicrobials (supernatant) were discarded, and the biofilm was washed twice with fresh BHIG broth. The biofilm was then disrupted by vigorous pipetting in order to determine the number of biofilm-associated cells by use of the spot plate method.
Checkerboard assay for antimicrobial combinations.
To evaluate the potential effectiveness of the CAms in combination with metronidazole on planktonic and biofilm cells, a checkerboard assay was performed as described by Algburi et al. (28), with minor modifications. For planktonic cells, a 24-h culture of G. vaginalis ATCC 14018 was diluted to achieve 106 CFU/ml. Each antimicrobial agent was diluted 2-fold with sBHI broth in two separate 96-well non-tissue-culture microplates. From each dilution of antimicrobial B, 50 μl was added horizontally over 50 μl of antimicrobial A. Next, 100 μl of bacterial suspension (106 CFU/ml) was separately added to an antimicrobial combination at predetermined concentrations. The MIC of each combination was determined after 24 h of incubation.
For biofilm formation, the overnight culture of G. vaginalis ATCC 14018 was diluted to approximately 107 CFU/ml, and 200 μl was transferred to a 96-well tissue culture microplate and incubated anaerobically at 37°C for 24 to 36 h. Following biofilm formation and removal of nonadherent cells by washing the biofilm twice with BHIG, each antimicrobial was diluted 2-fold separately with BHIG broth in two 96-well (deep well) microplates. From each dilution of antimicrobial B, 125 μl was added horizontally over 125 μl of antimicrobial A (see Fig. 1 of reference 21). From each combination, 200-μl samples were added to the biofilm in sequence, and the microplate was incubated for 24 h at 37°C under anaerobic conditions. The spot plate method was utilized for counting the number of CFU per milliliter and evaluating the bactericidal activity (MBC-B) of each antimicrobial combination against biofilm-associated G. vaginalis ATCC 14018. Isobolograms were used to analyze the nature of antimicrobial interactions, which were classified as synergistic, antagonistic, or additive, against the planktonic and biofilm cells.
Checkerboard assay data analysis.
Isobolograms were used to compare the MIC and MBC-B values of each antimicrobial alone to its MIC and MBC-B values in combination with other antimicrobials. The point on the x axis refers to the MIC or MBC-B value of the first antimicrobial, with the coordinates (0, x), and the point on the y axis represents the MIC or MBC-B value of the second antimicrobial, with the coordinates (y, 0), when the antimicrobials are used alone. The two MIC or MBC-B values are connected by a dashed line (50). The MIC or MBC-B values of each antimicrobial combination are plotted as dots on the graph. Results are expressed according to the locations of these dots relative to the line that connects the MIC or MBC-B values of the first and second antimicrobials. When the MIC or MBC-B values are located under the line, the combination of the two antimicrobials shows synergy, but when these dots of interaction are above the line, the combination of the two antimicrobials shows antagonism against the tested microorganism. An additive effect is observed when these dots are located on the line.
SEM.
SEM was used to visualize the antibiofilm activity of the AMP mimics. As in the biofilm formation assay, G. vaginalis ATCC 14018 cells were diluted to 107 CFU/ml before 2 ml was transferred to each well of a 6-well tissue culture plate (Falcon, BD, Franklin Lakes, NJ, USA), which was then incubated for 24 h at 37°C with a glass slide in each well (12 mm; Fisher Scientific, Waltham, MA). After incubation, the biofilms that formed on the glass slides were washed twice with fresh BHIG to remove the nonadherent bacteria. An antimicrobial(s) in BHIG was then added, and the plate was incubated again for 24 h. Each well was washed twice with BHIG and fixed with 2.5% glutaraldehyde at room temperature for 1 h. The biofilms were dehydrated with a graded series of ethanol solutions (50%, 70%, 80%, 95%, and 100%), followed by drying with graded hexamethyldisilazane (50% and 100%) in ethanol. Before being mounted on stubs and sputter coated with 20-nm gold, the samples were further air dried for an additional 2 days. SEM images were taken with a Zeiss Sigma field-emission SEM (Carl Zeiss, Ontario, Canada) at 5 kV.
Statistical analysis.
Each experiment was repeated at least three times in duplicate. The error bars in the provided figures represent the standard deviations of the data. All calculations were performed in Microsoft Excel, and then the statistical analysis was reshaped with SigmaPlot 11.0 (Systat Software Inc., Chicago, IL, USA). The Student t test (with significance for P values of ≤0.01 and P values of ≤0.05) was also performed using SigmaPlot 11.0.
ACKNOWLEDGMENT
We gratefully acknowledge the NIH (grant R21 AI126053) for financial support.
REFERENCES
- 1.Forsum U, Holst E, Larsson PG, Vasquez A, Jakobsson T, Mattsby-Baltzer I. 2005. Bacterial vaginosis, a microbiological and immunological enigma. APMIS 113:81–90. doi: 10.1111/j.1600-0463.2005.apm1130201.x. [DOI] [PubMed] [Google Scholar]
- 2.Machado D, Castro J, Palmeira-de-Oliveira A, Martinez-de-Oliveira J, Cerca N. 2016. Bacterial vaginosis biofilms: challenges to current therapies and emerging solutions. Front Microbiol 6:1528. doi: 10.3389/fmicb.2015.01528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spiegel CA. 1991. Bacterial vaginosis. Clin Microbiol Rev 4:485–502. doi: 10.1128/CMR.4.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar N, Behera B, Sagiri SS, Pal K, Ray SS, Roy S. 2011. Bacterial vaginosis: etiology and modalities of treatment—a brief note. J Pharm Bioallied Sci 3:496–503. doi: 10.4103/0975-7406.90102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allsworth JE, Peipert JF. 2007. Prevalence of bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey data. Obstet Gynecol 109:114–120. [DOI] [PubMed] [Google Scholar]
- 6.Goldenberg RL, Andrews WW, Yuan AC, MacKay HT, St Louis ME. 1997. Sexually transmitted diseases and adverse outcomes of pregnancy. Clin Perinatol 24:23–41. [PubMed] [Google Scholar]
- 7.Muller E, Berger K, Dennemark N, Oleen-Burkey M. 1999. Cost of bacterial vaginosis in pregnancy. Decision analysis and cost evaluation of a clinical study in Germany. J Reprod Med 44:807–814. [PubMed] [Google Scholar]
- 8.Machado A, Cerca N. 2015. Influence of biofilm formation by Gardnerella vaginalis and other anaerobes on bacterial vaginosis. J Infect Dis 212:1856–1861. doi: 10.1093/infdis/jiv338. [DOI] [PubMed] [Google Scholar]
- 9.Aroutcheva AA, Simoes JA, Behbakht K, Faro S. 2001. Gardnerella vaginalis isolated from patients with bacterial vaginosis and from patients with healthy vaginal ecosystems. Clin Infect Dis 33:1022–1027. doi: 10.1086/323030. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC). 2015. Sexually transmitted diseases treatment guidelines. Center for Surveillance, Epidemiology, and Laboratory Services, US Department of Health and Human Services, Atlanta, GA. [Google Scholar]
- 11.Löfmark S, Edlund C, Nord CE. 2010. Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin Infect Dis 50:S16–S23. doi: 10.1086/647939. [DOI] [PubMed] [Google Scholar]
- 12.Hardy L, Jespers V, Dahchour N, Mwambarangwe L, Musengamana V, Vaneechoutte M, Crucitti T. 2015. Unravelling the bacterial vaginosis-associated biofilm: a multiplex Gardnerella vaginalis and Atopobium vaginae fluorescence in situ hybridization assay using peptide nucleic acid probes. PLoS One 10:e0136658. doi: 10.1371/journal.pone.0136658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagaraja P. 2008. Antibiotics resistance of Gardnerella vaginalis in recurrent bacterial vaginosis. Indian J Med Microbiol 26:155–157. doi: 10.4103/0255-0857.40531. [DOI] [PubMed] [Google Scholar]
- 14.Bostwick DG, Woody J, Hunt C, Budd W. 2016. Antimicrobial resistance genes and modeling of treatment failure in bacterial vaginosis: clinical study of 289 symptomatic women. J Med Microbiol 65:377–386. doi: 10.1099/jmm.0.000236. [DOI] [PubMed] [Google Scholar]
- 15.Bradshaw C, Sobel J. 2016. Current treatment of bacterial vaginosis—limitations and need for innovation. J Infect Dis 214(Suppl 1):S14–S20. doi: 10.1093/infdis/jiw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eschenbach DA. 2007. Bacterial vaginosis: resistance, recurrence, and/or reinfection? Clin Infect Dis 44:220–221. doi: 10.1086/509584. [DOI] [PubMed] [Google Scholar]
- 17.Filho DSC, Diniz CG, da Silva VL. 2010. Bacterial vaginosis: clinical, epidemiologic and microbiological features. HU Rev 36:223–230. [Google Scholar]
- 18.Izadpanah A, Gallo RL. 2005. Antimicrobial peptides. J Am Acad Dermatol 52:381–390. doi: 10.1016/j.jaad.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 19.Bahar A, Ren D. 2013. Antimicrobial peptides. Pharmaceuticals 6:1543–1575. doi: 10.3390/ph6121543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wimley WC, Hristova K. 2011. Antimicrobial peptides: successes, challenges and unanswered questions. J Membr Biol 239:27–34. doi: 10.1007/s00232-011-9343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laverty G, Gorman SP, Gilmore BF. 2011. The potential of antimicrobial peptides as biocides. Int J Mol Sci 12:6566–6596. doi: 10.3390/ijms12106566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Algburi A, Wang N, Kholodovych V, Oh DO, Chikindas M, Uhrich KE. 2017. Self-assembled cationic amphiphiles as antimicrobial peptides mimics: role of hydrophobicity, linkage type, and assembly state. Nanomedicine 13:343–352. doi: 10.1016/j.nano.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saunders S, Bocking A, Challis J, Reid G. 2007. Effect of Lactobacillus challenge on Gardnerella vaginalis biofilms. Colloids Surf B Biointerfaces 55:138–142. doi: 10.1016/j.colsurfb.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 24.Nagaraja P. 2008. Antibiotic resistance of Gardnerella vaginalis in recurrent bacterial vaginosis. Indian J Med Microbiol 26:155. doi: 10.4103/0255-0857.40531. [DOI] [PubMed] [Google Scholar]
- 25.Findlay B, Zhanel GG, Schweizer F. 2010. Cationic amphiphiles, a new generation of antimicrobials inspired by the natural antimicrobial peptide scaffold. Antimicrob Agents Chemother 54:4049–4058. doi: 10.1128/AAC.00530-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spiegel CA. 1987. Susceptibility of Mobiluncus species to 23 antimicrobial agents and 15 other compounds. Antimicrob Agents Chemother 31:249–252. doi: 10.1128/AAC.31.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meltzer MC, Desmond RA, Schwebke JR. 2008. Association of Mobiluncus curtisii with recurrence of bacterial vaginosis. Sex Transm Dis 35:611–613. doi: 10.1097/OLQ.0b013e318167b105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Algburi A, Volski A, Chikindas ML. 2015. Natural antimicrobials subtilosin and lauramide arginine ethyl ester synergize with conventional antibiotics clindamycin and metronidazole against biofilms of Gardnerella vaginalis but not against biofilms of healthy vaginal lactobacilli. Pathog Dis 73:ftv018. doi: 10.1093/femspd/ftv018. [DOI] [PubMed] [Google Scholar]
- 29.Turovskiy Y, Cheryian T, Algburi A, Wirawan RE, Takhistov P, Sinko PJ, Chikindas ML. 2012. Susceptibility of Gardnerella vaginalis biofilms to natural antimicrobials subtilosin, ε-poly-l-lysine, and lauramide arginine ethyl ester. Infect Dis Obstet Gynecol 2012:284762. doi: 10.1155/2012/284762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, Mandaliya K, Ndinya-Achola JO, Bwayo J, Kreiss J. 1999. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 180:1863–1868. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 31.Delcour J, Ferain T, Deghorain M, Palumbo E, Hols P. 1999. The biosynthesis and functionality of the cell-wall of lactic acid bacteria. Antonie Van Leeuwenhoek 76:159–184. doi: 10.1023/A:1002089722581. [DOI] [PubMed] [Google Scholar]
- 32.Sovadinova I, Palermo EF, Urban M, Mpiga P, Caputo GA, Kuroda K. 2011. Activity and mechanism of antimicrobial peptide-mimetic amphiphilic polymethacrylate derivatives. Polymers 3:1512–1532. doi: 10.3390/polym3031512. [DOI] [Google Scholar]
- 33.Di Luca M, Maccari G, Nifosì R. 2014. Treatment of microbial biofilms in the post-antibiotic era: prophylactic and therapeutic use of antimicrobial peptides and their design by bioinformatics tools. Pathog Dis 70:257–270. doi: 10.1111/2049-632X.12151. [DOI] [PubMed] [Google Scholar]
- 34.Donlan RM. 2002. Biofilms: microbial life on surfaces. Emerg Infect Dis 8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parsek MR, Greenberg E. 2005. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol 13:27–33. doi: 10.1016/j.tim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Shah SR, Tatara AM, D'Souza RN, Mikos AG, Kasper FK. 2013. Evolving strategies for preventing biofilm on implantable materials. Mater Today 16:177–182. doi: 10.1016/j.mattod.2013.05.003. [DOI] [Google Scholar]
- 37.de la Fuente-Núñez C, Korolik V, Bains M, Nguyen U, Breidenstein EBM, Horsman S, Lewenza S, Burrows L, Hancock REW. 2012. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob Agents Chemother 56:2696–2704. doi: 10.1128/AAC.00064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Algburi A, Zehm S, Netrebov V, Bren AB, Chistyakov V, Chikindas ML. 2017. Subtilosin prevents biofilm formation by inhibiting bacterial quorum sensing. Probiotics Antimicrob Proteins 9:81–90. doi: 10.1007/s12602-016-9242-x. [DOI] [PubMed] [Google Scholar]
- 39.Segev-Zarko L, Saar-Dover R, Brumfeld V, Mangoni ML, Shai Y. 2015. Mechanisms of biofilm inhibition and degradation by antimicrobial peptides. Biochem J 468:259–270. doi: 10.1042/BJ20141251. [DOI] [PubMed] [Google Scholar]
- 40.Beckloff N, Laube D, Castro T, Furgang D, Park S, Perlin D, Clements D, Tang H, Scott RW, Tew GN, Diamond G. 2007. Activity of an antimicrobial peptide mimetic against planktonic and biofilm cultures of oral pathogens. Antimicrob Agents Chemother 51:4125–4132. doi: 10.1128/AAC.00208-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dover SE, Aroutcheva AA, Faro S, Chikindas ML. 2008. Natural antimicrobials and their role in vaginal health: a short review. Int J Probiotics Prebiotics 3:219–230. [PMC free article] [PubMed] [Google Scholar]
- 42.Jones SE, Versalovic J. 2009. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol 9:35. doi: 10.1186/1471-2180-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sobel R, Sobel JD. 2015. Metronidazole for the treatment of vaginal infections. Expert Opin Pharmacother 16:1109–1115. doi: 10.1517/14656566.2015.1035255. [DOI] [PubMed] [Google Scholar]
- 44.Rybak MJ, McGrath BJ. 1996. Combination antimicrobial therapy for bacterial infections. Drugs 52:390–405. doi: 10.2165/00003495-199652030-00005. [DOI] [PubMed] [Google Scholar]
- 45.National Center for Complementary and Integrative Health (NCCIH). 2014. Frequently asked questions: name change. https://nccih.nih.gov/news/name-change-faq. Accessed 5 October 2016.
- 46.CLSI. 2017. Performance standards for antimicrobial susceptibility testing, 27th ed CLSI supplement M100 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 47.Sandoe JA, Wysome J, West AP, Heritage J, Wilcox MH. 2006. Measurement of ampicillin, vancomycin, linezolid and gentamicin activity against enterococcal biofilms. J Antimicrob Chemother 57:767–770. doi: 10.1093/jac/dkl013. [DOI] [PubMed] [Google Scholar]
- 48.Borucki MK, Peppin JD, White D, Loge F, Call DR. 2003. Variation in biofilm formation among strains of Listeria monocytogenes. Appl Environ Microbiol 69:7336–7342. doi: 10.1128/AEM.69.12.7336-7342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aaron SD, Ferris W, Ramotar K, Vandemheen K, Chan F, Saginur R. 2002. Single and combination antibiotic susceptibilities of planktonic, adherent, and biofilm-grown Pseudomonas aeruginosa isolates cultured from sputa of adults with cystic fibrosis. J Clin Microbiol 40:4172–4179. doi: 10.1128/JCM.40.11.4172-4179.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turovskiy Y, Chikindas ML. 2011. Zinc lactate and sapindin act synergistically with lactocin 160 against Gardnerella vaginalis. Probiotics Antimicrob Proteins 3:144–149. doi: 10.1007/s12602-011-9068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]