ABSTRACT
To understand the epidemiological variation in bacteremia characteristics among differently aged populations, adults with community-onset bacteremia during a 6-year period were studied in a retrospective cohort. A total of 2,349 bacteremic patients were stratified into four age categories: young adults (18 to 44 years old; 196 patients; 8.3%), adults (45 to 64 years old; 707 patients; 30.1%), the elderly (65 to 84 years old; 1,098 patients; 46.7%), and the oldest old (≥85 years old; 348 patients; 14.8%). Age-related trends in critical illness (a Pitt bacteremia score of ≥4) at bacteremia onset, antibiotic-resistant pathogens (extended-spectrum β-lactamase [ESBL]-producing Escherichia coli, Klebsiella species, and Proteus mirabilis [EKP]; methicillin-resistant Staphylococcus aureus [MRSA]; and levofloxacin nonsusceptible EKP), inappropriate empirical antibiotic therapy (EAT), and 4-week mortality rate were observed. Using a multivariate regression model, critical illness at bacteremia onset (adjusted odds ratio [AOR], 9.03; P < 0.001) and inappropriate EAT (AOR, 2.67; P < 0.001) were the two leading predictors of 4-week mortality. Moreover, ESBL-producing EKP (AOR, 12.94; P < 0.001), MRSA (AOR, 8.66; P < 0.001), and levofloxacin-nonsusceptible EKP (AOR, 4.27; P < 0.001) were linked to inappropriate EAT. In conclusion, among adults with community onset bacteremia, significant positive age-related trends were noted in antibiotic-resistant pathogens and bacteremia severity, which were related to the increasing incidence of inappropriate EAT and 4-week mortality with age. Thus, different empirical antimicrobial regimens should be considered for distinct age groups.
KEYWORDS: community, appropriateness, empirical therapy, aging, bloodstream infections
INTRODUCTION
Bloodstream infection is a serious, life-threatening condition with a reported incidence of up to 16.9 cases per 1,000 hospital admissions (1–3). Importantly, the incidence of bacteremia and related mortality increases markedly with age (4), and elderly patients may present with nonspecific functional decline and atypical presentations, which make an accurate diagnosis challenging (5). Therefore, several studies have shown different clinical manifestations and outcomes between the elderly and younger adults in the literature (4, 6).
To achieve optimal outcomes, the successful management of elderly patients with bacteremia is required; this involves the elimination of the offending pathogen by the timely administration of appropriate antimicrobials and removal of the infection source (7, 8). To avoid a delay in administering appropriate antimicrobials to the elderly, understanding the differences in clinical characteristics, causative microorganisms, bacteremia severity, and antimicrobial susceptibility between young adults and the elderly is necessary. Although age-related differences in pathogen distribution and antimicrobial susceptibilities have been previously assessed (6, 9), no study has investigated age-related trends in clinical characteristics among adults with bloodstream infections in terms of comorbidity severity, bacteremia severity and sources, types and antimicrobial susceptibilities of causative microorganisms, and patient outcomes. To achieve the appropriateness of empirical antibiotic therapy (EAT) for the elderly, we investigated adult patients with community-onset bacteremia to compare the clinical manifestations and short-term outcomes of patients stratified by age groups and to describe the age-related trends in causative microorganisms and antimicrobial susceptibilities.
RESULTS
Clinical presentations, bacteremia severity, and outcomes in different age groups.
In accordance with the inclusion and exclusion criteria (see Fig. S1 in the supplemental material), the 2,349 patients with community-onset bacteremia were eligible and stratified into four categories by age: young adults (196 patients; 8.3%), adults (707 patients; 30.1%), the elderly (1,098 patients; 46.7%), and the oldest old (348 patients; 14.8%). Significantly positive linear-by-linear associations between age and the following variables were observed (Table 1): comorbidities with chronic kidney diseases, hypertension, neurological diseases, coronary artery diseases, or chronic obstructive pulmonary diseases; bacteremia due to pneumonia or biliary tract infections; critical illness (a Pitt bacteremia score of ≥4) at bacteremia onset; initial syndrome of severe sepsis; and the 2- and 4-week mortality rates. Otherwise, negative age-related trends in bacteremia due to intra-abdominal infections or bone and joint infections and mildly critical illness (a Pitt bacteremia score of 0) at bacteremia onset were observed.
TABLE 1.
Age-related trends in various clinical characteristics and outcomes of 2,349 patients with community-onset bacteremia
| Characteristic | No. (%) of patients |
P valuea | |||
|---|---|---|---|---|---|
| Young adults (18–44 yr; n = 196) |
Adults (45–64 yr; n = 707) |
Elderly (65–84 yr; n = 1,098) |
Oldest old (≥85 yr; n = 348) |
||
| Gender, male | 105 (53.8) | 397 (56.2) | 509 (46.4) | 198 (56.9) | 0.987 |
| Nursing home residents | 4 (2.0) | 22 (3.1) | 62 (5.6) | 45 (12.9) | 0.073 |
| Recent events within 4 weeks before ED arrival | |||||
| Hospitalization | 36 (18.4) | 179 (25.3) | 265 (24.1) | 71 (20.4) | 0.807 |
| Antimicrobial therapy | 27 (13.8) | 137 (19.4) | 227 (20.7) | 55 (15.8) | 0.704 |
| Invasive proceduresb or surgery | 6 (3.1) | 55 (7.8) | 93 (8.5) | 18 (5.2) | 0.636 |
| Polymicrobial bacteremia | 17 (8.7) | 55 (7.8) | 116 (10.6) | 52 (14.9) | 0.125 |
| Major comorbidity | |||||
| Malignancy | 35 (17.9) | 213 (30.1) | 345 (31.4) | 66 (19.0) | 0.917 |
| Liver cirrhosis | 27 (13.8) | 127 (18.0) | 133 (12.1) | 14 (4.0) | 0.223 |
| Chronic kidney disease | 25 (12.8) | 99 (14.0) | 221 (20.1) | 74 (21.3) | 0.045 |
| Hypertension | 15 (7.7) | 231 (32.7) | 656 (59.7) | 216 (62.1) | 0.042 |
| Diabetes mellitus | 10 (5.1) | 255 (36.1) | 491 (44.7) | 119 (34.2) | 0.282 |
| Neurological disease | 7 (3.6) | 56 (7.9) | 297 (27.0) | 162 (46.6) | 0.030 |
| Urological disease | 8 (3.1) | 23 (3.3) | 88 (8.0) | 45 (12.9) | 0.051 |
| Coronary artery disease | 1 (0.5) | 27 (3.8) | 144 (13.1) | 51 (14.7) | 0.046 |
| Chronic obstructive pulmonary disease | 0 (0) | 16 (2.3) | 56 (5.1) | 40 (11.5) | 0.032 |
| Ultimately or rapidly fatal comorbidity (McCabe classification) | 42 (21.4) | 197 (27.9) | 273 (24.9) | 52 (14.9) | 0.480 |
| Major sources of bacteremia | |||||
| Urinary tract infection | 46 (23.5) | 210 (29.7) | 379 (34.5) | 113 (32.5) | 0.143 |
| Intra-abdominal infection | 38 (19.4) | 108 (15.3) | 136 (12.4) | 30 (8.6) | 0.002 |
| Skin and soft-tissue infection | 27 (13.8) | 85 (12.0) | 86 (7.8) | 46 (13.2) | 0.714 |
| Primary bacteremia | 25 (12.8) | 62 (8.8) | 72 (6.6) | 22 (6.3) | 0.066 |
| Pneumonia | 19 (9.7) | 77 (10.9) | 162 (14.8) | 67 (19.3) | 0.026 |
| Bone and joint infection | 16 (8.2) | 34 (4.8) | 37 (3.4) | 5 (1.4) | 0.017 |
| Biliary tract infection | 7 (3.6) | 46 (6.5) | 121 (11.0) | 46 (13.2) | 0.008 |
| Severity-of-illness marker at bacteremia onset (Pitt bacteremia score) | |||||
| ≥4 points | 26 (13.3) | 121 (17.1) | 233 (21.2) | 96 (27.6) | 0.008 |
| 0 points | 66 (33.7) | 203 (28.7) | 289 (26.3) | 75 (21.6) | 0.008 |
| Initial syndrome | |||||
| Severe sepsis | 53 (27.0) | 286 (40.5) | 510 (46.4) | 174 (50.0) | 0.043 |
| Septic shock | 28 (14.3) | 142 (20.1) | 226 (20.6) | 83 (23.9) | 0.052 |
| Length (days, mean ± SD) | |||||
| Time to defervescencec | 6.9 ± 7.9 | 7.8 ± 8.8 | 7.5 ± 8.4 | 7.5 ± 8.8 | 0.487 |
| Total hospital stay | 16.9 ± 25.5 | 14.5 ± 15.7 | 15.1 ± 16.5 | 14.0 ± 15.5 | 0.174 |
| Crude mortality rate | |||||
| 2 wk | 10 (5.1) | 72 (10.2) | 116 (10.6) | 53 (15.2) | 0.040 |
| 4 wk | 19 (9.7) | 99 (14.0) | 158 (14.4) | 64 (18.4) | 0.038 |
Calculated by Pearson's correlation. Boldface indicates statistical significance, i.e., a P value of <0.05.
Including endoscopic retrograde cholangiopancreatography, bronchoscopy, panendoscopy, and colonoscopy.
Only 1,905 febrile patients at admission were calculated.
Causative microorganism and antimicrobial susceptibility in different age groups.
A total of 2,655 bacteremia isolates obtained from 2,349 patients were stratified into four categories: young adults (215 isolates; 8.1%), adults (779 isolates; 29.3%), the elderly (1,245 isolates; 46.9%), and the oldest old (416 isolates; 15.7%). Of 10 leading microorganisms, an age-related trend was not disclosed in all species but only in Proteus mirabilis (Table 2). However, certain age-related trends were observed in antimicrobial susceptibilities (Table 3). As age increased, susceptibilities to cefazolin, cefuroxime, cefotaxime, and levofloxacin decreased, and the percentages of extended-spectrum β-lactamase (ESBL) producers increased in two common Gram-negative pathogens, Escherichia coli and Klebsiella pneumoniae. Additionally, the proportions of methicillin-resistant Staphylococcus aureus (MRSA) increased, and susceptibilities to penicillin or levofloxacin in Streptococcus species decreased with age.
TABLE 2.
Age-related trends in species distribution of major pathogens in adults with community-onset bacteremia
| Microorganism (n) | No. (%) of isolates |
P valuea | |||
|---|---|---|---|---|---|
| Young adults (18–44 yr; n = 215) |
Adults (45–64 yr; n = 779) |
Elderly (65–84 yr; n = 1,245) |
Oldest old (≥85 yr; n = 416) |
||
| Escherichia coli (968) | 62 (28.8) | 264 (33.9) | 494 (39.7) | 148 (35.6) | 0.251 |
| Klebsiella pneumoniae (354) | 17 (7.9) | 123 (15.8) | 164 (13.2) | 50 (12.0) | 0.619 |
| Staphylococcus aureus (312) | 30 (14.0) | 114 (14.6) | 124 (10.0) | 44 (10.6) | 0.182 |
| Pseudomonas aeruginosa (84) | 9 (4.2) | 16 (2.1) | 46 (3.7) | 13 (3.1) | 0.757 |
| Proteus mirabilis (62) | 2 (0.9) | 16 (2.1) | 28 (2.2) | 16 (3.8) | 0.046 |
| Salmonella enteritidis (54) | 12 (5.6) | 18 (2.3) | 20 (1.6) | 4 (1.0) | 0.095 |
| Enterococcus faecalis (54) | 5 (2.3) | 7 (0.9) | 30 (2.4) | 12 (2.9) | 0.503 |
| Viridians streptococci (53) | 6 (2.8) | 21 (2.7) | 19 (1.5) | 7 (1.7) | 0.133 |
| Streptococcus agalactiae (52) | 3 (1.4) | 22 (2.8) | 19 (1.5) | 8 (1.9) | 0.960 |
| Streptococcus pneumoniae (22) | 5 (2.3) | 8 (1.0) | 12 (1.0) | 2 (0.5) | 0.095 |
Calculated by Pearson's correlation. Boldface indicates statistical significance, i.e., a P value of <0.05.
TABLE 3.
Trends in the susceptibility of the five leading microorganisms in patients with community-onset bacteremia
| Microorganisma | Susceptibilityb (%) |
P valuec | |||
|---|---|---|---|---|---|
| Young adults (18–44 yr) | Adults (45–64 yr) | The elderly (65–84 yr) | Oldest old (≥85 yr) | ||
| Escherichia coli | n = 62 | n = 264 | n = 494 | n = 148 | |
| Cefazolin-S | 64.5 | 56.8 | 55.3 | 48.6 | 0.027 |
| Cefuroxime-S | 79.0 | 78.0 | 72.1 | 66.2 | 0.035 |
| Cefotaxime-S | 98.4 | 90.2 | 88.9 | 83.1 | 0.034 |
| Levofloxacin-S | 90.3 | 84.1 | 82.0 | 68.9 | 0.049 |
| ESBL producer | 3.2 | 4.5 | 5.9 | 9.5 | 0.035 |
| Klebsiella pneumoniae | n = 17 | n = 123 | n = 164 | n = 50 | |
| Cefazolin-S | 88.2 | 79.7 | 73.2 | 62.0 | 0.006 |
| Cefuroxime-S | 94.1 | 93.5 | 86.0 | 82.0 | 0.041 |
| Cefotaxime-S | 100 | 95.9 | 91.5 | 88.0 | 0.001 |
| Levofloxacin-S | 100 | 95.1 | 90.9 | 86.0 | <0.001 |
| ESBL producer | 0 | 4.1 | 5.5 | 12.0 | 0.031 |
| Staphylococcus aureus | n = 30 | n = 114 | n = 124 | n = 44 | |
| Methicillin-R | 6.9 | 31.6 | 37.9 | 59.1 | 0.021 |
| Streptococcus species | n = 27 | n = 90 | n = 125 | n = 47 | |
| Penicillin-S | 96.3 | 94.4 | 93.6 | 89.4 | 0.047 |
| Cefotaxime-S | 96.3 | 94.4 | 94.4 | 97.9 | 0.673 |
| Levofloxacin-S | 100 | 98.9 | 96.8 | 93.6 | 0.023 |
| Pseudomonas aeruginosa | n = 9 | n = 16 | n = 46 | n = 13 | |
| Ceftazidime-S | 77.8 | 100 | 100 | 84.6 | 0.765 |
| Cefepime-S | 77.8 | 100 | 100 | 84.6 | 0.765 |
| Levofloxacin-S | 88.9 | 87.5 | 91.3 | 84.6 | 0.542 |
S, susceptible.
n indicates the number of isolates in various age groups.
Calculated by Pearson's correlation. Boldface indicates statistical significance, i.e., a P value of <0.05.
Empirical antimicrobial agents in different age groups.
Because 180 patients received combinative EAT and 22 were not treated by antimicrobials before the reporting of blood cultures, 2,507 antimicrobial agents prescribed to 2,349 patients in four age groups were displayed (Table 4). Cephalosporin was the most common class of antimicrobial therapy, accounting for nearly one-third (66.9%; 1,678/2,507) of all empirically administered drugs; third-generation cephalosporins composed nearly half (52.4%; 880/1,678) of all cephalosporins. Approximately 9% (209) of all cases were empirically treated by aminopenicillins/beta-lactamase inhibitors (BLIs), and there was an age-related trend with borderline significance (P = 0.057). Notably, there was a positive age-related trend in the incidence of inappropriate EAT.
TABLE 4.
Trends in empirical antimicrobial therapy in adults with community-onset bacteremia
| Antibiotic | No. (%) of patients |
P valuea | |||
|---|---|---|---|---|---|
| Young adults (18–44 yr; n = 216) |
Adults (45–64 yr; n = 762) |
Elderly (65–84 yr; n = 1,167) |
Oldest old (≥85 yr; n = 362) |
||
| Cephalosporins (n = 1,678) | 135 (62.5) | 528 (69.3) | 794 (68.0) | 221 (61.0) | 0.816 |
| First generation (n = 256) | 31 (14.4) | 85 (11.2) | 101 (8.7) | 39 (10.8) | 0.271 |
| Second generation (n = 316) | 24 (11.1) | 93 (12.2) | 161 (13.8) | 38 (10.5) | 0.982 |
| Third generation (n = 880) | 66 (30.6) | 272 (35.7) | 424 (36.3) | 118 (32.6) | 0.682 |
| Fourth generation (n = 226) | 14 (6.5) | 78 (10.2) | 108 (9.3) | 26 (7.2) | 0.911 |
| Aminopenicillins/BLIs (n = 209) | 10 (4.6) | 61 (8.0) | 102 (8.7) | 36 (9.9) | 0.057 |
| Fluoroquinolones (n = 182) | 17 (7.9) | 56 (7.3) | 83 (7.1) | 26 (7.2) | 0.174 |
| Glycopeptides (n = 144) | 24 (11.1) | 47 (6.2) | 59 (5.1) | 14 (3.9) | 0.072 |
| Ureidopenicillins/BLIs (n = 103) | 7 (3.2) | 17 (2.2) | 55 (4.7) | 24 (6.6) | 0.144 |
| Carbapenems (n = 77) | 8 (3.7) | 17 (2.2) | 37 (3.2) | 15 (4.1) | 0.654 |
| Ureidopenicillins (n = 49) | 3 (1.4) | 12 (1.6) | 17 (1.5) | 17 (4.7) | 0.210 |
| Inappropriate EATb | 28/196 (14.3) | 110/707 (15.6) | 206/1,098 (18.8) | 88/348 (25.3) | 0.049 |
Calculated by Pearson's correlation. Boldface indicates statistical significance, i.e., a P value of <0.05.
One hundred eighty patients received a combination of empirical antimicrobial therapy, and 22 were not treated by antimicrobials before blood culture results were available.
Clinical predictors linked to inappropriate EAT.
Several clinical and microbiological factors associated with inappropriate EAT were examined by univariate analysis (Table 5). The factors associated with inappropriate EAT included nursing home residents; previous antimicrobial use; polymicrobial bacteremia; ESBL-producing EKP, levofloxacin-nonsusceptible EKP, MRSA, or Pseudomonas species as causative microorganisms; bacteremia due to vascular-line infections; and preexisting neurological diseases, chronic kidney disease, or diabetes mellitus. In contrast, bacteremia due to liver abscess was often treated by appropriate EAT. The multivariate regression analyses identified six predictors of inappropriate EAT, including nursing home residents (adjusted odds ratio [AOR], 1.66; P = 0.049); previous antimicrobial use (AOR, 2.53; P < 0.001); polymicrobial bacteremia (AOR, 1.53; P = 0.01); causative microorganisms of ESBL-producing EKP (AOR, 11.83; P < 0.001), MRSA (AOR, 7.88 P < 0.001), or levofloxacin-nonsusceptible EKP (AOR, 4.21; P < 0.001); and underlying neurological diseases (AOR, 1.38; P = 0.03).
TABLE 5.
Clinical predictors of inappropriate empirical antimicrobial therapy in adults with community-onset bacteremia
| Variable | Analysis resulta |
|||
|---|---|---|---|---|
| Univariate |
Multivariateb |
|||
| OR (95% CI) | P value | AOR (95% CI) | P value | |
| Nursing home residents | 2.33 (1.60–3.40) | <0.001 | 1.66 (1.01–2.12) | 0.049 |
| Antimicrobial use within 4 wk before ED arrival | 2.28 (1.80–2.89) | <0.001 | 2.53 (1.60–4.02) | <0.001 |
| Polymicrobial bacteremia | 2.55 (1.91–3.41) | <0.001 | 1.53 (1.10–2.12) | 0.01 |
| Causative microorganism | ||||
| ESBL-producing EKP | 14.00 (8.39–23.35) | <0.001 | 11.83 (6.68–20.96) | <0.001 |
| Methicillin-resistant S. aureus | 10.90 (7.27–16.32) | <0.001 | 7.88 (5.12–12.15) | <0.001 |
| Levofloxacin nonsusceptible EKP | 7.30 (5.39–9.89) | <0.001 | 4.21 (3.04–5.85) | <0.001 |
| Pseudomonas species | 3.71 (2.40–5.71) | <0.001 | NS | NS |
| Bacteremia source | ||||
| Vascular-line infection | 2.56 (1.64–4.00) | <0.001 | NS | NS |
| Liver abscess | 0.17 (0.05–0.54) | 0.001 | NS | NS |
| Comorbidities | ||||
| Neurological disease | 1.85 (1.47–2.33) | <0.001 | 1.38 (1.03–1.86) | 0.03 |
| Chronic kidney disease | 1.41 (1.09–1.82) | 0.008 | NS | NS |
| Diabetes mellitus | 1.24 (1.00–1.54) | 0.046 | NS | NS |
Calculated by chi-square or multivariate regression tests. Boldface indicates statistical significance, i.e., a P value of <0.05.
NS, no significance (after processing the backward multivariate regression).
Clinical predictors of 4-week mortality.
For the included patients, the associations of clinical variables with 4-week mortality, in terms of patient demography, characteristics and severity of bacteremia, inappropriate EAT, resistant microorganisms, comorbidities, comorbidity severity (McCabe classification), and bacteremia source, were examined by univariate analysis (Table 6). A fatal outcome within 4 weeks after bacteremia onset was related to male gender; nursing home residency; ultimately or rapidly fatal comorbidities (McCabe classification); critical illness (a Pitt bacteremia score of ≥4) at bacteremia onset; polymicrobial bacteremia; causative microorganisms of K. pneumoniae, Pseudomonas species, or ESBL-producing EKP; bacteremic pneumonia; comorbidities with malignancies, neurological diseases, or liver cirrhosis; and inappropriate EAT. Furthermore, the following variables were negatively associated with 4-week mortality: E. coli as the causative microorganism; bacteremia due to urinary tract infections, biliary tract infections, or liver abscess; and comorbidities with hypertension.
TABLE 6.
Risk factors of 28-day mortality in adults with community-onset bacteremia
| Variable at bacteremia onset | Analysis resulta |
|||
|---|---|---|---|---|
| Univariate |
Multivariateb |
|||
| OR (95% CI) | P value | AOR (95% CI) | P value | |
| Gender, male | 1.52 (1.21–1.94) | <0.001 | NS | NS |
| Nursing home residents | 2.87 (1.95–4.23) | <0.001 | ||
| Ultimately or rapidly fatal comorbidity (McCabe classification) | 3.50 (2.76–4.45) | <0.001 | 2.13 (1.52–2.98) | <0.001 |
| Polymicrobial bacteremia | 2.30 (1.68–3.16) | <0.001 | ||
| Inappropriate EAT | 2.12 (1.63–2.75) | <0.001 | 2.60 (1.88–3.60) | <0.001 |
| Pitt bacteremia score of ≥4 | 11.82 (9.14–15.28) | <0.001 | 10.69 (8.00–14.28) | <0.001 |
| Causative microorganism | ||||
| ESBL-producing EKP | 3.25 (2.02–5.27) | <0.001 | ||
| Pseudomonas species | 2.88 (1.81–4.57) | <0.001 | NS | NS |
| Klebsiella pneumoniae | 1.83 (1.38–2.44) | <0.001 | NS | NS |
| Escherichia coli | 0.45 (0.35–0.58) | <0.001 | NS | NS |
| Bacteremia source | ||||
| Pneumonia | 4.74 (3.63–6.18) | <0.001 | 2.00 (1.41–2.82) | <0.001 |
| Urinary tract infection | 0.24 (0.17–0.34) | <0.001 | 0.37 (0.25–0.55) | <0.001 |
| Biliary tract infection | 0.57 (0.35–0.91) | 0.02 | NS | NS |
| Liver abscess | 0.31 (0.11–0.85) | 0.02 | NS | NS |
| Major comorbidities | ||||
| Malignancy | 2.67 (2.11–3.38) | <0.001 | 1.95 (1.34–2.83) | 0.001 |
| Neurological disease | 1.42 (1.09–1.84) | 0.008 | ||
| Liver cirrhosis | 2.00 (1.49–2.70) | <0.001 | 1.86 (1.36–2.26) | <0.001 |
| Hypertension | 0.74 (0.59–0.94) | 0.01 | NS | NS |
Calculated by chi-square or multivariate regression tests.
NS, no significance (after processing the backward multivariate regression).
With the exclusion of four predictors independently linked to inappropriate EAT (Table 5), such as nursing home residents, polymicrobial bacteremia, ESBL-producing EKP, and comorbidities with neurological diseases, significant predictors of 4-week mortality recognized by the univariate analyses were included in the multivariate regression. Consequently, seven independent predictors of 4-week mortality were identified: ultimately and rapidly fatal comorbidities (McCabe classification; AOR, 2.13; P < 0.001); inappropriate EAT (AOR, 2.60; P < 0.001); critical illness at bacteremia onset (a Pitt bacteremia score of ≥4; AOR, 10.69; P < 0.01); bacteremia because of pneumonia (AOR, 2.00; P < 0.001) or urinary tract infections (AOR, 0.37; P < 0.001); and comorbidities with malignancy (AOR, 1.95; P = 0.001) or liver cirrhosis (AOR, 1.86; P < 0.001). Notably, inappropriate EAT and bacteremia severity at onset were the two leading variables independently linked to 4-week mortality. Furthermore, of these significant independent predictors, age-related trends were only disclosed in critical illness at bacteremia onset, inappropriate EAT, and bacteremic pneumonia (Fig. 1).
FIG 1.
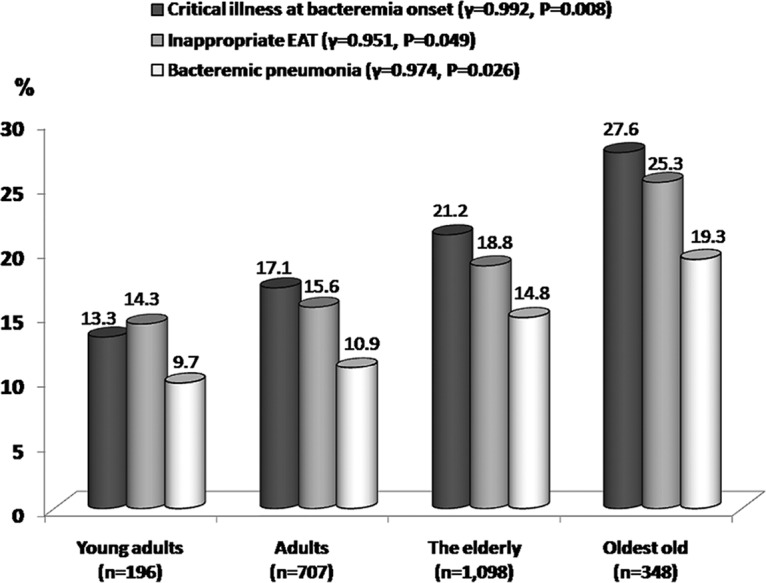
Positive age-related trends in three independent predictors of 4-week crude mortality. r indicates the correlation coefficients recognized by Pearson's correlation.
DISCUSSION
Although several studies questioned the survival benefit of early appropriate antimicrobial therapy (10), previous investigations have suggested that the early administration of appropriate antimicrobials could result in a favorable prognosis in bacteremia patients (11, 12); similarly, the importance of EAT appropriateness was highlighted in this cohort. Generally, with the increased severity of bacteremia, the importance of appropriate EAT should be vigorously emphasized (12), and the time to appropriate EAT administration should be as short as possible (13). With our study findings showing that bacteremic severity increased with age, the appropriateness of EAT should be strongly encouraged for the elderly.
Generally, the increasing incidence and adverse influence of multidrug-resistant microorganisms, such as ESBL producers and MRSA, have been documented in the community (14, 15). Similar to the SENTRY Antimicrobial Surveillance Program (9), the MRSA proportion here increased with age. Additionally, there were several reports that demonstrated a high colonization rate of MRSA in the elderly or nursing home residents (16, 17). Thus, the age-related correlation with MRSA bacteremia was not unexpected in our study. Moreover, positive age-related trends in ESBL producers and levofloxacin-nonsusceptible E. coli and K. pneumoniae were observed. Because the relationship of bacterial infections due to ESBL producers or fluoroquinolone-resistant pathogens and antimicrobial exposure has been well established (15, 18), it is our suspicion that the frequent emergency department (ED) visits or hospitalization of the elderly in the community and their subsequent antibiotic exposure contributed to a substantial risk of infection with multidrug-resistant organisms. More importantly, despite the occurrence of antimicrobial-resistant microorganisms increasing with age, the EAT profile was similar among the four age groups in the ED. Consequently, the age-related trend for inappropriate EAT was determined. The results indicated that for individuals with suspected or documented bacteremia in the ED, it is critical to consider patient age for EAT. For the elderly with risk factors of inappropriate EAT (i.e., recent antimicrobial therapy or underlying neurological disease), the spectrum of EAT may be broader than that for younger adults.
In this cohort, we focused on the cases of community-onset, rather than community-acquired, bacteremia for several reasons. First, community-onset bacteremia is not an uncommon and treatable infectious disease (19). Second, by definition, cases of community-acquired bacteremia should have no recent hospitalization within the past month, no recent invasive procedure before admission, no regular dialysis, and no indwelling intravascular devices (20). However, it is difficult to recognize the true category of patients from the community in overcrowded EDs (21). Accordingly, the patients visiting EDs with bacteremia can be regarded without argument as having community-onset bacteremia, as shown in our published ED-based studies (22–25).
In our cohort, of the seven independent predictors of 4-week mortality identified, the three leading predictors were inappropriate EAT, a critical illness at bacteremia onset, and bacteremic pneumonia, which all had a high AOR and a positive age-related trend. These significant prognostic variables may contribute to the positive age-related trend in 4-week mortality among adults with community-onset bacteremia.
There are several limitations inherent to the design of this study. First, multiple univariate hypothesis tests for age-related trends with no adjustment of covariates for multivariate testing were performed in the current study, increasing the likelihood that some trends are false positives. Therefore, the multivariate regression tests for 4-week mortality and inappropriate EAT were presented here. Second, despite the fact that a large cohort was analyzed using the linear-by-linear association test, the factors contributing to the age-related trend were complicated and not clarified as easily as the primary contributors. Using E. coli and K. pneumoniae as the samples, they were generally the leading pathogens linked to intra-abdominal, urinary, and biliary tract infections. Because of a positive age-related trend in biliary tract infections and a negative trend in intra-abdominal infections, we suspected that the contradictory age-related trends in the abovementioned bacteremic sources partially contribute to the absence of an age-related trend in E. coli or K. pneumoniae bacteremia. Third, the present study was a retrospective study; thus, there may have been some selection bias as a result of patient loss and incomplete clinical information. To diminish the patient number without complete clinical information and reduce the errors of information retrieval, two authors reviewed the medical records of eligible patients. Moreover, we used telephone follow-ups to collect outcome data after discharge. Only 16 (0.7% of 2,365) patients lacking outcome information were excluded, and such a selection bias was expected to minimally influence the study results. Finally, although the present study was conducted in a single center and our findings, particularly on antimicrobial susceptibility, may not be generalized to other areas, it included more than two thousand adults in a 6-year period and at least could be regarded as representative data of community-onset bacteremia in southern Taiwan.
In conclusion, for adults with community-onset bacteremia, age-related differences in bacteremia severity and antimicrobial-resistant microorganisms were evident and contributed to the risk of inappropriate EAT and subsequent mortality in the elderly. With regard to a positive age-related trend in bacteremia severity, the importance of appropriate EAT should be augmented with aging. To facilitate the early administration of broad-spectrum antimicrobials, clinically validated algorithms are warranted for the reliable identification of bacterial infections due to antimicrobial-resistant pathogens in the elderly.
MATERIALS AND METHODS
Study design.
This retrospective cohort study was conducted at the emergency department (ED) of the medical center. The study hospital is a tertiary-care, 1,300-bed, university-based medical center with an approximate annual ED census of 70,000 patients. This study was approved by the hospital's institutional review board (ER-100-182), and the requirement for informed consent was waived. This analysis was reported using the format recommended by STROBE (26). Partial clinical information in this study cohort has been published (13, 22–24).
Bacterial growth in blood cultures obtained from adults in the ED between January 2008 and December 2013 was screened in a computer database. Among adult patients with bacteremia, clinical information was retrieved from medical records and recorded in a predetermined case record form. Patients were excluded if they had hospital-onset bacteremia, contaminated blood cultures, bacteremia diagnosed prior to visiting the ED, inappropriate dosage or route of antimicrobial administration during all hospitalizations, or incomplete clinical information. The most inaccurate records of several variables, such as bacteremia severity, initial sepsis-related syndrome, and laboratory data, were captured within 24 h after ED arrival. Two authors reviewed the medical records of eligible patients for the above-described clinical information. If any discrepancies were observed, both authors inspected the medical records simultaneously, and a decision was reached through consensus. If there were multiple bacteremic episodes, only the first episode of each patient was included. The primary endpoint was crude 4-week mortality. To avoid underestimating the mortality rate, if a patient was not followed up at the outpatient clinic after discharge, outcome information was retrieved by telephone contact. Patients unable to be reached by telephone were excluded.
Microbiological methods.
Bacteremic aerobic isolates in the study period were prospectively collected. Bacteremic isolates were identified by the GNI card of the Vitek 2 system (bioMerieux, Lyon, France). For these stored isolates, antimicrobial susceptibility was determined by the disk diffusion method, based on the performance standards of the Clinical and Laboratory Standards Institute (CLSI) in 2016 (27). For Gram-negative bacilli, the tested drugs included cefazolin, cefuroxime, cefotaxime, ceftazidime, and levofloxacin. For streptococci, the tested drugs included penicillin, cefotaxime, and levofloxacin. If a patient was empirically treated with agents not described above, the susceptibility for these agents was measured. Cefoxitin susceptibility was measured to identify methicillin-resistant Staphylococcus aureus (MRSA). For Escherichia coli, Klebsiella species, and Proteus mirabilis (EKP), the presence of extended-spectrum β-lactamases (ESBLs) was detected by a phenotypic confirmatory test with cephalosporin-clavulanate combination disks, as recommended by the 2009 CLSI guidelines (28).
Definitions.
Similar to a previous categorization (4), our patients were stratified by age as young adults (18 to 44 years), adults (45 to 64 years), the elderly (65 to 84 years), and the oldest old (≥85 years). Community-onset bacteremia indicates that the place of onset of the bacteremic episode is the community and includes long-term health care facilities and community-acquired bacteremia, as previously described (22–25). Blood culture samples with potential contaminating pathogens were regarded as being contaminated according to previously described criteria (29). The sources of bacteremia were determined clinically based on the presence of an active infection site coincident with bacteremia or the isolation of a microorganism from other clinical specimens prior to or on the same date as the bacteremia onset.
As previously defined (13, 23), the antimicrobial therapy was considered appropriate when all of the following criteria were fulfilled: (i) antimicrobial agents were administered according to the route and dosage recommended in the Sanford guide (30) and (ii) bacteremic pathogens were susceptible in vitro to the administered antimicrobial agent based on the contemporary CLSI breakpoints (27). Inappropriate EAT was defined as >48 h for the time to appropriate antibiotic (13, 31), which was the period between ED arrival and the administration of appropriate antimicrobial therapy. The bacteremic severity was graded by the Pitt bacteremia score (13, 23). Patients with a high Pitt bacteremia score (≥4) were categorized as critically ill (13), whereas those with a Pitt bacteremia score of 0 and 1 to 3 were categorized as being mildly and moderately ill, respectively. Comorbidities were defined as in previous reports (32), and malignancies included hematological malignancies and solid tumors. The severity of underlying diseases was assessed by a previously delineated McCabe classification system (33).
Statistical analyses.
Statistical analyses were performed using the Statistical Package for the Social Sciences for Windows (Chicago, IL, USA), version 20.0. The age-related trends in clinical characteristics and the outcomes were analyzed by the equivalent linear-by-linear association test (i.e., Pearson's correlation). A univariate analysis for the predictors of 4-week mortality with odds ratios (ORs), 95% confident intervals (CIs), and P values was conducted using a chi-square test or Fisher's exact test. To assess the independent predictor (adjusted odds ratio [AOR] and 95% CI) of short-term mortality or inappropriate EAT, variables with a P value of less than 0.05 in the univariate analysis were included in a stepwise and backward multivariable logistic regression model. A P value of less than 0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We have no conflicts of interest to declare.
Financial support was received from the Ministry of Science and Technology (NSC102-2314-B-006-079), the Ministry of Health and Welfare (MOHW106-TDU-B-211-113003), and the National Cheng Kung University Hospital (NCKUH-10305018 and -10406029), Tainan, Taiwan.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01050-17.
REFERENCES
- 1.Javaloyas M, Garcia-Somoza D, Gudiol F. 2002. Epidemiology and prognosis of bacteremia: a 10-y study in a community hospital. Scand J Infect Dis 34:436–441. doi: 10.1080/00365540110080629. [DOI] [PubMed] [Google Scholar]
- 2.Kollef MH, Zilberberg MD, Shorr AF, Vo L, Schein J, Micek ST, Kim M. 2011. Epidemiology, microbiology and outcomes of healthcare-associated and community-acquired bacteremia: a multicenter cohort study. J Infect 62:130–135. doi: 10.1016/j.jinf.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Diekema DJ, Beekmann SE, Chapin KC, Morel KA, Munson E, Doern GV. 2003. Epidemiology and outcome of nosocomial and community-onset bloodstream infection. J Clin Microbiol 41:3655–3660. doi: 10.1128/JCM.41.8.3655-3660.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee CC, Chen SY, Chang IJ, Chen SC, Wu SC. 2007. Comparison of clinical manifestations and outcome of community-acquired bloodstream infections among the oldest old, elderly, and adult patients. Medicine (Baltimore, MD) 86:138–144. doi: 10.1097/SHK.0b013e318067da56. [DOI] [PubMed] [Google Scholar]
- 5.Berman P, Hogan DB, Fox RA. 1987. The atypical presentation of infection in old age. Age Ageing 16:201–207. doi: 10.1093/ageing/16.4.201. [DOI] [PubMed] [Google Scholar]
- 6.Gavazzi G, Mallaret MR, Couturier P, Iffenecker A, Franco A. 2002. Bloodstream infection: differences between young-old, old, and old-old patients. J Am Geriatr Soc 50:1667–1673. doi: 10.1046/j.1532-5415.2002.50458.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee CC, Chang CM, Hong MY, Hsu HC, Ko WC. 2013. Different impact of the appropriateness of empirical antibiotics for bacteremia among younger adults and the elderly in the ED. Am J Emerg Med 31:282–290. doi: 10.1016/j.ajem.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Girard TD, Ely EW. 2007. Bacteremia and sepsis in older adults. Clin Geriatr Med 23:633–647. doi: 10.1016/j.cger.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Diekema DJ, Pfaller MA, Jones RN, Group SP. 2002. Age-related trends in pathogen frequency and antimicrobial susceptibility of bloodstream isolates in North America: SENTRY Antimicrobial Surveillance Program, 1997-2000. Int J Antimicrob Agents 20:412–418. doi: 10.1016/S0924-8579(02)00204-2. [DOI] [PubMed] [Google Scholar]
- 10.Lin MY, Weinstein RA, Hota B. 2008. Delay of active antimicrobial therapy and mortality among patients with bacteremia: impact of severe neutropenia. Antimicrob Agents Chemother 52:3188–3194. doi: 10.1128/AAC.01553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. 2000. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146–155. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 12.Chen HC, Lin WL, Lin CC, Hsieh WH, Hsieh CH, Wu MH, Wu JY, Lee CC. 2013. Outcome of inadequate empirical antibiotic therapy in emergency department patients with community-onset bloodstream infections. J Antimicrob Chemother 68:947–953. doi: 10.1093/jac/dks475. [DOI] [PubMed] [Google Scholar]
- 13.Lee CC, Lee CH, Hong MY, Tang HJ, Ko WC. 2017. Timing of appropriate empirical antimicrobial administration and outcome of adults with community-onset bacteremia. Crit Care 21:119. doi: 10.1186/s13054-017-1696-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barr B, Wilcox MH, Brady A, Parnell P, Darby B, Tompkins D. 2007. Prevalence of methicillin-resistant Staphylococcus aureus colonization among older residents of care homes in the United Kingdom. Infect Control Hosp Epidemiol 28:853–859. doi: 10.1086/516795. [DOI] [PubMed] [Google Scholar]
- 15.Pitout JD, Laupland KB. 2008. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis 8:159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 16.O'Sullivan NR, Keane CT. 2000. The prevalence of methicillin-resistant Staphylococcus aureus among the residents of six nursing homes for the elderly. J Hosp Infect 45:322–329. doi: 10.1053/jhin.2000.0758. [DOI] [PubMed] [Google Scholar]
- 17.Lucet JC, Grenet K, Armand-Lefevre L, Harnal M, Bouvet E, Regnier B, Andremont A. 2005. High prevalence of carriage of methicillin-resistant Staphylococcus aureus at hospital admission in elderly patients: implications for infection control strategies. Infect Control Hosp Epidemiol 26:121–126. doi: 10.1086/502514. [DOI] [PubMed] [Google Scholar]
- 18.Lautenbach E, Strom BL, Bilker WB, Patel JB, Edelstein PH, Fishman NO. 2001. Epidemiological investigation of fluoroquinolone resistance in infections due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Clin Infect Dis 33:1288–1294. doi: 10.1086/322667. [DOI] [PubMed] [Google Scholar]
- 19.Laupland KB, Gregson DB, Flemons WW, Hawkins D, Ross T, Church DL. 2007. Burden of community-onset bloodstream infection: a population-based assessment. Epidemiol Infect 135:1037–1042. doi: 10.1017/S0950268806007631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, Reller LB, Sexton DJ. 2002. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 21.Lee CC, Lee NY, Chuang MC, Chen PL, Chang CM, Ko WC. 2012. The impact of overcrowding on the bacterial contamination of blood cultures in the ED. Am J Emerg Med 30:839–845. doi: 10.1016/j.ajem.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh CC, Lee CH, Li MC, Hong MY, Chi CH, Lee CC. 2016. Empirical third-generation cephalosporin therapy for adults with community-onset Enterobacteriaceae bacteraemia: impact of revised CLSI breakpoints. Int J Antimicrob Agents 47:297–303. doi: 10.1016/j.ijantimicag.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Lee CC, Wang JL, Lee CH, Hsieh CC, Hung YP, Hong MY, Tang HJ, Ko WC. 2017. Clinical benefit of appropriate empirical fluoroquinolone therapy for adults with community-onset bacteremia in comparison with third-generation-cephalosporin therapy. Antimicrob Agents Chemother 61:e02174-16. doi: 10.1128/AAC.02174-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh CC, Lee CH, Hong MY, Hung YP, Lee NY, Ko WC, Lee CC. 2016. Propensity score-matched analysis comparing the therapeutic efficacies of cefazolin and extended-spectrum cephalosporins as appropriate empirical therapy in adults with community-onset Escherichia coli, Klebsiella spp. and Proteus mirabilis bacteraemia. Int J Antimicrob Agents 48:712–718. doi: 10.1016/j.ijantimicag.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Lee CC, Lee NY, Chen PL, Hong MY, Chan TY, Chi CH, Ko WC. 2015. Impact of antimicrobial strategies on clinical outcomes of adults with septic shock and community-onset Enterobacteriaceae bacteremia: de-escalation is beneficial. Diagn Microbiol Infect Dis 82:158–164. doi: 10.1016/j.diagmicrobio.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 26.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, STROBE Initiative. 2007. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 27.Clinical and Laboratory Standards Institute. 2016. Performance standards for antimicrobial susceptibility testing; approved standard. 26th informational supplement. CLSI document M100-S26. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 28.Clinical and Laboratory Standards Institute. 2009. Performance standards for antimicrobial susceptibility testing; approved standard. 19th informational supplement. CLSI document M100-S19. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 29.Lee CC, Lin WJ, Shih HI, Wu CJ, Chen PL, Lee HC, Lee NY, Chang CM, Wang LR, Ko WC. 2007. Clinical significance of potential contaminants in blood cultures among patients in a medical center. J Microbiol Immunol Infect 40:438–444. [PubMed] [Google Scholar]
- 30.Gilbert DN, Moellering RC Jr, Eliopoulos GM, Chambers HF, Saag MSS (ed). 2009. Selected pharmacologic features of antimicrobial agents, p 78–82. In The Sanford guide to antimicrobial therapy, 39th ed Antimicrobial Therapy, Sperryville, VA. [Google Scholar]
- 31.Leibovici L, Shraga I, Drucker M, Konigsberger H, Samra Z, Pitlik SD. 1998. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J Intern Med 244:379–386. doi: 10.1046/j.1365-2796.1998.00379.x. [DOI] [PubMed] [Google Scholar]
- 32.Schellevis FG, van der Velden J, van de Lisdonk E, van Eijk JT, van Weel C. 1993. Comorbidity of chronic diseases in general practice. J Clin Epidemiol 46:469–473. doi: 10.1016/0895-4356(93)90024-U. [DOI] [PubMed] [Google Scholar]
- 33.McCabe WR. 1974. Gram-negative bacteremia. Adv Intern Med 19:135–158. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


