ABSTRACT
A low CD4/CD8 ratio during treated HIV infection reflects heightened immune activation and predicts death. The effects of different antiretroviral therapy regimens on CD4/CD8 ratio recovery remains unclear. We performed a post hoc analysis of the MERIT study, a randomized, double-blind trial of maraviroc versus efavirenz in combination with zidovudine-lamivudine in treatment-naive HIV-infected individuals. We found higher rates of CD4/CD8 ratio normalization with efavirenz, which was driven by a greater CD8+ T-cell decline.
KEYWORDS: HIV, antiretroviral therapy, CD4/CD8 ratio, T cells, T-cell activation, efavirenz, maraviroc
TEXT
Despite the unquestionable success of antiretroviral therapy (ART), HIV infection is still associated with an increased risk of death (1). A salient predictor of clinical progression is the activation of innate and adaptive immunity, as reflected by increased levels of markers of inflammation, coagulation, bacterial translocation, monocyte and macrophage activation, T-cell activation and T-cell senescence (2, 3). While these pathways are linked with the development of comorbidities during ART, a number of issues, including technical difficulties, economic constraints, and high intra- and interindividual variability, preclude their clinical use. The CD4/CD8 ratio has been demonstrated to correlate well with some of these pathways (4, 5) and has been shown to predict morbidity and death during ART (6, 7) despite adequate CD4+ recovery.
Although definitive evidence is lacking, the CCR5 antagonist maraviroc has been regarded as an antiretroviral agent with immunomodulatory properties, given its abilities to affect activation in peripheral blood and the gut mucosa (8), to improve HIV-associated neurocognitive disorder symptoms (9), and even to reactivate latent HIV (10). We hypothesized that first-line maraviroc-based therapy would result in a greater CD4/CD8 ratio recovery than nonnucleoside reverse transcriptase inhibitor-based therapy. For this purpose, we used the final 5-year data from a randomized, double blind, multicenter phase IIb/III (MERIT) study of treatment-naive patients.
The MERIT study (ClinicalTrials.gov no. NCT00098293 and NCT03178084) is a randomized, double-blind, multicenter, phase IIb/III clinical trial originally planned for 96 weeks but then extended to another 3 years of an open-label phase. The study design and details have been described elsewhere (11, 12). Briefly, treatment-naive R5 HIV-1 affected patients without baseline genotypic resistance to efavirenz, zidovudine, or lamivudine and with a minimum plasma HIV-1 RNA level of 2,000 copies/ml were considered eligible to receive maraviroc at 300 mg once a day (q.d.), maraviroc at 300 mg twice a day (b.i.d.), or efavirenz at 600 mg four times a day (q.d.) combined with zidovudine and lamivudine at 300 and 150 mg b.i.d. Treatment with maraviroc q.d. was discontinued in all of the patients included in this arm at week 16 for failure to meet the previously established efficacy criteria. Blinding was maintained for the first 96 weeks of the study, and then it was continued with a nominal 3-year open-label phase.
Ethics.
The protocol was approved by the appropriate review committee at each site and executed in accordance with good clinical practice guidelines. All participants provided written informed consent. This substudy was approved by the ethics committee of the Hospital Universitario Ramón y Cajal (approval no. 107/17).
Statistical methods.
The study sponsor shared the data with the investigators via the ClinicalStudyDataRequest.com consortium and had no role in the study design or analysis. Only patients treated with maraviroc at 300 mg b.i.d. or efavirenz at 600 mg q.d. in the MERIT study were included in this intention-to-treat post hoc analysis, since the maraviroc q.d. arm was abandoned at week 16. We aimed to assess the time to CD4/CD8 normalization at different cutoffs (>0.4 and >1.0). The rationale for this categorization was that (i) values of <1.0 correlate with immunosenescence in the general population (13) and HIV-infected individuals above this cutoff show levels of immunoactivation and immunosenescence similar to those of healthy controls (13) and (ii) a value of <0.4 (in a range of 0.3 and 0.5) appears to be the best cutoff to predict adverse outcomes in ART-treated HIV patients (5–7, 14).
For these cutoffs, we used Kaplan-Meier curves to calculate the time to and the rate of CD4/CD8 normalization and cumulative probabilities. Cox proportional-hazard models with Breslow methods for ties were used to compare the times to CD4/CD8 normalization by treatment arms. Then, we used generalized estimating equations (GEE) with an exchangeable correlation structure to compare longitudinal changes in the CD4/CD8 ratio taking into account repeated-measures and within-subject correlations, adjusting for the effects of age, sex, baseline CD4+ and CD8+ cell counts, and hepatitis C virus (HCV) serostatus. Interaction terms (time-versus-treatment arm) were created to assess whether these changes over time differed significantly between the two study arms. Continuous variables were log transformed when necessary to satisfy model assumptions. All statistical analyses were conducted with Stata v. 14.0 (StataCorp LP, College Station, TX, USA).
Characteristics of the study population and duration of follow-up.
A total of 721 subjects were included in this subanalysis, 361 treated with efavirenz and 360 treated with maraviroc at 300 mg b.i.d. Patients were representative of a medium-aged (37 ± 9.6 years) population with a higher prevalence of men (71.4%) and with baseline median CD4+ counts of 251.5 (range, 183 to 324) cells/mm3. One hundred eighty-one patients on maraviroc b.i.d. and 168 patients on efavirenz completed the 96-week blinded period and entered the open-label phase. The study population was comparable in both arms with respect to demographic and immunovirological parameters without statistically significant differences in the assessed parameters, as has been previously described (15, 16), with a median CD4/CD8 ratio of 0.3 (interquartile range, 0.2 to 0.4) in both groups. The study disposition has been previously published (15). The total duration of therapy (overall sum of all of the patients' treatment durations) was 1,243.3 years (median of 5.1 years per patient) in the maraviroc b.i.d. arm and 1,204.1 years (median of 3.9 years) in the efavirenz q.d. arm.
Changes in CD4/CD8 ratio normalization per treatment arm.
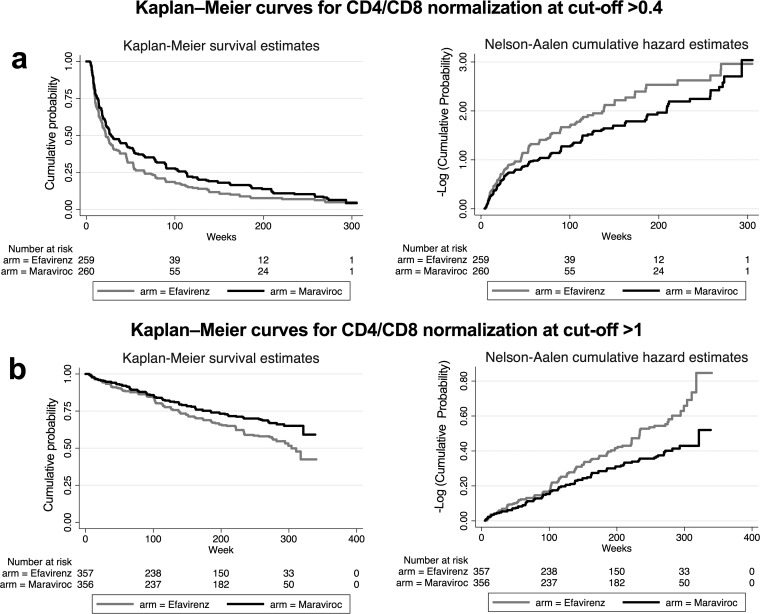
Using Kaplan-Meier methods, the median time to a CD4/CD8 cutoff of >0.4 was 10 (range, 0 to 37) weeks for efavirenz versus 14 (range, 0 to 47) weeks for maraviroc (log rank test P = 0.009). Similar findings were observed for the time to CD4/CD8 normalization at a cutoff of >1.0, with a median of 151 (range, 53 to 274) weeks for efavirenz versus 209 (range, 61 to 278) weeks for maraviroc (log rank test P < 0.001). Figure 1 shows Kaplan-Meier survival plots of estimated overall 5-year CD4/CD8 normalization at cutoffs of > 0.4 and >1.0.
FIG 1.
Kaplan-Meier analysis for the comparison of time to CD4/CD8 normalization at cutoffs of 0.4 (a) and 1.0 (b).
We explored the cumulative probabilities of CD4/CD8 ratio normalization in proportional-hazards models. The probability of normalizing the ratio above 0.4 over time was 25% higher with efavirenz than with maraviroc (hazard rate [HR] = 1.25, P = 0.020) (see Table S1 in the supplemental material). Similar results were obtained after adjustment for age, sex, the baseline CD4 count, the baseline CD8 count, and HCV serostatus, with an adjusted probability of normalizing the ratio 24% lower with maraviroc than with efavirenz (HR = 1.30, P = 0.007). For CD4/CD8 normalization at a cutoff of >1, we found a 42% greater probability of normalizing the ratio over time with efavirenz than with maraviroc (HR = 1.42, P = 0.010), which remained statistically significant in the adjusted model (HR = 1.43, P = 0.009) (Table S2).
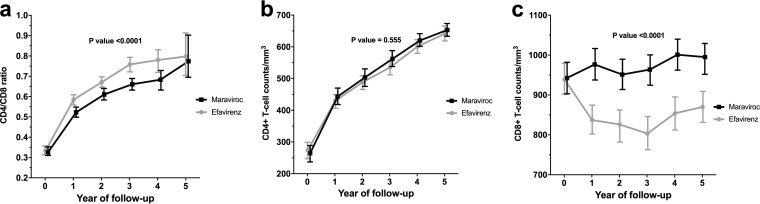
The linear CD4/CD8 ratio trajectories in the treatment arms were compared by using GEE, observing overall statistically significant changes (P < 0.001) (Table S3). The overall longitudinal changes in the CD4/CD8 ratio are represented in Fig. 2, which illustrates that differences were already apparent within the first year of the follow-up. Finally, using GEE modeling, we examined whether the overall CD4/CD8 ratio increase was driven by increases in CD4+ T-cell counts, decreases in CD8+ T-cell counts, or both (Table S3). While no statistically significant CD4+ T-cell count changes between treatment arms were found, the CD8+ counts showed a marked and significant decrease in the efavirenz arm, indicating that the better CD4/CD8 ratio recovery observed in the efavirenz arm was driven by a CD8+ T-cell count decline, rather than by a CD4+ T-cell count increase.
FIG 2.
Longitudinal trajectories of the CD4/CD8 ratio (a), the CD4+ T-cell count (b), and the CD8+ T-cell count (c). The lines represent estimated mean values over time from GEE for each group with the 95% confidence intervals.
Discussion.
In this post hoc analysis of a randomized, double-blind phase IIb/III clinical trial with a 5-year follow-up, efavirenz-based first-line ART was found to be more efficacious at improving the CD4/CD8 ratio than was maraviroc-based ART. Against our research hypothesis, patients treated with maraviroc showed slower CD4/CD8 ratio recovery and lesser CD8+ T-cell count declines. Some, but not all, studies of maraviroc intensification have shown a reduction of immune activation (17–20). In a substudy of the MERIT trial involving 57 subjects, there was an earlier decrease in the marker of percent CD4+ CD38+ T cells and a smaller proportion of patients with high-sensitivity C-reactive protein levels of >2 μg/ml, a threshold indicating low-grade inflammation. A recent comparison of maraviroc and tenofovir, each combined with boosted darunavir or emtricitabine, highlighted a greater capacity of maraviroc to increase the CD4/CD8 ratio after 48 weeks, although subjects treated with maraviroc showed smaller CD8+ T-cell count declines (21). We and others have shown that maraviroc exerts unique effects on the gut mucosa, implicating maturation and activation, likely explained via CCR5 signaling, increases in circulating CCR5 ligands, and higher drug concentrations in the gut tissue than with efavirenz (8, 22). Although unexpected, mechanistically, our results could be explained by the paradoxical increases in mucosal CD8+ T-cell activation that we (22) and others (8) have observed under maraviroc intensification, given the strong correlation between the CD4/CD8 ratio and CD8+ T-cell activation in the gut and blood (5, 22). It should be noted, however, that other studies have failed to demonstrate the putative beneficial effects of maraviroc on immunoactivation and inflammation (23–25), T-cell recovery (15, 26), or the development of immune reconstitution inflammatory syndrome (26).
Emerging data suggest that chronic inflammation, a hallmark of treated HIV infection, might be affected by the ART regiment selected (27–29). Given the correlation of the CD4/CD8 ratio with markers of activation of both the innate and adaptive immunity (5), we performed a subanalysis of the STARTMRK study. We observed a statistically significantly higher rate of CD4/CD8 ratio normalization with raltegravir than with efavirenz. In contrast to the present study, the differences between regimens were smaller and were explained by a greater CD4+ T-cell count increase in the raltegravir arm, without differences in the CD8+ T-cell count dynamics. To the best of our knowledge, this is the first report highlighting differential effects of two first-line ART regimens on CD8+ T-cell counts. Higher levels of circulating CD8+ T cells have been associated with adverse outcomes and seem to capture the spectrum of non-AIDS conditions linked with chronic immune activation that is captured by the CD4/CD8 ratio (5, 7, 30).
Maraviroc is now prescribed only in certain situations, so it is unlikely that these results affect clinical decisions. Moreover, zidovudine is a first-generation nucleoside reverse transcriptase inhibitor (NRTI) associated with bone marrow toxicity, which could have affected CD4/CD8 ratio dynamics. Given the lack of interaction of zidovudine with efavirenz or maraviroc and the randomized study design, it is unlikely that this effect has influenced our findings. Another important limitation of our work is that current NRTI backbone regimens in most countries no longer include zidovudine, which raises the question of whether similar findings would have been obtained with modern NRTIs.
There is now increasing awareness that ART might not be able to fully normalize health, and clinical research is shifting from strategies aimed at suppressing HIV replication and increasing CD4+ T-cell counts to evaluation of the impact of therapies designed to mitigate persistent immune defects. Our data highlight the need to evaluate new outcomes in ART trials, as a clear impact of surrogate markers linked with the risk of non-AIDS events such as the CD4/CD8 ratio might have been overlooked and should influence the positioning of ART regimens in clinical guidelines. The small variation in the effect of the treatment arm on the CD4/CD8 ratio after adjustment by factors known to influence the CD4/CD8 ratio indicates that the drug class used might affect this biomarker of clinical progression.
In conclusion, our data show, for the first time, distinct effects of two first-line ART regimens on CD8+ T-cell count dynamics and, secondarily, on the rates of CD4/CD8 ratio normalization at clinically meaningful cutoffs. These outcomes have been largely overlooked in clinical trials. Our findings provide a rationale for comparing the effects of different ART regimens on immune activation and for considering the CD8+ T-cell count and the CD4/CD8 ratio as outcomes in future studies.
Supplementary Material
ACKNOWLEDGMENTS
We thank all of the patients and their caregivers who participated in this MERIT study. The contributions of the many investigators are gratefully recognized. We thank ViiV Healthcare for sharing the data of the MERIT study for this analysis.
The data analyzed in this report were generated in a study sponsored and funded by ViiV, which manufactures maraviroc under the brand name CELSENTRI. S.S.-V. is funded by a grant of the Spanish Ministry of Economy and Competitiveness (Contratos Juan Rodés JR14/0004). This work has been partially funded by the SPANISH AIDS Research Network (RIS) RD16/0025/0001 project as part of the Plan Nacional R+D+I and cofinanced by ISCIII-Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER).
S.S.-V. has received grants from MSD and personal fees from ViiV, MSD, Gilead, and Janssen outside this study. J.A.P.-M. has received grants from MSD and personal fees from BMS, ViiV, Gilead, and MSD outside this study. S.M. has received grants from ViiV, MSD, and Gilead and personal fees from Abbvie, BMS, ViiV, Gilead, and MSD outside this study.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01763-17.
REFERENCES
- 1.Marcus JL, Chao CR, Leyden WA, Xu L, Quesenberry CP, Klein DB, Towner WJ, Horberg MA, Silverberg MJ. 2016. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr 73:39–46. doi: 10.1097/QAI.0000000000001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deeks SG. 2011. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, Plants J, Seth A, Wilson CC, Deeks SG, Lederman MM, Landay AL. 2014. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 210:1248–1259. doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sainz T, Serrano-Villar S, Díaz L, González-Tomé MI, Gurbindo MD, de José MI, Mellado MJ, Ramos JT, Zamora J, Moreno S, Muñoz-Fernández MA. 2013. The CD4/CD8 ratio as a marker T-cell activation, senescence and activation/exhaustion in treated HIV-infected children and young adults. AIDS 27:1513–1516. doi: 10.1097/QAD.0b013e32835faa72. [DOI] [PubMed] [Google Scholar]
- 5.Serrano-Villar S, Sainz T, Lee SA, Hunt PW, Sinclair E, Shacklett BL, Ferre AL, Hayes TL, Somsouk M, Hsue PY, Van Natta ML, Meinert CL, Lederman MM, Hatano H, Jain V, Huang Y, Hecht FM, Martin JN, McCune JM, Moreno S, Deeks SG. 2014. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 10:e1004078. doi: 10.1371/journal.ppat.1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.May MT, Trickey A, Costagliola D, Reiss P, Moreno S, Gill J, Smith C, Ingle SM, Sterne JA. 2015. Association of CD4:CD8 with cause-specific mortality in patients on long term ART, abstr 579. 22nd Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, 23 to 26 February 2015 http://www.croiconference.org/sites/default/files/posters-2015/579.pdf. [Google Scholar]
- 7.Mussini C, Lorenzini P, Cozzi-Lepri A, Lapadula G, Marchetti G, Nicastri E, Cingolani A, Lichtner M, Antinori A, Gori A, d'Arminio Monforte A, Icona Foundation Study Group. 2015. CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV 2:e98–e106. doi: 10.1016/S2352-3018(15)00006-5. [DOI] [PubMed] [Google Scholar]
- 8.Hunt PW, Shulman NS, Hayes TL, Dahl V, Somsouk M, Funderburg NT, McLaughlin B, Landay AL, Adeyemi O, Gilman LE, Clagett B, Rodriguez B, Martin JN, Schacker TW, Shacklett BL, Palmer S, Lederman MM, Deeks SG. 2013. The immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T-cell recovery: a randomized trial. Blood 121:4635–4646. doi: 10.1182/blood-2012-06-436345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gates TM, Cysique LA, Siefried KJ, Chaganti J, Moffat KJ, Brew BJ. 2016. Maraviroc-intensified combined antiretroviral therapy improves cognition in virally suppressed HIV-associated neurocognitive disorder. AIDS 30:591–600. doi: 10.1097/QAD.0000000000000951. [DOI] [PubMed] [Google Scholar]
- 10.López-Huertas MR, Jiménez-Tormo L, Madrid-Elena N, Gutiérrez C, Rodríguez-Mora S, Coiras M, Alcamí J, Moreno S. 2017. The CCR5-antagonist maraviroc reverses HIV-1 latency in vitro alone or in combination with the PKC-agonist bryostatin-1. Sci Rep 7:2385. doi: 10.1038/s41598-017-02634-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper DA, Heera J, Goodrich J, Tawadrous M, Saag M, Dejesus E, Clumeck N, Walmsley S, Ting N, Coakley E, Reeves JD, Reyes-Teran G, Westby M, Van Der Ryst E, Ive P, Mohapi L, Mingrone H, Horban A, Hackman F, Sullivan J, Mayer H. 2010. Maraviroc versus efavirenz, both in combination with zidovudine-lamivudine, for the treatment of antiretroviral-naive subjects with CCR5-tropic HIV-1 infection. J Infect Dis 201:803–813. doi: 10.1086/650697. [DOI] [PubMed] [Google Scholar]
- 12.Sierra-Madero J, Di Perri G, Wood R, Saag M, Frank I, Craig C, Burnside R, McCracken J, Pontani D, Goodrich J, Heera J, Mayer H. 2010. Efficacy and safety of maraviroc versus efavirenz, both with zidovudine/lamivudine: 96-week results from the MERIT study. HIV Clin Trials 11:125–132. doi: 10.1310/hct1103-125. [DOI] [PubMed] [Google Scholar]
- 13.Hadrup SR, Strindhall J, Køllgaard T, Johansson B, Pawelec G, Straten P, Wikby A, Seremet T. 2006. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol 176:2645–2653. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- 14.Serrano-Villar S, Pérez-Elías MJ, Dronda F, Casado JL, Moreno A, Royuela A, Pérez-Molina JA, Sainz T, Navas E, Hermida JM, Quereda C, Moreno S. 2014. Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PLoS One 9:e85798. doi: 10.1371/journal.pone.0085798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper DA, Heera J, Ive P, Botes M, Dejesus E, Burnside R, Clumeck N, Walmsley S, Lazzarin A, Mukwaya G, Saag M, van Der Ryst E. 2014. Efficacy and safety of maraviroc vs. efavirenz in treatment-naive patients with HIV-1: 5-year findings. AIDS 28:717–725. doi: 10.1097/QAD.0000000000000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gulick RM, Fatkenheuer G, Burnside R, Hardy WD, Nelson MR, Goodrich J, Mukwaya G, Portsmouth S, Heera JR. 2014. Five-year safety evaluation of maraviroc in HIV-1-infected treatment-experienced patients. J Acquir Immune Defic Syndr 65:78–81. doi: 10.1097/QAI.0b013e3182a7a97a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funderburg NT, Mayne E, Sieg SF, Asaad R, Jiang W, Kalinowska M, Luciano AA, Stevens W, Rodriguez B, Brenchley JM, Douek DC, Lederman MM. 2010. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood 115:161–167. doi: 10.1182/blood-2009-03-210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutiérrez C, Díaz L, Vallejo A, Hernández-Novoa B, Abad M, Madrid N, Dahl V, Rubio R, Moreno AM, Dronda F, Casado JL, Navas E, Pérez-Elías MJ, Zamora J, Palmer S, Muñoz E, Muñoz-Fernández MÁ, Moreno S. 2011. Intensification of antiretroviral therapy with a CCR5 antagonist in patients with chronic HIV-1 infection: effect on T cells latently infected. PLoS One 6:e27864. doi: 10.1371/journal.pone.0027864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkin TJ, Lalama CM, McKinnon J, Gandhi RT, Lin N, Landay A, Ribaudo H, Fox L, Currier JS, Mellors JW, Gulick R, Tenorio AR. 2012. A pilot trial of adding maraviroc to suppressive antiretroviral therapy for suboptimal CD4+ T-cell recovery despite sustained virologic suppression: ACTG A5256. J Infect Dis 206:534–542. doi: 10.1093/infdis/jis376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkin TJ, Ribaudo HR, Tenorio AR, Gulick RM. 2010. The relationship of CCR5 antagonists to CD4+ T-cell gain: a meta-regression of recent clinical trials in treatment-experienced HIV-infected patients. HIV Clin Trials 11:351–358. doi: 10.1310/hct1106-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan ES, Landay AL, Brown TT, Ribaudo HJ, Mirmonsef P, Ofotokun I, Weitzmann MN, Martinson J, Klingman KL, Eron JJ, Fichtenbaum CJ, Plants J, Taiwo BO. 2016. Differential CD4+ cell count increase and CD4+:CD8+ ratio normalization with maraviroc compared with tenofovir. AIDS 30:2091–2097. doi: 10.1097/QAD.0000000000001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serrano-Villar S, Sainz T, Ma Z-M, Utay NS, Wook-Chun T, Mann S, Kashuba AD, Siewe B, Albanese A, Troia-Cancio P, Sinclair E, Somasunderam A, Yotter T, Deeks SG, Landay A, Pollard RB, Miller CJ, Moreno S, Asmuth DM. 2016. Effects of combined CCR5/integrase inhibitors-based regimen on mucosal immunity in HIV-infected patients naïve to antiretroviral therapy: a pilot randomized trial. PLoS Pathog 12:e1005381. doi: 10.1371/journal.ppat.1005381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funderburg N, Kalinowska M, Eason J, Goodrich J, Heera J, Mayer H, Rajicic N, Valdez H, Lederman MM. 2010. Effects of maraviroc and efavirenz on markers of immune activation and inflammation and associations with CD4+ cell rises in HIV-infected patients. PLoS One 5:e13188. doi: 10.1371/journal.pone.0013188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim CJ, Rousseau R, Huibner S, Kovacs C, Benko E, Shahabi K, Kandel G, Ostrowski M, Kaul R. 2017. Impact of intensified antiretroviral therapy during early HIV infection on gut immunology and inflammatory blood biomarkers. AIDS 31:1529–1534. doi: 10.1097/QAD.0000000000001515. [DOI] [PubMed] [Google Scholar]
- 25.Tiraboschi J, Ray S, Patel K, Pace M, Phalora P, Robinson N, Hopkins E, Meyerowitz J, Wang Y, Davies O, Mant C, Cason J, Kaye S, Sanderson J, Fidler S, Klenerman P, Frater J, Fox J. 2017. Short communication: lack of effect of maraviroc intensification on blood and gut reservoir. AIDS Res Hum Retroviruses 33:143–146. doi: 10.1089/aid.2016.0198. [DOI] [PubMed] [Google Scholar]
- 26.Levy Y, Lelièvre J-D, Assoumou L, Aznar E, Pulido F, Tambussi G, Crespo M, Meybeck M, Molina J-M, Cardon F, Diallo A, Delaugerre C, Lancar R, Béniguel L, Costagliola D. ANRS 146-GeSIDA 7211 OPTIMAL phase III trial: maraviroc plus cART in advanced HIV-1-infected individuals. International AIDS Conference Paris, France, 23 to 26 July 2017 http://programme.ias2017.org/Abstract/Abstract/1800. [Google Scholar]
- 27.Martínez E, D'Albuquerque PM, Llibre JM, Gutierrez F, Podzamczer D, Antela A, Berenguer J, Domingo P, Moreno X, Perez I, Pich J, Gatell JM. 2012. Changes in cardiovascular biomarkers in HIV-infected patients switching from ritonavir-boosted protease inhibitors to raltegravir. AIDS 26:2315–2326. doi: 10.1097/QAD.0b013e328359f29c. [DOI] [PubMed] [Google Scholar]
- 28.Massanella M, Ouchi D, Marfil S, Llibre JM, Puertas MC, Buzón MJ, Richman DD, Orna E, Stevenson M, Gatell JM, Domingo P, Negredo E, Martinez-Picado J, Clotet B, Blanco J. 2014. Different plasma markers of inflammation are influenced by immune recovery and cART composition or intensification in treated HIV infected individuals. PLoS One 9:e114142. doi: 10.1371/journal.pone.0114142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hileman CO, Kinley B, Scharen-Guivel V, Melbourne K, Szwarcberg J, Robinson J, Lederman MM, McComsey GA. 2015. Differential reduction in monocyte activation and vascular inflammation with integrase inhibitor-based initial antiretroviral therapy among HIV-infected individuals. J Infect Dis 212:345–354. doi: 10.1093/infdis/jiv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helleberg M, Kronborg G, Ullum H, Ryder LP, Obel N, Gerstoft J. 2015. Course and clinical significance of CD8+ T-cell counts in a large cohort of HIV-infected individuals. J Infect Dis 211:1726–1734. doi: 10.1093/infdis/jiu669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.