Abstract
There is a lack of non-invasive screening modalities to diagnose chronic atrophic gastritis (CAG) and intestinal metaplasia (IM). Thus, the aim of the present study was to determine the sensitivity and specificity of serum pepsinogen I (PGI), PGI:II, the PGI:II ratio and gastrin-17 (G-17) in diagnosing CAG and IM, and the correlations between these serum biomarkers and pre-malignant gastric lesions. A cross-sectional study of 72 patients (82% of the calculated sample size) who underwent oesophageal-gastro-duodenoscopy for dyspepsia was performed in the present study. The mean age of the participants was 56.2±16.2 years. Serum PGI:I, PGI:II, G-17 and Helicobacter pylori antibody levels were measured by enzyme-linked immunosorbent assay. Median levels of PGI:I, PGI:II, the PGI:II ratio and G-17 for were 129.9 µg/l, 10.3 µg/l, 14.7 and 4.4 pmol/l, respectively. Subjects with corpus CAG/IM exhibited a significantly lower PGI:II ratio (7.2) compared with the control group (15.7; P<0.001). Histological CAG and IM correlated well with the serum PGI:II ratio (r=−0.417; P<0.001). The cut-off value of the PGI:II ratio of ≤10.0 demonstrated high sensitivity (83.3%), specificity (77.9%) and area under the receiver operating characteristic curve of 0.902 in detecting the two conditions. However, the sensitivity was particularly low at a ratio of ≤3.0. The serum PGI:II ratio is a sensitive and specific marker to diagnose corpus CAG/IM, but at a high cut-off value. This ratio may potentially be used as an outpatient, non-invasive biomarker for detecting corpus CAG/IM.
Keywords: gastritis, atrophic, metaplasia, pepsinogens, gastrin-17, ELISA
Introduction
Based on the National Cancer Registry Report 2007, gastric cancer was the ninth most common type of cancer in Malaysia and 3.5 cases per 100,000 population were reported (1). The highest percentage of cases were diagnosed at advanced stages (stage III and IV) due to non-specific symptoms and an inadequate screening program. Gastric carcinogenesis is a continuous process from non-atrophic gastritis leading to chronic atrophic gastritis (CAG), to intestinal metaplasia (IM) and dysplasia, and finally to intestinal-type gastric adenocarcinoma (2–4). A prospective 10-year follow-up study in Japan demonstrated that patients with Helicobacter pylori (H. pylori)-induced chronic active gastritis developed atrophic gastritis, which was observed endoscopically and histologically (5). CAG of the antrum and corpus are independent risk factors for gastric cancer, and the risk increases exponentially with the increase in grade and severity of atrophy (6,7). The progression of severe fundal atrophic gastritis to gastric cancer was documented to be ~3% from a total of 654 patients in the Japan (8). Annual surveillance of patients with IM or atrophic gastritis detected a higher percentage of gastric cancer, at 11% (9). A large retrospective study investigating the risk of gastric cancer in patients with pre-malignant gastric lesions has reported that ~6% of severe dysplastic patients developed gastric cancer within 5 years of diagnosis (10).
The utility of serum biomarkers in diagnosing these pre-malignant lesions in the stomach is an attractive alternative to oesophageal-gastro-duodenoscopy (OGD) with histopathological examination of gastric biopsies, which is considered to be the gold standard for diagnosing CAG and IM. This method is non-invasive, involves the use of blood samples and provides consistent results utilizing ELISA.
Serum levels of pepsinogen (PG)I, PGI:II, gastrin-17 (G-17) and the ratio of PGI:I to PGI:II (PGI:II) were investigated as alternative methods to diagnose CAG and IM. PGI:I is produced by chief and mucous neck cells in the gastric fundus mucosa, while G-17 is produced by G cells in the antrum. PGI:II, however, is secreted in the gastric cardia, fundus, antrum and proximal duodenum. The PGI:II ratio is greatly reduced in the corpus CAG due to replacement of chief cells by pyloric glands (6,11), while a low G-17 level was observed in H. pylori-infected patients with antral CAG (12,13). Previous studies, however, yielded conflicting results for serum PG (14–17) and G-17 values as potential biomarkers for CAG (18).
Various studies in different populations demonstrated different levels of serum PGI, PGII and the PGI:II ratio (Table I) (19–24). Serum PG concentrations measured in certain areas of Asia appear to be higher than those in Western countries. For example, the median PGI levels were >90 µg/l in Shandong, China (25), the mean PGI was >100 µg/l in Hunan, China (17) and Yazd, Iran (24), whereas values were usually <70 µg/l in European countries (4,26). However, a Japanese study by Inoue et al (19) involving 200 patients and a Korean study involving 2,558 patients by Lee et al (27) reported a mean serum PGI:I level of <70 µg/l (56.0 and 63.5 µg/l, respectively). Whether these variations in serum PG levels are due to different geographical locations (with ensuing different ethnicities), prevalence of the H. pylori infection, prevalence of CAG and gastric carcinoma or varying dietary patterns remains uncertain and requires further investigation. Furthermore, the cut-off points of serum PGI and PGI:II for the detection of CAG or IM may vary between populations resulting in requirements for validation in the local setting.
Table I.
Mean levels of serum PGI, PGII and the PGI:II ratio from various studies in Asia, America and Europe.
| Author/(Refs), year | Study location | Study population (n) | Mean PGI ± SD (µg/l) | Mean PGII ± SD (µg/l) | Mean PGI:II ratio ± SD |
|---|---|---|---|---|---|
| Inoue et al (19), 1998 | Nagoya, Japan | All subjects (200) | 56.0±2.2 | 19.6±0.8 | 3.2±0.1 |
| Ley et al (20), 2001 | Chiapas, Mexico | All subjects (148) | 95 (26–52)a | 15 (9–22)a | |
| Broutet et al (26), 2003 | 14 European countries | All subjects (381) | 77.4±41.2 | 13.2±9.0 | 6.7 ±2.6 |
| Väänänen et al (21), 2003 | Finland | Non CAG | 160±139 | ||
| Sierra et al (22), 2006 | San Jose, Costa Rica | Non CAG (291) | 53.1 | 18.9 | 3.3 |
| Oishi et al (23), 2006 | Hisayama, Japan | Male, Negative testb | 71.0 | 16.7 | 5.1 |
| Oishi et al (23), 2006 | Hisayama, Japan | Female, Negative testb | 64.0 | 16.3 | 5.1 |
| Mohamadkhani et al (24), 2013 | Ardabil, Northern Iran | All subjects (149) | 102.5±42.6 | 8.1±4.7 | 15.1±7.6 |
| Mohamadkhani et al (24), 2013 | Yazd, Sounthern Iran | All subjects (148) | 111.7±39 | 7.6±4.4 | 19.1±14.8 |
| Lee et al (27), 2014 | Seoul, Korea | All subjects (2,558) | 63.5±50.7 | 22.5±24.3 | 3.6±2.7 |
| Zhang et al (17), 2014 | Hunan, China | Healthy control (282) | 118.4±47.8 | 12.4±5.9 | 11.7±6.2 |
| Zhang et al (17), 2014 | Hunan, China | CAG (20) | 93.6±49.3 | 10.9±4.6 | 11.1±5.8 |
Median with interquartile range.
Negative test for serum PG: Serum PGI >70 µg/l or PGI:II >3.0. PG, pepsinogen; SD, standard deviation; CAG, chronic atrophic gastritis.
To date, little is known about the use of serum PG and gastrin levels in diagnosing CAG and IM in multi-racial nations in the Asia Pacific region. The present study aimed to determine the accuracy of serum PGI, PGII, G-17 and the PGI:II ratio as biomarkers of CAG and IM in Malaysia, as a country with different ethnicities. Measuring these serum biomarkers may facilitate with the early diagnosis of gastric cancer at the curable stage, particularly in patients with multiple risk factors, including the H. pylori infection (28,29), high intake of salted, pickled or smoked food (28,30), family history of gastric cancer (31), blood type A (32) and smoking (33). Thus, the aim of the present study was to determine the sensitivity and specificity of serum PGI, PGII, PGI:II ratio and serum G-17 levels in diagnosing pre-malignant lesions in the stomach, as well as to determine the correlation of these serum biomarkers with CAG and IM in patients with dyspeptic symptoms.
Materials and methods
A cross sectional observational study, involving 72 patients who underwent OGD for dyspepsia, was performed at the Pusat Perubatan Universiti Kebangsaan Malaysia (Kuala Lumpur, Malaysia) from June 2015 to March 2016. The sample size calculation was based upon the equation from power analysis in the diagnostic test. Based upon the study by Dinis-Ribeiro et al (14), the sensitivity and specificity of the serum PGI:II ratio ≤3 in diagnosing extensive IM were 66 and 78%, respectively. Thus, the calculated sample size required to assess the specificity of the serum PGI:II ratio was determined to be 88. The current study population was 82% of the calculated sample size. The present study was approved by the Research and Ethics Committee of the Faculty of Medicine, UKM (approval no. FF-2015-237). Patients enrolled in the current study provided written informed consent.
Patients were recruited according to certain inclusion and exclusion criteria. Exclusion criteria included patients aged <18 years, and those receiving proton pump inhibitor, histamine-2 receptor antagonist, bismuth or non-steroidal anti-inflammatory drugs two weeks prior to blood sampling for serum PGI, serum PGII and serum G-17. Patients who had received triple therapy for the H. pylori infection, partial or total gastrectomy, chemotherapy or radiotherapy involving the gastric field and upper gastrointestinal bleeding during OGD were also excluded. In addition, patients with gastric polyps and known case of gastric lymphoma or gastric carcinoid tumour were excluded. Their medical histories were recorded in a standardized data sheet through review of patients' clinical history and notes.
Endoscopic examination
OGD was performed using fibre optic white light endoscopy (Olympus GIF-Q260J or Fujifilm EG-530WR) and the gastric mucosa was visualized directly by experienced endoscopists. Patients with CAG were graded according to the location of the border between the fundic and pyloric gland regions, as proposed by Kimura and Takemoto (34) and Miike et al (35). If the border is on the lesser curvature of the stomach, it is defined as closed type (C-type)/mild extension of atrophy. Further divisions of C-type are as below: C1, at the angular part of the lesser curvature; C2, in the lower part of the lesser curvature and C3, in the middle part of the lesser curvature. If the atrophic border is located more caudally and not on the lesser curvature, it is defined as an open type (O-type)/severely extended atrophy. Subdivisions of O-type are: O1, all parts of the lesser curvature are pyloric; O2, the stage between O1 and O3; O3, all mucosa of the stomach are non-acid-secreting.
Histopathology examination of gastric mucosa biopsy
Random gastric mucosa biopsies were obtained from the antrum and corpus during OGD. At least two biopsies were obtained from each site in addition to biopsies from endoscopic visible mucosal lesions. The biopsy specimens were processed into paraffin blocks, and then histological sections (<4 mm) were obtained and stained with hematoxylin and eosin (20 min) and periodic acid Schiff reagent (10 min) at room temperature (25–27°C). Mononuclear cell infiltration, neutrophil infiltration, H. pylori, gastric glandular atrophy, and IM were identified and graded according to the principles of the updated Sydney system and the analogue visual scale (36).
Determination of serum PGI, PGII, G-17 and H. pylori antibody (HpAb) level
Fasting blood samples (8 ml) were obtained from subjects by venipuncture for the determination of serum PGI, serum PGII, serum G-17 and HpAb. The blood was placed into a plain tube and allowed to clot (for at least 30 min) at room temperature (25–27°C). After clotting, the serum was separated by centrifugation (4,000 × g) for 10 min at room temperature (25–27°C) and serum samples were stored at −70°C before use.
PGI, PGII, G-17 and HpAb levels in the serum samples were determined using a GastroPanel ELISA kit (cat. no. 601300; Biohit HealthCare, Helsinki, Finland) according to manufacturer's instructions. The absorbance of each serum biomarker was determined at a wavelength of 450 nm using a microplate reader (SpectraMax® Plus384; Molecular Devices, LLC, Sunnyvale, CA, USA). Assay results were then analysed using GastroSoft 1.51b for Excel (Biohit HealthCare) to obtain the serum sample concentrations.
Follow-up of patients
Subjects who were diagnosed with CAG and IM were given follow-up appointments at the outpatient gastroenterology clinic. Subjects who were diagnosed with gastric adenocarcinoma were sent for staging of the disease and referred to an upper gastrointestinal surgeon for further management. Those who exhibited the H. pylori infection received eradication therapy of amoxicillin, clarithromycin and proton pump inhibitors, and were followed up with a urea breath test to ensure successful eradication of the bacteria.
Statistical analysis
Normality tests were performed for all continuous study variables. Due to the small sample size, the data were not normally distributed and were expressed as the median interquartile range (IQR). The optimal serum PGI, PGII, G-17 and PGI:II ratio cut-off point was calculated using the area under the receiver operating characteristic (ROC) curve (AUC). Analysis was performed using SPSS version 23 (IBM Corp., Armonk, NY, USA) and P<0.05 was considered to indicate a statistically significant difference.
Results
Socio-demographic data of study subjects
A total of 72 subjects, including 35 controls without CAG, 34 subjects with CAG and three subjects with gastric adenocarcinoma (based upon endoscopic findings) were recruited. The ages (mean ± standard deviation) of subjects with CAG and gastric tumours were 57.1±16.6 and 64.3±8.5 years, respectively. No statistical differences were identified in age between the disease and control groups (Table II).
Table II.
Socio-demographic data of subjects according to histological findings.
| Endoscopic | ||||
|---|---|---|---|---|
| Variable | Non CAG (n=35) | CAG (n=34) | Gastric tumour (n=3) | Total (n=72) |
| Age, years (mean ± SD) | 54.6±16.3 | 57.1±16.6 | 64.3±8.5 | 56.2±16.2 |
| Sex, n | ||||
| Male | 17 | 14 | 2 | 33 |
| Female | 18 | 20 | 1 | 39 |
| Ethnicity, n | ||||
| Malay | 16 | 13 | 1 | 30 |
| Chinese | 17 | 18 | 1 | 36 |
| Indian | 2 | 3 | 1 | 6 |
| Level of education | ||||
| Primary | 9 | 11 | 1 | 21 |
| Secondary | 12 | 14 | 2 | 28 |
| Tertiary | 14 | 9 | 0 | 23 |
| BMI, kg/m2 (mean ± SD) | 23.5±4.2 | 24.4±4.6 | 21.0±6.0 | 23.7±4.5 |
| Medical history | ||||
| Diabetic | 10 | 11 | 1 | 22 |
| Not diabetic | 25 | 23 | 2 | 50 |
| Hypertension | 17 | 13 | 1 | 31 |
| Normotension | 18 | 21 | 2 | 41 |
| IHD | 3 | 6 | 0 | 9 |
| No IHD | 32 | 28 | 3 | 63 |
| Stroke | 3 | 1 | 0 | 4 |
| No stroke | 32 | 33 | 3 | 68 |
| ESRF | 4 | 0 | 1 | 5 |
| Non ESRF | 31 | 34 | 2 | 67 |
CAG, chronic atrophic gastritis; SD, standard deviation; IHD, ischemic heart disease; ESRF, end stage renal failure.
Serum biomarker levels among subjects with different sexes, ethnicities and risk factors of gastric cancer
The median levels of PGI, PGII, PGI:II ratio and G-17 for all subjects were 129.9 µg/l (IQR, 95.0–201.6), 10.3 µg/l (IQR, 6.0–13.0), 14.7 (IQR, 9.9–17.4) and 4.4 pmol/l (IQR, 1.6–15.0), respectively. The median values of serum PGI and PGII were significantly lower in females as compared to males (101.7 vs. 175.4 µg/l, P<0.01 and 8.0 vs. 12.1 µg/l, P<0.05, respectively).
Median PGI levels for smokers were significantly higher when compared with non-smokers (196.6 vs. 117.4 µg/l; P<0.05). The median value for the G-17 level in subjects with the H. pylori infection was significantly higher (5.7 pmol/l) than in those without the H. pylori infection (3.1 pmol/l; P<0.05). The H. pylori infection was associated with an increased risk of developing CAG and IM with an odds ratio of 5.0. Subjects with the H. pylori infection and CAG or IM had a significantly lower serum PGI:II ratio (9.9; P<0.05) when compared with those without the H. pylori infection and CAG/IM (15.4). Only one subject (1.4%) consumed >1.5 servings of smoked food per day.
Comparison of serum biomarker levels among subjects with different histological findings
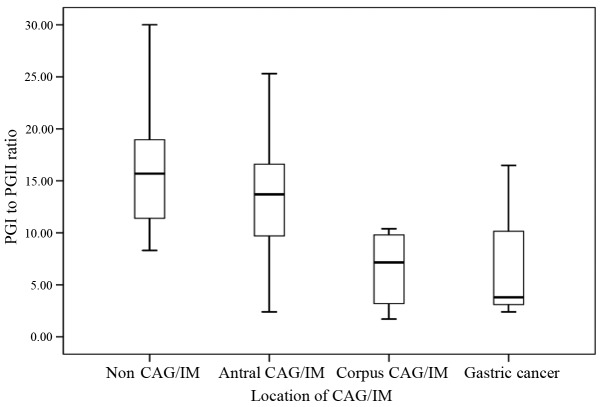
Median PGI levels for subjects without CAG, with CAG and with IM were 138.9, 108.7 and 116.6 µg/l, respectively. The PGI:II ratio was significantly lower in subjects with CAG (PGI:II=10.1) and IM (PGI:II=12.0) when compared with that of the control group (PGI:II=15.7; P<0.05; Table III). Based on the location of CAG or IM, subjects with CAG or IM involving the corpus (with or without antrum involvement) exhibited a significantly lower median serum PGI:II ratio of 7.2, compared with subjects without CAG (15.7; P<0.05) and subjects with CAG or IM confined to the antrum (13.7; P<0.05) (Fig. 1).
Table III.
Comparison of PGI, PGII, G-17 levels and the PGI:II ratio between subjects with histological non CAG, CAG, IM and gastric adenocarcinoma.
| Histological finding | |||||
|---|---|---|---|---|---|
| Serum biomarker | Non CAG (n=48) | CAG (n=12) | IM (n=9) | Gastric adenocarcinoma (n=3) | P-valuea |
| PG I, µg/l | 138.9 (98.0–218.0) | 108.7 (47.5–167.2) | 116.6 (76.9–167.2) | 191.3 | 0.261 |
| P-valueb | 0.100 | 0.175 | 0.925 | ||
| PG II, µg/l | 10.2 (6.0–12.7) | 7.7 (5.8–12.4) | 10.9 (8.3–13.1) | 17.6 | 0.096 |
| P-valueb | 0.657 | 0.470 | 0.012c | ||
| PGI:II ratio | 15.7 (11.4–19.2) | 10.1 (6.5–16.2) | 12.0 (8.6–15.4) | 3.8 | 0.016c |
| P-valueb | 0.027c | 0.031c | 0.075 | ||
| G-17, pmol/l | 3.75 (1.4–11.9) | 6.75 (2.8–57.4) | 5.1 (2.3–15.1) | 0.9 | 0.311 |
| P-valueb | 0.074 | 0.031 | 0.555 | ||
Data are provided as medians with interquartile range.
Kruskal-Wallis test
Mann-Whitney U test vs. histological non CAG
P<0.05 vs. histological non CAG. PG, pepsinogen; CAG, chronic atrophic gastritis; IM, intestinal metaplasia; G-17, gastrin-17; SD, standard deviation.
Figure 1.
Comparison of PGI:II ratio between subjects according to locations of CAG or IM, as illustrated by boxplots. PG, pepsinogen; CAG, chronic atrophic gastritis; IM, intestinal metaplasia.
In subjects with CAG, the median serum G-17 level was 6.75 pmol/l, with a wide quartile range of 2.8–57.4 pmol/l. This finding resulted from different serum G-17 levels in subjects with CAG in the antrum and CAG in the corpus. The median level of serum G-17 was significantly higher in subjects with CAG or IM involving the corpus (57.0 pmol/l) when compared with subjects without CAG (3.8 pmol/l; P<0.05) or subjects with CAG or IM involving the antrum only (5.0 pmol/l; P<0.05).
Correlation between serum biomarkers and histological findings
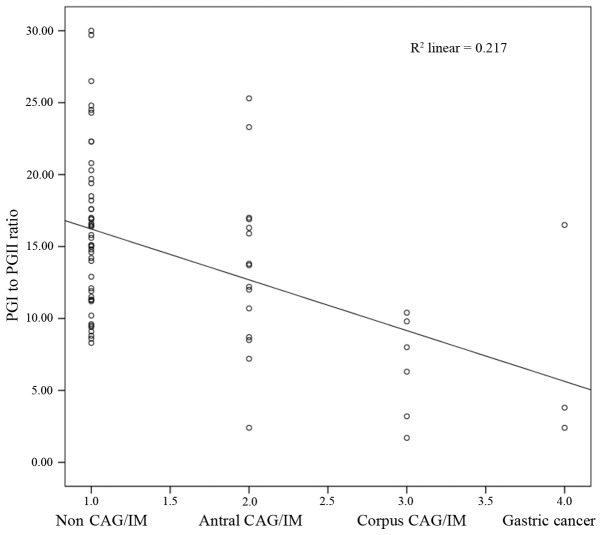
There was a significant negative correlation identified between histological CAG or IM and the serum PGI:II ratio (Spearman rank correlation coefficient, r=−0.417; P<0.001; Fig. 2). However, no statistically significant difference was identified for serum PGI (r =−0.198; P=0.095), PGI:II (r=0.146; P=0.222) or serum G-17 (r=0.165; P=0.166).
Figure 2.
Scatter plot demonstrating the correlation between the serum PGI:II ratio with CAG and IM. PG, pepsinogen; CAG, chronic atrophic gastritis; IM, intestinal metaplasia.
Sensitivity and specificity of white light endoscopy in diagnosing CAG
The results from OGD demonstrated 85.7% sensitivity, 66.7% specificity, 52.9% positive predictive value and 91.4% negative predictive value in diagnosing pre-malignant gastric lesions compared with the histology results as a gold standard (Table IV).
Table IV.
Cross-tabulation of endoscopy findings and histology results.
| Endoscopy | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Histology | Non CAG | C1 | C2 | C3 | O1 | O2 | O3 | Tumour | Total |
| Non CAG | 32 | 2 | 2 | 6 | 1 | 3 | 2 | 0 | 48 |
| CAG, antrum | 0 | 0 | 2 | 1 | 2 | 1 | 1 | 0 | 7 |
| CAG, corpus | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| CAG, antrum and corpus | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 4 |
| IM, antrum | 3 | 0 | 1 | 2 | 1 | 1 | 0 | 0 | 8 |
| IM, corpus | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| IM, antrum and corpus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastric adenocarcinoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 |
| Total | 35 | 2 | 7 | 10 | 6 | 5 | 4 | 3 | 72 |
CAG, chronic atrophic gastritis; IM, intestinal metaplasia; C-type, closed type; O-type, open type.
Sensitivity and specificity of serum biomarkers in diagnosing CAG and IM
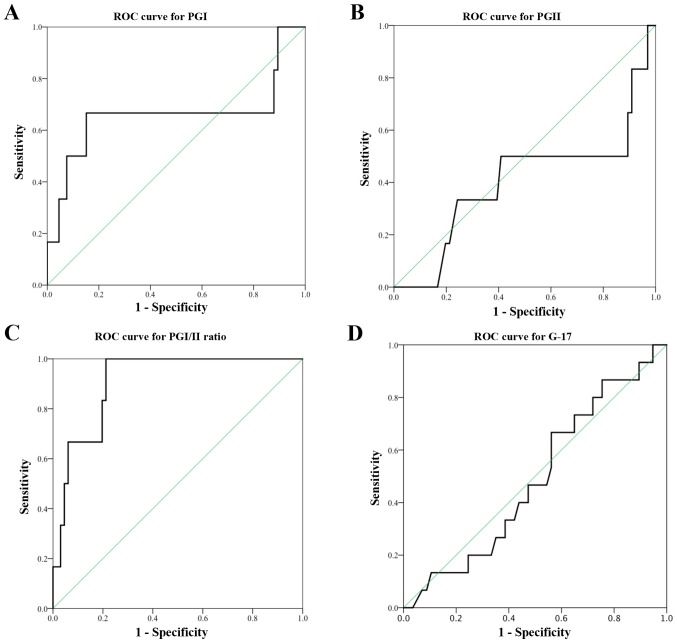
The serum PGI:II ratio was found to be accurate in diagnosing corpus CAG or IM with the AUC of 0.902. AUC for serum PGI was 0.659. Based on the ROC curve in the present study, a cut-off value of serum PGI ≤87.2 µg/l produced a sensitivity of 66.7% and specificity of 85.3%. A cut-off PGI:II ratio value of ≤10.0 detected corpus CAG or IM with a high sensitivity of 83.3% and specificity of 77.9%. The manufacturer recommended PGI cut-off value of ≤70 µg/l resulted in a low sensitivity of 50.0% but a high specificity of 91.2% in diagnosing corpus CAG or IM. Similarly, a cut-off PGI:II ratio of ≤3.0 had a low sensitivity of 16.7% in diagnosing corpus CAG or IM, even though the specificity was high (97.1%).
Poor test performances were observed in serum PGII level (cut-off value <8.8 µg/l, sensitivity 50.0% and specificity 60.3%) and serum G-17 level (cut-off <5.6 pmol/l, sensitivity 68.8% and specificity 44.8%). Each of the tests had AUC of <0.5 (Fig. 3).
Figure 3.
ROC curve for serum biomarker to diagnose corpus and antral CAG or IM. ROC, receiver operative characteristic; CAG, chronic atrophic gastritis; IM, intestinal metaplasia; PG, pepsinogen; G-17, gastrin-17.
Discussion
Gastric cancer is often diagnosed at an advanced stage due to non-specific symptoms during the early stages. The aim of the current study was to determine the sensitivity and specificity of serum PG and its ratio together with G-17 levels when diagnosing pre-malignant lesions in the stomach. It was successfully demonstrated that only the serum PGI:II ratio is potentially usable as a biomarker of corpus CAG and IM with high sensitivity of 83.3%, specificity of 77.9%, and AUC of 0.902 at a high cut-off value of ≤10.0.
The current study identified higher median PGI (129.9 µg/l; IQR, 95.0–201.6 µg/l) and PGII (10.3 µg/l; IQR, 6.0–13.0 µg/l) levels, and PGI:II ratio (14.7; IQR, 9.9–17.4) in the study population, when compared with other studies (19,22–23,26,27). A possible explanation is the different ethnicities involved, as the majority of subjects were Chinese and Malay. High serum PGI levels and PGI:II ratios were also reported in China (17) and Iran (24). The mean PGI level and PGI:II ratio were 118.4 µg/l and 11.7 respectively in Hunan, China (17). In Yazd, Southern Iran, the mean PGI level was 111.7 µg/l and the PGI:II ratio was 19.1 (24).
A more consistent and reliable biomarker for CAG and IM that could be used clinically is the serum PGI:I/II ratio. In addition, the importance of determining the location of CAG or IM was demonstrated, as there is a significant difference in serum PGI:II ratio between subjects with corpus CAG/IM and those with antral CAG/IM. When the histological subtypes were considered, regardless of the location of the lesions, no significant difference in serum PGI:II ratio was identified between patients with CAG and those with IM.
In previous studies that reported a correlation between serum PG level and pre-malignant lesions in the stomach, widely accepted cut-off values for the serum PGI concentration and PGI:II ratio were ≤70 µg/l and ≤3 respectively (14,37–39). At these cut-off values, Kitahara et al (37) reported a sensitivity of 84.6% and specificity of 73.5% in gastric cancer screening. In a study by Borch et al (40), serum PGI level of ≤71.6 µg/l had a sensitivity of 100% and specificity of 86%, while the serum PGI:II ratio of ≤5.5 had a sensitivity of 99% and specificity of 94% in diagnosing fundal CAG. A previous study reported that an accuracy of 83%, sensitivity of 83% and specificity of 95% using different serum PGI and G-17 cut-off values to diagnose CAG depending on the HpAb level (21). Another study demonstrated that the PGI:II ratio was a reliable marker of IM with a sensitivity of 66% and specificity of 78% at a cut-off value of 3.0 (14). Meanwhile, Broutet el at (26) reported that only the PGI:II ratio was accurate in diagnosing CAG at a cut-off value of 5.6.
Despite the above-mentioned good correlation, the present study identified a poor correlation of serum PG and G-17 in diagnosing CAG, which has also been reported in previous studies (17,23). In a prospective multicentre study in Spain, the AUC for serum PGI and PGI:II ratio were 0.6 and 0.66, respectively. In 2014, McNicholl et al (18) concluded that these serum biomarkers were not accurate enough to diagnose CAG. Zhang et al (17) reported that a low serum PGI level and PGI:II ratio may predict gastric cancer; however, no significant difference was observed in the serum PGI level and PGI:II ratio among subjects with or without CAG. Furthermore, the mean value of the PGI:II ratio for patients with CAG was higher, 11.07±5.78 when compared with 10.63±5.74 for patients with non-atrophic gastritis (17). The serum PGI and PGII alone or their combinations with a low PGI:II ratio were reported as not sensitive enough to diagnose CAG (24).
In the present study, using a cut-off value of serum PGI ≤70 or PGI:II ratio ≤3.0, the sensitivity of the test became very low in detecting corpus CAG, even though the specificity was 91.2 and 97.1%, respectively. Due to a small sample size, the median PGI:II ratio for subjects with corpus CAG or IM was 7.2 (IQR, 2.8–10.0). In order to improve the sensitivity and specificity of the serum PGI:II ratio in diagnosing corpus CAG and IM, the current study proposes to increase the cut-off value for the ratio. Based upon the ROC curve, the optimum cut-off value for the PGI:II ratio as a biomarker for corpus CAG and IM in the current population was ≤10.0 or serum PGI of ≤87.2 µg/l, which was higher than the values reported in previous studies. Therefore, a larger cohort study is required to determine the real sensitivity, specificity and cut-off value of serum PG for the diagnosis of corpus CAG or IM before it may be applied in clinical practice.
In the present study, subjects with the H. pylori infection were observed and identified to be associated with a high serum G-17 level. Furthermore, a higher level of G-17 was noted among Indian subjects as compared with Malay and Chinese subjects, while there was no difference in G-17 level among different age groups. This is in contrast to a large study in China (41), where subjects >60 years of age were observed to have higher serum G-17 levels compared with the younger age group. A previous study also demonstrated that a high serum G-17 level was detected in H. pylori-positive subjects (41). In addition, patients with increased gastric acidity, such as gastro-oesophageal reflux disease and Barrett's oesophagus were reported to exhibit a low fasting level of serum G-17 (42).
The serum concentration of G-17 level varies in association to the location of atrophic mucosa. A low level of serum G-17 is a sign of multifocal or antrum-limited gastritis in patients infected with H. pylori (13). The present results did not demonstrate low serum G-17 levels in subjects with antral CAG or IM irrespective of whether they were HpAb-positive or -negative. Conversely, subjects with corpus CAG or IM exhibited high serum G-17 levels. This is consistent with findings in other studies that high G-17 may be used as a biomarker in diagnosing corpus CAG (43,44). It has been hypothesized that atrophic gastric mucosa of the corpus results in hypochlorhydria and this stimulates gastrin production from the antrum.
To the best of our knowledge, this is the first study in Malaysia assessing serum PG, G-17 and HpAb levels in patients with CAG and IM. By assessing the clinical risk factors that are associated with CAG and IM, patients that are high-risk for gastric cancer may be identified by screening using serum biomarkers. The H. pylori infection was associated with an increased risk of developing premalignant gastric lesions. Subjects presenting with the H. pylori infection and CAG or IM exhibited a significantly lower serum PGI:II ratio compared with those without the H. pylori infection and CAG or IM. Therefore, it is proposed that the serum PGI:II ratio is a potential screening tool for gastric cancer, particularly in patients presenting with the H. pylori infection.
Long-term follow-up for subjects with CAG and IM enabled the determination of the incidence of gastric adenocarcinoma in the patient cohort. A population study is required to determine the prevalence of CAG, IM and gastric cancer in Malaysia. In a geographical area with a low prevalence of CAG, IM and gastric cancer, a surveillance programme using serum biomarkers or OGD with gastric biopsy may not be cost-effective. Although the serum PGI:II ratio demonstrated significant negative correlation with corpus CAG and IM, the best cut off value in these pre-malignant gastric lesions needs to be determined in a larger number of participants. The suitability of this serum biomarker may be improved by adding more biomarkers. More studies involving microRNA, the DNA methylation status of gastric cancer-associated genes or tissue-based proteomics using high-throughput technology are required. Furthermore, the utility of the serum PGI:II ratio should only be targeted to individuals with a high risk of gastric cancer or pre-malignant lesions, such as those with the H. pylori infection, in order to optimize the cost effectiveness.
In conclusion, the use of serum biomarkers in diagnosing pre-malignant lesions in the stomach presents an attractive alternative to OGD with histopathology examination of the gastric biopsy. It is non-invasive, involves simple blood taking and provides consistent results using ELISA, as compared with OGD. The current study identified that serum PGI, PGII and G-17 levels alone were not sensitive enough to diagnose CAG or IM. At a high cut-off value, the serum PGI:II ratio displayed high sensitivity and specificity, and a significant negative correlation in diagnosing corpus CAG or IM in the current population. The current findings are preliminary, thus further investigation with a larger cohort is required to determine the optimum cut-off value, in order to adopt this biomarker as an outpatient, non-invasive filter for detecting pre-malignant lesions in the stomach prior to endoscopy. Additional experiments, such as an immunohistochemistry study are required to support the current biomarker before it is used in clinical practice.
Acknowledgements
The authors would like to thank the Malaysian Society of Gastroenterology and Hepatology (MSGH) for its support and funding through the MSGH Research Project 2014 Grant Source of financial support (grant no. JJ-2015-004).
Glossary
Abbreviations
- CAG
chronic atrophic gastritis
- IM
intestinal metaplasia
- OGD
oesophageal-gastro-duodenoscopy
- G-17
gastrin-17
- PGI
pepsinogen I
- PGII
pepsinogen II
- HpAb
Helicobacter pylori antibody
- ROC
receiver operating characteristic
- IQR
interquartile range
References
- 1.Zainal AO, Saleha Nor IT. Ministry of Health Malaysia. Malaysia: 2007. National Cancer Registry Report. Malaysia Cancer Statistics - Data and Figure. [Google Scholar]
- 2.Correa P. Human gastric carcinogenesis: A multistep and multifactorial process - First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 3.Asaka M, Sugiyama T, Nobuta A, Kato M, Takeda H, Graham DY. Atrophic gastritis and intestinal metaplasia in Japan: Results of a large multicenter study. Helicobacter. 2001;6:294–299. doi: 10.1046/j.1523-5378.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 4.Weck MN, Brenner H. Prevalence of chronic atrophic gastritis in different parts of the world. Cancer Epidemiol Biomarkers Prev. 2006;15:1083–1094. doi: 10.1158/1055-9965.EPI-05-0931. [DOI] [PubMed] [Google Scholar]
- 5.Sakaki N, Kozawa H, Egawa N, Tu Y, Sanaka M. Ten-year prospective follow-up study on the relationship between Helicobacter pylori infection and progression of atrophic gastritis, particularly assessed by endoscopic findings. Aliment Pharmacol Ther. 2002;16(Suppl 2):198–203. doi: 10.1046/j.1365-2036.16.s2.13.x. [DOI] [PubMed] [Google Scholar]
- 6.Sipponen P. Update on the pathologic approach to the diagnosis of gastritis, gastric atrophy, and Helicobacter pylori and its sequelae. J Clin Gastroenterol. 2001;32:196–202. doi: 10.1097/00004836-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Park YH, Kim N. Review of atrophic gastritis and intestinal metaplasia as a premalignant lesion of gastric cancer. J Cancer Prev. 2015;20:25–40. doi: 10.15430/JCP.2015.20.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tatsuta M, Iishi H, Nakaizumi A, Okuda S, Taniguchi H, Hiyama T, Tsukuma H, Oshima A. Fundal atrophic gastritis as a risk factor for gastric cancer. Int J Cancer. 1993;53:70–74. doi: 10.1002/ijc.2910530114. [DOI] [PubMed] [Google Scholar]
- 9.Whiting JL, Sigurdsson A, Rowlands DC, Hallissey MT, Fielding JW. The long term results of endoscopic surveillance of premalignant gastric lesions. Gut. 2002;50:378–381. doi: 10.1136/gut.50.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, Kuipers EJ. Gastric cancer risk in patients with premalignant gastric lesions: A nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945–952. doi: 10.1053/j.gastro.2008.01.071. [DOI] [PubMed] [Google Scholar]
- 11.Hansen S, Vollset SE, Derakhshan MH, Fyfe V, Melby KK, Aase S, Jellum E, McColl KE. Two distinct aetiologies of cardia cancer; evidence from premorbid serological markers of gastric atrophy and Helicobacter pylori status. Gut. 2007;56:918–925. doi: 10.1136/gut.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallissey MT, Dunn JA, Fielding JW. Evaluation of pepsinogen A and gastrin-17 as markers of gastric cancer and high-risk pathologic conditions. Scand J Gastroenterol. 1994;29:1129–1134. doi: 10.3109/00365529409094899. [DOI] [PubMed] [Google Scholar]
- 13.Sipponen P, Ranta P, Helske T, Kääriäinen I, Mäki T, Linnala A, Suovaniemi O, Alanko A, Härkönen M. Serum levels of amidated gastrin-17 and pepsinogen I in atrophic gastritis: An observational case-control study. Scand J Gastroenterol. 2002;37:785–791. doi: 10.1080/gas.37.7.785.791. [DOI] [PubMed] [Google Scholar]
- 14.Dinis-Ribeiro M, da Costa-Pereira A, Lopes C, Barbosa J, Guilherme M, Moreira-Dias L, Lomba-Viana H, Silva R, Abreu N, Lomba-Viana R. Validity of serum pepsinogen I/II ratio for the diagnosis of gastric epithelial dysplasia and intestinal metaplasia during the follow-up of patients at risk for intestinal-type gastric adenocarcinoma. Neoplasia. 2004;6:449–456. doi: 10.1593/neo.03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Storskrubb T, Aro P, Ronkainen J, Sipponen P, Nyhlin H, Talley NJ, Engstrand L, Stolte M, Vieth M, Walker M, Agréus L. Serum biomarkers provide an accurate method for diagnosis of atrophic gastritis in a general population: The Kalixanda study. Scand J Gastroenterol. 2008;43:1448–1455. doi: 10.1080/00365520802273025. [DOI] [PubMed] [Google Scholar]
- 16.Lomba-Viana R, Dinis-Ribeiro M, Fonseca F, Vieira AS, Bento MJ, Lomba-Viana H. Serum pepsinogen test for early detection of gastric cancer in a European country. Eur J Gastroenterol Hepatol. 2012;24:37–41. doi: 10.1097/MEG.0b013e32834d0a0a. [DOI] [PubMed] [Google Scholar]
- 17.Zhang XM, Li JX, Zhang GY, Li XH, Gu H. The value of serum pepsinogen levels for the diagnosis of gastric diseases in Chinese Han people in midsouth China. BMC Gastroenterol. 2014;14:3. doi: 10.1186/1471-230X-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNicholl AG, Forné M, Barrio J, De la Coba C, González B, Rivera R, Esteve M, Fernandez-Bañares F, Madrigal B, Gras-Miralles B, et al. Helicobacter pylori Study Group of Asociación Española de Gastroenterología (AEG): Accuracy of GastroPanel for the diagnosis of atrophic gastritis. Eur J Gastroenterol Hepatol. 2014;26:941–948. doi: 10.1097/MEG.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue M, Kobayashi S, Matsuura A, Hamajima N, Tajima K, Tominaga S. Agreement of endoscopic findings and serum pepsinogen levels as an indicator of atrophic gastritis. Cancer Epidemiol Biomarkers Prev. 1998;7:261–263. [PubMed] [Google Scholar]
- 20.Ley C, Mohar A, Guarner J, Herrera-Goepfert R, Figueroa LS, Halperin D, Parsonnet J. Screening markers for chronic atrophic gastritis in Chiapas, Mexico. Cancer Epidemiol Biomarkers Prev. 2001;10:107–112. [PubMed] [Google Scholar]
- 21.Väänänen H, Vauhkonen M, Helske T, Kääriäinen I, Rasmussen M, Tunturi-Hihnala H, Koskenpato J, Sotka M, Turunen M, Sandström R, et al. Non-endoscopic diagnosis of atrophic gastritis with a blood test. Correlation between gastric histology and serum levels of gastrin-17 and pepsinogen I: A multicentre study. Eur J Gastroenterol Hepatol. 2003;15:885–891. doi: 10.1097/00042737-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Sierra R, Une C, Ramírez V, González MI, Ramírez JA, de Mascarel A, Barahona R, Salas-Aguilar R, Páez R, Avendaño G, et al. Association of serum pepsinogen with atrophic body gastritis in Costa Rica. Clin Exp Med. 2006;6:72–78. doi: 10.1007/s10238-006-0098-3. [DOI] [PubMed] [Google Scholar]
- 23.Oishi Y, Kiyohara Y, Kubo M, Tanaka K, Tanizaki Y, Ninomiya T, Doi Y, Shikata K, Yonemoto K, Shirota T, et al. The serum pepsinogen test as a predictor of gastric cancer: The Hisayama study. Am J Epidemiol. 2006;163:629–637. doi: 10.1093/aje/kwj088. [DOI] [PubMed] [Google Scholar]
- 24.Mohamadkhani A, Moghaddam Darvish S, Salmanroghani H, Allafsghari A, Yazdanbod A, Mirzaei M, Haj-sheykholeslami A, Bashiri J, Sadjadi A, Massarrat S. Are the serum biomarkers pepsinogen I and II good predictors for the detection of subjects with atrophic gastritis in areas that have different gastric cancer incidence? Arch Iran Med. 2013;16:208–212. [PubMed] [Google Scholar]
- 25.You WC, Blot WJ, Li JY, Chang YS, Jin ML, Kneller R, Zhang L, Han ZX, Zeng XR, Liu WD, et al. Precancerous gastric lesions in a population at high risk of stomach cancer. Cancer Res. 1993;53:1317–1321. [PubMed] [Google Scholar]
- 26.Broutet N, Plebani M, Sakarovitch C, Sipponen P, Mégraud F. Eurohepygast Study Group: Pepsinogen A, pepsinogen C, and gastrin as markers of atrophic chronic gastritis in European dyspeptics. Br J Cancer. 2003;88:1239–1247. doi: 10.1038/sj.bjc.6600877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JY, Kim N, Lee HS, Oh JC, Kwon YH, Choi YJ, Yoon KC, Hwang JJ, Lee HJ, Lee A, et al. Correlations among endoscopic, histologic and serologic diagnoses for the assessment of atrophic gastritis. J Cancer Prev. 2014;19:47–55. doi: 10.15430/JCP.2014.19.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Compare D, Rocco A, Nardone G. Risk factors in gastric cancer. Eur Rev Med Pharmacol Sci. 2010;14:302–308. [PubMed] [Google Scholar]
- 29.Ding SZ, Goldberg JB, Hatakeyama M. Helicobacter pylori infection, oncogenic pathways and epigenetic mechanisms in gastric carcinogenesis. Future Oncol. 2010;6:851–862. doi: 10.2217/fon.10.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang YW, Hwangbo Y, Lee JW, Jo SJ, Cho JH, Shim J, Jang JY, Kim HJ, Kim BH. Clinical parameters including serum pepsinogen level and management strategy in patients with premalignant gastric dysplasia. Eur J Gastroenterol Hepatol. 2011;23:405–410. doi: 10.1097/MEG.0b013e328346105a. [DOI] [PubMed] [Google Scholar]
- 31.Shin CM, Kim N, Yang HJ, Cho SI, Lee HS, Kim JS, Jung HC, Song IS. Stomach cancer risk in gastric cancer relatives: Interaction between Helicobacter pylori infection and family history of gastric cancer for the risk of stomach cancer. J Clin Gastroenterol. 2010;44:e34–e39. doi: 10.1097/MCG.0b013e3181a159c4. [DOI] [PubMed] [Google Scholar]
- 32.El Hajj II, Hashash JG, Baz EM, Abdul-Baki H, Sharara AI. ABO blood group and gastric cancer: Rekindling an old fire? South Med J. 2007;100:726–727. doi: 10.1097/SMJ.0b013e3180485d24. [DOI] [PubMed] [Google Scholar]
- 33.Mao Y, Hu J, Semenciw R, White K. Canadian Cancer Registries Epidemiology Research Group: Active and passive smoking and the risk of stomach cancer, by subsite, in Canada. Eur J Cancer Prev. 2002;11:27–38. doi: 10.1097/00008469-200202000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Kimura KTT, Takemoto T. An endoscopic recognization of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87–97. doi: 10.1055/s-0028-1098086. [DOI] [Google Scholar]
- 35.Miike T, Yamamoto S, Miyata Y, Hirata T, Noda Y, Noda T, Suzuki S, Takeda S, Natsuda S, Sakaguchi M, et al. Surrounding gastric mucosa findings facilitate diagnosis of gastric neoplasm as gastric adenoma or early gastric cancer. Gastroenterol Res Pract. 2016;2016:6527653. doi: 10.1155/2016/6527653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Kitahara F, Kobayashi K, Sato T, Kojima Y, Araki T, Fujino MA. Accuracy of screening for gastric cancer using serum pepsinogen concentrations. Gut. 1999;44:693–697. doi: 10.1136/gut.44.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miki K. Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer. 2006;9:245–53. doi: 10.1007/s10120-006-0397-0. [DOI] [PubMed] [Google Scholar]
- 39.Miki K, Fujishiro M, Kodashima S, Yahagi N. Long-term results of gastric cancer screening using the serum pepsinogen test method among an asymptomatic middle-aged Japanese population. Dig Endosc. 2009;21:78–81. doi: 10.1111/j.1443-1661.2009.00839.x. [DOI] [PubMed] [Google Scholar]
- 40.Borch K, Axelsson CK, Halgreen H, Nielsen Damkjaer MD, Ledin T, Szesci PB. The ratio of pepsinogen A to pepsinogen C: A sensitive test for atrophic gastritis. Scand J Gastroenterol. 1989;24:870–876. doi: 10.3109/00365528909089228. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z, Sun L, Gong YH, Wang XG, Zhang M, Yuan Y. Factors affecting the serum gastrin 17 level: An evidence-based analysis of 3906 serum samples among Chinese. J Dig Dis. 2007;8:72–76. doi: 10.1111/j.1443-9573.2007.00288.x. [DOI] [PubMed] [Google Scholar]
- 42.Sipponen P, Vauhkonen M, Helske T, Kaariainen I, Harkonen M. Low circulating levels of gastrin-17 in patients with Barrett's esophagus. World J Gastroenterol. 2005;11:5988–5992. doi: 10.3748/wjg.v11.i38.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan Y. A survey and evaluation of population-based screening for gastric cancer. Cancer Biol Med. 2013;10:72–80. doi: 10.7497/j.issn.2095-3941.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, Ling L, Li S, Qin G, Cui W, Li X, Ni H. The diagnostic value of gastrin-17 detection in atrophic gastritis: A meta-analysis. Medicine (Baltimore) 2016;95:e3599. doi: 10.1097/MD.0000000000003599. [DOI] [PMC free article] [PubMed] [Google Scholar]