ABSTRACT
The dose of trimethoprim-sulfamethoxazole (TMP-SMX) for the treatment of Pneumocystis pneumonia (PCP) in patients without human immunodeficiency virus (HIV) infection has not been verified. The aim of this study was to investigate the efficacy and toxicity of a low-dose TMP-SMX regimen in such patients. A retrospective study was conducted in four hospitals. We reviewed the medical records of patients with PCP but not HIV (non-HIV-PCP) who were treated with TMP-SMX between 2003 and 2016. The patients were divided into conventional-dose (TMP, 15 to 20 mg/kg/day) and low-dose (TMP, <15 mg/kg/day) groups after patients who received high-dose (TMP, >20 mg/kg/day) treatment were excluded. Grouping was done according to a correction dose, which was based on renal function. Eighty-two patients had non-HIV-PCP. The numbers of patients who received high-, conventional-, and low-dose treatments were 5, 36, and 41, respectively. Kaplan-Meier analysis for death associated with PCP showed no statistically significant difference in survival rates between the conventional- and low-dose groups. Ninety-day cause-specific mortality rates were 25.0% and 19.5% in the conventional-dose and low-dose groups (P = 0.76), respectively. Adverse events that were graded as ≥3 according to the Common Terminology Criteria for Adverse Events (version 4.0) (National Cancer Institute, 2010) were 41.7% and 17.1% in the conventional-dose and low-dose groups (P = 0.02), respectively. Moreover, vomiting (P = 0.03) and a decrease in platelet count (P = 0.03) occurred more frequently in the conventional-dose group. Treatment of non-HIV-PCP with low-dose or conventional-dose TMP-SMX produces comparable survival rates; however, the low-dose regimen is better tolerated and associated with fewer adverse effects.
KEYWORDS: low dose, Pneumocystis pneumonia, renal impairment, trimethoprim-sulfamethoxazole
INTRODUCTION
Pneumocystis pneumonia (PCP) is an opportunistic pulmonary infection in patients with AIDS. Antiretroviral therapy for human immunodeficiency virus (HIV) infection and chemoprophylaxis for Pneumocystis infection have reduced the frequency of PCP in HIV infection (HIV-PCP) (1). In contrast, having PCP but not HIV infection (non-HIV-PCP) is a growing concern as the number of patients receiving transplantation, corticosteroids, immunosuppressants, biological agents, and antitumor chemotherapy is increasing.
The clinical characteristics and immunological profiles of non-HIV-PCP are different from those of HIV-PCP. Non-HIV-infected patients do not usually show a decline in CD4+ cell counts. HIV-infected patients with CD4+ cell counts of less than 200 cells/μl have the highest risk of developing PCP (2, 3). Although non-HIV-infected patients typically have a smaller number of Pneumocystis organisms in their lungs than HIV-infected patients, non-HIV-infected patients usually have more severe bronchoalveolar lavage fluid neutrophilia and a greater inflammatory response (4, 5). The severity of non-HIV-PCP is higher with a more rapid and fulminant onset. The mortality rates associated with non-HIV-PCP and HIV-PCP are approximately 30 to 60% and 10 to 20%, respectively (3).
The treatment for PCP was recommended based on findings of randomized controlled trials conducted mostly in HIV-PCP patients. There is no well-established regimen for treating non-HIV-PCP. HIV-PCP and non-HIV-PCP are similarly treated although they are different in pathophysiology. According to current guidelines for the prevention and treatment of opportunistic infections in HIV-infected patients, the preferred treatment for PCP is oral or intravenous trimethoprim-sulfamethoxazole (TMP-SMX; TMP, 15 to 20 mg/kg/day; SMX, 75 to 100 mg/kg/day) for 21 days (6). However, clinicians often have to reduce the dose or switch to an alternative treatment due to the occurrence of adverse events (7).
A few studies have been conducted on the treatment of PCP with lower doses of TMP-SMX in order to reduce the occurrence of adverse events. Thomas et al. (8) reported that low-dose TMP-SMX (TMP, 10 mg/kg/day) and the conventional dose have comparable efficacies for HIV-PCP treatment. Moreover, the low-dose regimen is associated with fewer adverse effects. Creemers-Schild et al. (9) also reported that intermediate-dose TMP-SMX (TMP, 10 to 15 mg/kg/day) and the conventional dose have similar efficacies in the treatment of HIV-PCP and non-HIV-PCP. The authors reported that using low-dose TMP-SMX (TMP, 4 to 6 mg/kg/day), depending on the clinical course of the disease, did not compromise treatment outcome.
Patients with PCP and renal impairment have to be treated with a lower dose of TMP-SMX. To date, there are no reports on the appropriate dose of TMP-SMX for treating non-HIV-PCP with careful consideration of renal function. In the present study, we aimed to investigate the appropriate dose of TMP-SMX for treating non-HIV-PCP. We compared the efficacy and toxicity of a low-dose TMP-SMX regimen with those of the conventional-dose regimen, while making dose adjustments based on the renal function of each patient.
RESULTS
Characteristics of patients.
During the study period, 82 patients with non-HIV-PCP were identified. After adjustments were made based on renal function, five patients were excluded from the study because they received a high dose of the treatment, whereas the remaining patients were divided into conventional-dose (n = 36) and low-dose (n = 41) groups. The demographic and clinical features of patients in the two groups at the initiation of treatment are shown in Table 1. Body weight and creatinine clearance (CrCL), which affect calculation of the correction dose, were higher in the low-dose group. The differences in body mass indexes and parameters of severity, such as the arterial partial pressure of oxygen/fraction of inspiratory oxygen (PaO2/FiO2) ratio, alveolar-arterial oxygen difference (A-a DO2), and sequential organ failure assessment (SOFA) score, between the two treatment groups were not statistically significant. Of the 77 patients studied, 16 (20.8%) had CrCL values of ≥30 ml/min but <50 ml/min, 8 (10.4%) had CrCL values of ≥15 ml/min but <30 ml/min, 2 (2.6%) had CrCL values of <15 ml/min, and 4 (5.2%) were undergoing hemodialysis.
TABLE 1.
Demographic and clinical features of patients in the two treatment groups at the initiation of treatment
| Parametera | Value for the parameterb |
P value | ||
|---|---|---|---|---|
| Total patient population (n = 77) | Conventional-dose group (n = 36) | Low-dose group (n = 41) | ||
| Clinical characteristics | ||||
| Age (yr) | 66.1 ± 13.7 | 67.0 ± 14.1 | 65.4 ± 13.2 | 0.49 |
| Gender (no. of male patients/no. of female patients) | 47/30 | 19/17 | 28/13 | 0.16 |
| Body wt (kg) | 54.8 ± 10.5 | 51.2 ± 9.4 | 57.9 ± 10.4 | 0.008 |
| Body mass index (kg/m2) | 21.4 ± 3.4 | 20.9 ± 3.5 | 21.9 ± 3.3 | 0.4 |
| Underlying disease (no. of patients [%]) | ||||
| Collagen vascular disease | 33 (42.9) | 18 (50.0) | 15 (36.6) | 0.24 |
| Rheumatoid arthritis | 19 (24.7) | 11 (30.6) | 8 (19.5) | 0.26 |
| Hematological malignancy | 17 (22.1) | 8 (22.2) | 9 (22.0) | 0.98 |
| Solid tumor | 13 (16.9) | 5 (13.9) | 8 (19.5) | 0.51 |
| Interstitial lung disease | 3 (3.9) | 1 (2.8) | 2 (4.9) | 0.63 |
| Others | 11 (14.3) | 4 (11.1) | 7 (17.1) | 0.46 |
| Therapy for underlying disease (no. of patients [%]) | ||||
| Corticosteroid only | 35 (45.5) | 16 (44.4) | 19 (46.3) | 0.87 |
| Corticosteroid with other agents | 29 (37.7) | 13 (36.1) | 16 (39.0) | 0.79 |
| Without corticosteroids | 13 (16.9) | 7 (19.4) | 6 (14.6) | 0.57 |
| Primary prophylaxis with TMP-SMX (no. of patients [%]) | 1 (1.3) | 1 (2.8) | 0 (0) | 0.28 |
| Laboratory findings | ||||
| PaO2/FiO2 ratio | 231.0 ± 96.2 | 238.6 ± 94.1 | 223.9 ± 97.5 | 0.58 |
| A-a DO2 (torr) | 143.7 ± 129.8 | 119.0 ± 106.2 | 166.7 ± 144.7 | 0.35 |
| White blood cell count (×109/liter) | 8.4 ± 4.3 | 7.5 ± 3.6 | 9.3 ± 4.7 | 0.08 |
| Neutrophil count (×109/liter) | 7.2 ± 4.2 | 6.2 ± 3.1 | 8.1 ± 4.8 | 0.08 |
| Lymphocyte count (×109/liter) | 0.8 ± 0.5 | 0.8 ± 0.6 | 0.8 ± 0.5 | 0.55 |
| Hemoglobin (g/dl) | 10.8 ± 2.2 | 10.5 ± 2.1 | 11.1 ± 2.3 | 0.23 |
| Platelet count (×109/liter) | 191.2 ± 102.8 | 180.0 ± 88.8 | 201.0 ± 111.0 | 0.44 |
| Total protein (g/dl) | 5.5 ± 1.0 | 5.6 ± 1.0 | 5.4 ± 0.9 | 0.26 |
| Albumin (g/dl) | 2.7 ± 0.7 | 2.8 ± 0.7 | 2.7 ± 0.6 | 0.73 |
| Lactate dehydrogenase (IU/liter) | 431.5 ± 222.3 | 432.0 ± 250.6 | 431.1 ± 194.1 | 0.63 |
| Urea nitrogen (mEq/liter) | 28.3 ± 18.9 | 29.0 ± 21.2 | 27.8 ± 16.6 | 0.6 |
| Creatinine (mg/dl) | 1.4 ± 1.4 | 1.7 ± 1.9 | 1.2 ± 0.9 | 0.95 |
| C-reactive protein (mg/dl) | 8.9 ± 6.9 | 8.9 ± 5.8 | 8.9 ± 7.7 | 0.71 |
| KL-6 (IU/ml) | 1,171.9 ± 1,619.5 | 770.2 ± 526.7 | 1,487.5 ± 2,058.9 | 0.22 |
| β-d-Glucan (pg/ml) | 307.2 ± 1097.2 | 201.5 ± 248.1 | 400.0 ± 1,479.3 | 0.9 |
| Creatinine clearance (ml/min) | 59.3 ± 36.1 | 53.0 ± 38.0 | 64.8 ± 33.3 | 0.03 |
| SOFA score | 4.1 ± 2.7 | 4.2 ± 3.0 | 3.9 ± 2.3 | 0.91 |
A-a DO2, alveolar-arterial oxygen difference; PaO2/FiO2, arterial partial pressure of oxygen/fraction of inspiratory oxygen; SOFA, sequential organ failure assessment; TMP-SMX, trimethoprim-sulfamethoxazole.
Values are presented as means ± standard deviations unless otherwise indicated.
Collagen vascular disease (42.9%), particularly rheumatoid arthritis (24.7%), was the most frequent underlying disease, followed by hematological malignancies (22.1%), solid tumors (16.9%), and interstitial lung disease (3.9%). Several patients were undergoing long-term corticosteroid treatment with or without other agents such as immunosuppressants, biologics, and antitumor drugs. The remaining 16.9% of the patients were not receiving corticosteroid treatment; however, they were receiving treatment with other agents or had systemic or local immune system defects according to their underlying diseases. Only one patient with a malignant lymphoma and undergoing chemotherapy had received primary prophylaxis with TMP-SMX prior to being diagnosed with PCP.
Treatments and outcomes.
Analysis of the different treatments (Table 2) showed that the mean correction dose of TMP was 17.5 mg/kg in the conventional-dose group and 10.8 mg/kg in the low-dose group (P < 0.001). Except for this finding and the occurrence of adverse events, there were no differences in the details of treatment between the two groups. The mean duration from PCP onset to treatment initiation was 6.1 days, whereas the mean duration of treatment with TMP-SMX for PCP was 16.9 days. Although a 14-day or 21-day regimen is usually suggested for the treatment of non-HIV-PCP, 11 patients (14.3%) could not continue with treatment for 14 days due to the occurrence of adverse events or other reasons. Furthermore, seven patients (9.1%) required treatment for more than 21 days because recovery was prolonged. Twenty-three patients (29.9%) required admission into the intensive care unit. In addition, 25 patients (32.5%) required mechanical ventilation due to severe respiratory failure. Among them, 6 patients required noninvasive ventilation, whereas 19 patients required invasive ventilation. Most of the patients with moderate-to-severe respiratory failure were administered adjunctive corticosteroids according to the recommended regimen for treating HIV-PCP.
TABLE 2.
Analysis of the different treatments
| Treatment parametera | Value for the parameterc |
P value | ||
|---|---|---|---|---|
| Total patient population (n = 77) | Conventional-dose group (n = 36) | Low-dose group (n = 41) | ||
| Duration from PCP onset to treatment (days) | 6.1 ± 5.2 | 6.3 ± 4.3 | 5.9 ± 5.9 | 0.26 |
| TMP-SMX treatment | ||||
| Correction dose (mg/kg TMP) | 14.0 ± 4.1 | 17.5 ± 1.9 | 10.8 ± 2.8 | <0.001 |
| Duration of TMP-SMX therapy (days) | 16.9 ± 6.6 | 15.7 ± 6.1 | 18.1 ± 6.8 | 0.06 |
| Occurrence of adverse eventsb | 22 (28.6) | 15 (41.7) | 7 (17.1) | 0.02 |
| Dose reductiond | 15 (19.5) | 8 (22.2) | 7 (17.1) | 0.57 |
| Withdrawal or change to second-line regimend | 10 (13.0) | 7 (19.4) | 3 (7.3) | 0.22 |
| Second-line regimen | ||||
| Pentamidine | 6 (7.8) | 5 (13.9) | 1 (2.4) | 0.06 |
| Atovaquone | 1 (1.3) | 0 (0) | 1 (2.4) | 0.35 |
| ICU admission | 23 (29.9) | 9 (25.0) | 14 (34.1) | 0.38 |
| Mechanical ventilation | 25 (32.5) | 11 (30.6) | 14 (34.1) | 0.74 |
| Adjunctive corticosteroid therapy | 55 (71.4) | 26 (72.2) | 29 (70.7) | 0.89 |
CTCAE, Common Terminology Criteria for Adverse Events; ICU, intensive care unit; PCP, pneumocystis pneumonia; TMP-SMX, trimethoprim-sulfamethoxazole.
CTCAE grade of ≥3.
Unless noted otherwise, values are the number (percent) of patients. Other values are presented as means ± standard deviations.
Dose reduction and withdrawal or change to second-line regimen were due to the occurrence of adverse events.
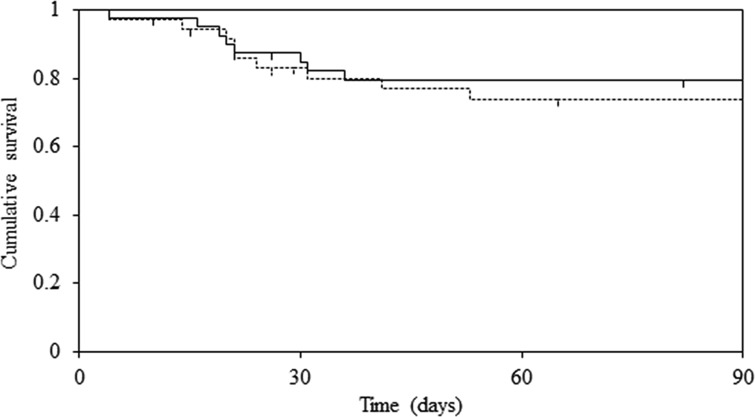
Kaplan-Meier analysis of death associated with PCP showed that there was no statistically significant difference in survival rates between the two groups (log rank test; P = 0.59) (Fig. 1). Moreover, the 90-day cause-specific mortality rate was 25.0% in the conventional-dose group and 19.5% in the low-dose group (P = 0.76). In addition, 90-day all-cause mortality rates were 27.8% and 24.4% in the conventional-dose and low-dose groups (P = 0.94), respectively. The results showed that mortality rates were not significantly different between the two groups.
FIG 1.
Kaplan-Meier survival curves for death associated with Pneumocystis pneumonia based on the dose of trimethoprim-sulfamethoxazole (solid line, low-dose group; dotted line, conventional-dose group). There was no statistically significant difference between the two groups. The survival curves were compared using log rank statistics. Censored subjects are indicated on the Kaplan-Meier curves as tick marks.
Toxicity.
The frequency of adverse events with grades of ≥3 according to the Common Terminology Criteria for Adverse Events (CTCAE) (10) was higher in the conventional-dose group (41.7%) than in the low-dose group (17.1%) (P = 0.02). Dose reduction due to adverse events was required in 15 patients (19.5%), whereas treatment withdrawal or change to a second-line regimen due to adverse events was required in 10 patients (13.0%). Pentamidine was used as an alternative agent in six patients, whereas atovaquone was used in one patient (Table 2). Vomiting of all grades (P = 0.03) and decreased platelet count of grade ≥3 (P = 0.03) were more frequent in the conventional-dose group (Table 3). Seven patients had grade ≥3 thrombocytopenia as follows: with grade 3 (platelet count, <50 ×109/liter), four patients in the conventional-dose group; with grade 4 (platelet count, <25 ×109/liter), two patients in the conventional-dose group and one patient in the low-dose group. Three patients with severe thrombocytopenia needed platelet transfusions, whereas one patient with grade 3 anemia (hemoglobin level, 5.6 g/dl) needed transfusions with packed red blood cells.
TABLE 3.
Adverse events and their CTCAE grades
| Adverse event | Frequency by CTCAE grade (no. of patients [%])a |
P value |
||||
|---|---|---|---|---|---|---|
| Conventional-dose group (n = 36) |
Low-dose group (n = 41) |
|||||
| All grades | Grade ≥3 | All grades | Grade ≥3 | All grades | Grade ≥3 | |
| Rash | 4 (11.1) | 3 (8.3) | 5 (12.2) | 1 (2.4) | 0.84 | 0.24 |
| Fever | 3 (8.3) | 1 (2.8) | 1 (2.4) | 0 (0) | 0.24 | 0.28 |
| Anorexia | 4 (11.1) | 2 (5.6) | 6 (14.6) | 0 (0) | 0.91 | 0.13 |
| Vomiting | 4 (11.1) | 1 (2.8) | 0 (0) | 0 (0) | 0.03 | 0.28 |
| Decreased white blood cell count | 3 (8.3) | 0 (0) | 4 (9.8) | 2 (4.9) | 0.83 | 0.18 |
| Decreased neutrophil count | 2 (5.6) | 1 (2.8) | 4 (9.8) | 2 (4.9) | 0.49 | 0.63 |
| Anemia | 8 (22.2) | 1 (2.8) | 7 (17.1) | 0 (0) | 0.57 | 0.28 |
| Decreased platelet count | 10 (27.8) | 6 (16.7) | 4 (9.8) | 1 (2.4) | 0.08 | 0.03 |
| Increased aspartate aminotransferase | 7 (19.4) | 0 (0) | 10 (24.4) | 0 (0) | 0.81 | NA |
| Increased alanine aminotransferase | 8 (22.2) | 1 (2.8) | 10 (24.4) | 0 (0) | 0.96 | 0.28 |
| Increased creatinine | 12 (33.3) | 0 (0) | 8 (19.5) | 0 (0) | 0.17 | NA |
| Hyponatremia | 12 (33.3) | 4 (11.1) | 11 (26.8) | 1 (2.4) | 0.53 | 0.12 |
| Hyperkalemia | 12 (33.3) | 4 (11.1) | 14 (34.1) | 5 (12.2) | 0.94 | 0.88 |
CTCAE, Common Terminology Criteria for Adverse Events; NA, not applicable.
DISCUSSION
The results of the present study suggest that low-dose TMP-SMX and the conventional-dose regimen have comparable efficacies in non-HIV-PCP treatment. Moreover, the low-dose regimen was associated with a decreased occurrence of adverse events. These findings are mostly in line with those reported by Thomas et al. (8) and Creemers-Schild et al. (9). Two important differences between the present study and the previous studies were the target of the analysis, which was treatment of non-HIV-PCP only, and the determination of a low or conventional dose based on careful consideration of renal function. In the present study, 39.0% of the patients had renal impairment (CrCL, <50 ml/min) and required dose adjustment. This suggests that renal impairment is a considerable factor in determining the dose of TMP-SMX for treating PCP. Although dose adjustment methods based on renal function differ slightly in various reports (11–13), we set the correction factor according to the value of CrCL, as reported in previous studies.
Conversely, Hughes et al. (14) showed that the efficacy of high-dose TMP-SMX was superior to that of the low-dose in the treatment of non-HIV-PCP patients. The difference in the results may be due to the difference in the doses studied. Hughes et al. (14) defined high- and low-dose TMP as 20 mg/kg and 4 to 7 mg/kg, respectively. In the present study, the mean correction dose of TMP was 17.5 mg/kg in the conventional-dose group and 10.8 mg/kg in the low-dose group. Although the efficacy of the treatment was not significantly decreased in the low-dose group in the present study, it might have declined if the administered dose had been lower. These findings indicate that the appropriate dosage of TMP in a TMP-SMX regimen for non-HIV-PCP is approximately 10 mg/kg/day.
Survival rates were not significantly different between the two treatment groups. The all-cause mortality rate in the low-dose group was 24.4%, which was lower than the 30 to 60% rate reported in several studies (3). Furthermore, the severity of PCP, which is defined by PaO2/FiO2 and A-a DO2 values and SOFA scores, was similar to that reported in other studies (15, 16). Collagen vascular disease, particularly rheumatoid arthritis, was the most prevalent underlying disease that led to PCP in the present study. In the case of non-HIV-PCP, the mortality rate was higher in patients with neoplastic disease (34 to 50%) (17, 18) and connective tissue disease (32%) (19) but lower in those with rheumatoid arthritis (10 to 29%) (20).
The frequency of adverse events with CTCAE grades of ≥3 was significantly higher in the conventional-dose group. Particularly, the incidences of vomiting and decreased platelet count were higher in this group. Increased serum creatinine levels, hyponatremia, hyperkalemia, anemia, and neutropenia are considered concentration-dependent adverse reactions of TMP-SMX, whereas rash, fever, gastrointestinal disorders, liver function abnormalities, and thrombocytopenia are considered concentration-independent reactions (21, 22). TMP-SMX-induced thrombocytopenia appears to be an immune-mediated process resulting in platelet destruction by drug-dependent platelet antibodies (23).
The present study had some limitations. First, it was a retrospective study; therefore, it is probable that there was selection bias regarding the various treatments. Physicians decide on the dose of TMP-SMX to administer to a patient after considering body weight, CrCL, and other factors such as complications and drug interactions. Such good decisions might have caused the survival rate in the low-dose group to be comparable with that in the conventional-dose group. Second, it was uncertain if efficacy and toxicity were dependent on serum concentrations of TMP-SMX as they were not measured. We believe that since grouping was done according to the correction dose, which was calculated based on renal function, the results of the present study will help physicians to decide the appropriate doses of TMP-SMX for patients. Third, the only data on survival rate was that the difference observed between the two treatment groups was not statistically significant. Further prospective randomized controlled studies are needed to clarify the equivalence of the two regimens.
In conclusion, the survival rate following treatment of non-HIV-PCP with low-dose TMP-SMX is similar to that with the conventional-dose regimen. Moreover, the low-dose regimen is well tolerated and associated with fewer adverse effects than the conventional-dose regimen. The present study is the first to investigate the possibility of using the low-dose regimen for treating non-HIV-PCP.
MATERIALS AND METHODS
Setting and study population.
A retrospective study was conducted in the following hospitals in Nagano Prefecture (Japan): Shinshu University Hospital, Nagano Municipal Hospital, Shinonoi General Hospital, and Suwa Red Cross Hospital. We reviewed the medical records of patients diagnosed with non-HIV-PCP who were treated with TMP-SMX at the participating hospitals between January 2003 and September 2016. Patients were excluded from the study if they had initially received treatments other than TMP-SMX for non-HIV-PCP.
The study was approved by the Ethics Review Board of Shinshu University (approval number 3133). Written informed consent was not obtained since patient information was anonymized. Moreover, the methods used in the study did not require obtaining informed consent under common law and ethics.
Patients were diagnosed with non-HIV-PCP if they satisfied all the following criteria: (i) an immunocompromised status, (ii) diffuse bilateral ground-glass opacity on chest radiography or computed tomography scans, (iii) detection of Pneumocystis jirovecii in respiratory specimens by traditional staining (Grocott, Diff-Quik, or Giemsa staining) or PCR assay, and (iv) significantly elevated plasma (1→3)-β-d-glucan levels. β-d-Glucan was measured using a β-d-glucan test kit (Wako Pure Chemical Industries, Osaka, Japan) or Fungitec G test MK (Seikagaku Corporation, Tokyo, Japan). Although there are various reports on the cutoff for the β-d-glucan level for the diagnosis of PCP (24–29), we defined values of at least 11 pg/ml (β-d-glucan test; Wako) or 20 pg/ml (Fungitec G test MK) as indicating infection with PCP.
Data collection.
Age, gender, body mass index, underlying diseases, primary prophylaxis with TMP-SMX, duration of symptoms prior to diagnosis with PCP, laboratory values, sequential organ failure assessment (SOFA) score (30) at the initiation of treatment, dose of TMP-SMX and duration of treatment, withdrawal or change to a second-line regimen, adjunctive corticosteroid therapy, mechanical ventilation, adverse events, and treatment outcome were obtained from medical records.
Adverse events occurring during treatment with TMP-SMX were extracted from the medical records and included rash, fever, anorexia, vomiting, decrease in white blood cell count, decrease in neutrophil count, anemia, decrease in platelet count, increased plasma levels of aspartate aminotransferase and alanine aminotransferase, increased serum creatinine level, hyponatremia, and hyperkalemia. Each event was graded according to the Common Terminology Criteria for Adverse Events ([CTCAE] version 4.0) (10) as mild (grade 1), moderate (grade 2), severe (grade 3), life-threatening (grade 4), or death related (grade 5).
Treatment outcome was assessed as death associated with PCP or all-cause mortality within 90 days after initiation of treatment. We used the survival rate to evaluate the efficacy of the treatment.
Definition of the two treatment groups.
Patients were divided into two groups: a conventional-dose group, in which patients were administered an initial dose of TMP-SMX according to current guidelines (6), and a low-dose group, in which patients were administered a dose lower than the conventional dose. Generally, if creatinine clearance (CrCL) is 15 to 30 ml/min, it is recommended to decrease the total daily dose by half (11). In addition, dose adjustments for patients with renal impairment are described in the Sanford guide (12). The Giusti-Hayton method (13), which involves calculations using CrCL and the fraction of the dose that is excreted unchanged in urine, is also used. Dosage was reduced for patients with moderate-to-severe renal impairment. If grouping had been done based on the actual dose, patients with poor renal function would have been assigned to the low-dose group. Therefore, we grouped the patients based on their renal function. Using the above-mentioned reports as references and based on expert opinions, we calculated the original correction dose from the actual dose using a correction factor based on renal function (Table 4). In order to calculate the correction dose of TMP (milligrams/kilogram), the correction factor was multiplied by the actual dose of TMP (milligrams/kilogram/day). We also used an estimated CrCL obtained from the Cockroft-Gault formula (31) as an indicator of renal function. The conventional dose was defined as the correction dose of TMP (15 to 20 mg/kg), whereas a low dose was defined as a TMP dose of <15 mg/kg. Patients who were treated with >20 mg/kg/day of TMP were excluded from the study.
TABLE 4.
Correction factor based on renal function
| CrCL (ml/min)a | Correction factor |
|---|---|
| ≥50 | 1.0 |
| 30 to <50 | 1.5 |
| 15 to <30 | 2.0 |
| <15 or hemodialysis | 3.0 |
CrCL, creatinine clearance.
Statistical analysis.
Data have been expressed as means ± standard deviations for continuous variables and as percentages for categorical variables. Categorical variables were compared using a chi-square test or Fisher's exact test, whereas continuous variables were analyzed using a Mann-Whitney U test. Time-to-event analysis was performed using Kaplan-Meier estimates and a log rank test. All tests were two-tailed, and P values of <0.05 were considered statistically significant.
REFERENCES
- 1.Buchacz K, Baker RK, Palella FJ Jr, Chmiel JS, Lichtenstein KA, Novak RM, Wood KC, Brooks JT, HOPS Investigators. 2010. AIDS-defining opportunistic illnesses in US patients, 1994–2007: a cohort study. AIDS 24:1549–1559. doi: 10.1097/QAD.0b013e32833a3967. [DOI] [PubMed] [Google Scholar]
- 2.Mansharamani NG, Balachandran D, Vernovsky I, Garland R, Koziel H. 2000. Peripheral blood CD4+ T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest 118:712–720. doi: 10.1378/chest.118.3.712. [DOI] [PubMed] [Google Scholar]
- 3.Thomas CF Jr, Limper AH. 2004. Pneumocystis pneumonia. N Engl J Med 350:2487–2498. doi: 10.1056/NEJMra032588. [DOI] [PubMed] [Google Scholar]
- 4.Limper AH, Offord KP, Smith TF, Martin WJ II. 1989. Pneumocystis carinii pneumonia: differences in lung parasite number and inflammation in patients with and without AIDS. Am Rev Respir Dis 140:1204–1209. doi: 10.1164/ajrccm/140.5.1204. [DOI] [PubMed] [Google Scholar]
- 5.Tokuda H, Sakai F, Yamada H, Johkoh T, Imamura A, Dohi M, Hirakata M, Yamada T, Kamatani N, Kikuchi Y, Sugii S, Takeuchi T, Tateda K, Goto H. 2008. Clinical and radiological features of Pneumocystis pneumonia in patients with rheumatoid arthritis, in comparison with methotrexate pneumonitis and Pneumocystis pneumonia in acquired immunodeficiency syndrome: a multicenter study. Intern Med 47:915–923. doi: 10.2169/internalmedicine.47.0702. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, Masur H, Centers for Disease Control and Prevention (CDC), National Institutes of Health, HIV Medicine Association of the Infectious Diseases Society of America. 2009. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 58(RR-4):1–207. [PubMed] [Google Scholar]
- 7.Chang HM, Tsai HC, Lee SS, Kunin C, Lin PC, Wann SR, Chen YS. 2016. High daily doses of trimethoprim/sulfamethoxazole are an independent risk factor for adverse reactions in patients with Pneumocystis pneumonia and AIDS. J Chin Med Assoc 79:314–319. doi: 10.1016/j.jcma.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Thomas M, Rupali P, Woodhouse A, Ellis-Pegler R. 2009. Good outcome with trimethoprim 10 mg/kg/day-sulfamethoxazole 50 mg/kg/day for Pneumocystis jirovecii pneumonia in HIV infected patients. Scand J Infect Dis 41:862–868. doi: 10.3109/00365540903214256. [DOI] [PubMed] [Google Scholar]
- 9.Creemers-Schild D, Kroon FP, Kuijper EJ, de Boer MG. 2016. Treatment of Pneumocystis pneumonia with intermediate-dose and step-down to low-dose trimethoprim-sulfamethoxazole: lessons from an observational cohort study. Infection 44:291–299. doi: 10.1007/s15010-015-0851-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Cancer Institute. 2010. Common terminology criteria for adverse events (CTCAE), version 4.0. National Cancer Institute, National Institutes of Health, Bethesda, MD: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. [Google Scholar]
- 11.Cockerill FR III, Edson RS. 1983. Trimethoprim-sulfamethoxazole. Mayo Clin Proc 58:147–153. [PubMed] [Google Scholar]
- 12.Gilbert DN, Eliopoulos GM, Chambers HF, Saag MS (ed). 2016. The Sanford guide to antimicrobial therapy, 46th ed Antimicrobial Therapy, Inc., Sperryville, VA. [Google Scholar]
- 13.Giusti DL, Hayton WL. 1973. Dosage regimen adjustments in renal impairment. Drug Intell Clin Pharm 7:382–387. [Google Scholar]
- 14.Hughes WT, Feldman S, Sanyal SK. 1975. Treatment of Pneumocystis carinii pneumonitis with trimethoprim-sulfamethoxazole. Can Med Assoc J 112:47–50. [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumura Y, Shindo Y, Iinuma Y, Yamamoto M, Shirano M, Matsushima A, Nagao M, Ito Y, Takakura S, Hasegawa Y, Ichiyama S. 2011. Clinical characteristics of Pneumocystis pneumonia in non-HIV patients and prognostic factors including microbiological genotypes. BMC Infect Dis 11:76. doi: 10.1186/1471-2334-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aoki Y, Iwamoto M, Kamata Y, Nagashima T, Yoshio T, Okazaki H, Minota S. 2009. Prognostic indicators related to death in patients with Pneumocystis pneumonia associated with collagen vascular diseases. Rheumatol Int 29:1327–1330. doi: 10.1007/s00296-009-0857-z. [DOI] [PubMed] [Google Scholar]
- 17.Torres HA, Chemaly RF, Storey R, Aguilera EA, Nogueras GM, Safdar A, Rolston KV, Raad II, Kontoyiannis DP. 2006. Influence of type of cancer and hematopoietic stem cell transplantation on clinical presentation of Pneumocystis jiroveci pneumonia in cancer patients. Eur J Clin Microbiol Infect Dis 25:382–388. doi: 10.1007/s10096-006-0149-4. [DOI] [PubMed] [Google Scholar]
- 18.Sepkowitz KA. 1992. Pneumocystis carinii pneumonia among patients with neoplastic disease. Semin Respir Infect 7:114–121. [PubMed] [Google Scholar]
- 19.Godeau B, Coutant-Perronne V, Le Thi Huong D, Guillevin L, Magadur G, De Bandt M, Dellion S, Rossert J, Rostoker G, Piette JC. 1994. Pneumocystis carinii pneumonia in the course of connective tissue disease: report of 34 cases. J Rheumatol 21:246–251. [PubMed] [Google Scholar]
- 20.Mori S, Sugimoto M. 2012. Pneumocystis jirovecii infection: an emerging threat to patients with rheumatoid arthritis. Rheumatology (Oxford) 51:2120–2130. doi: 10.1093/rheumatology/kes244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes WT, LaFon SW, Scott JD, Masur H. 1995. Adverse events associated with trimethoprim-sulfamethoxazole and atovaquone during the treatment of AIDS-related Pneumocystis carinii pneumonia. J Infect Dis 171:1295–1301. doi: 10.1093/infdis/171.5.1295. [DOI] [PubMed] [Google Scholar]
- 22.Brown GR. 2014. Cotrimoxazole—optimal dosing in the critically ill. Ann Intensive Care 4:13. doi: 10.1186/2110-5820-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamreudeewong W, Fosnocht BJ, Weixelman JM. 2002. Severe thrombocytopenia possibly associated with TMP/SMX therapy. Ann Pharmacother 36:78–82. doi: 10.1345/aph.1A188. [DOI] [PubMed] [Google Scholar]
- 24.Obayashi T, Yoshida M, Mori T, Goto H, Yasuoka A, Iwasaki H, Teshima H, Kohno S, Horiuchi A, Ito A, Yamaguchi H. 1995. Plasma (1→3)-beta-d-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes. Lancet 345:17–20. doi: 10.1016/S0140-6736(95)91152-9. [DOI] [PubMed] [Google Scholar]
- 25.Mori T, Ikemoto H, Matsumura M, Yoshida M, Inada K, Endo S, Ito A, Watanabe S, Yamaguchi H, Mitsuya M, Kodama M, Tani T, Yokota T, Kobayashi T, Kambayashi J, Nakamura T, Masaoka T, Teshima H, Yoshinaga T, Kohno S, Hara K, Miyazaki S. 1997. Evaluation of plasma (1→3)-beta-d-glucan measurement by the kinetic turbidimetric Limulus test, for the clinical diagnosis of mycotic infections. Eur J Clin Chem Clin Biochem 35:553–560. [PubMed] [Google Scholar]
- 26.Tasaka S, Hasegawa N, Kobayashi S, Yamada W, Nishimura T, Takeuchi T, Ishizaka A. 2007. Serum indicators for the diagnosis of Pneumocystis pneumonia. Chest 131:1173–1180. doi: 10.1378/chest.06-1467. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe T, Yasuoka A, Tanuma J, Yazaki H, Honda H, Tsukada K, Honda M, Gatanaga H, Teruya K, Kikuchi Y, Oka S. 2009. Serum (1→3) beta-d-glucan as a noninvasive adjunct marker for the diagnosis of Pneumocystis pneumonia in patients with AIDS. Clin Infect Dis 49:1128–1131. doi: 10.1086/605579. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura H, Tateyama M, Tasato D, Haranaga S, Yara S, Higa F, Ohtsuki Y, Fujita J. 2009. Clinical utility of serum beta-d-glucan and KL-6 levels in Pneumocystis jirovecii pneumonia. Intern Med 48:195–202. doi: 10.2169/internalmedicine.48.1680. [DOI] [PubMed] [Google Scholar]
- 29.Onishi A, Sugiyama D, Kogata Y, Saegusa J, Sugimoto T, Kawano S, Morinobu A, Nishimura K, Kumagai S. 2012. Diagnostic accuracy of serum 1,3-β-d-glucan for Pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. J Clin Microbiol 50:7–15. doi: 10.1128/JCM.05267-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. 1996. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710. [DOI] [PubMed] [Google Scholar]
- 31.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]