Abstract
Bariatric surgery is recognized as a highly effective therapy for obesity since it accomplishes sustained weight loss, reduction of obesity-related comorbidities and mortality, and improvement of quality of life. Overall, bariatric surgery is associated with a 42% reduction of the cardiovascular risk and 30% reduction of all-cause mortality. This review focuses on some nutritional consequences that can occur in bariatric patients that could potentially hinder the clinical benefits of this therapeutic option. All bariatric procedures, to variable degrees, alter the anatomy and physiology of the gastrointestinal tract; this alteration makes these patients more susceptible to developing nutritional complications, namely, deficiencies of macro- and micro-nutrients, which could lead to disabling diseases such as anemia, osteoporosis, protein malnutrition. Of note is the evidence that most obese patients present a number of nutritional deficits already prior to surgery, the most important being vitamin D and iron deficiencies. This finding prompts the need for a complete nutritional assessment and, eventually, an adequate correction of pre-existing deficits before surgery. Another critical issue that follows bariatric surgery is post-operative weight regain, which is commonly associated with the relapse of obesity-related co-morbidities. Nu-tritional complications associated with bariatric surgery can be prevented by life-long nutritional monitoring with the administration of multi-vitamins and mineral supplements according to the patient’s needs.
Keywords: Bariatric surgery, Nutrient deficiency, Roux-en-Y gastric bypass, Sleeve gastrectomy, Pre-operative deficit, Weight regain
Core tip: Bariatric surgery is increasingly and successfully applied for the treatment of morbid obesity. In spite of multiple clinical benefits, i.e., durable weight loss and improvement/reversal of many comorbidities, a number of nutritional complications can develop especially in the long term, which could cause serious detriment to patients’ health. We examine some important clinical conditions that are caused by the deficit of vitamins and micronutrients, such as anemia, osteoporosis, and malnutrition. We also discuss the importance of careful pre-operative assessments and the correction of pre-existing nutritional deficiencies, and present the current recommendations for an appropriate biochemical and nutritional monitoring in the long term.
INTRODUCTION
Obesity has become an important public health priority because it increases the risk of comorbid conditions, including diabetes, cardiovascular disease and several types of cancers. In addition, it affects life quality and expectancy[1]. The impact of obesity on life expectancy has been well documented. Worldwide, over 2.5 million deaths annually can be attributed to obesity. Of particular concern is the growing economic burden that the care of obesity and its complications imposes on society and the health care system[2].
The increasing prevalence of obesity and comorbid conditions worldwide prompts for effective strategies for both treatment and prevention[1]. The treatment of obesity includes lifestyle changes (dietary restrictions and increased physical activity), the use of medications, and in some cases, surgery. Lifestyle changes can cause a 2%-6% weight loss; however, after 1-5 years, almost 90% of the patients have returned to their original weight or might even gain some weight. Drug treatment in general leads to a 5%-15% weight loss and should be considered only as an adjunct to lifestyle changes. Unfortunately, with respect to lifestyle intervention, medical treatment rarely yields satisfactory results in the long term[1,3].
Bariatric surgery has proven to achieve greater weight loss than non-surgical management and, most importantly, has proven to maintain it in the long term[4]. Thus, in patients with morbid obesity, i.e., a body mass index of ≥ 40 or ≥ 35 kg/m2 with co-morbidities, bariatric surgery is presently considered to be the only effective therapy for obesity. Extensive data demonstrate that surgery can improve or even reverse many comorbidities, such as type 2 diabetes, hypertension, obstructive sleep apnea and steatohepatitis[5-7]. With regard to type 2 diabetes, observational and randomized controlled trials with a follow-up duration of up to 5 years have established the superiority of bariatric surgery over medical therapy at achieving remission of the disease and improvement of the overall cardiovascular risk profile[8-10]. One of the longest weight-loss studies - the Swedish Obese Subjects - evaluated the long-term effects of different bariatric procedures and demonstrated significant reductions in cardiovascular and cancer-related mortality as well as significant improvement in the quality of life[11-13].
In spite of multiple clinical benefits, a number of surgical and gastrointestinal complications can occur following bariatric procedures, although the diffusion of the laparoscopic approach and the expansion of centers of excellence have greatly reduced the rate of post-operative mortality and adverse events[14]. The mean mortality rate is 0.3% for all procedures, which is comparable to those for hip replacement (0.3%) or laparoscopic cholecystectomy (0.3%-0.6%). Indeed, even lower mortality rates (0.04-0.13) are achieved in high-volume obesity centers[14]. Among the possible complications, nutritional deficiencies deserve careful consideration. They can develop as a consequence of reduced intake and/or malabsorption of nutrients and are more commonly seen after malabsorptive or mixed procedures in comparison to the restrictive procedures. Other causal factors include pre-operative deficiencies, post-surgery food intolerance, changes in taste and eating patterns and non-adherence to dietary and supplement recommendations. Nutritional deficiencies can present with a wide range of clinical manifestations, depending on the specific nutrients/micronutrients that are involved, the severity, and the duration of the deficiency states. Because they could cause serious detriment to patients’ everyday lives and, in some instances, could result in life-threatening complications, a nutritional screening both before and after surgery is strongly recommended.
This review focuses on the main nutritional issues related to bariatric procedures by examining some important clinical conditions that are caused by the deficit of vitamins and micronutrients, such as anemia, osteoporosis, neurologic disorders, and malnutrition. We will also discuss the importance of careful pre-operative assessments and the correction of pre-existing nutritional deficiencies, which are quite common in obese patients. Last, recommendations for the prevention and treatment of nutritional deficiencies after bariatric surgery are presented.
CONVENTIONAL BARIATRIC SURGICAL PROCEDURES
Surgical procedures are generally classified into restrictive procedures, in which the stomach’s capacity is greatly reduced, malabsorptive procedures, in which malabsorption is the primary driver of the weight loss, or a combination of restrictive and malabsorptive elements (Figure 1). However, over the past few years, it has become clear that weight loss is not only due to reduced food intake and/or absorption induced by modification of gastrointestinal anatomy but also a consequence of changes in neural and gut hormonal signals that regulate hunger and satiety, gut microbiota, intestinal nutrient sensing, food preferences, and possibly energy expenditure[15]. These so-called weight-independent mechanisms contribute to a variable extent to weight loss and metabolic improvement, depending on the type of surgical technique.
Figure 1.
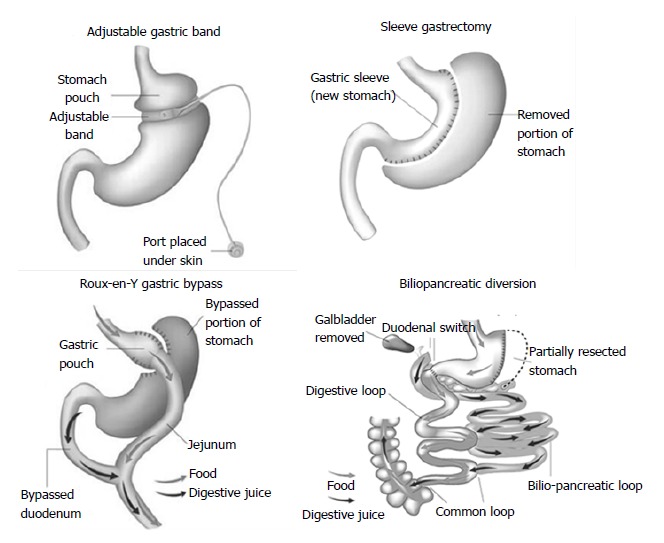
Commonly performed bariatric surgeries. Modified from http://www.bariatric-surgery-source.com/.
Laparoscopic adjustable gastric banding (AGB)
An adjustable silicone band is placed around the upper stomach, a few centimeters below the cardia, creating a 15 to 30 mL gastric pouch. The diameter of the outlet can be changed by injection of or removal of saline through a portal placed in the subcutaneous tissue that is connected to the band.
Roux-en-Y gastric bypass (RYGB)
A small, vertically oriented gastric pouch is created, which remains attached to the esophagus at one end and, at the other end, is connected to a small section of the small intestine, thus bypassing the remaining stomach and the initial loop of the small intestine.
Sleeve gastrectomy (SG)
The operation involves division of the stomach vertically, which reduces its size by 75%. The pyloric valve at the bottom of the stomach is preserved such that the stomach function and digestion remain unaltered. The procedure is not reversible and might be a first stage procedure to a RYGB or duodenal switch.
Biliopancreatic diversion (BPD)
The operation consists of a distal horizontal gastrectomy that leaves a 200-250 mL of upper stomach. This remnant stomach is anastomosed to the distal 250 cm of small intestine (alimentary limb). The excluded small intestine (carrying bile and pancreatic secretion), called the biliopancreatic limb, is connected to the small bowel 50 cm proximal to the ileocecal valve. The 50-cm common limb is the only segment where digestive secretions and nutrients mix, which causes a marked malabsorption, especially for fat and protein.
A recent survey by the International Federation for the Surgery of Obesity showed that RYGB and SG account for the large majority of bariatric procedures (45% and 37%, respectively). The use of ABG has drastically fallen during the last decade and currently accounts for 10% of all procedures. BPD and its duodenal switch (BPD/DS)variant, which are truly malabsorptive procedures, are rarely used (< 2%) to date given the high risk of nutritional complications[16].
NUTRITIONAL ISSUES AFTER BARIATRIC SURGERY
Anemia
According to a recent report from the American Society of Hematology, people who have undergone bariatric procedures show the highest risk for anemia, with 33%-49% of operated patients presenting anemia within 2 years after surgery[17]. As expected, the average prevalence of anemia is lower following LSG (17%) and reaches 45%-50% after RYGB and BPD. It should be noted that, as underlined for other nutrient deficiencies, up to 10%-12% of obese patients already have anemia before surgery[18]; thus, baseline screening for anemia is recommended in all patients who are scheduled for bariatric procedures.
Patients with mild anemia are most likely asymptomatic; however, when the anemia worsens, the patients could present with symptoms, such as fatigue, pallor, and dyspnea on exertion. Of note, the presence of anemia increases by twofold the risk of hospitalizations as well as the length of the in-hospital stay[19].
Post-bariatric anemia is in most cases due to iron deficiency, along with vitamin B12 deficiency as a secondary cause. Iron deficiency, expressed by low serum ferritin, occurs in more than 30% of patients after 5 years from surgery, with a similar rate after RYGB and SG, as recently reported by Alexandrou et al[20]. Iron-deficiency can be attributed to several causes. Reduced iron absorption due to hypocloridria and the bypassing of the duodenum and proximal jejunum (which are the main sites of iron absorption) are the primary mechanisms that lead to iron deficiency. Post-operative reduction in food intake and changes in food preferences, such as intolerance for meat and dairy products, are important contributory factors.
Measurement of serum ferritin is the best diagnostic test for detecting iron deficiency since it is a more specific and earlier indicator of iron body capacity and becomes abnormal prior to a decrease in serum iron con-centration. For this reason, ferritin and hemoglobin should be periodically monitored in bariatric patients. Current guidelines[21] recommend oral iron supplementation in all operated patients for preventive purposes. However, for the correction of iron deficiency (when iron deficiency sets in), oral supplementation is not sufficient, and intravenous iron administration is required.
Vitamin B12 deficiency is a major cause of anemia in patients who undergo BPD and RYGB, with a prevalence of 19%-35% after 5 years[22]. Purely restrictive procedures are usually not associated with vitamin B12 deficiency. Vitamin B12 deficiency can result from inadequate secretion of intrinsic factor, limited gastric acidity and, above all, the bypassing of the duodenum, which is the main site of vitamin B12 absorption. Since the human body has substantial reserves of vitamin B12, clinical manifestations of a deficit can appear after a certain time from surgery, when the body stores are depleted to as little as 5%-10%. In addition to anemia, a lack of vitamin B12 can lead to neurological and psychiatric symptoms, including paresthesia, numbness, disturbance of coordination, memory disturbance and, in some instances, dementia. Oral or intramuscular supplementation of vitamin B12 is recommended after malabsorptive procedures, while there is no evidence of benefits after restrictive surgery.
Folic acid deficiency is a potential complication of bariatric procedures that can contribute to anemia. The prevalence of this deficit after both restrictive and malabsorptive procedures ranges from 9% to 39%[23,24]. It can manifest as macrocytic anemia, piastrinopenia, leucopenia, or glossitis. It could cause growth retardation and, in pregnant women, congenital defects (neural tube). Since folate is absorbed throughout the small intestine, the deficiency is primarily induced by a shortage of dietary intake rather than malabsorption. Furthermore, folate deficiency can be aggravated by vitamin B12 deficiency since the latter is necessary for the conversion of inactive methyltetrahydrofolic acid to the active tetrahydrofolic acid. Folate deficiency can be easily corrected by oral supplementation.
Abnormalities of bone metabolism
Bariatric surgery could impact bone metabolism and induce significant changes, such as decreased mechanical loading, calcium/vitamin D malabsorption with secondary hyperparathyroidism, nutritional deprivations, changes in fat mass and alterations in fat- and gut-derived hormones[25-27].
In general, weight loss, achieved through dietary restriction, drugs or bariatric surgery, is associated with a significant reduction in bone mineral density (BMD) and increased bone turnover[28]. In particular, the bone loss reported after non-surgical weight loss is much lower (1%-2%)[29] than that found after bariatric procedures (8%-13%)[30,31] A recent meta-analysis of studies that compare bariatric vs a non-operated control group showed reduced BMD at the femoral neck but not at the lumbar spine[30]. However, it is important to note that the measurement error at the spine BMD is greater than at other sites, which could likely account for this discrepancy. In addition, there is high heterogeneity in the studies analyzed with regard to different surgery procedures, study design (most retrospective), and patient characteristics (ethnicity, sex, menopausal/postmenopausal stage, follow-up length), which could account for the differences between the two sites. Overall, the reductions in the BMD results are greater after malabsorptive or mixed than after restrictive procedures. Studies that compare RYGB and SG have shown a greater bone loss after RYBG than SG, especially at the hip and femoral neck[32]. Accordingly, bone turnover expressed by circulating markers such as CTX, PINP, TRAcP5b was significantly higher after RYGB than after SG[33]. The difference in the BMD between the two procedures could also be related to the different hormonal patterns induced by the two operations. Indeed, there is increasing evidence that many fat- and gut-derived hormones could affect bone health[25,33,34]. In particular, low levels of GIP, ghrelin, amylin, and insulin and high levels of PYY exert negative effects on the bone mass. In contrast, low serotonin and high GLP-1 levels appear to positively influence the bone metabolism[25]. However, further studies are needed to better define the role of these hormones in the regulation of bone metabolism.
Bariatric surgery is associated with an increased risk of fractures[35,36]. In a population-based study, the cumulative incidence of any new fracture at 15 years was 58% in bariatric patients compared to 24% in non-operated men and women of similar age. The relative risk for any fracture was increased by 2.3-fold both at the traditional osteoporotic (hip, spine, wrist) and at non-osteoporotic sites[35].
Calcium and vitamin D deficiencies are the main factors that are responsible for the accelerated bone loss after bariatric surgery. The incidence of calcium deficiency after surgery is almost 10%[37] and is caused by reduced calcium absorption that results from bypassing the duodenum and proximal jejunum, which are the main sites of absorption. In some cases, calcium deficiency could be exacerbated by low calcium intake due to the intolerance/exclusion of milk products.
The prevalence of hypovitaminosis D after surgery varies between 25% and 73%, depending on the duration of the follow-up and its defining parameters (25-OH-vitamin D < 20 or < 30 ng/mL). It is important to note that hypovitaminosis D exists in a large proportion of patients prior to surgery, with reports that range from 25% to 80%. However, bariatric surgery per se affects the vitamin D status[38]. Indeed, similar to calcium deficiency, hypovitaminosis D could be a consequence of fat malabsorption, due to the bypass of the primary absorption sites of liposoluble vitamins in the small intestine[39,40]. In fact, a duodenal surgical bypass decreases cholecystokinin secretion, which results in a reduction in pancreatic lipolytic enzyme secretions and alteration in biliary salts, which in turn leads to an alteration in fat digestion and steatorrhea[24]. In addition, after both malabsorptive and restrictive procedures, reduced intake of dairy products, vomiting, and non-adherence to supplement recommendations could worsen the vitamin D status[39,40].
These are no clear recommendations for vitamin D doses following bariatric surgery, since individual patients could require larger or smaller doses according to the degree of deficit. Current recommendations[21] indicate that at least 5000 IU/d is required to maintain adequate vitamin D levels after RYGB, while higher doses (up to 50000 IU) are required after BPD. Recent studies have suggested that the vitamin D level should be maintained at over 25-30 ng/mL for the effective prevention of osteoporosis and fracture risk. Daily calcium supplementation (preferably as calcium citrate) from 1200 to 2000 mg daily is recommended. It must be considered that oral calcium could interfere with the absorption of some essential minerals such as iron, zinc and copper.
Deficiencies of other vitamins and minerals
Low serum levels of fat-soluble vitamins (vitamin A, K and E) have been found to occur after malabsorptive procedures (BPD and long limb RYGB). However, the available data are largely based on clinical reports and, therefore, are insufficient to estimate the real prevalence of these deficiencies. In two series of studies, the incidence of vitamin A deficiency was 61%-69% at 2-4 year after BPD, with or without duodenal switch[41,42]. In a third series, the incidence was as low as 5% by 4 year[43]. Clinical manifestation of vitamin A deficits are night blindness, xerophthalmia and dry hair.
Low levels of vitamin K have been reported in 50%-60%[42] of patients who underwent BPD or BPD/DS, but no clinical symptoms such as easy bruising, increased bleeding, or clotting alterations were reported.
With regard to the water-soluble vitamins, thiamine (vitamin B1) deficiency can occur in up to 49% of patients after surgery as a result of bypass of the jejunum, where it is primarily absorbed, or in the presence of impaired nutritional intake from persistent, severe vomiting[44]. The early symptoms of thiamine deficiency are nausea and constipation, followed by neurological and psychiatric complications known as Wernicke-Korsakoff syndrome. The prevalence of vitamin C deficiency ranges from 10%-50%[45,46], but it rarely results in manifest clinical signs (poor wound healing, petechiae, bleeding gums).
Although most of the literature focuses on calcium and iron, deficiencies of other essential minerals such as magnesium, zinc, copper, and selenium have been reported in bariatric patients[47]. Essential minerals act as enzymatic cofactors in several biochemical pathways, and therefore, their deficiency could cause variable clinical manifestations that involve neurological, cardiac and gastrointestinal systems. Mineral deficiencies are more common after BPD and RYGB; however, the real prevalence of these disturbances cannot be precisely estimated since most deficiencies can be present already before surgery (see the next paragraph). In addition, for some minerals such as copper and magnesium, the circulating concentrations might not reflect their total body stores, thus leading to underestimation of the real deficit.
Protein malnutrition
Protein malnutrition remains the most severe macronutrient complication associated with malabsorptive surgical procedures. It has been reported in 7%-21% of patients who underwent BPD and is a consequence of poor protein digestion and absorption secondary to altered biliary and pancreatic function[48]. Protein malnutrition can also occur after RYGB, where the Roux limb exceeds 150 cm, with an incidence of 13% at the 2-year follow-up. SG and AGB can lead to protein malnutrition in patients who present maladaptive eating behaviors after surgery, those who avoid protein food sources and those who have protracted vomiting. The clinical signs of protein malnutrition include edema, hearing loss and low serum albumin level (< 3.5 g/dL). Protein malnutrition associated with malabsorptive procedures causes an annual hospitalization rate of 1% per year and leads to significant morbidity and poor outcomes[49,50]. Monitoring the serum albumin concentration is useful for the evaluation of the protein nutritional state, although the serum protein level often remains in the normal range until late. Measurement of lean body mass by means of dual X–ray absorptiometry or body bioimpedence assessment can be helpful for the evaluation of body composition, although their accuracy appears to be limited in bariatric patients.
According to consensus guidelines[21], the prevention of protein malnutrition requires an average daily protein intake of 60-120 g (1.1 g/kg of ideal body weight), which should be increased by 30% following BPD. Furthermore, great emphasis is posed on regular training and aerobic exercise as being essential to preserving lean mass and especially muscle mass. Patients with severe protein malnutrition should be managed with modular protein supplements that are rich in branch-chain amino acids and, eventually, enteral feeding.
Post-operative weight regain
The regain of the weight lost is one of the main concerns of bariatric patients over the long term. The incidence of this phenomenon is quite variable according to the type of procedure performed, the length of follow-up and, above all, the criteria to define weight regain. Among different definitions, the most widely accepted method refers to a regain of 25%-30% of the maximum weight lost, corresponding to the weight before surgery, with the subtraction of minimum weight or “nadir” after surgery[51-53]. A recent review has shown that the rates of weight regain for SG range from 5.7% at 2 years to 75.6% at 6 years[54]. For RYGB, the percentage of failure to maintain weight loss varies from 7% to 50% of the subjects and tends to be higher in superobese patients[55]. AGB is associated with the largest weight regain (35%-40% of the weight lost), as evidenced in several clinical studies[11,51].
The failure to maintain long-term weight loss has important consequences on the patients’ health, including the relapse of obesity-related co-morbidities[56]. Furthermore, it has substantial economic repercussions for the recurrent costs associated with the management of on-going obesity. Therefore, there have been many efforts to understand the biological and psychologic/behavioral bases that underlie this important phenomenon.
One of the major factors responsible for weight regain is the reduction in energy expenditure (EE), which is generally paralleled by the simultaneous loss of lean body mass[57]. Recently, Tam et al.[57] showed that EE is significantly reduced 1 year after RYGB (-124 ± 42 kcal/die) as well as after SG (-155 ± 118 kcal/die) compared to the baseline. These findings extend what was already known with diet-induced weight loss and give support to the view that the reduction in EE is a homeostatic mechanism that counteracts a reduction in the caloric intake, which is aimed at preventing excessive weight loss; however, in some conditions, it could favor weight regain.
Another factor that contributes to weight regain is the changes in entero-hormone and appetite regulation[56]. As widely demonstrated, BS is associated with a recovery of the postprandial response of GLP-1, which increases by 3- to 6-fold compared to pre-surgery levels[58]. Interestingly, it has been shown[52] that in patients operated by RYGB, the post-meal response of GLP1 was significantly greater in individuals who maintained weight loss compared to individuals who failed, which suggests that this hormone plays a role in the maintenance of a favorable weight outcome. With regard to ghrelin, the results are quite controversial, with some but not all[59] studies showing greater and more sustained suppression of ghrelin levels in bariatric patients who maintained appropriate weight loss compared to those who regained weight[60,61].
Moreover, mental health disorders, such as depression, alcohol and drug use, and food urges are predictive factors of weight regain[62,63]. Although binge eating is more frequent among obese patients who make recourse to BS (10%-50%), there is no doubt that its persistence after surgery is associated with a minor weight loss and an early weight regain[64].
Beyond all of the above-mentioned factors, the success of bariatric surgery is strongly influenced by the patients’ motivation to adhere to a healthier lifestyle, including controlled energy intake and physical activity[65]. In the Swedish Obese Subjects study[66], the reported mean energy intake was 2900 kcal/die before surgery, 1500 kcal/die 6 mo after surgery and approximately 2000 kcal/die 4-10 years after surgery, which demonstrates a progressive increase in calorie intake over the years. These data emphasize dietary counselling and the practice of physical exercise as fundamental measures to prevent weight recidivism.
PRE-OPERATIVE NUTRITIONAL STATE: A CRITICAL FACTOR
It is a common belief that nutritional deficiencies are rare in Western countries due to the availability of low cost and unlimited variety of food supply. However, obese subjects often adopt an unhealthy diet that is rich in high-calorie food with an unbalanced nutritional composition[67,68]. The concomitant presence of high calorie intake and nutrient deficiencies could impact the effectiveness of calorie utilization, which could determine a vicious cycle that leads to further weight gain, depression, eating disorders, metabolic syndrome, fatigue and more[67]. In support of these concepts, a growing number of studies in the literature attest to the frequent occurrence of nutrient and/or vitamin/mineral deficiencies in morbidly obese individuals prior to bariatric surgery, before weight loss and possible surgical-related malabsorption set in.
With regard to the vitamin status, most evidence refers to a 25(OH)vitamin D deficit. Vitamin D insufficiency (< 30 ng/dL) has been reported in approximately 90% of different study populations, and ranges from 65%[69] to 100%[70], while vitamin D deficiency (< 20 ng/dL) is observed in approximately 60% of the patients, ranging from 22%[71] to 83%[72]. The prevalence of severe deficit (< 10 ng/dL) could reach 25%[73]. The degree of deficiency is predicted by the degree of obesity and race, with African Americans being at higher risk[74].
Obese individuals are more likely to be deficient in vitamin D because of the higher volumetric dilution and sequestration of this fat-soluble hormone in the adipose tissue[75]. As the fat mass increases, an individual will require greater amounts of vitamin D (via photoproduction from sun exposure, dietary intake, and/or supplementation). Moreover, although there is no difference in the vitamin D3 production between obese and lean individuals, obese patients show an impaired release of vitamin D3 from the skin[76]. Genetic variation in the function of the vitamin D binding protein and vitamin D receptor could also influence the 25(OH)D levels, with some studies suggesting a higher frequency of the poorer functioning forms in obesity[77,78].
The prevalence of vitamin B12 deficiency in patients scheduled for BS is reported in approximately 18% of patients. Similarly, low levels of vitamin B1 (thiamine) are reported in up to 20% of bariatric candidates. Few studies have assessed the vitamin C status in bariatric candidates, with a prevalence that ranges from 15%[69] to 33%[79]. With regard to vitamins A and E, their deficiencies are less frequent[69,73]. In particular, vitamin A has been found to be inversely associated with BMI, age and number of comorbidities[73]. This finding most likely occurs because low vitamin A levels are related to increased oxidative stress, insulin resistance, impaired glucose metabolism, cancers, and age-related macular degeneration[80], all of which are commonly associated with morbid obesity.
Among the minerals, iron deficiency is the most common and ranges from 20% to 47%[81]. Iron and ferritin deficiency and iron-deficiency anemia are more frequent in younger patients (< 25 years) than in older patients and in women than in men, although this finding is not confirmed in all studies[82]. Iron deficiency in obese patients is likely related to the negative impact that chronic inflammation exerts on iron homeostasis. In particular, there is evidence that cytokines (TNFα and IFNγ) can induce the apoptosis of erythroid progenitor cells and increase hepcidin levels, which leads in turn, to reduced intestinal iron absorption and reduced bioavailability[83].
The prevalence of zinc deficiency prior to bariatric surgery amounts to 10.2%[84-86]. Interestingly, some studies have shown an inverse association of zinc levels, with C-reactive protein highlighting the adverse influence of systemic low-grade inflammation on the zinc status[84].
Overall, the high prevalence of pre-surgery nutritional deficiencies in bariatric candidates supports the need for a careful pre-operative evaluation of the nutritional status, to assess and adequately correct the pre-existing deficits.
CONCLUSION
Nutritional deficiencies represent a relevant long-term clinical problem in patients who underwent bariatric surgery as a result of modifications to the gastrointestinal anatomy and physiology, which could impact macro- and micro-nutrient absorption. Therefore, the best practices guidelines[21] highly recommend regular metabolic and nutritional monitoring after bariatric surgery, which frequency varies according to the type of procedure. In light of the high prevalence of nutrient deficiencies even prior to surgery, the current Guidelines also underscore the need for a complete pre-surgery nutritional assessment in all candidates for bariatric surgery. The schedule of the biochemical and nutritional monitoring for the different procedures is reported in Table 1. Although there are few studies with long-term nutritional follow-up, there is general agreement that nutritional assessments should be performed throughout life; furthermore, multivitamin and calcium supplementation with added vitamin D is recommended for all weight-loss surgery patients. In conclusion, nutritional surveillance is an essential component of the management of bariatric patients for the following reasons: (1) increases the patients’ adherence to healthy dietary habits and appropriate supplementation regimens; (2) prevents the risk of weight regain; (3) facilitates the detection of possible nutritional deficiencies that could develop despite medical therapy; and (4) contributes to maintaining a good quality of life.
Table 1.
Schedule of biochemical and nutritional assessments for the different bariatric procedures
| Assessments | Pre-operative | 1 mo | 3 mo | 6 mo | 12 mo | 18 mo | 24 mo | Annually |
| MOC DEXA | AGB, SG, RYGB, BPD1 | AGB3, SG, RYGB, BPD1 | ||||||
| Calcium | AGB, SG, RYGB, BPD2 | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 |
| Magnesium | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 | RYGB, BPD1 | RYGB, BPD1 | RYGB, BPD1 | ||
| Phosphorus | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 | ||||
| Zinc | AGB, SG, RYGB, BPD2 | RYGB, BPD1 | RYGB, BPD2 | AGB, SG, RYGB, BPD2 | AGB, SG, RYGB, BPD2 | AGB, SG, RYGB, BPD2 | ||
| Iron | AGB, SG, RYGB, BPD2 | RYGB, BPD1 | RYGB, BPD1 | AGB, SG, RYGB, BPD2 | RYGB, BPD1 | AGB, SG, RYGB, BPD2 | AGB, SG, RYGB, BPD2 | |
| Transferrin | AGB, SG, RYGB, BPD2 | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 | ||
| Ferritin | AGB, SG, RYGB, BPD2 | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 | ||
| Vitamin A | AGB, SG, RYGB, BPD2 | RYGB, BPD1 | RYGB, BPD1 | RYGB, BPD1 | RYGB, BPD1 | RYGB, BPD1 | ||
| Vitamin E | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 | ||||||
| Vitamin D | AGB, SG, RYGB, BPD2 | RYGB, BPD2 | RYGB, BPD2 | AGB, SG, RYGB, BPD2 | AGB, SG, RYGB, BPD2 | AGB, SG, RYGB, BPD2 | ||
| Vitamin B1 | AGB, SG, RYGB, BPD2 | AGB, SG, RYGB, BPD2 | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD1 | ||
| Vitamin B6 | AGB, SG, RYGB, BPD2 | AGB, SG, RYGB, BPD1 | AGB3, SG3, RYGB3, BPD1, 3 | |||||
| Vitamin B12 | AGB, SG, RYGB, BPD1 | AGB, SG, RYGB, BPD2 | AGB, SG, RYGB, BPD2 | AGB, SG, RYGB, BPD2 | AGB, SG, RYGB, BPD2 | AGB, SG, RYGB, BPD2 | ||
| Parathormone | AGB, SG, RYGB, BPD2 | AGB, SG, RYGB, BPD2 | AGB, SG, RYGB, BPD2 | AGB, SG, RYGB, BPD2 | AGB, SG, RYGB, BPD2 |
Useful, including all contents in the space;
Recommended, including all contents in the space;
Every 2-5 years. AGB: Laparoscopic adjustable gastric banding; SG: Sleeve gastrectomy; RYGB: Roux-en-Y gastric bypass; BPD: Biliopancreatic diversion.
Footnotes
Manuscript Source: Invited Manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare that there were no conflicts of interest.
Peer-review started: June 13, 2017
First decision: July 11, 2017
Article in press: September 5, 2017
P- Reviewer: Klimontov V, Panchu P, Saisho Y S- Editor: Ji FF L- Editor: A E- Editor: Zhao LM
Contributor Information
Roberta Lupoli, Department of Clinical Medicine and Surgery, Federico II University, 80131 Naples, Italy.
Erminia Lembo, Department of Clinical Medicine and Surgery, Federico II University, 80131 Naples, Italy.
Gennaro Saldalamacchia, Department of Clinical Medicine and Surgery, Federico II University, 80131 Naples, Italy.
Claudia Kesia Avola, Department of Clinical Medicine and Surgery, Federico II University, 80131 Naples, Italy.
Luigi Angrisani, Department of Public Health, Federico II University, 80131 Naples, Italy.
Brunella Capaldo, Department of Clinical Medicine and Surgery, Federico II University, 80131 Naples, Italy.
References
- 1.Bray GA, Frühbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet. 2016;387:1947–1956. doi: 10.1016/S0140-6736(16)00271-3. [DOI] [PubMed] [Google Scholar]
- 2.Center for Disease Control and Prevention. Overweight and obesity. Accessed Jan 7. 2015. Available from: https://www.cdc.gov/obesity/index.html. [Google Scholar]
- 3.Ludwig DS, Ebbeling CB. Weight-loss maintenance--mind over matter? N Engl J Med. 2010;363:2159–2161. doi: 10.1056/NEJMe1011361. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe BM, Kvach E, Eckel RH. Treatment of Obesity: Weight Loss and Bariatric Surgery. Circ Res. 2016;118:1844–1855. doi: 10.1161/CIRCRESAHA.116.307591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams TD, Davidson LE, Litwin SE, Kolotkin RL, LaMonte MJ, Pendleton RC, Strong MB, Vinik R, Wanner NA, Hopkins PN, et al. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012;308:1122–1131. doi: 10.1001/2012.jama.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caiazzo R, Lassailly G, Leteurtre E, Baud G, Verkindt H, Raverdy V, Buob D, Pigeyre M, Mathurin P, Pattou F. Roux-en-Y gastric bypass versus adjustable gastric banding to reduce nonalcoholic fatty liver disease: a 5-year controlled longitudinal study. Ann Surg. 2014;260:893–898; discussion 898-899. doi: 10.1097/SLA.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 7.Ashrafian H, Toma T, Rowland SP, Harling L, Tan A, Efthimiou E, Darzi A, Athanasiou T. Bariatric Surgery or Non-Surgical Weight Loss for Obstructive Sleep Apnoea? A Systematic Review and Comparison of Meta-analyses. Obes Surg. 2015;25:1239–1250. doi: 10.1007/s11695-014-1533-2. [DOI] [PubMed] [Google Scholar]
- 8.Cutolo PP, Nosso G, Vitolo G, Brancato V, Capaldo B, Angrisani L. Clinical efficacy of laparoscopic sleeve gastrectomy vs laparoscopic gastric bypass in obese type 2 diabetic patients: a retrospective comparison. Obes Surg. 2012;22:1535–1539. doi: 10.1007/s11695-012-0657-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotugno M, Nosso G, Saldalamacchia G, Vitagliano G, Griffo E, Lupoli R, Angrisani L, Riccardi G, Capaldo B. Clinical efficacy of bariatric surgery versus liraglutide in patients with type 2 diabetes and severe obesity: a 12-month retrospective evaluation. Acta Diabetol. 2015;52:331–336. doi: 10.1007/s00592-014-0644-5. [DOI] [PubMed] [Google Scholar]
- 10.Schauer PR, Mingrone G, Ikramuddin S, Wolfe B. Clinical Outcomes of Metabolic Surgery: Efficacy of Glycemic Control, Weight Loss, and Remission of Diabetes. Diabetes Care. 2016;39:902–911. doi: 10.2337/dc16-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 12.Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, Wedel H, Ahlin S, Anveden Å, Bengtsson C, Bergmark G, Bouchard C, Carlsson B, Dahlgren S, Karlsson J, Lindroos AK, Lönroth H, Narbro K, Näslund I, Olbers T, Svensson PA, Carlsson LM. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 13.Kwok CS, Pradhan A, Khan MA, Anderson SG, Keavney BD, Myint PK, Mamas MA, Loke YK. Bariatric surgery and its impact on cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Cardiol. 2014;173:20–28. doi: 10.1016/j.ijcard.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 14.Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Flum DR, Belle SH, King WC, Wahed AS, Berk P, Chapman W, Pories W, Courcoulas A, McCloskey C, Mitchell J, Patterson E, Pomp A, Staten MA, Yanovski SZ, Thirlby R, Wolfe B. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361:445–454. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miras AD, le Roux CW. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2013;10:575–584. doi: 10.1038/nrgastro.2013.119. [DOI] [PubMed] [Google Scholar]
- 16.Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric Surgery Worldwide 2013. Obes Surg. 2015;25:1822–1832. doi: 10.1007/s11695-015-1657-z. [DOI] [PubMed] [Google Scholar]
- 17.American Society of Hematology. Iron-deficiency anemia. Available from: http://www.hematology.org/patients/blood-disorders/anemia/5263.aspx.
- 18.Weng TC, Chang CH, Dong YH, Chang YC, Chuang LM. Anaemia and related nutrient deficiencies after Roux-en-Y gastric bypass surgery: a systematic review and meta-analysis. BMJ Open. 2015;5:e006964. doi: 10.1136/bmjopen-2014-006964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knight T, D’Sylva L, Moore B, Barish CF. Burden of Iron Deficiency Anemia in a Bariatric Surgery Population in the United States. J Manag Care Spec Pharm. 2015;21:946–954. doi: 10.18553/jmcp.2015.21.10.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexandrou A, Armeni E, Kouskouni E, Tsoka E, Diamantis T, Lambrinoudaki I. Cross-sectional long-term micronutrient deficiencies after sleeve gastrectomy versus Roux-en-Y gastric bypass: a pilot study. Surg Obes Relat Dis. 2014;10:262–268. doi: 10.1016/j.soard.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Mechanick JI, Youdim A, Jones DB, Garvey WT, Hurley DL, McMahon MM, Heinberg LJ, Kushner R, Adams TD, Shikora S, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient--2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity (Silver Spring) 2013;21 Suppl 1:S1–27. doi: 10.1002/oby.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blume CA, Boni CC, Casagrande DS, Rizzolli J, Padoin AV, Mottin CC. Nutritional profile of patients before and after Roux-en-Y gastric bypass: 3-year follow-up. Obes Surg. 2012;22:1676–1685. doi: 10.1007/s11695-012-0696-y. [DOI] [PubMed] [Google Scholar]
- 23.von Drygalski A, Andris DA, Nuttleman PR, Jackson S, Klein J, Wallace JR. Anemia after bariatric surgery cannot be explained by iron deficiency alone: results of a large cohort study. Surg Obes Relat Dis. 2011;7:151–156. doi: 10.1016/j.soard.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition. 2010;26:1031–1037. doi: 10.1016/j.nut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Hage MP, El-Hajj Fuleihan G. Bone and mineral metabolism in patients undergoing Roux-en-Y gastric bypass. Osteoporos Int. 2014;25:423–439. doi: 10.1007/s00198-013-2480-9. [DOI] [PubMed] [Google Scholar]
- 26.Scibora LM. Skeletal effects of bariatric surgery: examining bone loss, potential mechanisms and clinical relevance. Diabetes Obes Metab. 2014;16:1204–1213. doi: 10.1111/dom.12363. [DOI] [PubMed] [Google Scholar]
- 27.Costa TM, Paganoto M, Radominski RB, Borba VZ. Impact of deficient nutrition in bone mass after bariatric surgery. Arq Bras Cir Dig. 2016;29:38–42. doi: 10.1590/0102-6720201600010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harper C, Pattinson AL, Fernando HA, Zibellini J, Seimon RV, Sainsbury A. Effects of obesity treatments on bone mineral density, bone turnover and fracture risk in adults with overweight or obesity. Horm Mol Biol Clin Investig. 2016;28:133–149. doi: 10.1515/hmbci-2016-0025. [DOI] [PubMed] [Google Scholar]
- 29.Shapses SA, Riedt CS. Bone, body weight, and weight reduction: what are the concerns? J Nutr. 2006;136:1453–1456. doi: 10.1093/jn/136.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ko BJ, Myung SK, Cho KH, Park YG, Kim SG, Kim do H, Kim SM. Relationship Between Bariatric Surgery and Bone Mineral Density: a Meta-analysis. Obes Surg. 2016;26:1414–1421. doi: 10.1007/s11695-015-1928-8. [DOI] [PubMed] [Google Scholar]
- 31.Liu C, Wu D, Zhang JF, Xu D, Xu WF, Chen Y, Liu BY, Li P, Li L. Changes in Bone Metabolism in Morbidly Obese Patients After Bariatric Surgery: A Meta-Analysis. Obes Surg. 2016;26:91–97. doi: 10.1007/s11695-015-1724-5. [DOI] [PubMed] [Google Scholar]
- 32.Bredella MA, Greenblatt LB, Eajazi A, Torriani M, Yu EW. Effects of Roux-en-Y gastric bypass and sleeve gastrectomy on bone mineral density and marrow adipose tissue. Bone. 2017;95:85–90. doi: 10.1016/j.bone.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivaska KK, Huovinen V, Soinio M, Hannukainen JC, Saunavaara V, Salminen P, Helmiö M, Parkkola R, Nuutila P, Kiviranta R. Changes in bone metabolism after bariatric surgery by gastric bypass or sleeve gastrectomy. Bone. 2017;95:47–54. doi: 10.1016/j.bone.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Folli F, Sabowitz BN, Schwesinger W, Fanti P, Guardado-Mendoza R, Muscogiuri G. Bariatric surgery and bone disease: from clinical perspective to molecular insights. Int J Obes (Lond) 2012;36:1373–1379. doi: 10.1038/ijo.2012.115. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura KM, Haglind EG, Clowes JA, Achenbach SJ, Atkinson EJ, Melton LJ 3rd, Kennel KA. Fracture risk following bariatric surgery: a population-based study. Osteoporos Int. 2014;25:151–158. doi: 10.1007/s00198-013-2463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dix CF, Bauer JD, Wright OR. A Systematic Review: Vitamin D Status and Sleeve Gastrectomy. Obes Surg. 2017;27:215–225. doi: 10.1007/s11695-016-2436-1. [DOI] [PubMed] [Google Scholar]
- 37.Shah M, Sharma A, Wermers RA, Kennel KA, Kellogg TA, Mundi MS. Hypocalcemia After Bariatric Surgery: Prevalence and Associated Risk Factors. Obes Surg. 2017;27:2905–2911. doi: 10.1007/s11695-017-2705-7. [DOI] [PubMed] [Google Scholar]
- 38.Chakhtoura MT, Nakhoul NN, Shawwa K, Mantzoros C, El Hajj Fuleihan GA. Hypovitaminosis D in bariatric surgery: A systematic review of observational studies. Metabolism. 2016;65:574–585. doi: 10.1016/j.metabol.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saltzman E, Karl JP. Nutrient deficiencies after gastric bypass surgery. Annu Rev Nutr. 2013;33:183–203. doi: 10.1146/annurev-nutr-071812-161225. [DOI] [PubMed] [Google Scholar]
- 40.Shikora SA, Kim JJ, Tarnoff ME. Nutrition and gastrointestinal complications of bariatric surgery. Nutr Clin Pract. 2007;22:29–40. doi: 10.1177/011542650702200129. [DOI] [PubMed] [Google Scholar]
- 41.Dolan K, Hatzifotis M, Newbury L, Fielding G. A comparison of laparoscopic adjustable gastric banding and biliopancreatic diversion in superobesity. Obes Surg. 2004;14:165–169. doi: 10.1381/096089204322857500. [DOI] [PubMed] [Google Scholar]
- 42.Slater GH, Ren CJ, Siegel N, Williams T, Barr D, Wolfe B, Dolan K, Fielding GA. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg. 2004;8:48–55; discussion 54-55. doi: 10.1016/j.gassur.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 43.Brolin RE, Leung M. Survey of vitamin and mineral supplementation after gastric bypass and biliopancreatic diversion for morbid obesity. Obes Surg. 1999;9:150–154. doi: 10.1381/096089299765553395. [DOI] [PubMed] [Google Scholar]
- 44.Milone M, Di Minno MN, Lupoli R, Maietta P, Bianco P, Pisapia A, Gaudioso D, Taffuri C, Milone F, Musella M. Wernicke encephalopathy in subjects undergoing restrictive weight loss surgery: a systematic review of literature data. Eur Eat Disord Rev. 2014;22:223–229. doi: 10.1002/erv.2292. [DOI] [PubMed] [Google Scholar]
- 45.Clements RH, Katasani VG, Palepu R, Leeth RR, Leath TD, Roy BP, Vickers SM. Incidence of vitamin deficiency after laparoscopic Roux-en-Y gastric bypass in a university hospital setting. Am Surg. 2006;72:1196–1202; discussion 1203-1204. doi: 10.1177/000313480607201209. [DOI] [PubMed] [Google Scholar]
- 46.Riess KP, Farnen JP, Lambert PJ, Mathiason MA, Kothari SN. Ascorbic acid deficiency in bariatric surgical population. Surg Obes Relat Dis. 2009;5:81–86. doi: 10.1016/j.soard.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Stein J, Stier C, Raab H, Weiner R. Review article: The nutritional and pharmacological consequences of obesity surgery. Aliment Pharmacol Ther. 2014;40:582–609. doi: 10.1111/apt.12872. [DOI] [PubMed] [Google Scholar]
- 48.Faintuch J, Matsuda M, Cruz ME, Silva MM, Teivelis MP, Garrido AB Jr, Gama-Rodrigues JJ. Severe protein-calorie malnutrition after bariatric procedures. Obes Surg. 2004;14:175–181. doi: 10.1381/096089204322857528. [DOI] [PubMed] [Google Scholar]
- 49.Stocker DJ. Management of the bariatric surgery patient. Endocrinol Metab Clin North Am. 2003;32:437–457. doi: 10.1016/s0889-8529(03)00002-1. [DOI] [PubMed] [Google Scholar]
- 50.Skroubis G, Sakellaropoulos G, Pouggouras K, Mead N, Nikiforidis G, Kalfarentzos F. Comparison of nutritional deficiencies after Roux-en-Y gastric bypass and after biliopancreatic diversion with Roux-en-Y gastric bypass. Obes Surg. 2002;12:551–558. doi: 10.1381/096089202762252334. [DOI] [PubMed] [Google Scholar]
- 51.DiGiorgi M. Factors Associated With Long Term Weight Regain After Bariatric Surgery. Columbia University. 2012. Available from: https://academiccommons.columbia.edu/catalog/ac:174582. [Google Scholar]
- 52.Santo MA, Riccioppo D, Pajecki D, Kawamoto F, de Cleva R, Antonangelo L, Marçal L, Cecconello I. Weight Regain After Gastric Bypass: Influence of Gut Hormones. Obes Surg. 2016;26:919–925. doi: 10.1007/s11695-015-1908-z. [DOI] [PubMed] [Google Scholar]
- 53.Alvarez V, Carrasco F, Cuevas A, Valenzuela B, Muñoz G, Ghiardo D, Burr M, Lehmann Y, Leiva MJ, Berry M, et al. Mechanisms of long-term weight regain in patients undergoing sleeve gastrectomy. Nutrition. 2016;32:303–308. doi: 10.1016/j.nut.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 54.Lauti M, Kularatna M, Hill AG, MacCormick AD. Weight Regain Following Sleeve Gastrectomy-a Systematic Review. Obes Surg. 2016;26:1326–1334. doi: 10.1007/s11695-016-2152-x. [DOI] [PubMed] [Google Scholar]
- 55.Magro DO, Geloneze B, Delfini R, Pareja BC, Callejas F, Pareja JC. Long-term weight regain after gastric bypass: a 5-year prospective study. Obes Surg. 2008;18:648–651. [Google Scholar]
- 56.Karmali S, Brar B, Shi X, Sharma AM, de Gara C, Birch DW. Weight recidivism post-bariatric surgery: a systematic review. Obes Surg. 2013;23:1922–1933. doi: 10.1007/s11695-013-1070-4. [DOI] [PubMed] [Google Scholar]
- 57.Tam CS, Redman LM, Greenway F, LeBlanc KA, Haussmann MG, Ravussin E. Energy Metabolic Adaptation and Cardiometabolic Improvements One Year After Gastric Bypass, Sleeve Gastrectomy, and Gastric Band. J Clin Endocrinol Metab. 2016;101:3755–3764. doi: 10.1210/jc.2016-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nosso G, Griffo E, Cotugno M, Saldalamacchia G, Lupoli R, Pacini G, Riccardi G, Angrisani L, Capaldo B. Comparative Effects of Roux-en-Y Gastric Bypass and Sleeve Gastrectomy on Glucose Homeostasis and Incretin Hormones in Obese Type 2 Diabetic Patients: A One-Year Prospective Study. Horm Metab Res. 2016;48:312–317. doi: 10.1055/s-0041-111505. [DOI] [PubMed] [Google Scholar]
- 59.Abu Dayyeh BK, Jirapinyo P, Thompson CC. Plasma Ghrelin Levels and Weight Regain After Roux-en-Y Gastric Bypass Surgery. Obes Surg. 2017;27:1031–1036. doi: 10.1007/s11695-016-2418-3. [DOI] [PubMed] [Google Scholar]
- 60.Dirksen C, Jørgensen NB, Bojsen-Møller KN, Kielgast U, Jacobsen SH, Clausen TR, Worm D, Hartmann B, Rehfeld JF, Damgaard M, et al. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. Int J Obes (Lond) 2013;37:1452–1459. doi: 10.1038/ijo.2013.15. [DOI] [PubMed] [Google Scholar]
- 61.Bohdjalian A, Langer FB, Shakeri-Leidenmühler S, Gfrerer L, Ludvik B, Zacherl J, Prager G. Sleeve gastrectomy as sole and definitive bariatric procedure: 5-year results for weight loss and ghrelin. Obes Surg. 2010;20:535–540. doi: 10.1007/s11695-009-0066-6. [DOI] [PubMed] [Google Scholar]
- 62.Rutledge T, Groesz LM, Savu M. Psychiatric factors and weight loss patterns following gastric bypass surgery in a veteran population. Obes Surg. 2011;21:29–35. doi: 10.1007/s11695-009-9923-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Odom J, Zalesin KC, Washington TL, Miller WW, Hakmeh B, Zaremba DL, Altattan M, Balasubramaniam M, Gibbs DS, Krause KR, et al. Behavioral predictors of weight regain after bariatric surgery. Obes Surg. 2010;20:349–356. doi: 10.1007/s11695-009-9895-6. [DOI] [PubMed] [Google Scholar]
- 64.Marcus MD, Kalarchian MA, Courcoulas AP. Psychiatric evaluation and follow-up of bariatric surgery patients. Am J Psychiatry. 2009;166:285–291. doi: 10.1176/appi.ajp.2008.08091327. [DOI] [PubMed] [Google Scholar]
- 65.Sarwer DB, Wadden TA, Fabricatore AN. Psychosocial and behavioral aspects of bariatric surgery. Obes Res. 2005;13:639–648. doi: 10.1038/oby.2005.71. [DOI] [PubMed] [Google Scholar]
- 66.Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 67.Kaidar-Person O, Person B, Szomstein S, Rosenthal RJ. Nutritional deficiencies in morbidly obese patients: a new form of malnutrition? Part A: vitamins. Obes Surg. 2008;18:870–876. doi: 10.1007/s11695-007-9349-y. [DOI] [PubMed] [Google Scholar]
- 68.Kaidar-Person O, Person B, Szomstein S, Rosenthal RJ. Nutritional deficiencies in morbidly obese patients: a new form of malnutrition? Part B: minerals. Obes Surg. 2008;18:1028–1034. doi: 10.1007/s11695-007-9350-5. [DOI] [PubMed] [Google Scholar]
- 69.Schiavo L, Scalera G, Pilone V, De Sena G, Capuozzo V, Barbarisi A. Micronutrient Deficiencies in Patients Candidate for Bariatric Surgery: A Prospective, Preoperative Trial of Screening, Diagnosis, and Treatment. Int J Vitam Nutr Res. 2016;10:1–8. doi: 10.1024/0300-9831/a000282. [DOI] [PubMed] [Google Scholar]
- 70.Wang C, Guan B, Yang W, Yang J, Cao G, Lee S. Prevalence of electrolyte and nutritional deficiencies in Chinese bariatric surgery candidates. Surg Obes Relat Dis. 2016;12:629–634. doi: 10.1016/j.soard.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 71.Dagan SS, Zelber-Sagi S, Webb M, Keidar A, Raziel A, Sakran N, Goitein D, Shibolet O. Nutritional Status Prior to Laparoscopic Sleeve Gastrectomy Surgery. Obes Surg. 2016;26:2119–2126. doi: 10.1007/s11695-016-2064-9. [DOI] [PubMed] [Google Scholar]
- 72.Damms-Machado A, Friedrich A, Kramer KM, Stingel K, Meile T, Küper MA, Königsrainer A, Bischoff SC. Pre- and postoperative nutritional deficiencies in obese patients undergoing laparoscopic sleeve gastrectomy. Obes Surg. 2012;22:881–889. doi: 10.1007/s11695-012-0609-0. [DOI] [PubMed] [Google Scholar]
- 73.Lefebvre P, Letois F, Sultan A, Nocca D, Mura T, Galtier F. Nutrient deficiencies in patients with obesity considering bariatric surgery: a cross-sectional study. Surg Obes Relat Dis. 2014;10:540–546. doi: 10.1016/j.soard.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 74.Stein EM, Strain G, Sinha N, Ortiz D, Pomp A, Dakin G, McMahon DJ, Bockman R, Silverberg SJ. Vitamin D insufficiency prior to bariatric surgery: risk factors and a pilot treatment study. Clin Endocrinol (Oxf) 2009;71:176–183. doi: 10.1111/j.1365-2265.2008.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring) 2012;20:1444–1448. doi: 10.1038/oby.2011.404. [DOI] [PubMed] [Google Scholar]
- 76.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 77.Jiang H, Xiong DH, Guo YF, Shen H, Xiao P, Yang F, Chen Y, Zhang F, Recker RR, Deng HW. Association analysis of vitamin D-binding protein gene polymorphisms with variations of obesity-related traits in Caucasian nuclear families. Int J Obes (Lond) 2007;31:1319–1324. doi: 10.1038/sj.ijo.0803583. [DOI] [PubMed] [Google Scholar]
- 78.Ochs-Balcom HM, Chennamaneni R, Millen AE, Shields PG, Marian C, Trevisan M, Freudenheim JL. Vitamin D receptor gene polymorphisms are associated with adiposity phenotypes. Am J Clin Nutr. 2011;93:5–10. doi: 10.3945/ajcn.2010.29986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wolf E, Utech M, Stehle P, Büsing M, Stoffel-Wagner B, Ellinger S. Preoperative micronutrient status in morbidly obese patients before undergoing bariatric surgery: results of a cross-sectional study. Surg Obes Relat Dis. 2015;11:1157–1163. doi: 10.1016/j.soard.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 80.Facchini FS, Humphreys MH, DoNascimento CA, Abbasi F, Reaven GM. Relation between insulin resistance and plasma concentrations of lipid hydroperoxides, carotenoids, and tocopherols. Am J Clin Nutr. 2000;72:776–779. doi: 10.1093/ajcn/72.3.776. [DOI] [PubMed] [Google Scholar]
- 81.Ben-Porat T, Elazary R, Yuval JB, Wieder A, Khalaileh A, Weiss R. Nutritional deficiencies after sleeve gastrectomy: can they be predicted preoperatively? Surg Obes Relat Dis. 2015;11:1029–1036. doi: 10.1016/j.soard.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 82.Flancbaum L, Belsley S, Drake V, Colarusso T, Tayler E. Preoperative nutritional status of patients undergoing Roux-en-Y gastric bypass for morbid obesity. J Gastrointest Surg. 2006;10:1033–1037. doi: 10.1016/j.gassur.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 83.McClung JP, Karl JP. Iron deficiency and obesity: the contribution of inflammation and diminished iron absorption. Nutr Rev. 2009;67:100–104. doi: 10.1111/j.1753-4887.2008.00145.x. [DOI] [PubMed] [Google Scholar]
- 84.Gerig R, Ernst B, Wilms B, Thurnheer M, Schultes B. Preoperative nutritional deficiencies in severely obese bariatric candidates are not linked to gastric Helicobacter pylori infection. Obes Surg. 2013;23:698–702. doi: 10.1007/s11695-013-0878-2. [DOI] [PubMed] [Google Scholar]
- 85.Gobato RC, Seixas Chaves DF, Chaim EA. Micronutrient and physiologic parameters before and 6 months after RYGB. Surg Obes Relat Dis. 2014;10:944–951. doi: 10.1016/j.soard.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 86.Papamargaritis D, Aasheim ET, Sampson B, le Roux CW. Copper, selenium and zinc levels after bariatric surgery in patients recommended to take multivitamin-mineral supplementation. J Trace Elem Med Biol. 2015;31:167–172. doi: 10.1016/j.jtemb.2014.09.005. [DOI] [PubMed] [Google Scholar]


