ABSTRACT
The aim of this study was to investigate the variability of the voriconazole plasma level and its relationships with clinical outcomes and adverse events among liver transplant recipients to optimize the efficacy and safety of their treatment. Liver transplant recipients treated with voriconazole were included, and voriconazole trough levels were quantified by a validated high-performance liquid chromatography method. Cytochrome P450 genotypes for CYP2C19 were evaluated in allograft liver tissues. A total of 832 voriconazole trough levels from 104 patients were measured. Proven, probable, and possible invasive fungal infections were reported for 8/104 (7.7%), 42/104 (40.4%), and 54/104 (51.9%) patients, respectively. Receiver operating characteristic (ROC) curve analysis indicated that trough concentrations of ≥1.3 μg/ml minimized the incidence of treatment failure (95% confidence interval [CI], 0.68 to 0.91 μg/ml) (P < 0.001) and that those of <5.3 μg/ml minimized the incidence of any adverse events (95% CI, 0.83 to 0.97 μg/ml) (P < 0.001). Voriconazole trough levels were significantly higher for heterozygous extensive metabolizers, poor metabolizers, and individuals receiving coadministration with proton pump inhibitors. For ultrarapid metabolizers, oral administration of voriconazole, and concomitant use of glucocorticoids, voriconazole blood concentrations were significantly reduced. Furthermore, there was no statistically significant association of patient age, weight, or gender or coadministration of tacrolimus and cyclosporine with the voriconazole trough level. In conclusion, the results of our analysis indicate large inter- and intraindividual variabilities of voriconazole concentrations in liver transplant recipients. Voriconazole trough concentrations of ≥1.3 μg/ml and <5.3 μg/ml are optimal for treatment and for minimization of adverse events. Optimization of drug efficacy and safety requires the use of rational doses for voriconazole therapy.
KEYWORDS: CYP2C19 genotype, liver transplant, adverse events, fungal infection, treatment outcome, voriconazole
INTRODUCTION
Invasive fungal infections (IFIs) are common life-threatening complications in liver transplant recipients (LTRs), with incidence rates ranging from 4 to 50% (1). Systemic candidiasis accounts for over half of all IFIs (68 to 78.7%) in this population, and invasive aspergillosis occurs in 1 to 9.2% of cases (2–4). Voriconazole, a broad-spectrum triazole, is an effective agent for the treatment of invasive aspergillosis (5). It has potent activity against a broad range of clinically significant fungal pathogens (6–9). Smith et al. detected a relationship between disease progression and voriconazole drug concentration (P < 0.025) (10). Several factors may lead to large inter- and intraindividual variations in voriconazole plasma concentrations, including age, sex, weight, drug interactions, genetic polymorphisms in CYP2C19, and gastrointestinal abnormalities (11, 12). According to the literature, low voriconazole levels (below 1.0 μg/ml) are associated with therapeutic failure, and elevated levels (over 5.5 μg/ml) are correlated with an increased risk for toxicity (visual disturbance, skin rash, hallucination, and hepatotoxicity) (13, 14). Limited data have demonstrated the association between voriconazole plasma concentration and related factors among LTRs. The aim of this study was to investigate the variability of the voriconazole serum level and its relationships with clinical outcomes and adverse events among LTRs in order to optimize the use of voriconazole in such patients.
RESULTS
Patient characteristics.
During the observation period, 104 patients had suspected fungal infections and were treated with voriconazole and monitored by therapeutic drug monitoring. Demographic and clinical characteristics are summarized in Table 1. The mean patient age and weight were 36 ± 13.71 years and 61 ± 13.46 kg, respectively. The female-to-male ratio was 44/60. Proven, probable, and possible IFIs were reported for 8/104 (7.7%), 42/104 (40.4%), and 54/104 (51.9%) patients, respectively. Among patients receiving voriconazole for proven or probable IFIs (n = 50), Aspergillus species were the most common fungal pathogens (42/50 patients [84%]), and Aspergillus fumigatus was the most commonly identified species. Eight patients (8/50 patients [16%]) were treated for candidemia due to Candida krusei (n = 4) or Candida glabrata (n = 4).
TABLE 1.
Demographic, clinical, and laboratory data for 104 liver transplant recipients with therapeutic drug monitoring of voriconazole
| Variable | Value |
|---|---|
| Mean (range) age (yr) | 36 (18–62) |
| Sex (no. [%] of males, no. [%] of females) | 60 (58), 44 (42) |
| Mean (range) wt (kg) | 61 (34–90) |
| No. (%) of patients with invasive fungal infection | |
| Proven | 8 (7.7) |
| Probable | 42 (40.4) |
| Possible | 54 (51.9) |
| No. (%) of patients with route of administration | |
| Intravenous | 24 (23) |
| Oral | 80 (77) |
| No. (%) of patients with CYP2C19 genotypea | |
| Homozygous extensive metabolizer | 30 (40) |
| Heterozygous extensive metabolizer | 24 (32) |
| Poor metabolizer | 7 (9.3) |
| Ultrarapid metabolizer | 14 (18.7) |
| Mean (range) duration of therapy (days) | 54 (29–98) |
| Median (range) duration of voriconazole therapy posttransplantation (days)b | 39 (21–50) |
| Median (range) voriconazole daily dose (mg/kg/day) | |
| Intravenous | 8.0 (6–10) |
| Oral | 7.35 (4–9.4) |
| Median (range) voriconazole trough level (μg/ml) | 2.49 (0–11.86) |
Genotypes were evaluated by use of allograft liver biopsy specimens from 75 liver transplant recipients (72% of patients).
Median time interval between liver transplantation and the first voriconazole trough level measurement.
The median time interval between liver transplantation and the first voriconazole trough level measurement was 39 days (range, 21 to 50 days). The mean duration of treatment with voriconazole was 54 days (range, 29 to 98 days). The majority of patients (80/104 patients [77%]) received voriconazole orally; for 23% of patients, treatment was given by the intravenous route. The median voriconazole trough level was 2.49 μg/ml (range, 0 to 11.86 μg/ml).
Measurement of voriconazole trough concentration.
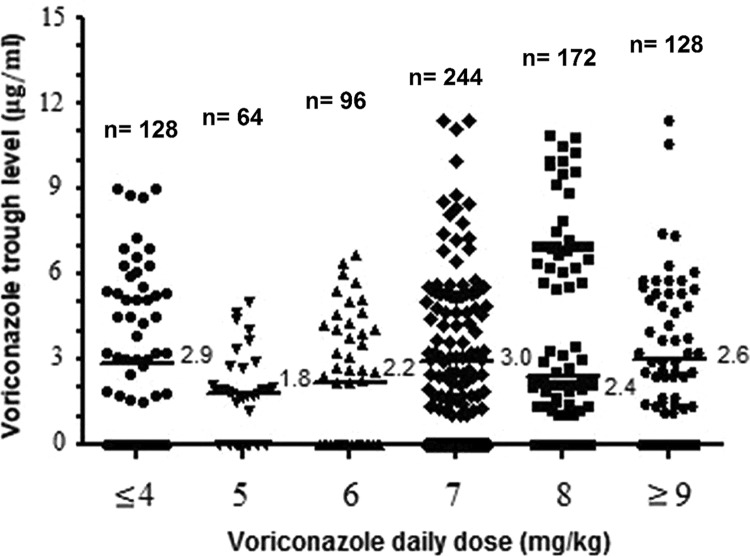
A total of 832 voriconazole trough levels from 104 patients were measured (median, 8 episodes per patient; range, 5 to 10 episodes per patient). Figure 1 shows the trough level and its relationship with voriconazole daily dose (standard dose/patient weight). A large intradose variability in voriconazole levels was observed, and no correlation was found between voriconazole trough levels and daily doses (r = 0.014). Variations in trough levels among patients and within the same patients were substantial for all dosage groups. The interindividual coefficient of variation (CV) was 87%, whereas the median intraindividual coefficient of variation was 38%. Intraindividual variability of the voriconazole trough level during therapy with identical daily doses was seen for 72/104 (69%) patients, among whom levels increased in 52 patients (median increase, 50%; range, 5% to 95%) and decreased in 20 patients (median decrease, 19.2%; range, 6% to 35%).
FIG 1.
Distribution of voriconazole trough levels over daily dosages. Numbers of measurements for each daily dose are reported. Median values of voriconazole trough levels for each dose group are reported to the right of the horizontal bars.
CYP2C19 genotyping.
Genotyping was performed for 72% of the patients (75/104 patients). The wild-type CYP2C19 genotype (homozygous extensive metabolizer) was the most commonly identified genotype (30/75 patients [40%]), followed by the mutant types heterozygous extensive metabolizer (24/75 patients [32%]), ultrarapid metabolizer (14/75 patients [18.7%]), and poor metabolizer (7/75 patients [9.3%]) (Table 1).
Relationships of voriconazole concentration to response to and safety of treatment.
Serum voriconazole concentrations were <1.3 μg/ml for 34 patients, 1.3 to 5.3 μg/ml for 46 patients, and >5.3 μg/ml for 24 patients. The relationships between median voriconazole trough levels and responses to treatment are shown in Table 2. The majority of patients had successful treatment (70/104 patients [67%]). Receiver operating characteristic (ROC) curve analysis (Fig. 2) indicated that the optimal cutoff value for voriconazole trough level associated with treatment success and with minimizing the incidence of treatment failure was ≥1.3 μg/ml. The area under the ROC curve was 0.81 (95% confidence interval [CI], 0.68 to 0.91) (P < 0.001). At the time of the clinical assessment process, the voriconazole concentration was <1.3 μg/ml in 34 cases (33%) and ≥1.3 μg/ml in 70 cases (67%). A lack of response to therapy was observed in 24 patients with levels of <1.3 μg/ml and 10 patients with levels of ≥1.3 μg/ml (P = 0.002) (Table 2).
TABLE 2.
Relationships of lower and upper limits of voriconazole trough concentration to outcomes and adverse events identified from ROC curve analysis
| Treatment outcome or adverse event | No. (%) of patients with voriconazole trough level of: |
P value | Odds ratio (95% CI) | |||
|---|---|---|---|---|---|---|
| <1.3 μg/ml (n = 34) | ≥1.3 μg/ml (n = 70) | ≤5.3 μg/ml (n = 80) | >5.3 μg/ml (n = 24) | |||
| Treatment outcomes | ||||||
| Success (n = 70) | 10 (29.4) | 60 (85.7) | 0.005 | |||
| Complete response | 4 (11.8) | 50 (71.4) | 0.004 | 0.05 (0.01–0.28) | ||
| Partial response | 6 (17.6) | 10 (14.3) | 0.79 | 1.29 (0.27–6.15) | ||
| Lack of response (n = 34) | 24 (70.6) | 10 (14.3) | 0.002 | |||
| Progression | 8 (23.5) | 2 (2.9) | 0.06 | 6.8 (0.71–65.16) | ||
| Persistent infection | 4 (11.8) | 2 (2.9) | 0.50 | 4.53 (0.38–53.93) | ||
| Death | 12 (35.3) | 6 (8.5) | 0.046 | 5.81 (1.24–27.3) | ||
| Adverse events | ||||||
| Anya (n = 28) | 10 (12.5) | 18 (75) | <0.001 | 0.05 (0.01–0.23) | ||
| Hallucination | 2 (2.5) | 12 (50) | <0.001 | 0.03 (0.002–0.25) | ||
| Skin rash | 2 (2.5) | 2 (8) | 0.250 | 0.28 (0.02–4.88) | ||
| Nervousness | 4 (5.0) | 6 (25) | 0.070 | 0.16 (0.02–1.09) | ||
| Visual disturbance | 2 (2.5) | 10 (42) | 0.005 | 0.04 (0.004–0.36) | ||
| Gastrointestinal syndrome | 2 (2.5) | 8 (33) | 0.030 | 0.05 (0.005–0.52) | ||
| Hepatotoxicityb | 4 (5.0) | 10 (42) | 0.001 | 0.07 (0.01–0.46) | ||
Eight patients had more than one adverse event.
Hepatotoxicity was defined as follows: grade 1, elevations in alanine transaminase, aspartate transaminase, and alkaline phosphatase levels of >3.0 times the upper limit of normal; and grade 2, elevations in alanine transaminase, aspartate transaminase, and alkaline phosphatase levels of 3.0 to 5.0 times the upper limit of normal and in the total bilirubin level of >3.0 times the upper limit of normal.
FIG 2.
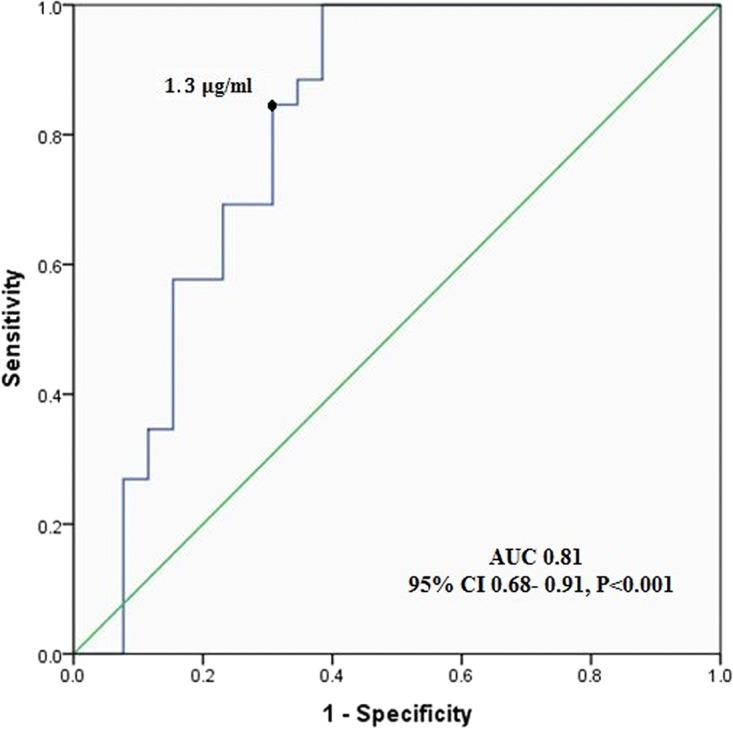
Receiver operating characteristic curve for predicting treatment success from voriconazole trough concentrations.
ROC curve analysis (Fig. 3) showed that the optimal cutoff value for voriconazole trough level associated with minimized adverse events was <5.3 μg/ml. The area under the curve was 0.90 (95% CI, 0.83 to 0.97) (P < 0.001) (Fig. 3). Voriconazole-related adverse events were observed in 28/104 (27%) patients, 18 with voriconazole concentrations of >5.3 μg/ml and 10 with concentrations of ≤5.3 μg/ml (Table 2). A significant proportion of patients with any adverse event (18/24 patients [75%]) had voriconazole levels of >5.3 μg/ml (P < 0.001). The median time from voriconazole administration to the onset of the adverse event was 7 days (range, 5 to 38 days). Fifty-eight (55%) patients showed baseline liver function test abnormalities due to hepatocellular injury before starting voriconazole (Common Terminology Criteria for Adverse Events [CTCAE] grade 1). During voriconazole therapy, 69% of patients (72/104 patients) had liver function test abnormalities (CTCAE grades 1 and 2). The most common adverse events were hallucination and hepatotoxicity (13.5% [14/104 patients]), followed by visual disturbance (11.5% [12/104 patients]) and nervousness (9.6% [10/104 patients]).
FIG 3.
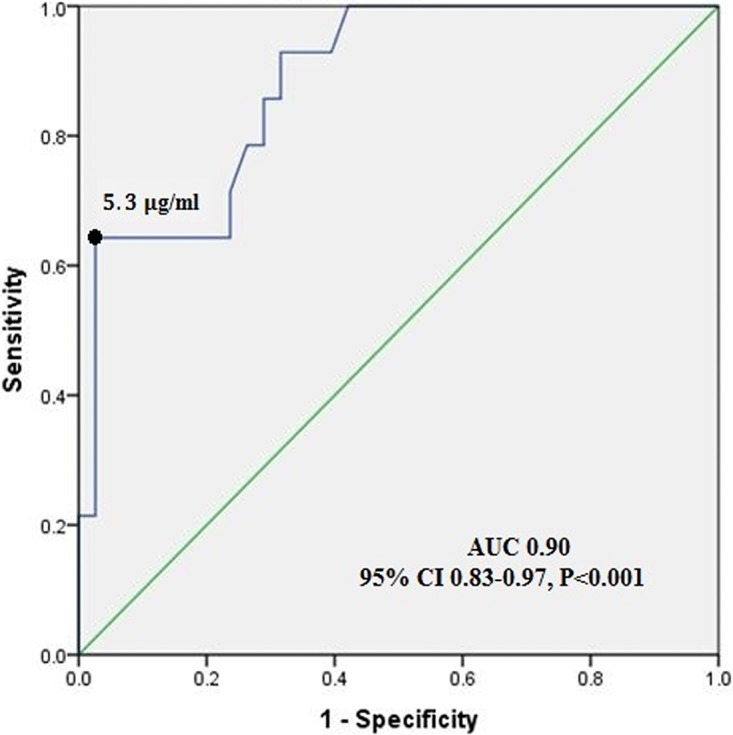
Receiver operating characteristic curve for predicting risk of toxic adverse events from voriconazole trough concentrations.
Factors affecting voriconazole concentration.
Diverse factors associated with variability of the voriconazole trough level were identified using multiple-linear-regression analysis (Table 3). Compared to those of homozygous extensive metabolizers, voriconazole trough levels were significantly higher in heterozygous extensive metabolizers (P = 0.045) or poor metabolizers (P = 0.002) and lower in ultrarapid metabolizers (P = 0.027).
TABLE 3.
Multivariate analysis of factors associated with low and potentially toxic voriconazole plasma concentrationse
| Variable | Coefficient | SE | P value |
|---|---|---|---|
| Age | −0.004 | 0.008 | 0.642 |
| Weight | −0.007 | 0.007 | 0.345 |
| Oral administrationa | −0.005 | 0.335 | 0.034 |
| Sex | −0.100 | 0.198 | 0.618 |
| CYP2C19 genotypeb | |||
| Heterozygous extensive metabolizer | 0.610 | 0.295 | 0.045 |
| Ultrarapid metabolizer | −0.661 | 0.288 | 0.027 |
| Poor metabolizer | 1.383 | 0.421 | 0.002 |
| Concomitant medication | |||
| Glucocorticoidsc | −3.175 | 0.433 | <0.001 |
| Tacrolimus/cyclosporine | −0.161 | 0.221 | 0.469 |
| Proton pump inhibitorsd | 1.291 | 0.309 | <0.001 |
Compared to intravenous administration.
Compared to homozygous extensive metabolizers.
Methylprednisolone, prednisone, and prednisolone.
Pantoprazole and omeprazole.
R2 = 0.932; n = 832 voriconazole trough measurements.
Coadministration of proton pump inhibitors (omeprazole and pantoprazole) resulted in significantly increased voriconazole serum concentrations (P < 0.001). The factor significantly associated with reduced voriconazole concentrations was found to be the concomitant administration of glucocorticoids (prednisone/prednisolone and methylprednisolone) (P < 0.001). Immunosuppressive therapies, including tacrolimus and cyclosporine, had no effect on subsequent voriconazole trough concentrations (P < 0.469). Similarly, there were no significant associations between patient age, weight, or gender and voriconazole trough levels. Lower concentrations of voriconazole were reported for oral administration of voriconazole than for intravenous administration (P = 0.034).
DISCUSSION
Voriconazole is an azole that is active against a large variety of fungi and is the drug of choice for the treatment of invasive aspergillosis and systemic candidiasis caused by resistant species (6, 8, 15). Its administration in therapeutic doses leads to extremely varied serum levels from patient to patient, and even in the same patient (16). The voriconazole serum concentration (measured by high-performance liquid chromatography [HPLC] or bioassay methods) plays an important role in patient outcomes (17). The present study investigated the factors associated with the variability of voriconazole serum concentrations. Despite the homogeneity of the population studied, significant variations in voriconazole trough level, clinical efficacy, and adverse events were demonstrated.
The incidences of proven, probable, and possible IFIs in LTRs using voriconazole in our study were 7.7%, 40.4%, and 51.9%, respectively. The corresponding rates in the study of Dolton et al. were 22%, 11.5%, and 29%, respectively (18).The differences may be due to the use of different diagnostic methods or public management strategies in each region.
In the present study, using ROC curve analysis, voriconazole trough levels of ≥1.3 μg/ml were demonstrated to be a significant predictor of treatment success, and those of >5.3 μg/ml were associated with an enhanced risk of adverse events. In a study of patients with hematological malignancy who received voriconazole for the treatment of known or suspected IFIs, a higher treatment success rate was reported for voriconazole concentrations of >1.7 μg/ml. All patients experiencing neurotoxic adverse events had voriconazole trough levels above 5 μg/ml (18). Pascual et al. noted that treatment failure was more frequent in cancer patients with voriconazole levels of <1 μg/ml and that neurological adverse events (encephalopathy) were reported among those with voriconazole concentrations of >5.5 μg/ml (19). Voriconazole monitoring for patients with hematological disorders revealed that successful treatment was more likely among patients with median voriconazole trough levels of >2 μg/ml, and a greater incidence of hepatotoxicity was reported for voriconazole concentrations of >6 μg/ml (20). The lower and upper limits of the voriconazole concentration for treatment in various studies were reported to be >1 to 2.2 and <4 to 6 μg/ml, respectively (21–23). The differences may be related to the populations in the studies. In our study, LTRs were investigated, while the most frequent underlying disease in other studies was cancer or hematologic malignancy.
Using multiple-linear-regression analysis of voriconazole concentrations, we found different factors contributing to changes in voriconazole trough level in this study. Voriconazole is a major substrate for the CYP2C19 enzyme and is metabolized by it. Polymorphic expression of the gene encoding the CYP2C19 enzyme may change the voriconazole pharmacokinetics and significantly affect its concentration (24). In LTRs, the polymorphisms of CYP2C19 found in liver tissue and the expression of the final liver graft genotypes are dependent on the donor graft (25, 26). Therefore, to determine patient CYP2C19 genotypes in our study, liver graft biopsy specimens were examined after transplantation. The results of the current study show significantly higher voriconazole trough levels in poor metabolizers and heterozygous extensive metabolizers, and lower levels in ultrarapid metabolizers, than those in homozygous extensive metabolizers. Our findings are in agreement with recently published data on a cohort of LTRs by Johnson et al., who reported significantly lower voriconazole blood levels in the presence of deficient CYP2C19*2 alleles (23), but the CYP2C19 genetic analysis in this population did not include ultrarapid metabolizers. Studies have shown that voriconazole concentrations were increased 4-fold in poor metabolizers and 2-fold in heterozygous metabolizers versus those in homozygous extensive metabolizers (27, 28).
Potential drug-drug interactions may also be another factor responsible for the interindividual variability of voriconazole exposure in LTRs. These patients receive many therapeutic agents for prophylaxis or treatment. Since these compounds are metabolized predominantly by a CYP2C19 enzyme, concomitant administration of medications which are inducers and/or inhibitors of CYP2C19 can influence the voriconazole pharmacokinetic profile (29). Our results suggest that receiving glucocorticoids (prednisone, prednisolone, and methylprednisolone) is associated with reduced voriconazole serum concentrations. Previous in vivo studies identified an association between glucocorticoid receptor binding sites in the CYP2C19 gene promoter and their important roles in the high expression of the CYP2C19 gene (30, 31). Dote et al. proposed that glucocorticoids can increase voriconazole metabolism as a result of CYP induction and thus reduce the voriconazole concentration (32). Our result is also consistent with the work of other studies which reported that coadministration of glucocorticoids significantly reduces the voriconazole exposure, to below the therapeutic range (18, 33). Data from other studies did not support such an interaction (27, 34), given the heterogeneity of the studied populations and the type and dose of the received glucocorticoids.
Conversely, coadministration of known CYP2C19 inhibitors, such as proton pump inhibitors (for the treatment of acid-related gastrointestinal disorders), was associated with increased concentrations of voriconazole in our population. Our results are in agreement with previous findings by Li et al., who demonstrated that all proton pump inhibitors are known to be able to affect voriconazole metabolism as competitive inhibitors (35). In contrast, Ueda et al. did not report proton pump inhibitors as a factor influencing voriconazole pharmacokinetics (20).
Based on our results, the voriconazole trough concentration was not influenced by comedication with tacrolimus in LTRs. This finding is consistent with the results of Gautier-Veyret et al., who revealed that immunosuppressive therapies, including calcineurin inhibitors, had no effect on voriconazole serum level (27). There was no relationship of age and weight with voriconazole serum concentration in the present analysis because there were limited overweight and no elderly patients in our study, consistent with the results of some other studies (20, 27). A study of a geriatric population showed that voriconazole concentrations in elderly patients aged >65 years were approximately 80 to 90% higher than those in younger patients (36).
According to previous studies, voriconazole oral bioavailability is 80% to 95% (37, 38). Dolton et al. showed reduced voriconazole trough levels following oral dosing (33). In the present study, significantly lower voriconazole concentrations were seen with oral than with intravenous administration (P = 0.034). Changes in motility of the gastrointestinal tract after any transplant surgery, mucositis, variations in bile flow (voriconazole is highly lipophilic, and its absorption is dependent on the secretion of bile), and diarrhea after use of some antirejection medications (tacrolimus) can cause a decrease in absorption, leading to the reduced voriconazole blood level (39, 40).
The present study had a few limitations. We were unable to evaluate the effects of other factors influencing voriconazole concentrations, e.g., dosing in relation to food or comedication with other drugs that many LTRs had received depending on their condition. These types of potential confounders were also difficult to determine because a limited number of patients received additional medications concurrently with voriconazole and most coadministered drugs were used only intermittently.
In conclusion, the results of our analysis indicated large inter- and intraindividual variabilities of voriconazole concentrations in LTRs. Optimization of drug efficacy and safety for this population demands rational doses for voriconazole therapy. Voriconazole trough levels of ≥1.3 μg/ml and <5.3 μg/ml are optimal for treatment and for minimizing the incidence of adverse events. Potential influencing factors, such as the type of administration, CYP2C19 genotype, and concomitant use of proton pump inhibitors and glucocorticoids, should be considered within the algorithm of voriconazole treatment for this population. Voriconazole therapeutic drug monitoring of LTRs is suggested as an important strategy to decrease adverse events and improve treatment outcomes.
MATERIALS AND METHODS
Study design and population.
This prospective study was conducted from January 2014 to April 2017 in Namazee Hospital, which is affiliated with the Shiraz University of Medical Sciences, Iran. This center is the largest liver transplant center in the country. Liver transplant recipients aged 18 years and older and treated with oral or intravenous voriconazole were eligible for this study. All patients received voriconazole by only one route, either intravenous or oral, based on the recommended dosing regimen during the study period. Patients receiving voriconazole prophylaxis or combination antifungal therapy (voriconazole and other antifungal agents) were excluded from the study. Combination therapy may affect sub- and supratherapeutic levels of voriconazole and may influence therapeutic outcomes.
Voriconazole (Vfend; Pfizer Inc., New York, NY) was prescribed for patients with known or suspected IFIs and symptoms, such as persistent fever for >72 h, not responsive to broad-spectrum antibacterial treatment. The definitions for proven, probable, and possible IFIs in immunocompromised patients are as follows. Proven IFI requires a positive culture for a pathogenic fungus from a biopsy specimen or normally sterile site, at least one positive blood culture for Candida species or other pathogenic fungi, and confirmation of fungal invasion by histopathology study. Probable IFI requires the isolation of fungi from nonsterile infected sites, radiological evidence of fungal infection (typical radiological shadows, halo sign, or air crescent sign), and/or positive blood samples for the galactomannan antigen test. Possible IFI is defined as the presence of immunocompromised host factors with sufficient clinical or radiological evidence consistent with IFIs but without mycological support (5, 41). For oral administration, a loading dose of 400 mg twice daily the first 24 h, followed by 200 mg every 12 h, was prescribed, and for intravenous therapy, 2 loading doses of 6 mg/kg of body weight at 12-h intervals for the first day, followed by 4 mg/kg every 12 h, were prescribed (42). Demographic information, including gender, age, weight, clinical characteristics, the time interval between liver transplantation and the first voriconazole trough level, and current comedications for each patient, was collected from patient medical records. Data on liver function tests prior to and after voriconazole treatment, histological biopsy studies, and immunosuppressive medications were available in the records for all recipients. The patients received corticosteroids (methylprednisolone, prednisone, or prednisolone), calcineurin inhibitors (tacrolimus/cyclosporine), and the antiproliferative agent mycophenolic acid (Cellcept), depending on their condition.
Ethical considerations.
This study was carried out in accordance with the guidelines of the Declaration of Helsinki as revised in Edinburgh (1975). The study protocol was approved by the ethics committee of the Shiraz University of Medical Sciences, Shiraz, Iran. Written informed consent was obtained from all patients for sample collection.
Examination for fungal infection.
As the clinical signs and symptoms of filamentous and yeast fungal infections are similar, the diagnosis was based on all mycological and serological methods (galactomannan test). The etiologic agents of fungal infections are yeasts (Candida species) and filamentous species (most Aspergillus species and other rare filamentous fungi). First, all clinical samples (urine, cerebrospinal fluid, pleural and abdominal fluids, bronchoalveolar lavage fluid, biopsy specimens, blood, and sputum) from the patients with clinically suspected fungal infections were collected under aseptic conditions. Blood samples were cultured by bedside inoculation onto Bactec medium (Becton Dickinson, Sparks, MD, USA). Specimens were examined by direct microscopic examination using potassium hydroxide and cultured on Sabouraud dextrose agar (Merck, Darmstadt, Germany) for 14 days at room temperature. Second, for patients with suspected invasive aspergillosis, the galactomannan test (Bio-Rad, France) was done on blood and bronchoalveolar lavage fluid.
Quantification of voriconazole trough level.
In this study, clinical care for all patients was done according to the guidelines, and dosing adjustments were not performed. Blood samples were taken on days 3, 5, and 7 following the initiation of voriconazole treatment and repeated once a week (43). Blood samples (3 ml) were collected 30 min before the next voriconazole dose and centrifuged at 3,000 rpm for 10 min. Serum was separated and frozen at −20°C until analysis. Voriconazole trough levels were quantified by a validated high-performance liquid chromatography (HPLC) method. Reversed-phase HPLC (RP-18) analyses were performed using a Knauer analytical HPLC (PDA 2800; Knauer, Berlin, Germany) with a K-1001 pump and a variable-wavelength UV spectrophotometric detector. The assay intraday and interday variability precisions were 0.8% to 6.0% and 3.01% to 6.54%, respectively. The linearity range was 0.25 to 16 μg/ml (R2 = 0.998).
Genotyping.
Genomic DNAs in allograft liver biopsy specimens from 75 LTRs with suspected acute rejection were extracted using an Invisorb Spin DNA microkit III (Invitek, Berlin, Germany) according to the manufacturer's protocol. Genotyping was performed using a TaqMan Drug Metabolism SNP genotyping assay kit (Applied Biosystems, USA) for the G681A, G636A, and C806T polymorphisms. Individuals with polymorphisms of CYP2C19 were classified as follows: homozygous extensive metabolizers (CYP2C19*1/*1), heterozygous extensive metabolizers (CYP2C19*1/*2, -*1/*3, -*2/*3, and *2/*17), ultrarapid metabolizers (CYP2C19*17/*17), and poor metabolizers (CYP2C19*2/*2 or -*3/*3). The homozygous extensive metabolizer genotype was considered wild type, and all other genotypes were considered mutant genotypes. Real-time PCR was done using an ABI 7500 Fast real-time PCR system (Applied Biosystems, USA).
Definition of treatment outcomes and adverse events.
A successful treatment was defined by partial or complete improvement in clinical symptoms (fever and/or blood markers), radiological signs (changes in chest X-ray, computed tomography, and magnetic resonance imaging findings), and evidence of mycological cure, such as negative results of culture and antigen assay. Treatment failure was defined by persistent or progressive infection based on the same parameters, continuing positive cultures, or death of the patient (44). Outcomes were analyzed at the following two points: 6 weeks of antifungal therapy for invasive filamentous fungal infection and 4 weeks for invasive candidiasis (44). Adverse events were monitored with a questionnaire and assessed by investigators blinded to the voriconazole level. The type and severity of adverse events, according to voriconazole therapy, were graded based on the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. In most of the LTRs, alanine transaminase, aspartate transaminase, alkaline phosphatase, and total bilirubin values were over the normal range (about CTCAE grade 1) before voriconazole initiation, and hepatotoxicity was defined as a level of grade 2 or higher within 14 days after commencing voriconazole therapy (45).
Statistical analysis.
The median voriconazole trough levels were used to assess the relationships between concentration and treatment outcome. The chi-square test or Fisher's exact test was used to compare proportions, as appropriate. The nonparametric Mann-Whitney U test and the Kruskal-Wallis test were used to compare continuous variables, including laboratory values during therapy. The inter- and intraindividual variabilities of voriconazole serum concentrations were determined by calculation of %CV. The nonparametric Spearman correlation was used to study the relationship between clinical or laboratory data and daily dose. The cutoff value for the voriconazole trough concentration (therapeutic or toxic level) was derived by receiver operating characteristic (ROC) curve analysis. Multiple-linear-regression analysis was used to identify factors that contribute to the variability in voriconazole trough level. Data analysis was performed using SPSS, version 18, and P values of <0.05 were considered significant.
ACKNOWLEDGMENTS
We thank H. Khajehei for copyediting the manuscript.
This work was based on the Ph.D. thesis of Zahra Hashemizadeh as a requirement for graduation in mycology from the Professor Alborzi Clinical Microbiology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
This study was financially supported by grant 92-6884 from the Shiraz University of Medical Sciences, Shiraz, Iran.
REFERENCES
- 1.Zicker M, Colombo AL, Ferraz-Neto BH, Camargo LF. 2011. Epidemiology of fungal infections in liver transplant recipients: a six-year study of a large Brazilian liver transplantation center. Mem Inst Oswaldo Cruz 106:339–345. doi: 10.1590/S0074-02762011000300014. [DOI] [PubMed] [Google Scholar]
- 2.Romero FA, Razonable RR. 2011. Infections in liver transplant recipients. World J Hepatol 3:83–92. doi: 10.4254/wjh.v3.i4.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badiee P, Alborzi A, Malekhosseini SA, Nikeghbalian S, Shakiba E. 2010. Determining the incidence of aspergillosis after liver transplant. Exp Clin Transplant 8:220–223. [PubMed] [Google Scholar]
- 4.Singh N, Husain S, AST Infectious Diseases Community of Practice. 2013. Aspergillosis in solid organ transplantation. Am J Transplant 13(Suppl 4):228–241. doi: 10.1111/ajt.12115. [DOI] [PubMed] [Google Scholar]
- 5.Patterson TF, Thompson GR III, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE. 2016. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 63:433–442. doi: 10.1093/cid/ciw444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badiee P, Alborzi A, Moeini M, Haddadi P, Farshad S, Japoni A, Ziyaeyan M. 2012. Antifungal susceptibility of the Aspergillus species by E test and CLSI reference methods. Arch Iran Med 15:429–432. [PubMed] [Google Scholar]
- 7.Troke P, Aguirrebengoa K, Arteaga C, Ellis D, Heath CH, Lutsar I, Rovira M, Nguyen Q, Slavin M, Chen SC, Global Scedosporium Study Group. 2008. Treatment of scedosporiosis with voriconazole: clinical experience with 107 patients. Antimicrob Agents Chemother 52:1743–1750. doi: 10.1128/AAC.01388-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badiee P, Alborzi A, Shakiba E, Farshad S, Japoni A. 2011. Susceptibility of Candida species isolated from immunocompromised patients to antifungal agents. East Mediterr Health J 17:425–430. [PubMed] [Google Scholar]
- 9.Kullberg BJ, Sobel JD, Ruhnke M, Pappas PG, Viscoli C, Rex JH, Cleary JD, Rubinstein E, Church LW, Brown JM, Schlamm HT, Oborska IT, Hilton F, Hodges MR. 2005. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: a randomised non-inferiority trial. Lancet 366:1435–1442. doi: 10.1016/S0140-6736(05)67490-9. [DOI] [PubMed] [Google Scholar]
- 10.Smith J, Safdar N, Knasinski V, Simmons W, Bhavnani SM, Ambrose PG, Andes D. 2006. Voriconazole therapeutic drug monitoring. Antimicrob Agents Chemother 50:1570–1572. doi: 10.1128/AAC.50.4.1570-1572.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyland R, Jones BC, Smith DA. 2003. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab Dispos 31:540–547. doi: 10.1124/dmd.31.5.540. [DOI] [PubMed] [Google Scholar]
- 12.Theuretzbacher U, Ihle F, Derendorf H. 2006. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin Pharmacokinet 45:649–663. doi: 10.2165/00003088-200645070-00002. [DOI] [PubMed] [Google Scholar]
- 13.Pasqualotto AC, Xavier MO, Andreolla HF, Linden R. 2010. Voriconazole therapeutic drug monitoring: focus on safety. Expert Opin Drug Saf 9:125–137. doi: 10.1517/14740330903485637. [DOI] [PubMed] [Google Scholar]
- 14.Ashbee HR, Barnes RA, Johnson EM, Richardson MD, Gorton R, Hope WW. 2014. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother 69:1162–1176. doi: 10.1093/jac/dkt508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badiee P, Alborzi A, Shakiba E, Ziyaeyan M, Rasuli M. 2009. Molecular identification and in-vitro susceptibility of Candida albicans and C. dubliniensis isolated from immunocompromised patients. Iran Red Crescent Med J 11:391–397. [Google Scholar]
- 16.Trifilio SM, Yarnold PR, Scheetz MH, Pi J, Pennick G, Mehta J. 2009. Serial plasma voriconazole concentrations after allogeneic hematopoietic stem cell transplantation. Antimicrob Agents Chemother 53:1793–1796. doi: 10.1128/AAC.01316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badiee P, Hashemizadeh Z, Montaseri H. Therapeutic drug monitoring of voriconazole: comparison of bioassay with high-performance liquid chromatography. Jundishapur J Microbiol, in press. [Google Scholar]
- 18.Dolton MJ, Ray JE, Chen SC, Ng K, Pont LG, McLachlan AJ. 2012. Multicenter study of voriconazole pharmacokinetics and therapeutic drug monitoring. Antimicrob Agents Chemother 56:4793–4799. doi: 10.1128/AAC.00626-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. 2008. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis 46:201–211. doi: 10.1086/524669. [DOI] [PubMed] [Google Scholar]
- 20.Ueda K, Nannya Y, Kumano K, Hangaishi A, Takahashi T, Imai Y, Kurokawa M. 2009. Monitoring trough concentration of voriconazole is important to ensure successful antifungal therapy and to avoid hepatic damage in patients with hematological disorders. Int J Hematol 89:592–599. doi: 10.1007/s12185-009-0296-3. [DOI] [PubMed] [Google Scholar]
- 21.Miyakis S, van Hal SJ, Ray J, Marriott D. 2010. Voriconazole concentrations and outcome of invasive fungal infections. Clin Microbiol Infect 16:927–933. doi: 10.1111/j.1469-0691.2009.02990.x. [DOI] [PubMed] [Google Scholar]
- 22.Imhof A, Schaer DJ, Schanz U, Schwarz U. 2006. Neurological adverse events to voriconazole: evidence for therapeutic drug monitoring. Swiss Med Wkly 136:739–742. [DOI] [PubMed] [Google Scholar]
- 23.Johnson HJ, Han K, Capitano B, Blisard D, Husain S, Linden PK, Marcos A, Kwak EJ, Potoski B, Paterson DL, Romkes M, Venkataramanan R. 2010. Voriconazole pharmacokinetics in liver transplant recipients. Antimicrob Agents Chemother 54:852–859. doi: 10.1128/AAC.00429-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss J, Ten Hoevel MM, Burhenne J, Walter-Sack I, Hoffmann MM, Rengelshausen J, Haefeli WE, Mikus G. 2009. CYP2C19 genotype is a major factor contributing to the highly variable pharmacokinetics of voriconazole. J Clin Pharmacol 49:196–204. doi: 10.1177/0091270008327537. [DOI] [PubMed] [Google Scholar]
- 25.Chiu KW, Nakano T, Hu TH, Tseng HP, Cheng YF, Jawan B, Eng HL, Goto S, Chen CL. 2012. Homogenous phenomenon of graft liver CYP2C19 genotypes after living donor liver transplantation. Eur J Clin Invest 42:352–356. doi: 10.1111/j.1365-2362.2011.02589.x. [DOI] [PubMed] [Google Scholar]
- 26.Hashemizadeh Z, Badiee P, Malek-Hosseini SA. Prevalence of CYP2C19 mutant alleles and genotypes among patients with hepatic disorders. Int J Organ Transplant Med, in press. [PMC free article] [PubMed] [Google Scholar]
- 27.Gautier-Veyret E, Fonrose X, Tonini J, Thiebaut-Bertrand A, Bartoli M, Quesada JL, Bulabois CE, Cahn JY, Stanke-Labesque F. 2015. Variability of voriconazole plasma concentrations after allogeneic hematopoietic stem cell transplantation: impact of cytochrome P450 polymorphisms and comedications on initial and subsequent trough levels. Antimicrob Agents Chemother 59:2305–2314. doi: 10.1128/AAC.04838-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfizer. 2008. VFend IV (voriconazole) for injection, VFend tablets, VFend for oral suspension. Pfizer, New York, NY. [Google Scholar]
- 29.Brüggemann RJ, Alffenaar JW, Blijlevens NM, Billaud EM, Kosterink JG, Verweij PE, Burger DM. 2009. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis 48:1441–1458. doi: 10.1086/598327. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Ferguson SS, Negishi M, Goldstein JA. 2003. Identification of constitutive androstane receptor and glucocorticoid receptor binding sites in the CYP2C19 promoter. Mol Pharmacol 64:316–324. doi: 10.1124/mol.64.2.316. [DOI] [PubMed] [Google Scholar]
- 31.Czock D, Keller F, Rasche FM, Häussler U. 2005. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet 44:61–98. doi: 10.2165/00003088-200544010-00003. [DOI] [PubMed] [Google Scholar]
- 32.Dote S, Sawai M, Nozaki A, Naruhashi K, Kobayashi Y, Nakanishi H. 2016. A retrospective analysis of patient-specific factors on voriconazole clearance. J Pharm Health Care Sci 2:10. doi: 10.1186/s40780-016-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dolton MJ, Mikus G, Weiss J, Ray JE, McLachlan AJ. 2014. Understanding variability with voriconazole using a population pharmacokinetic approach: implications for optimal dosing. Antimicrob Chemother 69:1633–1641. doi: 10.1093/jac/dku031. [DOI] [PubMed] [Google Scholar]
- 34.Hoenigl M, Duettmann W, Raggam RB, Seeber K, Troppan K, Fruhwald S, Prueller F, Wagner J, Valentin T, Zollner-Schwetz I, Wölfler A, Krause R. 2013. Potential factors for inadequate voriconazole plasma concentrations in intensive care unit patients and patients with hematological malignancies. Antimicrob Agents Chemother 57:3262–3267. doi: 10.1128/AAC.00251-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li XQ, Andersson TB, Ahlström M, Weidolf L. 2004. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos 32:821–827. doi: 10.1124/dmd.32.8.821. [DOI] [PubMed] [Google Scholar]
- 36.Pfizer Inc. 2011. Vfend prescribing information. Pfizer, New York, NY: http://labeling.pfizer.com/ShowLabeling.aspx?id=618. [Google Scholar]
- 37.Leveque D, Nivoix Y, Jehl F, Herbrecht R. 2006. Clinical pharmacokinetics of voriconazole. Int J Antimicrob Agents 27:274–284. doi: 10.1016/j.ijantimicag.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Purkins L, Wood N, Ghahramani P, Greenhalgh K, Allen MJ, Kleinermans D. 2002. Pharmacokinetics and safety of voriconazole following intravenous-to oral-dose escalation regimens. Antimicrob Agents Chemother 46:2546–2553. doi: 10.1128/AAC.46.8.2546-2553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herrero JI, Benlloch S, Bernardos A, Bilbao I, Castells L, Castroagudin JF, González L, Irastorza I, Navasa M, Otero A, Pons JA, Rimola A, Suárez F, Casanovas T, Otero E, Rodríguez M, Serrano T, Otero S, López I, Miras M, Prieto M, MITOS Study Group. 2007. Gastrointestinal complications in liver transplant recipients: MITOS study. Transplant Proc 39:2311–2313. doi: 10.1016/j.transproceed.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 40.Kalaitzakis E, Josefsson A, Castedal M, Henfridsson P, Bengtsson M, Andersson B, Björnsson E. 2013. Gastrointestinal symptoms in patients with cirrhosis: a longitudinal study before and after liver transplantation. Scand J Gastroenterol 48:1308–1316. doi: 10.3109/00365521.2013.836755. [DOI] [PubMed] [Google Scholar]
- 41.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE, European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group, National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.European Medicines Agency (EMA). 2014. Vfend: EPAR-product information. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Product_Information/human/002669/WC500144015.pdf Accessed 19 December 2014.
- 43.US Food and Drug Administration. 2001. Briefing document for voriconazole. http://www.fda.gov/ohrms/dockets/ac/01/briefing/3792b2.htm Accessed 15 July 2008.
- 44.Segal BH, Herbrecht R, Stevens DA, Ostrosky-Zeichner L, Sobel J, Viscoli C, Walsh TJ, Maertens J, Patterson TF, Perfect JR, Dupont B, Wingard JR, Calandra T, Kauffman CA, Graybill JR, Baden LR, Pappas PG, Bennett JE, Kontoyiannis DP, Cordonnier C, Viviani MA, Bille J, Almyroudis NG, Wheat LJ, Graninger W, Bow EJ, Holland SM, Kullberg BJ, Dismukes WE, De Pauw BE. 2008. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin Infect Dis 4:674–683. doi: 10.1086/590566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Cancer Institute, National Institutes of Health. 14 June 2010. Common terminology criteria for adverse events (CTCAE), version 4.03. NIH publication no. 09-5410. National Cancer Institute, National Institutes of Health, US Department of Health and Human Services, Bethesda, MD: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. [Google Scholar]