Abstract
Murine double minute clone 2 oncoprotein (MDM2) is a key component in the regulation of the tumour suppressor p53. The association between the MDM2 polymorphism and gastric cancer (GC) has been investigated in Turkish population. In the present case-control study, the aim was to investigate the association between genetic polymorphisms of the MDM2 gene (a major regulator of p53 function) and primary GC risk in a Turkish population. The polymorphism, T309G (rs2279744) in the MDM2 gene was determined in patients with GC (n=65) and in healthy control subjects (n=67) using the polymerase chain reaction-restriction fragment length polymorphism method. The findings were evaluated using logistic regression and χ2 tests. No statistically significant differences were observed between the control subjects and patients with GC regarding smoking status. A comparison between GC cases and control subjects indicated a statistically significant difference for family history of cancer [odds ratio (OR)=0.17; 95% confidence interval (CI), 0.05–0.56; χ2=0.19; P=0.01]. A significant difference was identified in the GG genotype distribution between GC patients and control subjects (OR=4.58; 95% CI, 1.18–17.79; P=0.022). Thus, the results of the present study indicate that the MDM2 gene T309G intron (GG) genotype may be an important risk factor for GC development in the Turkish population.
Keywords: gastric cancer, polymorphism, MDM2 gene T309G, Turkish population
Introduction
Genetic factors that may alter the repair of damaged gastric and colonic epithelial cell DNA, resulting in their transformation into cancer cells remain poorly understood. Accumulation of permanently generated reactive oxygen species during cellular metabolism and extracellular processes may lead to carcinogenesis caused by oxidative DNA damage. p53, a tumor suppressor protein encoded in humans by the p53 gene, regulates the cell cycle and preserves the stability of the human genome to prevent cancer initiation (1,2). Gastric cancer (GC) is the fourth most common malignancy and the second primary cause of cancer mortality, resulting in >800,000 mortalities worldwide annually (3,4). In addition to environmental factors, genetic variants are significant. As a crucial tumor suppressor gene, p53 is critical in cell-cycle regulation, DNA repair, cellular differentiation and apoptosis in various types of cancer. Furthermore, variants of p53 gene exert an important effect on tumor development and cancer risk (5–7).
Recently, numerous studies have evaluated the interaction between p53 and its regulators in carcinogenesis. Of these regulators, the murine double minute clone 2 (MDM2) proto-oncogene, an important negative regulator of the p53 gene, has become a point of interest (8). MDM2 is a major regulator of p53 function. p53 and MDM2 act in a feedback cycle whereby p53 activates MDM2 at the transcriptional level, while MDM2 binds, obstructs and degrades the p53 protein through E3 ligase activity (9–11). The human MDM2 gene is located on chromosome 12q13-14 (genomic size, 34 kb) and contains two promoters, the constituent promoter and the p53-responsive intronic promoter (12). The inherently occurring sequence variation in the MDM2 promoter results in a changed MDM2 protein expression leading to the alteration in p53 mediated tumor suppression activity. A single nucleotide polymorphism (SNP; T309G) located in the first intron of the core promoter region of the MDM2 gene influences binding of the transcription factor, Sp1. Sp1 binds with higher affinity to the G allele than to the T allele, which results in increased transcription of the MDM2 gene and higher expression levels of MDM2 protein, thereby obstructing the tumor suppressor function of p53. This SNP leads to increased MDM2 mRNA and protein expression levels and, as a result, p53 inhibition. Additionally, there is a disposition, which demonstrated earlier cancer inception in certain groups of patients harboring the GG or TG genotype for MDM2 T309G (13). The MDM2 T309G polymorphism has been reported to be associated with certain tumors, such as colon, gastric and hepatocellular cancer (14–16).
In the present study, the aim was to investigate the association between genetic polymorphisms of the MDM2 gene, a major regulator of p53 function, and primary GC risk in a Turkish population.
Materials and methods
Study population
The study was approved by the local ethic committee and informed consent was obtained from all patients. All subjects agreed to participate in the study and completed a short questionnaire, which included questions about their occupation, tobacco use, alcohol consumption and family history of cancer. In the present study, a total of 132 individuals (65 GC patients and 67 control subjects) were investigated. Blood samples were collected from the 65 patients who were diagnosed with GC at the Department of General Surgery, Cumhuriyet University, Faculty of Medicine (Sivas, Turkey). The diagnosis of GC was histologically confirmed and the tumor types were classified according to WHO guidelines, (https://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb2/bb2-chap3.pdf) No age and sex restrictions were applied for the selection of healthy volunteers, who were free of any chronic diseases, lived in the same geographic area, and had no history of cancer. All cases and controls were born and lived in Turkey.
DNA isolation
Peripheral blood samples (2 ml) were obtained and collected into citrate-containing tubes from all subjects. The DNA was extracted from whole blood using the salting out procedure as soon as the samples reached the laboratory (17).
MDM2 genotyping
The MDM2 T309G polymorphism was analyzed using the polymerase chain reaction (PCR)-restriction fragment length polymorphism method. The following primers were used: Forward, 5′-CGCGGGAGTTCAGGGTAAAG-3′ and reverse, 5′-CTGAGTCAACCTGCCCACTG-3′ to amplify the MDM2 polymorphism. Amplification was performed using the following: 25 pmol each primer, 200 mM total dNTP, 1.5 mM MgCI2, 1X PCR buffer and 2.5 U Taq DNA polymerase and 50–100 ng DNA in a total volume of 50 µl. The PCR program was initiated with denaturation at 95°C for 5 min, followed by 30 cycles of 94°C for 60 sec, 55°C for 60 sec (annealing) and 72°C for 60 sec (extension). The PCR was completed with a final extension cycle at 72°C for 5 min. Following confirmation of PCR amplification by 1.5% agarose gel electrophoresis, the amplified product was digested overnight with MspA1I restriction enzyme at 37°C and electrophoresed on 3% agarose gel stained with ethidium bromide and visualized under UV light. Genotypes were identified for the polymorphism as TT (157 bp), TG (157, 110 and 47 bp), or GG (110 and 47 bp) (18).
Statistical analysis
All statistical analyses were performed using SPSS version 11 (SPSS, Inc., Chicago, IL, USA). Genotype-associated odds ratios (ORs), their corresponding 95% confidence intervals (CIs), and associated P-values were estimated via unconditional logistic regression. Differences in the distributions of demographic characteristics between the cases and control subjects were evaluated using Student's t-test. χ2 or Fischer's exact test (two-sided) were used to compare the sex distribution, to test the association between the genotypes and alleles in relation to the controls, and to test for deviation of the genotype distribution from Hardy-Weinberg equilibrium. Pearson's χ2 test was used to determine whether there were any significant differences in allele and genotype frequencies between patients and control subjects. Logistic regression procedures were performed to assess the interaction between age, sex and all genotypes. In addition, the recessive or dominant effect of the MDM2 genotype on risk was estimated, and statistical analysis was performed on the relative risk of the GG genotype against the TG + TT genotype or the TG + GG genotype against the TT genotype.
Results
In the present study, the association between the MDM2 polymorphism and GC was investigated in a Turkish population. The polymorphism in the MDM2 gene, T309G has been determined in 65 patients with GC and in 67 healthy control subjects using the PCR-RFLP method.
The demographic characteristics of the study population are presented in Table I. The mean age of the GC patients and control subjects were 62.25±10.95 years (males, 62.32±10.55; females, 61.92±13.08) and 62.34±10.54 years (males, 62.58±9.86; females, 61.43±13.19), respectively. The percentage of males and females in the cases were 81.5 and 18.5%, respectively (Table I). No statistically significant differences were identified between the cases and control subjects by age and sex (P>0.05). Nor were there statistically significant associations between the cases and control subjects for smoking history (OR=0.99; 95% CI, 0.50–1.97; P=0.989; Table II). Furthermore, no statistically significant differences were identified regarding alcohol consumption among the cases and control subjects (OR=2.35; 95% CI, 0.88–6.27; P=0.082) (Table II). Comparison between GC cases and control subjects indicated statistically significant differences in family history of cancer (OR=0.17; 95% CI, 0.05–0.56; P=0.01; Table II). Individuals with the GG genotype were associated with a higher risk for GC and the GG polymorphism was significantly associated with this disease (OR=4.58; 95% CI, 1.18–17.79; P=0.022; Table III). Analysis of the MDM2 gene T309G polymorphisms is presented in Fig. 1.
Table I.
Demographics of control subjects (n=67) and gastric cancer cases (n=65) recruited in the present study.
| Variable | Control, n (%) | Gastric cancer, n (%) |
|---|---|---|
| Sex | ||
| Males | 53 (79.1) | 53 (81.5) |
| Females | 14 (20.9) | 12 (18.5) |
| Age (years) | ||
| Range | 40–85 | 40–85 |
| Means ± SD | 62.34±10.54 | 62.25±10.95 |
| Males | 62.58±9.86 | 62.32±10.55 |
| Females | 61.43±13.19 | 61.92±13.08 |
| Smoking history | ||
| Smoker | 31(46.3) | 30 (46.2) |
| Males | 30 (56.6) | 28 (52.8) |
| Females | 1 (7.1) | 2 (16.7) |
| Alcoholic drink consumption | ||
| Smoker | 7 (10.4) | 14 (21.5) |
| Males | 7 (13.2) | 11 (20.8) |
| Females | 0 | 3 (25.0) |
| Family history of cancer | 18 (26.9) | 4 (6.2) |
Table II.
Distribution of selected variables in gastric cancer cases and control subjects.
| Gastric cancer (n=65) | Control (n=67) | |||||
|---|---|---|---|---|---|---|
| Variable | n | % | n | % | P-value | Odds ratio |
| Smoking status | ||||||
| No | 35 | 53.8 | 36 | 53.7 | 0.989 | 0.99 (0.50–1.97) |
| Yes | 30 | 46.2 | 31 | 46.3 | ||
| Drinking status | ||||||
| No | 51 | 78.5 | 60 | 89.6 | 0.082 | 2.35 (0.88–6.27) |
| Yes | 14 | 21.5 | 7 | 10.4 | ||
| Family history of cancer | ||||||
| No | 61 | 93.8 | 41 | 73.1 | 0.010a | 0.17 (0.05–0.56) |
| Yes | 4 | 6.2 | 18 | 26.9 | ||
Fisher's exact test.
Table III.
Stratification analyses between murine double minute-2 T309G genotypes and gastric cancer risk.
| MDM2 T309G | Controls (n=67) | Gastric cancer (n=65) | χ2 | P-value | Crude odds ratio |
|---|---|---|---|---|---|
| Allele frequency | |||||
| T allele | 65 | 47 | Ref. | 0.042 | 1.66 (0.99–2.81) |
| G allele | 69 | 83 | 4.12 | ||
| Genotype frequency | |||||
| TT | 10 (14.9) | 4 (6.2) | Ref. | – | – |
| TG | 45 (67.2) | 39 (60.0) | 1.55 | 0.213a | 2.16 (0.62–7.45) |
| GG | 12 (7.9) | 22 (33.8) | 5.21 | 0.022 | 4.58 (1.18–17.79) |
| TG + GG | 57 (85.1) | 61 (93.8) | 2.67 | 0.102 | 2.65 (0.79–9.01) |
| TT + TG | 45 (67.2) | 39 (60.0) | 0.73 | 0.392 | 0.73 (0.36–1.49) |
Fisher's exact test. MDM2, murine double minute-2.
Figure 1.
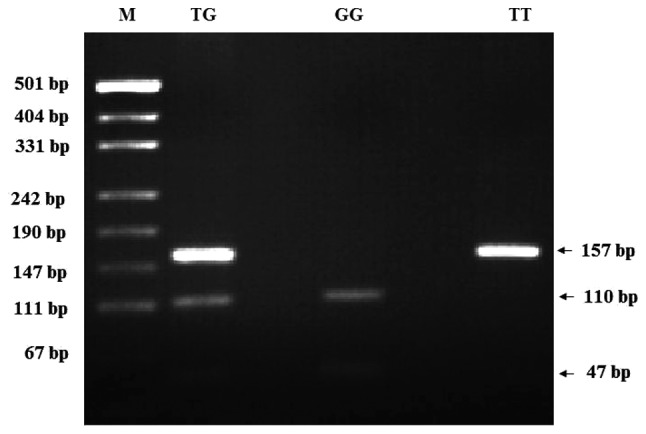
PCR-restriction fragment length polymorphism patterns of polymorphisms of MDM2 T309G. M: pUCI19/Msp I DNA ladder (501, 489, 404, 331, 242, 190, 147, 111 and 110 bp); MDM2 PCR product, 157 bp, TG (157, 110 and 47 bp); GG (110 and 47 bp); TT (157 bp). PCR, polymerase chain reaction; MDM2, murine double minute-2.
Discussion
GC is the fourth most common cancer and the second leading cause of cancer-associated mortality worldwide (4). GC is a complex trait caused by genetic and environmental factors (19). Genetic polymorphisms in the carcinogen detoxification, antioxidant protection, DNA repair and cell proliferation processes are also crucial in the development of GC (20).
In the current study, of 65 GC cases and 67 control subjects, the crude OR of GC patients for family history of cancer was 0.17, indicating an association between GC incidence and family history of cancer (P=0.01; Table II). To the best of our knowledge, GC and family history of cancer association has not been proposed in previous studies. In addition, GC patients and control subjects were evaluated by logistic regression analysis for smoking habits. No statistically significant difference was identified between GC patients and smoking habits in the Turkish population investigated in the current study (P=0.989; Table II).
The MDM2 T309G polymorphism in GC patients has been investigated in various different regions of the world; however, to the best of our knowledge, no research has been performed in Turkey. This study was the first study to evaluate the polymorphic variants of MDM2 T309G polymorphism and risk of GC. The MDM2 protein negatively regulates p53 function via multiple independent mechanisms, such as by binding to transcription activation domains, inhibiting acetylation, promoting nuclear export and, most importantly, promoting proteasomal degradation via ubiquitination (9,11,21,22). The MDM2 T309G polymorphism is a single nucleotide T to G polymorphism located at the 309th nucleotide in the MDM2 gene promoter. Over the last two decades, it has been confirmed that the MDM2 T309G polymorphism is capable of significantly altering the expression levels of MDM2, thereby suppressing the p53 signaling pathway. Furthermore, a number of epidemiological studies have investigated the association between the MDM2 T309G polymorphism and the risk of various types of cancer (23–26), including renal, hepatocellular, endometrial, bladder and stomach cancers. However, the results of previous studies regarding the association between the MDM2 T309G polymorphism and GC risk were different or even contradictory (27,28). Recently, numerous studies demonstrated that significant associations between the MDM2 T309G polymorphism and GC risk were identified for the G and T alleles, and the GG and TT genotypes. The G allele was revealed to act as a key factor for increasing the risk of GC, which is consistent with the study by Pan et al (29). In addition, in a meta-analysis, Chen et al (30) proposed that the G allele of the MDM2 T309G polymorphism is a high risk factor for developing GC. In the present study, a statistically significant difference was identified in the GG genotype distribution between GC patients and control subjects in the investigated Turkish population (OR=4.58; 95% CI, 1.18–17.79; P=0.022; Table III). MDM2 T309G GG genotype was associated with an increased risk of gastric carcinoma when compared with the TT genotype or T carriers (P<0.01), and a joint effect between MDM2 T309G GG and the Helicobacter pylori infection was observed to intensify the GC risk (31). Tian et al (32) proposed that the GG genotype is associated with a significantly increased risk of GC (OR=1.43; 95% CI, 1.08–1.91; P=0.013). Consistent with these studies, the current study identified that the overall GC risk for T309G GG was significantly increased when it was compared with T carriers (OR=4.58; 95% CI, 1.18–17.79; P=0.022), as presented in Table III. The distribution of MDM2 genotypes in different populations is provided in Table IV. While the genotype distribution of the control group of the present study was similar to those of the other studies (15,26–29,31,33) the case groups differed.
Table IV.
Distribution of polymorphisms of murine double minute-2 T309G genotype frequencies in different populations.
| Gastric cancer (%) | Control (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Country | TT | TG | GG | TT | TG | GG | Year | (Refs.) |
| Japan | 23.9 | 45.9 | 30.2 | 22.6 | 55.0 | 22.4 | 2006 | (15) |
| China | 21.4 | 50.0 | 28.6 | 29.8 | 49.8 | 20.4 | 2007 | (33) |
| Korea | 26.8 | 46.0 | 27.2 | 20.4 | 50.8 | 28.8 | 2008 | (28) |
| China | 21.7 | 48.1 | 30.2 | 36.8 | 43.4 | 19.8 | 2009 | (31) |
| China | 28.5 | 46.2 | 25.4 | 31.5 | 54.3 | 14.2 | 2009 | (27) |
| China | 20.9 | 54.5 | 24.6 | 20.5 | 51.6 | 27.9 | 2011 | (26) |
| China | 30.1 | 45.3 | 24.6 | 34.7 | 51.6 | 13.8 | 2013 | (29) |
| Turkey | 6.2 | 60.0 | 33.8 | 14.9 | 67.2 | 17.9 | 2017 | Current study |
In conclusion, the present results indicate a significant association between the MDM2 GG genotype and GC; although, it is not possible to conclude that a single polymorphism determines an individual's susceptibility to GC development. Furthermore, the MDM2 GG genotype may be defined as an independent marker for GC.
Acknowledgements
This study was funded by Cumhuriyet University (grant no. CÜBAP T-547).
References
- 1.McBride OW, Merry D, Givol D. The gene for human p53 cellular tumor antigen is located on chromosome 17 short arm (17p13) Proc Natl Acad Sci USA. 1986;83:130–134. doi: 10.1073/pnas.83.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strachan T, Read AP. Human molecular genetics. 2nd. Wiley-Liss; New York, NY: 1999. [Google Scholar]
- 3.Nakao M, Matsuo K, Ito H, Shitara K, Hosono S, Watanabe M, Ito S, Sawaki A, Iida S, Sato S, et al. ABO genotype and the risk of gastric cancer, atrophic gastritis, and Helicobacter pylori infection. Cancer Epidemiol Biomarkers Prev. 2011;20:1665–1672. doi: 10.1158/1055-9965.EPI-11-0213. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 5.Marte B. Cancer: Super p53. Nature. 2002;420:279. doi: 10.1038/420279a. [DOI] [PubMed] [Google Scholar]
- 6.Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R, Jacks T. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 7.Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Misra C, Majumder M, Bajaj S, Ghosh S, Roy B, Roychoudhury S. Polymorphisms at p53, p73, and MDM2 loci modulate the risk of tobacco associated leukoplakia and oral cancer. Mol Carcinog. 2009;48:790–800. doi: 10.1002/mc.20523. [DOI] [PubMed] [Google Scholar]
- 9.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 10.Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420:25–27. doi: 10.1016/S0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- 11.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 12.Momand J, Zambetti GP. Mdm-2: ‘big brother’ of p53. J Cell Biochem. 1997;64:343–352. doi: 10.1002/(SICI)1097-4644(19970301)64:3<343::AID-JCB1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 13.Bond GL, Hu W, Bond EE, Robins H, Lutzker SG, Arva NC, Bargonetti J, Bartel F, Taubert H, Wuerl P, et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell. 2004;119:591–602. doi: 10.1016/j.cell.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 14.Menin C, Scaini MC, De Salvo GL, Biscuola M, Quaggio M, Esposito G, Belluco C, Montagna M, Agata S, D'Andrea E, et al. Association between MDM2-SNP309 and age at colorectal cancer diagnosis according to p53 mutation status. J Natl Cancer Inst. 2006;98:285–288. doi: 10.1093/jnci/djj054. [DOI] [PubMed] [Google Scholar]
- 15.Ohmiya N, Taguchi A, Mabuchi N, Itoh A, Hirooka Y, Niwa Y, Goto H. MDM2 promoter polymorphism is associated with both an increased susceptibility to gastric carcinoma and poor prognosis. J Clin Oncol. 2006;24:4434–4440. doi: 10.1200/JCO.2005.04.1459. [DOI] [PubMed] [Google Scholar]
- 16.Yoon YJ, Chang HY, Ahn SH, Kim JK, Park YK, Kang DR, Park JY, Myoung SM, Kim DY, Chon CY, Han KH. MDM2 and p53 polymorphisms are associated with the development of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Carcinogenesis. 2008;29:1192–1196. doi: 10.1093/carcin/bgn090. [DOI] [PubMed] [Google Scholar]
- 17.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yilmaz M, Tas A, Kacan T, Sari M, Silig Y. Is there a relation between murine double minute 2 T309G polymorphism and lung cancer risk in the Turkish population? Turk J Biochem. 2017;42:123–129. [Google Scholar]
- 19.Crew KD, Neugut AI. Epidemiology of upper gastrointestinal malignancies. Semin Oncol. 2004;31:450–464. doi: 10.1053/j.seminoncol.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Geng JS, Song HT, Wang WR. Diversity of invasiveness and matrix metalloproteinases expression profile of human gastric carcinoma xenografted in different tissue environments. Zhonghua Bing Li Xue Za Zhi. 2004;33:53–56. (In Chinese) [PubMed] [Google Scholar]
- 21.Brady M, Vlatkovic N, Boyd MT. Regulation of p53 and MDM2 activity by MTBP. Mol Cell Biol. 2005;25:545–553. doi: 10.1128/MCB.25.2.545-553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freedman DA, Wu L, Levine AJ. Functions of the MDM2 oncoprotein. Cell Mol Life Sci. 1999;55:96–107. doi: 10.1007/s000180050273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirata H, Hinoda Y, Kikuno N, Kawamoto K, Suehiro Y, Tanaka Y, Dahiya R. MDM2 SNP309 polymorphism as risk factor for susceptibility and poor prognosis in renal cell carcinoma. Clin Cancer Res. 2007;13:4123–4129. doi: 10.1158/1078-0432.CCR-07-0609. [DOI] [PubMed] [Google Scholar]
- 24.Terry K, McGrath M, Lee IM, Buring J, De Vivo I. MDM2 SNP309 is associated with endometrial cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17:983–986. doi: 10.1158/1055-9965.EPI-07-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsson H, Hultman P, Rosell J, Söderkvist P, Jahnson S. MDM2 SNP309 promoter polymorphism and p53 mutations in urinary bladder carcinoma stage T1. BMC Urol. 2013;13:5. doi: 10.1186/1471-2490-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang GX, Li YQ, Pan XL. Polymorphism of MDM2 promoter, regulated by Helicobacter pylori lipopolysaccharide, is associated with both an increased susceptibility to gastric carcinoma and poor prognosis in Chinese patients. J Gastroenterol Hepatol. 2011;26:280. [Google Scholar]
- 27.Wang X, Yang J, Ho B, Yang Y, Huang Z, Zhang Z, Zhang G. Interaction of Helicobacter pylori with genetic variants in the MDM2 promoter, is associated with gastric cancer susceptibility in Chinese patients. Helicobacter. 2009;14:114–119. doi: 10.1111/j.1523-5378.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- 28.Cho YG, Choi BJ, Song JH, Kim CJ, Cao Z, Nam SW, Lee JY, Park WS. No association of MDM2 T309G polymorphism with susceptibility to Korean gastric cancer patients. Neoplasma. 2008;55:256–260. [PubMed] [Google Scholar]
- 29.Pan X, Li Y, Feng J, Wang X, Hao B, Shi R, Zhang G. A functional polymorphism T309G in MDM2 gene promoter, intensified by Helicobacter pylori lipopolysaccharide, is associated with both an increased susceptibility and poor prognosis of gastric carcinoma in Chinese patients. BMC Cancer. 2013;13:126. doi: 10.1186/1471-2407-13-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen W, Wu Q, Ren H. Meta-analysis of associations between MDM2 SNP309 polymorphism and gastric cancer risk. Biomed Rep. 2014;2:105–111. doi: 10.3892/br.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Er LM, Zhang LW, Niu WW, Xu ZB, Du CQ, Gao Y, Wang SP, Yuan L. Relation of MDM2 gene polymorphism and Helicobacter pylori infection to gastric cardiac carcinoma. Lin Chuang Hui Cui. 2009;24:1594–1597. (In Chinese) [Google Scholar]
- 32.Tian X, Tian Y, Ma P, Sui C-G, Meng F-D, Li Y, Fu L-Y, Jiang T, Wang Y, Ji FJ, et al. Association between MDM2 SNP309 T>G and risk of gastric cancer: A meta-analysis. Asian Pac J Cancer Prev. 2013;14:1925–1929. doi: 10.7314/APJCP.2013.14.3.1925. [DOI] [PubMed] [Google Scholar]
- 33.Yang M, Guo Y, Zhang X, Miao X, Tan W, Sun T, Zhao D, Yu D, Liu J, Lin D. Interaction of P53 Arg72Pro and MDM2 T309G polymorphisms and their associations with risk of gastric cardia cancer. Carcinogenesis. 2007;28:1996–2001. doi: 10.1093/carcin/bgm168. [DOI] [PubMed] [Google Scholar]


