ABSTRACT
The estimated attributable mortality rate for invasive candidiasis (IC) in the intensive care unit (ICU) setting varies from 30 to 40%. Physiological changes in critically ill patients may affect the distribution and elimination of micafungin, and therefore, dosing adjustments might be mandatory. The objective of this study was to determine the pharmacokinetic parameters of micafungin in critically ill patients and assess the probability of target attainment. Micafungin plasma concentrations were measured to estimate the pharmacokinetic properties of micafungin. MIC values for Candida isolates were determined to assess the probability of target attainment for patients. Data from 19 patients with suspected or proven invasive candidiasis were available for analysis. The median area under the concentration-time curve from 0 to 24 h at steady state (AUC0–24) was 89.6 mg · h/liter (interquartile range [IQR], 75.4 to 113.6 mg · h/liter); this was significantly lower than the median micafungin AUC0–24 values of 152.0 mg · h/liter (IQR, 136.0 to 162.0 mg · h/liter) and 134.0 mg · h/liter (IQR, 118.0 to 148.6 mg · h/liter) in healthy volunteers (P = <0.0001 and P = <0.001, respectively). All Candida isolates were susceptible to micafungin, with a median MIC of 0.016 mg/liter (IQR, 0.012 to 0.023 mg/liter). The median AUC0–24/MIC ratio was 5,684 (IQR, 4,325 to 7,578), and 3 of the 17 evaluable patients (17.6%) diagnosed with proven invasive candidiasis did not meet the AUC/MIC ratio target of 5,000. Micafungin exposure was lower in critically ill patients than in healthy volunteers. The variability in micafungin exposure in this ICU population could be explained by the patients' body weight. Our findings suggest that healthier patients (sequential organ failure assessment [SOFA] score of <10) weighing more than 100 kg and receiving 100 mg micafungin daily are at risk for inappropriate micafungin exposure and potentially inadequate antifungal treatment. (This study has been registered at ClinicalTrials.gov under identifier NCT01716988.)
KEYWORDS: micafungin, pharmacokinetics, invasive candidiasis, critically ill
INTRODUCTION
Candida bloodstream infections are associated with an increased length of stay in intensive care unit (ICUs) and hospitals. The estimated attributable mortality rate for invasive candidiasis in this setting varies from 30% to 40% (1). Early initiation of effective therapy and adequate dosing are critical for the successful treatment of invasive candidiasis, as demonstrated in several studies suggesting significantly higher survival rates among patients with invasive candidiasis for whom adequate antifungal therapy was promptly started (2–7). Once a bloodstream infection with a Candida species has been diagnosed, guidelines recommend the immediate initiation of therapy with an echinocandin combined with the prompt removal of central venous catheters (8, 9). Step-down therapy to an oral azole is advised once the patient is clinically stable and if the Candida isolate is susceptible to fluconazole. The efficacy of micafungin is concentration dependent and related mainly to the ratio of the area under the plasma concentration-time curve from 0 to 24 h at steady state (AUC0–24) to the MIC of the microorganism (AUC0–24/MIC ratio) (10). Andes et al. previously described three AUC/MIC ratio targets for a general Candida population, a non-Candida parapsilosis population, and a C. parapsilosis population (11).
Critically ill patients often have pathophysiological or iatrogenic conditions resulting in variations in extracellular volume and drug pharmacokinetics (12). These physiological changes may affect the distribution, metabolism, and elimination of micafungin, and therefore, dose adjustments might be mandatory. Standard dosages of echinocandins in ICU patients are frequently associated with lower drug exposure, which can result in subtherapeutic AUC0–24/MIC ratios (13–17). In an efficacy study of micafungin in ICU versus non-ICU patients, significantly lower treatment success rates were seen for ICU patients (62.5% success) than for non-ICU patients (85% success) (18). Disease severity as measured by the acute physiology and chronic health evaluation II (APACHE II) score was a potential explanatory factor associated with treatment success. In the ICU, disease scoring systems constructed from physical examination parameters and patient characteristics are used to assess disease severity to predict mortality. Although these scoring systems have been developed to predict mortality, they have been associated with pharmacokinetic variability in various studies (19–23). In systemic circulation, micafungin is highly bound to plasma protein (>99%), primarily albumin, and since critically ill patients are known to have decreased albumin concentrations, this may influence the efficacy of micafungin (24). Body weight is also a factor that might influence the pharmacokinetics of micafungin, as previously demonstrated in two different studies (25, 26). Continuous renal replacement therapy showed less of an effect on pharmacokinetic behavior, but dose elevation was possible in specific cases (27).
The objective of this study was to determine the pharmacokinetic parameters of micafungin in critically ill patients and assess the probability of target attainment based on the AUC0–24 h/MIC ratio for this ICU population. We try to associate micafungin exposure with patient variables that are likely to influence the micafungin plasma concentration. The findings of this study will be helpful in deriving practical decision rules and possibly influence future dosing schedules for critically ill patients treated with micafungin.
RESULTS
Study population.
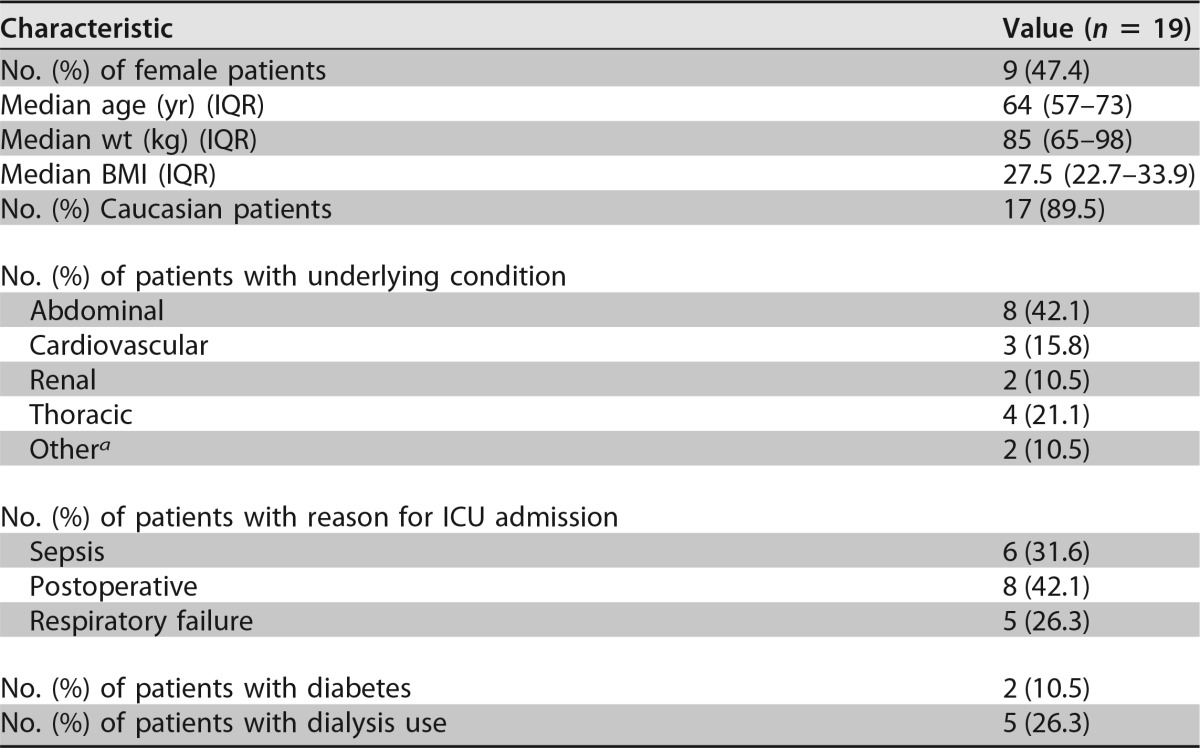
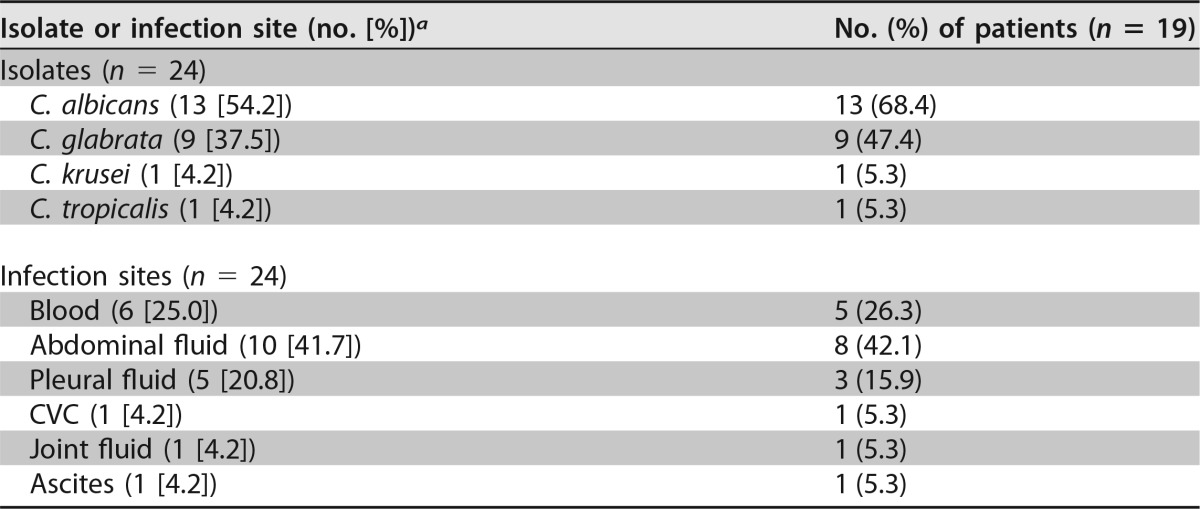
Twenty-two patients were enrolled in the study, resulting in 19 evaluable patients (see the flowchart in Fig. S1 in the supplemental material). Three patients were discharged to the ward before blood sampling on day 4 (±1 day) of micafungin therapy was possible. In Table 1, the most relevant patient characteristics are summarized. Approximately one-half the patients were female, and more than three-quarters of the patients (n = 15 [78.9%]) underwent major surgery. All patients had markedly reduced serum albumin levels (median, 19 g/liter [interquartile range {IQR}, 16 to 24 g/liter]), considering a reference range of 35 g/liter to 50 g/liter for healthy subjects. In total, 24 Candida isolates were cultured from a sterile site. The most prevalent pathogen was Candida albicans (n = 13 [54.2%]) in 13 patients, followed by C. glabrata (n = 9 [37.5%]) in 9 patients, and 4 patients were infected with mixed Candida species (C. albicans and C. glabrata [and one patient also with C. tropicalis]). Six Candida isolates (26.3%) in 5 patients were cultured from blood, and 18 isolates were cultured from another sterile site (Table 2). MIC values for 22 Candida isolates (91.7%) were determined, with a median MIC of 0.016 mg/liter (IQR, 0.012 to 0.023 mg/liter). The MIC values for C. albicans ranged from 0.008 to 0.023 mg/liter, those for C. glabrata ranged from 0.008 to 0.032 mg/liter, the MIC value for C. krusei (n = 1) was 0.125 mg/liter, and that for C. tropicalis (n = 1) was 0.016 mg/liter.
TABLE 1.
Patient characteristics
Rectal carcinoma and trauma.
TABLE 2.
Microbiological data
Three patients had mixed infections with C. albicans and C. glabrata, and 1 patient had a mixed infection with C. albicans, C. glabrata, and C. tropicalis. CVC, central venous catheter.
Pharmacokinetics.
Patients were treated with micafungin for a median time of 14 days (IQR, 11 to 17 days), resulting in 19 micafungin concentration-time curves (Fig. 1). In total, 197 samples were drawn from 19 patients for pharmacokinetic analysis. The median AUC0–24 on day 4 (±1 day) was 89.6 mg · h/liter (IQR, 75.4 to 113.6 mg · h/liter), the median maximum concentration of drug in serum (Cmax) was 7.7 mg/liter (IQR, 6.4 to 9.3 mg/liter), and the median C24 (concentration at 24 h after start of infusion) value was 1.8 mg/liter (IQR, 1.6 to 2.6 mg/liter). Both the micafungin peak concentration (r2 = 0.728; P = < 0.001 [as determined by Spearman correlations]) and trough concentration (r2 = 0.783; P = <0.001 [as determined by Spearman correlations]) (Fig. 2) showed a significant correlation with the micafungin AUC. The population pharmacokinetic parameters of micafungin are shown in Table S2 in the supplemental material. Fifty-seven trough concentrations were determined over time, with a median concentration of 1.9 mg/liter (IQR, 1.6 to 2.7 mg/liter). Trough concentrations were stable over time, and there was no significant difference between trough concentrations in individual patients.
FIG 1.
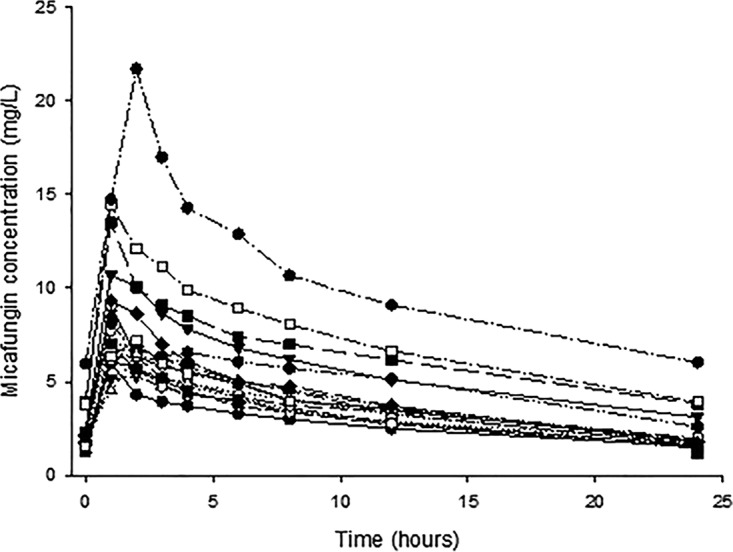
Micafungin concentration-time curves for 19 individual patients during steady state.
FIG 2.
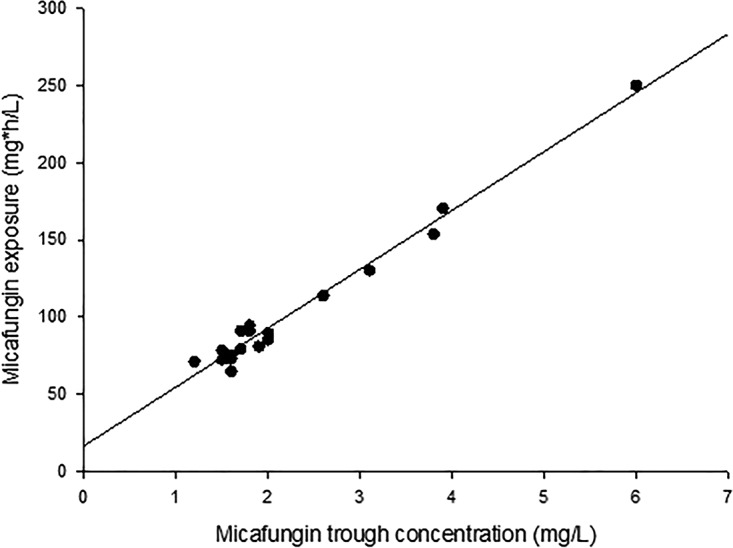
Micafungin exposure expressed as AUC0–24 correlated with the micafungin trough concentration (in steady state) expressed as C24 (y = 38,127x + 16,601; R2 = 0.9766).
The mean AUC0–24 of micafungin in this study population (AUC = 102.9 mg · h/liter [standard deviation {SD} = 45.5 mg · h/liter]) was significantly lower than the mean AUC0–24 of micafungin in two studies with healthy volunteers (AUC = 150.2 mg · h/liter [SD = 21.5 mg · h/liter] and 133.8 mg · h/liter [SD = 21.4 mg · h/liter] [P = <0.0001 and P = <0.001, respectively]) (28, 29) (see Fig. S1a and S1b in the supplemental material). The micafungin exposure expressed as AUC in our ICU population was comparable to that of another ICU population (17) (mean AUC = 102.9 mg · h/liter [SD = 45.5 mg · h/liter] versus 88.1 mg · h/liter [SD = 33.3 mg · h/liter] [P = 0.2457]).
Treatment and treatment outcome.
Thirteen patients (68.4%) had a complete response to micafungin therapy, three patients (15.8%) had a partial response to micafungin therapy, two patients (10.5%) had stable disease, and one patient (5.3%) died within 28 days after micafungin therapy was started. Micafungin therapy was stopped after successful treatment for the majority of patients (n = 15 [78.9%]). Micafungin was switched to fluconazole in one case (5.3%) because of suspected meningitis. Micafungin therapy was stopped in three patients (15.8%) because of an adverse event. All of these adverse events were scored with the Naranjo algorithm as possible adverse events (skin rash [Naranjo score of 2[, thrombocytopenia [Naranjo score of 3], and cardiac toxicity [Naranjo score of 2]). The patient that suffered from cardiac side effects stopped micafungin therapy after 14 days of treatment and died due to multiorgan failure caused by ongoing abdominal sepsis caused by C. albicans.
The median AUC0–24/MIC ratio was 5,684 (IQR, 4,325 to 7,578) for 22 Candida isolates from 17 patients. The median AUC0–24/MIC ratios were 6,221 (IQR, 4,576 to 7,582) for C. albicans (n = 12), 5,643 (IQR, 3,604 to 7,254) for C. glabrata (n = 8), 908 for C. krusei (n = 1), and 5,684 for C. tropicalis (n = 1).
Correlation of micafungin exposure with patient variables.
The micafungin exposure expressed as AUC correlated well with micafungin clearance (r2 = −0.992; P = <0.0001 [as determined by Spearman correlations]). The correlations between micafungin exposure, disease severity scores, and patient size descriptors are shown in Table 3. Only the multiple-organ dysfunction score (MODS) and the sequential organ failure assessment (SOFA) score showed significant positive correlations with micafungin exposure and significant negative correlations with micafungin clearance (r2 = −0.311 and P = 0.013 [as determined by Spearman correlations] for MODS and r2 = −0.308 and P = 0.014 [as determined by Spearman correlations] for SOFA). Patients with a MODS value of ≥5 or a SOFA score of ≥10 were associated with significantly lower micafungin clearance (P = 0.043 and P = 0.013, respectively, as determined by a Mann-Whitney U test).
TABLE 3.
Disease severity scores and patient size descriptors correlated with micafungin exposure (AUC)
| Parametera | Median value (IQR) | Spearman r | P value |
|---|---|---|---|
| APACHE II score | 15 (13–19) | 0.405 | 0.085 |
| APACHE IV score | 83 (51–107) | 0.339 | 0.156 |
| LODS score | 5 (4–7) | 0.339 | 0.156 |
| MODS | 4 (2–6) | 0.542 | 0.017 |
| MPMII (%) | 55.0 (30.0–66.0) | −0.035 | 0.886 |
| ODIN score | 3 (3–4) | 0.301 | 0.211 |
| SAPS III | 55 (38–66) | 0.421 | 0.072 |
| SOFA score | 4 (3–9) | 0.548 | 0.015 |
| Body wt (kg) | 85 (65–98) | −0.488 | 0.034 |
| BMI (kg m−2) | 27.5 (22.7–33.9) | −0.321 | 0.180 |
| BSA (m2) | 2.02 (1.81–2.20) | −0.545 | 0.016 |
| LBM (kg) | 64.1 (50.6–75.9) | −0.485 | 0.035 |
| FFM (kg) | 58.1 (45.3–69.5) | −0.546 | 0.016 |
APACHE II, acute physiology and chronic health evaluation II; LODS, logistic organ dysfunction system; MODS, multiple-organ dysfunction score; MPMII, mortality prediction model II; ODIN, organ dysfunctions and/or infection; SAPS III, simplified acute physiology score III; SOFA, sequential organ failure assessment; BMI, body mass index; BSA, body surface area; LBM, lean body mass; FFM, fat-free mass.
All patient size descriptors (body weight, body surface area [BSA], lean body mass [LBM], and fat-free mass [FFM]) were significantly negatively associated with micafungin exposure, except for the patients' body mass index (BMI). The patients' BSA and FFM showed the strongest association with micafungin exposure. Patients with a BSA of >2.10 m2 or a FFM of >62 kg were correlated with significantly lower micafungin exposure (P = 0.009 and P = 0.008, respectively, as determined by a Mann-Whitney U test). Patients with a body weight of >100 kg were also correlated with significantly lower micafungin exposure (P = 0.044). No significant correlation was found between micafungin exposure and albumin concentrations (P = 0.584, as determined by Spearman correlations). Data for multiple liver function test parameters, including ALP (alkaline phosphatase), ALAT (alanine transaminase), ASAT (aspartate aminotransferase), γGT (gamma-glutamyl transpeptidase), total bilirubin, and C-reactive protein (CRP), were collected but were not associated with micafungin exposure (P = 0.403, P = 0.634, P = 0.759, P = 0.298, P = 0.120, and P = 0.562, respectively, as determined by Spearman correlations).
In the multiple-linear-regression analysis, data for all ICU patients from two healthy-volunteer studies (n = 72) were included, and the SOFA score had the lowest P value in the univariate analysis. The adjusted R2 value of the model was 0.574, and the R2 change was −0.001, compared with the model with all variables. Body weight showed a significant and independent negative association with micafungin exposure expressed as the AUC (effect value of −0.934 [95.0% confidence interval, −1.310 to −0.576] and P = <0.0001), and the albumin plasma concentration and SOFA score showed significant and independent positive associations with micafungin exposure (effect value of 3.462 [95.0% confidence interval, 2.289 to 4.635] and P = <0.0001 for the albumin plasma concentration and effect value of 3.114 [95.0% confidence interval, 0.609 to 5.619] and P = 0.016 for the SOFA score).
The observed micafungin exposure for ICU patients and healthy volunteers with the corresponding patient body weights were used to predict micafungin exposure in these subjects using different dosing regiments. The box-and-whisker plots of the observed AUC0–24 values for subjects receiving 100 mg micafungin once daily and predicted values for fixed doses of 150 mg and 200 mg daily and weight-driven dosing of 1.5 mg/kg of body weight and 2 mg/kg are shown in Fig. 3. All subjects receiving 150 mg daily or 2 mg/kg daily achieved an AUC0–24 of >80 mg · h/liter and an AUC/MIC ratio of >5,000 in cases of C. albicans and C. glabrata infections.
FIG 3.
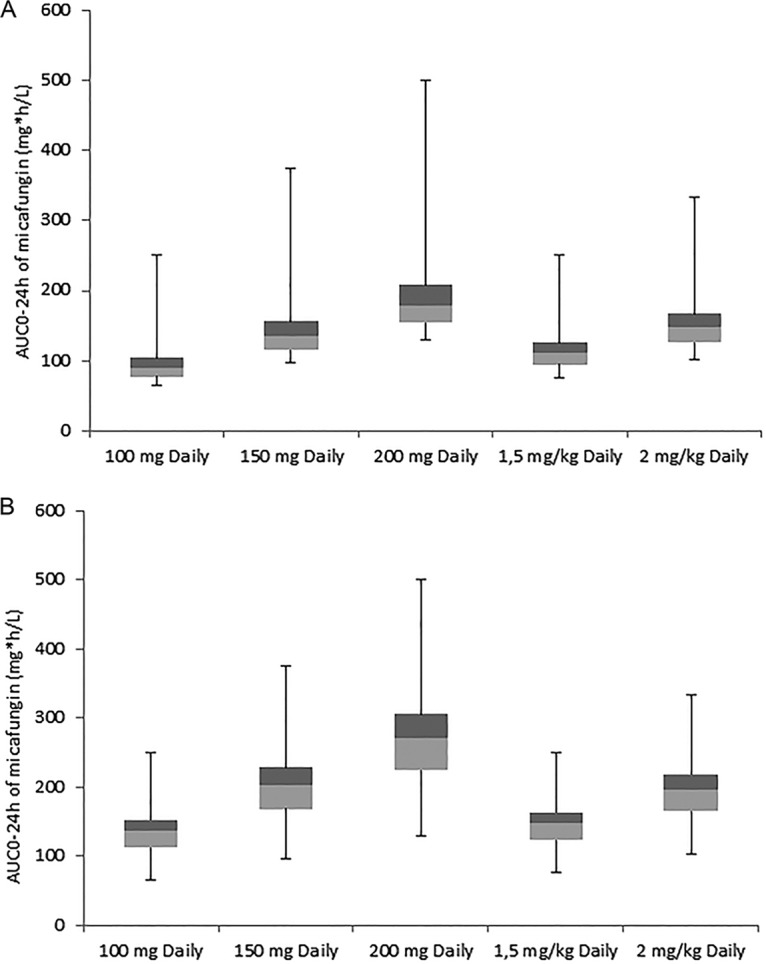
Box-and-whisker plots of the AUC0–24 of observed values for subjects receiving 100 mg micafungin once daily and predicted values for fixed doses and weight-driven dosing based on the observed values and a linear dosing-exposure relationship (38). (A) Data from ICU patients (n = 19); (B) data from both ICU patients and healthy volunteers (n = 72).
DISCUSSION
The median AUC0–24 of micafungin was 89.6 mg · h/liter and was considered low, although the standard dose of 100 mg daily was applied. All isolates of C. albicans and C. glabrata were susceptible to micafungin. Three of the 17 evaluable patients (17.6%) diagnosed with proven invasive candidiasis did not meet the proposed AUC/MIC ratio target of 5,000. Multiple-linear-regression analysis showed that micafungin exposure was negatively associated with body weight and positively associated with the albumin plasma concentration and SOFA score.
The level of micafungin exposure in our ICU population is significantly lower than the level of micafungin exposure in healthy volunteers (28, 29). Multiple factors in critically ill patients may influence micafungin clearance and the volume of distribution.
Multiple studies showed that the overall disease severity in patients may influence the pharmacokinetics of antimicrobial drugs (19–23). In the univariate analysis and in the multivariate analysis, the SOFA score showed a positive correlation with micafungin exposure, which is possibly explained by the hepatic component in the hybrid score. Hepatic function, reflected mainly by the bilirubin concentration, has a greater impact on the SOFA score than on the other ICU scoring systems. This is in accordance with the findings of Jullien et al., who found a decrease in micafungin clearance by 25% when the SOFA score was ≥10 (17). We found the exact same cutoff value for the SOFA score and variability in micafungin clearance. Despite this association, no correlation between micafungin exposure and individual hepatic parameters (ALP, ASAT, ALAT, γGT, and total bilirubin) was found. Identical associations with disease severity scores were investigated in several studies, but none of them were able to find a strong correlation between disease severity and echinocandin exposure in general (16, 30).
Factors that could explain the lower exposure of micafungin in this study confirmed previously reported findings from other studies on micafungin. Two studies showed that the patient's body weight affects micafungin exposure (25, 26). We found a significant negative correlation with body weight but also with other size descriptors such as BSA, LBM, and FFM. Dosing adjustments for heavier or larger patients might be mandatory, as with caspofungin, to achieve more appropriate micafungin concentrations. Micafungin is >99% bound to protein, mainly to albumin, and therefore, decreased albumin plasma concentrations may result in lower levels of micafungin exposure (26). We were not able to find a correlation between micafungin exposure and the albumin concentration in the univariate analysis, probably because our study participants were critically ill patients with a narrow range of low albumin concentrations (<30 g/liter). Although the total AUC of micafungin might be lower in patients with low albumin concentrations, the free fractions of micafungin might be comparable in patients with normal albumin concentrations. The efficacy of micafungin would be comparable in this situation. Nevertheless, unbound micafungin concentrations were not determined in this study, because the measurement of unbound micafungin is challenging, since micafungin is >99% bound to plasma proteins. To explore the effect of albumin, we included albumin concentrations of 35 g/liter (normal value, 35 to 55 g/liter) in the multiple-linear-regression analysis for a rough estimation. In this analysis, the albumin concentration showed an independent positive correlation with micafungin exposure. A change in the metabolic route in patients with liver impairment may decrease micafungin exposure. In these patients, an increase in the formation of the M5 metabolite results in decreased micafungin exposure (31). In this study, no patients suffered from liver failure or severe liver dysfunction. Besides, no concentrations of micafungin metabolites were measured.
The in vitro and in vivo efficacy of micafungin, expressed as the AUC/MIC ratio, is determined by micafungin exposure and the susceptibility of the fungal isolate (10, 32, 33). All isolates of C. albicans and C. glabrata were susceptible to micafungin, but no breakpoints for C. tropicalis and C. krusei are defined by the European Committee on Antimicrobial Susceptibility Testing (EUCAST). Andes et al. described three AUC/MIC ratio targets for a general Candida population, a non-C. parapsilosis population, and a C. parapsilosis population with cutoff values of 3,000, 5,000, and 285, respectively (11). This ICU population appeared to be a non-C. parapsilosis population with a corresponding AUC/MIC ratio target of 5,000. In three patients diagnosed with proven invasive candidiasis, the target of 5,000 was not attained, and these patients were infected with C. albicans (AUC/MIC ratio of 4,959), C. glabrata (AUC/MIC ratio of 3,152), and C. krusei (AUC/MIC ratio of 908). All three patients had a complete response, 28 days after the initiation of treatment with micafungin. A target of 5,000 and even a target of 3,000 might not be suitable for patients infected with C. krusei. As with C. parapsilosis, C. krusei isolates have higher MICs than those for other Candida species (34). In the absence of well-established AUC/MIC ratio target values, higher micafungin dosing to maximize exposure should be considered for those individuals at risk for microbiological failure or a lack of clinical improvement since micafungin is safe when administered at a higher-than-standard dose (35). Drug interactions with micafungin are uncommon, and repeated daily doses of up to 900 mg in adult patients have been administered, with no reported dose-limiting toxicity (36). In our cohort, three patients stopped micafungin therapy because of possible adverse events but had micafungin concentrations considered to be in the therapeutic range. These adverse events were more likely related to the underlying conditions of the patient and comedication. The treatment outcome was consistent with the current success rate of echinocandin therapy in the ICU, although the number of treated patients is too small for definitive conclusions to be drawn (37).
Further research on factors that contribute to the variability in micafungin exposure in which the suggested cutoff values can be validated with larger and more heterogeneous cohorts is mandatory. Besides the patient's body weight, all covariates that might influence micafungin exposure are theoretically explained. A fixed dose of 100 mg daily might not be sufficient for heavier patients, and therefore, multiple dosing regimens were simulated based on the linear dosage-exposure relationship over a dosing range of 0.15 to 8 mg/kg (38). A dosing regimen of 2 mg/kg daily was associated with 100% target attainment in cases of C. albicans and C. glabrata infections and showed less variability in micafungin exposure than a fixed dosage. The determination of unbound micafungin concentrations would give insight in the in vivo efficacy of micafungin and the impact of albumin concentration variability in patients on the efficacy of micafungin. The AUC/MIC ratio target of 5,000 for non-C. parapsilosis species was determined by using Monte Carlo simulations, and larger cohorts could provide a more real-life understanding of the association between patient outcomes and the AUC/MIC ratio for different Candida species.
Conclusion.
The mean level of micafungin exposure, expressed as the total AUC, was significantly lower in critically ill patients than in healthy volunteers. We can speculate about the suitability of a fixed 100-mg daily dose for every individual. Our findings on micafungin are in accordance with previously reported findings for heavier patients treated with echinocandins. Healthier patients (SOFA score of <10) weighing more than 100 kg and receiving 100 mg micafungin daily are at risk for inappropriate micafungin exposure and potentially inadequate antifungal treatment. Dosage adjustments might also applicable for patients with cultures positive for Candida species other than C. albicans (with MICs of ≤0.016 mg/liter) or C. glabrata (with MICs of ≤0.032 mg/liter), since information on the relationship between micafungin exposure and the MICs of less common Candida species is limited. Although the measurement of unbound micafungin concentrations is challenging, determination of the free fraction of micafungin would possibly be helpful for decision-making on dosages for hypoalbuminemic patients.
MATERIALS AND METHODS
Study design and study population.
This prospective pharmacokinetic study of micafungin in ICU patients was conducted at the University Medical Center Groningen (UMCG) from December 2012 to December 2016. This study was approved by the local ethics committee (Institutional Review Board approval no. 2012-189) and registered at ClinicalTrials.gov under identifier NCT01716988. Patients (aged 18 years and older) were eligible for inclusion as study participants if they were admitted to the ICU, treated with micafungin, and diagnosed with suspected or proven invasive candidiasis according to the 2008 definition of invasive fungal disease of the European Organization for Research and Treatment of Cancer and Mycoses Study Group (EORTC/MSG) consensus group (39). Patients were excluded if blood sampling was not possible. A sample size of 18 patients is needed to detect a clinically relevant correlation of plasma concentrations of micafungin with disease severity scores of 60% with 80% power and a significance level of an α value of 0.05 (two sided).
Study data.
Data on patient characteristics, disease severity scores, and laboratory parameters were collected from medical charts (see Table S1 in the supplemental material). Data on patient characteristics and disease severity scores were collected on the day when treatment with micafungin was started. Data on laboratory parameters were collected on the day when treatment with micafungin was started, the day of the pharmacokinetic curve, and the day when treatment with micafungin was stopped. The full pharmacokinetic profile of micafungin was obtained on day 4 (±1 day) after treatment initiation. Micafungin blood samples of 2 ml were taken from an indwelling vascular catheter prior to the administration of micafungin and 1, 2, 3, 4, 6, 8, 12, and 24 h after the start of the infusion. Trough plasma concentrations were measured every 3 days during treatment in the ICU, with a maximum of 28 days. Plasma concentrations of micafungin were determined by using assays validated in accordance with guidance for industry for bioanalytical method validation from the Food and Drug Administration (40). The precision and accuracy were within the ±15% limits over the calibration range of the methods. All yeast isolates were identified by using matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (Microflex LT mass spectrometer; Bruker Daltonik GmbH, Bremen, Germany). Susceptibility testing for micafungin was performed with the Etest (bioMérieux) on RPMI agar with 20% glucose (Mediaproducts, Groningen, the Netherlands). Interpretation of the MICs was done according to EUCAST breakpoints (41, 42). The microbiological data included Candida species, infection site (sterile), nonsterile infection site, time to culture conversion, preincubation time, follow-up cultures, and MIC.
Pharmacokinetic analysis.
The individual pharmacokinetic parameter estimates were calculated by fitting a two-compartment model to the plasma concentrations using MW\Pharm 3.82 (Mediware, the Netherlands) (43). A two-compartment pharmacokinetic model with intravenous infusion was selected to describe the pharmacokinetic behavior of micafungin (44, 45). For population pharmacokinetic analysis, an iterative two-stage Bayesian algorithm was used, equipped with the data from day 4 (±1 day) of treatment. The second approach was used to refine the individual estimates with the influence of covariates (weight and length) for a better estimation of the pharmacokinetic parameters (MW\Pharm 3.82). The following parameters were calculated by the data-adjusted model: systemic clearance (CL) (liters per hour), volume of distribution of compartment 1 (V1) (liters), V2 (liters), distribution half-life (T1/21) (hours), elimination half-life (T1/22) (hours), and AUC0–24 (milligrams per hour per liter). The AUC0–24 was calculated by using the log-linear trapezoidal rule from 0 h up to 24 h, the peak plasma concentration (Cmax) (milligrams per liter) was the highest observed plasma concentration, and the trough concentration (C24) (milligrams per liter) was the lowest observed plasma concentration 24 h after administration on day 4 (±1 day) of treatment. The pharmacokinetics of micafungin for this study population were compared with the pharmacokinetics of micafungin for healthy volunteers and another ICU population. Raw data from two healthy-volunteer studies were provided by Astellas, and the mean AUC and standard deviations from the other ICU populations were extracted from data reported in the literature (16, 28, 29).
Treatment and treatment outcome.
Initiation of treatment with micafungin for probable or proven infections was based on ESCMID guidelines (9). Micafungin was administered in a dosage of 100 mg once daily by intravenous infusion over 1 h. The response to antifungal therapy was determined 28 days after the initiation of antifungal treatment. The response to treatment was categorized as a successful response, partial response, stable disease, disease progression, or death; these criteria were derived from the EORTC/MSG consensus group (46). Possible reasons for treatment discontinuation were determined, including death, palliative care, lack of efficacy, successful treatment, or the onset of an adverse event. The potential causal relationship of an adverse event with the use of micafungin was analyzed by the attending physician and the local investigator using the Naranjo adverse drug reaction probability scale (47). Individual AUC/MIC ratios were calculated to determine the target attainment of micafungin.
In this study, we used the previously defined AUC/MIC ratio target value of 5,000 for non-C. parapsilosis species (11). If several identical species were isolated from a patient, the highest MIC value was used for analysis.
Statistical analysis.
Continuous data were expressed as numbers and percentiles, categorical data were expressed as medians with interquartile ranges, and all data were checked for normal distribution. The influence of patient characteristics on the pharmacokinetic parameters and plasma concentrations of micafungin was determined by using the Mann-Whitney U test. To assess correlations between patient variables not available in MW\Pharm, such as severity scores and albumin concentrations, and the pharmacokinetic properties of micafungin, a Spearman correlation coefficient was calculated. Multiple-linear-regression analysis was performed by using a backward-elimination strategy, keeping variables with P values of 0.1 in the model. The variables from this study as well as the variables from studies of healthy volunteers (28, 29) were included in the regression analysis. The variables that were included were gender (48), body weight (25, 26), albumin level (48), and the disease severity score with the lowest P value for the correlation. The albumin plasma concentrations of healthy volunteers were fixed at 35 g/liter, and the disease severity score was fixed at zero. All statistical analyses were performed by using SPSS version 20.0 (SPSS, Inc., Chicago, IL, USA). A P value of <0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Astellas Pharma Europe Ltd. for providing clinical data on healthy volunteers. We thank all patients who participated in this study. We also thank the medical and nursing staff of the ICU and the analytical staff of the pharmacy.
This study was financially supported by Astellas Pharma Europe Ltd.
R.J.B. has served as a consultant to and has received unrestricted and research grants from Astellas Pharma, Inc.; F2G; Gilead Sciences; Merck Sharpe and Dohme Corp.; and Pfizer, Inc. All contracts were through Radboudumc, and payments were invoiced by Radboudumc. None of the work is related to the manuscript. J.W.C.A. reports grants from Pfizer, Astellas, and MSD and personal fees from Pfizer, Astellas, MSD, and Gilead (all outside the submitted work).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01398-17.
REFERENCES
- 1.Kullberg BJ, Arendrup MC. 2015. Invasive candidiasis. N Engl J Med 373:1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- 2.Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. 2005. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis 41:1232–1239. doi: 10.1086/496922. [DOI] [PubMed] [Google Scholar]
- 3.Kollef M, Micek S, Hampton N, Doherty JA, Kumar A. 2012. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis 54:1739–1746. doi: 10.1093/cid/cis305. [DOI] [PubMed] [Google Scholar]
- 4.Ostrosky-Zeichner L, Kullberg BJ, Bow EJ, Hadley S, Leon C, Nucci M, Patterson TF, Perfect JR. 2011. Early treatment of candidemia in adults: a review. Med Mycol 49:113–120. doi: 10.3109/13693786.2010.512300. [DOI] [PubMed] [Google Scholar]
- 5.Morrell M, Fraser VJ, Kollef MH. 2005. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother 49:3640–3645. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labelle AJ, Micek ST, Roubinian N, Kollef MH. 2008. Treatment-related risk factors for hospital mortality in Candida bloodstream infections. Crit Care Med 36:2967–2972. doi: 10.1097/CCM.0b013e31818b3477. [DOI] [PubMed] [Google Scholar]
- 7.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, Bearden DT. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis 43:25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 8.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:e1–e50. doi: 10.1093/cid/civ1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, Bille J, Castagnola E, Cuenca-Estrella M, Donnelly JP, Groll AH, Herbrecht R, Hope WW, Jensen HE, Lass-Florl C, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ, ESCMID Fungal Infection Study Group. 2012. ESCMID guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 18(Suppl 7):S19–S37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 10.Andes DR, Diekema DJ, Pfaller MA, Marchillo K, Bohrmueller J. 2008. In vivo pharmacodynamic target investigation for micafungin against Candida albicans and C. glabrata in a neutropenic murine candidiasis model. Antimicrob Agents Chemother 52:3497–3503. doi: 10.1128/AAC.00478-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andes D, Ambrose PG, Hammel JP, Van Wart SA, Iyer V, Reynolds DK, Buell DN, Kovanda LL, Bhavnani SM. 2011. Use of pharmacokinetic-pharmacodynamic analyses to optimize therapy with the systemic antifungal micafungin for invasive candidiasis or candidemia. Antimicrob Agents Chemother 55:2113–2121. doi: 10.1128/AAC.01430-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith BS, Yogaratnam D, Levasseur-Franklin KE, Forni A, Fong J. 2012. Introduction to drug pharmacokinetics in the critically ill patient. Chest 141:1327–1336. doi: 10.1378/chest.11-1396. [DOI] [PubMed] [Google Scholar]
- 13.Bruggemann RJ, Middel-Baars V, de Lange DW, Colbers A, Girbes AR, Pickkers P, Swart EL. 2017. Pharmacokinetics of anidulafungin in critically ill intensive care unit patients with suspected or proven invasive fungal infections. Antimicrob Agents Chemother 61:e01894-16. doi: 10.1128/AAC.01894-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martial LC, Bruggemann RJ, Schouten JA, van Leeuwen HJ, van Zanten AR, de Lange DW, Muilwijk EW, Verweij PE, Burger DM, Aarnoutse RE, Pickkers P, Dorlo TP. 2016. Dose reduction of caspofungin in intensive care unit patients with child Pugh B will result in suboptimal exposure. Clin Pharmacokinet 55:723–733. doi: 10.1007/s40262-015-0347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Elst KC, Veringa A, Zijlstra JG, Beishuizen A, Klont R, Brummelhuis-Visser P, Uges DR, Touw DJ, Kosterink JG, van der Werf TS, Alffenaar JC. 2017. Low caspofungin exposure in patients in intensive care units. Antimicrob Agents Chemother 61:e01582-16. doi: 10.1128/AAC.01582-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lempers VJ, Schouten JA, Hunfeld NG, Colbers A, van Leeuwen HJ, Burger DM, Verweij PE, Pickkers P, Bruggemann RJ. 2015. Altered micafungin pharmacokinetics in intensive care unit patients. Antimicrob Agents Chemother 59:4403–4409. doi: 10.1128/AAC.00623-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jullien V, Azoulay E, Schwebel C, Le Saux T, Charles PE, Cornet M, Souweine B, Klouche K, Jaber S, Trouillet JL, Bruneel F, Cour M, Cousson J, Meziani F, Gruson D, Paris A, Darmon M, Garrouste-Orgeas M, Navellou JC, Foucrier A, Allaouchiche B, Das V, Gangneux JP, Ruckly S, Wolff M, Timsit JF, EMPIRICUS Trial Study Group. 2017. Population pharmacokinetics of micafungin in ICU patients with sepsis and mechanical ventilation. J. Antimicrob Chemother 72:181–189. doi: 10.1093/jac/dkw352. [DOI] [PubMed] [Google Scholar]
- 18.Dupont BF, Lortholary O, Ostrosky-Zeichner L, Stucker F, Yeldandi V. 2009. Treatment of candidemia and invasive candidiasis in the intensive care unit: post hoc analysis of a randomized, controlled trial comparing micafungin and liposomal amphotericin B. Crit Care 13:R159. doi: 10.1186/cc8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beloeil H, Mazoit JX, Benhamou D, Duranteau J. 2005. Norepinephrine kinetics and dynamics in septic shock and trauma patients. Br J Anaesth 95:782–788. doi: 10.1093/bja/aei259. [DOI] [PubMed] [Google Scholar]
- 20.Peeters MY, Bras LJ, DeJongh J, Wesselink RM, Aarts LP, Danhof M, Knibbe CA. 2008. Disease severity is a major determinant for the pharmacodynamics of propofol in critically ill patients. Clin Pharmacol Ther 83:443–451. doi: 10.1038/sj.clpt.6100309. [DOI] [PubMed] [Google Scholar]
- 21.Tod M, Padoin C, Minozzi C, Cougnard J, Petitjean O. 1996. Population pharmacokinetic study of isepamicin with intensive care unit patients. Antimicrob Agents Chemother 40:983–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Zanten AR, Polderman KH, van Geijlswijk IM, van der Meer GY, Schouten MA, Girbes AR. 2008. Ciprofloxacin pharmacokinetics in critically ill patients: a prospective cohort study. J Crit Care 23:422–430. doi: 10.1016/j.jcrc.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Marik PE. 1993. Aminoglycoside volume of distribution and illness severity in critically ill septic patients. Anaesth Intensive Care 21:172–173. [DOI] [PubMed] [Google Scholar]
- 24.Chandrasekar PH, Sobel JD. 2006. Micafungin: a new echinocandin. Clin Infect Dis 42:1171–1178. doi: 10.1086/501020. [DOI] [PubMed] [Google Scholar]
- 25.Gumbo T, Hiemenz J, Ma L, Keirns JJ, Buell DN, Drusano GL. 2008. Population pharmacokinetics of micafungin in adult patients. Diagn Microbiol Infect Dis 60:329–331. doi: 10.1016/j.diagmicrobio.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Grau S, Luque S, Campillo N, Samso E, Rodriguez U, Garcia-Bernedo CA, Salas E, Sharma R, Hope WW, Roberts JA. 2015. Plasma and peritoneal fluid population pharmacokinetics of micafungin in post-surgical patients with severe peritonitis. J Antimicrob Chemother 70:2854–2861. doi: 10.1093/jac/dkv173. [DOI] [PubMed] [Google Scholar]
- 27.Vossen MG, Knafl D, Haidinger M, Lemmerer R, Unger M, Pferschy S, Lamm W, Maier-Salamon A, Jager W, Thalhammer F. 2017. Micafungin plasma levels are not affected by continuous renal replacement therapy: experience in critically ill patients. Antimicrob Agents Chemother 61:e02425-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hebert MF, Townsend RW, Austin S, Balan G, Blough DK, Buell D, Keirns J, Bekersky I. 2005. Concomitant cyclosporine and micafungin pharmacokinetics in healthy volunteers. J Clin Pharmacol 45:954–960. doi: 10.1177/0091270005278601. [DOI] [PubMed] [Google Scholar]
- 29.Hebert MF, Blough DK, Townsend RW, Allison M, Buell D, Keirns J, Bekersky I. 2005. Concomitant tacrolimus and micafungin pharmacokinetics in healthy volunteers. J Clin Pharmacol 45:1018–1024. doi: 10.1177/0091270005279274. [DOI] [PubMed] [Google Scholar]
- 30.van Wanrooy MJ, Rodgers MG, Uges DR, Arends JP, Zijlstra JG, van der Werf TS, Kosterink JG, Alffenaar JW. 2014. Low but sufficient anidulafungin exposure in critically ill patients. Antimicrob Agents Chemother 58:304–308. doi: 10.1128/AAC.01607-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Undre N, Pretorius B, Stevenson P. 2015. Pharmacokinetics of micafungin in subjects with severe hepatic dysfunction. Eur J Drug Metab Pharmacokinet 40:285–293. doi: 10.1007/s13318-014-0204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andes D, Diekema DJ, Pfaller MA, Prince RA, Marchillo K, Ashbeck J, Hou J. 2008. In vivo pharmacodynamic characterization of anidulafungin in a neutropenic murine candidiasis model. Antimicrob Agents Chemother 52:539–550. doi: 10.1128/AAC.01061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louie A, Deziel M, Liu W, Drusano MF, Gumbo T, Drusano GL. 2005. Pharmacodynamics of caspofungin in a murine model of systemic candidiasis: importance of persistence of caspofungin in tissues to understanding drug activity. Antimicrob Agents Chemother 49:5058–5068. doi: 10.1128/AAC.49.12.5058-5068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meletiadis J, Geertsen E, Curfs-Breuker I, Meis JF, Mouton JW. 2016. Intra- and interlaboratory agreement in assessing the in vitro activity of micafungin against common and rare candida species with the EUCAST, CLSI, and Etest methods. Antimicrob Agents Chemother 60:6173–6178. doi: 10.1128/AAC.01027-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neofytos D, Huang YT, Cheng K, Cohen N, Perales MA, Barker J, Giralt S, Jakubowski A, Papanicolaou G. 2015. Safety and efficacy of intermittent intravenous administration of high-dose micafungin. Clin Infect Dis 61(Suppl 6):S652–S661. doi: 10.1093/cid/civ818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sirohi B, Powles RL, Chopra R, Russell N, Byrne JL, Prentice HG, Potter M, Koblinger S. 2006. A study to determine the safety profile and maximum tolerated dose of micafungin (FK463) in patients undergoing haematopoietic stem cell transplantation. Bone Marrow Transplant 38:47–51. doi: 10.1038/sj.bmt.1705398. [DOI] [PubMed] [Google Scholar]
- 37.Heimann SM, Cornely OA, Wisplinghoff H, Kochanek M, Stippel D, Padosch SA, Langebartels G, Reuter H, Reiner M, Vierzig A, Seifert H, Vehreschild MJ, Glossmann J, Franke B, Vehreschild JJ. 2015. Candidemia in the intensive care unit: analysis of direct treatment costs and clinical outcome in patients treated with echinocandins or fluconazole. Eur J Clin Microbiol Infect Dis 34:331–338. doi: 10.1007/s10096-014-2230-8. [DOI] [PubMed] [Google Scholar]
- 38.Wasmann RE, Muilwijk EW, Burger DM, Verweij PE, Knibbe CA, Bruggemann RJ. 8 August 2017. Clinical pharmacokinetics and pharmacodynamics of micafungin. Clin Pharmacokinet doi: 10.1007/s40262-017-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Munoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE, European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group, National Institute of Allergy and Infectious Diseases Mycoses Study Group Consensus Group. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine. 2001. Guidance for industry. Bioanalytical method validation. US Department of Health and Human Services, Washington, DC. [Google Scholar]
- 41.Arendrup MC, Cuenca-Estrella M, Lass-Florl C, Hope W, EUCAST-AFST . 2012. EUCAST technical note on the EUCAST definitive document EDef 7.2: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin Microbiol Infect 18:E246–E247. doi: 10.1111/j.1469-0691.2012.03880.x. [DOI] [PubMed] [Google Scholar]
- 42.Aigner M, Erbeznik T, Gschwentner M, Lass-Florl C. 2017. Etest and Sensititre YeastOne susceptibility testing of echinocandins against Candida species from a single center in Austria. Antimicrob Agents Chemother. 61:e00512-17. doi: 10.1128/AAC.00512-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Proost JH, Meijer DK. 1992. MW/Pharm, an integrated software package for drug dosage regimen calculation and therapeutic drug monitoring. Comput Biol Med 22:155–163. doi: 10.1016/0010-4825(92)90011-B. [DOI] [PubMed] [Google Scholar]
- 44.Groll AH, Stergiopoulou T, Roilides E, Walsh TJ. 2005. Micafungin: pharmacology, experimental therapeutics and clinical applications. Expert Opin Invest Drugs 14:489–509. doi: 10.1517/13543784.14.4.489. [DOI] [PubMed] [Google Scholar]
- 45.Hope WW, Kaibara A, Roy M, Arrieta A, Azie N, Kovanda LL, Benjamin DK Jr. 2015. Population pharmacokinetics of micafungin and its metabolites M1 and M5 in children and adolescents. Antimicrob Agents Chemother 59:905–913. doi: 10.1128/AAC.03736-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Segal BH, Herbrecht R, Stevens DA, Ostrosky-Zeichner L, Sobel J, Viscoli C, Walsh TJ, Maertens J, Patterson TF, Perfect JR, Dupont B, Wingard JR, Calandra T, Kauffman CA, Graybill JR, Baden LR, Pappas PG, Bennett JE, Kontoyiannis DP, Cordonnier C, Viviani MA, Bille J, Almyroudis NG, Wheat LJ, Graninger W, Bow EJ, Holland SM, Kullberg BJ, Dismukes WE, De Pauw BE. 2008. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin Infect Dis 47:674–683. doi: 10.1086/590566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naranjo CA. 1986. A clinical pharmacologic perspective on the detection and assessment of adverse drug reactions. Drug Infect J 20:387–393. doi: 10.1177/009286158602000403. [DOI] [PubMed] [Google Scholar]
- 48.Astellas. 2009. Mycamine annex 1 summary of product characteristics. Astellas, London, United Kingdom. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.